The Quantification of Vitamin D in Humans: A Promising, Non-Invasive and Cost-Effective Method to Measure 25-Hydroxyvitamin D
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Population
2.2. Biological Sample Collection, Processing, and Storage
2.3. 25(OH)D Quantification
2.4. Other Covariates and Potential Confounders
2.5. Statistical Analysis
3. Results
3.1. Sample Population Characteristics
3.2. Serum 25(OH)D Quantification by CMIA (Benchmark Measure)
3.3. Serum, Urine, and Saliva 25(OH)D Quantification by ELISA
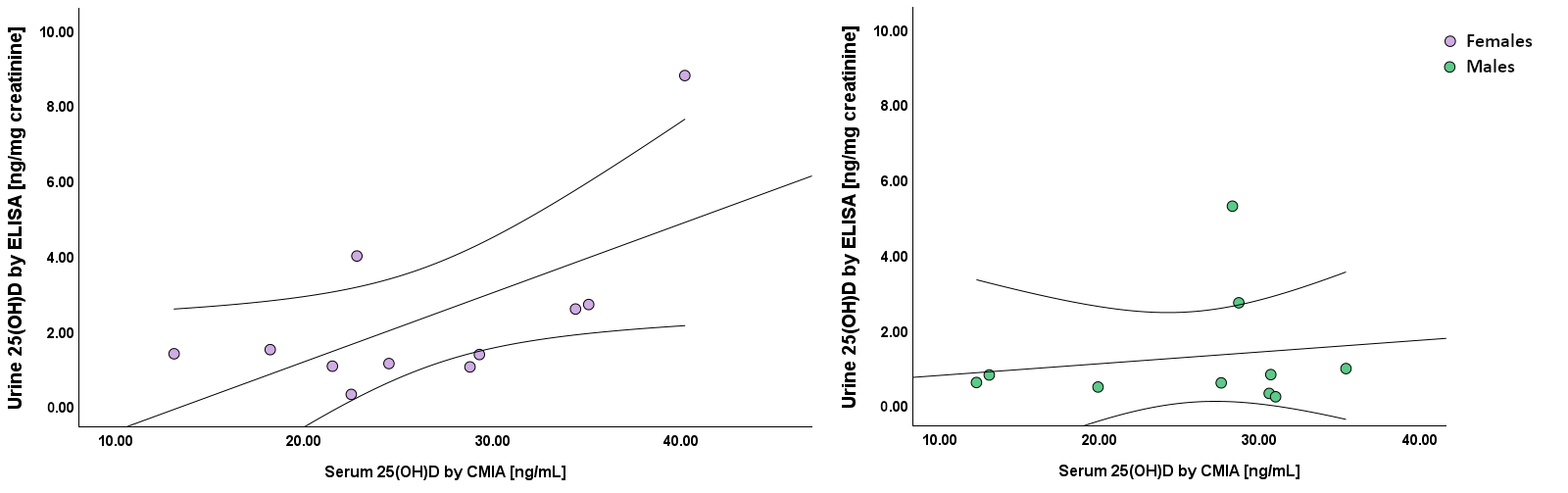
3.4. Correlation Between the Benchmark Measure and 25(OH)D, Measured by ELISA in Serum, Urine, and Saliva
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janoušek, J.; Pilařová, V.; Macáková, K.; Nomura, A.; Veiga-Matos, J.; da Silva, D.D.; Remião, F.; Saso, L.; Malá-Ládová, K.; Malý, J.; et al. Vitamin D: Sources, Physiological Role, Biokinetics, Deficiency, Therapeutic Use, Toxicity, and Overview of Analytical Methods for Detection of Vitamin D and Its Metabolites. Crit. Rev. Clin. Lab. Sci. 2022, 59, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Aiello, G.; Lombardo, M.; Baldelli, S. Exploring Vitamin D Synthesis and Function in Cardiovascular Health: A Narrative Review. Appl. Sci. 2024, 14, 4339. [Google Scholar] [CrossRef]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-Mediated Metabolism of Vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef]
- Jenkinson, C.; Desai, R.; McLeod, M.D.; Wolf Mueller, J.; Hewison, M.; Handelsman, D.J. Circulating Conjugated and Unconjugated Vitamin D Metabolite Measurements by Liquid Chromatography Mass Spectrometry. J. Clin. Endocrinol. Metab. 2022, 107, 435–449. [Google Scholar] [CrossRef]
- Schiffer, L.; Barnard, L.; Baranowski, E.S.; Gilligan, L.C.; Taylor, A.E.; Arlt, W.; Shackleton, C.H.L.; Storbeck, K.H. Human Steroid Biosynthesis, Metabolism and Excretion Are Differentially Reflected by Serum and Urine Steroid Metabolomes: A Comprehensive Review. J. Steroid Biochem. Mol. Biol. 2019, 194, 105439. [Google Scholar] [CrossRef] [PubMed]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The Serum Vitamin D Metabolome: What We Know and What Is Still to Discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Watkins, R.R.; Yamshchikov, A.V.; Lemonovich, T.L.; Salata, R.A. The Role of Vitamin D Deficiency in Sepsis and Potential Therapeutic Implications. J. Infect. 2011, 63, 321–326. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding Vitamin D Metabolism in Pregnancy: From Physiology to Pathophysiology and Clinical Outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Zhao, S.; Qian, F.; Wan, Z.; Chen, X.; Pan, A.; Liu, G. Vitamin D and Major Chronic Diseases. Trends Endocrinol. Metab. 2024, 35, 1050–1061. [Google Scholar] [CrossRef]
- D’avolio, A.; Avataneo, V.; Manca, A.; Cusato, J.; De Nicolò, A.; Lucchini, R.; Keller, F.; Cantù, M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Tsuprykov, O.; Chen, X.; Hocher, C.F.; Skoblo, R.; Yin, L.; Hocher, B. Why Should We Measure Free 25(OH) Vitamin D? J. Steroid Biochem. Mol. Biol. 2018, 180, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the Athlete: Current Perspectives and New Challenges. Sports Med. 2018, 48, 3–16. [Google Scholar] [CrossRef]
- Farrell, C.J.L.; Soldo, J.; McWhinney, B.; Bandodkar, S.; Herrmann, M. Impact of Assay Design on Test Performance: Lessons Learned from 25-Hydroxyvitamin D. Clin. Chem. Lab. Med. 2014, 52, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Fraser, W.D.; Milan, A.M. Vitamin D Assays: Past and Present Debates, Difficulties, and Developments. Calcif. Tissue Int. 2013, 92, 118–127. [Google Scholar] [CrossRef]
- Herrmann, M.; Harwood, T.; Gaston-Parry, O.; Kouzios, D.; Wong, T.; Lih, A.; Jimenez, M.; Janu, M.; Seibel, M.J. A New Quantitative LC Tandem Mass Spectrometry Assay for Serum 25-Hydroxy Vitamin D. Steroids 2010, 75, 1106–1112. [Google Scholar] [CrossRef]
- Bahramian, A.; Falsafi, P.; Abbasi, T.; Ghanizadeh, M.; Abedini, M.; Kavoosi, F.; Kouhsoltani, M.; Noorbakhsh, F.; Dabbaghi Tabriz, F.; Rajaeih, S.; et al. Comparing Serum and Salivary Levels of Vitamin D in Patients with Recurrent Aphthous Stomatitis and Healthy Individuals. J. Dent. 2018, 19, 295. [Google Scholar]
- Fairney, A.; Saphier, P.W. Studies on the Measurement of 25-Hydroxy Vitamin D in Human Saliva. Br. J. Nutr. 1987, 57, 13–25. [Google Scholar] [CrossRef]
- Hussein, A.S.; Almoudi, M.M.; Abu-Hassan, M.I.; Schroth, R.J.; Saripudin, B.; Mohamad, M.S.F. Serum and Saliva 25(OH)D Levels in Relation to Dental Caries in Young Children. J. Clin. Pediatr. Dent. 2021, 45, 414–420. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, M.R.; Mirzaii-Dizgah, M.H.; Mirzaii-Dizgah, I.; Koshkzari, R. The Association between Saliva and Serum Vitamin D with Knee Osteoarthritis. J. Nutr. Food Secur. 2022, 8, 108–113. [Google Scholar] [CrossRef]
- Sari, D.K.; Sari, L.M.; Laksmi, L.I. Farhat The Moderate Correlation Between 25(OH)D Serum and Saliva in Healthy People with Low Vitamin D Intake. Int. J. Gen. Med. 2021, 14, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Abdolsamadi, H.; Vahedi, M.; Fariba, F.; Soltanian, A.; Avval, M.Z.; Hosseini, A. Comparative Study of Salivary and Serum Levels of Vitamin D in Patients with a History of High Blood Pressure and Healthy People. J. Mol. Biol. Res. 2018, 8, p101. [Google Scholar] [CrossRef]
- Ogawa, S.; Ooki, S.; Shinoda, K.; Higashi, T. Analysis of Urinary Vitamin D3 Metabolites by Liquid Chromatography/Tandem Mass Spectrometry with ESI-Enhancing and Stable Isotope-Coded Derivatization. Anal. Bioanal. Chem. 2014, 406, 6647–6654. [Google Scholar] [CrossRef]
- Sato, K.A.; Gray, R.W.; Lemann, J. Urinary Excretion of 25-Hydroxyvitamin D in Health and the Nephrotic Syndrome. J. Lab. Clin. Med. 1982, 99, 325–330. [Google Scholar]
- Piccolini, A.; Grizzi, F.; Monari, M.; Hegazi, M.A.A.A.; Buffi, N.M.; Casale, P.; Fasulo, V.; Moretto, S.; Cella, L.; Vota, P.; et al. Preliminary Findings on Vitamin D 25-OH Levels in Urine Analysis: Implications for Clinical Practice. BJU Int. 2024, 134, 561–563. [Google Scholar] [CrossRef]
- McWhorter, C.A.; Mead, M.J.; Rodgers, M.D.; Ebeling, M.D.; Shary, J.R.; Gregoski, M.J.; Newton, D.A.; Baatz, J.E.; Hollis, B.W.; Hewison, M.; et al. Predicting Comorbidities of Pregnancy: A Comparison between Total and Free 25(OH)D and Their Associations with Parathyroid Hormone. J. Steroid Biochem. Mol. Biol. 2023, 235, 106420. [Google Scholar] [CrossRef] [PubMed]
- Tamblyn, J.A.; Jenkinson, C.; Larner, D.P.; Hewison, M.; Kilby, M.D. Serum and Urine Vitamin D Metabolite Analysis in Early Preeclampsia. Endocr. Connect. 2018, 7, 199–210. [Google Scholar] [CrossRef]
- Kushioka, T.; Mano, H.; Matsuoka, S.; Nishikawa, M.; Yasuda, K.; Ikushiro, S.; Sakaki, T. Analysis of Vitamin D Metabolites in Biological Samples Using a Nanoluc-Based Vitamin D Receptor Ligand Sensing System: NLucVDR. J. Steroid Biochem. Mol. Biol. 2023, 233, 106367. [Google Scholar] [CrossRef]
- Kushioka, T.; Mano, H.; Matsuoka, S.; Nishikawa, M.; Yasuda, K.; Ikushiro, S.; Sakaki, T. Association between Serum 25-Hydroxyvitamin D Concentrations and Urinary Vitamin D Metabolite Concentrations Measured by the NLucVDR Assay. J. Steroid Biochem. Mol. Biol. 2025, 247, 106678. [Google Scholar] [CrossRef]
- Simundic, A.M.; Bölenius, K.; Cadamuro, J.; Church, S.; Cornes, M.P.; Van Dongen-Lases, E.C.; Eker, P.; Erdeljanovic, T.; Grankvist, K.; Guimaraes, J.T.; et al. Joint EFLM-COLABIOCLI Recommendation for Venous Blood Sampling. Clin. Chem. Lab. Med. 2018, 56, 2015–2038. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Bilezikian, J.P.; Adler, R.A.; Banfi, G.; Bikle, D.D.; Binkley, N.C.; Bollerslev, J.; Bouillon, R.; Brandi, M.L.; Casanueva, F.F.; et al. Consensus Statement on Vitamin D Status Assessment and Supplementation: Whys, Whens, and Hows. Endocr. Rev. 2024, 45, 625–654. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a Literature-Based Adherence Score to Mediterranean Diet: The MEDI-LITE Score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef]
- Lahoz, R.; Sánchez, J.P.; Górriz, S.; Calmarza, P. Comparative Study of Two Immunoassays Used for the Determination of Serum Vitamin D. Pract. Lab. Med. 2021, 26, e00242. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Nafeesah, A.; AlEed, A.; Adam, I. Serum Level of 25-Hydroxyvitamin D and Symptoms of Pica Among Adolescent School Children in Northern Sudan: A Cross-Sectional Study. Glob. Pediatr. Health 2024, 11, 2333794X241242564. [Google Scholar] [CrossRef]
- Bakhuraysah, M.M.; Gharib, A.F.; Hassan, A.F.; Al Harthi, G.K.; Al Thobaiti, R.F.; Al Adwani, M.M.; Alharbi, A.D.; Alzahrani, A.S.; Alsubei, K.M.; Al-Asiri, R.F. Novel Insight Into the Relationship of Vitamin D Hydroxylase and Vitamin D With Obesity in Patients With Type 2 Diabetes Mellitus. Cureus 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- He, C.-S.; Gleeson, M.; Fraser, W.D. Measurement of Circulating 25-Hydroxy Vitamin d Using Three Commercial Enzyme-Linked Immunosorbent Assay Kits with Comparison to Liquid Chromatography: Tandem Mass Spectrometry Method. ISRN Nutr. 2013, 2013, 723139. [Google Scholar] [CrossRef]
- Sari, D.K.; Sari, L.M.; Laksmi, L.I.; Farhat, F. The Use of 25-Hydroxyvitamin D Saliva Test to Replace Vitamin D Serum Blood Test in Healthy People. Open Access Maced. J. Med. Sci. 2021, 9, 40–43. [Google Scholar] [CrossRef]
- Mantecón, L.; Agustina Alonso, M.; Moya, V.; Andrés, A.G.; Avello, N.; Martínez-Morillo, E.; Santos, F. Marker of Vitamin D Status in Healthy Children: Free or Total 25-Hydroxyvitamin D? PLoS ONE 2018, 13, e0202237. [Google Scholar] [CrossRef]
- Arshad, S.; Zaidi, S.J.A. Vitamin D Levels among Children, Adolescents, Adults, and Elders in Pakistani Population: A Cross-Sectional Study. BMC Public. Health 2022, 22, 2040. [Google Scholar] [CrossRef]
- Banerjee, S.; Basu, S.; Akhtar, S.; Sinha, R.; Sen, A.; Sengupta, J. Free Vitamin D Levels in Steroid-Sensitive Nephrotic Syndrome and Healthy Controls. Pediatr. Nephrol. 2020, 35, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Novotny, R.; Krueger, D.; Kawahara, T.; Daida, Y.G.; Lensmeyer, G.; Hollis, B.W.; Drezner, M.K. Low Vitamin D Status despite Abundant Sun Exposure. J. Clin. Endocrinol. Metab. 2007, 92, 2130–2135. [Google Scholar] [CrossRef]
- Bowles, S.D.; Basu, S.; Ranchordas, M.K.; Simper, T.; Lynn, A. Circulating Total 25(OH)D and Calculated Free 25(OH)D in Professional Academy Footballers at a Northerly Latitude in the UK. Biol. Sport. 2024, 41, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sollid, S.T.; Hutchinson, M.Y.S.; Berg, V.; Fuskevåg, O.M.; Figenschau, Y.; Thorsby, P.M.; Jorde, R. Effects of Vitamin D Binding Protein Phenotypes and Vitamin D Supplementation on Serum Total 25(OH)D and Directly Measured Free 25(OH)D. Eur. J. Endocrinol. 2016, 174, 445–452. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.B.; Ames, R.W.; Horne, A.M.; Mason, B.H.; Wattie, D.J.; Gamble, G.D.; Bouillon, R.; Reid, I.R. Age-, Gender-, and Weight-Related Effects on Levels of 25-Hydroxyvitamin D Are Not Mediated by Vitamin D Binding Protein. Clin. Endocrinol. 2007, 67, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Meschi, T.; Borghi, L. Vitamin D Concentration and Deficiency across Different Ages and Genders. Aging Clin. Exp. Res. 2012, 24, 548–551. [Google Scholar] [CrossRef]
- Lippi, G.; Nouvenne, A.; Ticinesi, A.; Bonelli, P.; Salvagno, G.L.; Cervellin, G.; Guidi, G.C. The Burden of Vitamin D Deficiency in a Mediterranean Country without a Policy of Food Fortification. Acta Biomed. 2015, 86, 59–62. [Google Scholar]
- Bani, P.; Cingari, S.; Mathis, B. Study on the Prevalence of Vitamin D Deficiency in the Population of Canton Ticino. Riv. Ital. Della Med. Lab. 2022, 18, 18–21. [Google Scholar] [CrossRef]
- AlQuaiz, A.J.M.; Kazi, A.; Fouda, M.; Alyousefi, N. Age and Gender Differences in the Prevalence and Correlates of Vitamin D Deficiency. Arch. Osteoporos. 2018, 13, 49. [Google Scholar] [CrossRef]
- Vallejo, M.S.; Blümel, J.E.; Arteaga, E.; Aedo, S.; Tapia, V.; Araos, A.; Sciaraffia, C.; Castelo-Branco, C. Gender Differences in the Prevalence of Vitamin D Deficiency in a Southern Latin American Country: A Pilot Study. Climacteric 2020, 23, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sanghera, D.K.; Sapkota, B.R.; Aston, C.E.; Blackett, P.R. Vitamin D Status, Gender Differences, and Cardiometabolic Health Disparities. Ann. Nutr. Metab. 2017, 70, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of Gender Difference on Vitamin D Status and Its Relationship with the Extent of Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Al-Hetar, M.; Al-Goshae, H.; Wahab, N.; Abdulghani, M.; Faez Baobaid, M.; Al-matary, A.M.; Al-hetar, Q.; Alezzy, M.; Senan, D.; Ruhi, S. Prevalence, Predictors, and Gender-Based Risk Factors of Vitamin D Deficiency: A Retrospective Cross-Sectional Study. J. Angiother. 2024, 8, 1–9. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Oczkowicz, M. Sex Differences in Vitamin D Metabolism, Serum Levels and Action. Br. J. Nutr. 2022, 128, 2115–2130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ouyang, P.; de Boer, I.H.; Lutsey, P.L.; Farag, Y.M.K.; Guallar, E.; Siscovick, D.S.; Post, W.S.; Kalyani, R.R.; Billups, K.L.; et al. Serum Vitamin D and Sex Hormones Levels in Men and Women: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2017, 96, 95–102. [Google Scholar] [CrossRef][Green Version]
- Harmon, Q.E.; Umbach, D.M.; Baird, D.D. Use of Estrogen-Containing Contraception Is Associated With Increased Concentrations of 25-Hydroxy Vitamin D. J. Clin. Endocrinol. Metab. 2016, 101, 3370–3377. [Google Scholar] [CrossRef]
- Harris, S.S.; Dawson-Hughes, B. The Association of Oral Contraceptive Use with Plasma 25-Hydroxyvitamin D Levels. J. Am. Coll. Nutr. 1998, 17, 282–284. [Google Scholar] [CrossRef]
- Knight, J.A.; Wong, J.; Blackmore, K.M.; Raboud, J.M.; Vieth, R. Vitamin D Association with Estradiol and Progesterone in Young Women. Cancer Causes Control 2010, 21, 479–483. [Google Scholar] [CrossRef]
- Lagunova, Z.; Porojnicu, A.C.; Lindberg, F.; Hexeberg, S.; Moan, J. The Dependency of Vitamin D Status on Body Mass Index, Gender, Age and Season. Anticancer. Res. 2009, 29, 3713–3720. [Google Scholar] [CrossRef]
- Rebel, H.; Dingemanse-Van Der Spek, C.; Salvatori, D.; Van Leeuwen, J.P.T.M.; Robanus-Maandag, E.C.; De Gruijl, F.R. UV Exposure Inhibits Intestinal Tumor Growth and Progression to Malignancy in Intestine-Specific Apc Mutant Mice Kept on Low Vitamin D Diet. Int. J. Cancer 2015, 136, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Crescioli, C.; Minisola, S. Vitamin D: Autoimmunity and Gender. Curr. Med. Chem. 2017, 24, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W. Assessment and Interpretation of Circulating 25-Hydroxyvitamin D and 1,25-Dihydroxyvitamin D in the Clinical Environment. Endocrinol. Metab. Clin. N. Am. 2010, 39, 271–286. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Overall 62 (100%) | Females 33 (53.2%) | Males 29 (46.8%) |
|---|---|---|---|
| Age, mean (SD) [years] | 31.5 (5.1) | 30.4 (4.1) | 32.8 (5.9) |
| BMI categories, n% | |||
| Normal weight | 44 (71.0) | 30 (48.4) | 14 (22.6) |
| Overweight | 13 (20.9) | 2 (3.2) | 11 (17.7) |
| Missing data | 5 (8.1) | 1 (1.6) | 4 (6.5) |
| Occupation, n (%) | |||
| Students | 22 (35.5) | 12 (19.4) | 10 (16.1) |
| Workers | 32 (51.6) | 18 (29.0) | 14 (22.6) |
| Missing data | 8 (12.9) | 3 (4.8) | 5 (8.1) |
| AMD, n (%) | |||
| Low (1st tertile) | 34 (54.8) | 20 (32.3) | 14 (22.6) |
| Medium (2nd tertile) | 9 (14.5) | 6 (9.7) | 3 (4.8) |
| High (3rd tertile) | 14 (22.6) | 6 (9.7) | 8 (12.9) |
| Missing data | 5 (8.1) | 1 (1.6) | 4 (6.5) |
| Tobacco smoking exposure, n (%) | |||
| Passive smokers | 9 (14.5) | 4 (6.5) | 5 (8.1) |
| Missing data | 5 (8.1) | 1 (1.6) | 4 (6.5) |
| Sun exposure behaviour, n (%) | |||
| Sun cream users | 45 (72.6) | 26 (41.9) | 19 (30.7) |
| Missing data | 5 (8.1) | 6 (9.7) | 4 (6.5) |
| Sun exposure < 15 min/day | 34 (54.8) | 22 (35.5) | 12 (19.4) |
| Missing data | 8 (12.9) | 2 (3.2) | 6 (9.7) |
| Phototype, n (%) | |||
| I. very light skin | 22 (35.5) | 15 (24.2) | 7 (11.3) |
| II. light eyes and skin | 23 (37.1) | 11 (17.7) | 12 (19.4) |
| III. light skin and brown hair | 5 (8.1) | 2 (3.2) | 3 (4.8) |
| IV. olive skin | 7 (11.3) | 4 (6.5) | 3 (4.8) |
| Missing data | 5 (8.1) | 1 (1.6) | 4 (6.5) |
| Vitamin D Benchmark Levels (CMIA) | Overall 62 (100%) | Females 33 (53.2%) | Males 29 (46.8%) | p-Value |
|---|---|---|---|---|
| Serum 25(OH)D, mean (SD) | ||||
| ng/mL | 26.1 (9.1) | 26.6 (9.6) | 25.6 (8.7) | 0.70 a |
| nmoL/L | 65.3 (22.7) | 66.4 (23.9) | 64.1 (21.7) | 0.70 a |
| Serum 25(OH)D, median (IQR) | ||||
| ng/mL | 25.2 (19.9–31.2) | 24.5 (20.8–32) | 25.2 (19.9–35.7) | 0.84 b |
| nmoL/L | 62.9 (49.8–78.0) | 61.3 (52–80) | 63 (49.8–77.5) | 0.84 b |
| Vitamin D deficiency, n (%) | ||||
| IOM | 16 (25.8) | 8 (12.9) | 8 (12.9) | 0.76 c |
| ES | 42 (67.7) | 23 (37.1) | 19 (30.6) | 0.73 c |
| Vitamin D ELISA Levels | Overall 62 (100%) | Females 33 (53.2%) | Males 29 (46.8%) | p-Value |
|---|---|---|---|---|
| Serum 25(OH)D, mean (SD) | ||||
| ng/mL | 6.54 (1.59) | 6.89 (3.31) | 6.13 (1.80) | 0.06 |
| nmoL/L | 16.34 (3.99) | 17.24 (3.31) | 15.32 (4.50) | 0.06 |
| Saliva 25(OH)D, mean (SD) | ||||
| ng/mL | 18.79 (6.21) | 18.89 (6.44) | 18.68 (6.10) | 0.89 |
| nmoL/L | 46.98 (15.53) | 47.23 (16.10) | 46.69 (15.14) | 0.89 |
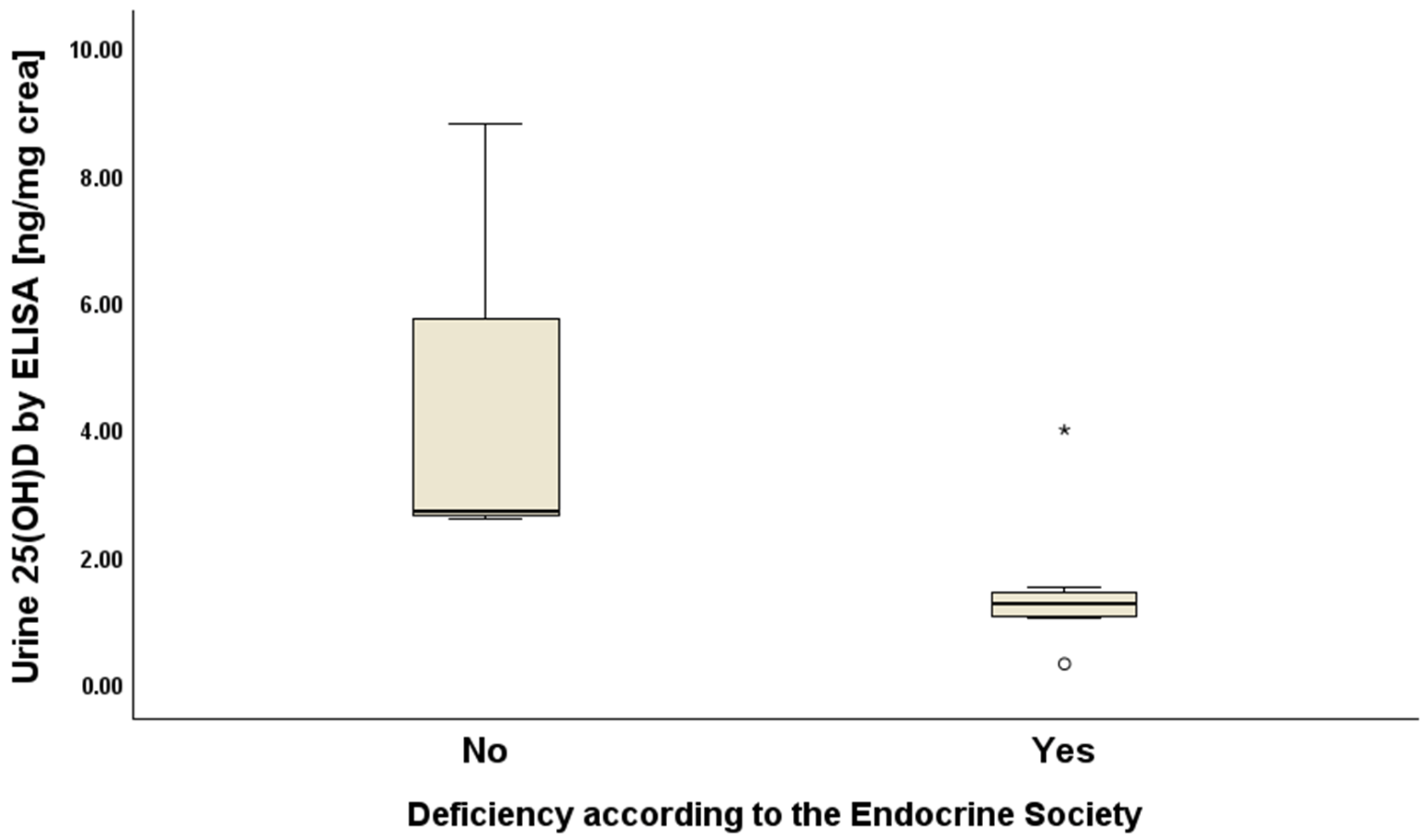
| Urine 25(OH)D, median (IQR) | ||||
| ng/mg creatinine | 1.05 (2.57–0.59) | 1.38 (2.69–1.05) | 0.69 (0.96–0.47) | 0.04 |
| nmoL/mmoL creatinine | 0.28 (0.68–0.18) | 0.44 (0.78–0.28) | 0.20 (0.26–0.14) | 0.04 |
| 25(OH)D | Serum Benchmark (CMIA) [ng/mL] | Serum ELISA [ng/mL] | Urine ELISA [ng/mg Creatine] |
|---|---|---|---|
| Serum ELISA [ng/mL] | r = −0.01 | ||
| p = 0.94 | |||
| n = 62 | |||
| Urine ELISA [ng/mg crea] | r = 0.44 | r = 0.11 | |
| p = 0.05 | p = 0.63 | ||
| n = 21 | n = 21 | ||
| Saliva ELISA [ng/mL] | r = 0.10 | r = −0.12 | r = 0.05 |
| p = 0.43 | p = 0.36 | p = 0.83 | |
| n = 62 | n =62 | n = 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Squillacioti, G.; El Sherbiny, S.; Lettico, V.; Ghelli, F.; Panizzolo, M.; Scaioli, G.; Martella, M.; Limoncelli, S.; Mengozzi, G.; Bono, R. The Quantification of Vitamin D in Humans: A Promising, Non-Invasive and Cost-Effective Method to Measure 25-Hydroxyvitamin D. Biomolecules 2025, 15, 560. https://doi.org/10.3390/biom15040560
Squillacioti G, El Sherbiny S, Lettico V, Ghelli F, Panizzolo M, Scaioli G, Martella M, Limoncelli S, Mengozzi G, Bono R. The Quantification of Vitamin D in Humans: A Promising, Non-Invasive and Cost-Effective Method to Measure 25-Hydroxyvitamin D. Biomolecules. 2025; 15(4):560. https://doi.org/10.3390/biom15040560
Chicago/Turabian StyleSquillacioti, Giulia, Samar El Sherbiny, Veronica Lettico, Federica Ghelli, Marco Panizzolo, Giacomo Scaioli, Manuela Martella, Selene Limoncelli, Giulio Mengozzi, and Roberto Bono. 2025. "The Quantification of Vitamin D in Humans: A Promising, Non-Invasive and Cost-Effective Method to Measure 25-Hydroxyvitamin D" Biomolecules 15, no. 4: 560. https://doi.org/10.3390/biom15040560
APA StyleSquillacioti, G., El Sherbiny, S., Lettico, V., Ghelli, F., Panizzolo, M., Scaioli, G., Martella, M., Limoncelli, S., Mengozzi, G., & Bono, R. (2025). The Quantification of Vitamin D in Humans: A Promising, Non-Invasive and Cost-Effective Method to Measure 25-Hydroxyvitamin D. Biomolecules, 15(4), 560. https://doi.org/10.3390/biom15040560








