Randomized Controlled Clinical Trial of Pediatric Pneumococcus and Hepatitis A Vaccinations With or Without a High-Dose Oral Vitamin A Supplement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Food Diary Acquisition and Analyses
2.3. Immune Assays
2.4. Total Serum Immunoglobulin Isotype Analyses
2.5. CRP Assay
2.6. Statistical Methods
3. Results
3.1. Vitamin A and D Levels in Young, Healthy Children in the PCVIT Study
3.2. Vitamin-Fortified Foods in Younger Children
3.3. Pneumococcus Vaccine-Induced Immune Responses
3.4. Hepatitis A Vaccine Immune Responses
3.5. Influences of Supplementation in This Predominantly Retinol-Sufficient Pediatric Population
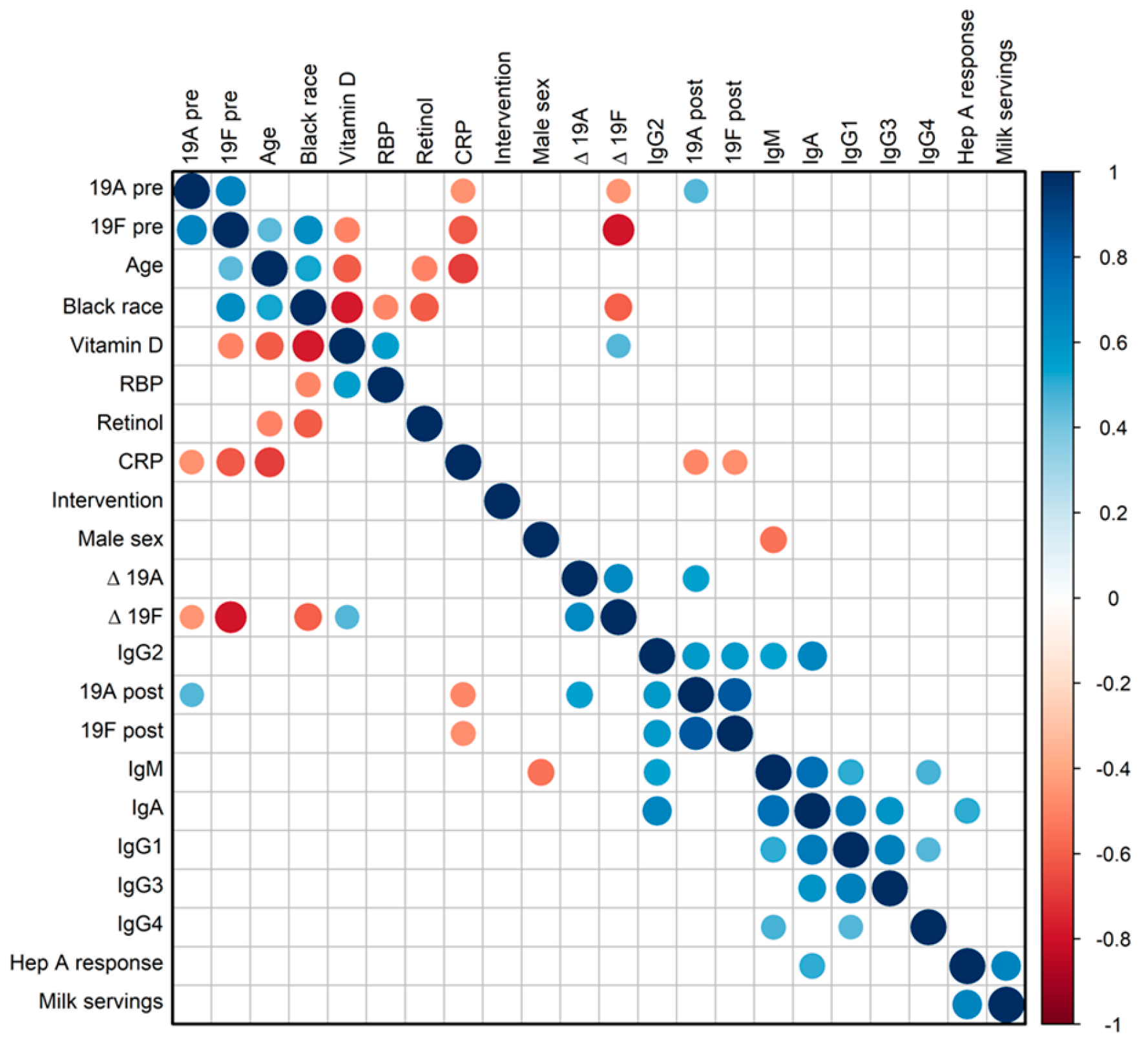
3.6. Total Immunoglobulin Isotypes and CRP Levels at Baseline
4. Discussion
4.1. Vitamin Levels Among PCVIT Participants
4.2. Vaccine-Induced Immune Responses Among PCVIT Participants
4.3. No Benefit of Vitamin A Supplementation Revealed Among Healthy 1–2 Year Old Children with Sufficient Levels of Retinol at Baseline
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudraraju, R.; Jones, B.G.; Surman, S.L.; Sealy, R.E.; Thomas, P.G.; Hurwitz, J.L. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS ONE 2014, 9, e86554. [Google Scholar] [CrossRef] [PubMed]
- Napoli, J.L. Functions of Intracellular Retinoid Binding-Proteins. Subcell. Biochem. 2016, 81, 21–76. [Google Scholar] [CrossRef] [PubMed]
- Mora, J.R.; Iwata, M.; Von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Dhokia, V.; Macip, S. A master of all trades—Linking retinoids to different signalling pathways through the multi-purpose receptor STRA6. Cell. Death Discov. 2021, 7, 358. [Google Scholar] [CrossRef]
- Berry, D.C.; Levi, L.; Noy, N. Holo-retinol-binding protein and its receptor STRA6 drive oncogenic transformation. Cancer Res. 2014, 74, 6341–6351. [Google Scholar] [CrossRef]
- Jones, B.G.; Penkert, R.R.; Surman, S.L.; Sealy, R.E.; Hurwitz, J.L. Nuclear Receptors, Ligands and the Mammalian B Cell. Int. J. Mol. Sci. 2020, 21, 4997. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, Y.; Tokuyama, H. Retinoic acid and steroid hormones regulate IgA production by LPS-stimulated murine spleen cells. Immunopharmacology 1994, 28, 145–151. [Google Scholar] [CrossRef]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef]
- Fadel, L.; Reho, B.; Volko, J.; Bojcsuk, D.; Kolostyak, Z.; Nagy, G.; Muller, G.; Simandi, Z.; Hegedus, E.; Szabo, G.; et al. Agonist binding directs dynamic competition among nuclear receptors for heterodimerization with retinoid X receptor. J. Biol. Chem. 2020, 295, 10045–10061. [Google Scholar] [CrossRef]
- Rastinejad, F. Allosteric communications between domains of nuclear receptors. Steroids 2025, 214, 109551. [Google Scholar] [CrossRef]
- Penkert, R.R.; Rowe, H.M.; Surman, S.L.; Sealy, R.E.; Rosch, J.; Hurwitz, J.L. Influences of Vitamin A on Vaccine Immunogenicity and Efficacy. Front. Immunol. 2019, 10, 1576. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Gangopadhyay, N.N.; Moldoveanu, Z.; Mestecky, J.; Stephensen, C.B. Vitamin A is required for regulation of polymeric immunoglobulin receptor (pIgR) expression by interleukin-4 and interferon-gamma in a human intestinal epithelial cell line. J. Nutr. 1998, 128, 1063–1069. [Google Scholar] [CrossRef]
- Cassani, B.; Villablanca, E.J.; De Calisto, J.; Wang, S.; Mora, J.R. Vitamin A and immune regulation: Role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Asp. Med. 2012, 33, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H. Retinoic acid, immunity, and inflammation. Vitam. Horm. 2011, 86, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.Y.; Jang, Y.S.; Kim, J.; Choe, J.; Han, H.J.; Lee, J.M.; Kang, S.H.; Rhee, K.J.; Park, S.R.; Kim, W.S.; et al. Retinoic acid acts as a selective human IgA switch factor. Hum. Immunol. 2014, 75, 923–929. [Google Scholar] [CrossRef]
- Aotsuka, Y.; Naito, M. Enhancing effects of retinoic acid on monoclonal antibody production of human-human hybridomas. Cell. Immunol. 1991, 133, 498–505. [Google Scholar] [CrossRef]
- Israel, H.; Odziemiec, C.; Ballow, M. The effects of retinoic acid on immunoglobulin synthesis by human cord blood mononuclear cells. Clin. Immunol. Immunopathol. 1991, 59, 417–425. [Google Scholar] [CrossRef]
- Treptow, S.; Grun, J.; Scholz, J.; Radbruch, A.; Heine, G.; Worm, M. 9-cis Retinoic acid and 1.25-dihydroxyvitamin D3 drive differentiation into IgA(+) secreting plasmablasts in human naive B cells. Eur. J. Immunol. 2021, 51, 125–137. [Google Scholar] [CrossRef]
- Penkert, R.R.; Jones, B.G.; Hacker, H.; Partridge, J.F.; Hurwitz, J.L. Vitamin A differentially regulates cytokine expression in respiratory epithelial and macrophage cell lines. Cytokine 2017, 91, 1–5. [Google Scholar] [CrossRef]
- Hurwitz, J.L.; Penkert, R.R.; Xu, B.; Fan, Y.; Partridge, J.F.; Maul, R.W.; Gearhart, P.J. Hotspots for Vitamin-Steroid-Thyroid Hormone Response Elements Within Switch Regions of Immunoglobulin Heavy Chain Loci Predict a Direct Influence of Vitamins and Hormones on B Cell Class Switch Recombination. Viral. Immunol. 2016, 29, 132–136. [Google Scholar] [CrossRef]
- Mader, S.; Leroy, P.; Chen, J.Y.; Chambon, P. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J. Biol. Chem. 1993, 268, 591–600. [Google Scholar] [PubMed]
- Mora, J.R.; von Andrian, U.H. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin. Immunol. 2009, 21, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, Y.; Tokuyama, H. Retinoids as Ig isotype-switch modulators. The role of retinoids in directing isotype switching to IgA and IgG1 (IgE) in association with IL-4 and IL-5. Cell. Immunol. 1996, 170, 230–234. [Google Scholar] [CrossRef]
- Jones, B.G.; Oshansky, C.M.; Bajracharya, R.; Tang, L.; Sun, Y.; Wong, S.S.; Webby, R.; Thomas, P.G.; Hurwitz, J.L. Retinol binding protein and vitamin D associations with serum antibody isotypes, serum influenza virus-specific neutralizing activities and airway cytokine profiles. Clin. Exp. Immunol. 2016, 183, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Surman, S.L.; Jones, B.G.; Sealy, R.E.; Rudraraju, R.; Hurwitz, J.L. Oral retinyl palmitate or retinoic acid corrects mucosal IgA responses toward an intranasal influenza virus vaccine in vitamin A deficient mice. Vaccine 2014, 32, 2521–2524. [Google Scholar] [CrossRef]
- Sommer, A. Vitamin A, infectious disease, and childhood mortality: A 2 cent solution? J. Infect. Dis. 1993, 167, 1003–1007. [Google Scholar] [CrossRef]
- Chesney, R.W. Vitamin D and The Magic Mountain: The anti-infectious role of the vitamin. J. Pediatr. 2010, 156, 698–703. [Google Scholar] [CrossRef]
- Patel, N.; Penkert, R.R.; Jones, B.G.; Sealy, R.E.; Surman, S.L.; Sun, Y.; Tang, L.; DeBeauchamp, J.; Webb, A.; Richardson, J.; et al. Baseline Serum Vitamin A and D Levels Determine Benefit of Oral Vitamin A&D Supplements to Humoral Immune Responses Following Pediatric Influenza Vaccination. Viruses 2019, 11, 907. [Google Scholar] [CrossRef]
- Surman, S.L.; Rudraraju, R.; Sealy, R.; Jones, B.; Hurwitz, J.L. Vitamin A deficiency disrupts vaccine-induced antibody-forming cells and the balance of IgA/IgG isotypes in the upper and lower respiratory tract. Viral. Immunol. 2012, 25, 341–344. [Google Scholar] [CrossRef]
- Haselow, N.J.; Joshi, V.; Bayo, P.N.; Murye, J.W.; Shaban, S.N.; Abebe, K.T.; Kassim, I.; Shiweredo, T.; Vinathan, H.; Jaiswal, C.P.; et al. A Review of Vitamin A Supplementation in South Sudan: Successes, Challenges, and Opportunities for the Way Forward. Glob. Health Sci. Pract. 2022, 10, e2100660. [Google Scholar] [CrossRef]
- Stephensen, C.B.; Lietz, G. Vitamin A in resistance to and recovery from infection: Relevance to SARS-CoV2. Br. J. Nutr. 2021, 126, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Dzopalic, T.; Bozic-Nedeljkovic, B.; Jurisic, V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Cent. Eur. J. Immunol. 2021, 46, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.A. Retinoic acid homeostasis and disease. Curr. Top. Dev. Biol. 2025, 161, 201–233. [Google Scholar] [CrossRef] [PubMed]
- Ramanarayanan, P.; Heine, G.; Worm, M. Vitamin A and vitamin D induced nuclear hormone receptor activation and its impact on B cell differentiation and immunoglobulin production. Immunol. Lett. 2023, 263, 80–86. [Google Scholar] [CrossRef]
- World Health Organization, Children 6–59 Months Receiving Vitamin A Supplements. Available online: https://www.who.int/data/nutrition/nlis/info/children-6-59-months-receiving-vitamin-a-supplements (accessed on 26 January 2025).
- Vitamin A supplementation: Who, when and how. Community Eye Health 2013, 26, 71.
- Imdad, A.; Ahmed, Z.; Bhutta, Z.A. Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age. Cochrane Database Syst. Rev. 2016, 9, CD007480. [Google Scholar] [CrossRef]
- Imdad, A.; Mayo-Wilson, E.; Haykal, M.R.; Regan, A.; Sidhu, J.; Smith, A.; Bhutta, Z.A. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst. Rev. 2022, 3, CD008524. [Google Scholar] [CrossRef]
- National Foundation for Infectious Diseases, Call to Action; Vitamin A for the Management of Measles in the United States. Available online: https://www.nfid.org/infectious-disease/measles (accessed on 26 January 2025).
- Manapurath, R.M.; Kumar, M.; Pathak, B.G.; Chowdhury, R.; Sinha, B.; Choudhary, T.; Chandola, N.; Mazumdar, S.; Taneja, S.; Bhandari, N.; et al. Enteral Low-Dose Vitamin A Supplementation in Preterm or Low Birth Weight Infants to Prevent Morbidity and Mortality: A Systematic Review and Meta-analysis. Pediatrics 2022, 150, e2022057092L. [Google Scholar] [CrossRef]
- Su, J.; Li, T.; Pan, H. Association of vitamin A supplementation with immune-related allergic diseases: A meta-analysis. Front. Nutr. 2022, 9, 984161. [Google Scholar] [CrossRef]
- Ishaq, M.U.; Kunwar, D.; Qadeer, A.; Komel, A.; Safi, A.; Malik, A.; Malik, L.; Akbar, A. Effect of vitamin A on maternal, fetal, and neonatal outcomes: An overview of deficiency, excessive intake, and intake recommendations. Nutr. Clin. Pract. 2024, 39, 373–384. [Google Scholar] [CrossRef]
- Semba, R.D.; Munasir, Z.; Beeler, J.; Akib, A.; Muhilal; Audet, S.; Sommer, A. Reduced seroconversion to measles in infants given vitamin A with measles vaccination. Lancet 1995, 345, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Bresee, J.S.; Fischer, M.; Dowell, S.F.; Johnston, B.D.; Biggs, V.M.; Levine, R.S.; Lingappa, J.R.; Keyserling, H.L.; Petersen, K.M.; Bak, J.R.; et al. Vitamin A therapy for children with respiratory syncytial virus infection: A multicenter trial in the United States. Pediatr. Infect. Dis. J. 1996, 15, 777–782. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Ross, A.C.; Moran, N.E. Our Current Dietary Reference Intakes for Vitamin A-Now 20 Years Old. Curr. Dev. Nutr. 2020, 4, nzaa096. [Google Scholar] [CrossRef]
- Di Muzio, E.; Polticelli, F.; di Masi, A.; Fanali, G.; Fasano, M.; Ascenzi, P. All-trans-retinoic acid and retinol binding to the FA1 site of human serum albumin competitively inhibits heme-Fe(III) association. Arch Biochem. Biophys. 2016, 590, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Almekinder, J.; Manda, W.; Soko, D.; Lan, Y.; Hoover, D.R.; Semba, R.D. Evaluation of plasma retinol-binding protein as a surrogate measure for plasma retinol concentrations. Scand. J. Clin. Lab. Investig. 2000, 60, 199–203. [Google Scholar] [CrossRef]
- Egea, P.F.; Rochel, N.; Birck, C.; Vachette, P.; Timmins, P.A.; Moras, D. Effects of ligand binding on the association properties and conformation in solution of retinoic acid receptors RXR and RAR. J. Mol. Biol. 2001, 307, 557–576. [Google Scholar] [CrossRef]
- de Pee, S.; Dary, O. Biochemical indicators of vitamin A deficiency: Serum retinol and serum retinol binding protein. J. Nutr. 2002, 132, 2895S–2901S. [Google Scholar] [CrossRef]
- Rosen, C.J.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; Kovacs, C.S.; et al. IOM committee members respond to Endocrine Society vitamin D guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1146–1152. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Schleicher, R.L.; Haynes, B.M.; Rybak, M.E.; Pirkle, J.L. The CDC’s Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population is a valuable tool for researchers and policy makers. J. Nutr. 2013, 143, 938S–947S. [Google Scholar] [CrossRef]
- McCartney, C.R.; McDonnell, M.E.; Corrigan, M.D.; Lash, R.W. Vitamin D Insufficiency and Epistemic Humility: An Endocrine Society Guideline Communication. J. Clin. Endocrinol. Metab. 2024, 109, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Torres-Gonzalez, M.; Cifelli, C.J.; Agarwal, S.; Fulgoni, V.L., 3rd. Association of Milk Consumption and Vitamin D Status in the US Population by Ethnicity: NHANES 2001–2010 Analysis. Nutrients 2020, 12, 3720. [Google Scholar] [CrossRef]
- Berger, R.; Just, M.; Althaus, B. Time course of hepatitis A antibody production after active, passive and active/passive immunisation: The results are highly dependent on the antibody test system used. J. Virol. Methods 1993, 43, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Forst, C.V.; Chung, M.; Hockman, M.; Lashua, L.; Adney, E.; Hickey, A.; Carlock, M.; Ross, T.; Ghedin, E.; Gresham, D. Vaccination History, Body Mass Index, Age, and Baseline Gene Expression Predict Influenza Vaccination Outcomes. Viruses 2022, 14, 2446. [Google Scholar] [CrossRef]
- Takano, T.; Morikawa, M.; Adachi, Y.; Kabasawa, K.; Sax, N.; Moriyama, S.; Sun, L.; Isogawa, M.; Nishiyama, A.; Onodera, T.; et al. Distinct immune cell dynamics correlate with the immunogenicity and reactogenicity of SARS-CoV-2 mRNA vaccine. Cell. Rep. Med. 2022, 3, 100631. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Scott, M.K.D.; Hagan, T.; Li, C.; Feng, Y.; Wimmers, F.; Grigoryan, L.; Trisal, M.; Edara, V.V.; Lai, L.; et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 2021, 596, 410–416. [Google Scholar] [CrossRef]
- Andersen-Nissen, E.; Fiore-Gartland, A.; Ballweber Fleming, L.; Carpp, L.N.; Naidoo, A.F.; Harper, M.S.; Voillet, V.; Grunenberg, N.; Laher, F.; Innes, C.; et al. Innate immune signatures to a partially-efficacious HIV vaccine predict correlates of HIV-1 infection risk. PLoS Pathog. 2021, 17, e1009363. [Google Scholar] [CrossRef]
- Wu, S.; Pushalkar, S.; Maity, S.; Pressler, M.; Rendleman, J.; Vitrinel, B.; Carlock, M.; Ross, T.; Choi, H.; Vogel, C. Proteomic Signatures of the Serological Response to Influenza Vaccination in a Large Human Cohort Study. Viruses 2022, 14, 2479. [Google Scholar] [CrossRef]
- Wu, S.; Ross, T.M.; Carlock, M.A.; Ghedin, E.; Choi, H.; Vogel, C. Evaluation of determinants of the serological response to the quadrivalent split-inactivated influenza vaccine. Mol. Syst. Biol. 2022, 18, e10724. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Fulbright, J.W.; Crowson, C.S.; Poland, G.A.; O’Fallon, W.M.; Weyand, C.M. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 2001, 75, 12182–12187. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Poland, G.A. Vaccinomics, predictive vaccinology and the future of vaccine development. Future Microbiol. 2010, 5, 1757–1760. [Google Scholar] [CrossRef]
- Team, H.-C.S.P.; Consortium, H.-I. Multicohort analysis reveals baseline transcriptional predictors of influenza vaccination responses. Sci. Immunol. 2017, 2, eaal4656. [Google Scholar] [CrossRef]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef]
- Patel, N.; Penkert, R.R.; Sealy, R.E.; Surman, S.L.; Jones, B.G.; Ringwald-Smith, K.; Ross, A.C.; Hurwitz, J.L. Retinol Binding Protein, Sunlight Hours, and the Influenza Virus-Specific Immune Response. Biomedicines 2022, 10, 2322. [Google Scholar] [CrossRef]
- Picciano, M.F.; Smiciklas-Wright, H.; Birch, L.L.; Mitchell, D.C.; Murray-Kolb, L.; McConahy, K.L. Nutritional guidance is needed during dietary transition in early childhood. Pediatrics 2000, 106, 109–114. [Google Scholar] [CrossRef]
- Mannino, M.L.; Lee, Y.; Mitchell, D.C.; Smiciklas-Wright, H.; Birch, L.L. The quality of girls’ diets declines and tracks across middle childhood. Int. J. Behav. Nutr. Phys. Act. 2004, 1, 5. [Google Scholar] [CrossRef]
- Hamner, H.C.; Moore, L.V. Dietary quality among children from 6 months to 4 years, NHANES 2011–2016. Am. J. Clin. Nutr. 2020, 111, 61–69. [Google Scholar] [CrossRef]
- Burnett, A.J.; Russell, C.G.; Farrow, C.; Spence, A.C.; Worsley, A.; Lacy, K.E. The effects of age on associations between pre-school children’s eating behaviour traits and diet quality. Appetite 2024, 203, 107675. [Google Scholar] [CrossRef]
- Penkert, R.R.; Patel, N.; Webby, R.J.; Ross, T.M.; Hurwitz, J.L. Month of Influenza Virus Vaccination Influences Antibody Responses in Children and Adults. Vaccines 2021, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Gavi, S.; Qurashi, S.; Stuart, L.M.; Lau, R.; Melendez, M.M.; Mynarcik, D.C.; McNurlan, M.A.; Gelato, M.C. Influence of age on the association of retinol-binding protein 4 with metabolic syndrome. Obesity 2008, 16, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, I.; Biebinger, R.; Lehmann, R.; L’Allemand, D.; Spinas, G.A.; Zimmermann, M.B. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J. Clin. Endocrinol. Metab. 2007, 92, 4359–4365. [Google Scholar] [CrossRef]
- Larson, L.M.; Guo, J.; Williams, A.M.; Young, M.F.; Ismaily, S.; Addo, O.Y.; Thurnham, D.; Tanumihardjo, S.A.; Suchdev, P.S.; Northrop-Clewes, C.A. Approaches to Assess Vitamin A Status in Settings of Inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) Project. Nutrients 2018, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Larson, L.M.; Namaste, S.M.; Williams, A.M.; Engle-Stone, R.; Addo, O.Y.; Suchdev, P.S.; Wirth, J.P.; Temple, V.; Serdula, M.; Northrop-Clewes, C.A. Adjusting retinol-binding protein concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am. J. Clin. Nutr. 2017, 106, 390S–401S. [Google Scholar] [CrossRef]
- Henze, A.; Frey, S.K.; Raila, J.; Scholze, A.; Spranger, J.; Weickert, M.O.; Tepel, M.; Zidek, W.; Schweigert, F.J. Alterations of retinol-binding protein 4 species in patients with different stages of chronic kidney disease and their relation to lipid parameters. Biochem. Biophys. Res. Commun. 2010, 393, 79–83. [Google Scholar] [CrossRef]
- Rosales, F.J.; Topping, J.D.; Smith, J.E.; Shankar, A.H.; Ross, A.C. Relation of serum retinol to acute phase proteins and malarial morbidity in Papua New Guinea children. Am. J. Clin. Nutr. 2000, 71, 1582–1588. [Google Scholar] [CrossRef]
- Chu, C.H.; Lam, H.C.; Lee, J.K.; Lu, C.C.; Sun, C.C.; Cheng, H.J.; Wang, M.C.; Chuang, M.J. Elevated serum retinol-binding protein 4 concentrations are associated with chronic kidney disease but not with the higher carotid intima-media thickness in type 2 diabetic subjects. Endocr. J. 2011, 58, 841–847. [Google Scholar] [CrossRef]
- Jones, B.G.; Penkert, R.R.; Xu, B.; Fan, Y.; Neale, G.; Gearhart, P.J.; Hurwitz, J.L. Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol. Immunol. 2016, 77, 97–102. [Google Scholar] [CrossRef]
- Scaggs Huang, F.; Bernstein, D.I.; Slobod, K.S.; Portner, A.; Takimoto, T.; Russell, C.J.; Meagher, M.; Jones, B.G.; Sealy, R.E.; Coleclough, C.; et al. Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults. Hum. Vaccin. Immunother. 2021, 17, 554–559. [Google Scholar] [CrossRef]
- Schlenz, H.; Intemann, T.; Wolters, M.; Gonzalez-Gil, E.M.; Nappo, A.; Fraterman, A.; Veidebaum, T.; Molnar, D.; Tornaritis, M.; Sioen, I.; et al. C-reactive protein reference percentiles among pre-adolescent children in Europe based on the IDEFICS study population. Int. J. Obes. 2014, 38 (Suppl. S2), S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.M.; Burke, J.S.t.; Roederer, A.L.; Gorman, M.J.; Mundle, S.; Lingwood, D.; Delagrave, S.; Sridhar, S.; Ross, T.M.; Kleanthous, H.; et al. Pre-existing Fc profiles shape the evolution of neutralizing antibody breadth following influenza vaccination. Cell. Rep. Med. 2023, 4, 100975. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, C.M.; Alter, G. Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine. Front. Immunol. 2019, 10, 440. [Google Scholar] [CrossRef]
- Dotzauer, A.; Brenner, M.; Gebhardt, U.; Vallbracht, A. IgA-coated particles of Hepatitis A virus are translocalized antivectorially from the apical to the basolateral site of polarized epithelial cells via the polymeric immunoglobulin receptor. J. Gen. Virol. 2005, 86, 2747–2751. [Google Scholar] [CrossRef]
- Tami, C.; Silberstein, E.; Manangeeswaran, M.; Freeman, G.J.; Umetsu, S.E.; DeKruyff, R.H.; Umetsu, D.T.; Kaplan, G.G. Immunoglobulin A (IgA) is a natural ligand of hepatitis A virus cellular receptor 1 (HAVCR1), and the association of IgA with HAVCR1 enhances virus-receptor interactions. J. Virol. 2007, 81, 3437–3446. [Google Scholar] [CrossRef]


| PCVIT Participant | Sex | Race # | Age (Years) | Enrollment Years * | Vaccine | Group & | Retinol (µg/mL) | RBP (ng/mL) | Vitamin D (ng/mL) | Daily Milk Servings in Liquid Cup Equivalents |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | B | 1.48 | 2019–2020 | Prevnar 13 | Control | 0.31 | 26,066 | 29 | 0 |
| 2 | M | B | 1.48 | 2019–2020 | Prevnar 13 | Test | 0.46 | 29,234 | 28 | 0 |
| 3 | M | B | 1.03 | 2019–2020 | Prevnar 13 | Test | 0.39 | 15,492 | 36 | NA |
| 4 | F | B | 1.23 | 2019–2020 | Prevnar 13 | Test | 0.36 | 34,045 | 33 | NA |
| 5 | M | W | 1.01 | 2019–2020 | Prevnar 13 | Control | 0.54 | 29,702 | 44 | NA |
| 6 | M | B | 1.08 | 2019–2020 | Prevnar 13 | Control | 0.27 | 24,149 | 30 | NA |
| 7 | M | B | 1.18 | 2019–2020 | Prevnar 13 | Test | 0.49 | 22,568 | 32 | NA |
| 8 | F | B | 3.01 | 2019–2020 | Prevnar 13 | Test | 0.47 | 21,425 | 20 | NA |
| 9 | F | B | 1.89 | 2019–2020 | Prevnar 13 | Control | 0.35 | 21,514 | 33 | NA |
| 10 | M | B | 2.66 | 2019–2020 | Prevnar 13 | Control | 0.49 | 19,480 | 16 | NA |
| 11 | M | B | 1.12 | 2023–2024 | Prevnar 13 | Test | 0.50 | 13,755 | 25 | 2.79 |
| 12 | M | B | 1.22 | 2023–2024 | Prevnar 20 | Control | 0.51 | 20,990 | 28 | 1.06 |
| 13 | F | B | 1.01 | 2023–2024 | Prevnar 20 | Control | 0.56 | 27,472 | 27 | 0.06 |
| 14 | M | W | 1.11 | 2023–2024 | Prevnar 20 | Test | 0.47 | 25,277 | 38 | 0.47 |
| 15 | M | W | 1.11 | 2023–2024 | Prevnar 20 | Test | 0.51 | 21,986 | 33 | 0.47 |
| 16 | M | W | 1.08 | 2023–2024 | Prevnar 20 | Control | 0.53 | 26,599 | 43 | 3.25 |
| 17 | F | W | 1.13 | 2023–2024 | Prevnar 20 | Test | 0.57 | 29,952 | 46 | 1.16 |
| 18 | F | W | 1.04 | 2023–2024 | Prevnar 20 | Control | 0.65 | 26,399 | 44 | 2.63 |
| 19 | M | W | 1.06 | 2023–2024 | Prevnar 20 | Test | 0.50 | 29,526 | 35 | 2.39 |
| 20 | F | B | 2.70 | 2023–2024 | Prevnar 20 | Control | NA | 17,931 | 16 | 2.39 |
| PCVIT Participant | Pre-19A | Post-19A | Δ19A * | Pre-19F | Post-19F | Δ19F * | Pre-Hep A | Post-Hep A | Δ-Hep A |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.57 | 10.74 | 18.84 (+) | 4.32 | 27.26 | 6.3 (+) | Negative | Negative | Negative |
| 2 | 1.34 | 3.09 | 2.31 (+) | 5.97 | 60.85 | 10.2 (+) | NA | NA | NA |
| 3 | 2.03 | 10.75 | 5.30 (+) | 3.8 | 96.6 | 25.4 (+) | Negative | Positive | Positive |
| 4 | 0.34 | 2.41 | 7.09 (+) | 0.4 | 21.95 | 54.9 (+) | Negative | Positive | Positive |
| 5 | 1.07 | 14.96 | 13.98 (+) | 0.87 | 112.96 | 129.8 (+) | Negative | Positive | Positive |
| 6 | 2.05 | 2.44 | 1.19 (+) | 15.48 | 15.19 | 1 (+) | Negative | Positive | Positive |
| 7 | 0.52 | 1.1 | 2.12 (+) | 1.51 | 5.12 | 3.4 (+) | NA | NA | NA |
| 8 | 0.85 | 15.45 | 18.18 (+) | 3.15 | 112.96 | 35.9 (+) | Positive | Positive | Unknown |
| 9 | 4.96 | 21.17 | 4.27 (+) | 58.55 | 112.96 | 1.9 (+) | Positive | Positive | Unknown |
| 10 | 0.86 | 15.59 | 18.13 (+) | 7.95 | 112.96 | 14.2 (+) | Negative | Positive | Positive |
| 11 | 0.55 | 3.68 | 6.69 (+) | 1.8 | 23.22 | 12.9 (+) | Negative | Positive | Positive |
| 12 | 1.41 | 12.49 | 8.86 (+) | 20.82 | 46.27 | 2.2 (+) | Negative | Negative | Negative |
| 13 | 1.46 | 2.11 | 1.45 (+) | 3.38 | 8.52 | 2.5 (+) | Negative | Negative | Negative |
| 14 | 1.06 | 7.29 | 6.88 (+) | 0.64 | 33 | 51.6 (+) | Negative | Positive | Positive |
| 15 | 0.45 | 11.2 | 24.89 (+) | 0.17 | 32.07 | 188.6 (+) | Negative | Negative | Negative |
| 16 | 1.35 | 12.82 | 9.50 (+) | 0.63 | 25.17 | 40 (+) | Negative | Positive | Positive |
| 17 | 3.47 | 12.14 | 3.50 (+) | 3.51 | 85.52 | 24.4 (+) | Negative | Negative | Negative |
| 18 | 0.22 | 7.41 | 33.68 (+) | 0.18 | 25.91 | 143.9 (+) | Negative | Positive | Positive |
| 19 | 0.24 | 0.49 | 2.04 (+) | 2.82 | 15.18 | 5.4 (+) | Negative | Negative | Negative |
| 20 | 2.56 | 18.74 | 7.32 (+) | 6.98 | 64.99 | 9.3 (+) | Negative | Positive | Positive |
| PCVIT Participant | IgM (mg/mL) | IgG1 (mg/mL) | IgG2 (mg/ml) | IgG3 (mg/mL) | IgG4 (mg/mL) | IgA (mg/mL) | CRP (mg/L) |
|---|---|---|---|---|---|---|---|
| 1 | 0.63 | 2.58 | 0.23 | 0.43 | 0.05 | 0.15 | 0.23 |
| 2 | 0.60 | 2.13 | 1.16 | 0.18 | 0.01 | 0.26 | 0.32 |
| 3 | 0.97 | 4.28 | 1.05 | 0.54 | 0.62 | 0.65 | 2.17 |
| 4 | 0.92 | 2.59 | 0.23 | 0.86 | 0.00 | 0.33 | 1.22 |
| 5 | 0.65 | 3.42 | 1.97 | 0.58 | 0.00 | 0.35 | 0.53 |
| 6 | 1.38 | 5.17 | 0.42 | 1.63 | 0.03 | 0.54 | 0.3 |
| 7 | 0.49 | 4.19 | 0.23 | 2.08 | 0.00 | 0.30 | 0.66 |
| 8 | 2.36 | 6.21 | 2.34 | 2.54 | 1.52 | 0.48 | 0.19 |
| 9 | 0.74 | 3.71 | 0.79 | 2.16 | 0.00 | 0.40 | 0.21 |
| 10 | 1.09 | 7.41 | 3.78 | 2.71 | 3.77 | 1.24 | 0.42 |
| 11 | 0.43 | 3.40 | 0.23 | 0.33 | 0.02 | 0.14 | 0.36 |
| 12 | 0.68 | 2.59 | 0.23 | 0.21 | 0.01 | 0.15 | 0.35 |
| 13 | 1.33 | 5.17 | 1.20 | 2.71 | 0.01 | 0.55 | 2.93 |
| 14 | 0.76 | 4.24 | 0.23 | 2.00 | 0.03 | 0.35 | 0.78 |
| 15 | 0.63 | 2.75 | 0.46 | 1.72 | 0.00 | 0.23 | 0.6 |
| 16 | 0.89 | 3.77 | 1.17 | 0.60 | 0.30 | 0.31 | 0.98 |
| 17 | 0.68 | 1.66 | 0.82 | 0.61 | 0.02 | 0.16 | 0.1 |
| 18 | 1.79 | 3.48 | 0.91 | 0.82 | 0.05 | 0.34 | 4.51 |
| 19 | 0.57 | 4.53 | 0.23 | 1.50 | 0.04 | 0.19 | 0.72 |
| 20 | 1.31 | 3.69 | 1.94 | 1.71 | 0.04 | 0.62 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, N.; Surman, S.L.; Jones, B.G.; Penkert, R.R.; Ringwald-Smith, K.; DeLuca, K.; Richardson, J.; Zheng, Y.; Tang, L.; Hurwitz, J.L. Randomized Controlled Clinical Trial of Pediatric Pneumococcus and Hepatitis A Vaccinations With or Without a High-Dose Oral Vitamin A Supplement. Biomolecules 2025, 15, 540. https://doi.org/10.3390/biom15040540
Patel N, Surman SL, Jones BG, Penkert RR, Ringwald-Smith K, DeLuca K, Richardson J, Zheng Y, Tang L, Hurwitz JL. Randomized Controlled Clinical Trial of Pediatric Pneumococcus and Hepatitis A Vaccinations With or Without a High-Dose Oral Vitamin A Supplement. Biomolecules. 2025; 15(4):540. https://doi.org/10.3390/biom15040540
Chicago/Turabian StylePatel, Nehali, Sherri L. Surman, Bart G. Jones, Rhiannon R. Penkert, Karen Ringwald-Smith, Kim DeLuca, Julie Richardson, Ying Zheng, Li Tang, and Julia L. Hurwitz. 2025. "Randomized Controlled Clinical Trial of Pediatric Pneumococcus and Hepatitis A Vaccinations With or Without a High-Dose Oral Vitamin A Supplement" Biomolecules 15, no. 4: 540. https://doi.org/10.3390/biom15040540
APA StylePatel, N., Surman, S. L., Jones, B. G., Penkert, R. R., Ringwald-Smith, K., DeLuca, K., Richardson, J., Zheng, Y., Tang, L., & Hurwitz, J. L. (2025). Randomized Controlled Clinical Trial of Pediatric Pneumococcus and Hepatitis A Vaccinations With or Without a High-Dose Oral Vitamin A Supplement. Biomolecules, 15(4), 540. https://doi.org/10.3390/biom15040540






