Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management
Abstract
1. Introduction
2. Classification of Polyphenols
3. The Dietary Sources of Different Polyphenol Compounds
4. Nutraceuticals and Pharmaceuticals Derived from Dietary Phenolic Compounds
| Class | Compound | Property(s) | Citation |
|---|---|---|---|
| 1. Flavonoids | Anthocyanins | Antioxidant and Anticancer | [14] |
| Flavanols | Anti-diabetic, Anti-inflammatory and Antioxidant | [15] | |
| Flavonols: Quercetin | Anti-inflammatory, Antioxidant, Antimicrobial, Anticancer, Antihypertensive, vasodilator, Antiobesity, Antiatherosclerosis | [16] | |
| Flavonols: Epigallocatechin-3-gallate | Antioxidant Anti-angiogenesis, Anti-inflammation and Anticancer | [17] | |
| Flavones; Luteolin | Anti-Inflammatory, Antioxidant, Antiallergy, Anticancer and Antibacterial | [18] | |
| Flavanones; Hesperetin | Anti-inflammatory, Antioxidant, Antibacterial and Anticancer | [18] | |
| Isoflavones; Genistein | Anti-inflammatory | [19] | |
| 2. Phenolic acids | Caffeic acid and its derivative caffeic acid phenethyl ester | Antioxidant, anti-inflammatory and anticancer | [20] |
| Gallic acids | Anticancer, Antioxidant, and Anti-inflammatory | [21] | |
| Rosmarinic acid | Anti-inflammatory, Antiviral, Antibacterial, Antidepressant and Anticancer | [22] | |
| Sinapic acid | Antioxidant, Antimicrobial, Anti-inflammatory, Anticancer, Antianxiety | [23] | |
| Hydroxy benzoic acid | Antimicrobial | [24] | |
| Hydroxycinnamic acid | Antioxidant | [25] | |
| Protocatechuic acid | Anti-inflammatory and Antimicrobial | [26] | |
| Syringic acid | Antioxidant, antimicrobial, anti-inflammatory, antiendotoxic, neuro and hepatoprotective | [27] | |
| Protocatechoic acid | Anti-inflammatory and Antimicrobial | [26] | |
| Synergic acid | Antioxidant, antimicrobial, anti-inflammatory, antiendotoxic, neuro and hepatoprotective | [27] | |
| Vanillic acid | Antioxidant, Anti-inflammatory and Neuroprotective | [28] | |
| 3. Curcuminoids | Curcumin | Anti-inflammatory, Antioxidant and Anticancer | [29] |
| 4. Stilbenes | Resveratrol | Antioxidant, Anti-inflammatory, Immunomodulatory, Neuroprotective, Cardiovascular protective and Anticancer | [30] |
| 5. Lignans | Dibenzocyclooctadiene lignans | Antioxidant, Antiviral, Anti-inflammatory and Anticancer | [31] |
5. Polyphenols and Their Anticancer Properties with Insights into Their Molecular Mechanisms, Preclinical Studies, and Clinical Applications
5.1. Flavonoids
5.1.1. Anthocyanins
| Anticancer Effect of Anthocyanins | Cancer Type | Citation |
|---|---|---|
| Anti-invasiveness and inhibition of the proliferation of MDA-MB-231 breast cancer cell lines. | Breast cancer | [43] |
| Reduction of the viability of breast cancer cell lines MCF-7, MDA-MB-231, and MDA-MB-453, in addition to induction of apoptosis in MDA-MB-453 cells via the intrinsic pathway (caspase cascade activation PARP cleavage and cytochrome C release) and suppression of tumour growth and angiogenesis via inhibiting MMP-9, MMP-2, and uPA expression in BALB/c naked mice with MDA-MB-453 cell xenografts. | Breast cancer | [44] |
| Inhibition of c-Jun N-terminal kinase, mitogen-activated protein kinase, and fibrosarcoma activation, downregulation of matrix metalloproteinase 2 secretion, and inhibition of cell migration and invasion in MDA-MB-453 breast cancer cells (HER2+). | Breast cancer | [45] |
| Inhibition of the development of abnormal crypt foci of colon in CF-1 mice. | Colon cancer | [46] |
| Induction of apoptosis in benign prostatic hyperplasia in rats. | Benign prostate hyperplasia | [47] |
| Triggering apoptotic factors such as TRAIL in cancer systems and suppression of Akt-mTOR signalling leading to maturation of acute myeloid leukaemia cells. | Leukaemia | [48] |
5.1.2. Flavanols
5.1.3. Flavonols
Quercetin
Epigallocatechin-3-Gallate (EGCG)
| Flavonols | Anticancer Effect | Cancer Type | Citation |
|---|---|---|---|
| Quercetin | Increase the expression of PTEN while downregulation of the phosphorylation of PI3K, Akt, ERK1/2, p38, NF-κB, and survivin proteins. | Prostate cancer | [65] |
| Induction of autophagy, inhibition of the Akt-mTOR pathway’s role in glycolysis and cell motility and reduction of the expression levels of VEGF, p-AKT, and PKM2 in tumour tissue. | Breast cancer | [67] | |
| Cell cycle arrest at the G1 phase that occurs due to the downregulation of cyclin-dependent kinase 1 (CDK1) and cyclin B1and Upregulation of Bax, Bcl-2, p21Cip1, p27Kip1, cyt-c, caspase 3, caspase 8, and p53. | Prostate cancer | [68] | |
| Inhibition of IL-6 and IL-10 cytokine production, resulting in cytotoxicity, in addition to downregulation of cell survival proteins, such as c-FLIP, cyclin D1, and cMyc via inhibiting the PI3K/AKT/mTOR and STAT3 pathways. | Primary Effusion Lymphoma | [69] | |
| Induction of apoptosis and anti-proliferation, with alteration of GSK3β/β-catenin signalling, in addition to reduction of COX-2 level, and downregulation of tumours’ VEGF-R and VEGF-A. | Human gastric cancer cell line AGS | [70] | |
| Epigallocatechin-3-Gallate (EGCG) | Decreased cell adhesion and downregulated the expression of VEGF, NF-κB, FAK, and MT1-MMP. | Breast cancer MCF-7 cells | [93] |
| Inhibition of cell division, migration, and Matrigel invasion. | TW01 and NA nasopharyngeal cancer cells | [85] | |
| Suppression of human umbilical vein endothelial cells (HUVECs’) migration, capillary tube formation, and cell proliferation and downregulation of MMP2, MMP7, MMP9, and MMP12 and reduction of the volume, angiogenesis, and metastasis of tumour, in addition to increased p38 and JNK activity and decreased ERK activity. | Pancreatic cancer xenograft AsPC-1 | [94] | |
| Inhibition of cells proliferation, induction of apoptosis, suppression of cell invasion and migration, in addition to suppression of MMP9 gene expression while upregulation of TIMP1 gene expression. | Cervical cancer HeLa cells | [87] | |
| Inhibition of the levels of MMP9 and VEGF expression. | Colon cancer HCT116 cells | [88] | |
| Induction of cytotoxicity, decrease Tax oncogene expression, and reduction of MMP9 activity, NF-κB activity, and MMP9 mRNA and protein levels. | ATL HuT-102 and C91-PL cells | [89] | |
| Prevention of cell invasion, migration, and proliferation, in addition to activation of caspases-8, -9, and -3, Bax and poly-ADP ribose. | Bladder cancer SW780 cells | [90] | |
| Reduction of the activation of MMP2 and MMP9 in patient sera in addition to lowering the serum levels of VEGF and hepatocyte growth factor (HGF). | Patients with breast cancer | [91] |
5.1.4. Flavones: Luteolin
| Anticancer Effect of Luteolin | Cancer Type | Citation |
|---|---|---|
| Inhibition of CDK2 activity and induction of induced G1 cell cycle arrest. | Colorectal cancer HT-29 and melanoma OCM-1 cells | [94] |
| Suppression of the expression of p-STAT3, p-EGFR, p-Akt, and p-Erk1/2, as well as inhibition of cell proliferation. | MCF-7 breast cancer cells | [94] |
| Increase of the mRNA levels of death receptors such as DR5 and caspase-9/-8/-3 activity, in addition to inhibition of poly-ADP ribose polymerase. Moreover, the induction of the release of cytochrome c after impairing the potential of the mitochondrial membrane. As a result, Bcl-2 expression was suppressed, and Bax expression rose. | MCF-7 breast cancer cells | [96] |
| Induction of cell cycle arrest in the S phase by lowering telomerase levels and preventing the phosphorylation of NF-κB inhibitor α, in addition to inhibition of the growth of breast malignant cells and induction of apoptosis. | MDA-MB-231 breast cancer cells | [97] |
| Akt inactivation and extracellular signal-regulated kinase (ERK) signalling inhibition. | MCF7/HER18 breast cancer cells | [98] |
| Induction of cell cycle arrest at the G2/M phase and inhibition of cyclin B1/CDC2. | LoVo human colon cells | [100] |
| Increased p53 phosphorylation and p53 target gene expression, which leads to cell cycle arrest, apoptosis, and autophagy. | HCT116 colon cells | [101] |
| Increased Nrf2 transcription by the DNA demethylation of its promoter, in addition to strengthening the interaction between Nrf2 and p53 that results in increased expression of antioxidant enzymes and apoptotic proteins. | Colorectal cancer cells | [102] |
| Inhibition of the proliferation of colorectal malignant cells, interruption of the cell cycle, damaged DNA and accelerated apoptosis through targeting the MAPK pathway. | Colorectal cancer cells | [103] |
| Decrease in the expression of p-PI3K, p-mTOR, p-AKT, p-STAT3, and Notch1, and an increase in the amount of p-P38 signal transduction. | Gastric tumour cells | [104] |
| Inhibition of the immune system and the expression of MMP-9 and VEGF-A, which stops cancer from growing, in addition to suppression of the expression of c-Met, ki-67, and MMP-9 that results in inhibition of tumour cells invasiveness and proliferation and induction of apoptosis. | Gastric tumour cells | [105] |
| EMT reversion by shrinking the cytoskeleton and increasing the expression of E-cadherin downstream of mesenchymal markers such as vimentin, N-cadherin, and Snail, in addition to prevention of the transduction of Notch1 signals. | Gastric tumour cells | [106] |
| Reduction of the expression of the target genes Mcl-1 and Bcl-xl and survival, in addition to inhibition of STAT3 phosphorylation. | Gastric cancer cells | [107] |
| Induction of ROS production, which in turn mediates the expression of tumour necrosis factor-activated cascade, in addition to upregulation of c-Jun N-terminal kinase expression and downregulation of NF-κB expression that results in promotion of tumour necrosis factor-induced apoptosis. | Lung cancer cells | [108] |
| Increase the synthesis of Bax, activation of JNK, and enhancing the cleavage of caspase-3 and procaspase-9, in addition to inhibition of trans-nuclear translocation controlled by TNF-α and NF-kB. | Lung cancer cells (A549) | [109] |
| Activation of caspase-9 and -3, inhibition of Bcl-2, increasing Bax expressions, phosphorylation of MEK and its downstream kinase ERK, activation of Akt, inhibition of cell growth, and induction of apoptosis. | Lung cancer cells (A549) | [110] |
| Increased cleaved caspase-3 levels and reduced cyclin D1 expression by decreasing the mRNA levels of LIM domain kinase signalling-related targets, such as p-cofilin and phosphorylated LIM domain kinase, that results into inhibition of tumour development. | Lung tumour xenograft | [111] |
| Reduction of miR-301-3p level results in inhibition of tumour cells proliferation and enhancing the antiproliferative effect of TRAIL on tumour cells. | Pancreatic ductal adenocarcinoma cells (PDAC) | [119] |
| Suppression of the expression of androgen receptors and prostate-specific antigen, in addition to the reduction of the mRNA levels of numerous genes involved in the cell cycle, cascades and the epidermal growth factor receptor signal transduction cascade that significantly promoted cell cycle arrest at the G2/M phase and triggered the production of p21 RNA and c-FOS. | Prostate cancer cells | [94] |
| Decreased Bcl-2 at the mRNA and protein levels, increased caspase 8, and caused G0-/G1-phase arrest. Additionally, it increased Beclin 1 expression, expedited the conversion of LC3B–I to LC3B–II, and increased the number of intracellular autophagosomes. | SMMC-7721 liver cancer cells | [122] |
| Inhibition of the activation of the PI3K/Akt and NF-κB signalling pathways, which are implicated in the growth and survival of cancer cells. | Kidney malignant cells | [124] |
5.1.5. Flavanones
| Anticancer Effect of Hesperetin (HSP) | Cancer Type | Citation |
|---|---|---|
| Increased the mRNA levels of p53, NOTCH1, and PPARG and decreased β-catenin, leading to apoptosis and cell cycle arrest in the G0/G1 phase. | MCF-7 breast cancer cells | [142] |
| Upregulation of tumour suppressor genes that can regulate cell cycle progression, induction of both intrinsic and extrinsic pathways that lead to cell death, inhibition of certain tumour-related growth factors which will prevent metastases, inhibition of MMP-9 production and induction of cell cycle arrest in the Sub G1 phase. | Breast cancer cells | [126] |
| Inhibition of the aryl hydrocarbon receptor (Ahr) and downregulation of the expression of CYP1A1, 1A2, and 1B1. | MCF-7 breast cancer cells | [143] |
| Inhibition of the activity of the aromatase enzyme, cyclin D1, CDK4, Bcl-xL, and pS2, while increasing the expression of CCAAT/C/EBP, pERK-1&-2, and p57Kip2 that results in decrease the tumour growth. | MCF-7 breast cancer cells and female athymic mice models | [144] |
| Reduction of HER2, MMP-9, Rac1 expression, lamellipodia formation, and induction of cell cycle at the G2/M phase, thereby lowering cell viability, invasion, migration, and promoting apoptosis. | HER2 overexpressed breast cancer cells (MCF-7/HER2) and MCF-7/EV cells | [145] |
| Increase ROS production, cyto-C release, the Bax/Bcl-2 ratio, PARP cleavage, caspase-9, -3, -7, JNK, and activation of sk1 and the ASK1/JNK pathway. | MCF-7, MCF-10A, HMEC, and MDA-MB 231 breast cancer cells | [146] |
| Suppression of insulin receptor-beta subunit (IR-beta) phosphorylation and Akt, which lowers glucose absorption, leading to decreased cell proliferation. | MDA-MB-231 breast cancer cells | [147] |
| Inhibition of HER2 Tyrosine Kinase (HER2-TK) activity, leading to MMP loss, chromatin condensation, and activating caspase-8 and-3 that resulted in cell cycle arrest at the G2 phase and lowered SKBR3. | MDA-MB-231 breast cancer cells | [148] |
| Induction of apoptosis and prevention of metastasis of tumour cells by downregulating MMP-9 production and stopping the cell cycle at the Sub G1 phase. | 4T1 murine breast cancer cells | [149] |
| G0/G1 phase arrest via increasing phosphorylation of the signal transducer and activator of transcription 3 (STAT 3), extracellular signal-regulated kinase ½ (ERK1/2), and AKT signalling pathways, as well as IL-6 gene expression. | PC-3 cells | [150] |
| Induction of apoptosis via initiating the Fas death receptor/extrinsic pathway, which led to the dose-dependent upregulation of Bax, caspase-3, and caspase-9. | H522 lung cancer cells | [151] |
| Blocking transforming growth factor β and decreasing glucose uptake in a cancer cell by downregulating glucose transporter expression. | H441 lung cancer cells | [152] |
| Activation of the c-Jun-N-Terminal kinase (JNK) pathway, leading to reduction of cell viability and induction of apoptosis. | Human cancer cell line HCT-116 | [157] |
| Elevation of Bax and caspase3, downregulation of the anti-apoptotic protein BCL-2, and induction of mitochondrial-mediated apoptosis. | Human cancer cell HT-29 | [158] |
| Repression of the TGF-β1/Smad pathway and reduction of the levels of AST, ALT, hydroxyproline (Hyp), HA, LN, TNF-α, IL-6, extracellular matrix (ECM) production, and Smad2/3 phosphorylation. | HSC-T6 cells and male C57 mice | [160] |
| Reduction of the expression levels of ALP, ALT, AST, TGF-β1, HA, Hyp, F4/80þ macrophage infiltration, MCP-1, TNF-α, IL-1β, IL-6, TNF-α, and IL-1β, Gli-1, and Shh. | Littermate male C57BL/6J mice | [160] |
| Decreasing the expression of α-SMA, Col1α1, Col3α1, TIMP-1, PAI-1, and Gli-1 and increasing the levels of Bax and Caspase-3 that results in apoptosis. | LX-2 liver cells | [161] |
| Inhibition of cells migration, decrease cell viability, and induction of apoptosis via obstructing the intracellular signalling, including focal adhesion kinase (FAK), p38 phosphorylation, and caspase-3 activation. | Miapaca-2, Panc-1, and SNU-213 pancreatic cancer cell lines | [129] |
| Reduction of renal fibrosis, normalising renal function, and decreasing the expression of fibronectin (FN), Collagen I, α-SMA, EMT, Shh, Gli-1, and E-cadherin. | Renal cancer (NRK)-52E cell line and UUO-mouse model | [163] |
| Induction of apoptosis through a dose-dependent increase in the Bax/Bcl-2 ratio, cyt-c, caspase-3, caspase-9, AIF, and Apaf-1 via a mitochondrial-dependent mechanism. | Gastric cancer cells | [156] |
| Reduction of cell migration and invasion by inhibiting the expression of genes linked to the metastasis and lowering disruptor of telemetric silencing 1-like (DOT1L) and histone H3K79 methylation by controlling CBP activity. | Gastric cancer cells | [164] |
| Reduction of cell viability by decreasing Bcl-2 and raising Bax protein expression, thus inducing apoptosis, in addition to cell cycle arrest by decreasing cyclin B1 CDK1 and enhancing tumour suppressor gene p21 activation p38 MAPK, which arrests the G2/M phase. | U251 and U87 glioblastoma cells | [131] |
| Prevention of tumour growth via activating caspase-3 and -9, raising the Bax/Bcl-2 ratio, which caused apoptosis, and downregulation of the HIF-1α, VEGF, and VEGFR2 signalling pathway. In addition, decreasing the expression of cyclin B1 and D1 while increasing the expression of Claudin-1 and ZO-1 decreased the growth of cancer cells. | C6 glioma cells implanted in Wister rats | [169] |
| Induction of apoptosis via raising caspase-3 activity, MMP loss, and cell cycle arrest in the G2/M and G0/G1 phases. | HL60 leukaemia cell lines | [141] |
| Induction of apoptosis and cell cycle arrest at the G0/G1 phase, in addition to increasing the expression of the DUSP1 (dual specificity phosphatase 1), DUSP3, DUSP5, CDK1A, CDK1B GADD45B, SPRR2D, MT1F, MT1A, p27Kip1, CASP4, and NFKBIA genes. Moreover, elevation of the production of BAD, caspase-3, luciferase activity, PARP cleavage, and Notch 1 signalling. | K562 leukaemia cells | [140,170] |
| Increasing ROS generation, JNK1/2, p38, Bax, and p21 expression, while suppressing ERK1/2, cyclin B1, D1, D3, and E1 expression, which resulted in apoptosis and decreased cell viability. | A431 human cancer cells | [171] |
| Reduction of the expression of GSH, Bcl-2, and survivin while increasing the generation of ROS, cyt-c, caspase-9, -3, Apaf-1, Bax, and Sufu (suppressor of fused protein), in addition to decreasing PI3K/AKT signalling pathway, cyclin D1, MMP-2,9, and PI3K-p85 expression, as well as increased PTEN phosphorylation and p21 expression, which results in cell cycle arrest at the G0/G1 phase. | Oesophageal Eca-109 cancer cells | [173] |
5.1.6. Isoflavones
| Anticancer Effect of Genistein | Cancer Type | Citation |
|---|---|---|
| Inhibition of NF-kB pathways and cell proliferation. | MDA-MB-231 | [196] |
| Suppression of Akt activity, which encourages the deactivation of downstream signalling pathways, such as NF-κB. | MDA-MB-231 | [197] |
| Reduction of the expression of MMPs 2, 3, 3, and 15, in addition to inhibition of angiogenesis and metastasis. | T47D cells | [198] |
| Dysregulation of the human oncoprotein, known as the carcinogenic inhibitor of protein phosphatase 2A (CIP2A), results in the induction of apoptosis and growth suppression. | MCF-7-C3 and T47D breast cancer cells | [192] |
| Induction of intracellular copper mobilisation leads to the production of ROS, which causes pro-oxidant cell death. | Prostate cancer cell lines LNCaP and DU145 | [199] |
| Reduction of serum levels of prostate-specific antigen (PSA). | Prostate cancer cell lines | [201] |
5.2. Phenolic Acids
5.2.1. Gallic Acid
5.2.2. Caffeic Acid
5.2.3. Rosmarinic Acid
5.2.4. Sinapic Acid
5.3. Curcuminoids: Curcumin
5.4. Stilbenes
5.5. Lignans
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ABCB8 | ATP-Binding Cassette subfamily B member 8 |
| ADAM17 | A Disintegrin And Metalloprotease 17 |
| ADP | Adenosine Diphosphate |
| AgNPs | Silver nanoparticles |
| Ahr | Aryl hydrocarbon receptor |
| AIF | Apoptosis Inducing Factor |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Transaminase |
| Antioxcin6 | Mitochondrial Targeted Antioxidant |
| AP-1 | Activator Protein-1 |
| APAF1 | Apoptotic Protease-Activating Factor 1 |
| ASK-1 | Apoptosis signal-regulating kinase 1 |
| AST | Aspartate aminotransferase |
| ATL | Adult T Cell Leukaemia |
| ATP | Adenosine Triphosphate |
| BAX | Bcl-2 Associated X Protein |
| B-CLL | B-cell Chronic Lymphocytic Leukaemia |
| Bcl-2 | B-Cell Lymphoma 2 |
| BNIP3 | BCL-2 Interacting Protein 3 |
| CA | Caffeic Acid |
| CASP | Caspase |
| CAT | Catalase |
| CCND1 | Cyclin D1 |
| CD44 | Cell surface adhesion receptor |
| CDC2 | Cell division cycle protein 2 |
| CDH1 | Cadherin 1 |
| CDH2 | Cadherin 2 |
| CDK1 | Cyclin-Dependent Kinase 1 |
| CDK2 | Cyclin-Dependent Kinase 2 |
| CDK4 | Cyclin-Dependent Kinase 4 |
| CDK1A | Cyclin-Dependent Kinase Inhibitor 1A |
| CDK1B | Cyclin-Dependent Kinase Inhibitor 1B |
| CEA | Carcinoembryonic Antigen |
| C/EBP | CCAAT/Enhancer Binding Protein |
| c-FLIP | Cellular FLICE (FADD-like IL-1β-converting enzyme)-Inhibitory Protein |
| cIAP1 | Cellular Inhibitor of Apoptosis Protein-1 |
| cIAP2 | Cellular Inhibitor of Apoptosis Protein-2 |
| CIS | Cisplatin |
| CIP2A | Carcinogenic Inhibitor of Protein Phosphatase 2A |
| CLNs | Curcumin-containing catanionic Lipid Nanosystems |
| Col1α1 | Collagen, type I, alpha 1 |
| Col3α1 | Collagen type III alpha 1 chain |
| ConA | Concanavalin A |
| COX-2 | Cyclooxygenase-2 |
| CSCs | Cancer Stem Cells |
| CTNNB | Catenin Beta 1 |
| CUR | Curcumin |
| CVD | Cardiovascular Disease |
| CYCS | Cytochrome C, Somatic |
| CYP1A1 | Cytochrome P450, family 1, subfamily A, Polypeptide 1 |
| CYP3A4 | Cytochrome P450 family 3, subfamily A, polypeptide 4 |
| Cyt-c | Cytochrome complex |
| DBCLS | Dibenzocyclooctadiene lignans |
| DENA | Diethylnitrosamine |
| DLC1 | DLC1 Rho GTPase Activating Protein |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| DMBS | 3,4-Dimethoxybenzenesulfonamide |
| DMH | 1,2-Dimethylhydrazine |
| DMSO | Dimethylsulfoxide |
| DNA | Deoxyribonucleic Acid |
| DOT1L | Disruptor of Telomeric Silencing 1-Like |
| DOX | Doxorubicin |
| DR4 | Death receptor 4 |
| DR5 | Death receptor 5 |
| DSS | Dextran Sulfate Sodium |
| DUSP1 | Dual specificity protein phosphatase 1 |
| DUSP3 | Dual specificity protein phosphatase 3 |
| DUSP5 | Dual specificity protein phosphatase 5 |
| D. caffra | Dovyalis caffra |
| EAFE | Ethyl Acetate Fractionated Extract |
| ECM | Extracellular Matrix |
| EGCG | Epigallocatechin-3-Gallate |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| EMT | Epithelial-Mesenchymal Transition |
| EPI | Epicatechin |
| ER | Endoplasmic Reticulum |
| ER | Estrogen Receptor |
| α-ER | Estrogen Receptor Alpha |
| β-ER | Estrogen Receptor Beta |
| ERK | Extracellular Signal-Regulated Proteinase |
| ERS | Endoplasmic Reticulum Stress |
| EZH2 | Enhancer of Zeste Homologue 2 |
| FAD | Flavin Adenine Dinucleotide |
| FAK | Focal Adhesion Kinase |
| FasL | Fas Ligand |
| FIH-1 | Factor-Inhibiting HIF-1 |
| FN | Fibronectin |
| FOXM1 | Forkhead Box M1 |
| 5-FU | 5-Fluorouracil |
| GA | Gallic Acid |
| GADD45A | Growth Inhibition And DNA-Damage-Inducible 45 Alpha |
| GADD45B | Growth Arrest and DNA-Damage Inducible Beta |
| GC | Gastric Cancer |
| Gli-1 | Glioma-associated oncogene |
| GSK3β | Glycogen Synthase Kinase-3 beta |
| GPx | Glutathione Peroxidase |
| GPx4 | Glutathione Peroxidase 4 |
| GRP78 | 78 kDa Glucose-Regulated Protein |
| GR | Glutathione Reductase |
| GSH | Glutathione |
| HA | Hyaluronic Acid |
| HC | Hepatic Cells |
| HCC | Hepatocellular Carcinoma |
| HDAC | Histone Deacetylase |
| HEPG2 | Human Hepatocellular Liver Cancer Cells |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HER2-TK | Human Epidermal Growth Factor Receptor 2-Tyrosine Kinase |
| HETNPs | Hesperetin-loaded Nanoparticles |
| HGF | Hepatocyte Growth Factor |
| HIF-α | Hypoxia-Inducible Factor 1-Alpha |
| HO-1 | Heme Oxygenase-1 |
| Hrk | HaraKiri |
| HSCs | Hepatic Stellate cells |
| HSP | Hesperetin |
| HTLV-1 | Human T-Lymphotropic Virus Type 1 |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| Hyp | Hydroxyproline |
| IC50: | Half maximal inhibitory concentration |
| IGF1R | Type 1 Insulin-Like Growth Factor Receptor |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IKKβ | Inhibitory kappa B Kinase Beta |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-12 | Interleukin 12 |
| IRAK4 | Interleukin 1 Receptor Associated Kinase 4 |
| IR-beta | Insulin Receptor-Beta Subunit |
| IUPAC | International Union of Pure and Applied Chemistry |
| JAK | Janus Kinase |
| JNK | Jun N-Terminal Kinases |
| LC3B | Light chain 3B |
| LPO | Lipid Peroxidation |
| 67LR | 67 kDa Laminin Receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| MARK4 | Microtubule Affinity-Regulating Kinase 4 |
| MBS | 4-Methoxybenzenesulfon |
| MD-2 | Myeloid Differentiation Factor 2 |
| MCL-1 | Myeloid Cell Leukaemia-1 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MDM2 | Mouse Double Minute 2 Homologue |
| MDR | MultiDrug Resistance |
| MEK | Mitogen-Activated protein kinase Kinse |
| MiR | MicroRNA |
| MMPBSA | Molecular Mechanics Poisson-Boltzmann Surface Area |
| MMP-2 | Matrix Metalloproteinase-2 |
| MMP-9 | Matrix Metalloproteinase-9 |
| MMP | Matrix Metalloproteinase |
| MM | Multiple myeloma |
| MPA | Medroxy Progesterone Acetate |
| mRNA | Messenger ribonucleic acid |
| MT1A | Metallothionein 1A |
| MT1F | Metallothionein 1F |
| mtFe | Mitochondrial iron (II) |
| mtNEET | Mitochondrial protein mitoNEET |
| MTX | Methotrexate |
| mTOR | Mammalian Target Of Rapamycin |
| M2-TAMs | Tumour-Associated Macrophages with the M2 phenotype |
| NAD | Nicotinamide Adenine Dinucleotide |
| NADPH | Reduced Nicotinamide Adenine Dinucleotide Phosphate |
| Nar | Naringenin |
| NFKBIA | Nuclear Factor-Kappa-B-Inhibitor Alpha |
| NF-Kb | Nuclear Factor Kappa B |
| NLRP3 | NLR Family Pyrin Domain-containing 3 |
| Nrf2 | Nuclear Factor Erythroid Related Factor 2 |
| NSCLC | Non-Small Cell Lung Carcinoma |
| OS | Oxidative Stress |
| P-AKT | Phospho-Akt |
| PAI | Plasminogen Activator Inhibitor-1 |
| PARP-1 | Poly-ADP-Ribose Polymerase 1 |
| PCNA | Proliferating Cell Nuclear Antigen |
| PC-3 | Prostate Cancer-3 |
| PBMC | Peripheral Blood Mononuclear Cells |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PDZD8 | PDZ domain-containing 8 |
| PEGFR | Phosphorylated-epidermal growth factor receptor |
| PEL | Primary Effusion Lymphoma |
| PERK | Phosphorylation of Extracellular Signal-Related Kinase |
| PGE2 | Prostaglandin E2 |
| P-gp | P-glycoprotein |
| PhK | Phosphorylase Kinase |
| PI3K | Phosphatidylinositol 3-kinase |
| PKA | Protein Kinase A |
| PKB | Protein Kinase B |
| PKM2 | Pyruvate Kinase Isozymes M1/M2 |
| PLGA | Poly (Lactic-co-Glycolic Acid) |
| PMA | Phorbol Myristate Acetate |
| PPARG | Peroxisome Proliferator-Activated Receptor Gamma |
| PRKCA | Protein Kinase C Alpha Gene |
| Prp4B | Pre-RNA processing factor 4B |
| PSA | Prostate-Specific Antigen |
| PTEN | Phosphatase and Tensin Homologue |
| PTE | Pterostilbene |
| PTX | Paclitaxel |
| RA | Rosmarinic Acid |
| RANK | Receptor Activator of Nuclear Factor Kappa-Β |
| RANKL | Receptor Activator of Nuclear Factor Kappa-Β Ligand |
| RASSF-1a | Ras-Association Domain Family-1a |
| Rb | Retinoblastoma protein |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| RSV | Resveratrol |
| SA | Sinapic Acid |
| SaNPs | Sinapic acid-loaded Nanoparticles |
| SCC | Squamous Cell Carcinoma |
| SCCHN | Squamous Cell Carcinoma of The Head and Neck |
| SCID | Severe Combined Immunodeficiency |
| Shh | Sonic hedgehog protein |
| SIRT1 | Sirtuin 1 |
| SKP2 | S Phase Kinase Associated Protein 2 |
| SLC7A11 | Solute Carrier Family 7 Member 11 |
| SLNs | Solid Lipid Nanoparticles |
| SOD | Superoxide Dismutase |
| SPRR2D | Small Proline Rich Protein 2D |
| STAT | Signal Transducer and Activator of Transcription |
| Sufu | Suppressor of Fused Protein |
| t-BHP | tert-Butyl Hydroperoxide |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TIMP-1 | Tissue Inhibitor of Metalloproteinases 1 |
| TIMP-2 | Tissue Inhibitor of Metalloproteinases 2 |
| TLR4 | Toll-Like Receptor 4 |
| TMBS | 3,4,5-Trimethoxybenzenesulfonamide |
| TME | Tumour Microenvironment |
| TNBS | 2,4,6-Trinitrobenzene Sulfonic Acid |
| TNFRSF11B | TNF Superfamily 11B Receptor |
| TNFRSF25 | Tumour Necrosis Factor Receptor Superfamily 25 |
| TNF-α | Tumour Nerosis Factor-Alpha |
| TOPK | T-LAK Cell-Originated Protein Kinase |
| TRAIL | Tumour Necrosis Factor (TNF)- Related Apoptosis-Inducing Ligand |
| TRIF | TIR-Domain-Containing Adapter-Inducing Interferon-β |
| UGT1A3 | UDP Glucuronosyltransferase Family 1 Member A3 |
| UPA | U-Plasminogen Activator |
| UV | Ultraviolet rays |
| UVB | Ultraviolet rays type B |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| VEGF | Vascular Endothelial Growth Factor |
| VV-IL-24 | Vaccinia Virus that encoded the IL-24 gene |
| WHO | World Health Organization |
| Wnt | Wingless-Related Integration Site |
| WT1 | Wilms Tumour Protein |
| XIAP | X-Linked Inhibitor of Apoptosis |
| α-SMA | Alpha-Smooth Muscle Actin |
| γCD | Gamma-Cyclodextrin |
References
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Tufarelli, V.; Casalino, E.; D’Alessandro, A.G.; Laudadio, V. Dietary phenolic compounds: Biochemistry, metabolism and significance in animal and human health. Curr. Drug Metab. 2017, 18, 905–913. [Google Scholar] [CrossRef]
- Konstantinou, E.K.; Panagiotopoulos, A.A.; Argyri, K.; Panoutsopoulos, G.I.; Dimitriou, M.; Gioxari, A. Molecular pathways of rosmarinic acid anticancer activity in triple-negative breast cancer cells: A literature review. Nutrients 2023, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Maruca, A.; Catalano, R.; Bagetta, D.; Mesiti, F.; Ambrosio, F.A.; Romeo, I.; Moraca, F.; Rocca, R.; Ortuso, F.; Artese, A.; et al. The Mediterranean Diet as source of bioactive compounds with multi-targeting anti-cancer profile. Eur. J. Med. Chem. 2019, 181, 111579. [Google Scholar] [CrossRef]
- Kumari, M.; Siddiqui, M.A.; Gupta, A. Recent Advancement and Novel Application of Natural Polyphenols for the Treatment of Allergy Asthma: From Phytochemistry to Biological Implications. Crit. Rev. Immunol. 2023, 43, 29–41. [Google Scholar] [CrossRef]
- Rauf, A.; Shariati, M.A.; Imran, M.; Bashir, K.; Khan, S.A.; Mitra, S.; Emran, T.B.; Badalova, K.; Uddin, M.S.; Mubarak, M.S.; et al. Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environ. Sci. Pollut. Res. 2022, 29, 31025–31041. [Google Scholar] [CrossRef]
- Yoganathan, S.; Alagaratnam, A.; Acharekar, N.; Kong, J. Ellagic acid and schisandrins: Natural biaryl polyphenols with therapeutic potential to overcome multidrug resistance in cancer. Cells 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi, S.; Abbaszadeh, H. Natural lignans honokiol and magnolol as potential anticarcinogenic and anticancer agents. A comprehensive mechanistic review. Nutr. Cancer 2022, 74, 761–778. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S. Plant polyphenols and human health: Novel findings for future therapeutic developments. Nutrients 2023, 15, 3764. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.H.; Bouyahya, A. Dietary phenolic compounds as anticancer natural drugs: Recent update on molecular mechanisms and clinical trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in colorectal cancer: Current state of knowledge including clinical trials and molecular mechanism of action. BioMed Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mohammed, A.H.; Auda, N.A.; Alsallameh, S.M.; Albekairi, N.A.; Muhseen, Z.T.; Butch, C.J. Network Pharmacology, Molecular Docking, and Molecular Dynamics Simulation Analysis Reveal Insights into the Molecular Mechanism of Cordia myxa in the Treatment of Liver Cancer. Biology 2024, 13, 315. [Google Scholar] [CrossRef]
- David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Kong, M.; Xie, K.; Lv, M.; Li, J.; Yao, J.; Yan, K.; Wu, X.; Xu, Y.; Ye, D. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed. Pharmacother. 2021, 133, 110975. [Google Scholar] [CrossRef]
- Chagas, M.D.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A review on its anti-inflammatory properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Espíndola, K.M.; Ferreira, R.G.; Narvaez, L.E.; Silva Rosario, A.C.; Da Silva, A.H.; Silva, A.G.; Vieira, A.P.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Therapeutic and nutraceutical potential of rosmarinic acid—Cytoprotective properties and pharmacokinetic profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 1799–1806. [Google Scholar] [CrossRef]
- Diederich, M.; Giblin, L.; Malki, M.C. Natural Products and the Hallmarks of Chronic Diseases. In Proceedings of the COST Action 16112—Personalized Nutrition in Ageing Society: Redox Control of Major Age-Related Diseases, Luxembourg, 25–27 March 2019. [Google Scholar]
- Kalinowska, M.; Gołębiewska, E.; Świderski, G.; Męczyńska-Wielgosz, S.; Lewandowska, H.; Pietryczuk, A.; Cudowski, A.; Astel, A.; Świsłocka, R.; Samsonowicz, M.; et al. Plant-derived and dietary hydroxybenzoic acids—A comprehensive study of structural, anti-/pro-oxidant, lipophilic, antimicrobial, and cytotoxic activity in MDA-MB-231 and MCF-7 cell lines. Nutrients 2021, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Polyphenols and intestinal health. In Nutrition and Functional Foods for Healthy Aging; Academic Press: Cambridge, MA, USA, 2017; pp. 191–210. [Google Scholar]
- Mahfuz, S.; Mun, H.S.; Dilawar, M.A.; Ampode, K.M.; Yang, C.J. Potential role of protocatechuic acid as natural feed additives in farm animal production. Animals 2022, 12, 741. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic acid, a bioactive phenolic compound, counteracts LPS-induced neurotoxicity by regulating c-Jun N-terminal kinase in mouse brain. Int. J. Mol. Sci. 2020, 22, 361. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef]
- Jafernik, K.; Motyka, S.; Calina, D.; Sharifi-Rad, J.; Szopa, A. Comprehensive review of dibenzocyclooctadiene lignans from the Schisandra genus: Anticancer potential, mechanistic insights and future prospects in oncology. Chin. Med. 2024, 19, 17. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Zou, H.; Wang, L.; Jin, B. Plant anthocyanins: Classification, biosynthesis, regulation, bioactivity, and health benefits. Plant Physiol. Biochem. 2024, 217, 109268. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.E.; Lee, S.; Mond, J.; Russell, J.; Mitchell, P.; Flood, V.M. Dietary flavonoid intake in older adults: How many days of dietary assessment are required and what is the impact of seasonality? Nutr. J. 2018, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Lage, N.N.; Layosa, M.A.; Arbizu, S.; Chew, B.P.; Pedrosa, M.L.; Mertens-Talcott, S.; Talcott, S.; Noratto, G.D. Dark sweet cherry (Prunus avium) phenolics enriched in anthocyanins exhibit enhanced activity against the most aggressive breast cancer subtypes without toxicity to normal breast cells. J. Funct. Foods 2020, 64, 103710. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-Inflammatory and Anticancer Effects of Anthocyanins in In Vitro and In Vivo Studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef]
- Li, G.; Ding, K.; Qiao, Y.; Zhang, L.; Zheng, L.; Pan, T.; Zhang, L. Flavonoids regulate inflammation and oxidative stress in cancer. Molecules 2020, 25, 5628. [Google Scholar] [CrossRef]
- Eskra, J.N.; Schlicht, M.J.; Bosland, M.C. Effects of black raspberries and their ellagic acid and anthocyanin constituents on taxane chemotherapy of castration-resistant prostate cancer cells. Sci. Rep. 2019, 9, 4367. [Google Scholar] [CrossRef]
- Mazewski, C.; Kim, M.S.; Gonzalez de Mejia, E. Anthocyanins, delphinidin-3-O-glucoside and cyanidin-3-O-glucoside, inhibit immune checkpoints in human colorectal cancer cells in vitro and in silico. Sci. Rep. 2019, 9, 11560. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Tuberoso, C.I.; Jerković, I. The Role of Rosmarinic Acid in Cancer Prevention and Therapy: Mechanisms of Antioxidant and Anticancer Activity. Antioxidants 2024, 13, 1313. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry anthocyanins and pyruvic acid adducts: Anticancer properties in breast cancer cell lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef]
- Hui, C.; Bin, Y.; Xiaoping, Y.; Long, Y.; Chunye, C.; Mantian, M.; Wenhua, L. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo. Nutr. Cancer 2010, 62, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Zhou, J.; Luo, L.P.; Han, B.; Li, F.; Chen, J.Y.; Zhu, Y.F.; Chen, W.; Yu, X.P. Black rice anthocyanins suppress metastasis of breast cancer cells by targeting RAS/RAF/MAPK pathway. BioMed Res. Int. 2015, 2015, 414250. [Google Scholar] [CrossRef]
- Lim, S.; Xu, J.; Kim, J.; Chen, T.Y.; Su, X.; Standard, J.; Carey, E.; Griffin, J.; Herndon, B.; Katz, B.; et al. Role of anthocyanin-enriched purple-fleshed sweet potato p40 in colorectal cancer prevention. Mol. Nutr. Food Res. 2013, 57, 1908–1917. [Google Scholar] [CrossRef]
- Jang, H.; Ha, U.S.; Kim, S.J.; Yoon, B.I.; Han, D.S.; Yuk, S.M.; Kim, S.W. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J. Agric. Food Chem. 2010, 58, 12686–12691. [Google Scholar] [CrossRef] [PubMed]
- Bontempo, P.; De Masi, L.; Carafa, V.; Rigano, D.; Scisciola, L.; Iside, C.; Grassi, R.; Molinari, A.M.; Aversano, R.; Nebbioso, A.; et al. Anticancer activities of anthocyanin extract from genotyped Solanum tuberosum L.“Vitelotte”. J. Funct. Foods 2015, 19, 584–593. [Google Scholar] [CrossRef]
- Martin, M.Á.; Ramos, S. Impact of cocoa flavanols on human health. Food Chem. Toxicol. 2021, 151, 112121. [Google Scholar] [CrossRef]
- Joshi, R.; Kulkarni, Y.A.; Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sci. 2018, 215, 43–56. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Xiao, B.; He, X.; Ying, G.; Zha, H.; Yang, C.; Jin, X.; Li, G.; Ping, L.; et al. Isorhamnetin alleviates cisplatin-induced acute kidney injury via enhancing fatty acid oxidation. Free Radic. Biol. Med. 2024, 212, 22–33. [Google Scholar] [CrossRef]
- Ismaeel, A.; McDermott, M.M.; Joshi, J.K.; Sturgis, J.C.; Zhang, D.; Ho, K.J.; Sufit, R.; Ferrucci, L.; Peterson, C.A.; Kosmac, K. Cocoa flavanols, Nrf2 activation, and oxidative stress in peripheral artery disease: Mechanistic findings in muscle based on outcomes from a randomized trial. Am. J. Physiol.-Cell Physiol. 2024, 326, C589–C605. [Google Scholar] [CrossRef]
- Vogiatzoglou, A.; Mulligan, A.A.; Luben, R.N.; Lentjes, M.A.; Heiss, C.; Kelm, M.; Merx, M.W.; Spencer, J.P.; Schroeter, H.; Kuhnle, G.G. Assessment of the dietary intake of total flavan-3-ols, monomeric flavan-3-ols, proanthocyanidins and theaflavins in the European Union. Br. J. Nutr. 2014, 111, 1463–1473. [Google Scholar] [CrossRef]
- Georgiou, N.; Kakava, M.G.; Routsi, E.A.; Petsas, E.; Stavridis, N.; Freris, C.; Zoupanou, N.; Moschovou, K.; Kiriakidi, S.; Mavromoustakos, T. Quercetin: A potential polydynamic drug. Molecules 2023, 28, 8141. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Kim, J.K.; Park, S.U. Quercetin and its role in biological functions: An updated review. EXCLI J. 2018, 17, 856. [Google Scholar] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, E.; Esmaeili, A.; Rahgozar, S.; Nourbakhshnia, M. Application of quercetin in neurological disorders: From nutrition to nanomedicine. Rev. Neurosci. 2019, 30, 555–572. [Google Scholar] [CrossRef]
- Sturza, A.; Pavel, I.; Ancușa, S.; Danciu, C.; Dehelean, C.; Duicu, O.; Muntean, D. Quercetin exerts an inhibitory effect on cellular bioenergetics of the B164A5 murine melanoma cell line. Mol. Cell. Biochem. 2018, 447, 103–109. [Google Scholar] [CrossRef]
- Pham, T.N.; Stempel, S.; Shields, M.A.; Spaulding, C.; Kumar, K.; Bentrem, D.J.; Matsangou, M.; Munshi, H.G. Quercetin enhances the anti-tumor effects of BET inhibitors by suppressing hnRNPA1. Int. J. Mol. Sci. 2019, 20, 4293. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Wu, L.; Li, J.; Liu, T.; Li, S.; Feng, J.; Yu, Q.; Zhang, J.; Chen, J.; Zhou, Y.; Ji, J.; et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med. 2019, 8, 4806–4820. [Google Scholar] [CrossRef]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M.; et al. Quercetin suppresses proliferation of liver cancer cell lines in vitro. Anticancer Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef]
- Erdogan, S.; Turkekul, K.; Dibirdik, I.; Doganlar, O.; Doganlar, Z.B.; Bilir, A.; Oktem, G. Midkine downregulation increases the efficacy of quercetin on prostate cancer stem cell survival and migration through PI3K/AKT and MAPK/ERK pathway. Biomed. Pharmacother. 2018, 107, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Lei, C.S.; Hou, Y.C.; Pai, M.H.; Lin, M.T.; Yeh, S.L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Pourmadadi, M.; Eshaghi, M.M.; Rahmani, E.; Shojaei, S.; Paiva-Santos, A.C.; Rahdar, A.; Behzadmehr, R.; García-Martín, M.L.; Díez-Pascual, A.M. Nanomaterials loaded with Quercetin as an advanced tool for cancer treatment. J. Drug Deliv. Sci. Technol. 2022, 78, 103938. [Google Scholar] [CrossRef]
- Bakun, P.; Mlynarczyk, D.T.; Koczorowski, T.; Cerbin-Koczorowska, M.; Piwowarczyk, L.; Kolasiński, E.; Stawny, M.; Kuźmińska, J.; Jelińska, A.; Goslinski, T. Tea-break with epigallocatechin gallate derivatives–Powerful polyphenols of great potential for medicine. Eur. J. Med. Chem. 2023, 261, 115820. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Shi, Y.Q.; Chen, X.; Dai, J.; Jiang, Z.F.; Li, N.; Zhang, Z.B. Protective effect of curcumin against formaldehyde-induced genotoxicity in A549 Cell Lines. J. Appl. Toxicol. 2013, 33, 1468–1473. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H.S.; Song, Y.S. Genistein as a potential anticancer agent against ovarian cancer. J. Tradit. Complement. Med. 2012, 2, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Human cancer stem cells are a target for cancer prevention using (−)-epigallocatechin gallate. J. Cancer Res. Clin. Oncol. 2017, 143, 2401–2412. [Google Scholar] [CrossRef]
- Yu, C.; Jiao, Y.; Xue, J.; Zhang, Q.; Yang, H.; Xing, L.; Chen, G.; Wu, J.; Zhang, S.; Zhu, W.; et al. Metformin sensitizes non-small cell lung cancer cells to an epigallocatechin-3-gallate (EGCG) treatment by suppressing the Nrf2/HO-1 signaling pathway. Int. J. Biol. Sci. 2017, 13, 1560. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Cortez Penso, N.E.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Epigallocatechin-3-gallate (EGCG) suppresses pancreatic cancer cell growth, invasion, and migration partly through the inhibition of Akt pathway and epithelial–mesenchymal transition: Enhanced efficacy when combined with gemcitabine. Nutrients 2019, 11, 1856. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: Recent trends and advancement. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 80, pp. 256–275. [Google Scholar]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-cancer effects of green tea epigallocatchin-3-gallate and coffee chlorogenic acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Ohishi, T.; Hayakawa, S.; Miyoshi, N. Involvement of microRNA modifications in anticancer effects of major polyphenols from green tea, coffee, wine, and curry. Crit. Rev. Food Sci. Nutr. 2023, 63, 7148–7179. [Google Scholar] [CrossRef]
- Bimonte, S.; Albino, V.; Piccirillo, M.; Nasto, A.; Molino, C.; Palaia, R.; Cascella, M. Epigallocatechin-3-gallate in the prevention and treatment of hepatocellular carcinoma: Experimental findings and translational perspectives. Drug Des. Dev. Ther. 2019, 12, 611–621. [Google Scholar] [CrossRef]
- Won, H.R.; Lee, P.; Oh, S.R.; Kim, Y.M. Epigallocatechin-3-gallate suppresses the expression of TNF-α-induced MMP-1 via MAPK/ERK signaling pathways in human dermal fibroblasts. Biol. Pharm. Bull. 2021, 44, 18–24. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Ding, Y.; Feng, G. CAMSAP2 promotes colorectal cancer cell migration and invasion through activation of JNK/c-Jun/MMP-1 signaling pathway. Sci. Rep. 2022, 12, 16899. [Google Scholar] [CrossRef]
- Fang, C.Y.; Wu, C.C.; Hsu, H.Y.; Chuang, H.Y.; Huang, S.Y.; Tsai, C.H.; Chang, Y.; Tsao, G.S.; Chen, C.L.; Chen, J.Y. EGCG inhibits proliferation, invasiveness and tumor growth by up-regulation of adhesion molecules, suppression of gelatinases activity, and induction of apoptosis in nasopharyngeal carcinoma cells. Int. J. Mol. Sci. 2015, 16, 2530–2558. [Google Scholar] [CrossRef]
- Djerir, D.; Iddir, M.; Bourgault, S.; Lamy, S.; Annabi, B. Biophysical evidence for differential gallated green tea catechins binding to membrane type-1 matrix metalloproteinase and its interactors. Biophys. Chem. 2018, 234, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Nusri Qel, A.; Begum, S.; Javed, E.; Rizvi, T.A.; Hussain, A. (-)-Epigallocatechin-3-gallate induces apoptosis and inhibits invasion and migration of human cervical cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 4815–4822. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jung, C.H.; Song, B.; Park, O.J.; Kim, Y.M. Pro-apoptotic and migration-suppressing potential of EGCG, and the involvement of AMPK in the p53-mediated modulation of VEGF and MMP-9 expression. Oncol. Lett. 2013, 6, 1346–1350. [Google Scholar] [CrossRef]
- Harakeh, S.; Diab-Assaf, M.; Azar, R.; Hassan, H.M.; Tayeb, S.; Abou-El-Ardat, K.; Damanhouri, G.A.; Qadri, I.; Abuzenadah, A.; Chaudhary, A.; et al. Epigallocatechin-3-gallate inhibits tax-dependent activation of nuclear factor kappa B and of matrix metalloproteinase 9 in human T-cell lymphotropic virus-1 positive leukemia cells. Asian Pac. J. Cancer Prev. 2014, 15, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Chen, W.; Lung, W.Y.; Wei, X.Y.; Cheng, B.H.; Cai, Z.M.; Huang, W.R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, K.; Liu, Q.; Yang, C.; et al. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Xie, P.; Li, H.; Zhang, X.; Sun, X.; Yu, J.; Xing, L. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother. Oncol. 2014, 110, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Suzuki, T.; Ohishi, T.; Isemura, M.; Nakamura, Y.; Unno, K. Effects of epigallocatechin-3-gallate on matrix metalloproteinases in terms of its anticancer activity. Molecules 2023, 28, 525. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Rauf, A.; Wilairatana, P.; Joshi, P.B.; Ahmad, Z.; Olatunde, A.; Hafeez, N.; Hemeg, H.A.; Mubarak, M.S. Revisiting luteolin: An updated review on its anticancer potential. Heliyon 2024, 10, e26701. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.Q.; Xie, K.P.; Xie, M.J. Inhibitory effect of luteolin on the proliferation of human breast cancer cell lines induced by epidermal growth factor. Sheng Li Xue Bao [Acta Physiol. Sin.] 2016, 68, 27–34. [Google Scholar]
- Lo, S.; Leung, E.; Fedrizzi, B.; Barker, D. Syntheses of mono-acylated luteolin derivatives, evaluation of their antiproliferative and radical scavenging activities and implications on their oral bioavailability. Sci. Rep. 2021, 11, 12595. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, B.; Gao, F.; Shi, R. Modulation of G2/M cell cycle arrest and apoptosis by luteolin in human colon cancer cells and xenografts. Oncol. Lett. 2018, 15, 1559–1565. [Google Scholar] [CrossRef]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer 2022, 74, 677–686. [Google Scholar] [CrossRef]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Li, L.; Wang, L.; Dong, L.; Xi, G.; Lu, Y.J.; Li, Z. Luteolin impacts deoxyribonucleic acid repair by modulating the mitogen-activated protein kinase pathway in colorectal cancer. Bioengineered 2022, 13, 10998–11011. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, T.; Wang, J.; Mao, Z.; Duan, B.; Long, Y.; Xue, F.; Liu, D.; Liu, S.; Gao, Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J. Cancer 2018, 9, 3669. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; He, K.; Jiang, W.; Xu, C.; Li, Z.; Wang, H.; Wang, W.; Wang, H.; Teng, X.; et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J. Transl. Med. 2015, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.D.; Hu, L.; Fan, Z.Y.; Wang, H.X.; Zhu, Z.L.; Cao, S.; Wu, X.Y.; Li, J.F.; Su, L.P.; Li, C.; et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 2017, 15, 52. [Google Scholar] [CrossRef]
- Song, S.; Su, Z.; Xu, H.; Niu, M.; Chen, X.; Min, H.; Zhang, B.; Sun, G.; Xie, S.; Wang, H.; et al. Luteolin selectively kills STAT3 highly activated gastric cancer cells through enhancing the binding of STAT3 to SHP-1. Cell Death Dis. 2017, 8, e2612. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. Vitr. 2011, 25, 1385–1391. [Google Scholar] [CrossRef]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem.-Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, H.S.; Lee, J.H.; Chi, G.Y.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Hyun, J.W.; Kim, W.J.; Choi, Y.H. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food Chem. Toxicol. 2013, 56, 100–109. [Google Scholar] [CrossRef]
- Ma, L.; Peng, H.; Li, K.; Zhao, R.; Li, L.; Yu, Y.; Wang, X.; Han, Z. Luteolin exerts an anticancer effect on NCI-H460 human non-small cell lung cancer cells through the induction of Sirt1-mediated apoptosis. Mol. Med. Rep. 2015, 12, 4196–4202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, R.; Tian, J.; Song, M.; Zhao, R.; Liu, K.; Zhu, F.; Shim, J.H.; Dong, Z.; Lee, M.H. Targeting LIMK1 with luteolin inhibits the growth of lung cancer in vitro and in vivo. J. Cell. Mol. Med. 2021, 25, 5560–5571. [Google Scholar] [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The role of tumor associated macrophages (TAMs) in cancer progression, chemoresistance, angiogenesis and metastasis-current status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef]
- Choi, H.J.; Choi, H.J.; Chung, T.W.; Ha, K.T. Luteolin inhibits recruitment of monocytes and migration of Lewis lung carcinoma cells by suppressing chemokine (C–C motif) ligand 2 expression in tumor-associated macrophage. Biochem. Biophys. Res. Commun. 2016, 470, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ahn, K.C.; Choi, J.Y.; Hwang, S.G.; Kim, W.J.; Um, H.D.; Park, J.K. Luteolin acts as a radiosensitizer in non-small cell lung cancer cells by enhancing apoptotic cell death through activation of a p38/ROS/caspase cascade. Int. J. Oncol. 2015, 46, 1149–1158. [Google Scholar] [CrossRef]
- Tu, D.G.; Lin, W.T.; Yu, C.C.; Lee, S.S.; Peng, C.Y.; Lin, T.; Yu, C.H. Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J. Formos. Med. Assoc. 2016, 115, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Kumar, D.R.; Suresh, P.K.; Kumar, S.; Kumar, R.A. Comparative studies to evaluate relative in vitro potency of luteolin in inducing cell cycle arrest and apoptosis in HaCaT and A375 cells. Asian Pac. J. Cancer Prev. 2013, 14, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Moeng, S.; Son, S.W.; Seo, H.A.; Lee, J.S.; Kim, C.K.; Kuh, H.J.; Park, J.K. Luteolin-regulated MicroRNA-301-3p targets caspase-8 and modulates TRAIL sensitivity in PANC-1 cells. Anticancer Res. 2020, 40, 723–731. [Google Scholar] [CrossRef]
- Kato, H.; Naiki-Ito, A.; Suzuki, S.; Inaguma, S.; Komura, M.; Nakao, K.; Naiki, T.; Kachi, K.; Kato, A.; Matsuo, Y.; et al. DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer. Carcinogenesis 2021, 42, 940–950. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Chen, L.; Li, H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018, 9, 3018–3027. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell. Physiol. Biochem. 2018, 43, 1803–1812. [Google Scholar] [CrossRef]
- Nazim, U.M.; Park, S.Y. Luteolin sensitizes human liver cancer cells to TRAIL-induced apoptosis via autophagy and JNK-mediated death receptor 5 upregulation. Int. J. Oncol. 2019, 54, 665–672. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Xiao, B.; Fang, H.; Huang, B.; Huang, F.; Wang, Y. Luteolin enhances the antitumor efficacy of oncolytic vaccinia virus that harbors IL-24 gene in liver cancer cells. J. Clin. Lab. Anal. 2021, 35, e23677. [Google Scholar] [CrossRef]
- Majumdar, D.; Jung, K.H.; Zhang, H.; Nannapaneni, S.; Wang, X.; Amin, A.R.; Chen, Z.; Chen, Z.G.; Shin, D.M. Luteolin nanoparticle in chemoprevention: In vitro and in vivo anticancer activity. Cancer Prev. Res. 2014, 7, 65–73. [Google Scholar] [CrossRef]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Amin, M.; Aktar, S.; Ali, M.C.; Rahim, Z.B.; Hossain, M.A.; Al Mamun, A.; Amin, M.N.; et al. Chemotherapeutic potential of hesperetin for cancer treatment, with mechanistic insights: A comprehensive review. Heliyon 2022, 8, e08815. [Google Scholar] [CrossRef] [PubMed]
- Babukumar, S.; Vinothkumar, V.; Ramachandhiran, D. Modulating effect of hesperetin on the molecular expression pattern of apoptotic and cell proliferative markers in 7, 12-dimethylbenz (a) anthracene-induced oral carcinogenesis. Arch. Physiol. Biochem. 2020, 126, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Chai, T.; Mohan, M.; Ong, H.; Wong, F. Antioxidant, iron-chelating and anti-glucosidase activities of Typha domingensis Pers (Typhaceae). Trop. J. Pharm. Res. 2014, 13, 67–72. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.H.; Kim, J.H. Combined administration of naringenin and hesperetin with optimal ratio maximizes the anti-cancer effect in human pancreatic cancer via down regulation of FAK and p38 signaling pathway. Phytomedicine 2019, 58, 152762. [Google Scholar] [CrossRef]
- Shirzad, M.; Beshkar, P.; Heidarian, E. The effects of hesperetin on apoptosis induction and inhibition of cell proliferation in the prostate cancer PC3 cells. J. HerbMed Pharmacol. 2015, 4, 121–124. [Google Scholar]
- Li, Q.; Miao, Z.; Wang, R.; Yang, J.; Zhang, D. Hesperetin induces apoptosis in human glioblastoma cells via p38 MAPK activation. Nutr. Cancer 2020, 72, 538–545. [Google Scholar] [CrossRef]
- Aboismaiel, M.G.; El-Mesery, M.; El-Karef, A.; El-Shishtawy, M.M. Hesperetin upregulates Fas/FasL expression and potentiates the antitumor effect of 5-fluorouracil in rat model of hepatocellular carcinoma. Egypt. J. Basic Appl. Sci. 2020, 7, 20–34. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem.-Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef]
- Nalini, N.; Aranganathan, S.; Kabalimurthy, J. Chemopreventive efficacy of hesperetin (citrus flavonone) against 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Toxicol. Mech. Methods 2012, 22, 397–408. [Google Scholar] [CrossRef]
- Elango, R.; Athinarayanan, J.; Subbarayan, V.P.; Lei, D.K.; Alshatwi, A.A. Hesperetin induces an apoptosis-triggered extrinsic pathway and a p53-independent pathway in human lung cancer H522 cells. J. Asian Nat. Prod. Res. 2018, 20, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Gohulkumar, M.; Gurushankar, K.; Prasad, N.R.; Krishnakumar, N. Enhanced cytotoxicity and apoptosis-induced anticancer effect of silibinin-loaded nanoparticles in oral carcinoma (KB) cells. Mater. Sci. Eng. C 2014, 41, 274–282. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumor Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.; Oliveira, H.; Pacheco, A.R.; Almeida, L.; Pimentel, F.; Santos, C.; de Oliveira, J.M. Hesperetin-etoposide combinations induce cytotoxicity in U2OS cells: Implications on therapeutic developments for osteosarcoma. DNA Repair 2017, 50, 36–42. [Google Scholar] [CrossRef]
- Mistry, B.; Patel, R.V.; Keum, Y.S. Access to the substituted benzyl-1, 2, 3-triazolyl hesperetin derivatives expressing antioxidant and anticancer effects. Arab. J. Chem. 2017, 10, 157–166. [Google Scholar] [CrossRef]
- Patel, P.N.; Yu, X.M.; Jaskula-Sztul, R.; Chen, H. Hesperetin activates the Notch1 signaling cascade, causes apoptosis, and induces cellular differentiation in anaplastic thyroid cancer. Ann. Surg. Oncol. 2014, 21, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Baran, Y. The pleiotropic effects of fisetin and hesperetin on human acute promyelocytic leukemia cells are mediated through apoptosis, cell cycle arrest, and alterations in signaling networks. Tumor Biol. 2015, 36, 8973–8984. [Google Scholar] [CrossRef]
- Hermawan, A.; Ikawati, M.; Khumaira, A.; Putri, H.; Jenie, R.I.; Angraini, S.M.; Muflikhasari, H.A. Bioinformatics and in vitro studies reveal the importance of p53, PPARG and notch signaling pathway in inhibition of breast cancer stem cells by hesperetin. Adv. Pharm. Bull. 2020, 11, 351. [Google Scholar] [CrossRef]
- Tan, Y.Q.; Chiu-Leung, L.C.; Lin, S.M.; Leung, L.K. The citrus flavonone hesperetin attenuates the nuclear translocation of aryl hydrocarbon receptor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 210, 57–64. [Google Scholar] [CrossRef]
- Ye, L.; Chan, F.L.; Chen, S.; Leung, L.K. The citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomized athymic mice. J. Nutr. Biochem. 2012, 23, 1230–1237. [Google Scholar] [CrossRef]
- Nurhayati, I.P.; Khumaira, A.; Ilmawati, G.P.; Meiyanto, E.; Hermawan, A. Cytotoxic and antimetastatic activity of hesperetin and doxorubicin combination toward Her2 expressing breast cancer cells. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1259. [Google Scholar] [CrossRef] [PubMed]
- Palit, S.; Kar, S.; Sharma, G.; Das, P.K. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. J. Cell. Physiol. 2015, 230, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wolfram, J.; Boom, K.; Fang, X.; Shen, H.; Ferrari, M. Hesperetin impairs glucose uptake and inhibits proliferation of breast cancer cells. Cell Biochem. Funct. 2013, 31, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, B.B.; Steephan, M.; Kumar, T.S.; Sabu, A.; Haridas, M. Hesperetin and naringenin sensitize HER2 positive cancer cells to death by serving as HER2 tyrosine kinase inhibitors. Life Sci. 2016, 160, 47–56. [Google Scholar] [CrossRef]
- Yunita, E.; Muflikhasari, H.A.; Ilmawati, G.P.; Meiyanto, E.; Hermawan, A. Hesperetin alleviates doxorubicin-induced migration in 4T1 breast cancer cells. Future J. Pharm. Sci. 2020, 6, 23. [Google Scholar] [CrossRef]
- Shirzad, M.; Heidarian, E.; Beshkar, P.; Gholami-Arjenaki, M. Biological effects of hesperetin on interleukin-6/phosphorylated signal transducer and activator of transcription 3 pathway signaling in prostate cancer PC3 cells. Pharmacogn. Res. 2017, 9, 188. [Google Scholar]
- Arya, A.; Khandelwal, K.; Ahmad, H.; Laxman, T.S.; Sharma, K.; Mittapelly, N.; Agrawal, S.; Bhatta, R.S.; Dwivedi, A.K. Co-delivery of hesperetin enhanced bicalutamide induced apoptosis by exploiting mitochondrial membrane potential via polymeric nanoparticles in a PC-3 cell line. RSC Adv. 2016, 6, 5925–5935. [Google Scholar] [CrossRef]
- Wolfram, J.; Scott, B.; Boom, K.; Shen, J.; Borsoi, C.; Suri, K.; Grande, R.; Fresta, M.; Celia, C.; Zhao, Y.; et al. Hesperetin liposomes for cancer therapy. Curr. Drug Deliv. 2016, 13, 711–719. [Google Scholar] [CrossRef]
- Tamayo, L.V.; Gouvea, L.R.; Sousa, A.C.; Albuquerque, R.M.; Teixeira, S.F.; de Azevedo, R.A.; Louro, S.R.; Ferreira, A.K.; Beraldo, H. Copper (II) complexes with naringenin and hesperetin: Cytotoxic activity against A 549 human lung adenocarcinoma cells and investigation on the mode of action. Biometals 2016, 29, 39–52. [Google Scholar] [CrossRef]
- Ramteke, P.; Yadav, U. Hesperetin, a Citrus bioflavonoid, prevents IL-1β-induced inflammation and cell proliferation in lung epithelial A549 cells. Indian J. Exp. Biol. 2019, 57, 7–14. [Google Scholar]
- Bodduluru, L.N.; Kasala, E.R.; Barua, C.C.; Karnam, K.C.; Dahiya, V.; Ellutla, M. Antiproliferative and antioxidant potential of hesperetin against benzo (a) pyrene-induced lung carcinogenesis in Swiss albino mice. Chem.-Biol. Interact. 2015, 242, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, J.; Wu, D.; Wang, J.; Dong, W. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Med. Oncol. 2015, 32, 101, Erratum in: Med. Oncol. 2019, 36, 38. https://doi.org/10.1007/s12032-019-1258-0. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.Y.; Park, J.; Han, Y.S.; Lee, Y.H.; Shin, S.Y.; Lim, Y. Synthesis and biological evaluation of hesperetin derivatives as agents inducing apoptosis. Bioorg. Med. Chem. 2017, 25, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sivagami, G.; Vinothkumar, R.; Preethy, C.P.; Riyasdeen, A.; Akbarsha, M.A.; Menon, V.P.; Nalini, N. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line–A comparative study. Food Chem. Toxicol. 2012, 50, 660–671. [Google Scholar] [CrossRef]
- Miler, M.; Živanović, J.; Ajdžanović, V.; Oreščanin-Dušić, Z.; Milenković, D.; Konić-Ristić, A.; Blagojević, D.; Milošević, V.; Šošić-Jurjević, B. Citrus flavanones naringenin and hesperetin improve antioxidant status and membrane lipid compositions in the liver of old-aged Wistar rats. Exp. Gerontol. 2016, 84, 49–60. [Google Scholar] [CrossRef]
- Kong, R.; Wang, N.; Luo, H.; Lu, J. Hesperetin mitigates bile duct ligation-induced liver fibrosis by inhibiting extracellular matrix and cell apoptosis via the TGF-β1/Smad pathway. Curr. Mol. Med. 2018, 18, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.F.; Chen, Y.; Zhu, S.; Li, H.D.; Chen, S.Y.; Wang, J.N.; Pan, X.Y.; Bu, F.T.; Huang, C.; et al. Hesperetin derivative attenuates CCl4-induced hepatic fibrosis and inflammation by Gli-1-dependent mechanisms. Int. Immunopharmacol. 2019, 76, 105838. [Google Scholar] [CrossRef]
- Kumar, M.; Dahiya, V.; Kasala, E.R.; Bodduluru, L.N.; Lahkar, M. The renoprotective activity of hesperetin in cisplatin induced nephrotoxicity in rats: Molecular and biochemical evidence. Biomed. Pharmacother. 2017, 89, 1207–1215. [Google Scholar] [CrossRef]
- Wang, H.W.; Shi, L.; Xu, Y.P.; Qin, X.Y.; Wang, Q.Z. Hesperetin alleviates renal interstitial fibrosis by inhibiting tubular epithelial-mesenchymal transition in vivo and in vitro. Exp. Ther. Med. 2017, 14, 3713–3719. [Google Scholar] [CrossRef]
- Wang, S.W.; Sheng, H.; Zheng, F.; Zhang, F. Hesperetin promotes DOT1L degradation and reduces histone H3K79 methylation to inhibit gastric cancer metastasis. Phytomedicine 2021, 84, 153499. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Gao, J.; De, Y. Hesperidin inhibits ovarian cancer cell viability through endoplasmic reticulum stress signaling pathways. Oncol. Lett. 2017, 14, 5569–5574. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Purushothaman, B.K. KMMS Magnetic casein-CaFe2O4 nanohybrid carrier conjugated with progesterone for enhanced cytotoxicity of citrus peel derived hesperidin drug towards breast and ovarian cancer. Int. J. Biol. Macromol. 2020, 151, 293–304. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chen, Y.H.; Huang, Y.C. Hesperetin induces autophagy and delayed apoptosis by modulating the AMPK/Akt/mTOR pathway in human leukemia cells in vitro. Curr. Issues Mol. Biol. 2023, 45, 1587–1600. [Google Scholar] [CrossRef] [PubMed]
- Ersoz, M.; Erdemir, A.; Duranoglu, D.; Uzunoglu, D.; Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative evaluation of hesperetin loaded nanoparticles for anticancer activity against C6 glioma cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, R.; Mir, R.H.; Sabreen, S.; Jan, R.; Pottoo, F.H.; Singh, I.P. Recent insights into therapeutic potential of plant-derived flavonoids against cancer. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2022, 22, 3343–3369. [Google Scholar] [CrossRef]
- Adan, A.; Baran, Y. Fisetin and hesperetin induced apoptosis and cell cycle arrest in chronic myeloid leukemia cells accompanied by modulation of cellular signaling. Tumor Biol. 2016, 37, 5781–5795. [Google Scholar] [CrossRef]
- Smina, T.P.; Mohan, A.; Ayyappa, K.A.; Sethuraman, S.; Krishnan, U.M. Hesperetin exerts apoptotic effect on A431 skin carcinoma cells by regulating mitogen activated protein kinases and cyclins. Cell. Mol. Biol. 2015, 61, 92–99. [Google Scholar]
- Jiang, S.; Wang, S.; Zhang, L.; Tian, L.; Li, L.; Liu, Z.; Dong, Q.; Lv, X.; Mu, H.; Zhang, Q.; et al. Hesperetin as an adjuvant augments protective anti-tumour immunity responses in B16F10 melanoma by stimulating cytotoxic CD8+ T cells. Scand. J. Immunol. 2020, 91, e12867. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Hu, X.; Ma, J.; Dong, W. Hesperetin inhibits Eca-109 cell proliferation and invasion by suppressing the PI3K/AKT signaling pathway and synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal cancer in vitro and in vivo. RSC Adv. 2018, 8, 24434–24443. [Google Scholar] [CrossRef]
- Gurushankar, K.; Nazeer, S.S.; Gohulkumar, M.; Jayasree, R.S.; Krishnakumar, N. Endogenous porphyrin fluorescence as a biomarker for monitoring the anti-angiogenic effect in antitumor response to hesperetin loaded nanoparticles in experimental oral carcinogenesis. RSC Adv. 2014, 4, 46896–46906. [Google Scholar]
- Torcasio, R.; Gallo Cantafio, M.E.; Veneziano, C.; De Marco, C.; Ganino, L.; Valentino, I.; Occhiuzzi, M.A.; Perrotta, I.D.; Mancuso, T.; Conforti, F.; et al. Targeting of mitochondrial fission through natural flavanones elicits anti-myeloma activity. J. Transl. Med. 2024, 22, 208. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, Y.; Hui, Q.; Wang, H.; Tao, K. Naringin suppresses the metabolism of A375 cells by inhibiting the phosphorylation of c-Src. Tumor Biol. 2016, 37, 3841–3850. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Dong, W.; Qu, X.; Huang, C.; Yan, T.; Du, J. Combination of hesperetin and platinum enhances anticancer effect on lung adenocarcinoma. Biomed. Pharmacother. 2019, 113, 108779. [Google Scholar] [CrossRef] [PubMed]
- Magura, J.; Moodley, R.; Mackraj, I. The effect of hesperidin and luteolin isolated from Eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2022, 40, 1791–1800. [Google Scholar] [CrossRef]
- Zare, M.; Norouzi Sarkati, M.; Tashakkorian, H.; Partovi, R.; Rahaiee, S.; Rezaei, P.; Razavi, S.A. Dextran–hesperetin conjugate as a novel biocompatible medicine for antimicrobial and anticancer applications. J. Polym. Environ. 2021, 29, 811–820. [Google Scholar] [CrossRef]
- Susidarti, R.A.; Nugroho, A.E.; Meiyanto, E. Increasing sensitivity of MCF-7/DOX cells towards doxorubicin by hesperetin through suppression of P-glycoprotein expression. Indones. J. Pharm. 2014, 25, 84. [Google Scholar]
- Vidal, S.J.; Rodriguez-Bravo, V.; Quinn, S.A.; Rodriguez-Barrueco, R.; Lujambio, A.; Williams, E.; Sun, X.; de la Iglesia-Vicente, J.; Lee, A.; Readhead, B.; et al. A targetable GATA2-IGF2 axis confers aggressiveness in lethal prostate cancer. Cancer Cell 2015, 27, 223–239. [Google Scholar] [CrossRef]
- Kong, W.; Ling, X.; Chen, Y.; Wu, X.; Zhao, Z.; Wang, W.; Wang, S.; Lai, G.; Yu, Z. Hesperetin reverses P-glycoprotein-mediated cisplatin resistance in DDP-resistant human lung cancer cells via modulation of the nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 1213–1224. [Google Scholar] [CrossRef]
- Gurushankar, K.; Gohulkumar, M.; Prasad, N.R.; Krishnakumar, N. Synthesis, characterization and in vitro anti-cancer evaluation of hesperetin-loaded nanoparticles in human oral carcinoma (KB) cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 5, 015006. [Google Scholar] [CrossRef]
- Gurushankar, K.; Nazeer, S.S.; Jayasree, R.S.; Krishnakumar, N. Evaluation of antitumor activity of hesperetin-loaded nanoparticles against DMBA-induced oral carcinogenesis based on tissue autofluorescence spectroscopy and multivariate analysis. J. Fluoresc. 2015, 25, 931–939. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Rangel, M.; Delgado-Zamarreño, M.M.; Pérez-Martín, L.; Rodríguez-Gonzalo, E.; Domínguez-Álvarez, J. Analysis of isoflavones in foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, C.W.; Jeon, S.Y.; Go, R.E.; Hwang, K.A.; Choi, K.C. Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Lab. Anim. Res. 2014, 30, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.P. Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac. J. Cancer Prev. 2014, 15, 7001–7010. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Yang, S.; Wang, Y.; Tang, Z.; Fu, X. Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 2018, 6, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Khongsti, K.; Das, K.B.; Das, B. MAPK pathway and SIRT1 are involved in the down-regulation of secreted osteopontin expression by genistein in metastatic cancer cells. Life Sci. 2021, 265, 118787. [Google Scholar] [CrossRef]
- Shin, H.R.; Joubert, C.; Boniol, M.; Hery, C.; Ahn, S.H.; Won, Y.J.; Nishino, Y.; Sobue, T.; Chen, C.J.; You, S.L.; et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control 2010, 21, 1777–1785. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, M.; Parris, A.B.; Xing, Y.; Yang, X. Genistein targets the cancerous inhibitor of PP2A to induce growth inhibition and apoptosis in breast cancer cells. Int. J. Oncol. 2016, 49, 1203–1210. [Google Scholar] [CrossRef]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A potent anti-breast cancer agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef]
- Islam, M.A.; Bekele, R.; vanden Berg, J.H.; Kuswanti, Y.; Thapa, O.; Soltani, S.; van Leeuwen, F.R.; Rietjens, I.M.; Murk, A.J. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicol. Vitr. 2015, 29, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Pavese, J.M.; Farmer, R.L.; Bergan, R.C. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010, 29, 465–482. [Google Scholar] [CrossRef]
- Mukund, V. Genistein: Its role in breast cancer growth and metastasis. Curr. Drug Metab. 2020, 21, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Latocha, M.; Płonka, J.; Kuśmierz, D.; Jurzak, M.; Polaniak, R.; Nowosad, A. Transcripional activity of genes encoding MMPs and TIMPs in breast cancer cells treated by genistein and in normal cancer-associated fibroblasts--in vitro studies. Acta Pol. Pharm. 2014, 71, 1095–1102. [Google Scholar]
- Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy isoflavones induce cell death by copper-mediated mechanism: Understanding its anticancer properties. Molecules 2023, 28, 2925. [Google Scholar] [CrossRef] [PubMed]
- Pavese, J.M.; Krishna, S.N.; Bergan, R.C. Genistein inhibits human prostate cancer cell detachment, invasion, and metastasis. Am. J. Clin. Nutr. 2014, 100, 431S–436S. [Google Scholar] [CrossRef]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W.; et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction-A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef]
- Süntar, I.; Yakıncı, Ö.F. Potential risks of phytonutrients associated with high-dose or long-term use. In Phytonutrients in Food; Woodhead Publishing: Sawston, UK, 2020; pp. 137–155. [Google Scholar]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Academic Press: Cambridge, MA, USA, 2020; pp. 341–361. [Google Scholar]
- Xie, J.; Wang, H.; Xie, W.; Liu, Y.; Chen, Y. Gallic acid promotes ferroptosis in hepatocellular carcinoma via inactivating Wnt/β-catenin signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2437–2445. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Martin, W.H.; Ras, B.A.; Isreb, M.; Jacob, B.; Aziz, A.; Adoul, Z.; Lagnado, R.; Bowen, R.D.; Najafzadeh, M. The anticancer/cytotoxic effect of a novel gallic acid derivative in non-small cell lung carcinoma A549 cells and peripheral blood mononuclear cells from healthy individuals and lung cancer patients. BioFactors 2024, 50, 201–213. [Google Scholar] [CrossRef]
- Patra, S.; Bhol, C.S.; Panigrahi, D.P.; Praharaj, P.P.; Pradhan, B.; Jena, M.; Bhutia, S.K. Gamma irradiation promotes chemo-sensitization potential of gallic acid through attenuation of autophagic flux to trigger apoptosis in an NRF2 inactivation signalling pathway. Free Radic. Biol. Med. 2020, 160, 111–124. [Google Scholar] [CrossRef]
- Ko, E.B.; Jang, Y.G.; Kim, C.W.; Go, R.E.; Lee, H.K.; Choi, K.C. Gallic acid hindered lung cancer progression by inducing cell cycle arrest and apoptosis in a549 lung cancer cells via PI3K/Akt pathway. Biomol. Ther. 2021, 30, 151. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F. TMA Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Sanchez-Martin, V.; Plaza-Calonge, M.D.; Soriano-Lerma, A.; Ortiz-Gonzalez, M.; Linde-Rodriguez, A.; Perez-Carrasco, V.; Ramirez-Macias, I.; Cuadros, M.; Gutierrez-Fernandez, J.; Murciano-Calles, J.; et al. Gallic acid: A natural phenolic compound exerting antitumoral activities in colorectal cancer via interaction with g-quadruplexes. Cancers 2022, 14, 2648. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.; Yu, Y.; Yang, Q.; Wang, Z.N. Synergistic anticancer activity of combined use of caffeic acid with paclitaxel enhances apoptosis of non-small-cell lung cancer H1299 cells in vivo and in vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Prasad, N.R. Anticancer effect of caffeic acid on human cervical cancer cells. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015; pp. 655–661. [Google Scholar]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.; Jernström, H. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor I receptor levels in human breast cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef]
- Chen, L.; Jin, Y.; Chen, H.; Sun, C.; Fu, W.; Zheng, L.; Lu, M.; Chen, P.; Chen, G.; Zhang, Y.; et al. Discovery of caffeic acid phenethyl ester derivatives as novel myeloid differentiation protein 2 inhibitors for treatment of acute lung injury. Eur. J. Med. Chem. 2018, 143, 361–375. [Google Scholar] [CrossRef]
- Kang, K.P.; Park, S.K.; Kim, D.H.; Sung, M.J.; Jung, Y.J.; Lee, A.S.; Lee, J.E.; Ramkumar, K.M.; Lee, S.; Park, M.H.; et al. Luteolin ameliorates cisplatin-induced acute kidney injury in mice by regulation of p53-dependent renal tubular apoptosis. Nephrol. Dial. Transplant. 2011, 26, 814–822. [Google Scholar] [CrossRef]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y.; et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef]
- Ozturk, G.; Ginis, Z.; Akyol, S.; Erden, G.; Gurel, A.; Akyol, O. The anticancer mechanism of caffeic acid phenethyl ester (CAPE): Review of melanomas, lung and prostate cancers. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2064–2068. [Google Scholar] [PubMed]
- Lin, H.P.; Lin, C.Y.; Huo, C.; Hsiao, P.H.; Su, L.C.; Jiang, S.S.; Chan, T.M.; Chang, C.H.; Chen, L.T.; Kung, H.J.; et al. Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1. Oncotarget 2015, 6, 6684. [Google Scholar] [CrossRef]
- Amorim, R.; Magalhães, C.C.; Benfeito, S.; Cagide, F.; Tavares, L.C.; Santos, K.; Sardão, V.A.; Datta, S.; Cortopassi, G.A.; Baldeiras, I.; et al. Mitochondria dysfunction induced by decyl-TPP mitochondriotropic antioxidant based on caffeic acid AntiOxCIN6 sensitizes cisplatin lung anticancer therapy due to a remodeling of energy metabolism. Biochem. Pharmacol. 2024, 219, 115953. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, K.; Kwon, D.Y.; Kim, J.K.; Sathasivam, R.; Park, S.U. Effects of Different Solvents on the Extraction of Phenolic and Flavonoid Compounds, and Antioxidant Activities, in Scutellaria baicalensis Hairy Roots. Horticulturae 2024, 10, 160. [Google Scholar] [CrossRef]
- Ishida, Y.; Gao, R.; Shah, N.; Bhargava, P.; Furune, T.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer activity in honeybee propolis: Functional insights to the role of caffeic acid phenethyl ester and its complex with γ-cyclodextrin. Integr. Cancer Ther. 2018, 17, 867–873. [Google Scholar] [CrossRef]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J. Cancer 2016, 7, 1755. [Google Scholar] [CrossRef]
- Gupta, S.; Tak, H.; Rathore, K.; Banavath, H.N.; Tejavath, K.K. Caffeic acid, a dietary polyphenol, pre-sensitizes pancreatic ductal adenocarcinoma to chemotherapeutic drug. J. Biomol. Struct. Dyn. 2024, 1–15, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Pagnan, A.L.; Pessoa, A.S.; Tokuhara, C.K.; Fakhoury, V.S.; Oliveira, G.S.; Sanches, M.L.; Inacio, K.K.; Ximenes, V.F.; Oliveira, R.C. Anti-tumour potential and selectivity of caffeic acid phenethyl ester in osteosarcoma cells. Tissue Cell 2022, 74, 101705. [Google Scholar] [CrossRef] [PubMed]
- Sehrawat, R.; Rathee, P.; Rathee, P.; Khatkar, S.; Küpelli Akkol, E.; Khatkar, A. In silico and in vitro analysis of phenolic acids for identification of potential dhfr inhibitors as antimicrobial and anticancer agents. Curr. Protein Pept. Sci. 2024, 25, 44–58. [Google Scholar] [CrossRef]
- Trócsányi, E.; György, Z.; Zámboriné-Németh, É. New insights into rosmarinic acid biosynthesis based on molecular studies. Curr. Plant Biol. 2020, 23, 100162. [Google Scholar] [CrossRef]
- Levsh, O.; Pluskal, T.; Carballo, V.; Mitchell, A.J.; Weng, J.K. Independent evolution of rosmarinic acid biosynthesis in two sister families under the Lamiids clade of flowering plants. J. Biol. Chem. 2019, 294, 15193–15205. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful plant antioxidants: A new biosustainable approach to the production of rosmarinic acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Hossan, M.S.; Rahman, S.; Bashar, A.B.; Jahan, R.; Al-Nahain, A.; Rahmatullah, M. Rosmarinic acid: A review of its anticancer action. World J. Pharm. Pharm. Sci. 2014, 3, 57–70. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in plants. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 263–284. [Google Scholar]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic acid exhibits anticancer effects via MARK4 inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef]
- Messeha, S.S.; Zarmouh, N.O.; Asiri, A.; Soliman, K.F. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020, 885, 173419. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Nazaruk, J.; Karna, E.; Popławska, B.; Bielawska, A.; Galicka, A. Rosmarinic acid influences collagen, MMPs, TIMPs, glycosylation and MUC1 in CRL-1739 gastric cancer cell line. Biomed. Pharmacother. 2018, 107, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Laila, F.; Fardiaz, D.; Yuliana, N.D.; Damanik, M.R.; Nur Annisa Dewi, F. Methanol extract of Coleus amboinicus (Lour) exhibited antiproliferative activity and induced programmed cell death in colon cancer cell WiDr. Int. J. Food Sci. 2020, 2020, 9068326. [Google Scholar] [CrossRef]
- Yang, K.; Shen, Z.; Zou, Y.; Gao, K. Rosmarinic acid inhibits migration, invasion, and p38/AP-1 signaling via miR-1225-5p in colorectal cancer cells. J. Recept. Signal Transduct. 2021, 41, 284–293. [Google Scholar] [CrossRef]
- Jin, B.R.; Chung, K.S.; Hwang, S.; Hwang, S.N.; Rhee, K.J.; Lee, M.; An, H.J. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the TLR4-mediated NF-κB-STAT3 axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, F.; Zhang, Y.; Shen, X. Protective effects of rosmarinic acid against radiation-induced damage to the hematopoietic system in mice. J. Radiat. Res. 2016, 57, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.G.; Hwang, K.A.; Choi, K.C. Rosmarinic acid, a component of rosemary tea, induced the cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer cell lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef]
- García-Sarrió, M.J.; Sanz, M.L.; Palá-Paúl, J.; Díaz, S.; Soria, A.C. Optimization of a Green Microwave-Assisted Extraction Method to Obtain Multifunctional Extracts of Mentha sp. Foods 2023, 12, 2039. [Google Scholar] [CrossRef]
- Chao, W.W.; Liou, Y.J.; Ma, H.T.; Chen, Y.H.; Chou, S.T. The Antitumor Mechanism of the Polyphenol-Enriched Ethyl Acetate Fraction Extract of Glechoma Hederacea (Lamiaceae) Against HepG2 Cells Involves Apoptosis Pathways; ResearchGate: Berlin, Germany, 2020. [Google Scholar]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic acid inhibits proliferation and invasion of hepatocellular carcinoma cells SMMC 7721 via PI3K/AKT/mTOR signal pathway. Biomed. Pharmacother. 2019, 120, 109443. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, J.; Yang, Y.; Wang, X.; Chen, G.; Shi, A.; Lu, Y.; Jia, S.; Kang, X.; Lu, L. Rosmarinic acid exerts an anticancer effect on osteosarcoma cells by inhibiting DJ-1 via regulation of the PTEN-PI3K-Akt signaling pathway. Phytomedicine 2020, 68, 153186. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer effects of rosemary (Rosmarinus officinalis L.) extract and rosemary extract polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef]
- Ghiulai, R.; Avram, S.; Stoian, D.; Pavel, I.Z.; Coricovac, D.; Oprean, C.; Vlase, L.; Farcas, C.; Mioc, M.; Minda, D.; et al. Lemon balm extracts prevent breast cancer progression in vitro and in ovo on chorioallantoic membrane assay. Evid.-Based Complement. Altern. Med. 2020, 2020, 6489159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Chen, H.H.; Huang, E.; Zhuang, H.; Li, D.; Ni, F. Rosmarinic acid inhibits stem-like breast cancer through hedgehog and Bcl-2/Bax signaling pathways. Pharmacogn. Mag. 2019, 15, 600–606. [Google Scholar] [CrossRef]
- Khanaree, C.; Punfa, W.; Tantipaiboonwong, P.; Nuntaboon, P.; Suttajit, M.; Topanurak, S.; Dukaew, N.; Mon, M.T.; Hu, R.; Pintha, K. In vitro anti-metastasis of Perilla frutescens leaf water extract on aggressive human breast cancer cells. J. Assoc. Med. Sci. 2022, 55, 51–59. [Google Scholar] [CrossRef]
- Cristy, G.P.; Liana, D.; Chatwichien, J.; Aonbangkhen, C.; Srisomsap, C.; Phanumartwiwath, A. Breast Cancer Prevention by Dietary Polyphenols: Microemulsion Formulation and In vitro Studies. Sci. Pharm. 2024, 92, 25. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Okda, T.M.; Omran, G.A.; Abd-Alhaseeb, M.M. Rosmarinic acid suppresses inflammation, angiogenesis, and improves paclitaxel induced apoptosis in a breast cancer model via NF3 κB-p53-caspase-3 pathways modulation. J. Appl. Biomed. 2021, 19, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, J.; Quan, J.; Xiang, D. Rosmarinic acid inhibits proliferation and migration, promotes apoptosis and enhances cisplatin sensitivity of melanoma cells through inhibiting ADAM17/EGFR/AKT/GSK3β axis. Bioengineered 2021, 12, 3065–3076. [Google Scholar] [CrossRef]
- Han, Y.; Ma, L.; Zhao, L.E.; Feng, W.; Zheng, X. Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108878. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Li, Z.; Chen, L.; Wen, C.; Ruan, Q.; Xu, Z.; Liu, R.; Xu, J.; Bai, Y.; et al. Rosmarinic acid decreases the malignancy of pancreatic cancer through inhibiting Gli1 signaling. Phytomedicine 2022, 95, 153861. [Google Scholar] [CrossRef]
- Cao, W.; Hu, C.; Wu, L.; Xu, L.; Jiang, W. Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κB signaling in H22 tumor-bearing mice. J. Pharmacol. Sci. 2016, 132, 131–137. [Google Scholar] [CrossRef]
- Li, Z.N.; Luo, Y. HSP90 inhibitors and cancer: Prospects for use in targeted therapies. Oncol. Rep. 2022, 49, 6. [Google Scholar] [CrossRef]
- Lim, S.H.; Nam, K.H.; Kim, K.; Yi, S.A.; Lee, J.; Han, J.W. Rosmarinic acid methyl ester regulates ovarian cancer cell migration and reverses cisplatin resistance by inhibiting the expression of Forkhead Box M1. Pharmaceuticals 2020, 13, 302. [Google Scholar] [CrossRef]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive effects of rosmarinic acid rich fraction from perilla on oxidative stress, inflammation and metastasis ability in A549 cells exposed to PM via C-Jun, P-65-Nf-Κb and Akt signaling pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, M.O.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Rosmarinic acid sensitizes cell death through suppression of TNF-α-induced NF-κB activation and ROS generation in human leukemia U937 cells. Cancer Lett. 2010, 288, 183–191. [Google Scholar] [CrossRef]
- Liao, X.Z.; Gao, Y.; Sun, L.L.; Liu, J.H.; Chen, H.R.; Yu, L.; Chen, Z.Z.; Chen, W.H.; Lin, L.Z. Rosmarinic acid reverses non-small cell lung cancer cisplatin resistance by activating the MAPK signaling pathway. Phytother. Res. 2020, 34, 1142–1153. [Google Scholar] [CrossRef]
- Highland, H.; Thakur, M.; Pandya, P.; Mankad, A.; George, L.B. Molecular Dynamics of A Biglycan-Rosmarinic Acid Complex with Focal Adhesion Kinase for Possible Arrest of Metastasis in Non-Small Cell Lung Cancer (NSCLC): An In-Silico Study. J. Drug Deliv. Ther. 2019, 9, 159–166. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, Z.; Ji, G.; Liu, J. Inhibition of bone metastasis from breast carcinoma by rosmarinic acid. Planta Med. 2010, 76, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Asai, M.; Jeon, S.K.; Iimura, T.; Yonezawa, T.; Cha, B.Y.; Woo, J.T.; Yamaguchi, A. Rosmarinic acid exerts an antiosteoporotic effect in the RANKL-induced mouse model of bone loss by promotion of osteoblastic differentiation and inhibition of osteoclastic differentiation. Mol. Nutr. Food Res. 2015, 59, 386–400. [Google Scholar] [CrossRef]
- Pagano, K.; Carminati, L.; Tomaselli, S.; Molinari, H.; Taraboletti, G.; Ragona, L. Molecular Basis of the Antiangiogenic Action of Rosmarinic Acid, a Natural Compound Targeting Fibroblast Growth Factor-2/FGFR Interactions. ChemBioChem 2021, 22, 160–169. [Google Scholar] [CrossRef]
- Rodríguez-Luna, A.; Ávila-Román, J.; Oliveira, H.; Motilva, V.; Talero, E. Fucoxanthin and rosmarinic acid combination has anti-inflammatory effects through regulation of NLRP3 inflammasome in UVB-exposed HaCaT keratinocytes. Mar. Drugs 2019, 17, 451. [Google Scholar] [CrossRef]
- Gupta, D.; Archoo, S.; Naikoo, S.H.; Abdullah, S.T. Rosmarinic acid: A naturally occurring plant based agent prevents impaired mitochondrial dynamics and apoptosis in ultraviolet-B-irradiated human skin cells. Photochem. Photobiol. 2022, 98, 925–934. [Google Scholar] [CrossRef]
- Fernando, P.M.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Hewage, S.R.; Chae, S.W.; Hyun, J.W. Rosmarinic acid attenuates cell damage against UVB radiation-induced oxidative stress via enhancing antioxidant effects in human HaCaT cells. Biomol. Ther. 2016, 24, 75. [Google Scholar] [CrossRef] [PubMed]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of Sinapic acid—An updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, L.Y.; He, W.; Li, W.Y.; Zeng, Z.; Qian, B.; Wang, C.; Song, J.L. Sinapic acid alleviated inflammation-induced intestinal epithelial barrier dysfunction in lipopolysaccharide-(LPS-) treated Caco-2 cells. Mediat. Inflamm. 2021, 2021, 5514075. [Google Scholar] [CrossRef]
- Bin Jardan, Y.A.; Ansari, M.A.; Raish, M.; Alkharfy, K.M.; Ahad, A.; Al-Jenoobi, F.I.; Haq, N.; Khan, M.R.; Ahmad, A. Sinapic acid ameliorates oxidative stress, inflammation, and apoptosis in acute doxorubicin-induced cardiotoxicity via the NF-κB-mediated pathway. BioMed Res. Int. 2020, 2020, 3921796. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y. Anti-inflammatory effects of sinapic acid on 2, 4, 6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch. Pharmacal Res. 2018, 41, 243–250. [Google Scholar] [CrossRef]
- Qian, B.; Wang, C.; Zeng, Z.; Ren, Y.; Li, D.; Song, J.L. Ameliorative effect of Sinapic acid on dextran sodium sulfate-(DSS-) induced ulcerative colitis in Kunming (KM) mice. Oxidative Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Ahad, A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M.; Khan, A.; Ali, N. Sinapic acid ameliorates bleomycin-induced lung fibrosis in rats. Biomed. Pharmacother. 2018, 108, 224–231. [Google Scholar] [CrossRef]
- Singh, A.K.; Bishayee, A.; Pandey, A.K. Targeting histone deacetylases with natural and synthetic agents: An emerging anticancer strategy. Nutrients 2018, 10, 731. [Google Scholar] [CrossRef]
- Huang, Z.W.; Tan, P.; Yi, X.K.; Chen, H.; Sun, B.; Shi, H.; Mou, Z.Q.; Cheng, Y.L.; Li, T.X.; Li, Q.; et al. Sinapic Acid Alleviates Acute Pancreatitis in Association with Attenuation of Inflammation, Pyroptosis, and the AMPK/NF-κ B Signaling Pathway. Am. J. Chin. Med. 2022, 50, 2185–2197. [Google Scholar] [CrossRef]
- Hu, X.; Geetha, R.V.; Surapaneni, K.M.; Veeraraghavan, V.P.; Chinnathambi, A.; Alahmadi, T.A.; Manikandan, V.; Manokaran, K. Lung cancer induced by Benzo (A) Pyrene: ChemoProtective effect of sinapic acid in swiss albino mice. Saudi J. Biol. Sci. 2021, 28, 7125–7133. [Google Scholar] [CrossRef]
- Janakiraman, K.; Kathiresan, S.; Mariadoss, A.V. Influence of sinapic acid on induction of apoptosis in human laryngeal carcinoma cell line. Int. J. Mod. Res. Rev. 2014, 2, 165–170. [Google Scholar]
- Eroğlu, C.; Avcı, E.; Vural, H.; Kurar, E. Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Balaji, C.; Muthukumaran, J.; Nalini, N. Chemopreventive effect of sinapic acid on 1, 2-dimethylhydrazine-induced experimental rat colon carcinogenesis. Hum. Exp. Toxicol. 2014, 33, 1253–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, H.; Li, W.; Wang, Z.; Dong, Z.; Lan, H.; Wang, C.; Song, J.L. Effects of Sinapic acid combined with cisplatin on the apoptosis and autophagy of the hepatoma cells HepG2 and SMMC-7721. Evid.-Based Complement. Altern. Med. 2021, 2021, 6095963. [Google Scholar] [CrossRef]
- Poyraz, F.S.; Akbaş, G.; Duranoğlu, D.; Acar, S.; Mansuroğlu, B.; Ersöz, M. Sinapic-Acid-Loaded Nanoparticles Optimized via Experimental Design Methods: Cytotoxic, Antiapoptotoic, Antiproliferative, and Antioxidant Activity. ACS Omega 2024, 9, 40329–40345. [Google Scholar] [CrossRef]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A review of recent curcumin analogues and their antioxidant, anti-inflammatory, and anticancer activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef]
- Vyas, A.; Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Curr. Pharm. Des. 2013, 19, 2047–2069. [Google Scholar]
- Haghi, A.; Azimi, H.; Rahimi, R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochemicals: Source of antioxidants and role in disease prevention. BoD—Books Demand 2018, 7, 49–74. [Google Scholar]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer activity of curcumin and its analogues: Preclinical and clinical studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Dai, F.; Chen, Z.; Wang, S.; Cheng, X.; Sheng, Q.; Lin, J.; Chen, W. Dimethoxy curcumin induces apoptosis by suppressing survivin and inhibits invasion by enhancing E-cadherin in colon cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 3215. [Google Scholar] [CrossRef] [PubMed]
- Masuelli, L.; Benvenuto, M.; Di Stefano, E.; Mattera, R.; Fantini, M.; De Feudis, G.; De Smaele, E.; Tresoldi, I.; Giganti, M.G.; Modesti, A.; et al. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget 2017, 8, 34405. [Google Scholar] [CrossRef]
- Weng, W.; Goel, A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 80, pp. 73–86. [Google Scholar]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Wong, S.C.; Kamarudin, M.N.; Naidu, R. Anticancer mechanism of curcumin on human glioblastoma. Nutrients 2021, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Tsai, T.H.; Hsu, C.W.; Hsu, Y.C. Curcuminoids suppress the growth and induce apoptosis through caspase-3-dependent pathways in glioblastoma multiforme (GBM) 8401 cells. J. Agric. Food Chem. 2010, 58, 10639–10645. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.M.; Chang, G.C.; Yu, S.L.; Hsieh, W.Y.; Chen, J.J.; Chen, H.W.; Yang, P.C. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef]
- Sultana, S.; Munir, N.; Mahmood, Z.; Riaz, M.; Akram, M.; Rebezov, M.; Kuderinova, N.; Moldabayeva, Z.; Shariati, M.A.; Rauf, A.; et al. Molecular targets for the management of cancer using Curcuma longa Linn. phytoconstituents: A Review. Biomed. Pharmacother. 2021, 135, 111078. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, X.; Jia, Y.; Huai, L.; He, K.; Yu, P.; Wang, M.; Xing, H.; Rao, Q.; et al. Role of the Wilms’ tumor 1 gene in the aberrant biological behavior of leukemic cells and the related mechanisms. Oncol. Rep. 2014, 32, 2680–2686. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.R.; Subramaniam, D.; Jensen, R.A.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2016; Volume 40, pp. 192–208. [Google Scholar]
- Fabianowska-Majewska, K.; Kaufman-Szymczyk, A.; Szymanska-Kolba, A.; Jakubik, J.; Majewski, G.; Lubecka, K. Curcumin from turmeric rhizome: A potential modulator of DNA methylation machinery in breast cancer inhibition. Nutrients 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular targets of curcumin in breast cancer. Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef]
- Zhou, X.; Jiao, D.; Dou, M.; Zhang, W.; Lv, L.; Chen, J.; Li, L.; Wang, L.; Han, X. Curcumin inhibits the growth of triple-negative breast cancer cells by silencing EZH2 and restoring DLC1 expression. J. Cell. Mol. Med. 2020, 24, 10648–10662. [Google Scholar] [CrossRef]
- Tu, Z.S.; Wang, Q.; Sun, D.D.; Dai, F.; Zhou, B. Design, synthesis, and evaluation of curcumin derivatives as Nrf2 activators and cytoprotectors against oxidative death. Eur. J. Med. Chem. 2017, 134, 72–85. [Google Scholar] [CrossRef] [PubMed]
- SDarvesh, A.; BAggarwal, B.; Bishayee, A. Curcumin and liver cancer: A review. Curr. Pharm. Biotechnol. 2012, 13, 218–228. [Google Scholar] [CrossRef]
- Moghtaderi, H.; Sepehri, H.; Attari, F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed. Pharmacother. 2017, 88, 582–594. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Wu, Z.; Xu, Y.; Jiang, R. Curcumin for Treating Breast Cancer: A Review of Molecular Mechanisms, Combinations with Anticancer Drugs, and Nanosystems. Pharmaceutics 2024, 16, 79. [Google Scholar] [CrossRef]
- Kong, W.Y.; Yee, Z.Y.; Mai, C.W.; Fang, C.M.; Abdullah, S.; Ngai, S.C. Zebularine and trichostatin A sensitized human breast adenocarcinoma cells towards tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced apoptosis. Heliyon 2019, 5, e02468. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Liu, C.; Zou, Y.; Huang, L.; Liang, Y.; Ren, J.; Liu, Y.; Lin, Q. Elaboration and characterization of curcumin-loaded soy soluble polysaccharide (SSPS)-based nanocarriers mediated by antimicrobial peptide nisin. Food Chem. 2021, 336, 127669. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Kumar, N.A.; Thakur, G. Developments in the anticancer activity of structurally modified curcumin: An up-to-date review. Eur. J. Med. Chem. 2019, 177, 76–104. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fang, C.; Zhang, J.; Liu, B.; Wei, Z.; Fan, X.; Sui, Z.; Tan, Q. Catanionic lipid nanosystems improve pharmacokinetics and anti-lung cancer activity of curcumin. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, T.; Xu, H.; Ren, B.; Cheng, X.; Qi, R.; Liu, H.; Wang, Y.; Yan, L.; Chen, S.; et al. Curcumin-loaded solid lipid nanoparticles enhanced anticancer efficiency in breast cancer. Molecules 2018, 23, 1578. [Google Scholar] [CrossRef]
- Shen, H.; Shen, J.; Pan, H.; Xu, L.; Sheng, H.; Liu, B.; Yao, M. Curcumin analog B14 has high bioavailability and enhances the effect of anti-breast cancer cells in vitro and in vivo. Cancer Sci. 2021, 112, 815–827. [Google Scholar] [CrossRef]
- Hanafy, N.A. Optimally designed theranostic system based folic acids and chitosan as a promising mucoadhesive delivery system for encapsulating curcumin LbL nano-template against invasiveness of breast cancer. Int. J. Biol. Macromol. 2021, 182, 1981–1993. [Google Scholar] [CrossRef] [PubMed]
- Ziasarabi, P.; Sahebkar, A.; Ghasemi, F. Evaluation of the effects of nanomicellar curcumin, berberine, and their combination with 5-fluorouracil on breast cancer cells. Nat. Prod. Hum. Dis. Pharmacol. Mol. Targets Ther. Benefits 2021, 1328, 21–35. [Google Scholar]
- Mukhopadhyay, R.; Sen, R.; Paul, B.; Kazi, J.; Ganguly, S.; Debnath, M.C. Gemcitabine co-encapsulated with curcumin in folate decorated PLGA nanoparticles; a novel approach to treat breast adenocarcinoma. Pharm. Res. 2020, 37, 56. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhang, Y.; Zhang, K.; Xie, J.; Liu, Y.; Li, W.; Feng, N. Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 921–935. [Google Scholar] [CrossRef]
- Yeap, S.K.; Mohd Ali, N.; Akhtar, M.N.; Razak, N.A.; Chong, Z.X.; Ho, W.Y.; Boo, L.; Zareen, S.; Kurniawan, T.A.; Avtar, R.; et al. Induction of apoptosis and regulation of microRNA expression by (2 E, 6 E)-2, 6-bis-(4-hydroxy-3-methoxybenzylidene)-cyclohexanone (BHMC) treatment on MCF-7 breast cancer cells. Molecules 2021, 26, 1277. [Google Scholar] [CrossRef]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly (butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef]
- Fathy Abd-Ellatef, G.E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.; et al. Curcumin-loaded solid lipid nanoparticles bypass p-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef]
- Lv, L.I.; Qiu, K.; Yu, X.; Chen, C.; Qin, F.; Shi, Y.; Ou, J.; Zhang, T.; Zhu, H.; Wu, J.; et al. Amphiphilic copolymeric micelles for doxorubicin and curcumin co-delivery to reverse multidrug resistance in breast cancer. J. Biomed. Nanotechnol. 2016, 12, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Darwish, N.H.; Mousa, S.A. Senescent colon and breast cancer cells induced by doxorubicin exhibit enhanced sensitivity to curcumin, caffeine, and thymoquinone. Integr. Cancer Ther. 2020, 19, 1534735419901160. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A comprehensive review on the benefits and problems of curcumin with respect to human health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Boon, E.A.; Croft, K.D.; Shinde, S.; Hodgson, J.M.; Ward, N.C. The acute effect of coffee on endothelial function and glucose metabolism following a glucose load in healthy human volunteers. Food Funct. 2017, 8, 3366–3373. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef]
- Wahab, A.; Gao, K.; Jia, C.; Zhang, F.; Tian, G.; Murtaza, G.; Chen, J. Significance of resveratrol in clinical management of chronic diseases. Molecules 2017, 22, 1329. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Sethi, G.; Diagaradjane, P.; Anand, P.; Pandey, M.K.; Gelovani, J.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. Int. J. Cancer 2010, 127, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.G.; D’Orazio, J.A.; Pearson, K.J. Resveratrol and cancer: Focus on in vivo evidence. Endocr.-Relat. Cancer 2014, 21, R209–R225. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Trung, L.Q.; Inaoka, P.T.; Yamada, K.; An, D.T.; Mizuno, S.; Nakao, S.; Takami, A. The repeated administration of resveratrol has measurable effects on circulating T-cell subsets in humans. Oxidative Med. Cell. Longev. 2017, 2017, 6781872. [Google Scholar] [CrossRef] [PubMed]
- Holcombe, R.F.; Martinez, M.; Planutis, K.; Planutiene, M. Effects of a grape-supplemented diet on proliferation and Wnt signaling in the colonic mucosa are greatest for those over age 50 and with high arginine consumption. Nutr. J. 2015, 14, 62. [Google Scholar] [CrossRef]
- Liu, P.; Tang, W.; Xiang, K.; Li, G. Pterostilbene in the treatment of inflammatory and oncological diseases. Front. Pharmacol. 2024, 14, 1323377. [Google Scholar] [CrossRef]
- Daniel, M.; Tollefsbol, T.O. Pterostilbene down-regulates hTERT at physiological concentrations in breast cancer cells: Potentially through the inhibition of cMyc. J. Cell. Biochem. 2018, 119, 3326–3337. [Google Scholar] [CrossRef]
- Mak, K.K.; Wu, A.T.; Lee, W.H.; Chang, T.C.; Chiou, J.F.; Wang, L.S.; Wu, C.H.; Huang, C.Y.; Shieh, Y.S.; Chao, T.Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-κ B/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef]
- Hsiao, P.C.; Chou, Y.E.; Tan, P.; Lee, W.J.; Yang, S.F.; Chow, J.M.; Chen, H.Y.; Lin, C.H.; Lee, L.M.; Chien, M.H. Pterostilbene simultaneously induced G0/G1-phase arrest and MAPK-mediated mitochondrial-derived apoptosis in human acute myeloid leukemia cell lines. PLoS ONE 2014, 9, e105342. [Google Scholar] [CrossRef]
- Tong, C.; Wang, Y.; Li, J.; Cen, W.; Zhang, W.; Zhu, Z.; Yu, J.; Lu, B. Pterostilbene inhibits gallbladder cancer progression by suppressing the PI3K/Akt pathway. Sci. Rep. 2021, 11, 4391. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, T.; Chen, X.; Cheng, J.; Wang, L. Pterostilbene regulates cell proliferation and apoptosis in non-small-cell lung cancer via targeting COX-2. Biotechnol. Appl. Biochem. 2023, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Kumar, A.; Li, K.; Tzivion, G.; Levenson, A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 265–275. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Y.; Fan, C.; Di, S.; Hu, W.; Jiang, S.; Li, T.; Ma, Z.; Chao, D.; Feng, X.; et al. Pterostilbene inhibits the growth of human esophageal cancer cells by regulating endoplasmic reticulum stress. Cell. Physiol. Biochem. 2016, 38, 1226–1244. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.M.; Liu, L.C.; Ho, C.T.; Lin, Y.C.; Way, T.D. Pterostilbene enhances TRAIL-induced apoptosis through the induction of death receptors and downregulation of cell survival proteins in TRAIL-resistance triple negative breast cancer cells. J. Agric. Food Chem. 2017, 65, 11179–11191. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Xiang, Y.; Mei, H.; Fang, B.; Wang, Q.; Cao, D.; Hu, Y.; Guo, T. Pterostilbene inhibits nutrient metabolism and induces apoptosis through AMPK activation in multiple myeloma cells. Int. J. Mol. Med. 2018, 42, 2676–2688. [Google Scholar] [CrossRef]
- Moon, D.; McCormack, D.; McDonald, D.; McFadden, D. Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. J. Surg. Res. 2013, 180, 208–215. [Google Scholar] [CrossRef]
- Bin, W.H.; Da, L.H.; Xue, Y.; Jing, B. Pterostilbene (3′, 5′-dimethoxy-resveratrol) exerts potent antitumor effects in HeLa human cervical cancer cells via disruption of mitochondrial membrane potential, apoptosis induction and targeting m-TOR/PI3K/Akt signalling pathway. JBUON 2018, 23, 1384–1389. [Google Scholar]
- Huang, W.C.; Chan, M.L.; Chen, M.J.; Tsai, T.H.; Chen, Y.J. Modulation of macrophage polarization and lung cancer cell stemness by MUC1 and development of a related small-molecule inhibitor pterostilbene. Oncotarget 2016, 7, 39363. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Li, M.; Li, S.; Zhao, H. Targeting cancer stem cells and signaling pathways by resveratrol and pterostilbene. Biofactors 2018, 44, 61–68. [Google Scholar] [CrossRef]
- Hojo, Y.; Kishi, S.; Mori, S.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Nishiguchi, Y.; Nakashima, C.; Luo, Y.; Shinohara, H.; et al. Sunitinib and pterostilbene combination treatment exerts antitumor effects in gastric cancer via suppression of PDZD8. Int. J. Mol. Sci. 2022, 23, 4002. [Google Scholar] [CrossRef] [PubMed]
- Roemhild, K.; von Maltzahn, F.; Weiskirchen, R.; Knüchel, R.; von Stillfried, S.; Lammers, T. Iron metabolism: Pathophysiology and pharmacology. Trends Pharmacol. Sci. 2021, 42, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hussain, A.; Guo, Q.Q.; Jin, X.W.; Wang, M.M. Oxygen and Iron Availability Shapes Metabolic Adaptations of Cancer Cells. World J. Oncol. 2024, 15, 28. [Google Scholar] [CrossRef]
- Nishiguch, Y.; Fujiwara-Tani, R.; Nukaga, S.; Nishida, R.; Ikemoto, A.; Sasaki, R.; Mori, S.; Ogata, R.; Kishi, S.; Hojo, Y.; et al. Pterostilbene induces apoptosis from endoplasmic reticulum stress synergistically with anticancer drugs that deposit iron in mitochondria. Int. J. Mol. Sci. 2024, 25, 2611. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene induces apoptosis and cell cycle arrest in diffuse large B-cell lymphoma cells. Sci. Rep. 2016, 6, 37417. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Xu, W.; Wang, Q.; Zhang, C.; Ding, Y.; Nie, W.; Lai, J.; Chen, Y.; Huang, H. Pterostilbene promotes mitochondrial apoptosis and inhibits proliferation in glioma cells. Sci. Rep. 2021, 11, 6381. [Google Scholar] [CrossRef]
- Tan, K.T.; Chen, P.W.; Li, S.; Ke, T.M.; Lin, S.H.; Yang, C.C. Pterostilbene inhibits lung squamous cell carcinoma growth in vitro and in vivo by inducing S phase arrest and apoptosis. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.M.; Sheweita, S.A.; Sultan, A.S. Pterostilbene as a phytochemical compound induces signaling pathways involved in the apoptosis and death of mutant P53-breast cancer cell lines. Nutr. Cancer 2021, 73, 1976–1984. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Fulda, S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov. Today 2010, 15, 757–765. [Google Scholar] [CrossRef]
- Yeo, S.C.; Ho, P.C.; Lin, H.S. Pharmacokinetics of pterostilbene in S prague-D awley rats: The impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013, 57, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Scherzberg, M.C.; Kiehl, A.; Zivkovic, A.; Stark, H.; Stein, J.; Fürst, R.; Steinhilber, D.; Ulrich-Rückert, S. Structural modification of resveratrol leads to increased anti-tumor activity, but causes profound changes in the mode of action. Toxicol. Appl. Pharmacol. 2015, 287, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, F.S.; Velázquez-Martínez, C.A. 3, 4′, 5-trans-Trimethoxystilbene; a natural analogue of resveratrol with enhanced anticancer potency. Investig. New Drugs 2015, 33, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Kempisty, H.; Ruciński, M.; Borys, S.; Kucińska, M.; Kaczmarek, M.; Zawierucha, P.; Wierzchowski, M.; Łażewski, D.; Murias, M.; Jodynis-Liebert, J. 3′-hydroxy-3, 4, 5, 4′-tetramethoxystilbene, the metabolite of resveratrol analogue DMU-212, inhibits ovarian cancer cell growth in vitro and in a mice xenograft model. Sci. Rep. 2016, 6, 32627. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin, C.L.; Tsai, J.H.; Ho, C.T.; Chen, W.J. 3, 5, 3′, 4′, 5′-pentamethoxystilbene (MR-5), a synthetically methoxylated analogue of resveratrol, inhibits growth and induces G1 cell cycle arrest of human breast carcinoma MCF-7 cells. J. Agric. Food Chem. 2010, 58, 226–234. [Google Scholar] [CrossRef]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.; Vijayaraghavan, K. A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, J.E.; Song, N.R.; Son, J.E.; Hwang, M.K.; Byun, S.; Kim, J.H.; Lee, K.W.; Lee, H.J. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc. Res. 2010, 85, 836–844. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- De Groote, D.; Van Belleghem, K.; Devière, J.; Van Brussel, W.; Mukaneza, A.; Amininejad, L. Effect of the intake of resveratrol, resveratrol phosphate, and catechin-rich grape seed extract on markers of oxidative stress and gene expression in adult obese subjects. Ann. Nutr. Metab. 2012, 61, 15–24. [Google Scholar] [CrossRef]
- Hwang, D.; Shin, S.Y.; Lee, Y.; Hyun, J.; Yong, Y.; Park, J.C.; Lee, Y.H.; Lim, Y. A compound isolated from Schisandra chinensis induces apoptosis. Bioorg. Med. Chem. Lett. 2011, 21, 6054–6057. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.S.; Wang, X.A.; Li, H.F.; Shu, Y.J.; Bao, R.F.; Zhang, F.; Cao, Y.; Ye, Y.Y.; Weng, H.; Wu, W.G.; et al. Schisandrin B induces apoptosis and cell cycle arrest of gallbladder cancer cells. Molecules 2014, 19, 13235–13250. [Google Scholar] [CrossRef]
- Ko, Y.H.; Jeong, M.; Jang, D.S.; Choi, J.H. Gomisin L1, a lignan isolated from Schisandra berries, induces apoptosis by regulating NADPH oxidase in human ovarian cancer cells. Life 2021, 11, 858. [Google Scholar] [CrossRef]
- Jung, S.; Moon, H.I.; Kim, S.; Quynh, N.T.; Yu, J.; Sandag, Z.; Le, D.D.; Lee, H.; Lee, H.; Lee, M.S. Anticancer activity of gomisin J from Schisandra chinensis fruit. Oncol. Rep. 2019, 41, 711–717. [Google Scholar] [CrossRef]
- Li, Y.J.; Liu, H.T.; Xue, C.J.; Xing, X.Q.; Dong, S.T.; Wang, L.S.; Ding, C.Y.; Meng, L.; Dong, Z.J. The synergistic anti-tumor effect of schisandrin B and apatinib. J. Asian Nat. Prod. Res. 2020, 22, 839–849. [Google Scholar] [CrossRef]
- Han, Y.H.; Mun, J.G.; Jeon, H.D.; Park, J.; Kee, J.Y.; Hong, S.H. Gomisin A ameliorates metastatic melanoma by inhibiting AMPK and ERK/JNK-mediated cell survival and metastatic phenotypes. Phytomedicine 2020, 68, 153147. [Google Scholar] [CrossRef] [PubMed]
- Waiwut, P.; Shin, M.S.; Yokoyama, S.; Saiki, I.; Sakurai, H. Gomisin A enhances tumor necrosis factor-α-induced G1 cell cycle arrest via signal transducer and activator of transcription 1-mediated phosphorylation of retinoblastoma protein. Biol. Pharm. Bull. 2012, 35, 1997–2003. [Google Scholar] [CrossRef]
- Maharjan, S.; Park, B.K.; Lee, S.I.; Lim, Y.; Lee, K.; Kwon, H.J. Gomisin G inhibits the growth of triple-negative breast cancer cells by suppressing AKT phosphorylation and decreasing cyclin D1. Biomol. Ther. 2018, 26, 322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, K.; Hu, Y.; Su, F.; Gao, Z.; Hu, T.; Yang, Y.; Cao, X.; Qian, F. Schisantherin A induces cell apoptosis through ROS/JNK signaling pathway in human gastric cancer cells. Biochem. Pharmacol. 2020, 173, 113673. [Google Scholar] [CrossRef]
- Jing, M.; Bi, X.J.; Yao, X.M.; Cai, F.; Liu, J.J.; Fu, M.; Kong, L.; Liu, X.Z.; Zhang, L.; He, S.Y.; et al. Enhanced antitumor efficacy using epirubicin and schisandrin B co-delivery liposomes modified with PFV via inhibiting tumor metastasis. Drug Dev. Ind. Pharm. 2020, 46, 621–634. [Google Scholar] [CrossRef]
- He, L.; Chen, H.; Qi, Q.; Wu, N.; Wang, Y.; Chen, M.; Feng, Q.; Dong, B.; Jin, R.; Jiang, L. Schisandrin B suppresses gastric cancer cell growth and enhances the efficacy of chemotherapy drug 5-FU in vitro and in vivo. Eur. J. Pharmacol. 2022, 920, 174823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Huang, Y.; Yang, S.; Tan, T.; Wang, N.; Zhang, J.; Ye, C.; Wei, M.; Luo, J.; et al. Schisandrin B suppresses osteosarcoma lung metastasis in vivo by inhibiting the activation of the Wnt/β-catenin and PI3K/Akt signaling pathways. Oncol. Rep. 2022, 47, 50. [Google Scholar] [CrossRef] [PubMed]
- Nasser, M.I.; Han, T.; Adlat, S.; Tian, Y.; Jiang, N. Inhibitory effects of Schisandrin B on human prostate cancer cells. Oncol. Rep. 2019, 41, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Shi, L.X.; Yao, X.M.; Jing, M.; Li, Q.Q.; Wang, Y.L.; Li, Q.S. Functional vinorelbine plus schisandrin B liposomes destroying tumor metastasis in treatment of gastric cancer. Drug Dev. Ind. Pharm. 2021, 47, 100–112. [Google Scholar] [CrossRef]
- Wang, S.; Wang, A.; Shao, M.; Lin, L.; Li, P.; Wang, Y. Schisandrin B reverses doxorubicin resistance through inhibiting P-glycoprotein and promoting proteasome-mediated degradation of survivin. Sci. Rep. 2017, 7, 8419. [Google Scholar] [CrossRef]
- Yan, C.; Gao, L.; Qiu, X.; Deng, C. Schisandrin B synergizes docetaxel-induced restriction of growth and invasion of cervical cancer cells in vitro and in vivo. Ann. Transl. Med. 2020, 8, 1157. [Google Scholar] [CrossRef]
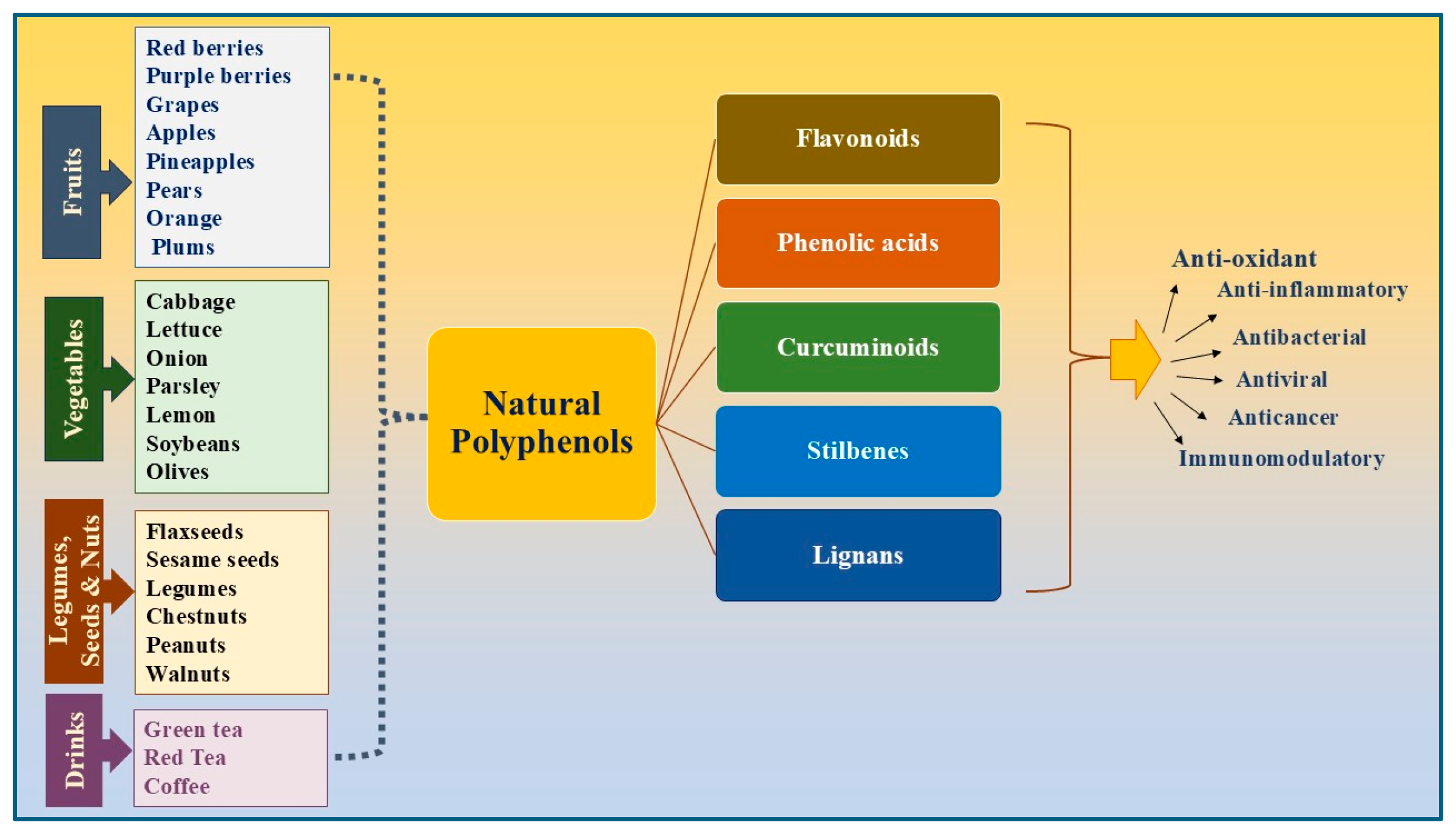

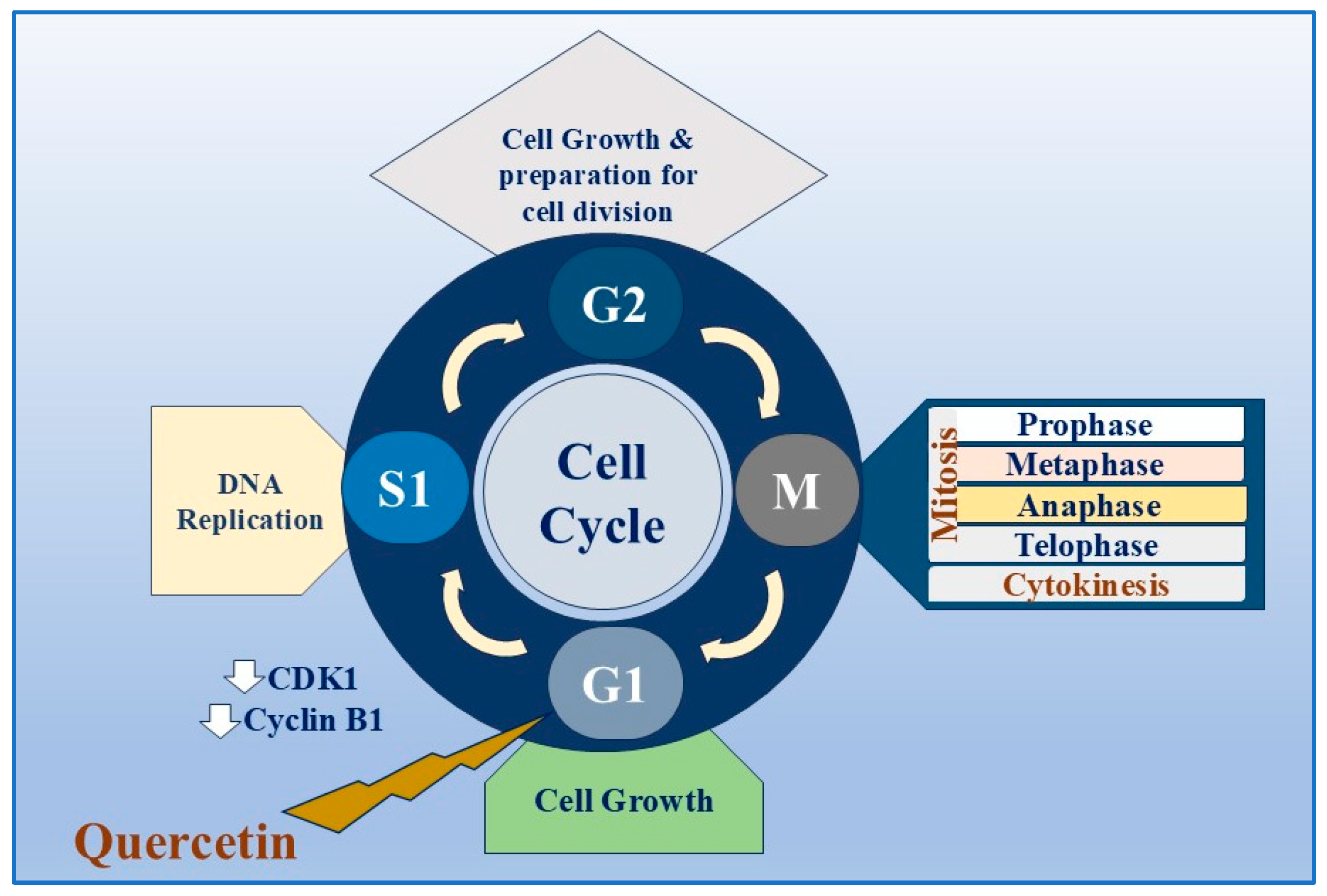

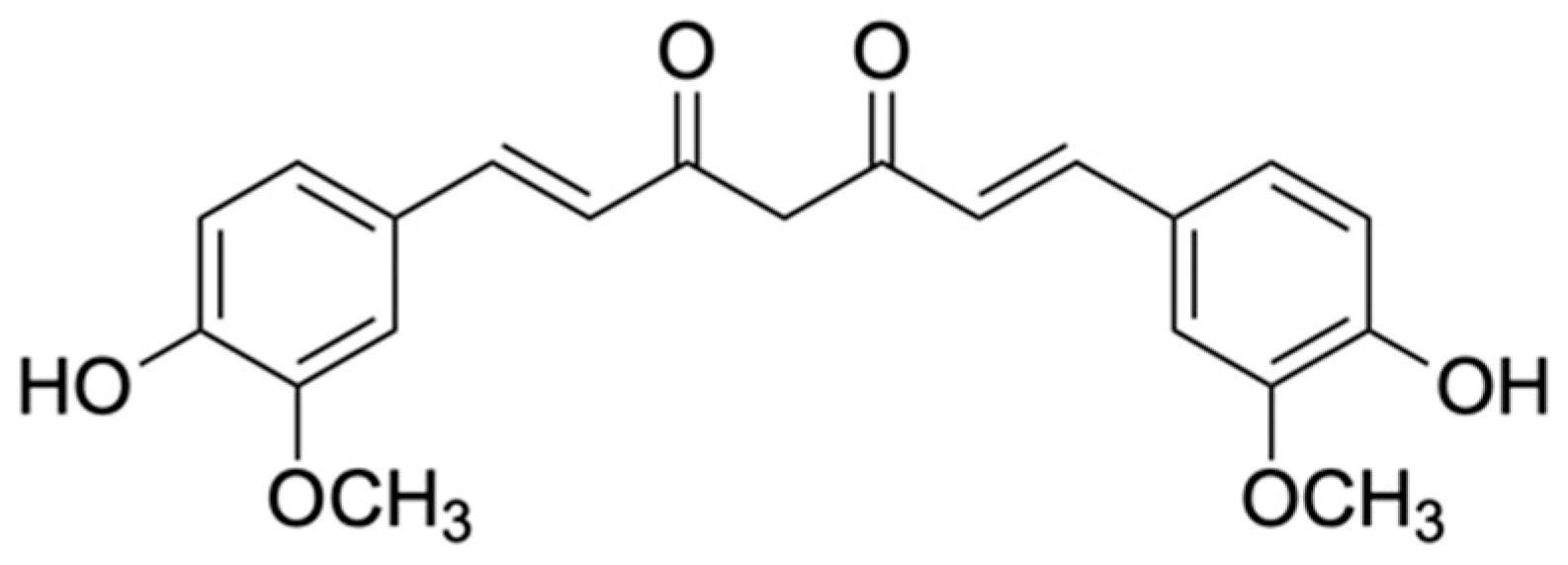

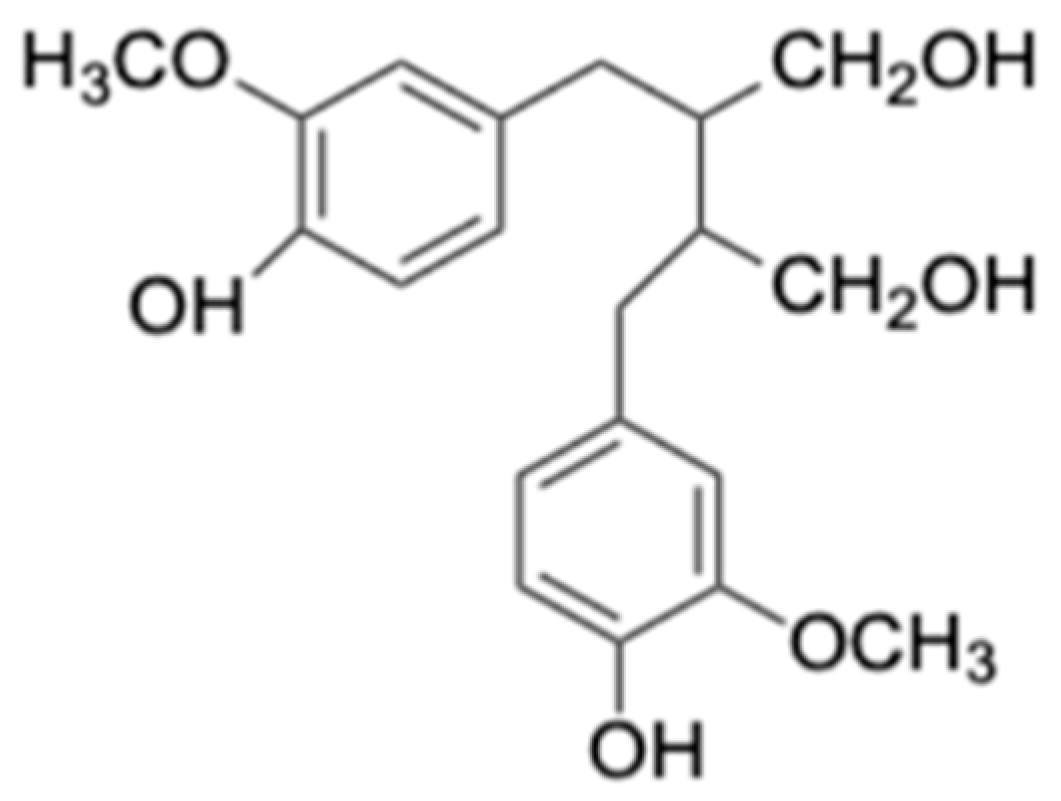
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, Z.S.O.; Khan, E.; Elias, N.; Elshebiny, A.; Dou, Q. Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management. Biomolecules 2025, 15, 629. https://doi.org/10.3390/biom15050629
Ahmed ZSO, Khan E, Elias N, Elshebiny A, Dou Q. Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management. Biomolecules. 2025; 15(5):629. https://doi.org/10.3390/biom15050629
Chicago/Turabian StyleAhmed, Zainab Sabry Othman, Elyas Khan, Nathan Elias, Alhussein Elshebiny, and Qingping Dou. 2025. "Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management" Biomolecules 15, no. 5: 629. https://doi.org/10.3390/biom15050629
APA StyleAhmed, Z. S. O., Khan, E., Elias, N., Elshebiny, A., & Dou, Q. (2025). Updated Review on Natural Polyphenols: Molecular Mechanisms, Biological Effects, and Clinical Applications for Cancer Management. Biomolecules, 15(5), 629. https://doi.org/10.3390/biom15050629


_Kwok.png)





