Ngn2-Induced Differentiation of the NG108-15 Cell Line Enhances Motor Neuronal Differentiation and Neuromuscular Junction Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. NG108-15 Cell Culture and Differentiation
2.2. C2C12 Cell Culture
2.3. Co-Culture of C2C12 and NG108-15 Cells
2.4. Genetic Modification of NG108-15 Cells
2.5. Fluorescence Activated Cell Sorting (FACS)
2.6. Viability Assay
2.7. Immunofluorescence
2.8. Immunocytochemistry Reagents
2.9. Neurite Quantification
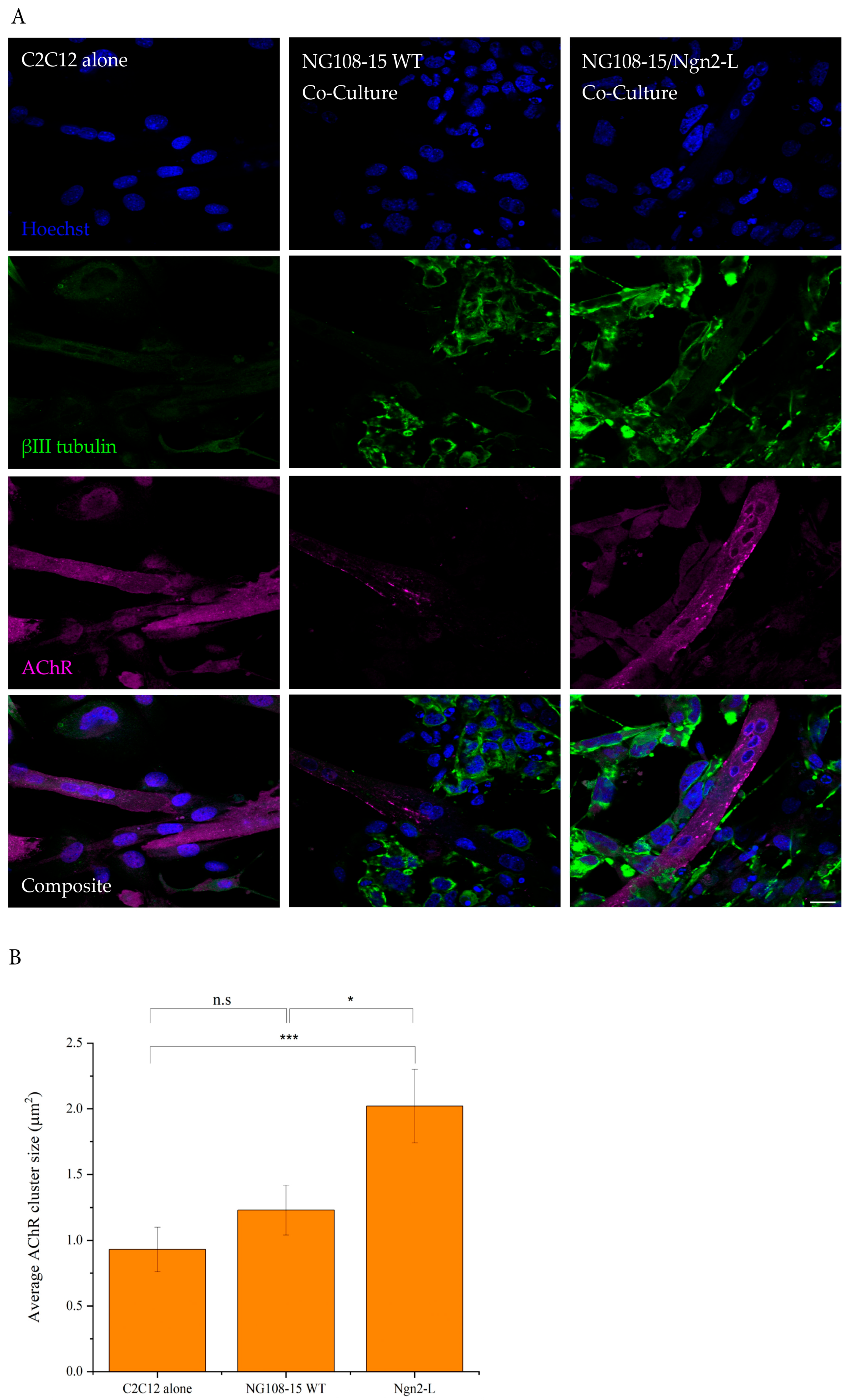
2.10. Quantification of Acetylcholine Receptor Clusters in Myotubes
2.11. mRNA Extraction, and mRNA Analysis
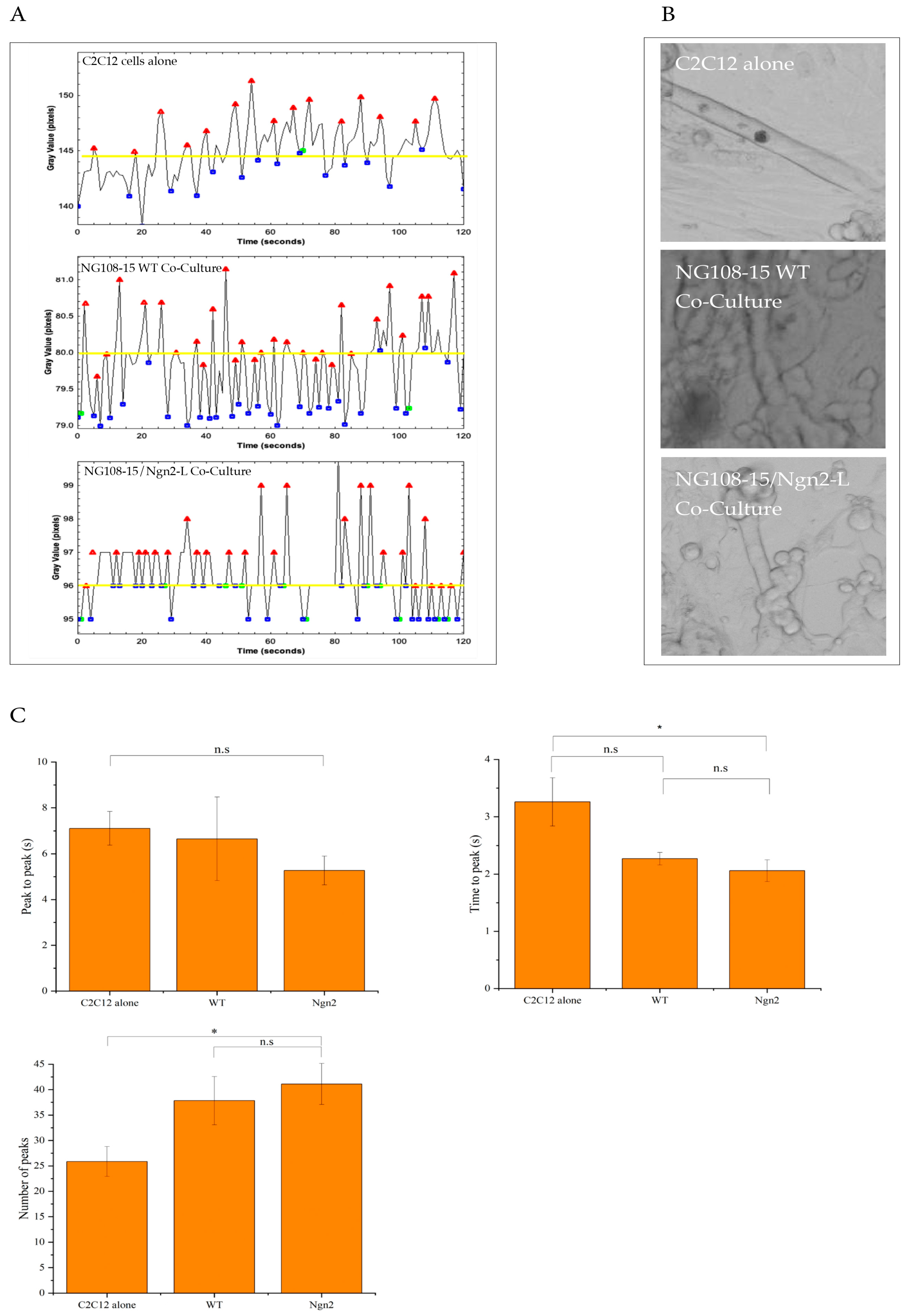
2.12. Quantification of Myotube Contraction
2.13. Graphs and Statistical Analysis
3. Results
3.1. Selecting for Various Gene Expression Levels in NG108-15/Ngn2 Cells According to Fluorescence
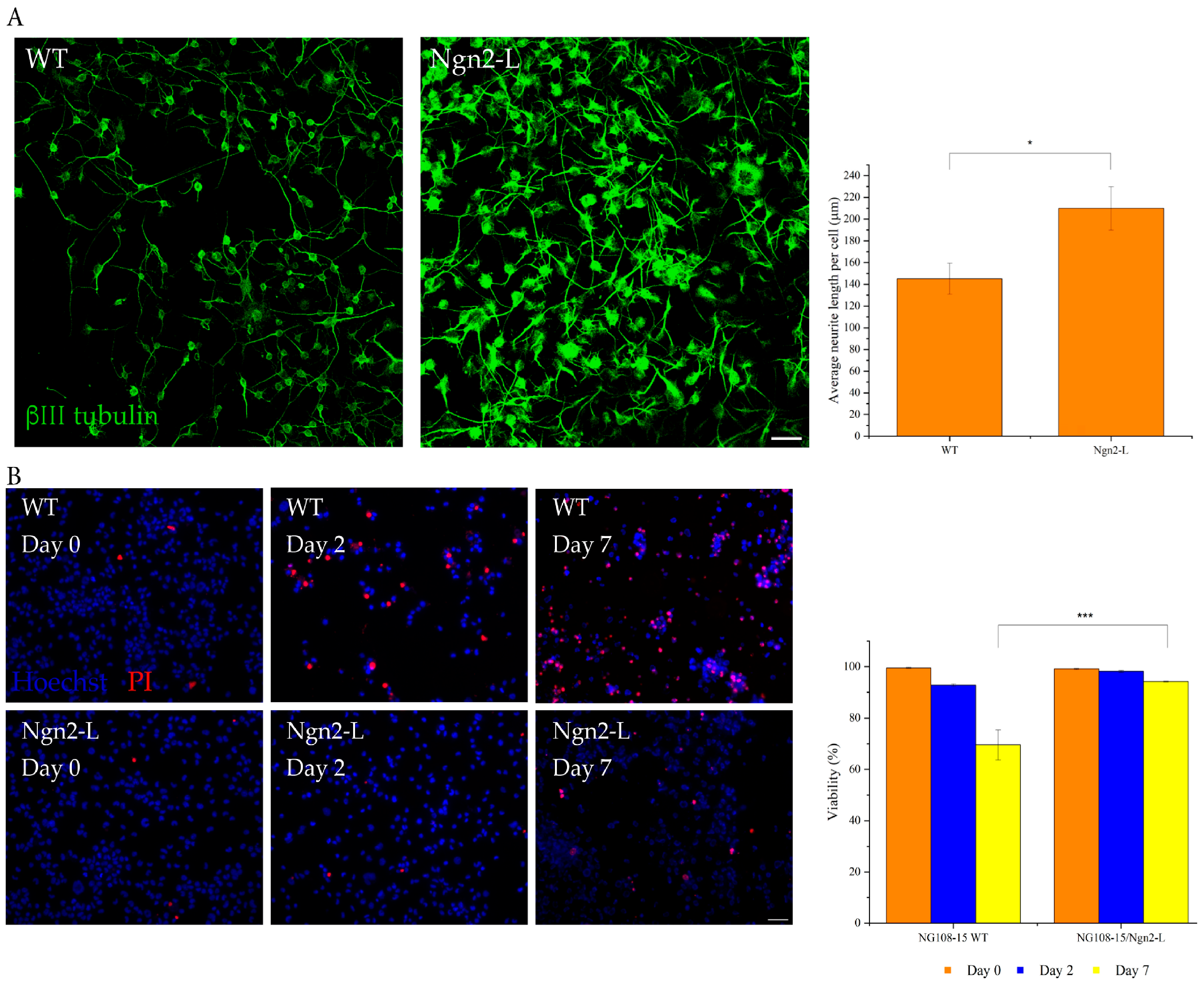
3.2. Ngn2 Improves Neuronal Morphology and Retains a Cholinergic Phenotype Following Induced Differentiation
3.3. Ngn2-Induced Differentiation Improves the Neurite Length of Differentiating Cultures
3.4. Ngn2-Induced Differentiation Improves the Viability of Differentiating Cultures
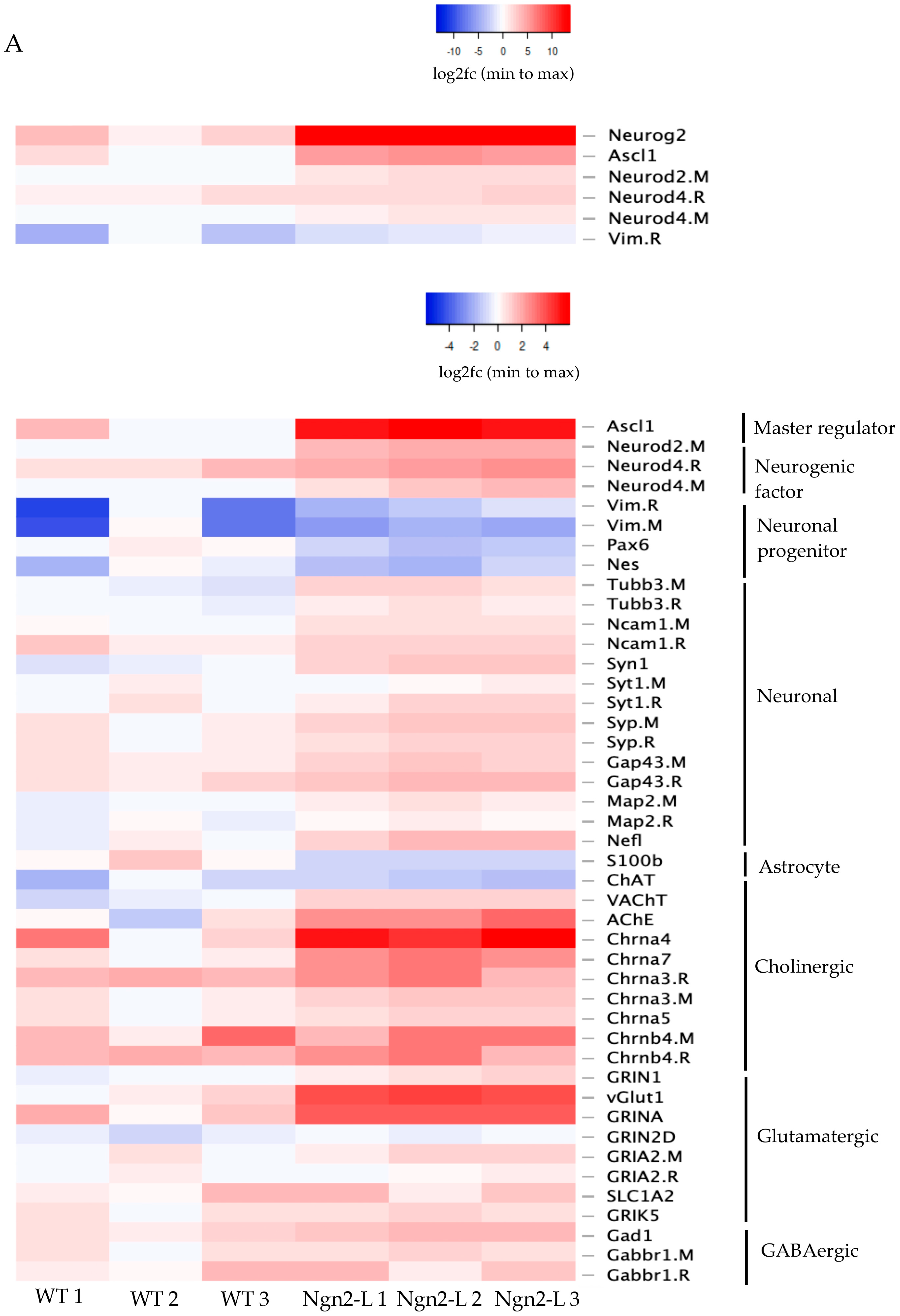
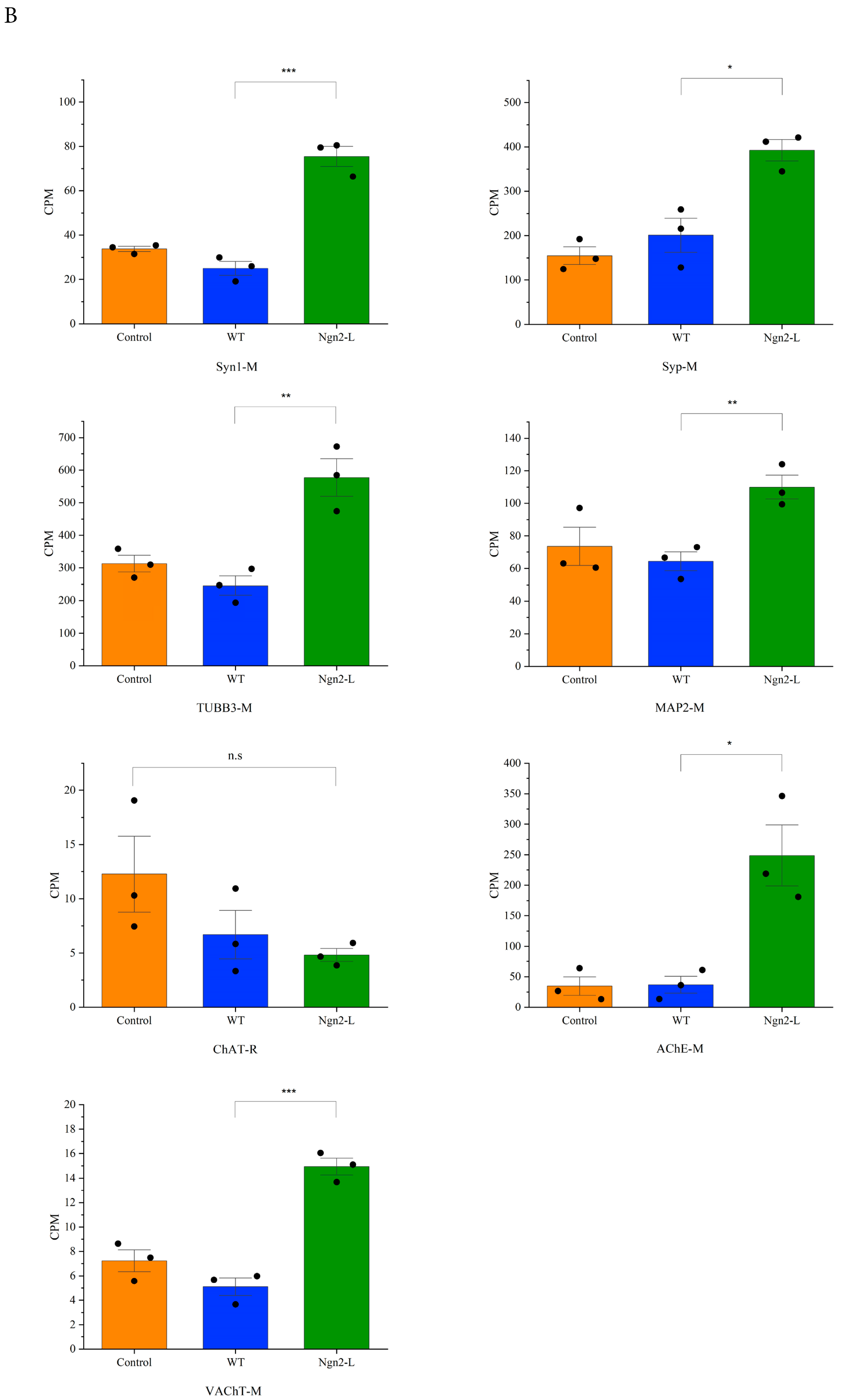
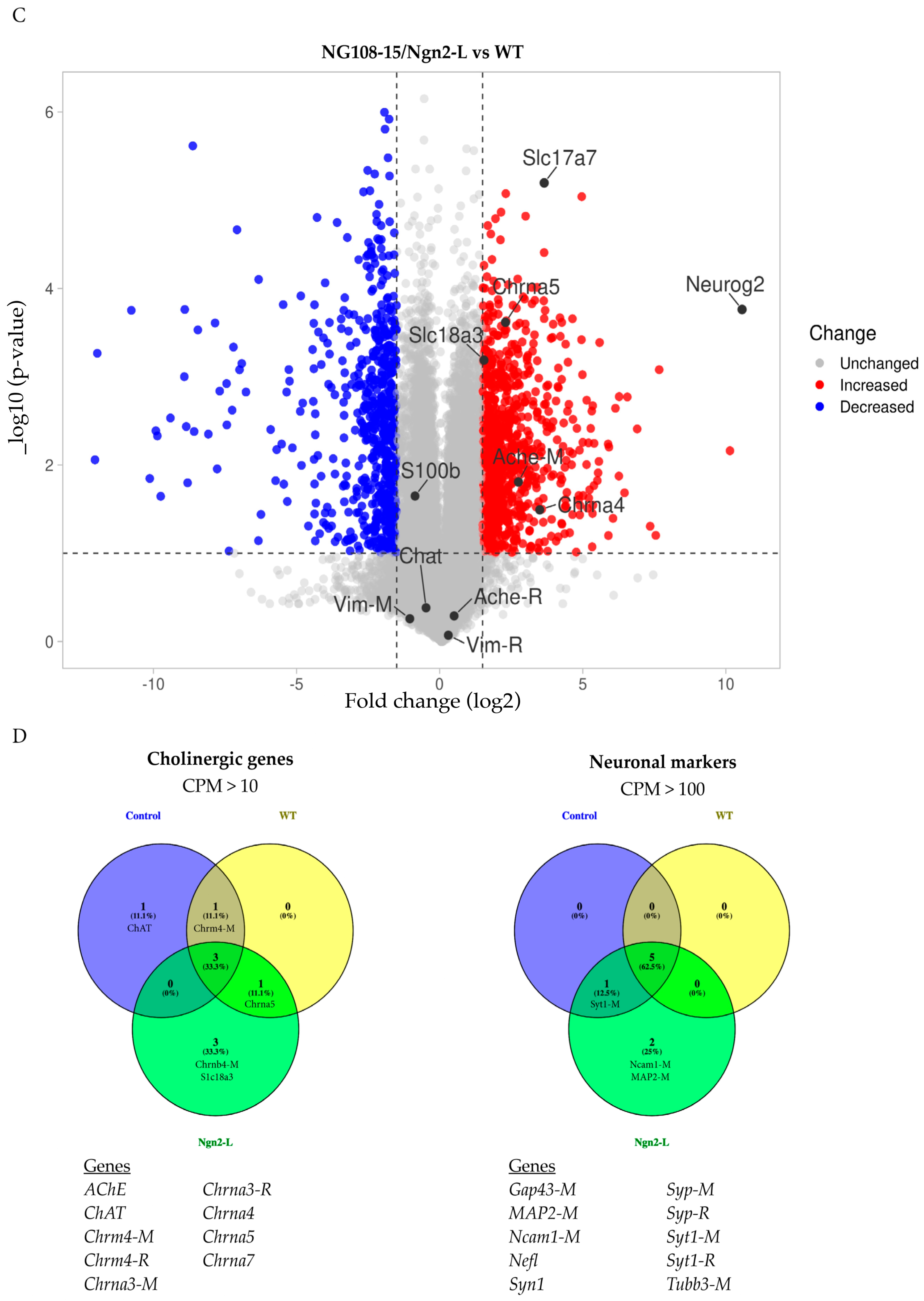
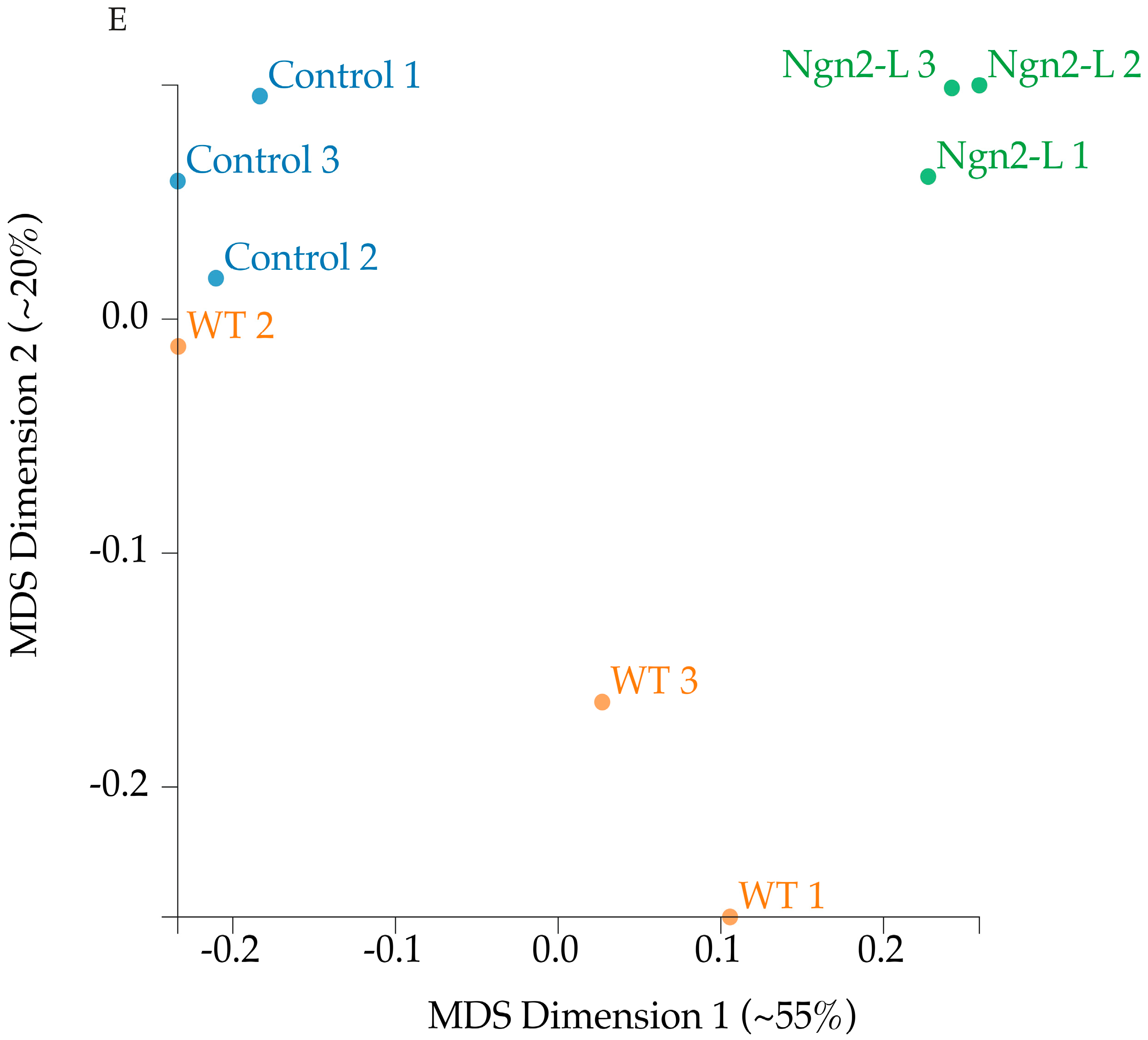
3.5. mRNA Sequencing Analysis of NG108-15/pBac_Ngn2-L Cells
3.6. Analysis of Neuromuscular Junction Formation and Myotube Contractile Dynamics in NG108-15/C2C12 Co-Cultures
3.7. Quantification of Myotube Contractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vila, O.F.; Qu, Y.; Vunjak-Novakovic, G. In vitro models of neuromuscular junctions and their potential for novel drug discovery and development. Expert Opin. Drug Discov. 2020, 15, 307–317. [Google Scholar] [CrossRef]
- Gordon, J.; Amini, S. General overview of neuronal cell culture. In Neuronal Cell Culture: Methods and Protocols; Humana: New York, NY, USA, 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Kondo, T. Stem cell-like cancer cells in cancer cell lines. Cancer Biomark. 2007, 3, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Dufour, J.M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis 2012, 2, 1–5. [Google Scholar] [CrossRef]
- Ho, S.-M.; Topol, A.; Brennand, K.J. From “directed differentiation” to “neuronal induction”: Modeling neuropsychiatric disease: Supplementary issue: Stem cell biology. Biomark. Insights 2015, 10, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Thoma, E.C.; Wischmeyer, E.; Offen, N.; Maurus, K.; Sirén, A.-L.; Schartl, M.; Wagner, T.U. Ectopic expression of neurogenin 2 alone is sufficient to induce differentiation of embryonic stem cells into mature neurons. PLoS ONE 2012, 7, e38651. [Google Scholar] [CrossRef]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; Marro, S.; Patzke, C.; Acuna, C.; Covy, J.; et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013, 78, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Hulme, A.J.; Maksour, S.; Glover, M.S.-C.; Miellet, S.; Dottori, M. Making neurons, made easy: The use of Neurogenin-2 in neuronal differentiation. Stem Cell Rep. 2021, 17, 14–34. [Google Scholar] [CrossRef]
- Gu, J.; Rollo, B.; Sumer, H.; Cromer, B. Targeting the AAVS1 site by CRISPR/Cas9 with an inducible transgene cassette for the neuronal differentiation of human pluripotent stem cells. In Applications of Genome Modulation and Editing; Springer: New York, NY, USA, 2022; pp. 99–114. [Google Scholar] [CrossRef]
- Liu, M.-L.; Zang, T.; Zou, Y.; Chang, J.C.; Gibson, J.R.; Huber, K.M.; Zhang, C.-L. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat. Commun. 2013, 4, 2183. [Google Scholar] [CrossRef]
- Xue, Y.; Zhan, X.; Sun, S.; Karuppagounder, S.S.; Xia, S.; Dawson, V.L.; Dawson, T.M.; Laterra, J.; Zhang, J.; Ying, M. Synthetic mRNAs drive highly efficient iPS cell differentiation to dopaminergic neurons. Stem Cells Transl. Med. 2019, 8, 112–123. [Google Scholar] [CrossRef]
- Goparaju, S.K.; Kohda, K.; Ibata, K.; Soma, A.; Nakatake, Y.; Akiyama, T.; Wakabayashi, S.; Matsushita, M.; Sakota, M.; Kimura, H. Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Hulme, A.J.; McArthur, J.R.; Maksour, S.; Miellet, S.; Ooi, L.; Adams, D.J.; Finol-Urdaneta, R.K.; Dottori, M. Molecular and functional characterization of neurogenin-2 induced human sensory neurons. Front. Cell. Neurosci. 2020, 14, 600895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, H.; Zhang, D.; Zheng, H.; Li, Y.L. Voltage-gated sodium channel expression and action potential generation in differentiated NG108-15 cells. BMC Neurosci. 2012, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Whitemarsh, R.C.; Pier, C.L.; Tepp, W.H.; Pellett, S.; Johnson, E.A. Model for studying Clostridium botulinum neurotoxin using differentiated motor neuron-like NG108-15 cells. Biochem. Biophys. Res. Commun. 2012, 427, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Kiris, E.; Nuss, J.E.; Burnett, J.C.; Kota, K.P.; Koh, D.C.; Wanner, L.M.; Torres-Melendez, E.; Gussio, R.; Tessarollo, L.; Bavari, S. Embryonic stem cell-derived motoneurons provide a highly sensitive cell culture model for botulinum neurotoxin studies, with implications for high-throughput drug discovery. Stem Cell Res. 2011, 6, 195–205. [Google Scholar] [CrossRef]
- Seidman, K.J.N.; Barsuk, J.H.; Johnson, R.F.; Weyhenmeyer, J.A. Differentiation of NG108-15 neuroblastoma cells by serum starvation or dimethyl sulfoxide results in marked differences in angiotensin II receptor subtype expression. J. Neurochem. 1996, 66, 1011–1018. [Google Scholar] [CrossRef]
- Ho, S.-Y.; Chao, C.-Y.; Huang, H.-L.; Chiu, T.-W.; Charoenkwan, P.; Hwang, E. NeurphologyJ: An automatic neuronal morphology quantification method and its application in pharmacological discovery. BMC Bioinform. 2011, 12, 1–18. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. C2C12 cell model: Its role in understanding of insulin resistance at the molecular level and pharmaceutical development at the preclinical stage. J. Pharm. Pharmacol. 2020, 72, 1667–1693. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 12 April 2025).
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, 147–153. [Google Scholar] [CrossRef]
- Goedhart, J.; Luijsterburg, M.S. VolcaNoseR is a web app for creating, exploring, labeling and sharing volcano plots. Sci. Rep. 2020, 10, 20560. [Google Scholar] [CrossRef]
- Hong, W.S. Microsystems Engineering Assisted Cell and Tissue Culture Models: Detection and Therapeutic Applications of Botulinum Neurotoxin Type A. Ph.D. Thesis, University of Wisconsin–Madison, Madison, WI, USA, 2015. [Google Scholar]
- QIAGEN QIAGEN RNeasy(R) Mini Handbook. Available online: https://www.qiagen.com/us/resources/download.aspx?id=f646813a-efbb-4672-9ae3-e665b3045b2b&lang=en (accessed on 9 May 2024).
- Langhammer, C.G.; Zahn, J.D.; Firestein, B.L. Identification and quantification of skeletal myotube contraction and association in vitro by video microscopy. Cytoskeleton 2010, 67, 413–424. [Google Scholar] [CrossRef]
- Pasqualin, C.; Gannier, F.; Yu, A.; Benoist, D.; Findlay, I.; Bordy, R.; Bredeloux, P.; Maupoil, V. Spiky: An ImageJ plugin for data analysis of functional cardiac and cardiomyocyte studies. J. Imaging 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Rollo, B.; Berecki, G.; Petrou, S.; Kwan, P.; Sumer, H.; Cromer, B. Generation of a stably transfected mouse embryonic stem cell line for inducible differentiation to excitatory neurons. Exp. Cell Res. 2024, 435, 113902. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H.; Coates, C.J.; George, A.L. PiggyBac transposon-mediated gene transfer in human cells. Mol. Ther. 2007, 15, 139–145. [Google Scholar] [CrossRef]
- Soboleski, M.R.; Oaks, J.; Halford, W.P. Green fluorescent protein is a quantitative reporter of gene expression in individual eukaryotic cells. FASEB J. 2005, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kolacsek, O.; Krízsik, V.; Schamberger, A.; Erdei, Z.; Apáti, Á.; Várady, G.; Mátés, L.; Izsvák, Z.; Ivics, Z.; Sarkadi, B.; et al. Reliable transgene-independent method for determining Sleeping Beauty transposon copy numbers. Mob. DNA 2011, 2, 5. [Google Scholar] [CrossRef]
- Lauer, S.; Avecilla, G.; Spealman, P.; Sethia, G.; Brandt, N.; Levy, S.F.; Gresham, D. Single-cell copy number variant detection reveals the dynamics and diversity of adaptation. PLoS Biol. 2018, 16, e3000069. [Google Scholar] [CrossRef]
- Shupliakov, O.; Haucke, V.; Pechstein, A. How synapsin I may cluster synaptic vesicles. Semin. Cell Dev. Biol. 2011, 22, 393–399. [Google Scholar] [CrossRef]
- Fried, G.; Han, H.Q. Increase in synaptic vesicle proteins in synapsin-transfected NG108-15 cells: A subcellular fractionation study. Synapse 1995, 20, 44–53. [Google Scholar] [CrossRef]
- Chelladurai, K.S.; Christyraj, J.D.S.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Vasantha, N.C.; Christyraj, J.R.S.S. Alternative to FBS in animal cell culture—An overview and future perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef]
- Halder, N.; Lal, G. Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front. Immunol. 2021, 12, 660342. [Google Scholar] [CrossRef]
- Rodríguez Cruz, P.M.; Cossins, J.; Beeson, D.; Vincent, A. The neuromuscular junction in health and disease: Molecular mechanisms governing synaptic formation and homeostasis. Front. Mol. Neurosci. 2020, 13, 610964. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, A.A.; Willmann, R.; Sadasivam, G.; Lin, S.; Rüegg, M.A.; Gesemann, M.; Fuhrer, C. Tyrosine phosphatases such as SHP-2 act in a balance with Src-family kinases in stabilization of postsynaptic clusters of acetylcholine receptors. BMC Neurosci. 2007, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Gajsek, N.; Jevsek, M.; Mars, T.; Mis, K.; Pirkmajer, S.; Brecelj, J.; Grubic, Z. Synaptogenetic mechanisms controlling postsynaptic differentiation of the neuromuscular junction are nerve-dependent in human and nerve-independent in mouse C2C12 muscle cultures. Chem.-Biol. Interact. 2008, 175, 50–57. [Google Scholar] [CrossRef]
- Wang, J.; Fu, X.-Q.; Lei, W.-L.; Wang, T.; Sheng, A.-L.; Luo, Z.-G. Nuclear factor κB controls acetylcholine receptor clustering at the neuromuscular junction. J. Neurosci. 2010, 30, 11104–11113. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.K.; Siow, N.L.; Choi, R.C.; Tsim, K.W. ATP potentiates the formation of AChR aggregate in the co-culture of NG108-15 cells with C2C12 myotubes. FEBS Lett. 2005, 579, 2469–2474. [Google Scholar] [CrossRef]
- Grubišić, V.; Gottipati, M.K.; Stout Jr, R.F.; Grammer, J.R.; Parpura, V. Heterogeneity of myotubes generated by the MyoD and E12 basic helix-loop-helix transcription factors in otherwise non-differentiation growth conditions. Biomaterials 2014, 35, 2188–2198. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meli, M.; Swiderski, K.; Gu, J.; Rollo, B.; Bartlett, B.; Caldow, M.K.; Lynch, G.S.; Kwan, P.; Sumer, H.; Cromer, B. Ngn2-Induced Differentiation of the NG108-15 Cell Line Enhances Motor Neuronal Differentiation and Neuromuscular Junction Formation. Biomolecules 2025, 15, 637. https://doi.org/10.3390/biom15050637
Meli M, Swiderski K, Gu J, Rollo B, Bartlett B, Caldow MK, Lynch GS, Kwan P, Sumer H, Cromer B. Ngn2-Induced Differentiation of the NG108-15 Cell Line Enhances Motor Neuronal Differentiation and Neuromuscular Junction Formation. Biomolecules. 2025; 15(5):637. https://doi.org/10.3390/biom15050637
Chicago/Turabian StyleMeli, Madeline, Kristy Swiderski, Jinchao Gu, Ben Rollo, Ben Bartlett, Marissa K. Caldow, Gordon S. Lynch, Patrick Kwan, Huseyin Sumer, and Brett Cromer. 2025. "Ngn2-Induced Differentiation of the NG108-15 Cell Line Enhances Motor Neuronal Differentiation and Neuromuscular Junction Formation" Biomolecules 15, no. 5: 637. https://doi.org/10.3390/biom15050637
APA StyleMeli, M., Swiderski, K., Gu, J., Rollo, B., Bartlett, B., Caldow, M. K., Lynch, G. S., Kwan, P., Sumer, H., & Cromer, B. (2025). Ngn2-Induced Differentiation of the NG108-15 Cell Line Enhances Motor Neuronal Differentiation and Neuromuscular Junction Formation. Biomolecules, 15(5), 637. https://doi.org/10.3390/biom15050637







