A Study of the Effects of Oleuropein and Polydatin Association on Muscle and Bone Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Human Cell Lines
2.3. MTT Assay
2.4. Alkaline Phosphatase Staining
2.5. Alizarin Red Staining
2.6. Cell Cycle Analysis
2.7. Gene Expression Analysis
2.8. Western Blot
2.9. Cell Death Analysis
2.10. Statistical Analysis
3. Results
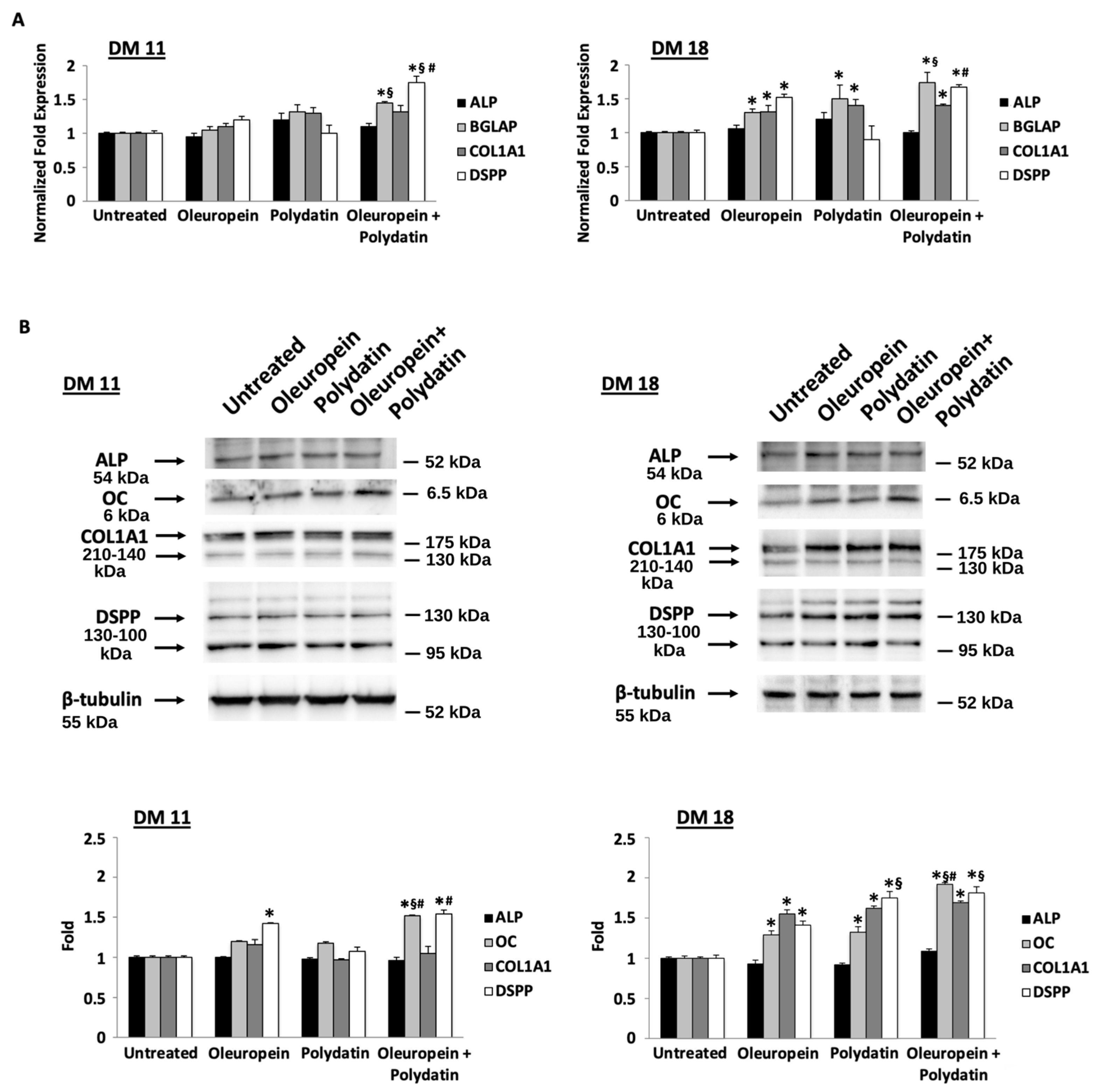
3.1. Low Concentration of Oleuropein and Polydatin Stimulates Osteoblast Differentiation
3.2. Low Concentration of Oleuropein and Polydatin Stimulates Myoblast Differentiation
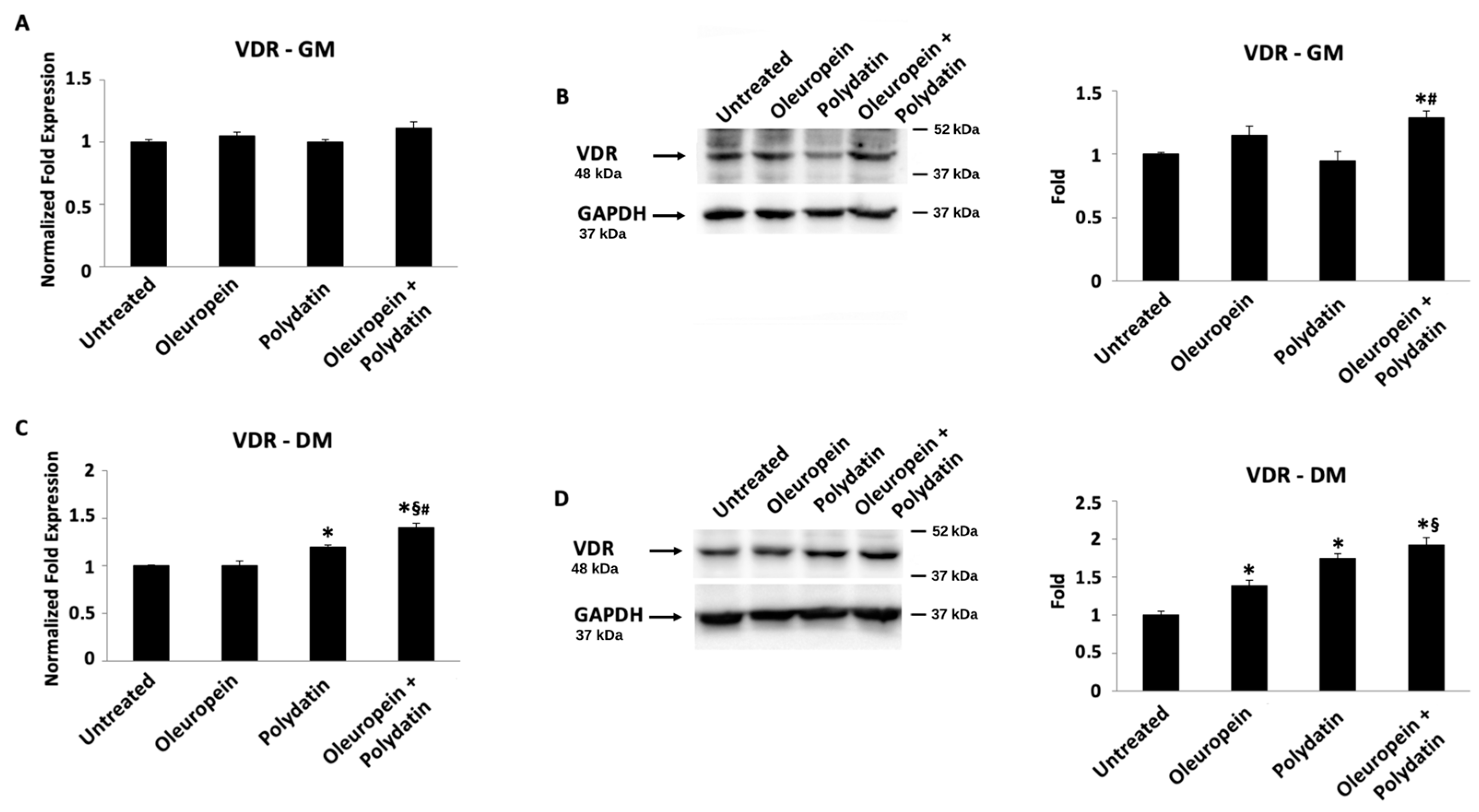
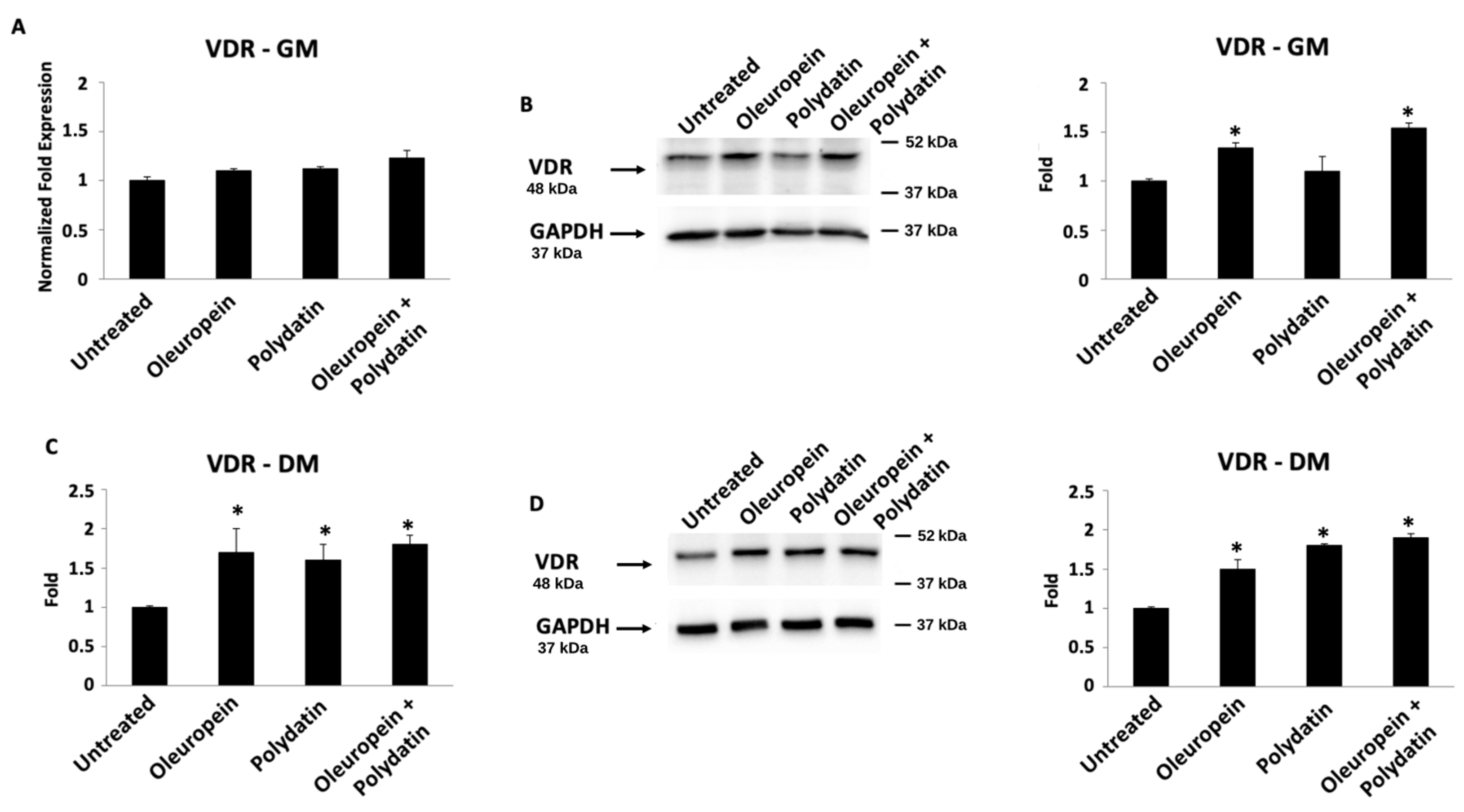
3.3. Oleuropein–Polydatin Combination Stimulates Vitamin D3 Receptor Expression
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greising, S.M.; Baltgalvis, K.A.; Lowe, D.A.; Warren, G.L. Hormone therapy and skeletal muscle strength: A meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Critchlow, A.J.; Hiam, D.; Williams, R.; Scott, D.; Lamon, S. The role of estrogen in female skeletal muscle aging: A systematic review. Maturitas. 2023, 178, 107844. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Crosstalk between muscle and bone. J. Bone Miner. Metab. 2024, 42, 391–398. [Google Scholar] [CrossRef]
- Avin, K.G.; Bloomfield, S.A.; Gross, T.S.; Warden, S.J. Biomechanical apsects of the muscle-bone interaction. Curr. Osteoporos. Rep. 2015, 13, 1–8. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Reginster, J.; Beaudart, C.; Buckinx, F.; Bruyere, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef]
- Li, G.B.; Zhang, L.; Wang, D.E.; AlQudsy, L.X.; Jiang, J.; Xu, H.Y.; Shang, P. Muscle-bone crosstalk and potential therapies for sarco-osteoporosis. J. Cell Biochem. 2019, 120, 14262–14273. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Levinger, I.; Scott, D.; Nicholson, G.C.; Stuart, A.L.; Duque, G.; McCorquodale, T.; Herrmann, M.; Ebeling, P.R.; Sanders, K.M. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone 2014, 64, 8–12. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, K.; Guan, Q.; Guo, Q.; Zhao, C. Mechanism and physical activities in bone-skeletal muscle crosstalk. Front. Endocrinol. 2024, 3, 1287972. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Ferron, M.; Confavreaux, C.; Galan-Diez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; Bacchetta, J. Osteocalcin signaling in myofibres is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef]
- Coll, P.P.; Phu, S.; Hajjar, S.H.; Kirk, B.; Duque, G.; Taxel, P. The prevention of osteoporosis and sarcopenia in older adults. J. Am. Geriatr. Soc. 2021, 69, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.-R.; Youn, S.; Kim, D.H.; Pack, S.P. Natural Compounds for Preventing Age-Related Diseases and Cancers. Int. J. Mol. Sci. 2024, 25, 7530. [Google Scholar] [CrossRef]
- Puel, C.M.; Coxam, V.; Davicco, M.-J. Mediterranean diet and osteoporosis prevention. Med. Sci. 2007, 23, 756–760. [Google Scholar] [CrossRef]
- Garcìa-Martìnez, O.; Rivas, A.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Ruiz, C. The effect of olive oil on osteoporosis prevention. Int. J. Food Sci. Nutr. 2014, 65, 834–840. [Google Scholar] [CrossRef]
- Andreo-López, M.C.; Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients 2023, 15, 3224. [Google Scholar] [CrossRef]
- Gotsis, E.; Anagnostis, P.; Mariolis, A.; Vlachou, A.; Katsiki, N.; Karagiannis, A. Health benefits of the Mediterranean Diet: An update of research over the last 5 years. Angiology 2015, 66, 304–318. [Google Scholar] [CrossRef]
- Garcìa Pérez de Sevilla, G.; Sànchez-Pinto Pinto, B. Effectiveness of Workplace Mediterranean Diet Interventions on Cardiometabolic Risk Factors: A Systematic Review. Workplace Health Saf. 2022, 70, 73–80. [Google Scholar] [CrossRef]
- Cannataro, R.; Fazio, A.; La Torre, C.; Caroleo, M.C.; Cione, E. Polyphenols in the Mediterranean Diet: From Dietary Sources to microRNA Modulation. Antioxidants 2021, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Castejòn, M.L.; Montoya, T.; Alarcòn-de-Lastra, C.; Sànchez-Hidalgo, M. Potential Protective Role Exerted by Secoiridoids from Olea eiropaea L. in Cancer, Cardiovascular, Neurodegenerative, Aging-Related, and Neuroinflammatory Diseases. Antioxidants 2020, 10, 149. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in human. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Phenolic compounds and antioxidant activity of olive leaf extract. Nat. Prod. Res. 2012, 26, 186–189. [Google Scholar] [CrossRef]
- Santiago-Mora, R.; Casado-Diaz, A.; De Castro, M.D.; Quesada-Gomez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef]
- Drira, R.; Chen, S.; Sakamoto, K. Oleuropein and hydroxytyrosol inhibit adipocyte differentiation in 3 T3-L1 cells. Life Sci. 2011, 89, 708–716. [Google Scholar] [CrossRef]
- Pérez-Rey, J.; Roncero-Martín, R.; Rico-Martín, S.; Rey-Sánchez, P.; Pedrera-Zamorano, J.D.; Pedrera-Canal, M.; López-Espuela, F.; Lavado-García, J.M. Adherence to a Mediterranean Diet and Bone Mineral Density in Spanish Premenopausal Women. Nutrients 2019, 11, 555. [Google Scholar] [CrossRef]
- Quattrini, S.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Brandi, M.L. The Mediterranean Diet in Osteoporosis Prevention: An Insight in a Peri- and Post-Menopausal Population. Nutrients 2021, 13, 531. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Jennings, A.; Steves, C.J.; Skinner, J.; Cassidy, A.; MacGregor, A.J.; Welch, A.A. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef]
- Jennings, A.; Mulligan, A.A.; Khaw, K.T.; Luben, R.N.; Welch, A.A. A Mediterranean Diet Is Positively Associated with Bone and Muscle Health in a Non-Mediterranean Region in 25,450 Men and Women from EPIC-Norfolk. Nutrients 2020, 12, 1154. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.H.; Peng, C.; Zhang, H. Polydatin: A review of pharmacology and pharmacokinetics. Pharm. Biol. 2013, 51, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Andres-Lacueva, C.; Lamuela-Raventòs, R.M.; Berenguer, T.; Jakszyn, P.; Martínez, C.; Sanchez, M.J.; Navarro, C.; Chirlaque, M.D.; Tormo, M.J.; et al. Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br. J. Nutr. 2008, 100, 188–196. [Google Scholar] [CrossRef]
- Chen, M.; Hou, Y.; Lin, D. Polydatin protects bone marrow stem cells against oxidative injury: Involvement of Nrf 2/ARE patways. Stem Cells Int. 2016, 2016, 9394150. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Z.; Jia, R.; Jiang, D.; Xu, Z.; Zhang, Y.; Wu, Y.Q.; Wang, X. Protective effects of polydatin against bone and joint disorders: The in vitro and in vivo evidence so far. Nutr. Res. Rev. 2024, 37, 96–107. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, Q.; Hong, G.; Chen, D.; Liang, J.; He, W.; Chen, M.H. Polydatin induces bone marrow stromal cells migration by activation of ERK1/2. Biomed. Pharmacother. 2016, 82, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Cai, Z.; Li, D.; Zhang, Y.; He, M.; Yang, Y.; Liu, D.; Xie, W.; Li, Y.; Xiao, W. Myogenic Differentiation of Stem Cells for Skeletal Muscle Regeneration. Stem Cells Int. 2021, 2021, 8884283. [Google Scholar] [CrossRef]
- Stevenson, J.C. A woman’s journey through the reproductive, transitional and postmenopausal periods of life: Impact on cardiovascular and musculo-skeletal risk and the role of estrogen replacement. Maturitas 2011, 70, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Shin, S. Association of Sarcopenia with Osteopenia and Osteoporosis in Community-Dwelling Older Korean Adults: A Cross-Sectional Study. J. Clin. Med. 2021, 11, 129. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Ferguson, L.D.; Gray, S.R.; Rodrìguez-Gòmez, I.; Sattar, N.; Siebert, S.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Association of sarcopenia with incident osteoporosis: A prospective study of 168,682 UK biobank participants. J. Cachexia Sarcopenia Muscle 2021, 12, 1179–1188. [Google Scholar] [CrossRef]
- Clynes, M.A.; Greson, C.L.; Bruyère, O.; Cooper, C.; Dennison, E.M. Osteosarcopenia: Where osteoporosis and sarcopenia collide. Rheumatology 2021, 60, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Sjoblom, S.; Suuronen, J.; Rikkonen, T.; Honkanen, R.; Kroger, H.; Sirola, J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 2013, 75, 175–180. [Google Scholar] [CrossRef]
- Słupski, W.; Jawień, P.; Nowak, B. Botanicals in Postmenopausal Osteoporosis. Nutrients 2021, 13, 1609. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, S.; Li, P.; Liu, C.; Yuan, B.; Zhang, S.; Liu, A. Natural products and skeletal muscle health. J. Nutr. Biochem. 2021, 93, 108619. [Google Scholar] [CrossRef]
- Zheng, X.; Lee, S.K.; Chun, O.K. Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J. Med. Food. 2016, 19, 1–14. [Google Scholar] [CrossRef]
- Zare, R.; Devrim-Lanpir, A.; Guazzotti, S.; Ali Redha, A.; Prokopidis, K.; Spadaccini, D.; Cannataro, R.; Cione, E.; Henselmans, M.; Aragon, A.A. Effect of Soy Protein Supplementation on Muscle Adaptations, Metabolic and Antioxidant Status, Hormonal Response, and Exercise Performance of Active Individuals and Athletes: A Systematic Review of Randomised Controlled Trials. Sports Med. 2023, 53, 2417–2446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hengevoß, J.; Soukup, S.T.; Kulling, S.E.; Xie, M.; Diel, P. An isoflavone enriched diet increases skeletal muscle adaptation in response to physical activity in ovariectomized rats. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 collagen: Synthesis, structure and key functions in bone mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Tirachaimongkol, C.; Pothacharoen, P.; Reichart, P.A.; Khongkhunthian, P. Relation between the stability of dental implants and two biological markers during the healing period: A prospective clinical study. Int. J. Implant. Dent. 2016, 2, 27. [Google Scholar] [CrossRef]
- Caetano-Lopes, J.; Canhão, H.; Fonseca, J.E. Osteoblasts and bone formation. Acta Reumatol. Port. 2007, 32, 103–110. [Google Scholar] [PubMed]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Biro, R.; Kovacova, V.; Babikova, M.; Zemanova, N.; Mondockova, V.; Omelka, R. Current knowledge of bone-derived factor osteocalcin: Its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases. J. Mol. Med. 2024, 102, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Pi, M.; Nishimoto, S.K.; Quarles, L.D. Explaining Divergent Observations Regarding Osteocalcin/GPRC6A Endocrine Signaling. Endocrinology 2021, 162, bqab011. [Google Scholar] [CrossRef]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.E.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef]
- Rached, M.T.; Kode, A.; Silva, B.C.; Jung, D.Y.; Gray, S.; Ong, H.; Paik, J.H.; DePinho, R.A.; Kim, J.K.; Karsenty, G.; et al. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J. Clin. Investig. 2010, 120, 357–368. [Google Scholar] [CrossRef]
- Ferron, M.; McKee, M.D.; Levine, R.L.; Ducy, P.; Karsenty, G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 2012, 50, 568–575. [Google Scholar] [CrossRef]
- Qin, C.; Brunn, J.C.; Cadena, E.; Ridall, A.; Butler, W.T. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect. Tissue Res. 2003, 44 (Suppl. S1), 179–183. [Google Scholar] [CrossRef]
- Chen, S.; Rani, S.; Wu, Y.; Unterbrink, A.; Gu, T.T.; Gluhak-Heinrich, J.; Chuang, H.H.; MacDougall, M. Differential regulation of dentin sialophosphoprotein expression by Runx2 during odontoblast cytodifferentiation. J. Biol. Chem. 2005, 280, 29717–29727. [Google Scholar] [CrossRef]
- Jia, J.; Bian, Z.; Song, Y. The Role of DSPP in Dentine Formation and Hereditary Dentine Defects. Chin. J. Dent. Res. 2024, 27, 17–28. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Moncaut, N.; Rigby, P.W.; Carvajal, J.J. Dial M(RF) for myogenesis. FEBS J. 2013, 280, 3980–3990. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; von Essen, M.R.; Boding, L.; Levring, T.B.; Schjerling, P.; Lauritsen, J.P.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D up-regulates the vitamin D receptor by protecting it from proteasomal degradation in human CD4+ T cells. PLoS ONE 2014, 9, e96695. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Yoshizawa, T.; Fukuda, T.; Shirode-Fukuda, Y.; Yu, T.; Sekine, K.; Sato, T.; Kawano, H.; Aihara, K.; Nakamichi, Y.; et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology 2013, 154, 1008–1020. [Google Scholar] [CrossRef]
- Bishoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Durmuller, U.; Stahlen, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Rejnmark, L. Effects of vitamin D on muscle function and performance: A review of evidence from randomized controlled trials. The Adv. Chronic Dis. 2011, 2, 25–37. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle 2019, 10, 1228–1240. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, M.B.; Aguzzi, C.; Rascioni, R.; Mignini, F. A Study of the Effects of Oleuropein and Polydatin Association on Muscle and Bone Metabolism. Biomolecules 2025, 15, 628. https://doi.org/10.3390/biom15050628
Morelli MB, Aguzzi C, Rascioni R, Mignini F. A Study of the Effects of Oleuropein and Polydatin Association on Muscle and Bone Metabolism. Biomolecules. 2025; 15(5):628. https://doi.org/10.3390/biom15050628
Chicago/Turabian StyleMorelli, Maria Beatrice, Cristina Aguzzi, Riccardo Rascioni, and Fiorenzo Mignini. 2025. "A Study of the Effects of Oleuropein and Polydatin Association on Muscle and Bone Metabolism" Biomolecules 15, no. 5: 628. https://doi.org/10.3390/biom15050628
APA StyleMorelli, M. B., Aguzzi, C., Rascioni, R., & Mignini, F. (2025). A Study of the Effects of Oleuropein and Polydatin Association on Muscle and Bone Metabolism. Biomolecules, 15(5), 628. https://doi.org/10.3390/biom15050628






