Evaluation of the Effects of Thymoquinone on RAGE/NOX4 Expressions and Brain Tissue Morphometry in Experimental Alzheimer’s Disease Induced by Amyloid Beta 1–42 Peptide
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Reagents
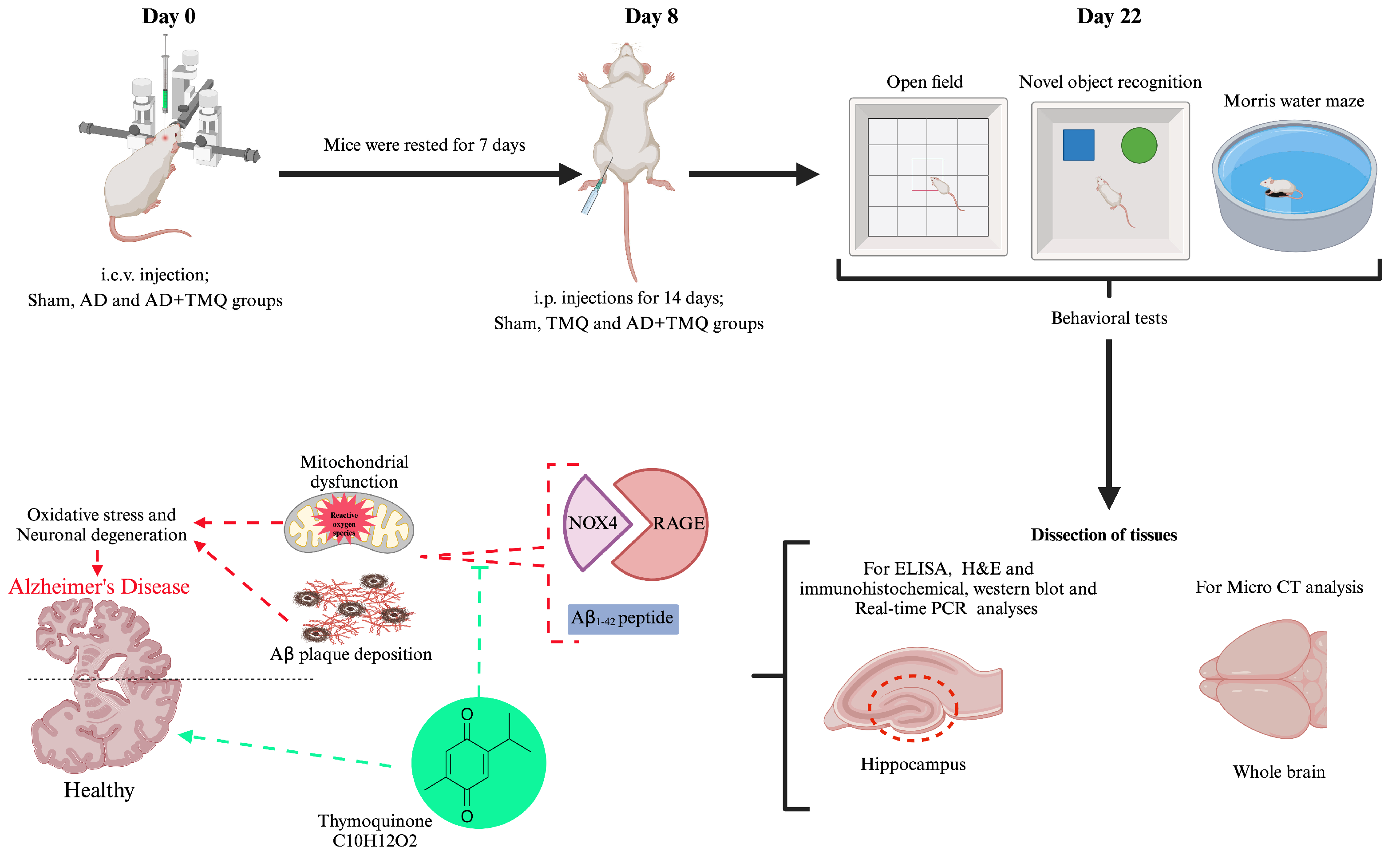
2.3. Experimental Design
2.4. Induction of Alzheimer’s Disease and Administration of Thymoquinone
2.5. Behavioral Tests
2.5.1. Open Field Test
2.5.2. Novel Object Recognition Test
2.5.3. Morris Water Maze Test
2.6. Micro CT
2.7. ELISA Analysis
2.8. Hematoxylin and Eosin Staining
2.9. Immunohistochemical Analysis
2.10. Western Blot Analysis
2.11. RNA Isolation and Real-Time PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. Behavioral Tests Results
3.1.1. Open Field Test Results
3.1.2. Novel Object Recognition Test Results
3.1.3. Morris Water Maze Test Results
3.2. Micro CT Results
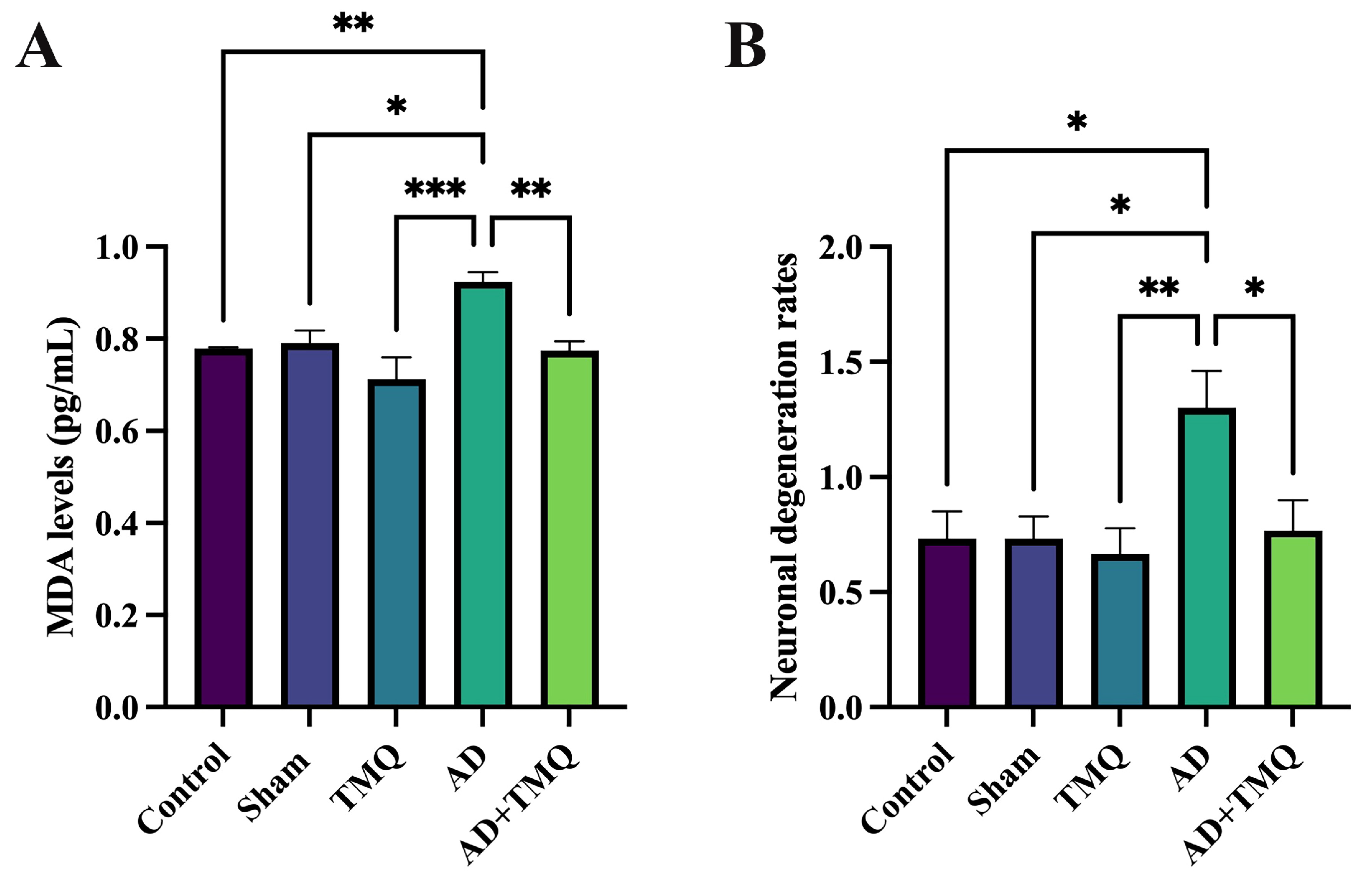
3.3. ELISA Results
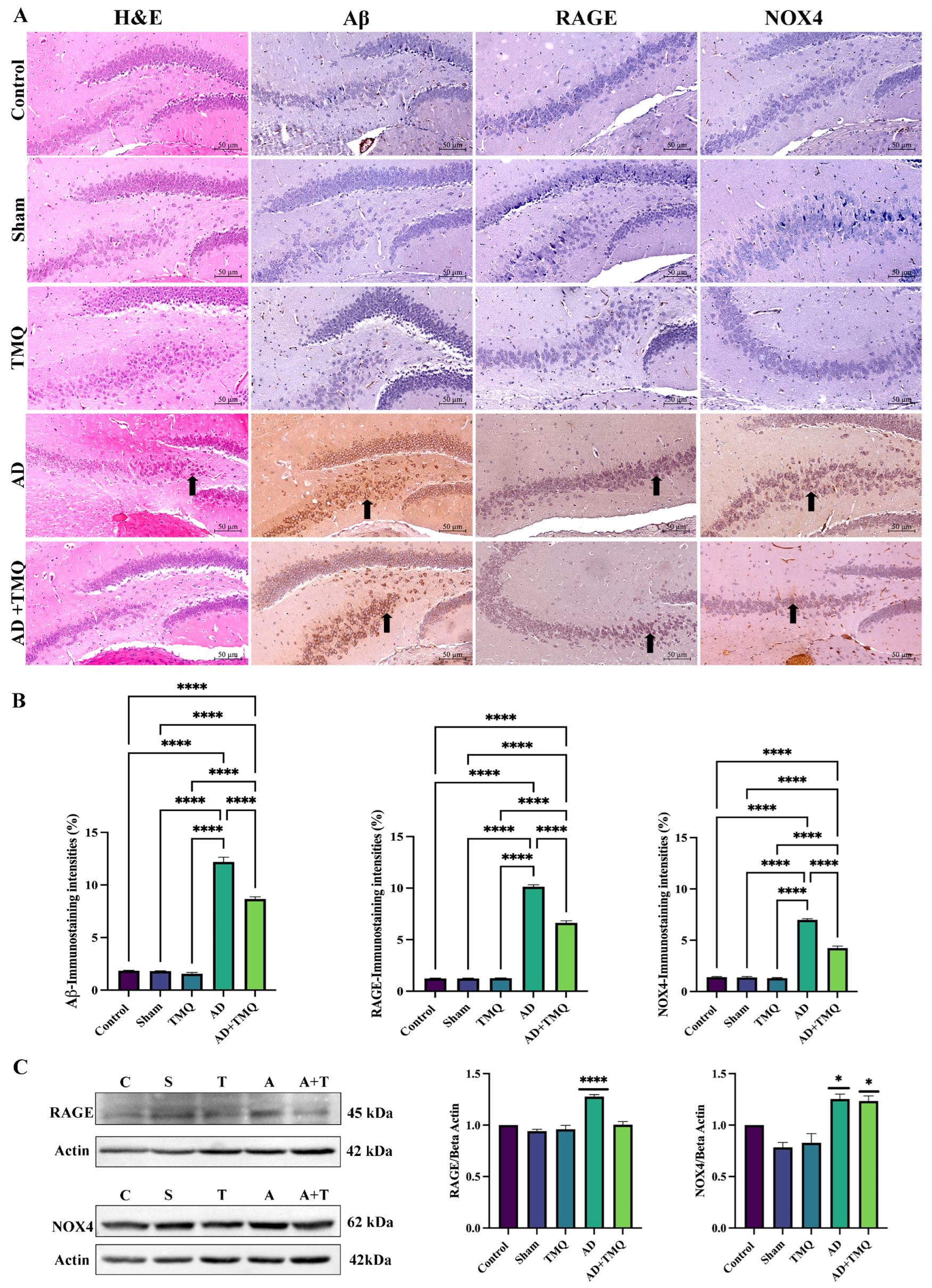
3.4. Histological Analysis
3.5. Immunohistochemical Analysis Results
3.6. Western Blot Analysis Results
3.7. Real-Time PCR Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | amyloid beta |
| APP | amyloid beta precursor protein |
| Micro CT | micro-computed tomography |
| MDA | malondialdehyde |
| H&E | hematoxylin and eosin |
| IHC | immunohistochemical |
| WB | Western blot |
| PCR | polymerase chain reaction |
| TMQ | thymoquinone |
| RAGE | receptor for advanced glycation end products |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NOX4 | NADPH oxidase 4. |
References
- 2024 Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- John, A.; Reddy, P.H. Synaptic Basis of Alzheimer’s Disease: Focus on Synaptic Amyloid Beta, P-Tau and Mitochondria. Ageing Res. Rev. 2021, 65, 101208. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Tripathi, R.; Troung, Q.; Tirumala, K.; Reddy, T.P.; Anekonda, V.; Shirendeb, U.P.; Calkins, M.J.; Reddy, A.P.; Mao, P.; et al. Abnormal Mitochondrial Dynamics and Synaptic Degeneration as Early Events in Alzheimer’s Disease: Implications to Mitochondria-Targeted Antioxidant Therapeutics. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2012, 1822, 639–649. [Google Scholar] [CrossRef]
- Lazarov, O.; Gupta, M.; Kumar, P.; Morrissey, Z.; Phan, T. Memory Circuits in Dementia: The Engram, Hippocampal Neurogenesis and Alzheimer’s Disease. Prog. Neurobiol. 2024, 236, 102601. [Google Scholar] [CrossRef]
- Lang, M.; Colby, S.; Ashby-Padial, C.; Bapna, M.; Jaimes, C.; Rincon, S.P.; Buch, K. An Imaging Review of the Hippocampus and Its Common Pathologies. J. Neuroimaging 2024, 34, 5–25. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef]
- Guan, L.; Mao, Z.; Yang, S.; Wu, G.; Chen, Y.; Yin, L.; Qi, Y.; Han, L.; Xu, L. Dioscin Alleviates Alzheimer’s Disease through Regulating RAGE/NOX4 Mediated Oxidative Stress and Inflammation. Biomed. Pharmacother. 2022, 152, 113248. [Google Scholar] [CrossRef]
- Evering, T.H.; Marston, J.L.; Gan, L.; Nixon, D.F. Transposable Elements and Alzheimer’s Disease Pathogenesis. Trends Neurosci. 2023, 46, 170–172. [Google Scholar] [CrossRef]
- Hroudová, J.; Fišar, Z. Alzheimer’s Disease Approaches—Focusing on Pathology, Biomarkers and Clinical Trial Candidates. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 134, 111069. [Google Scholar] [CrossRef]
- Atwood, C.S.; Martins, R.N.; Smith, M.A.; Perry, G. Senile Plaque Composition and Posttranslational Modification of Amyloid-Beta Peptide and Associated Proteins. Peptides 2002, 23, 1343–1350. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Sasanian, N.; Bernson, D.; Horvath, I.; Wittung-Stafshede, P.; Esbjörner, E.K. Redox-Dependent Copper Ion Modulation of Amyloid-β (1-42) Aggregation In Vitro. Biomolecules 2020, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Isaev, N.K.; Genrikhs, E.E.; Stelmashook, E.V. Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants 2023, 12, 433. [Google Scholar] [CrossRef]
- Kinscherf, N.A.; Pehar, M. Role and Therapeutic Potential of RAGE Signaling in Neurodegeneration. Curr. Drug Targets 2022, 23, 1191–1209. [Google Scholar] [CrossRef] [PubMed]
- Verdile, G.; Keane, K.N.; Cruzat, V.F.; Medic, S.; Sabale, M.; Rowles, J.; Wijesekara, N.; Martins, R.N.; Fraser, P.E.; Newsholme, P. Inflammation and Oxidative Stress: The Molecular Connectivity Between Insulin Resistance, Obesity, and Alzheimer’s Disease. Mediat. Inflamm. 2015, 2015, 105828. [Google Scholar] [CrossRef]
- Liang, E.; Ma, M.; Wang, L.; Liu, X.; Xu, J.; Zhang, M.; Yang, R.; Zhao, Y. The BET/BRD Inhibitor JQ1 Attenuates Diabetes-Induced Cognitive Impairment in Rats by Targeting Nox4-Nrf2 Redox Imbalance. Biochem. Biophys. Res. Commun. 2018, 495, 204–211. [Google Scholar] [CrossRef]
- Tao, W.; Yu, L.; Shu, S.; Liu, Y.; Zhuang, Z.; Xu, S.; Bao, X.; Gu, Y.; Cai, F.; Song, W.; et al. miR-204-3p/Nox4 Mediates Memory Deficits in a Mouse Model of Alzheimer’s Disease. Mol. Ther. 2021, 29, 396–408. [Google Scholar] [CrossRef]
- Gola, L.; Bierhansl, L.; Csatári, J.; Schroeter, C.B.; Korn, L.; Narayanan, V.; Cerina, M.; Abdolahi, S.; Speicher, A.; Hermann, A.M.; et al. NOX4-Derived ROS Are Neuroprotective by Balancing Intracellular Calcium Stores. Cell. Mol. Life Sci. 2023, 80, 127. [Google Scholar] [CrossRef]
- Lozhkin, A.; Vendrov, A.E.; Pan, H.; Wickline, S.A.; Madamanchi, N.R.; Runge, M.S. NADPH Oxidase 4 Regulates Vascular Inflammation in Aging and Atherosclerosis. J. Mol. Cell. Cardiol. 2017, 102, 10–21. [Google Scholar] [CrossRef]
- Sarkar, S.; Malovic, E.; Harishchandra, D.S.; Ghaisas, S.; Panicker, N.; Charli, A.; Palanisamy, B.N.; Rokad, D.; Jin, H.; Anantharam, V.; et al. Mitochondrial Impairment in Microglia Amplifies NLRP3 Inflammasome Proinflammatory Signaling in Cell Culture and Animal Models of Parkinson’s Disease. NPJ Park. Dis. 2017, 3, 30. [Google Scholar] [CrossRef]
- Boonpraman, N.; Yi, S.S. NADPH Oxidase 4 (NOX4) as a Biomarker and Therapeutic Target in Neurodegenerative Diseases. Neural Regen. Res. 2023, 19, 1961–1966. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Liu, B.; Wang, N.; Liao, Z.; Wu, B.; He, B.; Jia, Y. The Flavonoids of Okra Insulates against Oxidative Stress, Neuroinflammation and Restores BDNF Levels in Aβ1-42 Induced Mouse Model of Alzheimer’s Disease. Exp. Gerontol. 2021, 147, 111263. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, X. Protective Effect of Flavonoids on Oxidative Stress Injury in Alzheimer’s Disease. Nat. Prod. Res. 2024, 39, 1272–1299. [Google Scholar] [CrossRef]
- Hosni, A.; Abdel-Moneim, A.; Hussien, M.; Zanaty, M.I.; Eldin, Z.E.; El-Shahawy, A.A.G. Therapeutic Significance of Thymoquinone-Loaded Chitosan Nanoparticles on Streptozotocin/Nicotinamide-Induced Diabetic Rats: In Vitro and in Vivo Functional Analysis. Int. J. Biol. Macromol. 2022, 221, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, E.; Öztürk, E.; Akİn, A.T.; Karabulut, D.; Yakan, B. Thymoquinone Alleviates Doxorubicin Induced Acute Kidney Injury by Decreasing Endoplasmic Reticulum Stress, Inflammation and Apoptosis. Biotech. Histochem. 2022, 97, 622–634. [Google Scholar] [CrossRef]
- Özsoy, Ş.; Çakir, Z.; Akçay, E.; Gevrek, F. Effects of Thymoquinone and Memantine Alone and in Combination on Memory and Hippocampal Morphology in Rats with Streptozotocin-Induced Alzheimer’s Disease. Turk. J. Med. Sci. 2023, 53, 894–901. [Google Scholar] [CrossRef]
- Sakib, R.; Caruso, F.; Aktar, S.; Belli, S.; Kaur, S.; Hernandez, M.; Rossi, M. Antioxidant Properties of Thymoquinone, Thymohydroquinone and Black Cumin (Nigella sativa L.) Seed Oil: Scavenging of Superoxide Radical Studied Using Cyclic Voltammetry, DFT and Single Crystal X-Ray Diffraction. Antioxidants 2023, 12, 607. [Google Scholar] [CrossRef]
- Mokarizadeh, N.; Karimi, P.; Erfani, M.; Sadigh-Eteghad, S.; Fathi Maroufi, N.; Rashtchizadeh, N. β-Lapachone Attenuates Cognitive Impairment and Neuroinflammation in Beta-Amyloid Induced Mouse Model of Alzheimer’s Disease. Int. Immunopharmacol. 2020, 81, 106300. [Google Scholar] [CrossRef]
- Sharma, S.; Saini, A.; Nehru, B. Neuroprotective Effects of Carbenoxolone against Amyloid-Beta 1-42 Oligomer-Induced Neuroinflammation and Cognitive Decline in Rats. Neurotoxicology 2021, 83, 89–105. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Z.-Y.; Li, Y.-F.; Liu, C.; Wang, C.; Gong, X.-J.; He, L. Dopamine D2 Receptor Agonist Bromocriptine Ameliorates Aβ1-42-Induced Memory Deficits and Neuroinflammation in Mice. Eur. J. Pharmacol. 2023, 938, 175443. [Google Scholar] [CrossRef]
- Sener, E.F.; Dana, H.; Tahtasakal, R.; Hamurcu, Z.; Taheri, S.; Delibasi, N.; Mehmetbeyoglu, E.; Sukranli, Z.Y.; Dal, F.; Tufan, E.; et al. Heterozygous Cc2d1a Mice Show Sex-Dependent Changes in the Beclin-1/P62 Ratio with Impaired Prefrontal Cortex and Hippocampal Autophagy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 125, 110764. [Google Scholar] [CrossRef]
- Fu, X.-X.; Wei, B.; Cao, H.-M.; Duan, R.; Deng, Y.; Lian, H.-W.; Zhang, Y.-D.; Jiang, T. Telmisartan Alleviates Alzheimer’s Disease-Related Neuropathologies and Cognitive Impairments. J. Alzheimers Dis. 2023, 94, 919–933. [Google Scholar] [CrossRef]
- Gordon, B.A.; McCullough, A.; Mishra, S.; Blazey, T.M.; Su, Y.; Christensen, J.; Dincer, A.; Jackson, K.; Hornbeck, R.C.; Morris, J.C.; et al. Cross-Sectional and Longitudinal Atrophy Is Preferentially Associated with Tau Rather than Amyloid β Positron Emission Tomography Pathology. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 245–252. [Google Scholar] [CrossRef]
- Ossenkoppele, R.; Smith, R.; Ohlsson, T.; Strandberg, O.; Mattsson, N.; Insel, P.S.; Palmqvist, S.; Hansson, O. Associations between Tau, Aβ, and Cortical Thickness with Cognition in Alzheimer Disease. Neurology 2019, 92, e601–e612. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Kalinowski, P.; Ayton, S. Accelerated Brain Volume Loss Caused by Anti-β-Amyloid Drugs: A Systematic Review and Meta-Analysis. Neurology 2023, 100, e2114–e2124. [Google Scholar] [CrossRef]
- Feng, Y.; Laraib, A.; Lin, X.; Li, Q.; Zhan, J.; Li, X. Associations of Tau, Aβ, and Brain Volume of the Papez Circuit with Cognition in Alzheimer’s Disease. Eur. Arch. Psychiatry Clin. Neurosci. 2025, 275, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Doğanyiğit, Z.; Ocak, M.; Söylemez, E.S.A.; Oflamaz, A.O.; Uçar, S.; Ateş, Ş.; Farooqi, A.A. Inhibition of Ehrlich Ascites Carcinoma Growth by Melatonin: Studies with Micro-CT. Oncol. Res. 2024, 32, 175–185. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Advances in Micro-CT Imaging of Small Animals. Phys. Medica 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Pinto, R.; Matula, J.; Gomez-Lazaro, M.; Sousa, M.; Lobo, A.; Zikmund, T.; Kaiser, J.; Gomes, J.R. High-Resolution Micro-CT for 3D Infarct Characterization and Segmentation in Mice Stroke Models. Sci. Rep. 2022, 12, 17471. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of Lipid Peroxidation by Measuring Malondialdehyde (MDA) and Relatives in Biological Samples: Analytical and Biological Challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Shao, S.; Ye, X.; Su, W.; Wang, Y. Curcumin Alleviates Alzheimer’s Disease by Inhibiting Inflammatory Response, Oxidative Stress and Activating the AMPK Pathway. J. Chem. Neuroanat. 2023, 134, 102363. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Kocki, J.; Bogucki, J.; Bogucka-Kocka, A.; Czuczwar, S.J. LRP1 and RAGE Genes Transporting Amyloid and Tau Protein in the Hippocampal CA3 Area in an Ischemic Model of Alzheimer’s Disease with 2-Year Survival. Cells 2023, 12, 2763. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, H.; Sato, Y.; Yamazaki, M.; Yoshizawa, T.; Ando, Y.; Ueda, M.; Yamagata, K. SIRT7 Deficiency Protects against Aβ42-Induced Apoptosis through the Regulation of NOX4-Derived Reactive Oxygen Species Production in SH-SY5Y Cells. Int. J. Mol. Sci. 2022, 23, 9027. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, X.; Zhang, J.; Shen, Z.; Li, X.; Yang, Y. Chuanxiong Renshen Decoction Inhibits Alzheimer’s Disease Neuroinflammation by Regulating PPARγ/NF-κB Pathway. Drug Des. Dev. Ther. 2024, 18, 3209–3232. [Google Scholar] [CrossRef]
- Doğanyiğit, Z.; Okan, A.; Akyüz, E.; Yılmaz, S.; Ateş, Ş.; Taheri, S.; Yılmaz, Z.; Shaikh, M.F. Can Endoplasmic Reticulum Stress Observed in the PTZ-Kindling Model Seizures Be Prevented with TUDCA and 4-PBA? Eur. J. Pharmacol. 2023, 960, 176072. [Google Scholar] [CrossRef]
- Okan, A.; Doğanyiğit, Z.; Eroğlu, E.; Akyüz, E.; Demir, N. Immunoreactive Definition of TNF- α, HIF-1 α, Kir6.2, Kir3.1 and M2 Muscarinic Receptor for Cardiac and Pancreatic Tissues in a Mouse Model for Type 1 Diabetes. Life Sci. 2021, 284, 119886. [Google Scholar] [CrossRef]
- Qian, X.; Liu, X.; Chen, G.; Chen, S.; Tang, H. Injection of Amyloid-β to Lateral Ventricle Induces Gut Microbiota Dysbiosis in Association with Inhibition of Cholinergic Anti-Inflammatory Pathways in Alzheimer’s Disease. J. Neuroinflamm. 2022, 19, 236. [Google Scholar] [CrossRef]
- Isaev, N.; Chetverikov, N.; Stelmashook, E.; Genrikhs, E.; Khaspekov, L.; Illarioshkin, S. Thymoquinone as a Potential Neuroprotector in Acute and Chronic Forms of Cerebral Pathology. Biochemistry 2020, 85, 167–176. [Google Scholar] [CrossRef]
- Wei, J.; Wen, W.; Dai, Y.; Qin, L.; Wen, Y.; Duan, D.D.; Xu, S. Drinking Water Temperature Affects Cognitive Function and Progression of Alzheimer’s Disease in a Mouse Model. Acta Pharmacol. Sin. 2021, 42, 45–54. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Ni, Y.; Qi, C.; Bai, S.; Xu, Q.; Fan, Y.; Ma, X.; Lu, C.; Du, G.; et al. Maternal BPAF Exposure Impaired Synaptic Development and Caused Behavior Abnormality in Offspring. Ecotoxicol. Environ. Saf. 2023, 256, 114859. [Google Scholar] [CrossRef]
- Gök, D.K.; Erdoğan, F.F.; Göl, M.F.; Taheri, S.; Önal, M.G.; Şükranlı, Z.Y.; Güvenilir, E.; Yora, S. Effects of Valproic Acid on Transcript Levels in Neurotrophin Signaling Pathway in Mice Hippocampus According to the Implementation Period. EuroBiotech J. 2024, 8, 103–114. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris Water Maze: Procedures for Assessing Spatial and Related Forms of Learning and Memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Ocak, M.; Ateş, Ş.; Kahveci, S.; Okan, A.; Doğanyiğit, Z.; Uçar, S.; Yılmaz, S. Evaluation of the Anticarcinogenic Effects of Rutin on Brain Tissue in Mice with Ehrlich Ascites Carcinoma by Micro-Computed Tomography and Histological Methods. Asia-Pac. J. Clin. Oncol. 2025, 21, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, O.; Dikici, Y.; Gür, Ö.; Ocak, M.; Doğanyiğit, Z.; Okan, A.; Arıkan Söylemez, E.S.; Ateş, Ş.; Uçar, S.; Unal, M.; et al. Evaluation of the Effect of Tartrazine on the Offspring Rats in an in Vivo Experimental Model. Food Sci. Nutr. 2024, 12, 9162–9174. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, E.; Akin, A.T.; Öztürk, E.; Karabulut, D.; Kuloğlu, N.; Yakan, B. Thymoquinone Has a Neuroprotective Effect against Inflammation, Oxidative Stress, and Endoplasmic Reticulum Stress in the Brain Cortex, Medulla, and Hippocampus Due to Doxorubicin. J. Biochem. Mol. Toxicol. 2021, 35, e22888. [Google Scholar] [CrossRef]
- Arslan, D.; Ekinci, A.; Arici, A.; Bozdemir, E.; Akil, E.; Ozdemir, H.H. Effects of Ecballium Elaterium on Brain in a Rat Model of Sepsis-Associated Encephalopathy. Libyan J. Med. 2017, 12, 1369834. [Google Scholar] [CrossRef]
- Tambe, R.; Jain, P.; Patil, S.; Ghumatkar, P.; Sathaye, S. Antiepileptogenic Effects of Borneol in Pentylenetetrazole-Induced Kindling in Mice. Naunyn Schmiedeberg’s Arch. Pharmacol. 2016, 389, 467–475. [Google Scholar] [CrossRef]
- Okan, A.; Demir, N.; Doğanyiğit, Z. Linagliptin in Combination with Insulin Suppresses Apoptotic Unfolded Protein Response in Ovaries Exposed to Type 1 Diabetes. Cell Biochem. Funct. 2024, 42, e3898. [Google Scholar] [CrossRef]
- Okan, A.; Demir, N.; Sozen, B. Unfolded Protein Response Triggers Differential Apoptotic Mechanisms in Ovaries and Early Embryos Exposed to Maternal Type 1 Diabetes. Sci. Rep. 2021, 11, 12759. [Google Scholar] [CrossRef]
- Taheri, S.; Karaca, Z.; Mehmetbeyoglu, E.; Hamurcu, Z.; Yilmaz, Z.; Dal, F.; Çınar, V.; Ulutabanca, H.; Tanriverdi, F.; Unluhizarci, K.; et al. The Role of Apoptosis and Autophagy in the Hypothalamic-Pituitary-Adrenal (HPA) Axis after Traumatic Brain Injury (TBI). Int. J. Mol. Sci. 2022, 23, 15699. [Google Scholar] [CrossRef]
- Abyadeh, M.; Gupta, V.; Paulo, J.A.; Mahmoudabad, A.G.; Shadfar, S.; Mirshahvaladi, S.; Gupta, V.; Nguyen, C.T.O.; Finkelstein, D.I.; You, Y.; et al. Amyloid-Beta and Tau Protein beyond Alzheimer’s Disease. Neural Regen. Res. 2024, 19, 1262–1276. [Google Scholar] [CrossRef] [PubMed]
- Kocahan, S.; Doğan, Z. Mechanisms of Alzheimer’s Disease Pathogenesis and Prevention: The Brain, Neural Pathology, N-Methyl-D-Aspartate Receptors, Tau Protein and Other Risk Factors. Clin. Psychopharmacol. Neurosci. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Perneczky, R.; Dom, G.; Chan, A.; Falkai, P.; Bassetti, C. Anti-Amyloid Antibody Treatments for Alzheimer’s Disease. Eur. J. Neurol. 2024, 31, e16049. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.Z.; Mostaid, M.S.; Apu, M.N.H. Molecular Signaling Pathway Targeted Therapeutic Potential of Thymoquinone in Alzheimer’s Disease. Heliyon 2022, 8, e09874. [Google Scholar] [CrossRef]
- Gülşen, İ.; Ak, H.; Çölçimen, N.; Alp, H.H.; Akyol, M.E.; Demir, İ.; Atalay, T.; Balahroğlu, R.; Rağbetli, M.Ç. Neuroprotective Effects of Thymoquinone on the Hippocampus in a Rat Model of Traumatic Brain Injury. World Neurosurg. 2016, 86, 243–249. [Google Scholar] [CrossRef]
- Lotfi, M.; Kazemi, S.; Ebrahimpour, A.; Pourabdolhossein, F.; Satarian, L.; Eghbali, A.; Moghadamnia, A.A. Thymoquinone Improved Nonylphenol-Induced Memory Deficit and Neurotoxicity Through Its Antioxidant and Neuroprotective Effects. Mol. Neurobiol. 2022, 59, 3600–3616. [Google Scholar] [CrossRef]
- Bellio, T.A.; Laguna-Torres, J.Y.; Campion, M.S.; Chou, J.; Yee, S.; Blusztajn, J.K.; Mellott, T.J. Perinatal Choline Supplementation Prevents Learning and Memory Deficits and Reduces Brain Amyloid Aβ42 Deposition in AppNL-G-F Alzheimer’s Disease Model Mice. PLoS ONE 2024, 19, e0297289. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, D.; Ma, D.-N.; Hou, Y.-F.; Zhuang, Q.-Q.; Gong, Y.-L.; Sun, L.-H.; Zhao, H.-Y.; Tao, B.; Yang, Y.-Y.; et al. Osteocalcin Ameliorates Cognitive Dysfunctions in a Mouse Model of Alzheimer’s Disease by Reducing Amyloid β Burden and Upregulating Glycolysis in Neuroglia. Cell Death Discov. 2023, 9, 46. [Google Scholar] [CrossRef]
- Day, S.M.; Gironda, S.C.; Clarke, C.W.; Snipes, J.A.; Nicol, N.I.; Kamran, H.; Vaughan, W.; Weiner, J.L.; Macauley, S.L. Ethanol Exposure Alters Alzheimer’s-Related Pathology, Behavior, and Metabolism in APP/PS1 Mice. Neurobiol. Dis. 2023, 177, 105967. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Ye, Q.; Fu, Y.; Li, X.; Yang, K.; Gao, F.; Zhou, A.; Wei, Y.; Tian, S.; et al. Histone Deacetylase Inhibitors VPA and WT161 Ameliorate the Pathological Features and Cognitive Impairments of the APP/PS1 Alzheimer’s Disease Mouse Model by Regulating the Expression of APP Secretases. Alzheimer’s Res. Ther. 2024, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Wang, Z.; Zhou, F.; Yuan, X.; Xia, C.; Wang, W.; Yan, Y.; Cheng, Y.; Yang, H.; Xu, J.; et al. Cornuside Ameliorates Cognitive Impairments via RAGE/TXNIP/NF-κB Signaling in Aβ1-42 Induced Alzheimer’s Disease Mice. J. Neuroimmune Pharmacol. 2024, 19, 24. [Google Scholar] [CrossRef]
- Singh, A.; Rakshit, D.; Kumar, A.; Mishra, A.; Shukla, R. Vitamin E Modified Polyamidoamine Dendrimer for Piperine Delivery to Alleviate Aβ1–42 Induced Neurotoxicity in Balb/c Mice Model. J. Biomater. Sci. Polym. Ed. 2023, 34, 2232–2254. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Hang, Q.; Si-Yue, C.; Hui-Dong, T. Multi-Strain Probiotics Ameliorate Alzheimer’s-like Cognitive Impairment and Pathological Changes Through the AKT/GSK-3β Pathway in Senescence-Accelerated Mouse Prone 8 Mice. Brain Behav. Immun. 2024, 119, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chun, H.; Kim, Y.; Kim, Y.; Park, U.; Chu, J.; Bhalla, M.; Choi, S.-H.; Yousefian-Jazi, A.; Kim, S.; et al. Astrocytic Autophagy Plasticity Modulates Aβ Clearance and Cognitive Function in Alzheimer’s Disease. Mol. Neurodegener. 2024, 19, 55. [Google Scholar] [CrossRef]
- Elibol, B.; Beker, M.; Terzioglu-Usak, S.; Dalli, T.; Kilic, U. Thymoquinone Administration Ameliorates Alzheimer’s Disease-like Phenotype by Promoting Cell Survival in the Hippocampus of Amyloid Beta1–42 Infused Rat Model. Phytomedicine 2020, 79, 153324. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Farghaly, H.S.M.; Farrag, M.M.Y.; Abdel-Rahman, M.S.; Abdel-Wahab, B.A. A Potential Mechanism for the Ameliorative Effect of Thymoquinone on Pentylenetetrazole-Induced Kindling and Cognitive Impairments in Mice. Biomed. Pharmacother. 2017, 88, 553–561. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Badary, O.A. Protective Effects of Thymoquinone on D-Galactose and Aluminum Chloride Induced Neurotoxicity in Rats: Biochemical, Histological and Behavioral Changes. Neurol. Res. 2018, 40, 324–333. [Google Scholar] [CrossRef]
- Akarsu, G.D.; Çetin, A. The Effect of Thymoquinone on Oxidative Stress Parameters and Apolipoprotein E in Alzheimer Model in Rats. Dement. Geriatr. Cogn. Disord. 2022, 51, 297–309. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, X.; Hu, C.; Gao, Y.; Zhao, Y.; Hei, M.; Wang, Z.; Guo, N.; Zhu, H. QKL Injection Ameliorates Alzheimer’s Disease-like Pathology by Regulating Expression of RAGE. Exp. Gerontol. 2024, 190, 112422. [Google Scholar] [CrossRef]



| Primer (Gen) Name | Primers’ Sequences |
|---|---|
| APP-F | GTCGCCAAAGAGACATGCAG |
| APP-R | CCCCTCG GAACTTGTCGATG |
| RAGE-F | CCGAGTCCGAGTCTACCAGA |
| RAGE-R | CGCAGTGTAAAGAGTCCCGT |
| NOX4-F | TCACCCTCGCTGCATTA |
| NOX4-R | ACTTGGGTTCTTCCAGGCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ateş, Ş.; Ülger, H.; Uçar, S.; Okan, A.; Ocak, M.; Güvenilir, E.; Şükranlı, Z.Y.; Kaymak, E.; Doğanyiğit, Z.; Taheri, S.; et al. Evaluation of the Effects of Thymoquinone on RAGE/NOX4 Expressions and Brain Tissue Morphometry in Experimental Alzheimer’s Disease Induced by Amyloid Beta 1–42 Peptide. Biomolecules 2025, 15, 543. https://doi.org/10.3390/biom15040543
Ateş Ş, Ülger H, Uçar S, Okan A, Ocak M, Güvenilir E, Şükranlı ZY, Kaymak E, Doğanyiğit Z, Taheri S, et al. Evaluation of the Effects of Thymoquinone on RAGE/NOX4 Expressions and Brain Tissue Morphometry in Experimental Alzheimer’s Disease Induced by Amyloid Beta 1–42 Peptide. Biomolecules. 2025; 15(4):543. https://doi.org/10.3390/biom15040543
Chicago/Turabian StyleAteş, Şükrü, Harun Ülger, Sümeyye Uçar, Aslı Okan, Mert Ocak, Ecma Güvenilir, Zeynep Yılmaz Şükranlı, Emin Kaymak, Züleyha Doğanyiğit, Serpil Taheri, and et al. 2025. "Evaluation of the Effects of Thymoquinone on RAGE/NOX4 Expressions and Brain Tissue Morphometry in Experimental Alzheimer’s Disease Induced by Amyloid Beta 1–42 Peptide" Biomolecules 15, no. 4: 543. https://doi.org/10.3390/biom15040543
APA StyleAteş, Ş., Ülger, H., Uçar, S., Okan, A., Ocak, M., Güvenilir, E., Şükranlı, Z. Y., Kaymak, E., Doğanyiğit, Z., Taheri, S., & Yilmaz, S. (2025). Evaluation of the Effects of Thymoquinone on RAGE/NOX4 Expressions and Brain Tissue Morphometry in Experimental Alzheimer’s Disease Induced by Amyloid Beta 1–42 Peptide. Biomolecules, 15(4), 543. https://doi.org/10.3390/biom15040543







