Altering the Properties of Laccases from Ensifer meliloti (Sinorhizobium meliloti) and Cerrena unicolor by Chemical Modifications of Proteins
Abstract
1. Introduction
2. Materials and Methods
2.1. Medium, Growth Conditions, and Preparation of Enzymes
2.2. Chemical Modifications of Laccases
2.2.1. EGNHS (Ethylene Glycol Bis-(Succinimidyl Succinate))
2.2.2. CA (Citraconic Anhydride)
2.2.3. GA (Glutaraldehyde) and CDI (Carbodiimide)
2.2.4. N-HSP (Palmitic Acid N-Hydroxysuccinimide Ester)
2.2.5. Mono and Disaccharides (Glc—Glucose, Gal—Galactose, Cel—Cellobiose, Lac—Lactose)
2.2.6. PS (Polymeric Sucrose)
2.3. Characterization of Modified Laccases
2.3.1. Optimum pH Ranges and pH-Stability of Modified Laccases
2.3.2. Optimum Temperature and Thermal Stability of Modified Laccases
2.3.3. Laccase Inhibitors
2.4. Prediction of Ensifer Meliloti L3.8 and Cerrena Unicolor C-139 Laccase Structure
3. Results
3.1. Influence of Modification on the Activity and Stability of E. Meliloti L3.8 and C. Unicolor C-139 Laccases
3.2. Comparative Analysis of the Structural Properties of E. Meliloti L3.8 and C. Unicolor C-139 Laccase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Institutional Review Board Statement:
Informed Consent Statement:
Appendix A
| Sample Shortcut (Name) | Description of Modifications |
|---|---|
| Ensifer meliloti laccase | |
| EM_control | Control experiment for untreated E. meliloti laccase |
| EGNHS-EM | EGNHS-modified E. meliloti laccase |
| CA-EM | CA-modified E. meliloti laccase |
| GA-EM | GA-modified E. meliloti laccase |
| CDI-EM | CDI-modified E. meliloti |
| GA-CDI-EM | GA-CDI-modified E. meliloti laccase |
| GA-ver-EM | GA-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| CDI-ver-EM | CDI-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| GA-CDI-ver-EM | GA/CDI-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| NHSP-EM | NHSP-modified E. meliloti laccase |
| NHSP-ver-EM | NHSP-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| Glc-EM | Glucose-modified E. meliloti laccase |
| Gal-EM | Galactose-modified E. meliloti laccase |
| Cel-EM | Cellobiose-modified E. meliloti laccase |
| Lac-EM | Lactose-modified E. meliloti laccase |
| Glc-ver-EM | Glucose-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| Gal-ver-EM | Galactose-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| Cel-ver-EM | Cellobiose-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| Lac-ver-EM | Lactose-modified E. meliloti laccase having inactivated enzyme catalytic site with veratric acid |
| PS-EM | Polymeric sucrose-modified E. meliloti laccase |
| Cerrena unicolor laccase | |
| CU_control | Control experiment for untreated C. unicolor laccase |
| EGNHS-CU | EGNHS-modified C. unicolor laccase |
| CA-SM | CA-modified C. unicolor laccase |
| PS-CU | Polymeric sucrose-modified C. unicolor laccase |
References
- Roy, J.J.; Abraham, T.E. Strategies in making cross-linked enzyme crystals. Chem. Rev. 2004, 104, 3705–3721. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.; Davis, B.G. Chemical modification in the creation of novel biocatalysts. Curr. Opin. Chem. Biol. 2011, 15, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Giri, P.; Pagar, A.D.; Patil, M.D.; Yun, H. Chemical modification of enzymes to improve biocatalytic performance. Biotechnol. Adv. 2021, 53, 107868. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Kunamneni, A.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Laccases and their applications: A patent review. Recent Pat. Biotechnol. 2008, 2, 10–24. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-A resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [PubMed]
- Xu, F.; Shin, W.; Brown, S.H.; Wahleithner, J.A.; Sundaram, U.M.; Solomon, E.I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta 1996, 1292, 303–311. [Google Scholar]
- Sakurai, T. Anaerobic reactions of Rhus vernicifera laccase and its type-2 copper-depleted derivatives with hexacyanoferrate(II). Biochem. J. 1992, 284 Pt 3, 681–685. [Google Scholar]
- Loncar, N.; Bozic, N.; Lopez-Santin, J.; Vujcic, Z. Bacillus amyloliquefaciens laccase--from soil bacteria to recombinant enzyme for wastewater decolorization. Bioresour. Technol. 2013, 147, 177–183. [Google Scholar]
- Virk, A.P.; Sharma, P.; Capalash, N. Use of laccase in pulp and paper industry. Biotechnol. Prog. 2012, 28, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, R.; Belbahri, L.; Woodward, S.; Ellouz, M.; Dhouib, A.; Sayadi, S.; Mechichi, T. Decolourization and detoxification of textile industry wastewater by the laccase-mediator system. J. Hazard. Mater. 2010, 175, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Benzina, O.; Daassi, D.; Zouari-Mechichi, H.; Frikha, F.; Woodward, S.; Belbahri, L.; Rodriguez-Couto, S.; Mechichi, T. Decolorization and detoxification of two textile industry effluents by the laccase/1-hydroxybenzotriazole system. Environ. Sci. Pollut. Res. Int. 2013, 20, 5177–5187. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.; Kato, C.G.; Corrêa, R.C.G.; Peralta Muniz Moreira, R.D.F.; Peralta, R.A.; Barros, L.; Ferreira, I.C.F.R.; Zanin, G.M.; Bracht, A.; Peralta, R.M. Laccases in food processing: Current status, bottlenecks and perspectives. Trends Food Sci. Technol. 2021, 115, 445–460. [Google Scholar] [CrossRef]
- Saito, K.; Ikeda, R.; Endo, K.; Tsujino, Y.; Takagi, M.; Tamiya, E. Isolation of a novel alkaline-induced laccase from Flammulina velutipes and its application for hair coloring. J. Biosci. Bioeng. 2012, 113, 575–579. [Google Scholar] [CrossRef]
- Bolli, A.; Galluzzo, P.; Ascenzi, P.; Del Pozzo, G.; Manco, I.; Vietri, M.T.; Mita, L.; Altucci, L.; Mita, D.G.; Marino, M. Laccase treatment impairs bisphenol A-induced cancer cell proliferation affecting estrogen receptor alpha-dependent rapid signals. IUBMB Life 2008, 60, 843–852. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Chen, Q.; Wang, H.; Zhang, J. A novel laccase with inhibitory activity towards HIV-I reverse transcriptase and antiproliferative effects on tumor cells from the fermentation broth of mushroom Pleurotus cornucopiae. Biomed. Chromatogr. 2013, 28, 548–553. [Google Scholar] [CrossRef]
- Ren, X.J.; Fan, C.Z.; Lu, L.H.; Wang, C.; Zeng, G.M. Isolation, identification and enzymological characterization of a new fungal with high laccase production from agricultural waste composting. Huan Jing Ke Xue 2012, 33, 3220–3227. [Google Scholar]
- Chen, X.; Zhu, Y.; Chen, F.; Li, Z.; Zhang, X.; Wang, G.; Ji, J.; Guan, C. The role of microplastics in the process of laccase-assisted phytoremediation of phenanthrene-contaminated soil. Sci. Total Environ. 2023, 905, 167305. [Google Scholar] [CrossRef]
- Margot, J.; Bennati-Granier, C.; Maillard, J.; Blanquez, P.; Barry, D.A.; Holliger, C. Bacterial versus fungal laccase: Potential for micropollutant degradation. AMB Express 2013, 3, 63. [Google Scholar] [CrossRef]
- Uthandi, S.; Prunetti, L.; De Vera, I.M.; Fanucci, G.E.; Angerhofer, A.; Maupin-Furlow, J.A. Enhanced archaeal laccase production in recombinant Escherichia coli by modification of N-terminal propeptide and twin arginine translocation motifs. J. Ind. Microbiol. Biotechnol. 2012, 39, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.F.; Yang, D.J.; Bai, M.X.; Qiu, X.Q. The influence of laccase modification on the adsorption of lignosulfonate. Adv. Mater. Res. 2012, 550–553, 1266–1269. [Google Scholar] [CrossRef]
- Theerachat, M.; Emond, S.; Cambon, E.; Bordes, F.; Marty, A.; Nicaud, J.M.; Chulalaksananukul, W.; Guieysse, D.; Remaud-Simeon, M.; Morel, S. Engineering and production of laccase from Trametes versicolor in the yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 125, 267–274. [Google Scholar] [CrossRef]
- Camarero, S.; Pardo, I.; Canas, A.I.; Molina, P.; Record, E.; Martinez, A.T.; Martinez, M.J.; Alcalde, M. Engineering platforms for directed evolution of laccase from Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 2012, 78, 1370–1384. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, H.; Dhoke, G.V.; Zou, Z.; Sauer, D.F.; Davari, M.D.; Schwaneberg, U. Engineering of laccase CueO for improved electron transfer in bioelectrocatalysis by semi-rational design. Chem-Eur. J. 2020, 26, 4974–4979. [Google Scholar] [CrossRef]
- Mateljak, I.; Alcalde, M. Engineering a highly thermostable high-redox potential laccase. ACS Sustain. Chem. Eng. 2021, 9, 9632–9637. [Google Scholar] [CrossRef]
- Danait-Nabar, S.; Singhal, R.S. Chemical modification of laccase using phthalic and 2-octenyl succinic anhydrides: Enzyme characterization, stability, and its potential for clarification of cashew apple juice. Process Biochem. 2022, 122, 181–195. [Google Scholar] [CrossRef]
- DeSantis, G.; Jones, J.B. Chemical modification of enzymes for enhanced functionality. Curr. Opin. Biotechnol. 1999, 10, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Bhardwaj, P.; Ishqi, H.M.; Shahid, M.; Islam, A. Laccase engineering: Redox potential is not the only activity-determining feature in the metalloproteins. Molecules 2023, 28, 6209. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef]
- Schroeder, M.; Heumann, S.; Silva, C.J.; Cavaco-Paulo, A.; Guebitz, G.M. Specificities of a chemically modified laccase from Trametes hirsuta on soluble and cellulose-bound substrates. Biotechnol. Lett. 2006, 28, 741–747. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hassani, T.; Ba, S.; Cabana, H. Formation of enzyme polymer engineered structure for laccase and cross-linked laccase aggregates stabilization. Bioresour. Technol. 2013, 128, 640–645. [Google Scholar] [CrossRef]
- Steffensen, C.L.; Andersen, M.L.; Degn, P.E.; Nielsen, J.H. Cross-linking proteins by laccase-catalyzed oxidation: Importance relative to other modifications. J. Agric. Food Chem. 2008, 56, 12002–12010. [Google Scholar] [CrossRef]
- Kucharzyk, K.H.; Janusz, G.; Karczmarczyk, I.; Rogalski, J. Chemical modifications of laccase from white-rot basidiomycete Cerrena unicolor. Appl. Biochem. Biotechnol. 2012, 168, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Lindeberg, G.; Holm, G. Occurrence of tyrosinase and laccase in fruit bodies and mycelia of some Hymenomycetes. Physiol. Plant. 1952, 5, 100–114. [Google Scholar] [CrossRef]
- Janusz, G.; Rogalski, J.; Szczodrak, J. Increased production of laccase by Cerrena unicolor in submerged liquid cultures. World J. Microbiol. Biotechnol. 2007, 23, 1459–1464. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root-Nodule Bacteria; IBP Handbk 15 Oxford and Edinburgh; Blackwell Scientific: Oxford, UK, 1970; p. 164. [Google Scholar]
- Castro-Sowinski, S.; Martinez-Drets, G.; Okon, Y. Laccase activity in melanin-producing strains of Sinorhizobium meliloti. FEMS Microbiol. Lett. 2002, 209, 119–125. [Google Scholar] [PubMed]
- Rosconi, F.; Fraguas, L.F.; Martinez-Drets, G.; Castro-Sowinski, S. Purification and characterization of a periplasmic laccase produced by Sinorhizobium meliloti. Enzym. Microb. Technol. 2005, 36, 800–807. [Google Scholar] [CrossRef]
- Pawlik, A.; Wojcik, M.; Rulka, K.; Motyl-Gorzel, K.; Osinska-Jaroszuk, M.; Wielbo, J.; Marek-Kozaczuk, M.; Skorupska, A.; Rogalski, J.; Janusz, G. Purification and characterization of laccase from Sinorhizobium meliloti and analysis of the lacc gene. Int. J. Biol. Macromol. 2016, 92, 138–147. [Google Scholar] [CrossRef]
- Habeeb, A.F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 1966, 14, 328–336. [Google Scholar]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzym. Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Forde, J.; Tully, E.; Vakurov, A.; Gibson, T.D.; Millner, P.; O’Fagain, C. Chemical modification and immobilisation of laccase from Trametes hirsuta and from Myceliophthora thermophila. Enzym. Microb. Technol. 2010, 46, 430–437. [Google Scholar] [CrossRef]
- Sundaram, P.V.; Venkatesh, R. Retardation of thermal and urea induced inactivation of alpha-chymotrypsin by modification with carbohydrate polymers. Protein Eng. 1998, 11, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Lüthy, R.; Bowie, J.U.; Eisenberg, D. Assessment of protein models with three-dimensional profiles. Nature 1992, 356, 83–85. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Cavallo, L.; Kleinjung, J.; Fraternali, F. POPS: A fast algorithm for solvent accessible surface areas at atomic and residue level. Nucl. Acids Res. 2003, 31, 3364–3366. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef]
- Senior, A.W.; Evans, R.; Jumper, J.; Kirkpatrick, J.; Sifre, L.; Green, T.; Qin, C.; Žídek, A.; Nelson, A.W.R.; Bridgland, A.; et al. Improved protein structure prediction using potentials from deep learning. Nature 2020, 577, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Bertoline, L.M.F.; Lima, A.N.; Krieger, J.E.; Teixeira, S.K. Before and after AlphaFold2: An overview of protein structure prediction. Front. Bioinform. 2023, 3, 1120370. [Google Scholar] [CrossRef]
- Cretin, G.; Galochkina, T.; Vander Meersche, Y.; de Brevern, A.G.; Postic, G.; Gelly, J.-C. SWORD2: Hierarchical analysis of protein 3D structures. Nucl. Acids Res. 2022, 50, W732–W738. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucl. Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef] [PubMed]
- Tantipanjaporn, A.; Wong, M.-K. Development and recent advances in lysine and N-terminal bioconjugation for peptides and proteins. Molecules 2023, 28, 1083. [Google Scholar] [CrossRef]
- Salem, M.; Mauguen, Y.; Prange, T. Revisiting glutaraldehyde cross-linking: The case of the Arg-Lys intermolecular doublet. Acta Crystallogr. F 2010, 66, 225–228. [Google Scholar] [CrossRef]
- Pessatti, T.B.; Terenzi, H.; Bertoldo, J.B. Protein modifications: From chemoselective probes to novel biocatalysts. Catalysts 2021, 11, 1466. [Google Scholar] [CrossRef]
- Matos, M.J.; Oliveira, B.L.; Martínez-Sáez, N.; Guerreiro, A.; Cal, P.M.S.D.; Bertoldo, J.; Maneiro, M.; Perkins, E.; Howard, J.; Deery, M.J.; et al. Chemo- and Regioselective Lysine Modification on Native Proteins. J. Am. Chem. Soc. 2018, 140, 4004–4017. [Google Scholar] [CrossRef]
- Liu, R.; Yue, Z.; Tsai, C.-C.; Shen, J. Assessing Lysine and Cysteine Reactivities for Designing Targeted Covalent Kinase Inhibitors. J. Am. Chem. Soc. 2019, 141, 6553–6560. [Google Scholar] [CrossRef]
- Shin-ya, Y.; Aye, H.N.; Hong, K.-J.; Kajiuchi, T. Efficacy of amphiphile-modified laccase in enzymatic oxidation and removal of phenolics in aqueous solution. Enzym. Microb. Technol. 2005, 36, 147–152. [Google Scholar] [CrossRef]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera de los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Fact. 2019, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Gräff, M.; Buchholz, P.C.F.; Le Roes-Hill, M.; Pleiss, J. Multicopper oxidases: Modular structure, sequence space, and evolutionary relationships. Proteins Struct. Funct. Bioinform. 2020, 88, 1329–1339. [Google Scholar] [CrossRef]
- Komori, H.; Higuchi, Y. Structure and molecular evolution of multicopper blue proteins. Biomol. Concepts 2010, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Larrondo, L.F.; Salas, L.; Melo, F.; Vicuña, R.; Cullen, D. A novel extracellular multicopper oxidase from Phanerochaete chrysosporium with ferroxidase activity. Appl. Environ. Microbiol. 2003, 69, 6257–6263. [Google Scholar] [CrossRef]
- Polyansky, A.A.; Zagrovic, B. Protein electrostatic properties predefining the level of surface hydrophobicity change upon phosphorylation. J. Phys. Chem. Lett. 2012, 3, 973–976. [Google Scholar] [CrossRef]
- Naowarojna, N.; Cheng, R.; Lopez, J.; Wong, C.; Qiao, L.; Liu, P. Chemical modifications of proteins and their applications in metalloenzyme studies. Synth. Syst. Biotechnol. 2021, 6, 32–49. [Google Scholar] [CrossRef]
- Piersimoni, L.; Kastritis, P.L.; Arlt, C.; Sinz, A. Cross-linking mass spectrometry for investigating protein conformations and protein–protein interactions—A method for all seasons. Chem. Rev. 2022, 122, 7500–7531. [Google Scholar] [CrossRef]
- Haque, M.; Forte, N.; Baker, J.R. Site-selective lysine conjugation methods and applications towards antibody–drug conjugates. Chem. Commun. 2021, 57, 10689–10702. [Google Scholar] [CrossRef]
- Kamyshny, A.; Feldman, A.; Baszkin, A.; Boissonnade, M.M.; Rosilio, V.; Magdassi, S. Chemically modified glucose oxidase with enhanced hydrophobicity: Adsorption at polystyrene, silica, and silica coated by lipid monolayers. J. Colloid Interface Sci. 1999, 218, 300–308. [Google Scholar] [CrossRef]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 790–802. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.M.; Ó’Fágáin, C.; Nielsen, P.F.; Welinder, K.G. Location of crosslinks in chemically stabilized horseradish peroxidase: Implications for design of crosslinks. Biotechnol. Bioeng. 2001, 76, 277–284. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Yin, D.; Xu, X.; Tan, T.; Lv, Y. Boosted activity by engineering the enzyme microenvironment in cascade reaction: A molecular understanding. Synth. Syst. Biotechnol. 2021, 6, 163–172. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, C.-Y.; Branford-White, C.J.; Ning, X.; Nie, H.-L.; Zhu, L.-M. Chemical modification of stem bromelain with anhydride groups to enhance its stability and catalytic activity. J. Mol. Catal. B Enzym. 2010, 63, 188–193. [Google Scholar] [CrossRef]
- Jaiswal, N.; Jaiswal, P. Thermostable α-amylases and laccases: Paving the way for sustainable industrial applications. Processes 2024, 12, 1341. [Google Scholar] [CrossRef]
- Strong, P.J.; Claus, H. Laccase: A review of its past and its future in bioremediation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 373–434. [Google Scholar] [CrossRef]
- Majeau, J.-A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef]
- Buzzo, B.B.; Lima, N.S.M.; Pereira, P.A.M.; Gomes-Pepe, E.S.; Sartini, C.C.F.; Lemos, E.G.d.M. Lignin degradation by a novel thermophilic and alkaline yellow laccase from Chitinophaga sp. Microbiol. Spectr. 2024, 12, e04013-23. [Google Scholar] [CrossRef]
- Liu, N.; Shen, S.; Jia, H.; Yang, B.; Guo, X.; Si, H.; Cao, Z.; Dong, J. Heterologous expression of Stlac2, a laccase isozyme of Setosphearia turcica, and the ability of decolorization of malachite green. Int. J. Biol. Macromol. 2019, 138, 21–28. [Google Scholar] [CrossRef]
- Ayodeji, F.D.; Shava, B.; Iqbal, H.M.N.; Ashraf, S.S.; Cui, J.; Franco, M.; Bilal, M. Biocatalytic versatilities and biotechnological prospects of laccase for a sustainable industry. Catal. Lett. 2023, 153, 1932–1956. [Google Scholar] [CrossRef]
- Nwagu, T.N.; Okolo, B.; Aoyagi, H.; Yoshida, S. Chemical modification with phthalic anhydride and chitosan: Viable options for the stabilization of raw starch digesting amylase from Aspergillus carbonarius. Int. J. Biol. Macromol. 2017, 99, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tsai, C.-J.; Ma, B.; Nussinov, R. Contribution of salt bridges toward protein thermostability. J. Biomol. Struct. Dyn. 2000, 17, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Díaz, R.; Díaz-Godínez, G.; Anducho-Reyes, M.A.; Mercado-Flores, Y.; Herrera-Zúñiga, L.D. In silico design of laccase thermostable mutants from Lacc 6 of Pleurotus ostreatus. Front. Microbiol. 2018, 9, 2743. [Google Scholar] [CrossRef]
- Abdoul, R.; Peters, G.H.; Karl, J.J.; Westh, P. Effects of mannose, fructose, and fucose on the structure, stability, and hydration of lysozyme in aqueous solution. Curr. Phys. Chem. 2013, 3, 113–125. [Google Scholar] [CrossRef]
- Nwagu, T.N.; Aoyagi, H.; Okolo, B.; Moneke, A.; Yoshida, S. Citraconylation and maleylation on the catalytic and thermodynamic properties of raw starch saccharifying amylase from Aspergillus carbonarius. Heliyon 2020, 6, e04351. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nakagawa, T.; Uchida, Y.; Seki, K.; Ohba, M.; Kondo, K. Effect of modification of citraconic anhydrides on catalytic activity and thermostability of enzymes. J. Chem. Technol. Biotechnol. 2016, 91, 59–64. [Google Scholar] [CrossRef]
- Xu, W.; He, K.; Lin, Z.; McClements, D.J.; Jin, Z.; Chen, L. Progress in using cross-linking technologies to increase the thermal stability of colloidal delivery systems. Crit. Rev. Food Sci. Nutr. 2024, 1–15. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial structure and stability of food emulsions as affected by protein–polysaccharide interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. Protein-polysaccharide interactions for stabilization of food emulsions. J. Dispers. Sci. Technol. 2002, 23, 93–123. [Google Scholar] [CrossRef]
- González-González, P.; Gómez-Manzo, S.; Tomasini, A.; Martínez y Pérez, J.L.; García Nieto, E.; Anaya-Hernández, A.; Ortiz Ortiz, E.; Castillo Rodríguez, R.A.; Marcial-Quino, J.; Montiel-González, A.M. Laccase production from Agrocybe pediades: Purification and functional characterization of a consistent laccase isoenzyme in liquid culture. Microorganisms 2023, 11, 568. [Google Scholar] [CrossRef]
- Janusz, G.; Skwarek, E.; Pawlik, A. Potential of laccase as a tool for biodegradation of wastewater micropollutants. Water 2023, 15, 3770. [Google Scholar] [CrossRef]
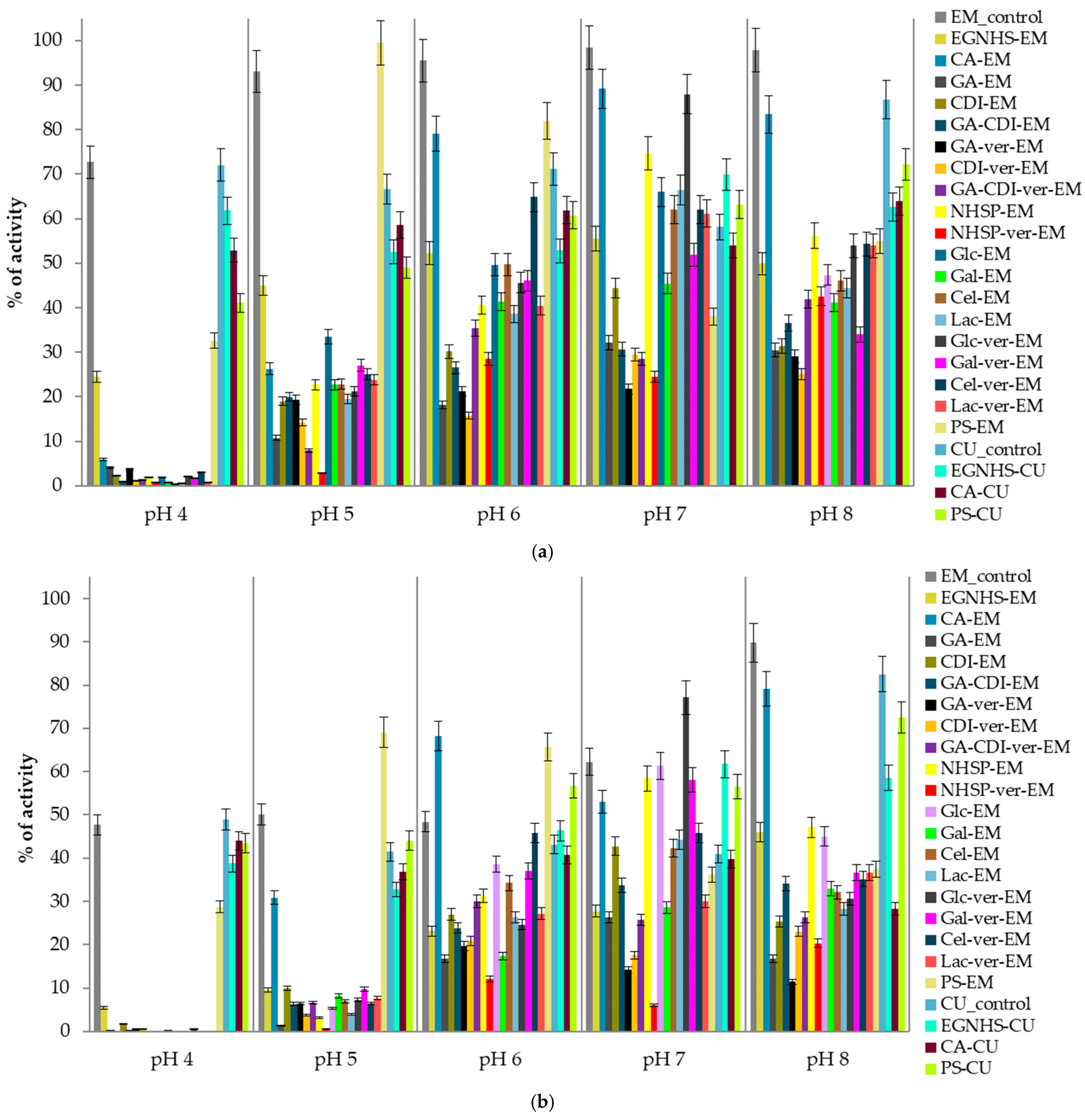
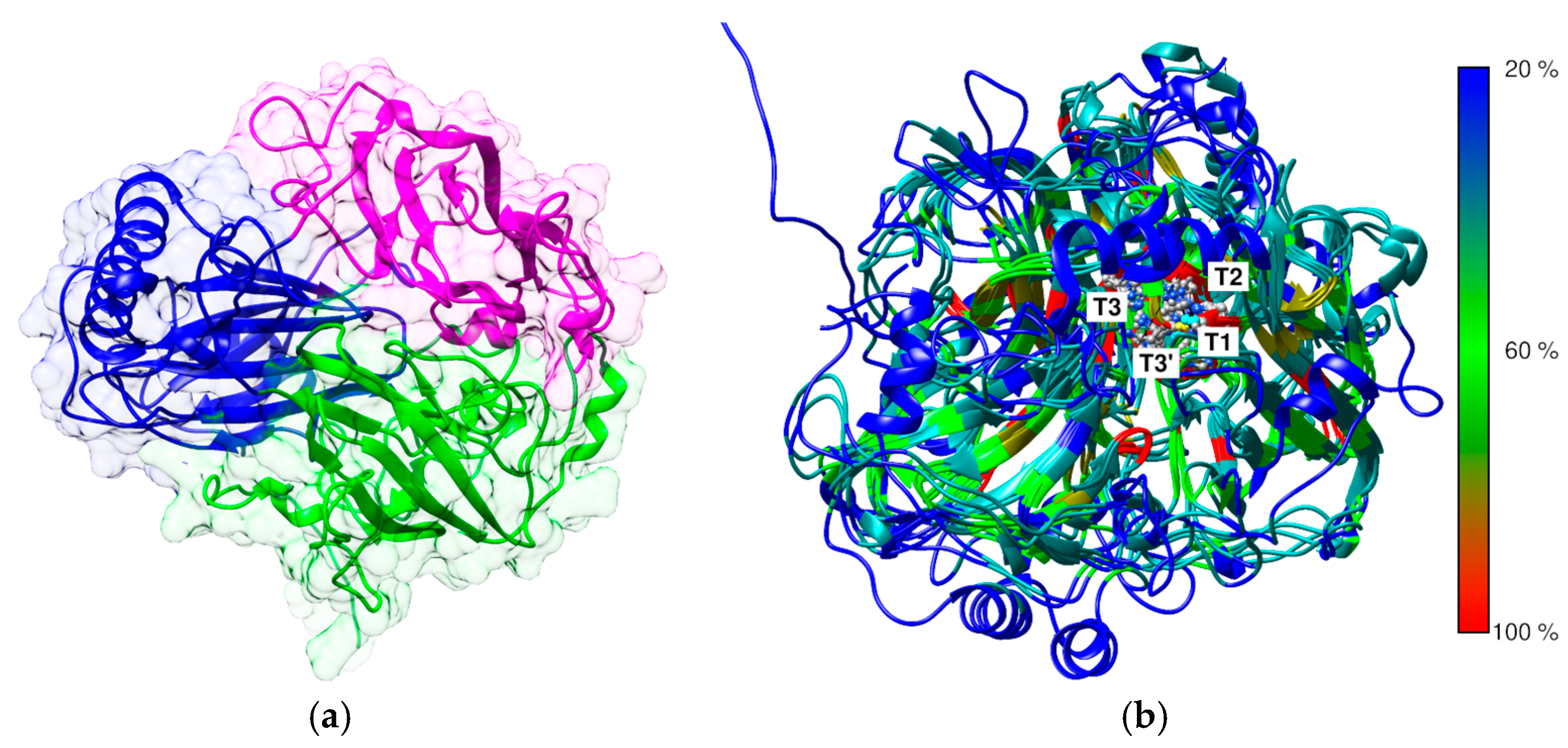

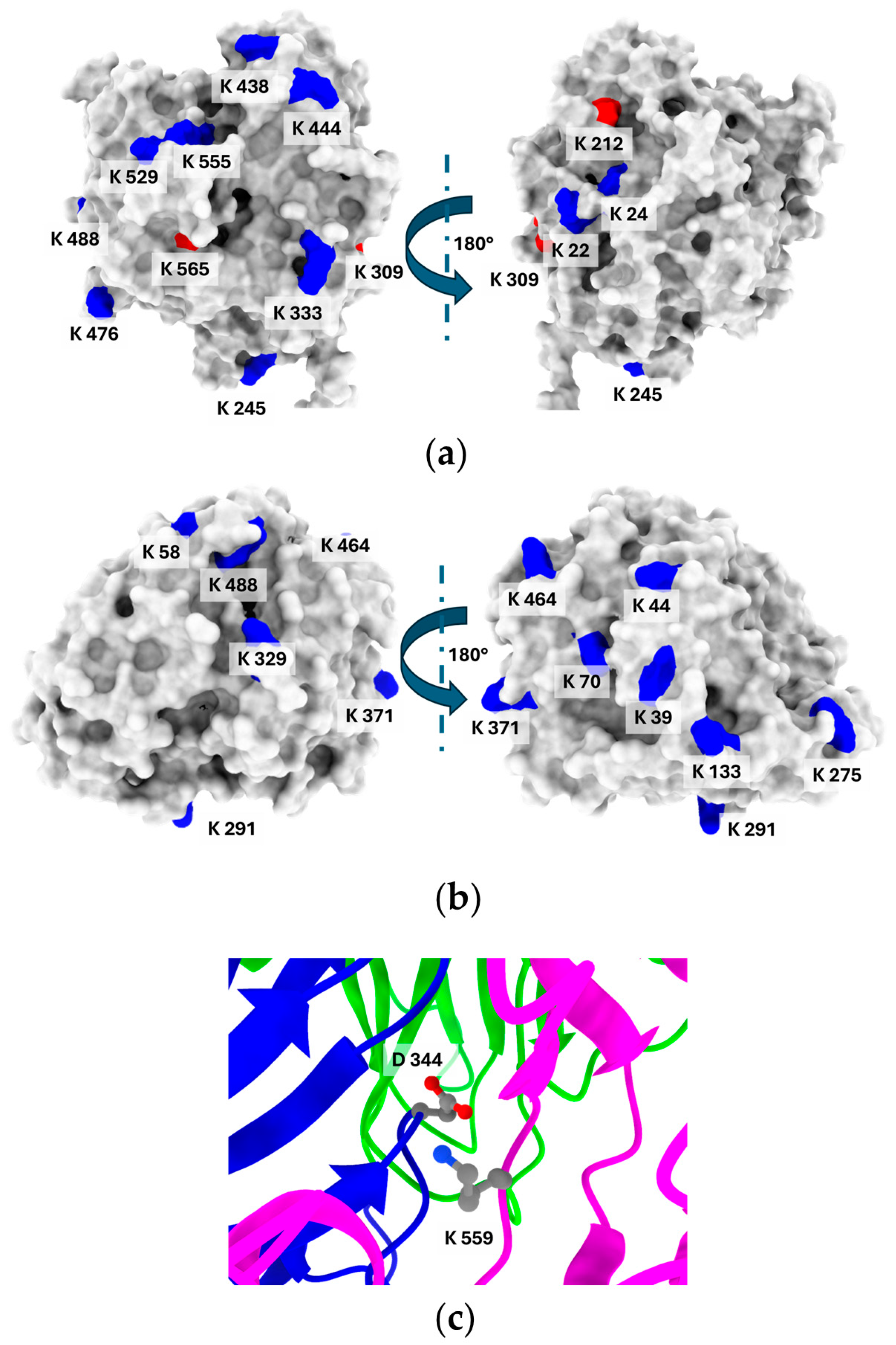
| Modification | Recovery of Initial Activity [%] | DM * [%] | pH Optimum | Temperature Optimum [°C] |
|---|---|---|---|---|
| EM_control | 100 | - | 6.0 | 80 |
| EGNHS-EM | 82.0 ± 1.24 | 30.78 ± 0.8 | 6.5 | 80 |
| CA-EM | 90.1 ± 4.16 | 28.64 ± 1.13 | 6.5 | 80 |
| GA-EM | 97.5 ± 1.25 | 22.07 ± 0.86 | 6.5 | 80 |
| CDI-EM | 98.3 ± 5 | 13.76 ± 0.48 | 6.5 | 80 |
| GA-CDI-EM | 105.0 ± 0 | 5.53 ± 0.3 | 6.5 | 60 |
| GA-ver-EM | 84.2 ± 2.92 | 43.47 ± 1.34 | 6.5 | 80 |
| CDI-ver-EM | 104.2 ± 1.25 | 18.72 ± 0.63 | 6.5 | 80 |
| GA-CDI-ver-EM | 95.8 ± 1.25 | 33.08 ± 0.49 | 6.0 | 60 |
| NHSP-EM | 42.2 ± 2.32 | 59.18 ± 0.57 | 6.5 | 60 |
| NHSP-ver-EM | 40.7 ± 1.58 | 62.94 ± 0.77 | 6.0 | 80 |
| Glc-EM | 87.8 ± 0.46 | 0.00 | 7.0 | 80 |
| Gal-EM | 77.2 ± 5.36 | 3.48 ± 0.1 | 6.5 | 80 |
| Cel-EM | 88.3 ± 0.2 | 11.97 ± 0.3 | 6.0 | 80 |
| Lac-EM | 92.1 ± 4.44 | 3.81 ± 0.12 | 6.0 | 80 |
| Glc-ver-EM | 80.4 ± 2.85 | 0.00 | 6.5 | 80 |
| Gal-ver-EM | 85.6 ± 1.19 | 0.00 | 6.0 | 80 |
| Cel-ver-EM | 103.7 ± 0.46 | 0.97 ± 0.7 | 6.5 | 70 |
| Lac-ver-EM | 106.2 ± 3.05 | 8.33 ± 0.29 | 6.5 | 80 |
| PS-EM | 119.7 ± 2.3 | 0.69 ± 0.13 | 7.0 | 80 |
| CU_control | 100 | - | 5.5 | 80 |
| EGNHS-CU | 115.6 ± 0.91 | 57.01 ± 1.45 | 5.5 | 80 |
| CA-CU | 105.0 ± 1.63 | 0.00 | 6.0 | 80 |
| PS-CU | 100.7 ± 0.53 | 35.02 ± 0.69 | 5.5 | 80 |
| Modification | kd (min−1) * | t1/2 (Min) † | R # |
|---|---|---|---|
| EM_control | 31.55 × 10−4 | 219.7 | - |
| Glc-ver-EM | 44.81 × 10−4 | 154.7 | 0.7 |
| Glc-EM | 53.36 × 10−4 | 129.9 | 0.6 |
| Gal-ver-EM | 27.45 × 10−4 | 252.5 | 1.1 |
| Gal-EM | 59.55 × 10−4 | 116.4 | 0.5 |
| Cel-ver-EM | 39.54 × 10−4 | 175.3 | 0.8 |
| Cel-EM | 48.10 × 10−4 | 144.1 | 0.7 |
| Lac-ver-EM | 38.90 × 10−4 | 178.2 | 0.8 |
| Lac-EM | 70.87 × 10−4 | 97.8 | 0.4 |
| GA-ver-EM | 45.27 × 10−4 | 153.1 | 0.7 |
| GA-EM | 62.17 × 10−4 | 111.5 | 0.5 |
| CDI-ver-EM | 50.30 × 10−4 | 137.8 | 0.6 |
| CDI-EM | 26.18 × 10−4 | 264.8 | 1.2 |
| GA-CDI-ver-EM | 7.38 × 10−4 | 938.7 | 4.3 |
| GA-CDI-EM | 20.70 × 10−4 | 334.9 | 1.5 |
| NHSP-ver-EM | 110.02 × 10−4 | 63.0 | 0.3 |
| NHSP-EM | 108.99 × 10−4 | 63.6 | 0.3 |
| CA-EM | 116.11 × 10−4 | 59.7 | 0.3 |
| EGNHS-EM | 25.46 × 10−4 | 272.2 | 1.2 |
| PS-EM | 15.85 × 10−4 | 437.3 | 2.0 |
| CU_control | 145.31 × 10−4 | 47.7 | - |
| CA-CU | 3.88 × 10−4 | 1787.4 | 37.5 |
| EGNHS-CU | 62.39 × 10−4 | 111.1 | 2.3 |
| PS-CU | 11.54 × 10−4 | 600.8 | 12.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, A.; Drozd, R.; Janusz, G. Altering the Properties of Laccases from Ensifer meliloti (Sinorhizobium meliloti) and Cerrena unicolor by Chemical Modifications of Proteins. Biomolecules 2025, 15, 531. https://doi.org/10.3390/biom15040531
Pawlik A, Drozd R, Janusz G. Altering the Properties of Laccases from Ensifer meliloti (Sinorhizobium meliloti) and Cerrena unicolor by Chemical Modifications of Proteins. Biomolecules. 2025; 15(4):531. https://doi.org/10.3390/biom15040531
Chicago/Turabian StylePawlik, Anna, Radosław Drozd, and Grzegorz Janusz. 2025. "Altering the Properties of Laccases from Ensifer meliloti (Sinorhizobium meliloti) and Cerrena unicolor by Chemical Modifications of Proteins" Biomolecules 15, no. 4: 531. https://doi.org/10.3390/biom15040531
APA StylePawlik, A., Drozd, R., & Janusz, G. (2025). Altering the Properties of Laccases from Ensifer meliloti (Sinorhizobium meliloti) and Cerrena unicolor by Chemical Modifications of Proteins. Biomolecules, 15(4), 531. https://doi.org/10.3390/biom15040531









