Sustainable, Targeted, and Cost-Effective Laccase-Based Bioremediation Technologies for Antibiotic Residues in the Ecosystem: A Comprehensive Review
Abstract
1. Introduction
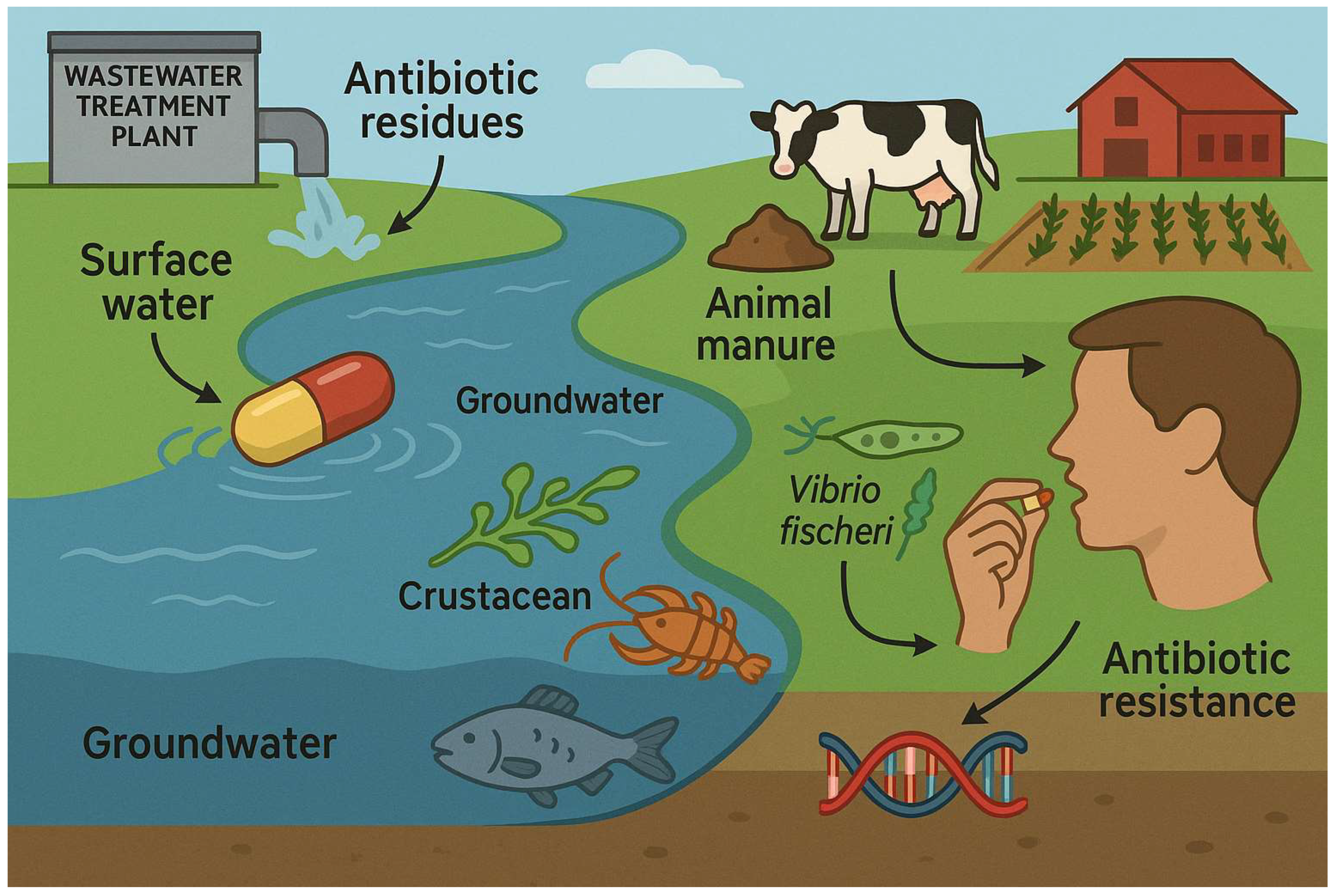
2. The Occurrence of Antibiotic Compounds in the Environment
3. Antibiotic Compounds’ Effects on the Ecosystem and Non-Target Organisms Including the Increase in Resistance Mechanisms
4. Biotransformation, Degradation, and Elimination of Antibiotic Residues in the Ecosystem
5. Antibiotic Bioremediation by Fungal Laccase
5.1. Enzyme-Mediated Bioremediation with a Focus on Fungal Laccase
5.1.1. Structural and Catalytic Characteristics of Laccases in Antibiotic-Based Bioremediation
5.1.2. Laccase Antibiotic Degradation Efficiency
5.2. Laccase Immobilization Methods
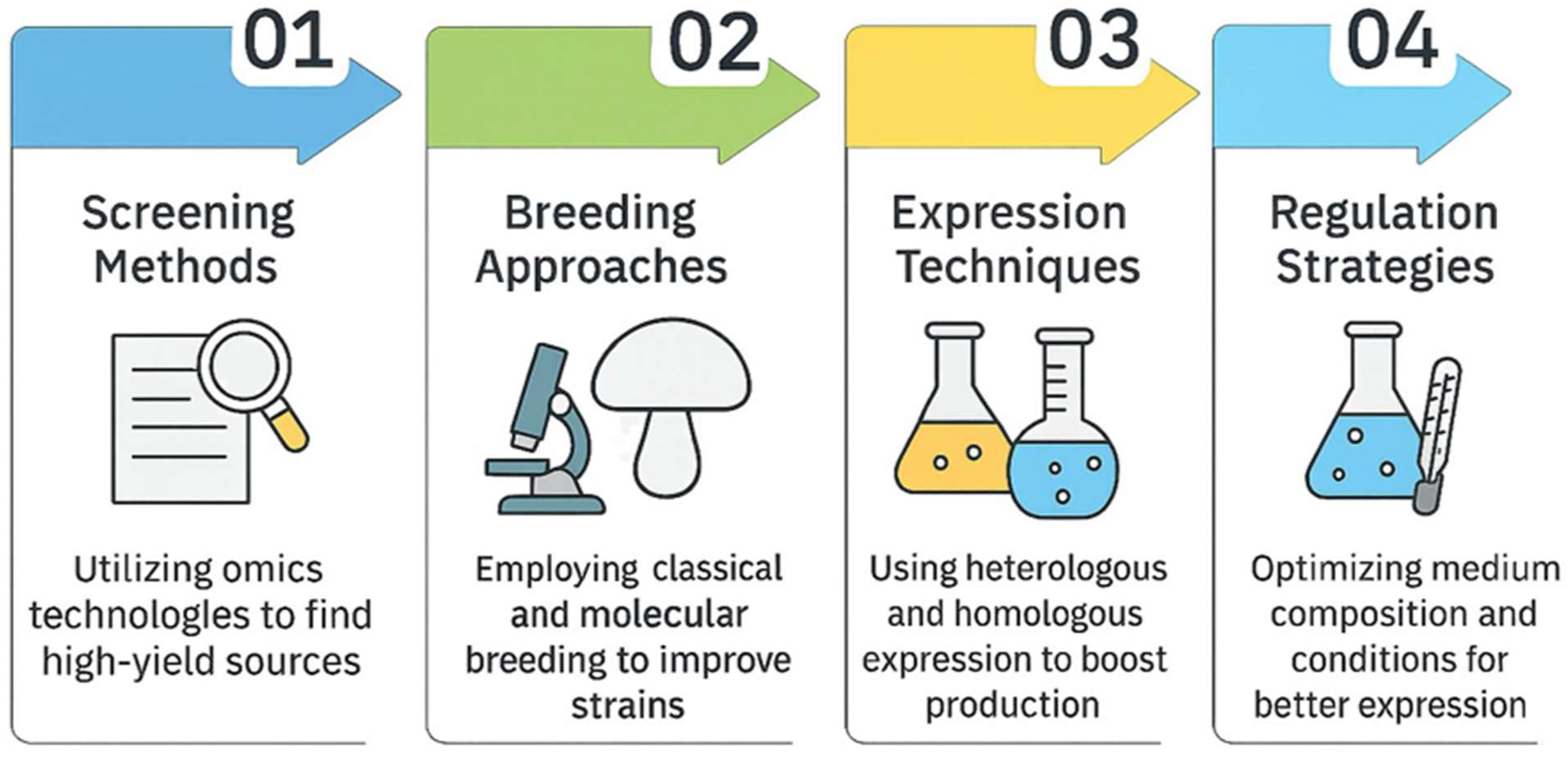
6. Enhancing Laccase Production and Laccase Optimization
6.1. Screening for High-Laccase-Producing Sources and Optimizing Cultivation Conditions
6.2. Optimizing Laccase Production via Classical and Molecular Breeding Approaches
- B.1. Heterologous Expression:
- B.2. Homologous Expression
6.3. Identifying Potential Elements Regulating Laccase Expression
6.4. Other Emerging Methods Enhancing Laccase Activity
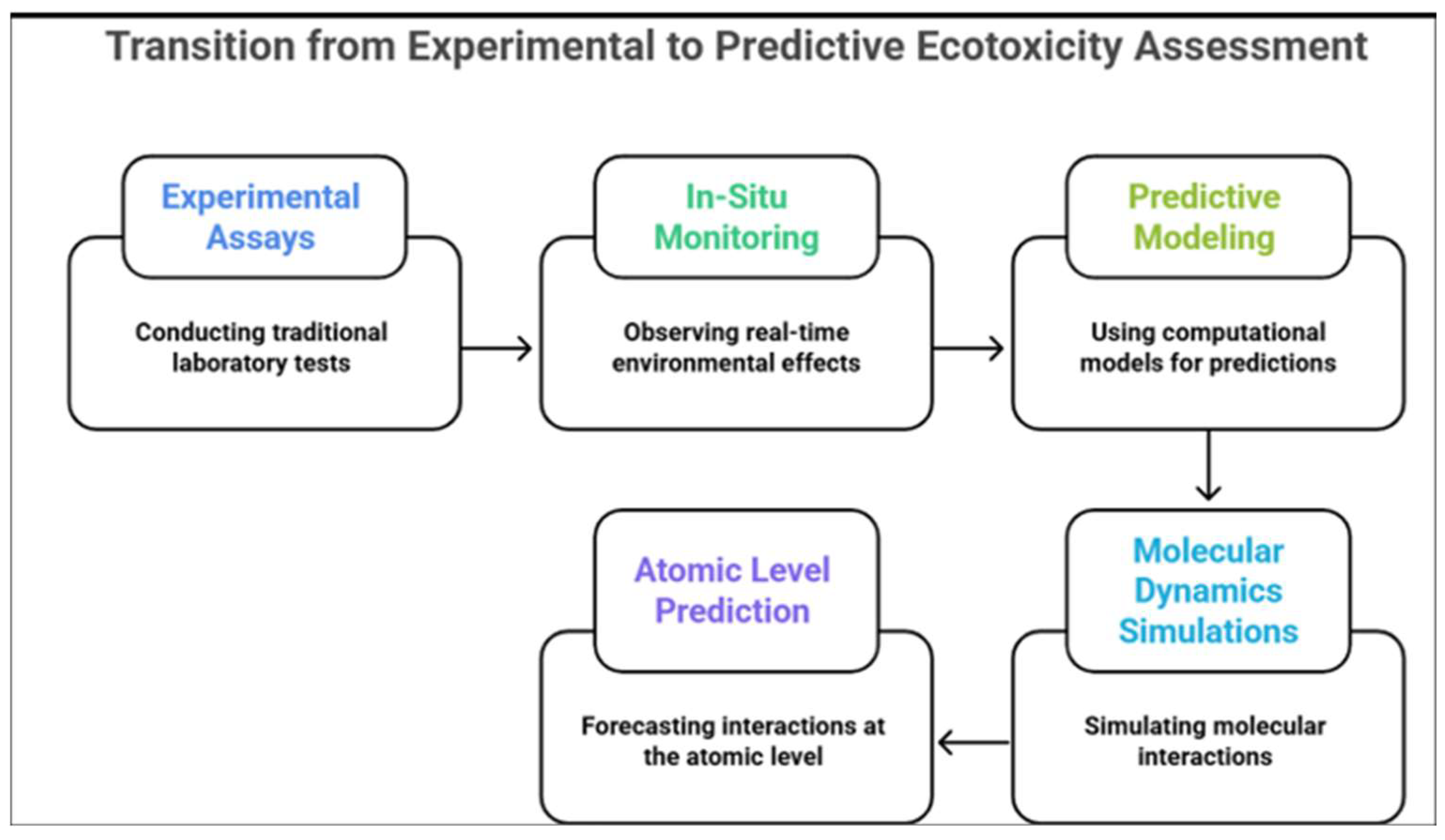
7. Shifting Towards Real-World Laccase-Based Bioremediation and Ecotoxicity Testing of Its Byproducts
7.1. Large-Scale and Cost-Effective Laccase-Based Applications
7.2. Laccase Bioremediation and Residual Toxicity
7.3. Exploring Alternative Eco-Friendly and Cost-Effective Enzymatic Systems
8. Improving Laccase-Based Bioremediation and Environmental and Economic Sustainability
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Tawfiq, J.A.; Momattin, H.; Al-Ali, A.Y.; Eljaaly, K.; Tirupathi, R.; Haradwala, M.B.; Schlagenhauf, P. Antibiotics in the pipeline: A literature review (2017–2020). Infection 2022, 50, 553–564. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Nat. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Moga, A.; Vergara-Barberán, M.; Lerma-García, M.J.; Carrasco-Correa, E.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Determination of antibiotics in meat samples using analytical methodologies: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1681–1716. [Google Scholar] [CrossRef] [PubMed]
- Välitalo, P.; Kruglova, A.; Mikola, A.; Vahala, R. Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hygiene Environ. Health 2017, 220, 558–569. [Google Scholar] [CrossRef]
- Axel, M.; Ewelina, K.; Jenny-Maria, B.; Leif, K. An online SPE LC-MS/MS method for the analysis of antibiotics in environmental water. Environ. Sci. Pollut. Res. 2017, 24, 8692–8699. [Google Scholar] [CrossRef]
- Borghi, A.A.; Palma, M.S.A. Tetracycline: Production, waste treatment and environmental impact assessment. Braz. J. Pharm. Sci. 2014, 50, 25–40. [Google Scholar] [CrossRef]
- Suzuki, S.; Hoa, P.T.P. Distribution of quinolones, sulfonamides, tetracyclines in aquatic environment and antibiotic resistance in Indochina. Front. Microbiol. 2012, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Cheong, M.S.; Seo, K.H.; Chohra, H.; Yoon, Y.E.; Choe, H.; Kantharaj, V.; Lee, Y.B. Influence of sulfonamide contamination derived from veterinary antibiotics on plant growth and development. Antibiotics 2020, 9, 456. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M. Biocatalytic degradation/redefining “removal” fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211. [Google Scholar] [CrossRef]
- Awakawa, T.; Barra, L.; Abe, I. Biosynthesis of sulfonamide and sulfamate antibiotics in actinomycete. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab001. [Google Scholar] [CrossRef]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef]
- Blondeau, J.M. Immunomodulatory Effects of Macrolides Considering Evidence from Human and Veterinary Medicine. Microorganisms 2022, 10, 2438. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J.; Li, P. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment. J. Hazar. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment-occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef] [PubMed]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, C.; Yin, Y.; Zheng, G.; Shan, Q.; Zhang, M.; Wei, L.; Shi, X.; Huang, H.; Zhang, W.; et al. Simultaneous Determination of Active Clinical Components of Teicoplanin and Ramoplanin in Environmental Water by LC-MS/MS Coupled With Cascade Elution. Front. Environ. Sci. 2021, 9, 785408. [Google Scholar] [CrossRef]
- Obayiuwana, A.; Ogunjobi, A.; Yang, M.; Ibekwe, M. Characterization of bacterial communities and their antibiotic resistance profiles in wastewaters obtained from pharmaceutical facilities in Lagos and Ogun States, Nigeria. Inter. J. Environ. Res. Public Health 2018, 15, 1365. [Google Scholar] [CrossRef]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Jendrzejewska, N.; Karwowska, E. The influence of antibiotics on wastewater treatment processes and the development of antibiotic-resistant bacteria. Water Sci. Technol. 2018, 77, 2320–2326. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. Rev. Sci. Tech. Off. Int. Epizoot. 2012, 31, 199–210. [Google Scholar] [CrossRef]
- Ana, K.M.S.; Madriaga, J.; Espino, M.P. β-Lactam antibiotics and antibiotic resistance in Asian lakes and rivers: An overview of contamination, sources and detection methods. Environ. Pollut. 2021, 275, 116624. [Google Scholar] [CrossRef]
- Sanganyado, E.; Gwenzi, W. Antibiotic resistance in drinking water systems: Occurrence, removal, and human health risks. Sci. Total Environ. 2019, 669, 785–797. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.R.; Sarri, E.; Kelch, S.E.; Basinski, J.J.; Vaidya, S.; Aristilde, L. Probing the fate of different structures of beta-lactam antibiotics: Hydrolysis, mineral capture, and influence of organic matter. ACS Earth Space Chem. 2021, 5, 1511–1524. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Giri, B.S.; Shukla, P.; Gupta, P. Recent advancement in remediation of synthetic organic antibiotics from environmental matrices: Challenges and perspective. Bioresour. Technol. 2021, 319, 124161. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Conde-Cid, M.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Tetracycline and sulfonamide antibiotics in soils: Presence, fate and environmental risks. Processes 2020, 8, 1479. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment—Degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Luo, Y.; Wu, L.; Huang, Y.; Christie, P. Residues and potential ecological risks of veterinary antibiotics in manures and composts associated with protected vegetable farming. Environ. Sci. Pollut. Res. 2015, 22, 5908–5918. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial resistance: Impacts, challenges, and future prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Barathe, P.; Kaur, K.; Reddy, S.; Shriram, V.; Kumar, V. Antibiotic pollution and associated antimicrobial resistance in the environment. J. Hazar. Mater. Lett. 2024, 5, 100105. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineering 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Gen. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Alexy, R.; Schöll, A.; Kümpel, T.; Kümmerer, K. What do we know about antibiotics in the environment? In Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks; Springer: Berlin/Heidelberg, Germany, 2004; pp. 209–221. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Helbling, D.E. Biotransformation of antibiotics: Exploring the activity of extracellular and intracellular enzymes derived from wastewater microbial communities. Water Res. 2019, 155, 115. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Kulik, K.; Lenart-Boroń, A.; Wyrzykowska, K. Impact of antibiotic pollution on the bacterial population within surface water with special focus on mountain rivers. Water 2023, 15, 975. [Google Scholar] [CrossRef]
- Mora-Gamboa, M.P.; Rincón-Gamboa, S.M.; Ardila-Leal, L.D.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. Impact of antibiotics as waste, physical, chemical, and enzymatical degradation: Use of laccases. Molecules 2022, 27, 4436. [Google Scholar] [CrossRef]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases-A review. J. Cleaner Product. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Liu, G.H.; Wang, H.Y.; Chen, D.H.; Dai, C.C.; Zhang, Z.H.; Feng, Y.J. Photodegradation performances and transformation mechanism of sulfamethoxazole with CeO2/CN heterojunction as photocatalyst. Sep. Purif. Technol. 2020, 237, 116329. [Google Scholar] [CrossRef]
- Yang, L.H.; Qiao, B.; Xu, Q.M.; Liu, S.; Yuan, Y.; Cheng, J.S. Biodegradation of sulfonamide antibiotics through the heterologous expression of laccases from bacteria and investigation of their potential degradation pathways. J. Hazar. Mater. 2021, 416, 125815. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The effect of bio-electrochemical systems on antibiotics removal and antibiotic resistance genes: A review. Chem. Engin. J. 2019, 358, 1421–1437. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, C.K. Recent development in the sustainable remediation of antibiotics: A review. Total Environ. Res. Themes 2022, 3, 100008. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Degórska, O.; Kaźmierczak, K.; Nguyen, L.N.; Jesionowski, T. Enhanced wastewater treatment by immobilized enzymes. Curr. Pollut. Rep. 2021, 7, 167–179. [Google Scholar] [CrossRef]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: Mechanisms, challenges, and future prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- El Yagoubi, Y.; Lemieux, B.; Segura, P.A.; Cabana, H. Characterization of laccases from Trametes hirsuta in the context of bioremediation of wastewater treatment plant effluent. Enz. Microb. Technol. 2023, 171, 110308. [Google Scholar] [CrossRef]
- Kumar, L.; Bharadvaja, N. Chapter 6: Enzymatic bioremediation: A smart tool to fight environmental pollutants. In Smart Bioremediation Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 99–118. [Google Scholar] [CrossRef]
- Singh, S.K.; Khajuria, R.; Kaur, L. Biodegradation of ciprofloxacin by white rot fungus Pleurotus ostreatus. 3 Biotech 2017, 7, 69. [Google Scholar] [CrossRef]
- Ben Ayed, A.; Akrout, I.; Albert, Q.; Greff, S.; Simmler, C.; Armengaud, J.; Kielbasa, M.; Turbé-Doan, A.; Chaduli, D.; Navarro, D.; et al. Biotransformation of the Fluoroquinolone, Levofloxacin, by the White-Rot Fungus Coriolopsis gallica. J. Fungi 2022, 8, 965. [Google Scholar] [CrossRef]
- Prieto, A.; Möder, M.; Rodil, R.; Adrian, L.; Marco-Urrea, E. Degradation of the antibiotics norfloxacin and ciprofloxacin by a white-rot fungus and identification of degradation products. Bioresour. Technol. 2011, 102, 10987–10995. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Masís-Mora, M.; Corcellas, C.; Eljarrat, E.; Barceló, D.; Sarrà, M.; Caminal, G.; Vicent, T.; Rodríguez-Rodríguez, C.E. Degradation of selected agrochemicals by the white rot fungus Trametes versicolor. Sci. Total Environ. 2014, 500, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Park, B.J.; Jeong, H.R.; Lee, J.T.; Cho, J.Y. Biodegradation of B-lactam antibiotic” ampicillin” by white rot fungi from aqueous solutions. J. Pure Appl. Microbiol. 2013, 7, 3163–3169. [Google Scholar]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef]
- Kupski, L.; Salcedo, G.M.; Caldas, S.S.; de Souza, T.D.; Furlong, E.B.; Primel, E.G. Optimization of a laccase-mediator system with natural redox-mediating compounds for pesticide removal. Environ. Sci. Pollut. Res. 2019, 26, 5131–5139. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Nasseri, S.; Alimohammadi, M.; Mahvi, A.H.; Faramarzi, M.A. Application of free and immobilized laccase for removal and detoxification of fluoroquinolones from aqueous solution. Global Nest. J. 2020, 22, 240–249. [Google Scholar] [CrossRef]
- Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: Production and role from in lignin degradation. FEMS Microbiol. Rev. 1994, 13, 125–135. [Google Scholar] [CrossRef]
- Masaphy, S.; Levanon, D. The effect of lignocellulose on lignocellulolytic activity of Pleurotus pulmonarius in submerged culture. Appl. Microbiol. Biotechnol. 1992, 36, 828–832. [Google Scholar] [CrossRef]
- Pointing, S. Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar] [CrossRef]
- Agarwal, N.; Solanki, V.S.; Gacem, A.; Hasan, M.A.; Pare, B.; Srivastava, A.; Jeon, B.H. Bacterial Laccases as Biocatalysts for the Remediation of Environmental Toxic Pollutants: A Green and Eco-Friendly Approach-A Review. Water 2022, 14, 4068. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, A.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S.; Garcia, O.; Santos, A.; Téllez, C. Laccase-catalyzed degradation of anti-inflammatories and antibiotics. Chemosphere 2007, 67, 793–799. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Lú-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Engin. J. 2010, 51, 124–131. [Google Scholar] [CrossRef]
- Camarero, S.; Garcia, O.; Vidal, T.; Colom, J.F.; del Río, J.C.; Gutiérrez, A.; Martínez, Á.T. Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzym. Microb. Technol. 2005, 35, 113–120. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of estrogens by laccase from Trametes versicolor under batch and continuous conditions. Chemosphere 2010, 78, 101–106. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, D.; Wang, Q.; Liu, C.; Wang, C. Electro-Fenton process and its application in hospital wastewater treatment: A review. Chem Engin. J. 2021, 409, 128212. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, L.; Yang, M.; Zhang, C. Enhanced removal of antibiotics by a novel bioelectro-Fenton system: Performance and mechanism. Bioresour. Technol. 2020, 310, 123433. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A prom-ising technology for wastewater treatment and bioenergy. Sci. Total Environ. 2017, 595, 567–581. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L.; Zhang, Z.; Guo, S.; Shang, H.; Li, Y.; Liu, J. Wearable biofuel cells based on the classification of enzyme for high power outputs and lifetimes. Biosens. Bioelectron. 2019, 124, 40–52. [Google Scholar] [CrossRef]
- Couto, S.R.; Herrera, J.L.T. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Couto, S.R.; Toca-Herrera, J.L. Laccases in the textile industry. Biotechnol. Mol. Biol. Rev. 2006, 1, 115–120. [Google Scholar]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazar. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.M.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.C.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Kim, Y.J.; Nicell, J.A. Laccase-catalysed oxidation of aqueous triclosan. J. Chem. Technol. Biotechnol. Int. Res. Proc. Environ. Clean Technol. 2006, 81, 1344–1352. [Google Scholar] [CrossRef]
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A review of the fate of micropollutants in wastewater treatment plants. Wiley Interdiscip. Rev. Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G.; Freiermuth, B.; Bodie, E.; Borneman, S. Reactivities of Various Mediators and Laccases with Kraft Pulp and Lignin Model Compounds. Appl. Environ. Microbiol. 1997, 63, 4627–4632. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, D.; Jiang, S.; Yu, H.; Cheng, Y.; Zhang, S.; Zhang, X.; Chen, W. The study of laccase immobilization optimization and stability improvement on CTAB-KOH modified biochar. BMC Biotechnol. 2021, 21, 47. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Laccase immobilization for water purification: A comprehensive review. Chem. Engin. J. 2021, 403, 126272. [Google Scholar] [CrossRef]
- Fernandez-Fernandez, M.; Sanromán, M.Á.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- Olshansky, Y.; Masaphy, S.; Root, R.A.; Rytwo, G. Immobilization of Rhus vernicifera laccase on sepiolite; effect of chitosan and copper modification on laccase adsorption and activity. Appl. Clay Sci. 2018, 152, 143–147. [Google Scholar] [CrossRef]
- Cabana, H.; Ahamed, A.; Leduc, R. Conjugation of laccase from the white rot fungus Trametes versicolor to chitosan and its utilization for the elimination of triclosan. Bioresour. Technol. 2011, 102, 1656–1662. [Google Scholar] [CrossRef]
- Aricov, L.; Leonties, A.R.; Gîfu, I.C.; Preda, D.; Raducan, A.; Anghel, D.F. Enhancement of laccase immobilization onto wet chitosan microspheres using an iterative protocol and its potential to remove micropollutants. J. Environ. Manag. 2020, 276, 111326. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.P.; Hung, Y.; Chang, J.H.; Lin, C.H.; Mou, C.Y. Enzyme encapsulated hollow silica nanospheres for intracellular biocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 6883–6890. [Google Scholar] [CrossRef]
- Chang, B.V.; Ren, Y.L. Biodegradation of three tetracyclines in river sediment. Ecol. Eng. 2015, 75, 272–277. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresour. Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Arca-Ramos, A.; Kumar, V.V.; Eibes, G.; Moreira, M.T.; Cabana, H. Recyclable cross-linked laccase aggregates coupled to magnetic silica microbeads for elimination of pharmaceuticals from municipal wastewater. Environ. Sci. Pollut. Res. 2016, 23, 8929–8939. [Google Scholar] [CrossRef]
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Covalent immobilization of laccase onto nanofibrous membrane for degradation of pharmaceutical residues in water. ACS Sustain. Chem. Engin. 2017, 5, 10430–10438. [Google Scholar] [CrossRef]
- Kwak, J.; Yoon, S.; Mahanty, B.; Kim, C.G. Redox-mediator-free degradation of sulfathiazole and tetracycline using Phanerochaete chrysosporium. J. Environ. Sci. Health Part A 2017, 52, 1211–1217. [Google Scholar] [CrossRef]
- Garcia-Delgado, C.; Eymar, E.; Camacho-Arévalo, R.; Petruccioli, M.; Crognale, S.; D’Annibale, A. Degradation of tetracyclines and sulfonamides by stevensite biochar-immobilized laccase systems and impact on residual antibiotic activity. J. Chem. Technol. Biotechnol. 2018, 93, 3394–3409. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 2019, 222, 865–871. [Google Scholar] [CrossRef]
- Jeong, D.; Choi, K.Y. Biodegradation of tetracycline antibiotic by laccase biocatalyst immobilized on chitosan-tripolyphosphate beads. Appl. Biochem. Microbiol. 2020, 56, 306–312. [Google Scholar] [CrossRef]
- Tian, Q.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.; Shen, Y.; You, C.; Guan, Z.; Liao, X. Characterization of a robust cold-adapted and thermostable laccase from Pycnoporus sp. SYBC-L10 with a strong ability for the degradation of tetracycline and oxytetracycline by laccase-mediated oxidation. J. Hazar. Mater. 2020, 382, 121084. [Google Scholar] [CrossRef]
- Navada, K.K.; Kulal, A. Enzymatic degradation of chloramphenicol by laccase from Trametes hirsuta and comparison among mediators. Int. Biodeter. Biodegrad. 2019, 138, 63–69. [Google Scholar] [CrossRef]
- Xu, X.; Feng, L.; Han, Z.; Luo, S.; Wu, A.; Xie, J. Selection of high laccase-producing Coriolopsis gallica strain T906: Mutation breeding, strain characterization, and features of the extracellular laccases. J. Microbiol. Biotechnol. 2016, 26, 1570–1578. [Google Scholar] [CrossRef]
- Ren, D.; Wang, Z.; Jiang, S.; Yu, H.; Zhang, S.; Zhang, X. Recent environmental applications of and development prospects for immobilized laccase: A review. Biotechnol. Genetic Engineer. Rev. 2020, 36, 81–131. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zeng, G.; Liu, Y.; Yang, X.; Zhou, C.; Chen, M.; Liu, Y.; Jiang, Y.; Yan, M. Immobilization of laccase on hollow mesoporous carbon nanospheres: Noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal. J. Hazar. Mater. 2019, 362, 318–326. [Google Scholar] [CrossRef]
- Kadam, A.A.; Shinde, S.K.; Ghodake, G.S.; Saratale, G.D.; Saratale, R.G.; Sharma, B.; Hyun, S.; Sung, J.S. Chitosan-grafted halloysite nanotubes-Fe3O4 composite for laccase-immobilization and sulfamethoxazole-degradation. Polymers 2020, 12, 2221. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Bachosz, K.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Kaczorek, E.; Jesionowski, T. A promising laccase immobilization using electrospun materials for biocatalytic degradation of tetracycline: Effect of process conditions and catalytic pathways. Catal. Today 2020, 348, 127–136. [Google Scholar] [CrossRef]
- Lou, X.; Zhi, F.; Sun, X.; Wang, F.; Hou, X.; Lv, C.; Hu, Q. Construction of co-immobilized laccase and mediator based on MOFs membrane for enhancing organic pollutants removal. Chem. Eng. J. 2023, 451, 138080. [Google Scholar] [CrossRef]
- Zhang, L.B.; Qiu, T.T.; Qiu, X.G.; Yang, W.W.J.; Ye, X.Y.; Meng, C. Transcriptomic and metabolomic analysis unveils a negative effect of glutathione metabolism on laccase activity in Cerrena unicolor 87613. Microbiol. Spect. 2024, 12, e03405–e03423. [Google Scholar] [CrossRef]
- Ghai, M.K.; Khatri, A.; Kumar, K.; Thakur, I.S. Multi-omics and advance technologies in biodegradation of emerging contaminants and eco-estrogens in environmental waste. Total Environ. Adv. J. 2024, 11, 200113. [Google Scholar] [CrossRef]
- Beloqui, A.; Pita, M.; Polaina, J.; Martínez-Arias, A.; Golyshina, O.V.; Zumárraga, M.; Golyshin, P.N. Novel polyphenol oxidase mined from a metagenome expression library of bovine rumen: Biochemical properties, structural analysis, and phylogenetic relationships. J. Biol. Chem. 2006, 281, 22933–22942. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, T.; Wang, Q.; Zhang, X.; Peng, H.; Fang, W.; Hong, Y.; Ge, H.; Xiao, Y. A bacterial laccase from marine microbial metagenome exhibiting chloride tolerance and dye decolorization ability. Appl. Microbiol. Biotechnol. 2011, 89, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, expression, regulation, and applications in pharmaceutical biodegradation. Front. Microbiol. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Chica, R.A.; Doucet, N.; Pelletier, J.N. Semi-rational approaches to engineering enzyme activity: Combining the benefits of directed evolution and rational design. Curr. Opin. Biotechnol. 2005, 16, 378–384. [Google Scholar] [CrossRef]
- Alcalde, M. Engineering the ligninolytic enzyme consortium. Trends Biotechnol. 2015, 33, 155–162. [Google Scholar] [CrossRef]
- Risso, V.A.; Gavira, J.A.; Gaucher, E.A.; Sanchez-Ruiz, J.M. Phenotypic comparisons of consensus variants versus laboratory resurrections of Precambrian proteins. Proteins 2014, 82, 887–896. [Google Scholar] [CrossRef]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martínez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Sci 2012, 336, 1715–1719. [Google Scholar] [CrossRef]
- Yang, J.; Ng, T.B.; Lin, J.; Ye, X. A novel laccase from basidiomycete Cerrena sp.: Cloning, heterologous expression, and characterization. Int. J. Biol. Macromol. 2015, 77, 344–349. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, G.; Lin, Y.; Xu, X.; Yang, J. A highly thermotolerant laccase produced by Cerrena unicolor strain CGMCC 5.1011 for complete and stable malachite green decolorization. AMB Express 2020, 10, 178. [Google Scholar] [CrossRef]
- Iimura, Y.; Sonoki, T.; Habe, H. Heterologous expression of Trametes versicolor laccase in Saccharomyces cerevisiae. Protein Exp. Purif. 2018, 141, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.F.; Hu, J.H.; Guo, C.; Liu, C.Z. Scale-up laccase production from Trametes versicolor stimulated by vanillic acid. Bioproc. Biosyst. Engin. 2016, 39, 1041–1049. [Google Scholar] [CrossRef]
- Yuliana, T.; Komara, D.Z.; Saripudin, G.L.U.; Subroto, E.; Safitri, R. Potential of Lignocellulosic Waste for Laccase Production by Trametes versicolor under Submerged Fermentation. Pak. J. Biol. Sci. 2021, 24, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.M.; Record, E.; Lomascolo, A.; Scholtmeijer, K.; Asther, M.; Wessels, J.G.; Wösten, H.A. Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 2000, 70, 6379–6384. [Google Scholar] [CrossRef]
- Mate, D.M.; Alcalde, M. Laccase engineering: From rational design to directed evolution. Biotechnol. Adv. 2015, 33, 25–40. [Google Scholar] [CrossRef]
- Forootanfar, H.; Faramarzi, M.A. Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol. Prog. 2015, 31, 1443–1463. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E. Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J. Biotechnol. 2009, 144, 37–42. [Google Scholar] [CrossRef]
- Weenink, X.O.; Punt, P.J.; Van Den Hondel, C.A.; Ram, A.F. A new method for screening and isolation of hypersecretion mutants in Aspergillus niger. Appl. Microbiol. Biotechnol. 2006, 69, 711–717. [Google Scholar] [CrossRef]
- Bailey, M.J.; Adamitsch, B.; Rautio, J.; von Weymarn, N.; Saloheimo, M. Use of a growth-associated control algorithm for efficient production of a heterologous laccase in Trichoderma reesei in fed-batch and continuous cultivation. Enzyme Microb. Technol. 2007, 41, 484–491. [Google Scholar] [CrossRef]
- Choudhary, A.; Tiwari, A.; Bansal, H. Molecular docking analysis of laccase mediated bioremediation of pharmaceutical compounds from wastewater. Syst. Microbiol. Biomanuf. 2025, 5, 854–864. [Google Scholar] [CrossRef]
- Mora-Gamboa, M.P.; Ardila-Leal, L.D.; Galindo, J.F.; Poutou-Piñales, R.A.; Quevedo-Hidalgo, B.E. “In Silico” prediction of antibiotics biodegradation by Ganoderma lucidum GILCC 1 laccase. Discover. Appl. Sci. 2024, 6, 418. [Google Scholar] [CrossRef]
- Martin, E.; Dubessay, P.; Record, E.; Audonnet, F.; Michaud, P. Recent advances in laccase activity assays: A crucial challenge for applications on complex substrates. Enzyme Microbial Technol. 2024, 173, 110373. [Google Scholar] [CrossRef]
- Shraddha Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Dey, P. Optimization of growth conditions for enhancing the production of microbial laccase and its application in treating antibiotic contamination in wastewater. 3 Biotech. 2021, 11, 81. [Google Scholar] [CrossRef]
- Llorca, M.; Rodríguez-Mozaz, S.; Couillerot, O.; Panigoni, K.; de Gunzburg, J.; Bayer, S.; Czaja, R.; Barceló, D. Identification of new transformation products during enzymatic treatment of tetracycline and erythromycin antibiotics at laboratory scale by an on-line turbulent flow liquid-chromatography coupled to a high resolution mass spectrometer LTQ-Orbitrap. Chemosphere 2015, 119, 90–98. [Google Scholar] [CrossRef] [PubMed]
- De Cazes, M.D.; Belleville, M.P.; Mougel, M.; Kellner, H.; Sanchez-Marcano, J. Characterization of laccase-grafted ceramic membranes for pharmaceuticals degradation. J. Membr. Sci. 2015, 476, 384–393. [Google Scholar] [CrossRef]
- Becker, D.; Della Giustina, S.V.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase–degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, M.H.; Qi, X.M.; Hong, D.; Dai, L.Z.; Li, S.Y.; Lu, Y.C.; Yu, H.Q. Laccase-evoked removal of antibiotics: Reaction kinetics, conversion mechanisms, and ecotoxicity assessment. Crit. Rev. Environ. Sci. Technol. 2024, 54, 162–183. [Google Scholar] [CrossRef]
- Fillat, A.; Colom, J.F.; Vidal, T. A new approach to the biobleaching of flax pulp with laccase using natural mediators. Bioresour. Technol. 2010, 101, 4104–4110. [Google Scholar] [CrossRef]
- Weng, S.S.; Liu, S.M.; Lai, H.T. Application parameters of laccase-mediator systems for treatment of sulfonamide antibiotics. Bioresour. Technol. 2013, 141, 152–159. [Google Scholar] [CrossRef]
- Wei, J.; Ma, D.; Ma, X.; Sheng, Q.; Sun, X.; Li, J.; Liu, X.; Shen, J.; Zheng, M.; Wang, L. New insight into increased toxicity during ozonation of chlorophenol: The significant contribution of oxidizing intermediate. Sci. Total Environ. 2021, 769, 144569. [Google Scholar] [CrossRef]
- Zhang, C.; You, S.; Zhang, J.; Qi, W.; Su, R.; He, Z. An effective in-situ method for laccase immobilization: Excellent activity, effective antibiotic removal rate and low potential ecological risk for degradation products. Bioresour. Technol. 2020, 308, 123271. [Google Scholar] [CrossRef]
- Liang, S.; Luo, Q.; Huang, Q. Degradation of sulfadimethoxine catalyzed by laccase with soybean meal extract as natural mediator: Mechanism and reaction pathway. Chemosphere 2017, 181, 320–327. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Aptitude of oxidative enzymes for treatment of wastewater pollutants: A laccase perspective. Molecules 2019, 24, 2064. [Google Scholar] [CrossRef] [PubMed]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Johannes, C.; Majcherczyk, A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 2000, 66, 524–528. [Google Scholar] [CrossRef]
- D’Acunzo, F.; Galli, C. First evidence of catalytic mediation by phenolic compounds in the laccase-induced oxidation of lignin models. Eur. J. Biochem. 2003, 270, 3634–3640. [Google Scholar] [CrossRef]
- Eggert, C.; Temp, U.; Dean, J.F.D.; Eriksson, K.E.L. A fungal metabolite mediates degradation of non-phenoliclignin structures and synthetic lignin by laccase. Febs Lett. 1996, 391, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Li, K. Degradation of non-phenolic lignin by the white-rot fungus Pycnoporus cinnabarinus. Appl. Microbiol. Biotechnol. 2002, 60, 342–346. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” Laccases. J. Biochem. 2007, 72, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Iqhrammullah, M.; Fahrina, A.; Chiari, W.; Ahmad, K.; Fitriani, F.; Suriaini, N.; Safitri, E.; Puspita, K. Laccase immobilization using polymeric supports for wastewater treatment: A critical review. Macromol. Chem. Phys. 2023, 224, 2200461. [Google Scholar] [CrossRef]
- Najafvand, S.; Motamedi, E.; Ghollasi, M.; Irian, S.; Ariaeenejad, S. Performance improvement of metagenomic laccase immobilized on nanocellulose-reinforced hydrogel nanocomposites for enhanced delignification and detoxification. Ind. Crops Prod. 2024, 208, 117840. [Google Scholar] [CrossRef]
- Motamedi, E.; Kavousi, K.; Motahar, S.F.S.; Ghaffari, M.R.; Mamaghani, A.S.A.; Salekdeh, G.H.; Ariaeenejad, S. Efficient removal of various textile dyes from wastewater by novel thermo-halotolerant laccase. Bioresour. Technol. 2021, 337, 125468. [Google Scholar] [CrossRef]
- Zhao, L.H.; Ma, Q.Q.; Nie, F.; Chen, W.; Sun, H.J. Increasing laccase activity of white rot fungi by mutagenesis and treating papermaking wastewater. IOP Conf. Ser. Earth Environ. Sci. 2018, 191, 012053. [Google Scholar] [CrossRef]
- Bu, T.; Yang, R.; Zhang, Y.; Cai, Y.; Tang, Z.; Li, C.; Wu, Q.; Chen, H. Improving decolorization of dyes by laccase from Bacillus licheniformis by random and site-directed mutagenesis. Peer J. 2020, 8, e10267. [Google Scholar] [CrossRef]
- Ariaeenejad, S.; Kavousi, K.; Jahanshahi, D.A.; Mamaghani, A.S.A.; Ghasemitabesh, R.; Moosavi-Movahedi, A.A.; Salekdeh, G.H. Enzymatically triggered delignification through a novel stable laccase: A mixed in-silico/in-vitro exploration of a complex environmental microbiota. Int. J. Biol. Macromol. 2022, 211, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Sanyal, D. Removal of emerging contaminants from pharmaceutical waste through application of bio-nanotechnology. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 1 January 2022; pp. 485–500. [Google Scholar] [CrossRef]

| Antibiotics | Origins | Applications | Concentration in the Environment | References |
|---|---|---|---|---|
| ꞵ-Lactams | Fungi and bacteria | Humans, veterinary | 1.67 µg/L (effluent) | [3,4] |
| Tetracyclines | Bacteria | Humans, veterinary, agriculture | 6.8 mg/L | [5,6] |
| Quinolones | Synthetic | Humans, veterinary, agriculture, aquaculture | 3.35 µg/L (effluent) | [4,7,8] |
| Sulfonamides | Synthetic | Humans, veterinary | 0.326 µg/L (river water) | [9,10,11] |
| Macrolides | Bacteria | Humans, veterinary | 3.847 µg/L (wastewater treatment plants) | [12,13,14] |
| Amino-Glycosides | Bacteria | Humans, veterinary | 3.4 µg/L | [15] |
| Glycopeptides | Bacteria | Humans | 0.0127 µg/L | [16,17] |
| Antibiotic Class | Antibiotic Group | White-Rot Fungi Used to Degrade Antibiotics | Reference |
|---|---|---|---|
| Fluoroquinolone | Ciprofloxacin | Pleurotus ostreatus | [60] |
| Fluoroquinolone | Levofloxacin | Coriolopsis gallica | [61] |
| Quinolones | Norfloxacin | Trametes versicolor | [62] |
| Tetracycline | Oxytetracycline | Trametes versicolor | [63] |
| β-Lactams | Ampicillin | Verticillium leptobactrum | [64] |
| Sulfonamides | Sulfamethoxazole | Phanerochaete chrysosporium | [65] |
| Aminoglycoside | Neomycin | Trametes versicolor | [61] |
| Characteristics | Bioremediation Methods: | |||
|---|---|---|---|---|
| Electro-Fenton (EF) | Bioelectro-Fenton (BEF) | Microbial Fuel Cells (MFCs) | Fungal Laccase Bioremediation | |
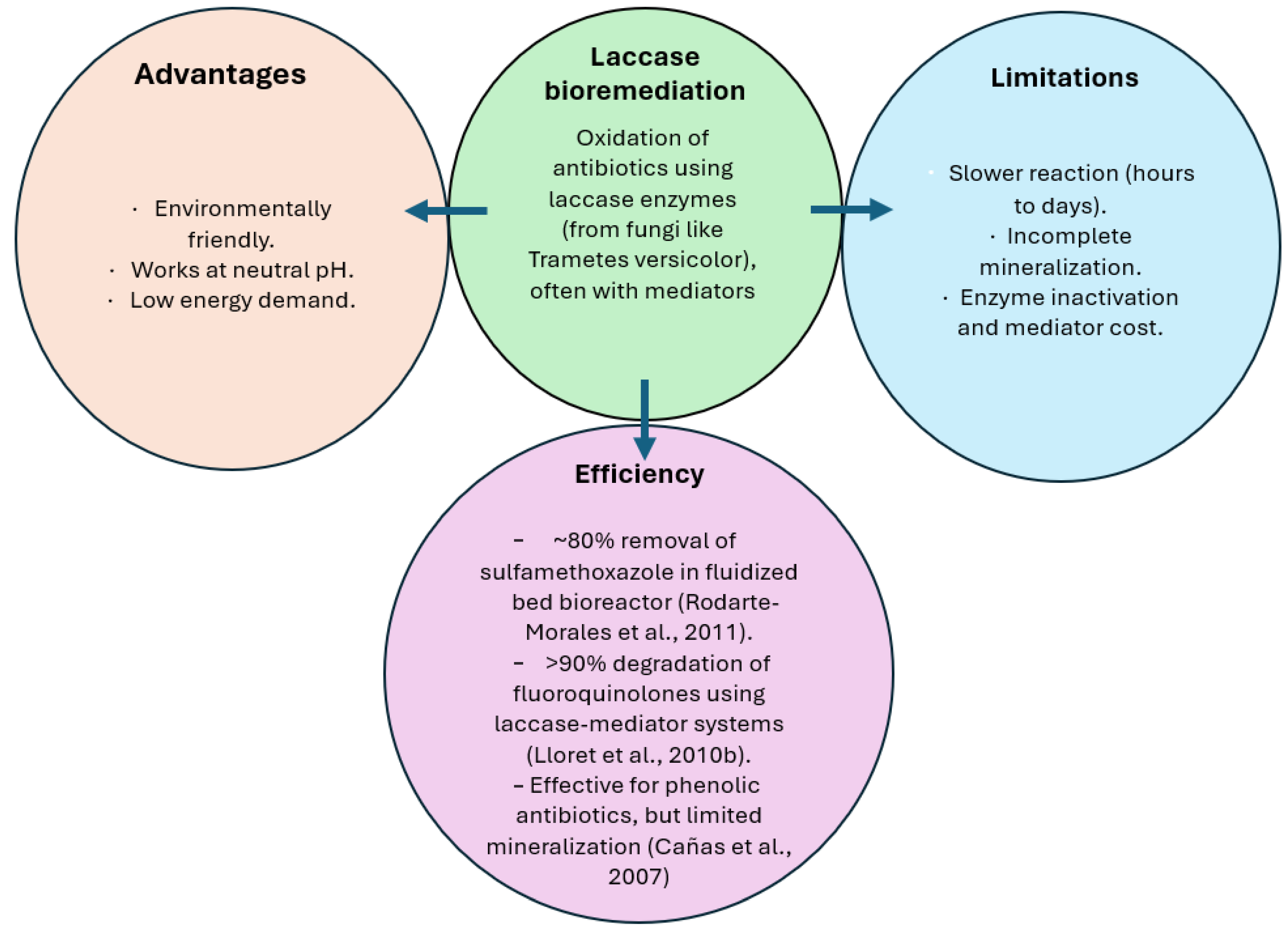
| Mode of action/ mechanism: | EF generates hydroxyl radicals (•OH) from electro-generated H2O2 and Fe2+ in acidic conditions. These radicals degrade antibiotics non-selectively. | Similar to EF, but H2O2 is produced biologically by microbes at the cathode, reducing external chemical input. | Electroactive bacteria degrade antibiotics while generating electricity. Oxygen or alternative acceptors reduce compounds at the cathode. | Oxidation of antibiotics using laccase enzymes (from fungi like Trametes versicolor), often with mediators. |
| Efficiency: | Removal rates >90% for many antibiotics (ciprofloxacin, sulfamethoxazole, amoxicillin) in <1–2 h. · High mineralization >70% total organic carbon (TOC) removal. Hospital wastewater treated via EF achieved 93% removal of antibiotics and a substantial reduction in resistance genes [80]. | Achieved 85–95% removal of tetracycline and oxytetracycline using BEF in <3 h [81]. · Less energy-intensive than EF. · Capable of treating low-concentration antibiotics in real wastewater. | MFCs removed ~70–80% of sulfamethoxazole and tetracycline over several days [82]. · Removal efficiencies vary but can reach 90% with optimized biofilms and operating conditions [83]. | ~80% removal of sulfamethoxazole in fluidized bed bioreactor [75]. >90% degradation of fluoroquinolones using laccase-mediator systems [79]. Effective for phenolic antibiotics, but limited mineralization [76]. |
| Advantages: | · Lower energy and chemical cost. · Integrates biological and electrochemical processes. · Better suited for longer treatment times and eco-friendly applications. | · Simultaneous energy production. · Sustainable and low-energy. · Less chemical input compared to EF. | ·Environmentally friendly. · Works at neutral pH. · Low energy demand. | |
| Limitations: | · Slower than EF/BEF. · Lower mineralization. · Sensitive to environmental fluctuations. | · Slower reaction (hours to days). · Incomplete mineralization. · Enzyme inactivation and mediator cost. | ||
| Fungi | Enzyme | Matrix | Method | Compound | References |
|---|---|---|---|---|---|
| Pleurotus eryngii | Laccase | Microcapsule | Immobilization (encapsulation) | Tetracycline | [97,98] |
| Aspergillus oryzae | Laccase | Granular activated carbon | Immobilization (adsorption) | Sulfamethoxazole | [99] |
| Trametes versicolor | Laccase | Magnetic silica microbeads | Immobilization (covalent binding) | Acetaminophen (Paracetamol) | [100] |
| Trametes versicolor | Laccase | Polyacrylonitrile-biochar composite nanofibrous membrane | Immobilization (adsorption) | Chlortetracycline | [101] |
| Cerrena unicolor | Laccase | Magnetic nanoparticles cross-linked to laccase | Immobilization (covalent binding) | Tetracycline, Oxytetracycline, Ampicillin, Sulfamethoxazole Erythromycin | [86] |
| Phanerochaete chrysosporium | Laccase | - | Liquid-phase batch experiments | Tetracycline and sulfathiazole | [102] |
| Trametes versicolor | Laccase | Biochar and stevensite | Immobilization (covalent binding) | Tetracycline | [103] |
| Trametes versicolor | Laccase | Bentonite-derived mesoporous material | Immobilization (adsorption) | Tetracycline | [104] |
| Trametes versicolor | Laccase | Chitosan tripolyphosphate beads | Immobilization (covalent binding) | Tetracycline | [105] |
| Pycnoporus sp. | Laccase | - | Batch culture | Tetracycline | [106] |
| Trametes hirsuta | Laccase | - | Batch culture | Chloramphenicol | [107] |
| Coriolopsis gallica | Laccase | - | Solid and liquid media | Levofloxacin | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezra, R.; Vanti, G.; Masaphy, S. Sustainable, Targeted, and Cost-Effective Laccase-Based Bioremediation Technologies for Antibiotic Residues in the Ecosystem: A Comprehensive Review. Biomolecules 2025, 15, 1138. https://doi.org/10.3390/biom15081138
Ezra R, Vanti G, Masaphy S. Sustainable, Targeted, and Cost-Effective Laccase-Based Bioremediation Technologies for Antibiotic Residues in the Ecosystem: A Comprehensive Review. Biomolecules. 2025; 15(8):1138. https://doi.org/10.3390/biom15081138
Chicago/Turabian StyleEzra, Rinat, Gulamnabi Vanti, and Segula Masaphy. 2025. "Sustainable, Targeted, and Cost-Effective Laccase-Based Bioremediation Technologies for Antibiotic Residues in the Ecosystem: A Comprehensive Review" Biomolecules 15, no. 8: 1138. https://doi.org/10.3390/biom15081138
APA StyleEzra, R., Vanti, G., & Masaphy, S. (2025). Sustainable, Targeted, and Cost-Effective Laccase-Based Bioremediation Technologies for Antibiotic Residues in the Ecosystem: A Comprehensive Review. Biomolecules, 15(8), 1138. https://doi.org/10.3390/biom15081138







