Immune Checkpoint Inhibitor Therapy for Prostate Cancer: Present and Future Prospectives
Abstract
1. Introduction
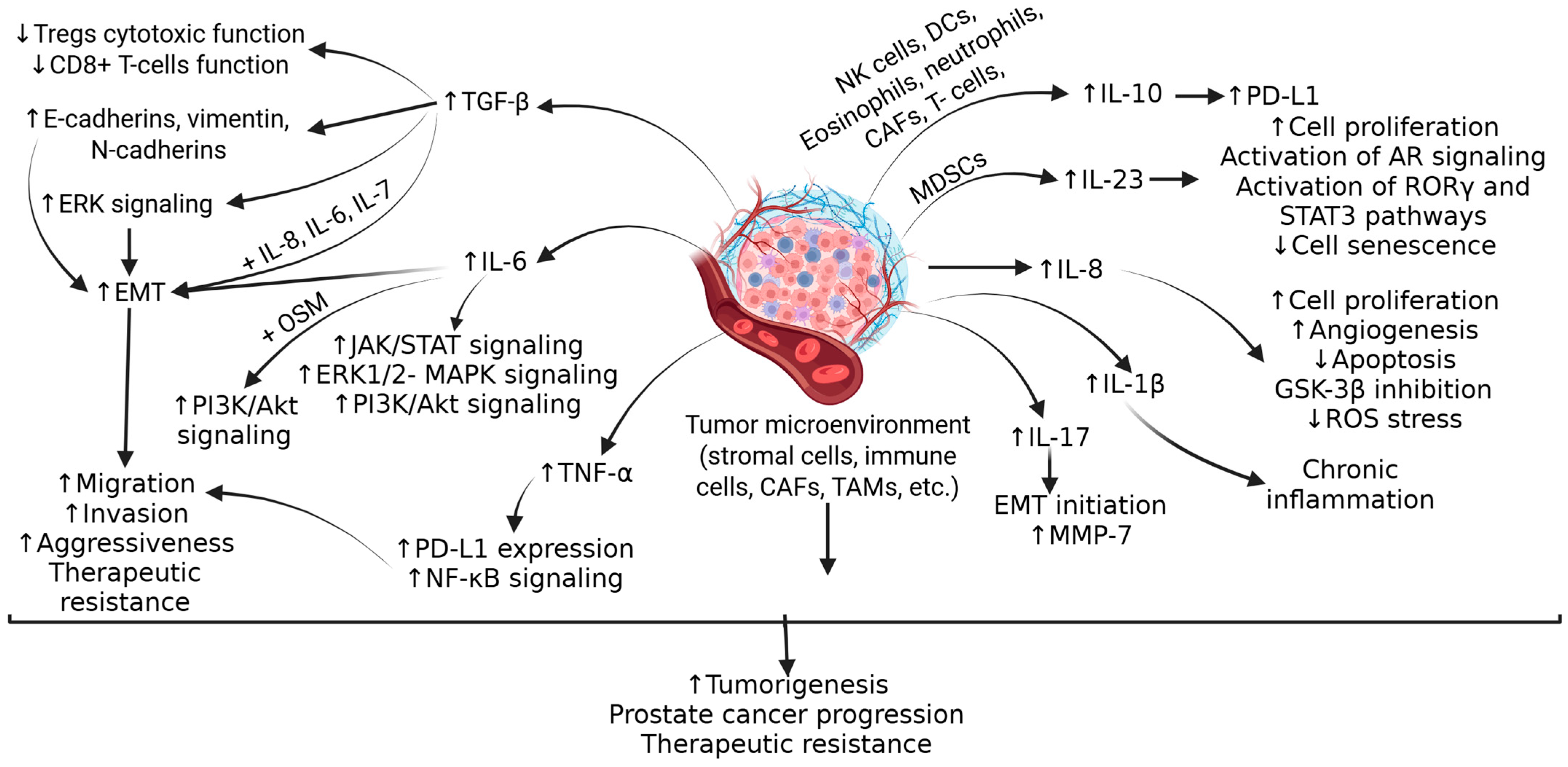
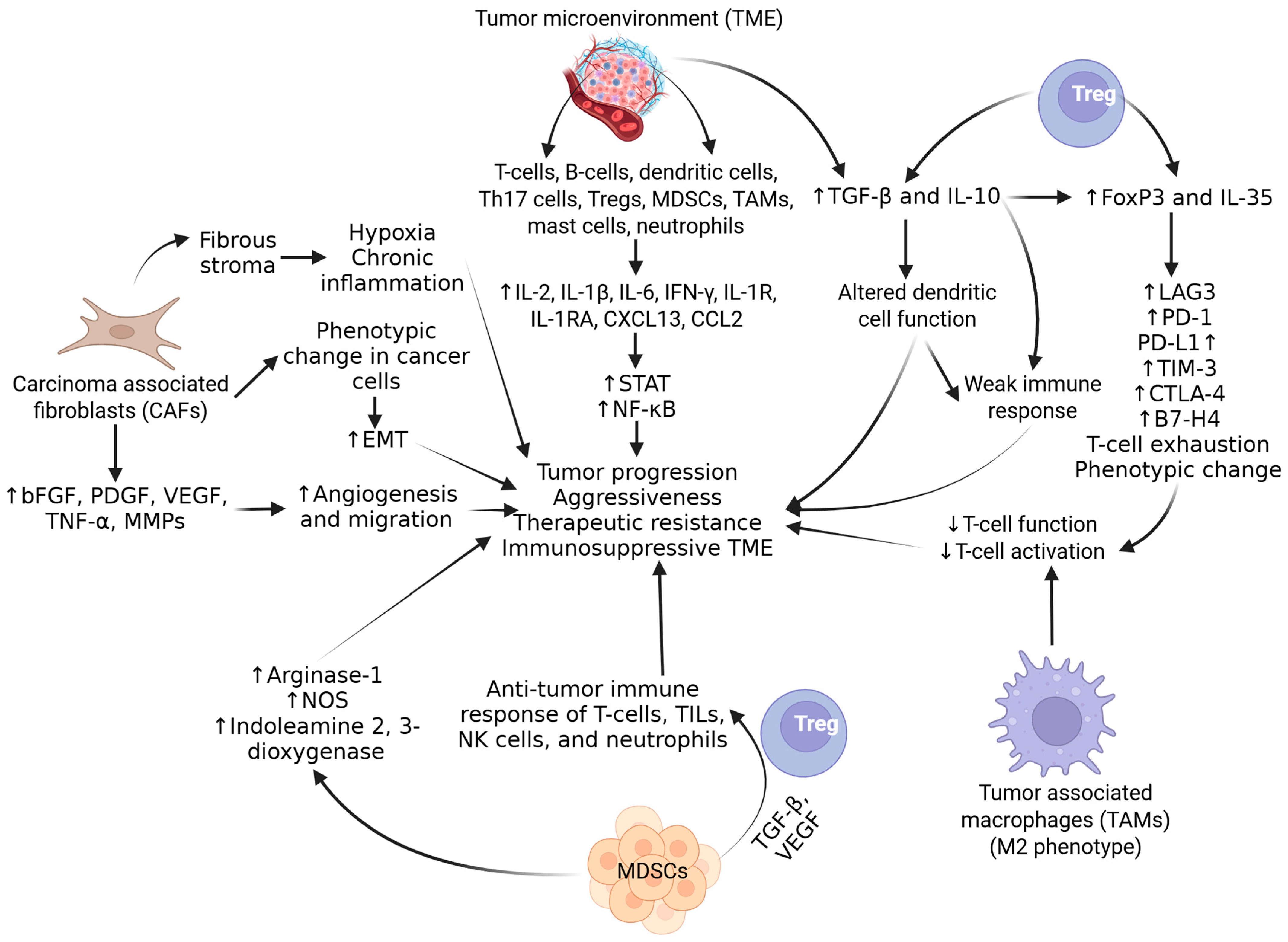
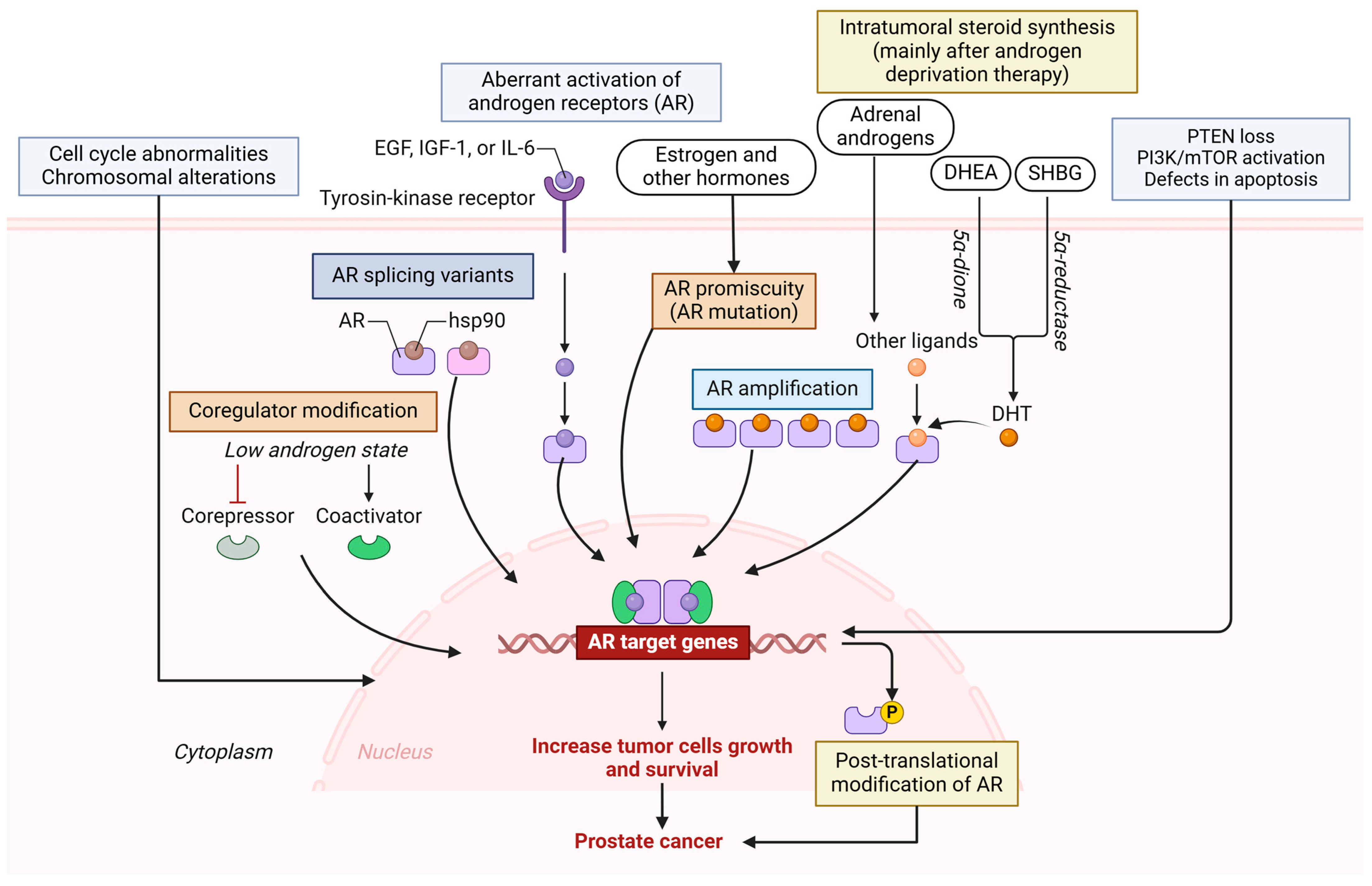
2. Molecular Mechanisms of PCa Progression and Immunosuppression
3. Immunotherapy for PCa
3.1. Cytotoxic T Lymphocyte-Associated Protein 4
3.2. Programmed Death 1
3.3. Anti-Tumor Vaccines
4. Combination Therapies
5. Therapies for Neuroendocrine Prostate Cancer and Castration-Resistant Prostate Cancer
6. Limitations of Immunotherapy
7. Future Prospectives
8. Completed and Ongoing Clinical Trials for PCa Therapy
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Androgen receptor |
| CDK12 | Cyclin-dependent kinase 12 |
| CAR-T cells | Chimeric antigen receptor -T cell |
| CAFs | Cancer-associated fibroblasts |
| HDAC | Histone deacetylases |
| HMTs | Histone methyltransferase |
| HDMs | Histone demethylase |
| ICIs | Immune checkpoint inhibitors |
| mTOR | Mammalian target of rapamycin |
| mCRPC | Metastatic castration-resistant prostate cancer |
| MHC | Major histocompatibility complex |
| MDSCs | Myeloid-derived suppressor cells |
| PI3K | Phosphoinositide 3-kinase |
| PTEN | Phosphatase and tensin homolog |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death ligand 1 |
| PSA | Prostate specific antigen |
| STAT3 | Signal transducer and activator of transcription 3 |
| TMB | Tumor mutational burden |
| TAA | Tumor-associated antigens |
| TILs | Tumor-infiltrating lymphocytes |
| TAMs | Tumor-associated macrophages |
References
- Lowrance, W.; Dreicer, R.; Jarrard, D.F.; Scarpato, K.R.; Kim, S.K.; Kirkby, E.; Buckley, D.I.; Griffin, J.C.; Cookson, M.S. Updates to Advanced Prostate Cancer: AUA/SUO Guideline (2023). J. Urol. 2023, 209, 1082–1090. [Google Scholar] [CrossRef]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Gann, P.H. Risk factors for prostate cancer. Rev. Urol. 2002, 4 (Suppl. 5), S3–S10. [Google Scholar]
- Cicione, A.; Brassetti, A.; Lombardo, R.; Franco, A.; Turchi, B.; D’Annunzio, S.; Nacchia, A.; Tubaro, A.; Simone, G.; De Nunzio, C. Metabolic Syndrome and Physical Inactivity May Be Shared Etiological Agents of Prostate Cancer and Coronary Heart Diseases. Cancers 2022, 14, 936. [Google Scholar] [CrossRef]
- Dunn, M.W.; Kazer, M.W. Prostate cancer overview. Semin. Oncol. Nurs. 2011, 27, 241–250. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.-J. The diagnosis and treatment of prostate cancer: A review. Jama 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Board, P.A.T.E. Prostate cancer treatment (PDQ®). In PDQ Cancer Information Summaries [Internet]; National Cancer Institute (US): Bethesda, MD, USA, 2024. [Google Scholar]
- Wang, F.; Li, Z.; Feng, X.; Yang, D.; Lin, M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 11–26. [Google Scholar] [CrossRef]
- Cicione, A.; Nacchia, A.; Guercio, A.; Gravina, C.; Franco, A.; Grimaldi, M.C.; Tema, G.; Lombardo, R.; Tubaro, A.; De Nunzio, C. Cardiovascular adverse events-related to GnRH agonists and GnRH antagonists: Analysis of real-life data from Eudra-Vigilance and Food and Drug Administration databases entries. Prostate Cancer Prostatic Dis. 2023, 26, 765–771. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Ratta, R.; Matsubara, N.; Korbenfeld, E.; Gafanov, R.; Mourey, L.; Todenhofer, T.; Gurney, H.; Kramer, G.; Bergman, A.M.; et al. Pembrolizumab Plus Docetaxel Versus Docetaxel for Previously Treated Metastatic Castration-Resistant Prostate Cancer: The Randomized, Double-Blind, Phase III KEYNOTE-921 Trial. J. Clin. Oncol. 2025, 43, JCO2401283. [Google Scholar] [CrossRef]
- Onishi, K.; Nakai, Y.; Tachibana, A.; Nishimura, N.; Maesaka, F.; Tomizawa, M.; Morizawa, Y.; Hori, S.; Gotoh, D.; Miyake, M.; et al. Testosterone Recovery and Quality of Life of Japanese Patients After Short-Term Neoadjuvant Androgen Deprivation Therapy With Low-Dose-Rate Brachytherapy for Prostate Cancer. Int. J. Urol. 2025. [Google Scholar] [CrossRef]
- Strasner, A.; Karin, M. Immune Infiltration and Prostate Cancer. Front. Oncol. 2015, 5, 128. [Google Scholar] [CrossRef]
- Palano, M.T.; Gallazzi, M.; Cucchiara, M.; Deho, F.; Capogrosso, P.; Bruno, A.; Mortara, L. The tumor innate immune microenvironment in prostate cancer: An overview of soluble factors and cellular effectors. Explor. Target. Anti-Tumor Ther. 2022, 3, 694–718. [Google Scholar] [CrossRef]
- Harder, J.L.; Linden, P.; Jahn, L.; Aslan, M.; Schmucker, V. [Cross-regional telemedicine services as a supplement to rural primary care: A mixed-methods analysis]. Z. Evidenz Fortbild. Qual. Gesundheitswesen 2022, 169, 67–74. [Google Scholar] [CrossRef]
- Kaminski, A.; Hahne, J.C.; Haddouti, E.M.; Florin, A.; Wellmann, A.; Wernert, N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int. J. Mol. Med. 2006, 18, 941–950. [Google Scholar] [CrossRef]
- Stultz, J.; Fong, L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 697–717. [Google Scholar] [CrossRef]
- Lin, D.; Wang, X.; Choi, S.Y.C.; Ci, X.; Dong, X.; Wang, Y. Immune phenotypes of prostate cancer cells: Evidence of epithelial immune cell-like transition? Asian J. Urol. 2016, 3, 195–202. [Google Scholar] [CrossRef]
- Karpisheh, V.; Mousavi, S.M.; Naghavi Sheykholeslami, P.; Fathi, M.; Mohammadpour Saray, M.; Aghebati-Maleki, L.; Jafari, R.; Majidi Zolbanin, N.; Jadidi-Niaragh, F. The role of regulatory T cells in the pathogenesis and treatment of prostate cancer. Life Sci. 2021, 284, 119132. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Cellular and Molecular Mechanisms Underlying Prostate Cancer Development: Therapeutic Implications. Medicines 2019, 6, 82. [Google Scholar] [CrossRef]
- Maekawa, S.; Takata, R.; Obara, W. Molecular Mechanisms of Prostate Cancer Development in the Precision Medicine Era: A Comprehensive Review. Cancers 2024, 16, 523. [Google Scholar] [CrossRef]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular mechanisms and genetic alterations in prostate cancer: From diagnosis to targeted therapy. Cancer Lett. 2022, 534, 215619. [Google Scholar] [CrossRef]
- Shtivelman, E.; Beer, T.M.; Evans, C.P. Molecular pathways and targets in prostate cancer. Oncotarget 2014, 5, 7217–7259. [Google Scholar] [CrossRef]
- Perdomo, H.A.G.; Zapata-Copete, J.A.; Sanchez, A. Molecular alterations associated with prostate cancer. Cent. Eur. J. Urol. 2018, 71, 168–176. [Google Scholar] [CrossRef]
- Paltoglou, S.; Das, R.; Townley, S.L.; Hickey, T.E.; Tarulli, G.A.; Coutinho, I.; Fernandes, R.; Hanson, A.R.; Denis, I.; Carroll, J.S.; et al. Novel Androgen Receptor Coregulator GRHL2 Exerts Both Oncogenic and Antimetastatic Functions in Prostate Cancer. Cancer Res. 2017, 77, 3417–3430. [Google Scholar] [CrossRef]
- Ying, Y.; Wu, Y.; Zhang, F.; Tang, Y.; Yi, J.; Ma, X.; Li, J.; Chen, D.; Wang, X.; Liu, X.; et al. Co-transcriptional R-loops-mediated epigenetic regulation drives growth retardation and docetaxel chemosensitivity enhancement in advanced prostate cancer. Mol. Cancer 2024, 23, 79. [Google Scholar] [CrossRef]
- Cai, C.; Chen, S.; Ng, P.; Bubley, G.J.; Nelson, P.S.; Mostaghel, E.A.; Marck, B.; Matsumoto, A.M.; Simon, N.I.; Wang, H.; et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011, 71, 6503–6513. [Google Scholar] [CrossRef]
- Monge, A.; Jagla, M.; Lapouge, G.; Sasorith, S.; Cruchant, M.; Wurtz, J.M.; Jacqmin, D.; Bergerat, J.P.; Ceraline, J. Unfaithfulness and promiscuity of a mutant androgen receptor in a hormone-refractory prostate cancer. Cell. Mol. Life Sci. 2006, 63, 487–497. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Shiota, M.; Akamatsu, S.; Tsukahara, S.; Nagakawa, S.; Matsumoto, T.; Eto, M. Androgen receptor mutations for precision medicine in prostate cancer. Endocr. Relat. Cancer 2022, 29, R143–R155. [Google Scholar] [CrossRef]
- Schulz, W.A.; Burchardt, M.; Cronauer, M.V. Molecular biology of prostate cancer. Mol. Hum. Reprod. 2003, 9, 437–448. [Google Scholar] [CrossRef][Green Version]
- Fordyce, C.A.; Heaphy, C.M.; Joste, N.E.; Smith, A.Y.; Hunt, W.C.; Griffith, J.K. Association between cancer-free survival and telomere DNA content in prostate tumors. J. Urol. 2005, 173, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, C.M.; Yoon, G.S.; Peskoe, S.B.; Joshu, C.E.; Lee, T.K.; Giovannucci, E.; Mucci, L.A.; Kenfield, S.A.; Stampfer, M.J.; Hicks, J.L.; et al. Prostate cancer cell telomere length variability and stromal cell telomere length as prognostic markers for metastasis and death. Cancer Discov. 2013, 3, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, C.; Bastian, P.J.; Bjartell, A.; Carbone, G.M.; Catto, J.W.; Clark, S.J.; Henrique, R.; Nelson, W.G.; Shariat, S.F. Epigenetics in prostate cancer: Biologic and clinical relevance. Eur. Urol. 2011, 60, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Ragavi, R.; Muthukumaran, P.; Nandagopal, S.; Ahirwar, D.K.; Tomo, S.; Misra, S.; Guerriero, G.; Shukla, K.K. Epigenetics regulation of prostate cancer: Biomarker and therapeutic potential. Urol. Oncol. 2023, 41, 340–353. [Google Scholar] [CrossRef]
- Bilusic, M.; Madan, R.A.; Gulley, J.L. Immunotherapy of Prostate Cancer: Facts and Hopes. Clin. Cancer Res. 2017, 23, 6764–6770. [Google Scholar] [CrossRef]
- Markowski, M.C.; Shenderov, E.; Eisenberger, M.A.; Kachhap, S.; Pardoll, D.M.; Denmeade, S.R.; Antonarakis, E.S. Extreme responses to immune checkpoint blockade following bipolar androgen therapy and enzalutamide in patients with metastatic castration resistant prostate cancer. Prostate 2020, 80, 407–411. [Google Scholar] [CrossRef]
- Venkatachalam, S.; McFarland, T.R.; Agarwal, N.; Swami, U. Immune Checkpoint Inhibitors in Prostate Cancer. Cancers 2021, 13, 2187. [Google Scholar] [CrossRef]
- Adamaki, M.; Zoumpourlis, V. Immunotherapy as a Precision Medicine Tool for the Treatment of Prostate Cancer. Cancers 2021, 13, 173. [Google Scholar] [CrossRef]
- Wang, I.; Song, L.; Wang, B.Y.; Rezazadeh Kalebasty, A.; Uchio, E.; Zi, X. Prostate cancer immunotherapy: A review of recent advancements with novel treatment methods and efficacy. Am. J. Clin. Exp. Urol. 2022, 10, 210–233. [Google Scholar]
- Patel, D.; McKay, R.; Parsons, J.K. Immunotherapy for Localized Prostate Cancer: The Next Frontier? Urol. Clin. N. Am. 2020, 47, 443–456. [Google Scholar] [CrossRef]
- Fellner, C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. Pharm. Ther. 2012, 37, 503–530. [Google Scholar]
- Jeong, S.H.; Kwak, C. Immunotherapy for prostate cancer: Requirements for a successful regime transfer. Investig. Clin. Urol. 2022, 63, 3–13. [Google Scholar] [CrossRef]
- Witt, K.; Evans-Axelsson, S.; Lundqvist, A.; Johansson, M.; Bjartell, A.; Hellsten, R. Inhibition of STAT3 augments antitumor efficacy of anti-CTLA-4 treatment against prostate cancer. Cancer Immunol. Immunother. 2021, 70, 3155–3166. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Antonarakis, E.S.; Piulats, J.M.; Gross-Goupil, M.; Goh, J.; Ojamaa, K.; Hoimes, C.J.; Vaishampayan, U.; Berger, R.; Sezer, A.; Alanko, T.; et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J. Clin. Oncol. 2020, 38, 395–405. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Loriot, Y.; Shaffer, D.R.; Braiteh, F.; Powderly, J.; Harshman, L.C.; Conkling, P.; Delord, J.P.; Gordon, M.; Kim, J.W.; et al. Safety and Clinical Activity of Atezolizumab in Patients with Metastatic Castration-Resistant Prostate Cancer: A Phase I Study. Clin. Cancer Res. 2021, 27, 3360–3369. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Gillessen, S.; Rathkopf, D.; Matsubara, N.; Drake, C.; Fizazi, K.; Piulats, J.M.; Wysocki, P.J.; Buchschacher, G.L., Jr.; Doss, J. IMbassador250: A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). Cancer Res. 2020, 80, TPS5090. [Google Scholar] [CrossRef]
- Yu*, E.Y.; Fong, P.; Piulats, J.M.; Appleman, L.; Conter, H.; Feyerabend, S.; Shore, N.; Gravis, G.; Laguerre, B.; Gurney, H. PD16-12 pembrolizumab plus enzalutamide in abiraterone-pretreated patients with metastatic castration-resistant prostate cancer: Updated results from KEYNOTE-365 cohort C. J. Urol. 2020, 203, e368. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Graff, J.N.; Tagawa, S.T.; Hwang, C.; Kilari, D.; Ten Tije, A.; Omlin, A.; McDermott, R.S.; Vaishampayan, U.N.; Elliott, T. KEYNOTE-199 cohorts (C) 4 and 5: Phase II study of pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 5543. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M. Peptide-based vaccine for cancer therapies. Front. Immunol. 2023, 14, 1210044. [Google Scholar] [CrossRef]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef]
- Sasada, T.; Noguchi, M.; Yamada, A.; Itoh, K. Personalized peptide vaccination: A novel immunotherapeutic approach for advanced cancer. Hum. Vaccines Immunother. 2012, 8, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Lanka, S.M.; Zorko, N.A.; Antonarakis, E.S.; Barata, P.C. Metastatic Castration-Resistant Prostate Cancer, Immune Checkpoint Inhibitors, and Beyond. Curr. Oncol. 2023, 30, 4246–4256. [Google Scholar] [CrossRef] [PubMed]
- Karzai, F.; VanderWeele, D.; Madan, R.A.; Owens, H.; Cordes, L.M.; Hankin, A.; Couvillon, A.; Nichols, E.; Bilusic, M.; Beshiri, M.L.; et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 2018, 6, 141. [Google Scholar] [CrossRef]
- Yu, E.Y.; Piulats, J.M.; Gravis, G.; Laguerre, B.; Arranz Arija, J.A.; Oudard, S.; Fong, P.C.; Kolinsky, M.P.; Augustin, M.; Feyerabend, S. KEYNOTE-365 cohort A updated results: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 100. [Google Scholar] [CrossRef]
- Kolinsky, M.P.; Gravis, G.; Mourey, L.; Piulats, J.M.; Sridhar, S.S.; Romano, E.; Berry, W.R.; Gurney, H.; Retz, M.; Appleman, L.J. KEYNOTE-365 cohort B updated results: Pembrolizumab (pembro) plus docetaxel and prednisone in abiraterone (abi) or enzalutamide (enza)-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38, 103. [Google Scholar] [CrossRef]
- Morris, M.J.; Fong, L.; Petrylak, D.P.; Sartor, A.O.; Higano, C.S.; Pagliaro, L.C.; Alva, A.S.; Appleman, L.J.; Tan, W.; Vaishampayan, U.N. Safety and clinical activity of atezolizumab (atezo)+ radium-223 dichloride (r-223) in 2L metastatic castration-resistant prostate cancer (mCRPC): Results from a phase Ib clinical trial. J. Clin. Oncol. 2020, 38, 5565. [Google Scholar] [CrossRef]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013, 24, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Drake, C.G.; Beer, T.M.; Kwon, E.D.; Scher, H.I.; Gerritsen, W.R.; Bossi, A.; den Eertwegh, A.; Krainer, M.; Houede, N.; et al. Final Analysis of the Ipilimumab Versus Placebo Following Radiotherapy Phase III Trial in Postdocetaxel Metastatic Castration-resistant Prostate Cancer Identifies an Excess of Long-term Survivors. Eur. Urol. 2020, 78, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Hurley, P.J.; Tran, P.T.; Rowe, S.P.; Benzon, B.; Neal, T.O.; Chapman, C.; Harb, R.; Milman, Y.; Trock, B.J.; et al. A pilot trial of pembrolizumab plus prostatic cryotherapy for men with newly diagnosed oligometastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis. 2020, 23, 184–193. [Google Scholar] [CrossRef]
- Rosser, C.J.; Hirasawa, Y.; Acoba, J.D.; Tamura, D.J.; Pal, S.K.; Huang, J.; Scholz, M.C.; Dorff, T.B. Phase Ib study assessing different sequencing regimens of atezolizumab (anti-PD-L1) and sipuleucel-T (SipT) in patients who have asymptomatic or minimally symptomatic metastatic castrate resistant prostate cancer. J. Clin. Oncol. 2020, 38, e17564. [Google Scholar] [CrossRef]
- Tuthill, M.; Cappuccini, F.; Carter, L.; Pollock, E.; Poulton, I.; Verrill, C.; Evans, T.; Gillessen, S.; Attard, G.; Protheroe, A. 682P Results from ADVANCE: A phase I/II open-label non-randomised safety and efficacy study of the viral vectored ChAdOx1-MVA 5T4 (VTP-800) vaccine in combination with PD-1 checkpoint blockade in metastatic prostate cancer. Ann. Oncol. 2020, 31, S543. [Google Scholar] [CrossRef]
- Madan, R.A.; Mohebtash, M.; Arlen, P.M.; Vergati, M.; Rauckhorst, M.; Steinberg, S.M.; Tsang, K.Y.; Poole, D.J.; Parnes, H.L.; Wright, J.J.; et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012, 13, 501–508. [Google Scholar] [CrossRef]
- Boudadi, K.; Suzman, D.L.; Anagnostou, V.; Fu, W.; Luber, B.; Wang, H.; Niknafs, N.; White, J.R.; Silberstein, J.L.; Sullivan, R.; et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 2018, 9, 28561–28571. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef]
- Hotte, S.; Winquist, E.; Chi, K.; Ellard, S.; Sridhar, S.; Emmenegger, U.; Salim, M.; Iqbal, N.; Canil, C.; Kollmannsberger, C. CCTG IND 232: A phase II study of durvalumab with or without tremelimumab in patients with metastatic castration resistant prostate cancer (mCRPC). Ann. Oncol. 2019, 30, v885. [Google Scholar] [CrossRef]
- Subudhi, S.K.; Siddiqui, B.A.; Aparicio, A.M.; Yadav, S.S.; Basu, S.; Chen, H.; Jindal, S.; Tidwell, R.S.S.; Varma, A.; Logothetis, C.J.; et al. Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naive metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J. Immunother. Cancer 2021, 9, e002919. [Google Scholar] [CrossRef]
- Agarwal, N.; Loriot, Y.; McGregor, B.A.; Dreicer, R.; Dorff, T.B.; Maughan, B.L.; Kelly, W.K.; Pagliaro, L.C.; Srinivas, S.; Squillante, C.M. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: Results of cohort 6 of the COSMIC-021 study. J. Clin. Oncol. 2020, 38, 5564. [Google Scholar] [CrossRef]
- Fei, X.; Xue, J.W.; Wu, J.Z.; Yang, C.Y.; Wang, K.J.; Ma, Q. Promising therapy for neuroendocrine prostate cancer: Current status and future directions. Ther. Adv. Med. Oncol. 2024, 16, 17588359241269676. [Google Scholar] [CrossRef] [PubMed]
- Kulakowska, W.; Taylor, R.; Ranasinghe, W.; Darcy, P.K.; Trapani, J.; Risbridger, G. Lewis Y antigen as a novel target for CAR (chimeric antigen receptor) T-cell therapy in patients with neuroendocrine prostate cancer. J. Clin. Oncol. 2025, 43, 217. [Google Scholar] [CrossRef]
- Wee, C.E.; Costello, B.A.; Orme, J.J.; Quevedo, J.F.; Pagliaro, L.C. Chemotherapy with atezolizumab for small cell or neuroendocrine carcinoma of the prostate: A single institution experience. Prostate 2021, 81, 938–943. [Google Scholar] [CrossRef]
- Salhab, M.; Donahue, M.; Walsh, W. Pembrolizumab for platinum refractory small cell carcinoma of the prostate: Case report. Hematol. Med. Oncol. 2018, 3, 1–3. [Google Scholar]
- Puca, L.; Gavyert, K.; Sailer, V.; Conteduca, V.; Dardenne, E.; Sigouros, M.; Isse, K.; Kearney, M.; Vosoughi, A.; Fernandez, L.; et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl. Med. 2019, 11, eaav0891. [Google Scholar] [CrossRef]
- Haque Chowdhury, H.; Hawlina, S.; Gabrijel, M.; Trkov Bobnar, S.; Kreft, M.; Lenart, G.; Cukjati, M.; Kopitar, A.N.; Kejzar, N.; Ihan, A.; et al. Survival of castration-resistant prostate cancer patients treated with dendritic-tumor cell hybridomas is negatively correlated with changes in peripheral blood CD56(bright) CD16(-) natural killer cells. Clin. Transl. Med. 2021, 11, e505. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, G.B.; Lucien-Matteoni, F.; Chaudhuri, A.A.; Orme, J.J.; Childs, D.S.; Rincon, M.M.; Li, G.G.; Chauhan, P.S.; Lee, S.; Gupta, S. Current and future directions in theranostics for neuroendocrine prostate cancer. Cancer Treat. Rev. 2025, 136, 102941. [Google Scholar] [CrossRef]
- Bernal, A.; Bechler, A.J.; Mohan, K.; Rizzino, A.; Mathew, G. The Current Therapeutic Landscape for Metastatic Prostate Cancer. Pharmaceuticals 2024, 17, 351. [Google Scholar] [CrossRef]
- Chen, J.; Ma, N.; Chen, B.; Huang, Y.; Li, J.; Li, J.; Chen, Z.; Wang, P.; Ran, B.; Yang, J. Synergistic effects of immunotherapy and adjunctive therapies in prostate cancer management. Crit. Rev. Oncol./Hematol. 2024, 207, 104604. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.F.; Lawrence, M.S.; Demichelis, F.; Drier, Y.; Cibulskis, K.; Sivachenko, A.Y.; Sboner, A.; Esgueva, R.; Pflueger, D.; Sougnez, C.; et al. The genomic complexity of primary human prostate cancer. Nature 2011, 470, 214–220. [Google Scholar] [CrossRef]
- Hua, L.; Xia, H.; Zheng, W. The Association between Immune Infiltration and Clinical Phenotypes and Prognosis of Prostate Cancer. Iran. J. Biotechnol. 2020, 18, e2538. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, T.; Ma, L. Correlation analysis between immune-related genes and cell infiltration revealed prostate cancer immunotherapy biomarkers linked to T cells gamma delta. Sci. Rep. 2023, 13, 2459. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Ai, M.; Liu, A.; Budhani, P.; Bartkowiak, T.; Sheng, J.; Ager, C.; Nicholas, C.; Jaiswal, A.R.; Sun, Y.; et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J. Clin. Investig. 2018, 128, 5137–5149. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.; Gatenbee, C.; Robertson-Tessi, M.; Bravo, R.; Dhillon, J.; Balagurunathan, Y.; Berglund, A.; Vishvakarma, N.; Ibrahim-Hashim, A.; Choi, J.; et al. Acidity promotes tumour progression by altering macrophage phenotype in prostate cancer. Br. J. Cancer 2019, 121, 556–566. [Google Scholar] [CrossRef]
- Bishop, J.L.; Sio, A.; Angeles, A.; Roberts, M.E.; Azad, A.A.; Chi, K.N.; Zoubeidi, A. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 2015, 6, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ni, Y.; Liang, X.; Lin, Y.; An, B.; He, X.; Zhao, X. Mechanisms of tumor resistance to immune checkpoint blockade and combination strategies to overcome resistance. Front. Immunol. 2022, 13, 915094. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Cheteh, E.H.; Augsten, M.; Rundqvist, H.; Bianchi, J.; Sarne, V.; Egevad, L.; Bykov, V.J.; Ostman, A.; Wiman, K.G. Human cancer-associated fibroblasts enhance glutathione levels and antagonize drug-induced prostate cancer cell death. Cell Death Dis. 2017, 8, e2848. [Google Scholar] [CrossRef]
- Wang, T.Y.; Wang, L.; Alam, S.K.; Hoeppner, L.H.; Yang, R. ScanNeo: Identifying indel-derived neoantigens using RNA-Seq data. Bioinformatics 2019, 35, 4159–4161. [Google Scholar] [CrossRef] [PubMed]
- Maselli, F.M.; Giuliani, F.; Laface, C.; Perrone, M.; Melaccio, A.; De Santis, P.; Santoro, A.N.; Guarini, C.; Iaia, M.L.; Fedele, P. Immunotherapy in Prostate Cancer: State of Art and New Therapeutic Perspectives. Curr. Oncol. 2023, 30, 5769–5794. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Qian, X.; Liu, J.; Xue, F.; Luo, J.; Yao, G.; Yan, J.; Liu, X.; Xiao, B.; Li, J. Radiotherapy plus immune checkpoint inhibitor in prostate cancer. Front. Oncol. 2023, 13, 1210673. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Lee, K.; Kim, I.Y. Current update on prostate cancer immunotherapy. Korean J. Urol. Oncol. 2023, 21, 14–22. [Google Scholar] [CrossRef]
- Lampe, H.; Tam, L.; Hansen, A.R. Bi-specific T-cell engagers (BiTEs) in prostate cancer and strategies to enhance development: Hope for a BiTE-r future. Front. Pharmacol. 2024, 15, 1399802. [Google Scholar] [CrossRef]
- Aggarwal, R.; Starzinski, S.; de Kouchkovsky, I.; Koshkin, V.; Bose, R.; Chou, J.; Desai, A.; Kwon, D.; Kaushal, S.; Trihy, L.; et al. Single-dose (177)Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: An open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023, 24, 1266–1276. [Google Scholar] [CrossRef]
- Grover, P.; Nunez-Cruz, S.; Leferovich, J.; Wentz, T.; Bagchi, A.; Milone, M.C.; Greene, M.I. F77 antigen is a promising target for adoptive T cell therapy of prostate cancer. Biochem. Biophys. Res. Commun. 2023, 680, 51–60. [Google Scholar] [CrossRef]
- Wei, Q.; Costanzi, S.; Balasubramanian, R.; Gao, Z.G.; Jacobson, K.A. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal 2013, 9, 271–280. [Google Scholar] [CrossRef]
- Sandhu, S.; Subramaniam, S.; Hofman, M.S.; Stockler, M.R.; Martin, A.J.; Pokorski, I.; Goh, J.C.; Pattison, D.A.; Dhiantravan, N.; Gedye, C. Evolution: Phase II study of radionuclide 177Lu-PSMA-617 therapy versus 177Lu-PSMA-617 in combination with ipilimumab and nivolumab for men with metastatic castration-resistant prostate cancer (mCRPC; ANZUP 2001). J. Clin. Oncol. 2023, 41, TPS271. [Google Scholar] [CrossRef]
- Huang, L.; Xie, Y.; Jiang, S.; Dai, T.; Xu, Z.; Shan, H. Insights into immune microenvironment and therapeutic targeting in androgen-associated prostate cancer subtypes. Sci. Rep. 2024, 14, 18036. [Google Scholar] [CrossRef]
- Meng, L.; Yang, Y.; Mortazavi, A.; Zhang, J. Emerging Immunotherapy Approaches for Treating Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 14347. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Lopez, J.I.; Nunes-Xavier, C.E. B7-H3: A robust target for immunotherapy in prostate cancer. Trends Cancer 2024, 10, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, M.; Wang, M.; Wang, H.; Wu, H.; Mao, L.; Zhang, M.; Li, H.; Zheng, J.; Ma, P.; et al. B7-H3 specific CAR-T cells exhibit potent activity against prostate cancer. Cell Death Discov. 2023, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Winter, J.N.; Giulino-Roth, L.; Longley, J.; Lopez, J.; Michot, J.M.; Leonard, J.P.; Ribrag, V.; McCabe, M.T.; Creasy, C.L.; et al. Phase I Study of the Novel Enhancer of Zeste Homolog 2 (EZH2) Inhibitor GSK2816126 in Patients with Advanced Hematologic and Solid Tumors. Clin. Cancer Res. 2019, 25, 7331–7339. [Google Scholar] [CrossRef]
- Deng, K.; Zou, Y.; Zou, C.; Wang, H.; Xiang, Y.; Yang, X.; Yang, S.; Cui, C.; Yang, G.; Huang, J. Study on pharmacokinetic interactions between SHR2554 and itraconazole in healthy subjects: A single-center, open-label phase I trial. Cancer Med. 2023, 12, 1431–1440. [Google Scholar] [CrossRef]
- Choudhury, A.D.; Kwak, L.; Cheung, A.; Allaire, K.M.; Marquez, J.; Yang, D.D.; Tripathi, A.; Kilar, J.M.; Flynn, M.; Maynard, B.; et al. Randomized Phase II Study Evaluating the Addition of Pembrolizumab to Radium-223 in Metastatic Castration-resistant Prostate Cancer. Cancer Immunol. Res. 2024, 12, 704–718. [Google Scholar] [CrossRef]
- Agarwal, N.; Azad, A.; Carles, J.; Matsubara, N.; Oudard, S.; Saad, F.; Merseburger, A.S.; Soares, A.; McGregor, B.A.; Zurawski, B. CONTACT-02: Phase 3 study of cabozantinib (C) plus atezolizumab (A) vs second novel hormonal therapy (NHT) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2024, 42, 18. [Google Scholar] [CrossRef]
- Fan, Z.; Oh, D.Y.; Wong, A.; Shinohara, K.; Nguyen, H.; Hwang, C.; Chang, H.; Starzinski, A.; Li, T.; De Leon, C. 862 TLR9 agonism promotes cytotoxic T cell persistence and myeloid remodeling when combined with radiation therapy and PD-1 blockade in oligometastatic prostate cancer patients. J. ImmunoTher. Cancer 2023, 11. [Google Scholar] [CrossRef]
- Linch, M.D.; Leone, G.; Wong, Y.N.S.; Jones, R.J.; Sankey, P.; Josephs, D.H.; Crabb, S.J.; Harris, L.; Tasnim, A.; Rashid, M. Nivolumab and ipilimumab for metastatic prostate cancer with an immunogenic signature: The NEPTUNES multi-centre two-cohort, biomarker-selected phase 2 trial. J. Clin. Oncol. 2024, 42, 5013. [Google Scholar] [CrossRef]
- Sharma, P.; Krainer, M.; Saad, F.; Castellano, D.; Bedke, J.; Kwiatkowski, M.; Patnaik, A.; Procopio, G.; Wiechno, P.; Kochuparambil, S.T. Nivolumab plus ipilimumab for the treatment of post-chemotherapy metastatic castration-resistant prostate cancer (mCRPC): Additional results from the randomized phase 2 CheckMate 650 trial. J. Clin. Oncol. 2023, 41, 22. [Google Scholar] [CrossRef]
- Tsimberidou, A.M.; Drakaki, A.; Khalil, D.N.; Okrah, K.; Schachter, M.; Gautam, S.; Da Silva, D.M.; Alayli, F.A.; Spencer, C.N.; Foo, W.C. Evaluating nivolumab (nivo) plus ipilimumab (ipi) in patients with metastatic castration-resistant prostate cancer (mCRPC): Clinical and translational results from the AMADEUS prostate expansion cohort. In Proceedings of the American Association for Cancer Research Annual Meeting 2024, San Diego, CA, USA, 5–10 April 2024. [Google Scholar]
- Kelly, W.; Danila, D.; Lin, C.; Lee, J.; Matsubara, N.; Ward, P.; Armstrong, A.; Pook, D.; Kim, M.; Dorff, T. 1765O Interim results from a phase I study of AMG 509 (xaluritamig), a STEAP1 x CD3 XmAb 2+1 immune therapy, in patients with metastatic castration-resistant prostate cancer (mCRPC). Ann. Oncol. 2023, 34, S953–S954. [Google Scholar] [CrossRef]


| Type of Vaccine | Source | Immune Response to | Against | Effects |
|---|---|---|---|---|
| Cell-based vaccines | ||||
| Sipuleucel-T (the only FDA-approved therapeutic cancer vaccine) | Autologous dendritic cell vaccine | Prostatic acid phosphatase (PAP) antigen | Asymptomatic or minimally symptomatic mCRPC patients with no visceral metastases | Significantly improved OS (up to 20 months) |
| DCVAC/PCa | Dendritic cell vaccine | Induction of immune response | mCRPC | Mixed results on OS |
| GVAX (irradiated whole tumor cells) | Tumor cell-based vaccine | Induce immune response to multiple TAA without the need for HLA-matching | mCRPC | Enhanced median survival |
| Vector-based vaccine | ||||
| PROSTVAC-VF (PSA-TRICOM) | Contain plasmid carrying transgenes coding for PSA and a triad of T cell co-stimulatory molecules LFA-3, B7.1, and ICAM-1 | Elicits a strong immune response against PSA and the viral protein | Localized and advanced PCa | Inconclusive results in terms of OS or progression-free survival Better results with combination therapy |
| Ad5-PSA | Derived from replication-deficient recombinant adenovirus type 5 | Substantial anti-PSA immune responses | mCRPC | Prolonged survival Prolonged metastasis-free survival |
| DNA/mRNA-Based Vaccines | ||||
| CV9103 | mRNA-based vaccine encodes for PSA, PSMA, PSCA, and STEAP1 | High level of cellular immunogenicity | CRPC | Safe and well-tolerated Prolonged survival |
| Study | # of Patients | Pre-Treatment/ Therapy Strategy | Outcome |
|---|---|---|---|
| Immune Checkpoint Blockade with PARP Inhibitors | |||
| Durvalumab plus olaparib trial [58] | 17 mCRPC patients | Progression in androgen receptor blockade therapy | Median rPFS: 16.1 months PSA decline of ≥50%: 53% radiographic response: 44.4% Patients with DDR gene mutations showed better progression-free survival than those with no mutation (83.3% vs. 36.4%) |
| Olaparib with pembrolizumab (KEYNOTE-365, cohort A) [59] | Molecularly unselected mCRPC patients | Docetaxel-pretreated | OS of 14 months PSA response rate of 9% ORR of 8% with two partial responses Median response duration: not reached |
| Immune Checkpoint Blockade with Chemotherapy | |||
| Chemotherapy plus pembrolizumab + docetaxel and prednisone [60] | 104 mCRPC patients | Pembrolizumab 200 mg IV + docetaxel 75 mg/m2 IV Q3W and prednisone 5 mg orally twice daily | ORR-18%; median DOR of 6.7 months; PSA response rate of 28% Radiological PFS was 8.3 months OS-20.4 months |
| Immune Checkpoint Blockade with Radiotherapy or Cryotherapy | |||
| Atezolizumab plus radium-223 [61] | 44 mCRPC | Treatment with one 2nd generation androgen pathway inhibitor | Failed to show clinical benefit ORR: 6.8% Median radiological PFS: 3 months Median OS: 16.3 months |
| Ipilimumab with or without radiotherapy [62] | 28 mCRPC patients were evaluated | ipilimumab alone-16 ipilimumab + radiotherapy: 34 | 1 patient—complete response 6 patients—stable disease 8/50 patient—>50% PSA decline |
| Ipilimumab against placebo following Radiotherapy CA184-043 (a phase III RCT) | 799 mCRPC (1:1 randomization) | Progressed on docetaxel therapy | Median OS: 11.2 months (both groups) [63] OS was 25.2% vs. 16.6% at 2 years and 7.9% vs. 2.7% at 5 years: Ipilimumab vs. placebo [64] Median OS: 22.7 vs. 15.8 months in Ipilimumab vs placebo [63] |
| Pembrolizumab + cryotherapy [65] | 12 newly diagnosed metastatic PC | Cryoablation with short-term androgen deprivation (8 months) and pembrolizumab (6 doses) | PFS was 14 months and PSA responses were 92%. Median systemic therapy-free survival: 17.5 months 42% of patients had PSA < 0.6 ng/ml |
| Immune Checkpoint Blockade with Tumor Vaccines | |||
| Atezolizumab + sipuleucel-T [66] | 37 asymptomatic or minimally symptomatic progressive mCRPC patients | Atezolizumab 1200 mg IV every 3 weeks for 2 doses SipT IV every 2 weeks | PFS: 8.2 months (atezolizumab followed by sipuleucel-T) and 5.8 months (sipuleucel-T followed by atezolizumab) Manageable safety profile |
| ChAdOx1-MVA 5T4 vaccine + nivolumab (ADVANCE trial) [67] | 23 mCRPC patients | Two cycles of ChAdOx1-MVA 5T4 (VTP-800) vaccination and three nivolumab | 22% patients—>50% reduction in PSA level Therapy well tolerated |
| PSA-Tricom vaccine + Ipilimumab and GM-CSF [68] | 30 mCRPC patients | Chemotherapy | A decline in PSA in chemotherapy-naïve (14/24) and chemotherapy (1/6) patients Chemotherapy-naïve patients (6/14) had a median OS of 34.4 months. |
| CTLA-4 and PD-1/PD-L1 Combination Therapy | |||
| Nivolumab plus ipilimumab (STARVE-PC) [69] | 15 patients with mCRPC expressing AR-V7 isoform | Nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four doses, then maintenance nivolumab 3 mg/kg every 2 weeks. | Promising results in patients with DDR mutations but not in others. PSA response rate (33% vs. 0%), ORR(40% vs. 0%), and OS (9.04 vs. 7.23 months) in the two subsets (DDR+ vs. DDR-) No safety concerns |
| Ipilimumab and nivolumab (CheckMate 650) [70] | 90 mCRPC patients | 45 pre-chemotherapy 45 post-chemotherapy | ORR 25% vs. 10% PSA response 17.6% vs. 10% Median OS 19.0 vs. 15.2-months Four treatment-related deaths |
| Durvalumab alone or durvalumab plus tremelimumab [71] | 52 mCRPC patients | Previously progressed on prior abiraterone and/or enzalutamide | Durvalumab + tremelimumab: 16% vs. 0% in durvalumab alone |
| Tremelimumab plus durvalumab followed by durvalumab [72] | 26 mCRPC patients | Tremelimumab (75 mg) plus durvalumab (1500 mg) every 4 weeks × 4 doses, followed by durvalumab (1500 mg) maintenance every 4 weeks × 9 doses | Tremelimumab plus durvalumab was safe and well tolerated. PSA declined 50% in three patients (12%). Stable disease for >6 months in six patients (24%). Median rPFS was 3.7 at a median follow-up of 43.6 months, Median overall survival was 28.1 months. |
| Tyrosine Kinase Inhibitors with Immune Checkpoint Blockade | |||
| Cabozantinib with atezolizumab [73] | 44 mCRPC patients in cohort 6 of the COSMIC-021 trial | Oral cabozantinib 40 mg per day and I.V. atezolizumab 1200 mg once every 3 weeks | ORR was 32% 80% disease control rate Tolerable side effects |
| Neuroendocrine Prostate Cancer | ||
|---|---|---|
| Therapeutic Agent | Patient population | Outcome |
| Atezolizumab [76] | Seven patients with de novo small cell or neuroendocrine tumor or transformation from preexisting adenocarcinoma | Median PFS: 3.4 months OS: 8.4 months |
| Pembrolizumab [77] | One patient with a refractory tumor after transformation from a preexisting adenocarcinoma after hormonal therapy- | Substantial improvement in tumor burden after 4 therapy cycles and stable disease after 21 cycles of therapy |
| Rova-T (DLL3 targeted antibody–drug conjugate) [78] | Rova-T was intravenously administered at a dose of 3 mg/kg to a DLL3-positive mNEPC patient every 6 weeks | A significant decrease in the size of metastatic lymph nodes with complete or partial responses in other metastatic lesion was obsereved. |
| YL212 (DLL3-targeted ADC) | YL212 has both extracellular and intracellular cleavage mechanisms and overcomes drug toxicity. | Clinical application is currently underway |
| Castration resistant prostate cancer | ||
| Dendritic tumor cell hybridomas (aHyC) [79] | 22 men with CRPC were included and 19 of them were treated with aHyC vaccine. | aHyC treatment attenuates an increase in CD56brightCD16− NK cell and benefits CRPC patient survival. Median OS was 58.5 months and cancer-specific survival was 75.7 months. |
| Identifier | Aim of the Study | Status | Results |
|---|---|---|---|
| NCT02082977 | To investigate the safety, pharmacokinetics, pharmacodynamics, and clinical activity of EZH2 inhibitor GSK2816126 | Terminated | Phase 1 clinical trial showed insufficient evidence of clinical activity of GSK2816126 [105] |
| NCT03480646 | A study evaluating EZH2-inhibitor CPI-1205 in patients with metastatic CRPC: ProSTAR- A phase 1b/2 study | Active, not recruiting | No results posted on Clinicaltrials.gov |
| NCT03741712 | To study the tolerance, pharmacokinetics (PK), and efficacy of EZH2 inhibitor SHR2554, alone or in combination with SHR3680, in the treatment of patients with metastatic CRPC. | Terminated | A single dose of SHR2554 50 mg was well tolerated and had a good safety profile, and its effect was significantly affected by the combined administration of itraconazole [106]. |
| NCT03460977 | A Phase 1 dose escalation and expanded cohort study of EZH2 inhibitor PF-06821497 (Mevrometostat) in CRPC. The primary aim is to confirm the safety and tolerability of PF-06821497 in combination with enzalutamide plus androgen deprivation therapy. | Recruiting patients | No results posted on Clinicaltrials.gov |
| NCT03093428 | To study the safety and tolerability of a combination of radium-223 plus pembrolizumab, a Phase II clinical trial. | Completed | 42 patients received the treatment. After 8 weeks, there was no evidence of increased CD4+ or CD8+ T-cell infiltration with treatment. However, treatment did not lead to prolonged rPFS or OS [107]. |
| NCT05150236 | To investigate the activity and safety of radionuclide 177Lu-PSMA therapy versus 177Lu-PSMA in combination with Ipilimumab and Nivolumab in patients with mCRPC- an open label, randomized, stratified, multicentre phase 2 clinical trial. | Active but not recruiting patients. | Recruiting 110 participants. No results posted on Clinicaltrials.gov |
| NCT05766371 | A single-center, open-label study of prostate-specific membrane antigen (PSMA)-targeted radionuclide therapy with 177Lu-PSMA-617 in combination with pembrolizumab in mCRPC previously progressed on at least one prior androgen pathway inhibitor (e.g., abiraterone, enzalutamide, apalutamide). | Recruiting | A single priming dose of 177Lu-PSMA-617 followed by pembrolizumab maintenance is safe with encouraging preliminary activity in mCRPC patients [97]. |
| NCT04446117 | A Phase 3, multi-center, randomized, open-label, controlled study designed to evaluate the safety and efficacy of cabozantinib, given in combination with atezolizumab (PD-L1 inhibitor), versus a second novel hormonal therapy in men with mCRPC (CONTACT-02) | Active but not recruiting patients. | With 507 patients, cabozantinib with atezolizumab significantly improved PFS compared to second hormonal therapy in mCRPC patients with visceral metastasis [108]. |
| NCT03007732 | A non-comparative open-label multicenter Phase 2 clinical trial combining stereotactic body radiation therapy and pembrolizumab (anti-PD-1) with or without intratumoral SD-101 (TLR9 agonist) in patients with newly diagnosed hormone-naive oligometastatic prostate cancer. | Active but not recruiting patients. | TLR9 agonism, in combination with radiation and PD-1 blockade, amplifies T cell and myeloid compartments remodeling in prostate tumors and this may guide future immunotherapy strategies [109]. |
| NCT03061539 | To test the hypothesis that patients with mCRPC that have progressed following at least one line of therapy and have an immunogenic signature will respond to combined PD-1 and CTLA4 inhibition (nivolumab + ipilimumab). | Active but not recruiting patients. | Phase 2 trial results showed that nivolumab 1 mg/kg + ipilimumab 3 mg/kg had more toxicities than nivolumab 3 mg/kg + ipilimumab 1 mg/kg; the efficacy results were consistently better, suggesting the need to test a later dose schedule in a phase 3 clinical trial [110]. |
| NCT01688492 | To determine the effects of taking ipilimumab with abiraterone acetate plus prednisone in patients and prostate cancer. A phase 2 study combining ipilimumab with abiraterone acetate plus prednisone in chemotherapy and immunotherapy-naïve patients with progressive mCRPC. | Active but not recruiting patients. | No results are posted on Clinicaltrials.gov. |
| NCT02985957 | A phase 2 trial to evaluate the effectiveness, safety, and tolerability of nivolumab followed by ipilimumab in subjects with mCRPC (CheckMate 650). | Completed | Preliminary analyses showed no clear and consistent association between efficacy and tissue or blood tumor mutational burden [70,111]. |
| NCT03651271 | An open-label, exploratory study to evaluate nivolumab with or without ipilimumab based on the percentage of tumoral CD8 cells at the time of treatment in participants with varying advanced solid tumors, including mCRPC (AMADEUS primary cohort). | Completed | Nivolumab/ipilimumab induced clinical responses and increased %CD8 in a subset of “cold” mCRPC tumors with low CD8 cells [112]. |
| NCT05502315 | A multicenter, single-arm, two-stage open-label phase 2 study of the combination of cabozantinib + nivolumab in subjects with advanced mCRPC (CANOPY). | Recruiting patients | No results are posted on Clinicaltrials.gov. |
| NCT05806814 | Sipuleucel-T-based autologous cellular immunotherapy for advanced prostate cancer (OU-SCC-EXCITE). To evaluate the feasibility of Sipuleucel-T, given in three doses at weeks 0, 2, and 12–14, and to investigate the changes in immune response in mCRPC patients who are receiving an extended course of sipuleucel-T treatment. | Recruiting patients | No results are posted on Clinicaltrials.gov. |
| NCT06782555 | Phase 1/2 study to test the overall safety, tolerability, and effectiveness of the combination of investigational drugs evofosfamide, zalifrelimab, and balstilimab in treating advanced or mCRPC. | Recruiting patients | No results are posted on Clinicaltrials.gov. |
| NCT04221542 | A phase 1 study evaluating the safety, tolerability, pharmacokinetics, and efficacy of AMG 509 (xaluritamig), a STEAP1 × CD3 XmAb 2+1 immune therapy, in subjects with mCRPC. | Recruiting patients | Xaluritamig was tolerable with low-grade cytokine release syndrome (occurring primarily in cycle 1) and showed encouraging preliminary efficacy in heavily pretreated pts with mCRPC [113]. |
| NCT06100705 | An open-label, single-arm phase II study of bipolar androgen therapy, given in addition to standard-of-care Sipuleucel-T to determine the interferon gamma Enzyme-linked Immunospot (ELISPOT) response rate to PA2024 (an engineered fusion protein of prostatic acid phosphatase and granulocyte-macrophage colony-stimulating factor, which the activated autologous dendritic cells in the Sipuleucel-T vaccine are loaded with) in mCRPC patients. | Recruiting patients | No results are posted on Clinicaltrials.gov. |
| NCT06555796 | A phase 1b, open-label, multicenter study evaluating the safety, tolerability, and efficacy of xaluritamig in subjects with high-risk biochemical recurrence of nonmetastatic castration-sensitive prostate cancer after definitive therapy | Recruiting patients | No results are posted on Clinicaltrials.gov. |
| NCT03866382 | A phase II trial testing the effectiveness of two immunotherapy drugs (nivolumab and ipilimumab) with one anti-cancer-targeted drug (cabozantinib) for rare genitourinary tumors. | Recruiting patients | No results are posted on Clinicaltrials.gov. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rai, V. Immune Checkpoint Inhibitor Therapy for Prostate Cancer: Present and Future Prospectives. Biomolecules 2025, 15, 751. https://doi.org/10.3390/biom15060751
Rai V. Immune Checkpoint Inhibitor Therapy for Prostate Cancer: Present and Future Prospectives. Biomolecules. 2025; 15(6):751. https://doi.org/10.3390/biom15060751
Chicago/Turabian StyleRai, Vikrant. 2025. "Immune Checkpoint Inhibitor Therapy for Prostate Cancer: Present and Future Prospectives" Biomolecules 15, no. 6: 751. https://doi.org/10.3390/biom15060751
APA StyleRai, V. (2025). Immune Checkpoint Inhibitor Therapy for Prostate Cancer: Present and Future Prospectives. Biomolecules, 15(6), 751. https://doi.org/10.3390/biom15060751






