1. Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), first reported in 1951 by Dr. Churg and Dr. Strauss, is one of the types of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis that affect small- to medium-sized vessels [
1]. Traditional treatments for EGPA have primarily consisted of immunosuppressive therapies centered on glucocorticoids (GC) and cyclophosphamide. However, despite achieving disease activity control, relapse rates remain notably high following dose reduction or discontinuation of these medications. In cases with poor prognosis (five-factor score ≥ 1), the relapse rate was significantly higher in the GC monotherapy group compared to the group receiving combination therapy with cyclophosphamide [
2]. Furthermore, severe adverse effects associated with long-term use of these treatments, such as infections and malignancies, cannot be overlooked [
3,
4]. These challenges underscore the urgent need to develop molecularly targeted therapies with fewer side effects, based on the underlying disease mechanisms.
In 2017, the efficacy of mepolizumab, an interleukin-5 (IL-5) inhibitor, was demonstrated for EGPA in the MIRRA trial [
5]. This drug not only controlled disease activity in EGPA but also enabled a reduction in GC use [
5]. Subsequently, mepolizumab was approved for use in refractory and relapsing EGPA and has since become widely utilized. Furthermore, in 2024, benralizumab, an IL-5 receptor inhibitor, was shown to be non-inferior to mepolizumab in terms of efficacy for EGPA in the MANDARA trial, leading to its approval for use in this condition [
6].
The introduction of IL-5-targeted therapies has significantly advanced the treatment strategy for EGPA. This review provides an overview of the role of eosinophils and IL-5 in the pathophysiology of EGPA, incorporating the latest research findings. Furthermore, it organizes the existing evidence on mepolizumab and benralizumab in asthma and EGPA, while summarizing the pharmacological characteristics, similarities, and differences between these two agents. The goal of this review is to offer insights into the optimal utilization of these therapies based on their distinct features.
2. Pathophysiological Differences in EGPA Based on ANCA Status: The Roles of Eosinophils and IL-5
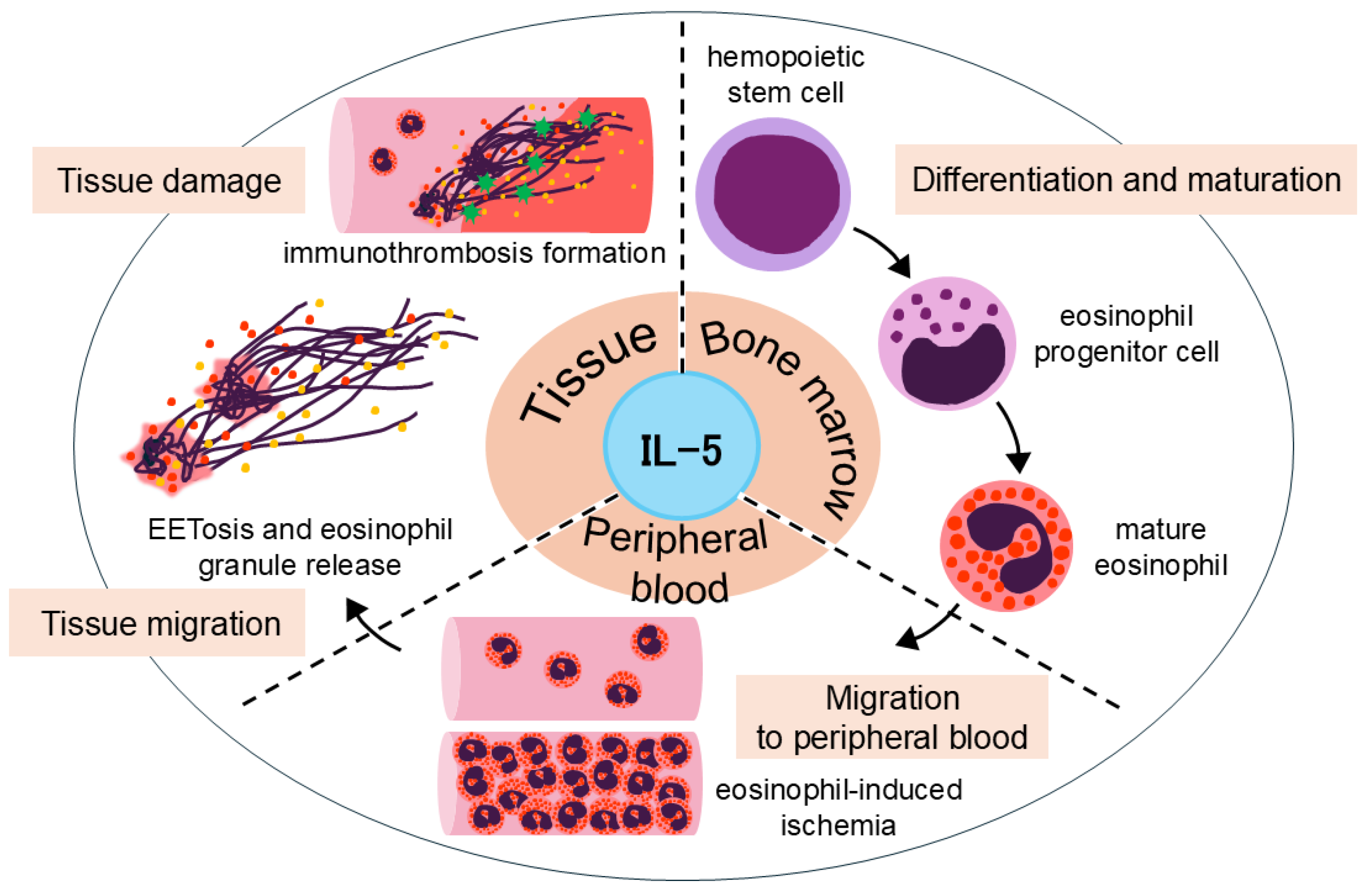
The typical progression of EGPA can be broadly categorized into three stages. It begins with a prodromal phase characterized by asthma and eosinophilic sinusitis, followed by a phase of peripheral blood and tissue eosinophilia. Ultimately, it progresses to the vasculitic phase, which is pathologically marked by extensive eosinophilic infiltration (
Figure 1), leading to systemic organ damage including mononeuritis multiplex, purpura, and renal impairment [
4,
7].
The myeloperoxidase (MPO)-ANCA positivity rate in EGPA is approximately 30–40%, and differences in genetic background, pathophysiology, and organ involvement have been reported based on ANCA status (
Table 1) [
8,
9,
10,
11]. In ANCA-positive cases,
HLA-DQ has been identified as a risk allele, sharing a genetic background with MPO-positive ANCA-associated vasculitis. Conversely, ANCA-negative cases are characterized by mutations in
IRF1/
IL5, and
GPA33, which are primarily associated with impaired airway mucosal barrier function.
Reflecting these genetic backgrounds, ANCA-positive EGPA is predominantly characterized by neutrophil-driven vasculitis, with a higher likelihood of organ involvement such as glomerulonephritis and cutaneous purpura. On the other hand, in ANCA-negative EGPA, the vasculitic phase is thought to be primarily mediated by eosinophilic inflammation, leading to a greater propensity for eosinophilic myocarditis and eosinophilic gastroenteritis. The detailed mechanisms underlying these differences remain unclear; however, recent studies have increasingly elucidated the role of eosinophils in the pathophysiology of EGPA.
2.1. Pathophysiology of Eosinophilic Inflammation
The well-known roles of eosinophils include their involvement in allergic reactions and immune responses to parasitic infections. However, it has been revealed that eosinophils are present in tissues such as the skin, lungs, and gastrointestinal tract at levels approximately 100 times higher than in peripheral blood. Within these tissues, they contribute to maintaining homeostasis, including tissue repair and the lipid and glucose metabolism, and are even implicated in the suppression or promotion of malignant tumors [
12,
13,
14,
15].
In conditions such as eosinophilic gastrointestinal disorders, hypereosinophilic syndrome (HES), and EGPA, the hallmark of eosinophilic inflammation is degranulation, during which eosinophils release cytotoxic granules. Eosinophils store at least four types of granule proteins in their cytoplasm, each of which plays a role such as antimicrobial activity or the activation of Type 2 (T2) inflammation [
16,
17]. The mechanisms of eosinophil degranulation can be broadly categorized into two types: those involving cell lysis and those that do not [
18]. In the organs of EGPA patients, a form of programmed eosinophil cell death known as eosinophil extracellular trap cell death (EETosis) has been observed. This process involves the release of eosinophilic granules, which are thought to contribute to the development of vasculitis [
19].
2.2. The Multifaceted Role of IL-5 in Eosinophil Biology and EETosis
IL-5 is a cytokine of critical importance that exerts multifaceted effects on the life cycle of eosinophils (
Figure 2). IL-5 contributes to eosinophil differentiation and maturation in the bone marrow, migration to peripheral blood and tissues, and the prolongation of eosinophil survival [
11,
20,
21,
22].
Notably, IL-5 stimulation, along with other factors, plays a key role in inducing EETosis, potentially leading to the release of galectin-10 from the eosinophil cytoplasm. Galectin-10 levels have been shown to correlate with disease activity in EGPA and are positively associated with IL-5 [
19]. Furthermore, the administration of mepolizumab in patients with severe asthma has been shown to reduce serum galectin-10 levels [
23]. These findings suggest that IL-5-mediated eosinophil activation triggers a cascade involving EETosis and galectin-10 release. Moreover, the net-like structures formed during EETosis have been shown to promote platelet adhesion, implicating their role in immunothrombosis formation [
24].
Based on the above molecular and biological findings, targeting IL-5 as a therapeutic strategy is highly rational from a pathophysiological perspective and is now regarded as an indispensable approach in the treatment of EGPA.
3. Mechanism of Action of Mepolizumab and Benralizumab
IL-5R is expressed not only on eosinophils but also on basophils, mast cells, and CD34+ progenitor cells [
25,
26,
27]. The IL-5Rα subunit specifically binds to IL-5, while the βc subunit is involved in signal transduction [
25,
28]. There are three monoclonal antibodies targeting IL-5: mepolizumab, reslizumab, and benralizumab.
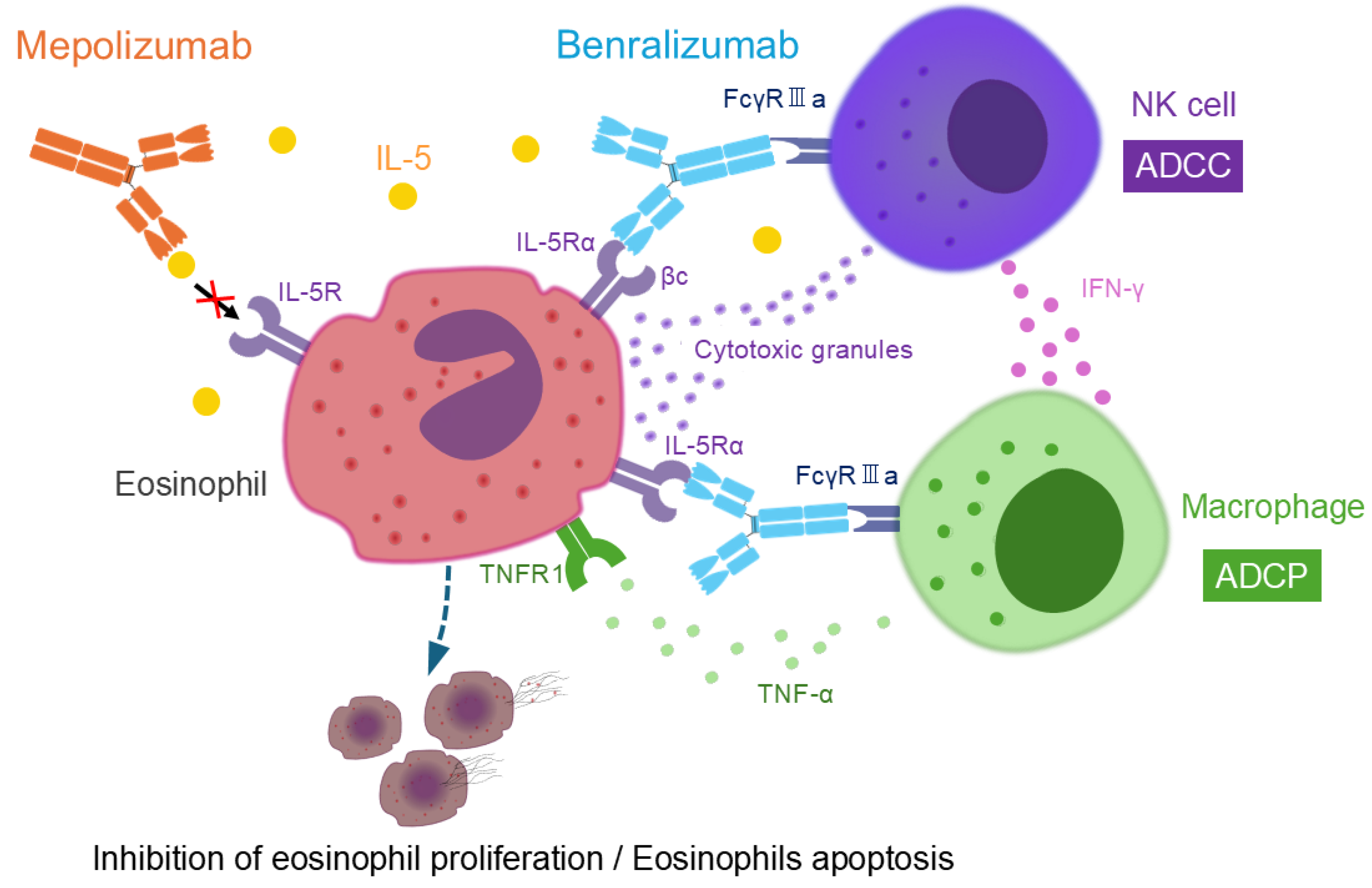
Mepolizumab, a monoclonal antibody against IL-5, inhibits IL-5 from binding to the IL-5 receptor α (IL-5Rα) on the eosinophil surface, thereby suppressing eosinophil proliferation. It has a high affinity for IL-5 and does not interfere with the biological activity of other cytokines [
29].
Similarly, reslizumab, another IL-5 monoclonal antibody, shares a comparable mechanism of action with mepolizumab. It is approved for the treatment of severe eosinophilic asthma in the United States and Europe [
29,
30,
31], but not in Japan. Since this review focuses on the comparison between mepolizumab and benralizumab, which is approved for EGPA, a detailed discussion of reslizumab falls beyond the scope of this article.
In contrast, benralizumab is a humanized monoclonal antibody targeting IL-5Rα. Unlike mepolizumab and reslizumab, which block IL-5 itself, benralizumab directly binds to IL-5Rα, preventing IL-5 from interacting with its receptor. Its most notable pharmacological feature lies in its glycosylation modification. Specifically, the Fc region of benralizumab, particularly the CH2 domain, has been engineered to lack fucose, enhancing its ability to bind strongly to eosinophils via FcγRIIIa receptors (also known as CD16) on natural killer (NK) cells. This modification amplifies antibody-dependent cellular cytotoxicity (ADCC) activity by 10–100 times [
32,
33,
34].
Furthermore, benralizumab promotes antibody-dependent cellular phagocytosis (ADCP) through FcγRIIIa on macrophages. NK cells activated by benralizumab release interferon (IFN)-γ, which stimulates tumor necrosis factor (TNF)-α secretion from macrophages. TNF-α binds to TNF receptor 1 expressed on eosinophils, inducing eosinophil apoptosis and consequently enhancing ADCP. Through these immune cell interactions involving NK cells, macrophages, and eosinophils, benralizumab effectively depletes eosinophils, leading to their near-complete depletion [
35]. (
Figure 3). In in vitro experiments, benralizumab strongly promoted ADCC and ADCP; however, complement-dependent cytotoxicity activity was not observed, presumably due to insufficient activation of the complement system [
35]. Importantly, in vitro experiments have shown that eosinophil granules such as eosinophil cationic protein and eosinophil-derived neurotoxin are not released during benralizumab-induced eosinophil apoptosis, supporting the safety profile of benralizumab [
36].
4. Comparison of the Characteristics of Mepolizumab and Benralizumab in Asthma
4.1. Asthma Exacerbations and Lung Function
The IL-5 pathway has been proven effective in suppressing asthma exacerbations in clinical trials. In the MENSA trial, mepolizumab reduced the annual exacerbation rate by 53% [
37]. Similarly, benralizumab demonstrated a 45% and 35% reduction in exacerbations in the SIROCCO and CALIMA trials, respectively [
38,
39] and reduced the annual exacerbation rate by 74% in the MIRACLE trial conducted in Asia [
40]. Furthermore, the XALOC-1 trial, a large-scale, multinational, retrospective observational study investigating benralizumab in adults with severe eosinophilic asthma, showed that benralizumab was associated with a reduction in exacerbation rates even in patients with a prior history of omalizumab or mepolizumab treatment [
41]. A real-world comparative study of benralizumab and mepolizumab demonstrated significant reductions in the Asthma Control Questionnaire (ACQ) scores and asthma exacerbation rates with both therapies, indicating comparable efficacy [
42].
Blood and sputum eosinophil counts have been shown to significantly correlate with worsening forced expiratory volume in 1 s (FEV1), a key marker of asthma control [
43,
44]. Both therapies demonstrated significant improvements in FEV1 after 12 months of treatment; the median FEV1 increased from 59% to 74% with mepolizumab and from 63% to 72% with benralizumab [
45]. In real-world clinical practice, no clinically meaningful differences have been observed between mepolizumab and benralizumab in terms of their effects on lung function.
4.2. Glucocorticoid-Sparing Effects
In the SIRIUS trial, mepolizumab reduced GC doses by 50% while maintaining asthma control [
46]. Similarly, benralizumab demonstrated numerically superior GC-sparing capabilities, achieving a 75% reduction in GC doses in the ZONDA trial [
47]. Additionally, in the PONENTE trial, 81.9% of patients treated with benralizumab were able to reduce their prednisolone dose to ≤5 mg, and 62.9% achieved GC-free status [
48]. The GC-sparing effects of benralizumab have also been supported by the large-scale XALOC-1 study, in which 47.4% of patients with severe eosinophilic asthma who received benralizumab were able to completely discontinue GC therapy after 48 weeks [
41].
In real-world clinical practice, the proportion of patients with severe T2-high asthma who achieved remission—defined as the absence of exacerbations, no requirement for oral corticosteroids, an Asthma Control Test score of ≥20, and an ACQ-6 score of <1.5—was 29% with mepolizumab and 44% with benralizumab [
49].
Both agents demonstrate GC-sparing effects in asthma management. However, as no large-scale trials have directly compared their efficacy, it remains premature to determine their relative superiority. Large-scale comparative studies are needed to further elucidate the clinical impact of their GC-sparing effects in asthma management.
4.3. Airway Remodeling Effect
Airway remodeling is a key feature of severe eosinophilic asthma, driving persistent airflow limitation and disease progression. IL-5R is expressed on bronchial fibroblasts, and, importantly, its expression is significantly elevated in fibroblasts from asthma patients compared to those from healthy controls [
50]. Mepolizumab has demonstrated significant improvements in airway remodeling markers after 12 months, including reductions in sub-basement membrane thickness, airway smooth muscle (ASM) layer thickness, and epithelial damage, indicating structural restoration [
51,
52]. Benralizumab, which depletes eosinophils via ADCC and ADCP, reduces ASM mass by targeting TGF-β1-expressing eosinophils but has minimal impact on myofibroblast numbers, reflecting differences in remodeling effects [
53]. These findings underscore the potential of IL-5-targeted therapies to address airway remodeling in severe eosinophilic asthma, while highlighting the need for further research on their differential impacts and long-term outcomes.
4.4. Safety Profiles
Headache (20%) and injection site reactions (8%) are the most commonly reported adverse reactions associated with mepolizumab treatment, based on clinical trial data. In contrast, headache (8%) and pharyngitis (5%) are more frequently observed with benralizumab [
29]. Notably, long-term studies of benralizumab have identified no new adverse reactions attributable to eosinophil depletion, further supporting its safety profile [
54,
55]. Both therapies have demonstrated acceptable safety profiles in long-term use, making them suitable for the management of severe eosinophilic asthma. Continued monitoring in real-world clinical settings is warranted to confirm these findings and ensure the safe application of these therapies in broader patient populations.
5. Efficacy of Mepolizumab and Benralizumab in EGPA
5.1. Clinical Trial Evidence for Mepolizumab
The MIRRA trial, a pivotal international Phase 3 study, evaluated the efficacy and safety of mepolizumab in 136 patients with relapsing or refractory EGPA. In this study, remission was defined as a Birmingham Vasculitis Activity Score (BVAS) of 0 and a GC dose of ≤4 mg. The results demonstrated a significant difference in remission rates at weeks 36 and 48 between the mepolizumab group and the placebo group [
5]. The trial also confirmed the drug’s effectiveness in reducing relapses and enabling GC tapering. Regarding safety, although injection site reactions and headaches were observed in approximately 10% of patients receiving mepolizumab, no serious adverse events were reported [
5].
However, the MIRRA trial excluded patients with organ/life-threatening EGPA, and the proportion of ANCA-positive patients was relatively low, at 10%. The inclusion criteria resulted in a study population with limited external validity. Moreover, the majority of patients were already receiving GC therapy at baseline, and these factors may have influenced the outcomes. In a subsequent post hoc analysis of the MIRRA trial, remission was achieved and relapse was suppressed regardless of ANCA status or disease severity [
56]. Another post hoc analysis demonstrated that remission was achieved and GC-sparing effects were observed regardless of the number of relapses at baseline, disease duration, or baseline GC dose [
57]. These findings suggest that the body of evidence supporting the efficacy of mepolizumab continues to grow.
Currently, mepolizumab is recommended as a remission induction therapy in combination with GC for relapsing or refractory EGPA, in the absence of active organ/life-threatening disease. Evidence supporting the efficacy of mepolizumab in severe EGPA remains insufficient. As a remission-maintenance therapy, it is recommended regardless of the presence of poor prognostic factors and is considered a first-line option, particularly for maintaining remission in relapsing EGPA [
3,
4]. Although the current evidence indicates that mepolizumab is effective regardless of ANCA status [
4,
56], ANCA status may be a relevant factor in guiding treatment decisions in clinical practice, particularly as more real-world data become available.
5.2. Long-Term Efficacy of Mepolizumab in Real-World Clinical Practice
In the MIRRA trial’s Long-Term Access Programme (LAP), the retention rate of mepolizumab treatment was 71% at up to 89 months [
58]. Similarly, in the Open-Label Extension (OLE) of the MIRRA trial, the retention rate for up to 7.6 years was 68% [
59], indicating a high level of tolerability.
In the OLE, the median GC decreased from 10.0 mg/day at the OLE baseline to 5.0 mg/day at the end of the study [
60]. The GC-sparing effects of mepolizumab was also demonstrated in a separate study using LAP data from the MIRRA trial, with an observation period of up to 7.4 years. Notably, patients who had already received mepolizumab during the MIRRA trial and had been on treatment for a longer duration were able to reduce their GC dose earlier [
61].
Long-term outcomes in real-world clinical settings are gradually being elucidated. In a two-year multicenter observational study conducted in Europe, mepolizumab demonstrated an improvement in BVAS and a glucocorticoid-sparing effects [
62]. We previously demonstrated in a multicenter cohort study that the five-year drug retention rate of mepolizumab in 60 patients with EGPA was 78.7%, indicating high efficacy and tolerability [
63]. Additionally, we compared the clinical course between the mepolizumab-treated group and the non-treated group. Propensity score matching was used to adjust for baseline characteristics of the two groups. The results showed that the BVAS at the last observation was significantly lower, and the GC dose was significantly reduced in the mepolizumab group. Although there was no significant difference in the rate of achieving GC-free status, the proportion of patients achieving a GC dose of ≤4 mg was significantly higher in the mepolizumab group. Moreover, the use of mepolizumab was associated with achieving a GC dose of ≤4 mg, whereas poor asthma control was identified as a factor associated with failure to achieve this dose reduction. These findings suggest that mepolizumab contributes to reduce disease activity and GC tapering in EGPA in real-world clinical practice [
64].
In another study of EGPA patients who had received mepolizumab treatment for more than 48 weeks, an additional 96 weeks of long-term treatment resulted in further reductions in GC dose. This study also suggested that suppressing EGPA relapses may help prevent the progression of neurological symptoms associated with peripheral neuropathy, potentially improving patient outcomes [
65]. These findings support the long-term efficacy and safety of mepolizumab in the treatment of EGPA.
5.3. Clinical Outcomes of Benralizumab
An international Phase 3 trial (MANDARA trial) was conducted in patients with relapsing or refractory EGPA. Remission was defined in the same manner as in the MIRRA trial: BVAS of 0 and GC dose of ≤4 mg. There was no significant difference in remission rates at weeks 36 and 48 between the benralizumab group and the mepolizumab group [
6]; however, benralizumab demonstrated a more potent reduction in eosinophil counts than mepolizumab. The proportion of patients achieving GC-free status during the 52-week observation period was 41% in the benralizumab group and 27% in the mepolizumab group, suggesting that benralizumab may have superior GC-sparing effects. In this trial, the most frequently reported adverse events were COVID-19 infections (benralizumab/mepolizumab: 21%/27%), headaches (17%/16%), and arthralgia (17%/11%). The incidence of serious adverse events was 6% in the benralizumab group and 13% in the mepolizumab group [
6]. During the open-label extension phase of the MANDARA trial, the safety profile of benralizumab was consistent with that observed in the double-blind phase [
66].
Based on the positive results of the MANDARA trial, benralizumab has been approved as an additional therapeutic option for EGPA. Given the study design, benralizumab is expected to be used in a manner similar to mepolizumab for remission induction therapy. However, definitive evidence regarding its efficacy in remission maintenance therapy has not yet been established.
It is important to note, though, that the MANDARA trial had several important limitations. As in the MIRRA trial, patients with organ/life-threatening EGPA were excluded, and the sample size was limited to 140, resulting in restricted follow-up data. Furthermore, secondary endpoints were not adjusted for multiplicity, and the discussion section did not adequately address the methodological limitations of the trial.
In the subgroup analysis of the MANDARA trial, no significant association was observed between ANCA status and the proportion of patients achieving remission; however, given the aforementioned limitations, further case accumulation and subsequent analyses are warranted.
In real-world clinical practice, benralizumab has been increasingly reported to effectively suppress disease activity, as measured by BVAS, while enabling GC dose reduction in patients with EGPA [
67,
68,
69,
70,
71,
72,
73]. The accumulation of long-term clinical data on benralizumab will be essential for a better understanding of its efficacy and safety profile, particularly in diverse patient populations and under real-world clinical conditions.
6. Optimizing the Use of Mepolizumab and Benralizumab: Tailoring Treatment Strategies
6.1. Rapid and Comprehensive Eosinophil Suppression by Benralizumab
Subgroup analyses from the MANDARA trial confirmed that benralizumab rapidly and significantly reduced blood eosinophil counts as early as the first week of treatment. This effect may surpass that of mepolizumab [
74]. Proteomic analysis using randomized trial samples further demonstrated that benralizumab, compared to mepolizumab, suppresses pathways related to eosinophil activation and migration at an earlier stage. This finding suggests the potential for rapid inflammation control in T2 inflammatory diseases involving T2 cytokines (e.g., IL-4, IL-5, IL-13) [
75].
Benralizumab (30 mg subcutaneous injection every four weeks) has been demonstrated to significantly reduce eosinophils in peripheral blood, bone marrow, and gastrointestinal tissues in patients with HES involving organ symptoms [
76]. Furthermore, it has been reported that benralizumab achieves a greater reduction in sputum eosinophil counts compared to mepolizumab [
77]. In contrast, while mepolizumab reduces peripheral blood eosinophil counts, its efficacy in reducing tissue eosinophils appears to be limited. Furthermore, its ability to reduce eosinophil progenitor cells in the bone marrow is considered less pronounced [
78,
79]. In real-world clinical settings, further case accumulation is warranted to determine how this rapid eosinophil depletion translates into tangible benefits for patients.
6.2. Glucocorticoid-Sparing Effects
In the MANDARA trial, the GC-free achievement rate was 41% in the benralizumab group compared to 27% in the mepolizumab group, suggesting that benralizumab may have superior GC-sparing effects [
6,
80]. Furthermore, a subgroup analysis of the MANDARA trial demonstrated that among EGPA patients using medium- or high-dose inhaled corticosteroids (ICS), the proportion of patients achieving GC-free status was significantly higher in the benralizumab group compared to the mepolizumab group [
81].
In real-world clinical practice, benralizumab also led to greater reductions in GC use compared to mepolizumab, with a higher proportion of patients achieving complete discontinuation [
69]. The superior eosinophil-suppressing effect of benralizumab is hypothesized to contribute to its enhanced GC-sparing effects; however, additional evidence is needed to validate this hypothesis.
6.3. Eosinophil Depletion and Immune Homeostasis
It is important to emphasize that complete depletion of eosinophils is not always advantageous in treatment. Eosinophils have traditionally been understood as inflammatory subtypes (inflammatory eosinophils: iEos) involved in inflammatory diseases such as asthma. However, recent studies have identified a novel eosinophil subtype in the peripheral blood of asthma patients, known as homeostatic eosinophils (hEos), which contribute to the maintenance of immune homeostasis [
14,
82,
83]. iEos and hEos exhibit distinct gene expression profiles, with iEos being highly IL-5 dependent, whereas hEos is relatively less dependent on IL-5. Since benralizumab exerts its effects through immunological mechanisms, it fully depletes both iEos and hEos, regardless of their IL-5 dependence. In contrast, mepolizumab effectively reduces IL-5-dependent iEos while partially preserving hEos. This approach facilitates the restoration of eosinophil balance to a state closer to that of healthy individuals. This unique property of mepolizumab enables symptom improvement in asthma and EGPA while maintaining a balance between inflammation suppression and immune homeostasis, underscoring its significant therapeutic advantage.
With regard to the immunological changes induced by mepolizumab, a comprehensive analysis of bioactive mediators in the serum of patients with EGPA demonstrated that proteins involved in the Th17/innate lymphoid cells (ILC) 3 axis, angiogenesis, and adhesion molecule pathways were suppressed following mepolizumab treatment [
84]. These findings suggest that mepolizumab may exert effects beyond the IL-5–eosinophil axis. However, the potential long-term effects of these changes remain unclear, highlighting the need for further fundamental research into the immunological effects of IL-5 biologics, including benralizumab.
6.4. Eosinophil Depletion and Its Potential Link to Infections
Eosinophils play a critical role in immune responses against bacteria, viruses, and fungi [
12], and the potential link between eosinophil suppression and an increased risk of infection remains a highly important subject of discussion. In patients with GC-dependent severe asthma, most respiratory infections during benralizumab treatment were non-eosinophilic. Specifically, the increased incidence of infections did not correlate with peripheral blood or airway eosinophil counts but was significantly associated with elevated neutrophil counts in sputum [
85,
86]. This is a highly intriguing finding, especially when considered in the context of the reported dysfunction of NK cell activity under immunosuppressive conditions.
In the context of viral infections, IL-5 transgenic mice exhibit eosinophilia accompanied by reduced Toll-like receptor (TLR)7 expression in the lungs. In a TLR7-deficient asthma model, decreased interferon production and an increased rhinovirus load were observed [
87]. These findings suggest that IL-5 inhibition may positively influence the immune response against rhinovirus. Conversely, a recent study investigating the role of mast cells in viral infections demonstrated that IL-5-treated mast cells produced higher levels of interferons in response to viral challenge. Notably, the study also suggested that IL-5 may contribute to mast cell survival. These findings imply that anti-IL-5 therapy may potentially impair antiviral immune responses [
88].
In a real-world study comparing the five-year long-term safety of mepolizumab and benralizumab in patients with severe eosinophilic asthma, no significant differences in safety profiles were observed between the two agents, and no increased risk of serious infections was reported [
89]. Furthermore, a meta-analysis of randomized controlled trials evaluating infection-related adverse events suggested that IL-5 biologics may reduce the incidence of serious bacterial infections, pneumonia, and influenza [
90]. Taken together, the current evidence remains insufficient to determine whether eosinophil suppression is ultimately beneficial or detrimental with respect to infection risk, underscoring the need for further research, including case accumulation and mechanistic studies.
6.5. Eosinophil Suppression and Malignancy Risk
Eosinophils have been implicated in cancer, exerting not only direct antitumor effects through the release of cytotoxic granule proteins but also indirect antitumor effects through interactions with immune cells such as CD8⁺ T cells and macrophages. Conversely, eosinophils have also been suggested to contribute to the formation of an immunosuppressive tumor microenvironment, potentially promoting tumor progression [
12,
91]. These observations indicate that the role of eosinophils in cancer is heterogeneous and may depend on tumor type and microenvironmental context.
The impact of anti-IL-5 biologics on malignancy risk, as well as treatment strategies for patients with EGPA who develop malignancies, remains critical for clinical practice. However, clear evidence addressing these concerns is currently lacking.
At present, there is no conclusive evidence that treatment with mepolizumab or benralizumab increases the risk of malignancy [
89,
92,
93]. Continued accumulation of case reports and long-term safety data is strongly warranted.
6.6. NK Cell Function and the Efficacy of Benralizumab: Implications for Anti–IL-5 Biologics and Immunosuppressant Use
Human NK cells are classified into two major subsets based on the relative expression of CD56 and CD16: CD56brightCD16− and CD56dimCD16+ [
94,
95]. CD56dimCD16+ NK cells differentiate from CD56brightCD16− NK cells and constitute 90% of NK cells in peripheral blood. This subset exhibits high cytotoxic activity, contains abundant perforin, granzyme, and cytotoxic granules, and has the ability to induce ADCC.
The therapeutic efficacy of benralizumab may depend on NK cell function. It has been suggested that patients with impaired NK cell function due to concomitant use of immunosuppressants (ISs) may experience limited treatment effects. In patients with severe asthma, responders to benralizumab demonstrated preserved ADCC function, whereas non-responders showed reduced IFN-γ secretion from NK cells and impaired ADCC function compared to responders [
96]. Indeed, in vitro experiments showed that dexamethasone administration to NK cells resulted in reduced IFN-γ expression. This suggests a potential decrease in ADCC activity and eosinophil apoptosis mediated by macrophages [
96]. Furthermore, in patients with GC-dependent severe asthma who exhibited an insufficient response to benralizumab, a reduction in NK cell counts and a decreased proportion of CD16-positive NK cells, which are responsible for inducing ADCC activity, have been observed [
85].
Recent studies on severe eosinophilic asthma have revealed an imbalance between mature CD16-positive NK cells and immature CD16-negative NK cells in asthma patients compared to healthy controls. After 24 months of benralizumab treatment, an increase in mature NK cells, specifically the CD56dimCD16bright subset, was observed, which correlated with changes in FEV1 and GC use [
97]. The mechanisms by which benralizumab alters NK cell subsets remain unclear, and further research is warranted.
Regarding the combination of anti–IL-5 biologics and ISs, a post hoc analysis of the MIRRA trial demonstrated that mepolizumab was effective regardless of baseline IS use, including agents such as azathioprine and mycophenolate mofetil [
57]. In contrast, a subgroup analysis of the MANDARA trial suggested that the remission rate with benralizumab appeared to be higher in patients without baseline IS use than in those receiving ISs at baseline [
6]. However, given the small sample size and the major limitations previously noted in the MANDARA trial, this finding should be interpreted with caution. Currently, there is no established evidence regarding the concomitant use of these two IL-5–targeted agents with ISs. Nevertheless, as such combinations may have clinical implications for patient outcomes in real-world practice, the generation of further evidence is critically needed.
6.7. Effects of Benralizumab on Basophils
IL-5R is expressed on basophils and mast cells, suggesting that benralizumab can also exert effects on these immune cells, a characteristic not observed with mepolizumab [
25,
26,
27]. In real-world clinical practice, benralizumab has been shown to reduce not only eosinophils but also basophils and mast cells [
69,
98,
99]. Notably, studies evaluating basophils in severe eosinophilic asthma have demonstrated that benralizumab reduces basophil counts without inducing degranulation, highlighting its potential therapeutic utility [
100].
Human basophils express the thymic stromal lymphopoietin receptor (TSLPR), which contributes to the pathogenesis of T2 inflammation [
101,
102,
103]. Basophils can also be induced by IL-3, and TSLP-induced basophils exhibit distinct gene expression profiles compared to IL-3-induced basophils, suggesting heterogeneity [
103,
104]. Specifically, TSLP-induced basophils predominantly express genes related to fatty acid metabolism and cell adhesion molecules, whereas IL-3-induced basophils express genes associated with TNF-α signaling and the maturation of monocytes and dendritic cells [
103].
A study comparing the characteristics of basophils before and after six months of benralizumab treatment in patients with severe eosinophilic asthma found that the baseline basophil count in asthma patients was significantly higher than that in healthy subjects. Interestingly, after benralizumab administration, the number of basophils expressing IL-3R and IL-5R significantly decreased, whereas there was no significant effect on basophils expressing TSLPR [
105]. These findings suggest the presence of basophil subsets that respond differently to benralizumab; however, the clinical significance of this differential response remains unclear. Moreover, the roles of basophils and mast cells in the pathogenesis of EGPA remain largely unclear, emphasizing the need for further investigations to elucidate their contributions.
6.8. Cardiac and Thrombotic Implications of IL-5–Targeted Therapy in EGPA
Cardiac involvement is relatively common in EGPA and has been associated with a poor prognosis [
4]. The role of eosinophils and IL-5 in cardiac pathophysiology remains inconclusive and debated. A study in the general population demonstrated that lower peripheral blood eosinophil counts were associated with an elevated short-term risk of mortality due to heart failure and coronary artery disease [
106]. Furthermore, experimental studies using mouse models have suggested that IL-5 may promote tissue repair and improve cardiac function following myocardial infarction by inducing M2-type macrophages through eosinophil activation [
107]. In contrast, clinical case reports have indicated that mepolizumab contributed to improved cardiac function and enabled GC tapering in patients with eosinophilic myocarditis associated with HES or EGPA [
108].
In addition, EGPA has also been reported to be associated with both arterial and venous thrombosis. Interestingly, this thrombotic risk does not appear to be related to traditional cardiovascular risk factors such as hypertension, hyperlipidemia, or smoking, but rather to increased disease activity [
109]. In real-world clinical practice, a low incidence of thrombotic events has been observed in patients with EGPA treated with mepolizumab. Patients who developed thrombosis had higher BVAS at the onset and were more likely to exhibit cardiac involvement compared to those without thrombosis [
110]. Furthermore, a real-world study comparing the cardiovascular adverse effects of anti-IL-5/IL-5Rα antibody therapies with those of the anti-immunoglobulin E (IgE) antibody omalizumab found that cardiovascular events were reported less frequently with anti-IL-5/IL-5Rα therapies [
111]. These findings suggest that thrombotic events in EGPA may be associated with underlying vasculitic activity, and that treatment with IL-5-targeted agents may help mitigate this risk. However, the current evidence is limited, and further accumulation of clinical data is warranted.
6.9. Paradoxical Arthritis Under Anti–IL-5 Biologics
Arthralgia has been reported as a paradoxical reaction associated with IL-5 antibody therapies [
112,
113]. Recent studies have identified a subset of eosinophils known as regulatory eosinophils, which play a role in suppressing inflammation in arthritis [
114]. Interestingly, in this study, rheumatoid arthritis patients with comorbid asthma experienced a recurrence of arthritis following mepolizumab treatment. In the MANDARA trial, joint pain was observed in 17% of patients treated with benralizumab and 11% of those treated with mepolizumab [
6]. However, progression to rheumatoid arthritis or inflammatory arthritis as a side effect of IL-5 antibody therapies is very rare [
115]. The long-term adverse effects of these therapies remain largely unknown, underscoring the need for further case accumulation and studies.
6.10. Immunogenicity and Clinical Impact of Anti-Drug Antibodies
A systematic review and meta-analysis investigating the incidence of anti-drug antibodies (ADA) in biologics approved for asthma treatment reported an ADA incidence of 8.35% for benralizumab and 3.63% for mepolizumab [
116]. In the MANDARA trial, ADAs and neutralizing antibodies were observed in 9% and 1.5% of patients in the benralizumab group, respectively. However, these antibodies did not have a significant impact on achieving remission [
6]. Similarly, a study evaluating the immunogenicity of mepolizumab in 915 patients across five major clinical trials (DREAM, MENSA, SIRIUS, COSMOS, and COLUMBA) detected ADAs in less than 1–9% of patients, with half of these cases being transient. Furthermore, neutralizing antibodies were identified in only a small number of patients, and no effect on treatment efficacy or adverse events was observed. No differences in adverse events or therapeutic outcomes were noted between ADA-positive and ADA-negative patients [
117].
These findings suggest that there is no evidence that ADAs or neutralizing antibodies associated with either benralizumab or mepolizumab affect their efficacy and safety. Nonetheless, further data collection is necessary to comprehensively evaluate their clinical significance.
The unique characteristics of mepolizumab and benralizumab described above are summarized in
Table 2.
7. Proposals for Drug Selection and Decision-Making in Clinical Practice
Thus far, we have outlined and summarized the respective characteristics and distinctions between mepolizumab and benralizumab. In this section, we explore their practical application and differential use in clinical settings.
In eosinophil-associated disorders, the number of affected organs has been reported to increase with rising eosinophil counts [
118]. In real-world practice, 54.1% of EGPA cases have been diagnosed during the eosinophilic phase, and 30.7% during the vasculitic phase [
119]. Early initiation of anti-IL-5 biologics during the prodromal or eosinophilic phase may be considered to prevent progression to the vasculitic phase.
The therapeutic efficacy of IL-5 biologics may vary among individuals. Regarding the relationship between blood eosinophil counts and treatment response, it has been reported that EGPA patients with higher baseline eosinophil levels are more likely to achieve remission following treatment with mepolizumab or benralizumab [
69,
70]. In a transcriptomic analysis of mepolizumab responsiveness in patients with EGPA, those who exhibited a favorable clinical response demonstrated an upregulated expression of genes associated with AP-1 transcription factors and IL-1β signaling pathways [
120]. This finding suggests that interindividual differences in gene expression profiles may underlie the heterogeneous responses to IL-5–targeted therapies. Continued advances in research will be essential for the implementation of precision medicine in EGPA.
With regard to the switching of biologic agents, several case reports have described patients with severe eosinophilic asthma who achieved improved disease control after transitioning from mepolizumab to benralizumab due to an inadequate response [
121]. Furthermore, in cases where EGPA developed during treatment with anti–T2 inflammatory monoclonal antibodies, the majority of patients were reinitiated on anti-T2 agents following the induction of remission. It has been reported that switching from benralizumab to mepolizumab, or vice versa, was associated with sustained remission without relapse in EGPA [
122]. However, these observations are based on a limited number of cases, and high-quality evidence regarding the switching of anti–IL-5 biologics in EGPA remains insufficient. Further accumulation of clinical data and the establishment of robust evidence are warranted.
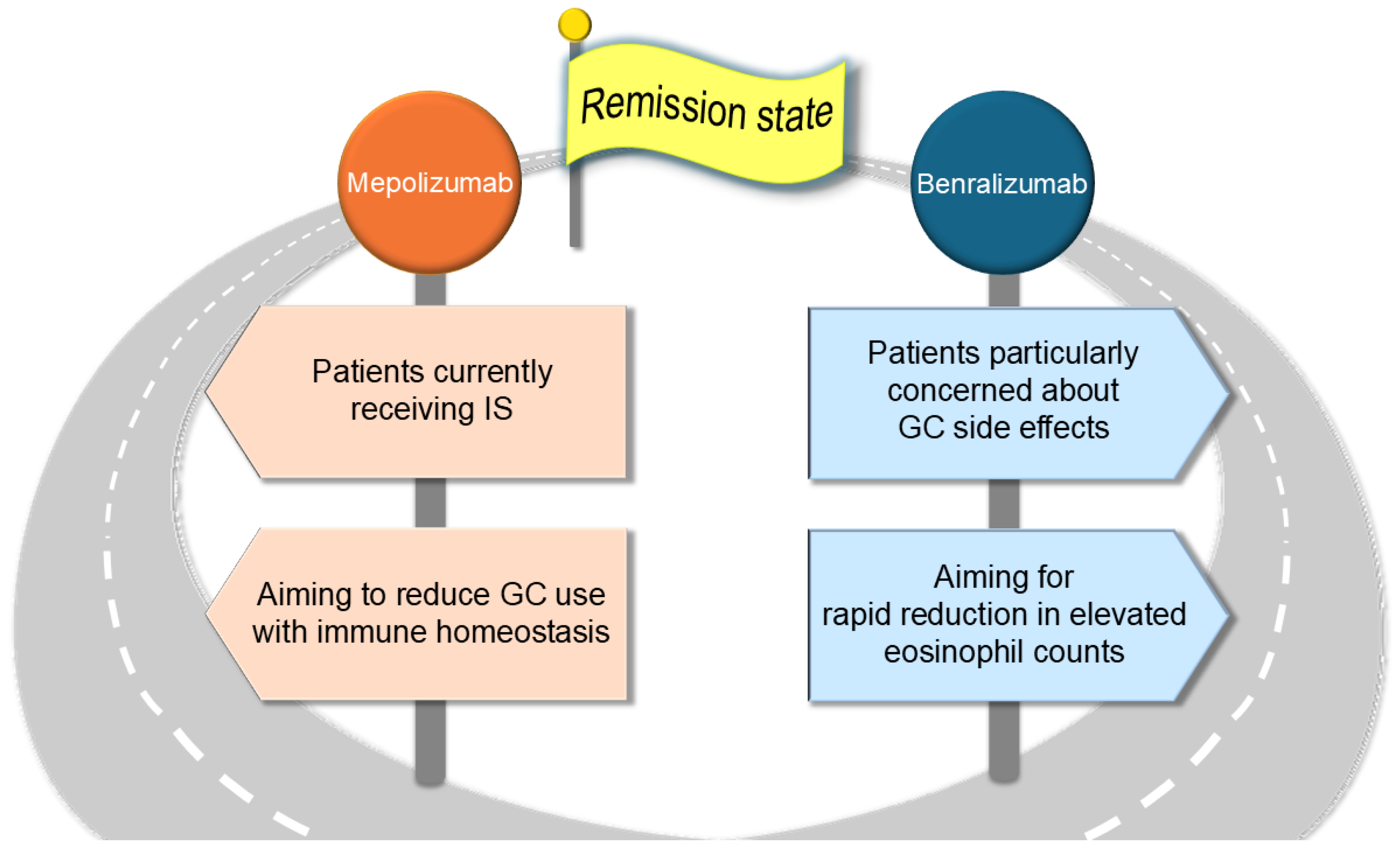
Based on the points discussed in this review, key considerations for the clinical use and differentiation of the two agents in real-world practice are summarized below. Mepolizumab might be effective even in patients currently receiving ISs, as its efficacy is less likely to be influenced by NK cell dysfunction. Furthermore, by selectively reducing inflammatory eosinophils while preserving eosinophil homeostasis, mepolizumab may contribute to long-term immune stability and facilitate GC tapering. In contrast, benralizumab may induce a rapid reduction in eosinophil counts, particularly in cases with markedly elevated eosinophil levels. Additionally, for patients with substantial concerns regarding GC-related adverse effects, benralizumab may help mitigate GC dependency (
Figure 4).
Moving forward, it will be essential to accumulate evidence on how the differences between these two agents translate into clinical benefits for patients in real-world settings, and to develop a decision-making algorithm to guide their optimal use.
8. Exploring Alternative Therapeutic Targets Beyond IL-5 in EGPA
This review provides a comparative analysis of the pharmacological properties and clinical utility of mepolizumab and benralizumab. By systematically evaluating the latest scientific evidence, it provides practical insights to guide the optimal selection of IL-5 antibody therapies in real-world clinical settings. Furthermore, this review examines novel therapeutic target molecules identified through recent research beyond the IL-5 signaling pathway and discusses their therapeutic significance and potential for clinical application.
8.1. Proinflammatory Role of Charcot–Leyden Crystals
Charcot–Leyden crystals (CLCs) have long been recognized as a characteristic histopathological finding in eosinophilic inflammation. Recent studies have revealed that CLCs are produced during the process of EETosis [
19]. The protein constituting CLCs, galectin-10, does not exhibit biological activity in its non-crystalline state. However, upon crystallization to form CLCs, it induces the production of inflammatory cytokines and amplifies T2 inflammatory responses [
123]. Antibody therapies targeting and dissolving CLCs have been shown to suppress airway inflammation and IgE production [
124].
Research on the regulatory mechanisms of EETosis has also progressed. An essential molecular mechanism in the EETosis is the function of peptidylarginine deiminase 4 (PAD4), which catalyzes histone citrullination. Experimental studies have demonstrated that inhibiting PAD4 suppresses EETosis [
125]. Furthermore, in chronic eosinophilic rhinosinusitis, extracellular traps released during EETosis have been shown to degrade through the combined use of heparin and DNA-degrading enzymes [
126].
The development of novel therapies targeting EETosis, a critical process in the pathogenesis of vasculitis in EGPA, is being actively pursued. The clinical application of innovative treatment strategies based on these fundamental research findings is highly anticipated.
8.2. IL-33: A Key Alarmin of ILC2 Activation
Group 2 ILC2 are rapidly activated by epithelial-derived alarmins such as IL-33 and TSLP, primarily releasing IL-5 and IL-13. IL-5 produced by ILC2 is essential for the recruitment of eosinophils to tissues and subsequent tissue damage [
127,
128,
129,
130]. A Phase 2 trial evaluated the efficacy of itepekimab, an IL-33-targeting therapy, in asthma [
131]. Itepekimab improved asthma control, enhanced lung function, and demonstrated a favorable safety profile. In mouse studies, IL-33 was shown to induce and exacerbate EGPA in normal mice, independently of systemic eosinophilia [
132]. IL-33 has gathered attention as an alarmin that plays a crucial role in the pathogenesis of EGPA, and its clinical application as a novel therapeutic target is highly anticipated.
8.3. NMU-NMUR1 Pathway
Neuromedin U (NMU) is a neuropeptide that acts on ILC2 to promote T2 inflammation. Its receptor, neuromedin U receptor 1 (NMUR1), is specifically expressed on ILC2 [
125]. In patients with asthma, NMU activates ILC2 more rapidly than IL-33, highlighting the importance of the NMU–NMUR1 pathway in the early activation of ILC2 [
133]. However, it has been shown that NMUR1 expression is suppressed under high-dose ICS treatment, suggesting that the NMU–NMUR1 pathway may be insufficiently functional in severe asthma or GC-resistant conditions. Future studies are required to comprehensively evaluate the role of NMUR1 and explore its potential as a novel therapeutic target.
8.4. HIF-1α/Glycolysis Axis in ILC2 Metabolism
Hypoxia-inducible factor-1α (HIF-1α) plays a central role in the metabolic regulation of ILC2s and is a critical factor in the pathogenesis of allergic airway inflammation [
134]. In ILC2s stimulated by IL-33, upregulation of HIF-1α, a key regulator of glycolysis, was observed [
135]. The administration of glycolysis inhibitors significantly suppressed ILC2 proliferation and the production of IL-5 and IL-13. Furthermore, in patients with asthma, decreased activity of the HIF-1α/glycolysis signaling pathway was positively correlated with the suppression of ILC2 function. These findings suggest that the HIF-1α/glycolysis signaling pathway plays a pivotal role in regulating ILC2 responses in allergic inflammation and may represent a novel therapeutic target for EGPA.
9. Conclusions
This review highlights the molecular roles of eosinophils and IL-5 in EGPA pathophysiology and summarizes the mechanisms and clinical benefits of the anti-IL-5 therapies mepolizumab and benralizumab. The approval of benralizumab has expanded the treatment options for EGPA, underscoring the need for personalized therapy based on disease activity, immunological phenotype, and comorbidities. Future research should focus on diverse patient populations and the optimization of treatment strategies.
Author Contributions
M.S., writing—original draft preparation; R.W., writing—review and editing; R.I., creating figures; S.T., providing pathological images and offering expert pathological insights; T.N., providing general support for the study; M.H., supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our sincere gratitude to the faculty members of the Department of Clinical Immunology at Osaka Metropolitan University for their valuable advice and guidance throughout this research. We are also deeply thankful to the staff at Nakatsu Hospital for their kind support and cooperation, which greatly contributed to the completion of this study.
Conflicts of Interest
R.W. received a research grant from AbbVie and speaker’s fee from AbbVie, Asahi Kasei, Chugai, Eli Lilly, GSK, and UCB Japan. MH received research grants and/or speaker’s fee from AbbVie, Asahi Kasei, Astellas, Bristol Meyers, Chugai, EA Pharma, Eisai, Daiichi Sankyo, Eli Lilly, Novartis Pharma, Taisho Toyama, Tanabe Mitsubishi, Towa Pharma and UCB Japan. These pharmaceutical companies had no role in the writing of the manuscript.
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 37715. [Google Scholar] [CrossRef]
- Sorin, B.; Papo, M.; Sinico, R.A.; Teixeira, V.S.; Venhoff, N.; Urban, M.-L.; Iudici, M.; Mahrhold, J.; Locatelli, F.; Cassone, G.; et al. Glucocorticoids versus glucocorticoids plus cyclophosphamide in eosinophilic granulomatosis with polyangiitis with poor-prognosis factors. J. Autoimmun. 2024, 149, 103338. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, B.; Sanchez-Alamo, B.; Schirmer, J.H.; Berti, A.; Blockmans, D.; Cid, M.C.; Holle, J.U.; Hollinger, N.; Karadag, O.; Kronbichler, A.; et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann. Rheum. Dis. 2024, 83, 30–47. [Google Scholar] [CrossRef]
- Emmi, G.; Bettiol, A.; Gelain, E.; Bajema, I.M.; Berti, A.; Burns, S.; Cid, M.C.; Tervaert, J.W.C.; Cottin, V.; Durante, E.; et al. Evidence-Based Guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat. Rev. Rheumatol. 2023, 19, 378–393. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Nair, P.; Terrier, B.; Walz, B.; Bourdin, A.; Jayne, D.R.; Jackson, D.J.; Roufosse, F.; Sjö, L.B.; Fan, Y.; et al. Benralizumab versus Mepolizumab for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2024, 390, 911–921. [Google Scholar] [CrossRef]
- Vega Villanueva, K.L.; Espinoza, L.R. Eosinophilic Vasculitis. Curr. Rheumatol. Rep. 2020, 22, 5. [Google Scholar] [CrossRef]
- Lyons, P.A.; Peters, J.E.; Alberici, F.; Liley, J.; Coulson, R.M.R.; Astle, W.; Baldini, C.; Bonatti, F.; Cid, M.C.; Elding, H.; et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat. Commun. 2019, 10, 5120. [Google Scholar] [CrossRef]
- Furuta, S.; Iwamoto, T.; Nakajima, H. Update on eosinophilic granulomatosis with polyangiitis. Allergol. Int. 2019, 68, 430–436. [Google Scholar] [CrossRef]
- Nishi, R.; Koike, H.; Ohyama, K.; Fukami, Y.; Ikeda, S.; Kawagashira, Y.; Iijima, M.; Katsuno, M.; Sobue, G. Differential clinicopathologic features of EGPA-associated neuropathy with and without ANCA. Neurology 2020, 94, e1726–e1737. [Google Scholar] [CrossRef]
- Watanabe, R.; Hashimoto, M. Eosinophilic Granulomatosis with Polyangiitis: Latest Findings and Updated Treatment Recommendations. J. Clin. Med. 2023, 12, 5996. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.C.; Munitz, A. Spatial adaptation of eosinophils and their emerging roles in homeostasis, infection and disease. Nat. Rev. Immunol. 2024, 24, 858–877. [Google Scholar] [CrossRef] [PubMed]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar] [CrossRef]
- Fricker, M.; Harrington, J.; Hiles, S.A.; Gibson, P.G. Mepolizumab depletes inflammatory but preserves homeostatic eosinophils in severe asthma. Allergy 2024, 79, 3118–3128. [Google Scholar] [CrossRef]
- Xenakis, J.J.; Howard, E.D.; Smith, K.M.; Olbrich, C.L.; Huang, Y.; Anketell, D.; Maldonado, S.; Cornwell, E.W.; Spencer, L.A. Resident intestinal eosinophils constitutively express antigen presentation markers and include two phenotypically distinct subsets of eosinophils. Immunology 2018, 154, 298–308. [Google Scholar] [CrossRef]
- Fagni, F.; Bello, F.; Emmi, G. Eosinophilic Granulomatosis With Polyangiitis: Dissecting the Pathophysiology. Front. Med. 2021, 8, 627776. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef]
- Spencer, L.A.; Bonjour, K.; Melo, R.C.N.; Weller, P.F. Eosinophil Secretion of Granule-Derived Cytokines. Front. Immunol. 2014, 5, 496. [Google Scholar] [CrossRef]
- Fukuchi, M.; Kamide, Y.; Ueki, S.; Miyabe, Y.; Konno, Y.; Oka, N.; Takeuchi, H.; Koyota, S.; Hirokawa, M.; Yamada, T.; et al. Eosinophil ETosis–Mediated Release of Galectin-10 in Eosinophilic Granulomatosis With Polyangiitis. Arthritis Rheumatol. 2021, 73, 1683–1693. [Google Scholar] [CrossRef]
- Nagase, H.; Ueki, S.; Fujieda, S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: Asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol. Int. 2020, 69, 178–186. [Google Scholar] [CrossRef]
- Roufosse, F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front. Med. 2018, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Hellmich, B.; Cid, M.C.; Jayne, D.; Tian, X.; Baylis, L.; Roufosse, F. Unmet needs and evidence gaps in hypereosinophilic syndrome and eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. 2023, 151, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nagase, H.; Sugimoto, N.; Yamamoto, S.; Tanaka, A.; Fukunaga, K.; Atsuta, R.; Tagaya, E.; Hojo, M.; Gon, Y.; et al. Mepolizumab decreased the levels of serum galectin-10 and eosinophil cationic protein in asthma. Asia Pac. Allergy 2021, 11, e31. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ueki, S.; Kamide, Y.; Miyabe, Y.; Fukuchi, M.; Yokoyama, Y.; Furukawa, T.; Azuma, N.; Oka, N.; Takeuchi, H.; et al. Increased Circulating Cell-Free DNA in Eosinophilic Granulomatosis With Polyangiitis: Implications for Eosinophil Extracellular Traps and Immunothrombosis. Front. Immunol. 2021, 12, 801897. [Google Scholar] [CrossRef]
- Antosz, K.; Batko, J.; Błażejewska, M.; Gawor, A.; Sleziak, J.; Gomułka, K. Insight into IL-5 as a Potential Target for the Treatment of Allergic Diseases. Biomedicines 2024, 12, 1531. [Google Scholar] [CrossRef]
- Molfino, N.A.; Gossage, D.; Kolbeck, R.; Parker, J.M.; Geba, G.P. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin. Exp. Allergy 2012, 42, 712–737. [Google Scholar] [CrossRef]
- Dahl, C.; Hoffmann, H.J.; Saito, H.; Schiøtz, P.O. Human mast cells express receptors for IL-3, IL-5 and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy 2004, 59, 1087–1096. [Google Scholar] [CrossRef]
- Ghazi, A.; Trikha, A.; Calhoun, W.J. Benralizumab—A humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity—A novel approach for the treatment of asthma. Expert Opin. Biol. Ther. 2012, 12, 113–118. [Google Scholar] [CrossRef]
- Jackson, D.J.; Wechsler, M.E.; Brusselle, G.; Buhl, R. Targeting the IL-5 pathway in eosinophilic asthma: A comparison of anti-IL-5 versus anti-IL-5 receptor agents. Allergy 2024, 79, 2943–2952. [Google Scholar] [CrossRef]
- Pavord, I.D.; Bel, E.H.; Bourdin, A.; Chan, R.; Han, J.K.; Keene, O.N.; Liu, M.C.; Martin, N.; Papi, A.; Roufosse, F.; et al. From DREAM to REALITI-A and beyond: Mepolizumab for the treatment of eosinophil-driven diseases. Allergy 2022, 77, 778–797. [Google Scholar] [CrossRef]
- Pitlick, M.M.; Li, J.T.; Pongdee, T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ. J. 2022, 15, 100676. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Fieldes, M.; Bourguignon, C.; Assou, S.; Nasri, A.; Fort, A.; Vachier, I.; De Vos, J.; Ahmed, E.; Bourdin, A. Targeted therapy in eosinophilic chronic obstructive pulmonary disease. ERJ Open Res. 2021, 7, 00437–2020. [Google Scholar] [CrossRef]
- Koga, Y.; Aoki-Saito, H.; Kamide, Y.; Sato, M.; Tsurumaki, H.; Yatomi, M.; Ishizuka, T.; Hisada, T. Perspectives on the Efficacy of Benralizumab for Treatment of Eosinophilic Granulomatosis with Polyangiitis. Front. Pharmacol. 2022, 13, 865318. [Google Scholar] [CrossRef]
- Dagher, R.; Kumar, V.; Copenhaver, A.M.; Gallagher, S.; Ghaedi, M.; Boyd, J.; Newbold, P.; Humbles, A.A.; Kolbeck, R. Novel mechanisms of action contributing to benralizumab’s potent anti-eosinophilic activity. Eur. Respir. J. 2022, 59, 2004306. [Google Scholar] [CrossRef]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’acqua, W.W.; Stephens, G.L.; et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353.e2. [Google Scholar]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; Fitzgerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; Yancey, S.W.; et al. Mepolizumab Treatment in Patients with Severe Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef]
- Bleecker, E.R.; FitzGerald, J.M.; Chanez, P.; Papi, A.; Weinstein, S.F.; Barker, P.; Sproule, S.; Gilmartin, G.; Aurivillius, M.; Werkström, V.; et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β(2)-agonists (SIROCCO): A randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016, 388, 2115–2127. [Google Scholar] [CrossRef]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar]
- Lai, K.; Sun, D.; Dai, R.; Samoro, R.; Park, H.-S.; Åstrand, A.; Cohen, D.; Jison, M.; Shih, V.H.; Werkström, V.; et al. Benralizumab efficacy and safety in severe asthma: A randomized trial in Asia. Respir. Med. 2024, 229, 107611. [Google Scholar] [CrossRef]
- Jackson, D.J.; Pelaia, G.; Emmanuel, B.; Tran, T.N.; Cohen, D.; Shih, V.H.; Shavit, A.; Arbetter, D.; Katial, R.; Rabe, A.P.J.; et al. Benralizumab in severe eosinophilic asthma by previous biologic use and key clinical subgroups: Real-world XALOC-1 programme. Eur. Respir. J. 2024, 64, 2301521. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Smidt-Hansen, T.; Pedersen, L.M.; Skjold, T.; Schmid, J. A real-life com-pari-son of patients with severe eosinophilic asthma treated with benralizumab or mepoli-zumab. Eur. Respir. J. 2023, 62 (Suppl. 67), PA631. [Google Scholar]
- Coumou, H.; Westerhof, G.A.; de Nijs, S.B.; Zwinderman, A.H.; Bel, E.H. Predictors of accelerated decline in lung function in adult-onset asthma. Eur. Respir. J. 2018, 51, 1701785. [Google Scholar] [CrossRef]
- Hancox, R.J.; Pavord, I.D.; Sears, M.R. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur. Respir. J. 2018, 51, 1702536. [Google Scholar] [CrossRef]
- Kayser, M.Z.; Drick, N.; Milger, K.; Fuge, J.; Kneidinger, N.; Korn, S.; Buhl, R.; Behr, J.; Welte, T.; Suhling, H. Real-World Multicenter Experience with Mepolizumab and Benralizumab in the Treatment of Uncontrolled Severe Eosinophilic Asthma Over 12 Months. J. Asthma Allergy 2021, 14, 863–871. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. Oral Glucocorticoid-Sparing Effect of Mepolizumab in Eosinophilic Asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M. Oral Glucocorticoid–Sparing Effect of Benralizumab in Severe Asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Gurnell, M.; Heaney, L.G.; Corren, J.; Bel, E.H.; Maspero, J.; Harrison, T.; Jackson, D.J.; Price, D.; Lugogo, N.; et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): A multicentre, open-label, single-arm study. Lancet Respir. Med. 2022, 10, 47–58. [Google Scholar] [CrossRef]
- Vervier, J.; Schleich, F.; Sabbe, M. Clinical Remission in Severe T2 high Asthma: A re-al-word analysis with Omalizumab, Mepolizumab and Benralizumab. Eur. Respir. J. 2024, 64 (Suppl. 68), PA1191. [Google Scholar]
- Bajbouj, K.; AbuJabal, R.; Sahnoon, L.; Olivenstein, R.; Mahboub, B.; Hamid, Q. IL-5 receptor expression in lung fibroblasts: Potential role in airway remodeling in asthma. Allergy 2023, 78, 882–885. [Google Scholar] [CrossRef]
- Domvri, K.; Tsiouprou, I.; Bakakos, P.; Steiropoulos, P.; Katsoulis, K.; Antoniou, K.M.; Papaioannou, A.I.; Rovina, N.; Katsaounou, P.; Papamitsou, T.; et al. Effect of mepolizumab in airway remodeling in patients with late-onset severe asthma with an eosinophilic phenotype. J. Allergy Clin. Immunol. 2024, 155, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Flood-Page, P.; Menzies-Gow, A.; Phipps, S.; Ying, S.; Wangoo, A.; Ludwig, M.S.; Barnes, N.; Robinson, D.; Kay, A.B. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J. Clin. Investig. 2003, 112, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Brightling, C.E.; Grainge, C.; Lambrecht, B.N.; Chanez, P. Airway remodelling in asthma and the epithelium: On the edge of a new era. Eur. Respir. J. 2024, 63, 2301619. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Marchi, M.; Caminati, M.; Romagnoli, M.; Micheletto, C.; Bonato, M.; Idotta, G.; Nizzetto, M.; D’alba, G.; Cavenaghi, M.; et al. Long-Term Eosinophil Depletion: A Real-World Perspective on the Safety and Durability of Benralizumab Treatment in Severe Eosinophilic Asthma. J. Clin. Med. 2024, 14, 191. [Google Scholar] [CrossRef]
- Busse, W.W.; Bleecker, E.R.; FitzGerald, J.M.; Ferguson, G.T.; Barker, P.; Sproule, S.; Olsson, R.; Martin, U.J.; Goldman, M.; Yañez, A.; et al. Long-term safety and efficacy of benralizumab in patients with severe, uncontrolled asthma: 1-year results from the BORA phase 3 extension trial. Lancet Respir. Med. 2019, 7, 46–59. [Google Scholar] [CrossRef]
- Terrier, B.; Jayne, D.R.; Hellmich, B.; Bentley, J.H.; Steinfeld, J.; Yancey, S.W.; Kwon, N.; Akuthota, P.; Khoury, P.; Baylis, L.; et al. Clinical Benefit of Mepolizumab in Eosinophilic Granulomatosis With Polyangiitis for Patients With and Without a Vasculitic Phenotype. ACR Open Rheumatol. 2023, 5, 354–363. [Google Scholar] [CrossRef]
- Jayne, D.R.; Terrier, B.; Hellmich, B.; Khoury, P.; Baylis, L.; Bentley, J.H.; Steinfeld, J.; Yancey, S.W.; Kwon, N.; Wechsler, M.E.; et al. Mepolizumab has clinical benefits including oral corticosteroid sparing irrespective of baseline EGPA characteristics. ERJ Open Res. 2024, 10, 00509–2023. [Google Scholar] [CrossRef]
- Khoury, P.; Silver, J.; Wolff, G.; Verghis, R.; Wechsler, M. Long-Term Safety of Mepolizumab in EGPA: Results From the Long-Term Access Programme. J. Allergy Clin. Immunol. 2024, 153 (Suppl. S2), AB372. [Google Scholar] [CrossRef]
- Wolff, G.; Wechsler, M.; Silver, J.; Price, R.; Verghis, R.; Weller, P.; Merkel, P.; Corbridge, T.; Khoury, P. Long-Term Safety of Mepolizumab in Eosinophilic Granulomatosis with Polyangiitis (EGPA): Pooled Results from Two Open-Label Extension Studies [abstract]. Arthritis Rheumatol. 2024, 76 (Suppl. S9), 5048–5050. [Google Scholar]
- Wechsler, M.E.; Silver, J.; Wolff, G.; Price, R.G.; Verghis, R.; Weller, P.F.; Merkel, P.A.; Khoury, P. Long-term use of mepolizumab in eosinophilic granulomatosis with polyangiitis: An open-label exten-sion study of the MIRRA trial. Arthritis Rheumatol. 2025, 77. in press. [Google Scholar]
- Khoury, P.; Silver, J.; Wolff, G.; Price, R.G.; Verghis, R.; Wechsler, M.E. Oral corticoster-oid-sparing effects of mepolizumab in eosinophilic granulomatosis with polyangiitis (EGPA): Results up to 7.4 years from the long-term access programme. Am. J. Respir. Crit. Care Med. 2024, 209, A1382. [Google Scholar]
- Bettiol, A.; Urban, M.L.; Dagna, L.; Cottin, V.; Franceschini, F.; Del Giacco, S.; Schiavon, F.; Neumann, T.; Lopalco, G.; Novikov, P.; et al. Mepolizumab for Eosinophilic Granulomatosis With Polyangiitis: A European Multicenter Observational Study. Arthritis Rheumatol. 2022, 74, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Watanabe, R.; Matsuda, S.; Kotani, T.; Okazaki, A.; Masuda, Y.; Yoshida, T.; Shoji, M.; Tsuge, R.; Kadoba, K.; et al. Factors associated with drug retention of mepolizumab in patients with eosinophilic granulomatosis with polyangiitis: A multicentre REVEAL cohort study. Mod. Rheumatol. 2024, 35, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Watanabe, R.; Matsuda, S.; Kotani, T.; Okazaki, A.; Masuda, Y.; Yoshida, T.; Shoji, M.; Tsuge, R.; Kadoba, K.; et al. Long-term efficacy of mepolizumab in patients with eosinophilic granulomatosis with polyangiitis: A propensity score matching analysis in the multicenter REVEAL cohort study. Front. Immunol. 2024, 15, 1457202. [Google Scholar] [CrossRef]
- Ishii, T.; Kunishige, H.; Kobayashi, T.; Hayashi, E.; Komatsubara, M.; Alfonso-Cristancho, R.; Tamaoki, J.; Howarth, P. Real-world safety and effectiveness of mepolizumab for patients with eosinophilic granulomatosis with polyangiitis in Japan: Long-term observation of the MARS study. Mod. Rheumatol. 2024, 2024, roae100. [Google Scholar] [CrossRef]
- Specks, U.; Wechsler, M.; Jackson, D.; Roufosse, F.; Egan, A.; Ivanov, S.; Jison, M.; Shavit, A.; Walton, C.; Merkel, P. Safety of Benralizumab in the treatment of eosinophilic granulomatosis with polyangiitis during the open-label extension period of the Phase 3 MANDARA study. Am. J. Respir. Crit. Care Med. 2024, 209, A5358. [Google Scholar]
- Bettiol, A.; Urban, M.L.; Padoan, R.; Groh, M.; Lopalco, G.; Egan, A.; Cottin, V.; Fraticelli, P.; Crimi, C.; Del Giacco, S.; et al. Benralizumab for eosinophilic granulomatosis with polyangiitis: A retrospective, multicentre, cohort study. Lancet Rheumatol. 2023, 5, e707–e715. [Google Scholar] [CrossRef]
- Spataro, F.; Solimando, A.G.; Di Girolamo, A.; Vacca, A.; Ria, R. Efficacy and safety of benralizumab in eosinophilic granulomatosis with polyangiitis: A meta-analysis of eight studies. Eur. J. Clin. Investig. 2025, 55, e14333. [Google Scholar] [CrossRef]
- Nolasco, S.; Portacci, A.; Campisi, R.; Buonamico, E.; Pelaia, C.; Benfante, A.; Triggiani, M.; Spadaro, G.; Caiaffa, M.F.; Scioscia, G.; et al. Effectiveness and safety of anti-IL-5/Rα biologics in eosinophilic granulomatosis with polyangiitis: A two-year multicenter observational study. Front. Immunol. 2023, 14, 1204444. [Google Scholar]
- Portacci, A.; Campisi, R.; Buonamico, E.; Nolasco, S.; Pelaia, C.; Crimi, N.; Benfante, A.; Triggiani, M.; Spadaro, G.; Caiaffa, M.F.; et al. Real-world characteristics of “super-responders” to mepolizumab and benralizumab in severe eosinophilic asthma and eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2023, 9, 00419–2023. [Google Scholar] [CrossRef]
- Nanzer, A.M.; Maynard-Paquette, A.-C.; Alam, V.; Green, L.; Thomson, L.; Lam, J.; Fernandes, M.; Roxas, C.; D’ancona, G.; Hearn, A.; et al. Long-Term Effectiveness of Benralizumab in Eosinophilic Granulomatosis with Polyangiitis. J. Allergy Clin. Immunol. Pract. 2024, 12, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Cottu, A.; Groh, M.; Desaintjean, C.; Marchand-Adam, S.; Guillevin, L.; Puechal, X.; Beaumesnil, S.; Lazaro, E.; Samson, M.; Taille, C.; et al. Benralizumab for eosinophilic granulomatosis with polyangiitis. Ann. Rheum. Dis. 2023, 82, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Desaintjean, C.; Ahmad, K.; Traclet, J.; Gerfaud-Valentin, M.; Durel, C.-A.; Glerant, J.-C.; Hot, A.; Lestelle, F.; Mainbourg, S.; Nasser, M.; et al. Mepolizumab and benralizumab in patients with severe asthma and a history of eosinophilic granulomatosis with polyangiitis. Front. Med. 2024, 11, 1341310. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Nair, P.; Terrier, B.; Walz, B.; Bourdin, A.; Jayne, D.R.W.; Jackson, D.J.; Roufosse, F.; Fan, Y.; Jison, M.; et al. The ef-fect of benralizumab versus mepolizumab on the depletion of eosinophils in the treat-ment of eosinophilic granulomatosis with polyangiitis during the Phase 3 MANDARA study. Am. J. Respir. Crit. Care Med. 2024, 209, A5364. [Google Scholar]
- Rodriguez-Suarez, E.; Cardner, M.; Wechsler, M.E.; Merkel, P.A.; Terrier, B.; Jayne, D.R.W.; Mccrae, C. Effect of treatment with benralizumab or mepolizumab on the serum proteome in eo-sinophilic granulomatosis with polyangiitis. Eur. Respir. J. 2024, 64 (Suppl. 68), PA828. [Google Scholar]
- Kuang, F.L.; Legrand, F.; Makiya, M.; Ware, J.; Wetzler, L.; Brown, T.; Magee, T.; Piligian, B.; Yoon, P.; Ellis, J.H.; et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N. Engl. J. Med. 2019, 380, 1336–1346. [Google Scholar] [CrossRef]
- Mukherjee, M.; Tan, N.S.; Huang, C.; Kim, B.; Radford, K.; Garrido, C.V.; Bhalla, A.; Rodríguez-Suárez, E.; Wechsler, M.; Walker, B.; et al. Late Breaking Abstract-Benralizumab reduces eosinophils and ANCA in sputum in EGPA. Eur. Respir. J. 2024, 64 (Suppl. S68), PA5372. [Google Scholar]
- Gigon, L.; Fettrelet, T.; Yousefi, S.; Simon, D.; Simon, H. Eosinophils from A to Z. Allergy 2023, 78, 1810–1846. [Google Scholar] [CrossRef]
- Jorssen, J.; Van Hulst, G.; Mollers, K.; Pujol, J.; Petrellis, G.; Baptista, A.P.; Schetters, S.; Baron, F.; Caers, J.; Lambrecht, B.N.; et al. Single-cell pro-teomics and transcriptomics resolve the development of human and murine eosino-phils and the impact of IL-5 neutralization on the expansion of their lineage. Eur. Respir. J. 2024, 64 (Suppl. S68), PA3527. [Google Scholar]
- Hellmich, B.; Wechsler, M.; Merkel, P.A.; Nair, P.; Bourdin, A.; Jayne, D.R.W.; Roufosse, F.; Sjö, L.B.; Fan, Y.; Maho, E.; et al. OP0188 Ef-fect of benralizumab versus mepolizumab on reduction in oral glucocorticoid use in patients with eosinophilic granulomatosis with polyangiitis. Ann. Rheum. Dis. 2024, 83, 186–187. [Google Scholar] [CrossRef]
- Wechsler, M.; Bourdin, A.; Chanez, P.; Jackson, D.; Siddiqui, S.; Specks, U.; Baudy, P.; Necander, S. Efficacy of benralizumab and mepolizumab on asthma outcomes in patients with eosinophilic granulomatosis with polyangiitis. Ann. Allergy Asthma Immunol. 2024, 133 (Suppl. S6), S49–S50. [Google Scholar] [CrossRef]
- Vultaggio, A.; Accinno, M.; Vivarelli, E.; Mecheri, V.; Maggiore, G.; Cosmi, L.; Parronchi, P.; Rossi, O.; Maggi, E.; Gallo, O.; et al. Blood CD62Llow inflammatory eosinophils are related to the severity of asthma and reduced by mepolizumab. Allergy 2023, 78, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E. A hidden residential cell in the lung. J. Clin. Investig. 2016, 126, 3185–3187. [Google Scholar] [CrossRef] [PubMed]
- Kamide, Y.; Sonehara, K.; Sekiya, K.; Ueki, S.; Nakamura, Y.; Okada, Y.; Taniguchi, M. Bioactive Mediator Profile of Mepolizumab-Treated Eosinophilic Granulomatosis With Polyangiitis. Allergy 2025, 80, 882–885. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Mukherjee, M.; Zhao, N.; Huang, C.; Radford, K.; Ashkar, A.A.; Nair, P. Asthma exacerbations on benralizumab are largely non-eosinophilic. Allergy 2021, 76, 375–379. [Google Scholar]
- Logan, J.; Wetherall, K.; Gillespie, L.; Mcconnachie, A.; Lee, W.T.; Burhan, H.; Brown, T.; Faruqi, S.; Jackson, D.J.; Kurukulaaratchy, R.; et al. Asthma Exacerbation Profile on open-label treatment with Benralizumab for severe eo-sino-philic asthma [BenRex]. Eur. Respir. J. 2024, 64 (Suppl. S68), PA5356. [Google Scholar]
- Hatchwell, L.; Collison, A.; Girkin, J.; Parsons, K.; Li, J.; Zhang, J.; Phipps, S.; Knight, D.; Bartlett, N.W.; Johnston, S.L.; et al. Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia. Thorax 2015, 70, 854–861. [Google Scholar] [CrossRef]
- Barra, J.; Liwski, C.R.; Phonchareon, P.; Portales-Cervantes, L.; Gaston, D.; Karakach, T.K.; Haidl, I.D.; Marshall, J.S. Interleukin-5 enhances human mast cell survival and interferon responses to viral infection. J. Allergy Clin. Immunol. 2025. [Google Scholar] [CrossRef]
- Bourdin, A.; Chupp, G.; Jackson, D.J.; Cohen, D.; Emerath, U.; Shavit, A.; Kurdyukova, Y.; Menzies-Gow, A. MELTEMI and COLUMBA: 5-Year Comparative Safety Analysis of Benralizumab and Mepolizumab. J. Allergy Clin. Immunol. Pract. 2024, 12, 2074–2083.e4. [Google Scholar] [CrossRef]
- Lustberg, M.E.; Clark, B. Anti-IL-5 treatment reduces infection-related adverse events: A meta-analysis of phase 3 clinical trials. J. Allergy Clin. Immunol. Pract. 2025, in press. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology 2018, 7, e1393134. [Google Scholar] [CrossRef] [PubMed]
- Sitek, A.; Chiarella, S.E.; Pongdee, T. Adverse effects of biologics used to treat asthma. Ther. Adv. Respir. Dis. 2025, 19, 17534666251319175. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Silver, J.; Wolff, G.; Price, R.G.; Verghis, R.; Weller, P.F.; A Merkel, P.; Khoury, P. EGPA Mepolizumab Open-Label Extension study group. Long-Term Safety and Efficacy of Mepolizumab in Eosinophilic Granulomatosis with Polyangiitis. Arthritis Rheumatol. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lepretre, F.; Gras, D.; Chanez, P.; Duez, C. Natural killer cells in the lung: Potential role in asthma and virus-induced exacerbation? Eur. Respir. Rev. 2023, 32, 230036. [Google Scholar]
- Poli, A.; Michel, T.; Thérésine, M.; Andrès, E.; Hentges, F.; Zimmer, J. CD56bright natural killer (NK) cells: An important NK cell subset. Immunology 2009, 126, 458–465. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Portillo, A.; Mukherjee, M.; Bhalla, A.; Son, K.; Ashkar, A.A.; Khalidi, N.; Nair, P. Benralizumab’s anti-eosinophil efficacy may be decreased by impaired NK cell activity. Eur. Respir. J. 2021, 59, 2102210. [Google Scholar] [CrossRef]
- Mehta, R.S.; Rezvani, K. Chimeric Antigen Receptor Expressing Natural Killer Cells for the Immunotherapy of Cancer. Front. Immunol. 2018, 9, 283. [Google Scholar] [CrossRef]
- Alvarado-Vazquez, P.A.; Mendez-Enriquez, E.; Salomonsson, M.; Kopac, P.; Koren, A.; Bidovec-Stojkovic, U.; Škrgat, S.; Simonson, O.E.; Yasinska, V.; Dahlén, S.-E.; et al. Targeting of the IL-5 pathway in severe asthma reduces mast cell progenitors. J. Allergy Clin. Immunol. 2024, 155, 1310–1320. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Marchewski, H.; Schwefel, G.; Stoll, P.; Virchow, J.C.; Bratke, K. Benralizumab strongly reduces blood basophils in severe eosinophilic asthma. Clin. Exp. Allergy 2020, 50, 1267–1269. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Rodrigo-Muñoz, J.M.; Lorente-Sorolla, C.; de Castro, Z.G.; Mínguez, P.; Cañas, J.A.; Valverde-Monge, M.; Bernaola, J.; Pinillos-Robles, E.J.; Betancor, D.; et al. Benralizumab reduces blood basophil percentage and activation in vitro without eliciting degranulation. Allergy 2024, 79, 2551–2553. [Google Scholar] [CrossRef]
- Theofani, E.; Tsitsopoulou, A.; Morianos, I.; Semitekolou, M. Severe Asthmatic Responses: The Impact of TSLP. Int. J. Mol. Sci. 2023, 24, 7581. [Google Scholar] [CrossRef] [PubMed]
- Poto, R.; Gambardella, A.R.; Marone, G.; Schroeder, J.T.; Mattei, F.; Schiavoni, G.; Varricchi, G. Basophils from allergy to cancer. Front. Immunol. 2022, 13, 1056838. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, M.C.; Saenz, S.A.; Hill, D.A.; Kim, B.S.; Headley, M.B.; Doering, T.A.; Wherry, E.J.; Jessup, H.K.; Siegel, L.A.; Kambayashi, T.; et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011, 477, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Brown, M.A.; Legros, G. Understanding the roles of basophils: Breaking dawn. Immunology 2012, 135, 192–197. [Google Scholar] [CrossRef]
- Calabrese, C.; Massaro, F.; Sola, A.; Balestrino, M.; De Falco, C.; Garziano, F.; Labruna, G.; Perna, F. The effects of Benralizumab on blood basophils in severe asthmatic patients: Preliminary results. Eur. Respir. J. 2024, 64 (Suppl. S68), PA2181. [Google Scholar]
- Shah, A.D.; Denaxas, S.; Nicholas, O.; Hingorani, A.D.; Hemingway, H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A CALIBER cohort study. Open Heart 2016, 3, e000477. [Google Scholar] [CrossRef]
- Xu, J.Y.; Xiong, Y.Y.; Tang, R.J.; Jiang, W.Y.; Ning, Y.; Gong, Z.T.; Huang, P.S.; Chen, G.H.; Wu, C.X.; Hu, M.J.; et al. Interleukin-5-induced eosinophil population improves cardiac function after myocardial infarction. Cardiovasc. Res. 2022, 118, 2165–2178. [Google Scholar] [CrossRef]
- Trovato, V.; Asada, A.; Fussner, L.; Curtis, C.; Kahwash, R. Interleukin-5 Antagonist Monoclonal Antibody Therapy Improves Symptoms and Reduces Steroid Dependence in Eosinophilic Myocarditis Patients. JACC Case Rep. 2024, 29, 102267. [Google Scholar] [CrossRef]
- Bettiol, A.; Sinico, R.A.; Schiavon, F.; Monti, S.; Bozzolo, E.P.; Franceschini, F.; Govoni, M.; Lunardi, C.; Guida, G.; Lopalco, G.; et al. Risk of acute arterial and venous thromboembolic events in eosinophilic granulomatosis with polyangiitis (Churg–Strauss syndrome). Eur. Respir. J. 2021, 57, 2004158. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Cariddi, A.; Lanzillotta, M.; Benanti, G.; Batani, V.; Moroni, L.; Matucci-Cerinic, M.; Dagna, L. IL-5 modu-lation and thrombotic risk in patients with eosinophilic granulomatosis with polyan-giitis: A proof-of-concept monocentric study. Ann. Rheum. Dis. 2024, 83 (Suppl. S1), POS1101. [Google Scholar]
- Quinta, J.-B.; Montastruc, F.; Sommet, A.; Touafchia, A.; Galinier, M.; Reber, L.; Rousseau, V.; Guilleminault, L. Cardiovascular adverse effects of anti–IL-5/IL-5Rα therapies: A real-world study. J. Allergy Clin. Immunol. Pract. 2021, 9, 1411–1413. [Google Scholar] [CrossRef]
- Harrison, T.; Canonica, G.W.; Chupp, G.; Lee, J.; Schleich, F.; Welte, T.; Valero, A.; Gemzoe, K.; Maxwell, A.; Joksaite, S.; et al. Real-world mepolizumab in the prospective severe asthma REALITI-A study: Initial analysis. Eur. Respir. J. 2020, 56, 2000151. [Google Scholar] [CrossRef]
- Liu, W.; Ma, X.; Zhou, W. Adverse events of benralizumab in moderate to severe eosinophilic asthma: A meta-analysis. Medicine 2019, 98, e15868. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.; Liu, M.; Kachler, K.; Perez, M.L.; Kirchner, P.; Kölle, J.; Gießl, A.; Rauber, S.; Song, R.; Aust, O.; et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 451–468. [Google Scholar] [CrossRef]
- Dean, N.J.; Clifton, I.J.; Salman, R.; Bridgewood, C.; Nam, J.; Macleod, T.; McGonagle, D.G. Anti-IL-5 biologics and rheumatoid arthritis: A single-centre 500 patient year exposure analysis. RMD Open 2023, 9, e003583. [Google Scholar] [CrossRef]
- Chen, M.-L.; Nopsopon, T.; Akenroye, A. Incidence of Anti-Drug Antibodies to Monoclonal Antibodies in Asthma: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2023, 11, 1475–1484.e20. [Google Scholar] [CrossRef]
- Ortega, H.G.; Meyer, E.; Brusselle, G.; Asano, K.; Prazma, C.M.; Albers, F.C.; Mallett, S.A.; Yancey, S.W.; Gleich, G.J. Update on immunogenicity in severe asthma: Experience with mepolizumab. J. Allergy Clin. Immunol. Pract. 2019, 7, 2469–2475.e1. [Google Scholar] [CrossRef]
- Lombardi, C.; Comberiati, P.; Ridolo, E.; Cottini, M.; Yacoub, M.R.; Casagrande, S.; Riccò, M.; Bottazzoli, M.; Berti, A. Anti-IL-5 Pathway Agents in Eosinophilic-Associated Disorders Across the Lifespan. Drugs 2024, 84, 661–684. [Google Scholar] [CrossRef]
- Jakes, R.W.; Kwon, N.; Huynh, L.; Hwee, J.; Baylis, L.; Alfonso-Cristancho, R.; Du, S.; Khanal, A.; Duh, M.S.; Terrier, B. Burden of eosinophilic granulomatosis with polyangiitis in Europe. ERJ Open Res. 2024, 10, 00912–2023. [Google Scholar] [CrossRef]
- Baek, S.; Khoury, P.; Maiorino, E.; Glass, K.; Carey, V.; Chase, R.; Stankiewicz, S.; Weller, P.; Langford, C.; Dyer, A.-M.; et al. A Transcriptomic Signature of Mepolizumab Response in Eosinophilic Granulomatosis With Polyangiitis (EGPA). Eur. Respir. J. 2023, 62 (Suppl. 67), A1318. [Google Scholar]
- O’leary, R.; Philipenko, B.; Ramsahai, J.M.; Mitchell, P. Mepolizumab to Benralizumab: A Case Series of a Beneficial Switch in Severe Asthma. Am. J. Respir. Crit. Care Med. 2024, 209, A5381. [Google Scholar] [CrossRef]
- Caminati, M.; Fassio, A.; Alberici, F.; Baldini, C.; Bello, F.; Cameli, P.; Conticini, E.; Cottin, V.; Crimi, C.; Dagna, L.; et al. Eosinophilic granulomatosis with polyangiitis onset in severe asthma patients on monoclonal antibodies targeting type 2 inflammation: Report from the European EGPA study group. Allergy 2023, 79, 516–519. [Google Scholar] [CrossRef]
- Rodríguez-Alcázar, J.F.; Ataide, M.A.; Engels, G.; Schmitt-Mabmunyo, C.; Garbi, N.; Kastenmüller, W.; Latz, E.; Franklin, B.S. Charcot–Leyden Crystals Activate the NLRP3 Inflammasome and Cause IL-1β Inflammation in Human Macrophages. J. Immunol. 2019, 202, 550–558. [Google Scholar] [CrossRef]
- Persson, E.K.; Verstraete, K.; Heyndrickx, I.; Gevaert, E.; Aegerter, H.; Percier, J.-M.; Deswarte, K.; Verschueren, K.H.G.; Dansercoer, A.; Gras, D.; et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science 2019, 364, 751. [Google Scholar] [CrossRef]
- Sim, M.S.; Kim, H.J.; Bae, I.; Kim, C.; Chang, H.S.; Choi, Y.; Lee, D.-H.; Park, H.-S.; Chung, I.Y. Calcium ionophore-activated platelets induce eosinophil extracellular trap formation. Allergol. Int. 2022, 72, 466–476. [Google Scholar] [CrossRef]
- Miyabe, Y.; Fukuchi, M.; Tomizawa, H.; Nakamura, Y.; Jikei, M.; Matsuwaki, Y.; Arima, M.; Konno, Y.; Moritoki, Y.; Takeda, M.; et al. Aggregated eosinophils and neutrophils characterize the properties of mucus in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2024, 153, 1306–1318. [Google Scholar] [CrossRef]
- Jarick, K.J.; Topczewska, P.M.; Jakob, M.O.; Yano, H.; Arifuzzaman, M.; Gao, X.; Boulekou, S.; Stokic-Trtica, V.; Leclère, P.S.; Preußer, A.; et al. Non-redundant functions of group 2 innate lymphoid cells. Nature 2022, 611, 794–800. [Google Scholar] [CrossRef]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef]
- Kotas, M.E.; Dion, J.; Van Dyken, S.; Ricardo-Gonzalez, R.R.; Danel, C.J.; Taillé, C.; Mouthon, L.; Locksley, R.M.; Terrier, B. A role for IL-33–activated ILC2s in eosinophilic vasculitis. J. Clin. Investig. 2021, 6, 143366. [Google Scholar] [CrossRef]
- Buchheit, K.M.; Shaw, D.; Chupp, G.; Lehtimaki, L.; Heffler, E.; Finney-Hayward, T.; Zangrilli, J.; Kwiatek, J.; Siddiqui, S.; Roufosse, F.; et al. Interleukin-5 as a pleiotropic cytokine orchestrating airway type 2 inflammation: Effects on and beyond eosinophils. Allergy 2024, 79, 2662–2679. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.-C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-L.; Qin, D.-Y.; Mo, Z.-M.; Li, Y.; Wei, W.; Wang, Y.-S.; Wang, W.; Wei, Y.-Q. Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci. China Life Sci. 2016, 59, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Kabata, H.; Ueki, S. Unveiling the Neuron-mediated Group 2 Innate Lymphoid Cell Activation in Human Asthma. Am. J. Respir. Crit. Care Med. 2024, 210, 701–703. [Google Scholar] [CrossRef]
- Han, J.; Wan, Q.; Seo, G.-Y.; Kim, K.; el Baghdady, S.; Lee, J.H.; Kronenberg, M.; Liu, Y.-C. Hypoxia induces adrenomedullin from lung epithelia, stimulating ILC2 inflammation and immunity. J. Exp. Med. 2022, 219, e20211985. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Li, X.; Zheng, G.; Wang, T.; Sun, H.; Huang, Z.; He, J.; Qiu, J.; Zhao, Z.; et al. Blocking the HIF-1α/glycolysis axis inhibits allergic airway inflammation by reducing ILC2 metabolism and function. Allergy 2024. ahead of print. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).