Baicalin, Amoxicillin, and Probenecid Provide Protection in Mice Against Glaesserella parasuis Challenge
Abstract
1. Introduction
2. Results
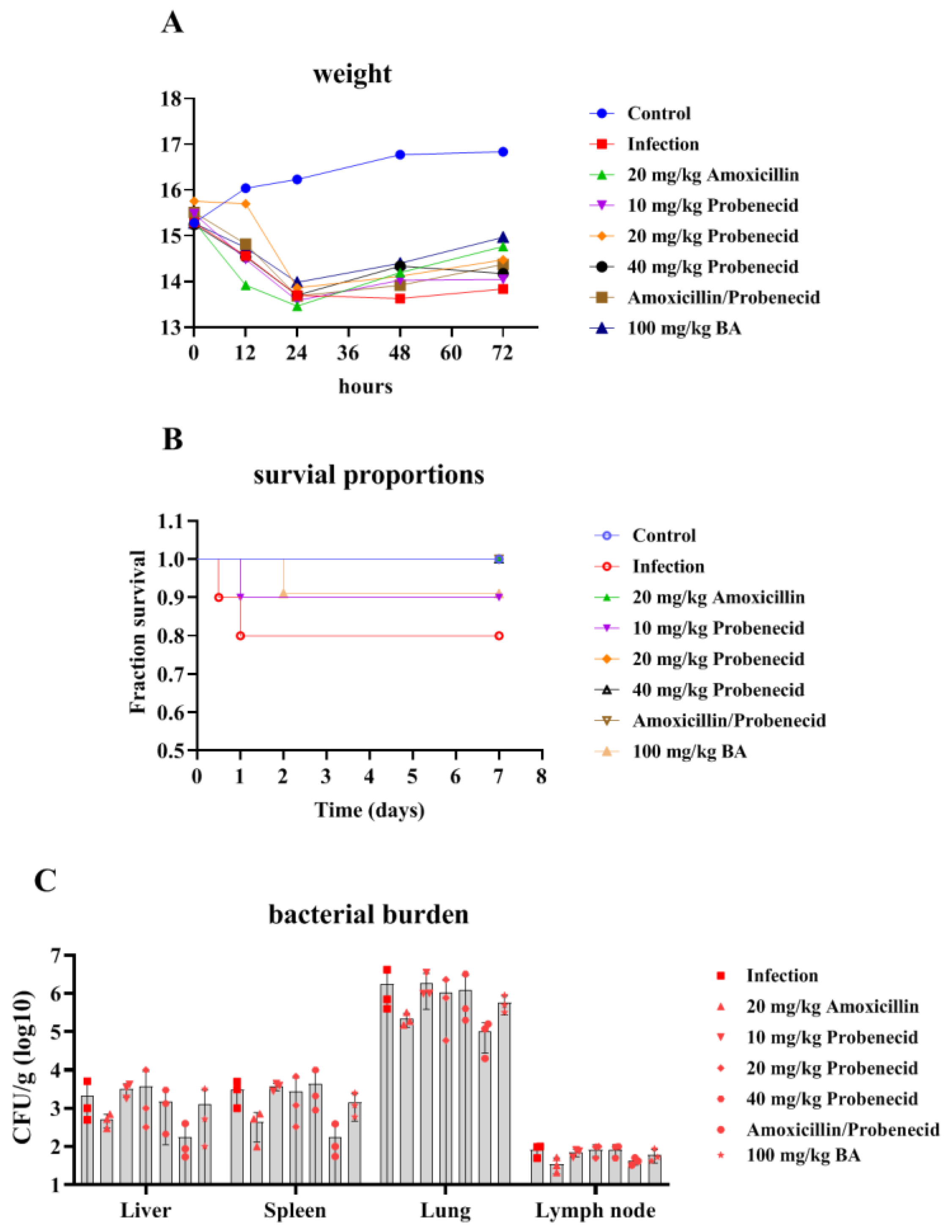
2.1. The Effects of Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin on the Body Weight (BW), Survival Rate, and Bacterial Burden of G. parasuis-Challenged Mice
2.2. Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin Altered the Blood Biochemical Parameters and Routine Blood Test Indicators in G. parasuis-Challenged Mice
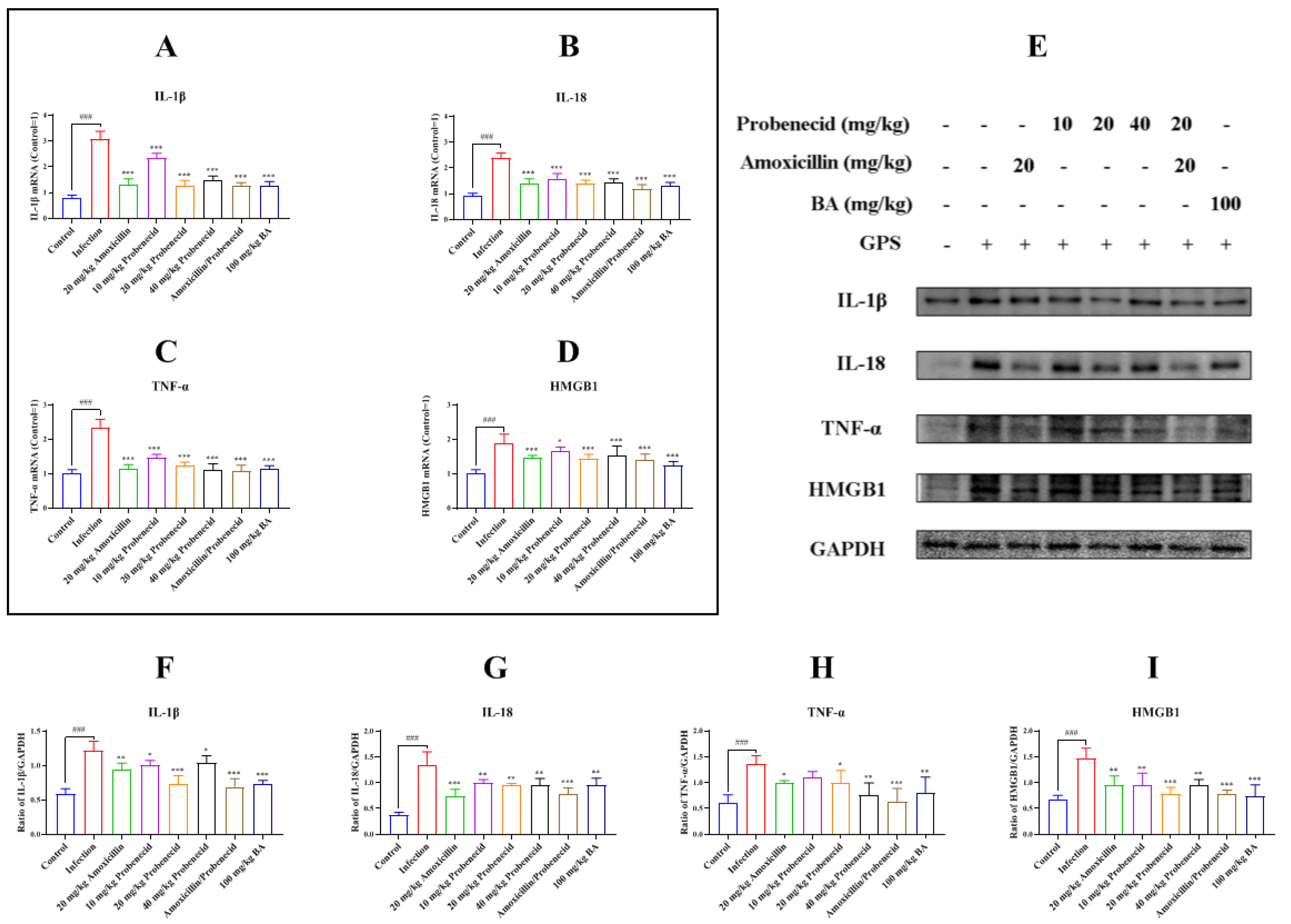
2.3. Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin Inhibited Inflammatory Cytokines and High Mobility Group Box 1 Protein (HMGB1) Production in G. parasuis-Challenged Mice
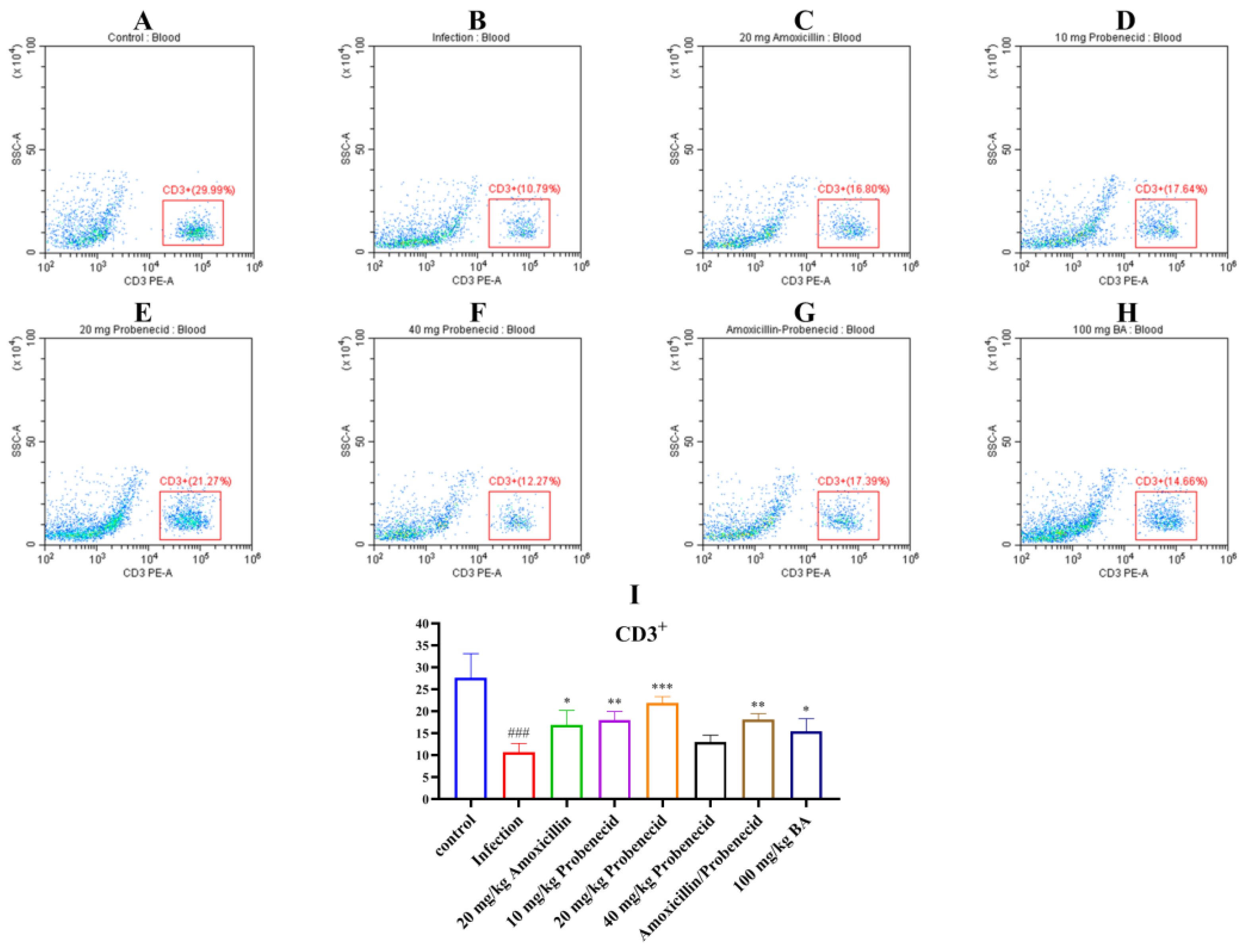
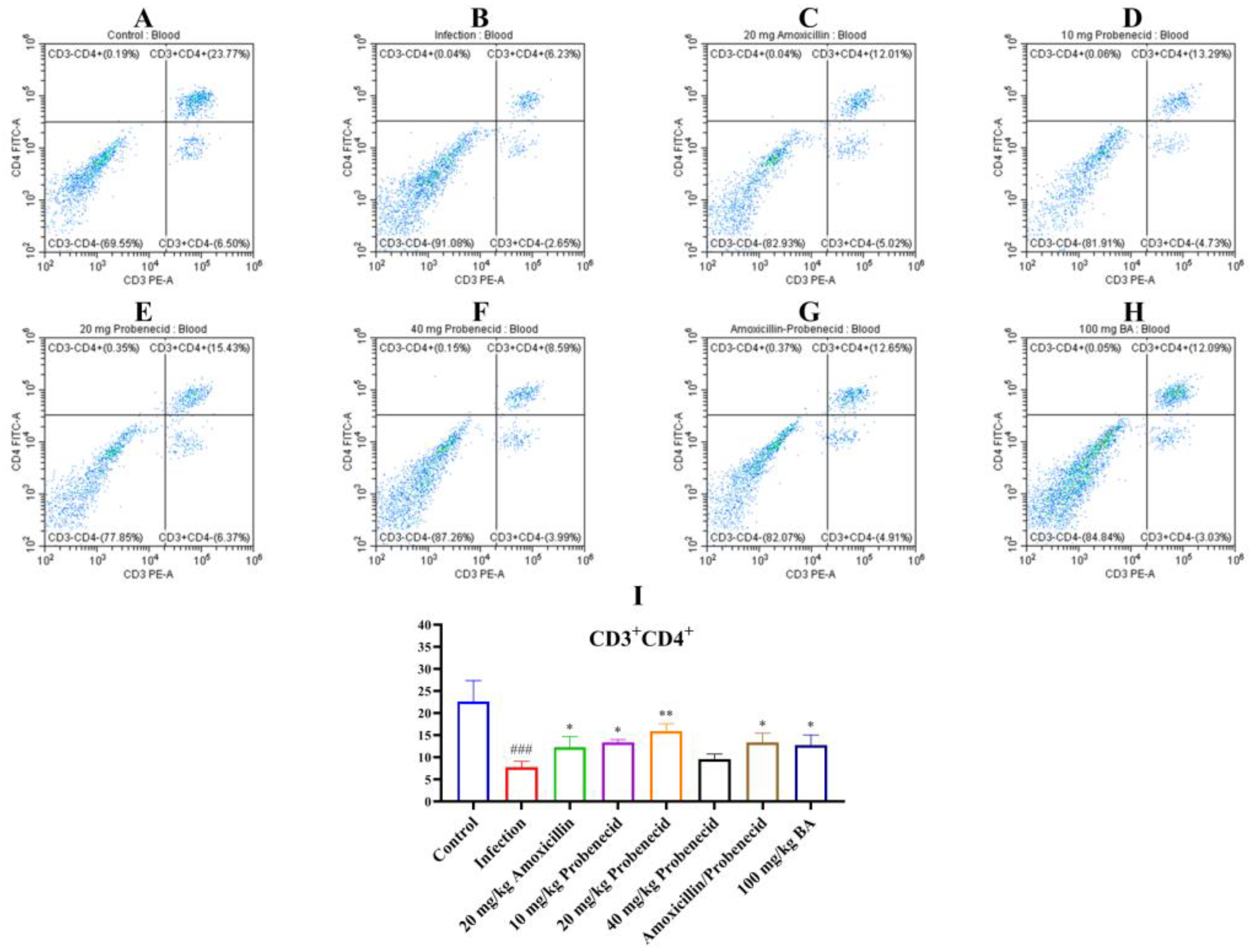
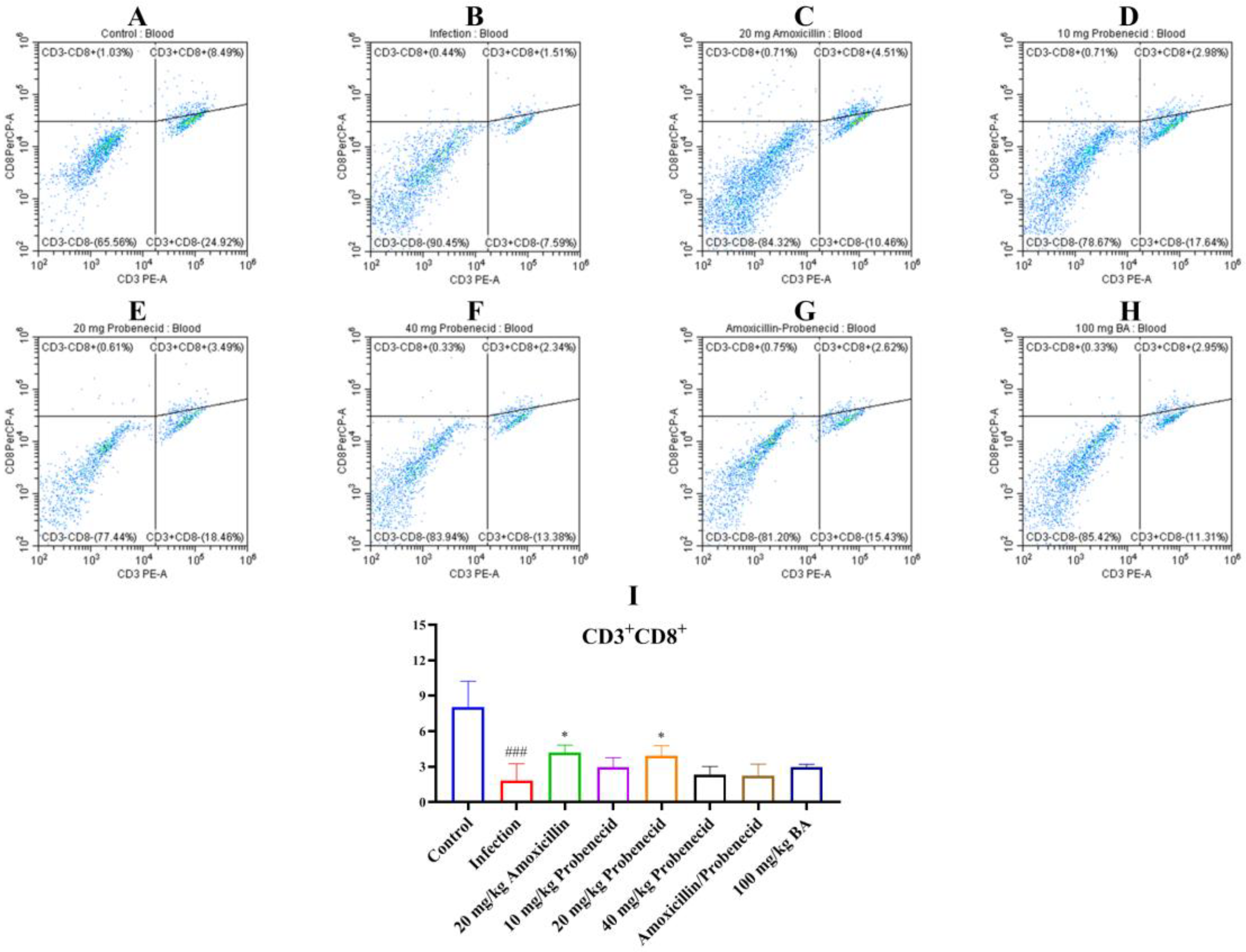
2.4. Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin Modified the Proportion of CD3+, CD4+, and CD8+ T Cells in the Blood and Spleen of G. parasuis-Challenged Mice
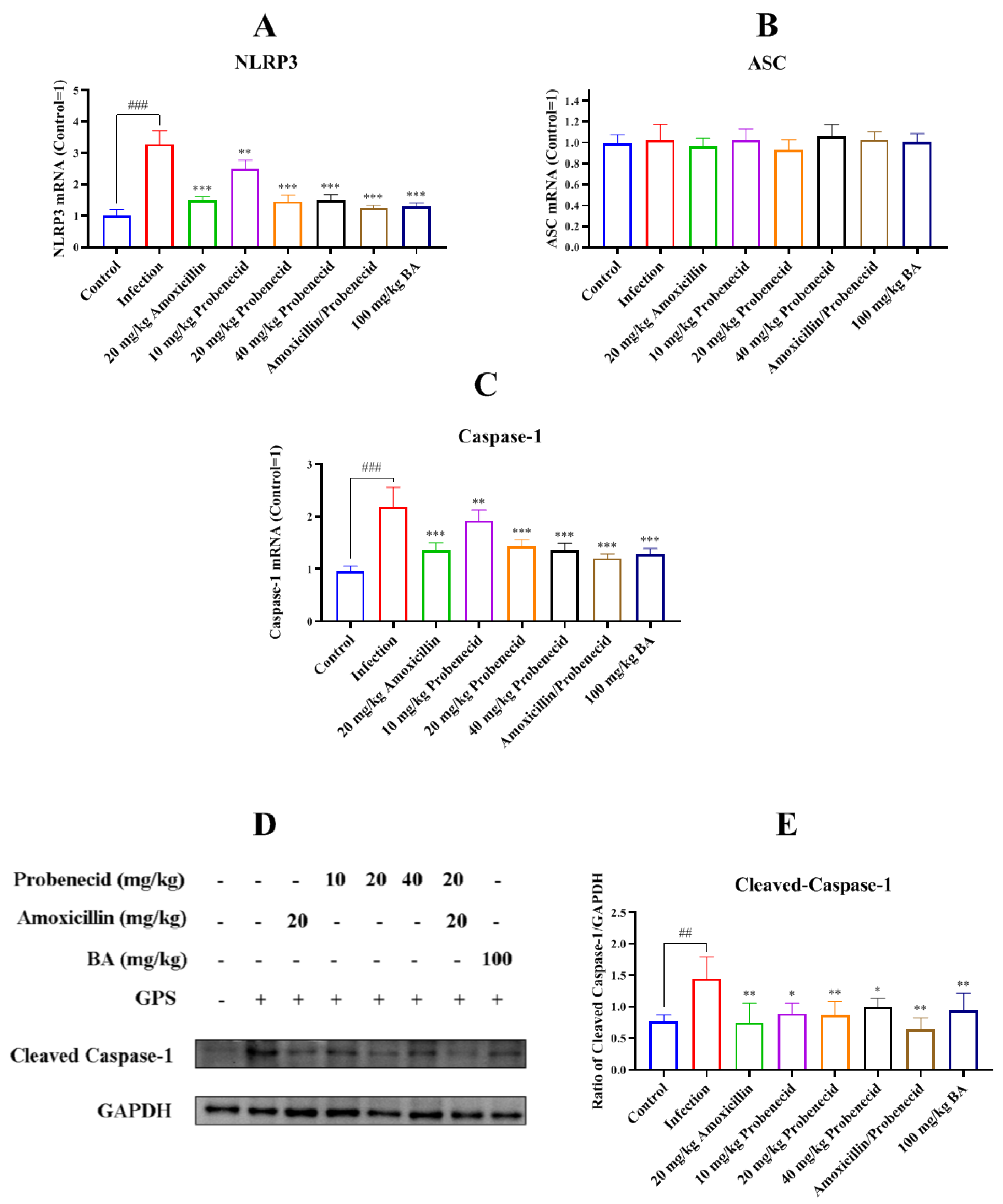
2.5. Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin Attenuated NLR Family Pyrin Domain Containing 3 (NLRP3) Inflammasome Activation in Spleen of G. parasuis-Challenged Mice
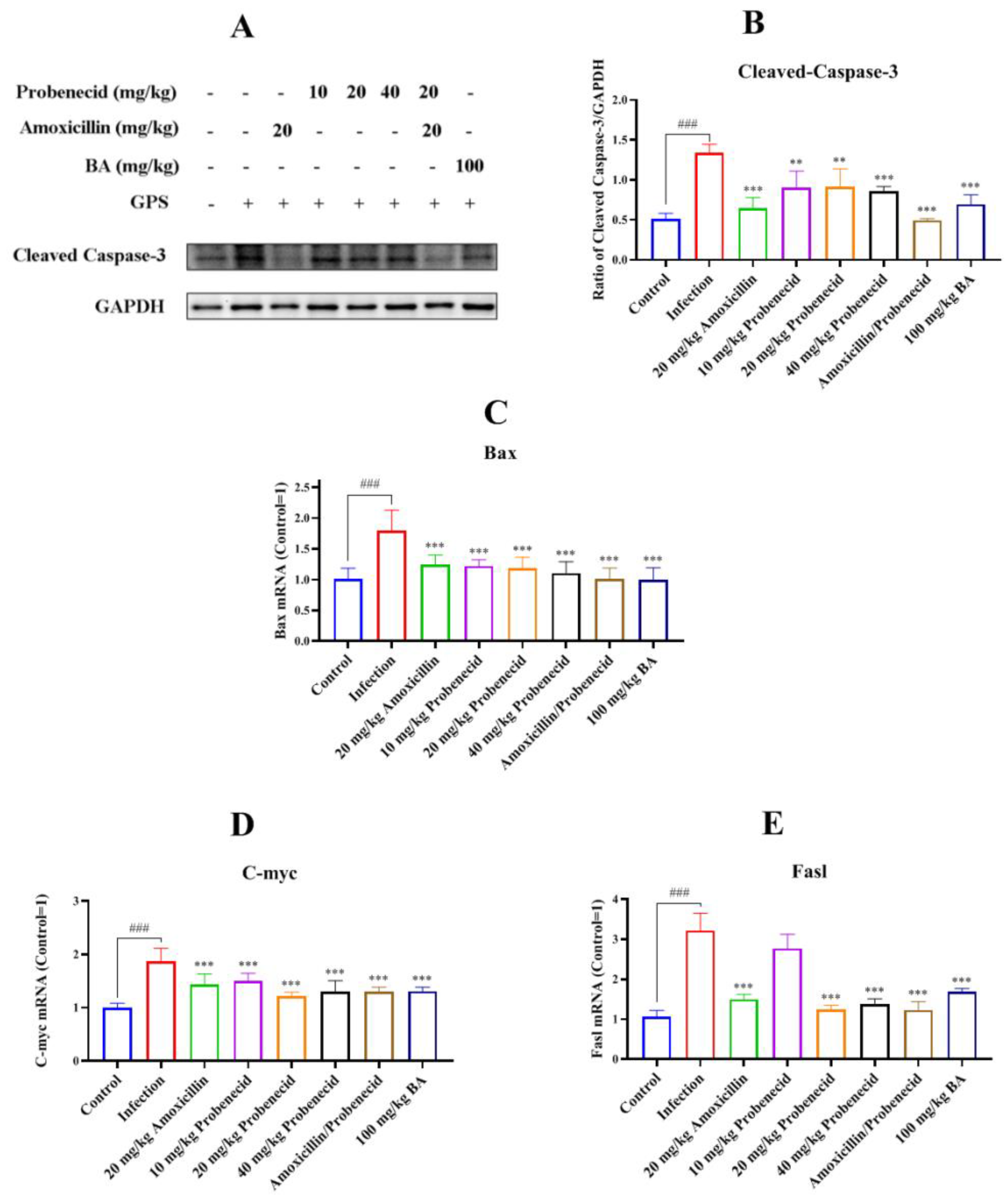
2.6. Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin Inhibited Apoptosis in the Spleen of G. parasuis-Challenged Mice
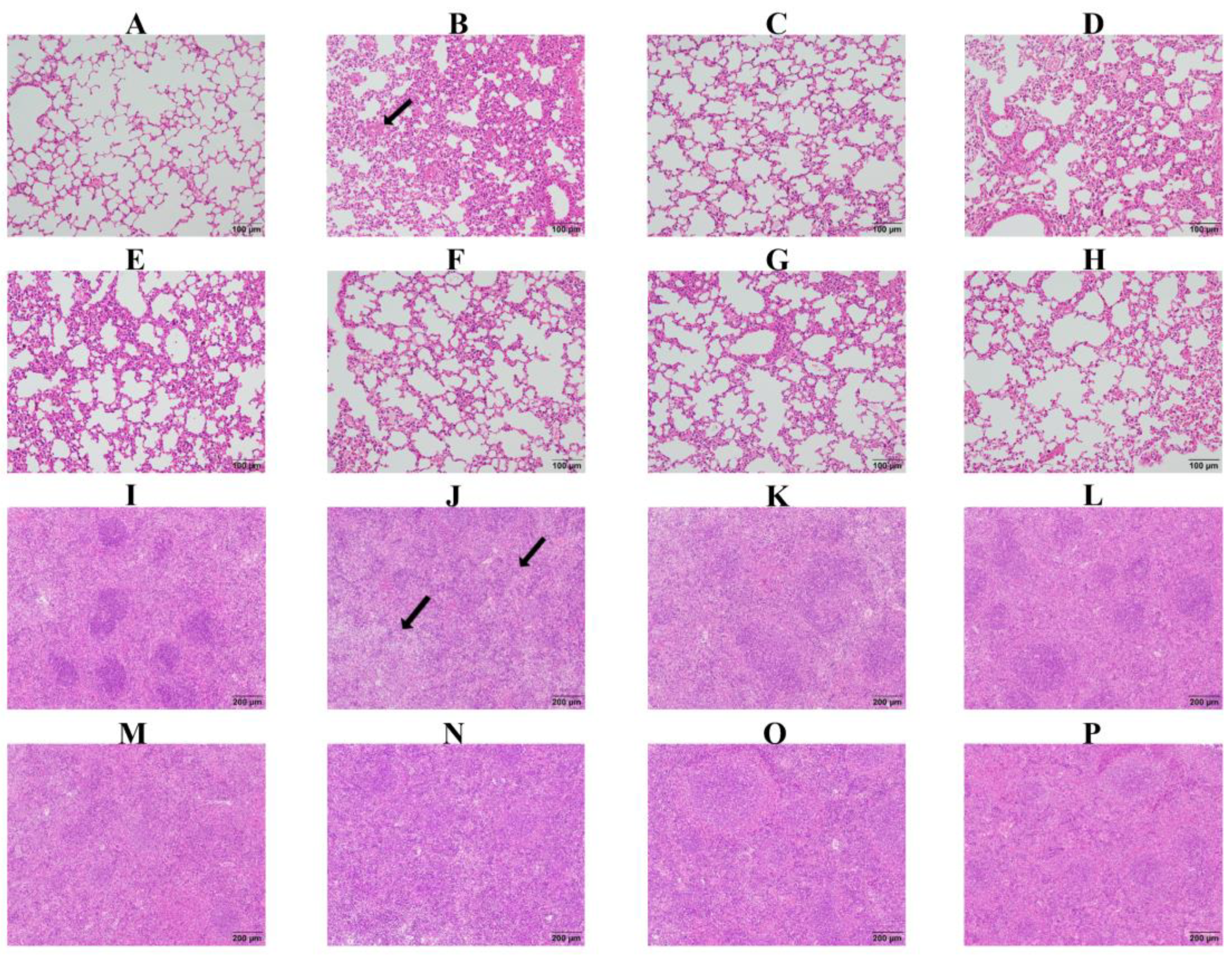
2.7. Baicalin, Probenecid, Amoxicillin, and the Combination of Probenecid and Amoxicillin Relieved Pathological Lung and Spleen Tissue Damage in G. parasuis-Challenged Mice
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Bacterial and Culture Conditions
4.3. Drugs and the Mice
4.4. Experimental Design
4.5. Blood Biochemical Parameters and Routine Blood Test Indicators
4.6. Flow Cytometry
4.7. Bacterial Burden
4.8. RT-qPCR
4.9. Western Blotting
4.10. Immunohistochemistry
4.11. Histopathological Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hua, K.; Li, T.; He, Y.; Guan, A.; Chen, L.; Gao, Y.; Xu, Q.; Wang, H.; Luo, R.; Zhao, L.; et al. Resistin secreted by porcine alveolar macrophages leads to endothelial cell dysfunction during Haemophilus parasuis infection. Virulence 2023, 14, 2171636. [Google Scholar] [PubMed]
- Mugabi, R.; Silva, A.; Hu, X.; Gottschalk, M.; Aragon, V.; Macedo, N.R.; Sahin, O.; Harms, P.; Main, R.; Tucker, A.W.; et al. Molecular characterization of Glaesserella parasuis strains circulating in North American swine production systems. BMC Vet. Res. 2023, 19, 135. [Google Scholar]
- Duan, Y.; Hao, Y.; Feng, H.; Shu, J.; He, Y. Research progress on Haemophilus parasuis vaccines. Front. Vet. Sci. 2025, 12, 1492144. [Google Scholar]
- Kielstein, P.; Rapp-Gabrielson, V.J. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992, 30, 862–865. [Google Scholar]
- Yan, P.; Jia, Y.C.; Zhang, X.L.; Zhou, Y.Y.; Guo, Y.; Yin, R.L.; Yuan, J.; Wang, L.X.; Guo, Z.B.; Wang, J.Y.; et al. Virulence assessment of four Glaesserella parasuis strains isolated in Liaoning province of China. Res. Vet. Sci. 2023, 158, 226–234. [Google Scholar]
- Zhang, X.; Lin, Y.; Xu, X.; Wen, S.; Wang, Z.; Gu, J.; He, Q.; Cai, X. HtrA is involved in stress response and adhesion in Glaesserella parasuis serovar 5 strain Nagasaki. Vet. Microbiol. 2023, 282, 109748. [Google Scholar]
- An, J.; Cai, J.; Zhang, B.; Li, Y. Pili Subunit PilA Contributes to the Cytoadhesion of Glaesserella Parasuis to Host Cells and Provides Immunoprotection. Appl. Environ. Microbiol. 2023, 89, e0200222. [Google Scholar]
- Yan, X.; Dai, K.; Gu, C.; Yu, Z.; He, M.; Xiao, W.; Zhao, M.; He, L. Deletion of two-component system QseBC weakened virulence of Glaesserella parasuis in a murine acute infection model and adhesion to host cells. PeerJ 2022, 10, e13648. [Google Scholar]
- Rochegüe, T.; Haenni, M.; Mondot, S.; Astruc, C.; Cazeau, G.; Ferry, T.; Madec, J.Y.; Lupo, A. Impact of Antibiotic Therapies on Resistance Genes Dynamic and Composition of the Animal Gut Microbiota. Animals 2021, 11, 3280. [Google Scholar] [CrossRef]
- Elsbroek, L.; Amiteye, D.; Schreiber, S.; Herrmann, F. Molecular Imaging of Isolated Escherichia coli DH5α Peptidoglycan Sacculi Identifies the Mechanism of Action of Cell Wall-Inhibiting Antibiotics. ACS Chem. Biol. 2023, 18, 848–860. [Google Scholar]
- El Garch, F.; de Jong, A.; Simjee, S.; Moyaert, H.; Klein, U.; Ludwig, C.; Marion, H.; Haag-Diergarten, S.; Richard-Mazet, A.; Thomas, V.; et al. Monitoring of antimicrobial susceptibility of respiratory tract pathogens isolated from diseased cattle and pigs across Europe, 2009–2012: VetPath results. Vet. Microbiol. 2016, 194, 11–22. [Google Scholar] [PubMed]
- An, J.; Zhang, C.; Li, Y. Outer Membrane Vesicles Associated with the Delivery of Amoxicillin Resistance in Glaesserella parasuis. Microb. Drug Resist. 2023, 29, 263–270. [Google Scholar] [PubMed]
- Clark, R.S.B.; Empey, P.E.; Kochanek, P.M.; Bell, M.J. N-Acetylcysteine and Probenecid Adjuvant Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1529–1537. [Google Scholar] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar]
- Onódi, Z.; Koch, S.; Rubinstein, J.; Ferdinandy, P.; Varga, Z.V. Drug repurposing for cardiovascular diseases: New targets and indications for probenecid. Br. J. Pharmacol. 2023, 180, 685–700. [Google Scholar]
- Fu, S.; Zhang, M.; Ou, J.; Liu, H.; Tan, C.; Liu, J.; Chen, H.; Bei, W. Construction and immune effect of Haemophilus parasuis DNA vaccine encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in mice. Vaccine 2012, 30, 6839–6844. [Google Scholar]
- Singh, H.P.; Wilkinson, S.; Kamran, S. Decreasing Antibiotic Use in a Community Neonatal Intensive Care Unit: A Quality Improvement Initiative. Am. J. Perinatol. 2024, 41 (Suppl. 1), e2767–e2775. [Google Scholar]
- Gao, L.; Zhao, J.X.; Qin, X.M.; Zhao, J. The ethanol extract of Scutellaria baicalensis Georgi attenuates complete Freund’s adjuvant (CFA)-induced inflammatory pain by suppression of P2X3 receptor. J. Ethnopharmacol. 2023, 317, 116762. [Google Scholar]
- Du, Z.; Han, J.; Luo, J.; Bi, G.; Liu, T.; Kong, J.; Chen, Y. Combination effects of baicalin with linezolid against Staphylococcus aureus biofilm-related infections: In vivo animal model. N. Microbiol. 2023, 46, 258–263. [Google Scholar]
- Bai, X.; Yao, M.; Zhu, X.; Lian, Y.; Zhang, M. Baicalin suppresses interleukin-1β-induced apoptosis, inflammatory response, oxidative stress, and extracellular matrix degradation in human nucleus pulposus cells. Immunopharmacol. Immunotoxicol. 2023, 45, 433–442. [Google Scholar]
- Ning, X.; Luo, D.; Chen, Y.; Shao, Y.; Xu, J. Baicalin Reduces Renal Inflammation in Mesangial Proliferative Glomerulonephritis through Activation of Nrf2/ARE and PI3K/AKT Pathways. Discov. Med. 2023, 35, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhang, H.; Zhu, Z.; Ling, Z.; Zeng, F.; Wang, Y.; Wang, J. Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways. Molecules 2023, 28, 1873. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Meng, F.; Ma, Y.; Zhang, C.; Zhang, Z.; Yang, Z.; Li, Y.; Hou, L.; Xu, Y.; Liang, X.; et al. In situ immunomodulation of tumors with biosynthetic bacteria promote anti-tumor immunity. Bioact. Mater. 2024, 32, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Z.; Gao, W.; Wang, H.; Wang, Z.; Tian, C.; Chen, G. Detection of the CD8(+) T cell immune response in mice infected with OVA-Listeriamonocytogenes. STAR Protoc. 2023, 4, 102582. [Google Scholar] [CrossRef]
- Schardey, J.; Lu, C.; Neumann, J.; Wirth, U.; Li, Q.; Jiang, T.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; et al. Differential Immune Infiltration Profiles in Colitis-Associated Colorectal Cancer versus Sporadic Colorectal Cancer. Cancers 2023, 15, 4743. [Google Scholar] [CrossRef]
- Reynolds, G.; Cliff, E.R.S.; Mohyuddin, G.R.; Popat, R.; Midha, S.; Liet Hing, M.N.; Harrison, S.J.; Kesselheim, A.S.; Teh, B.W. Infections following bispecific antibodies in myeloma: A systematic review and meta-analysis. Blood Adv. 2023, 7, 5898–5903. [Google Scholar] [CrossRef]
- Chen, Y.; Zander, R.A.; Wu, X.; Schauder, D.M.; Kasmani, M.Y.; Shen, J.; Zheng, S.; Burns, R.; Taparowsky, E.J.; Cui, W. BATF regulates progenitor to cytolytic effector CD8(+) T cell transition during chronic viral infection. Nat. Immunol. 2021, 22, 996–1007. [Google Scholar] [CrossRef]
- Smyk-Pearson, S.; Tester, I.A.; Klarquist, J.; Palmer, B.E.; Pawlotsky, J.M.; Golden-Mason, L.; Rosen, H.R. Spontaneous recovery in acute human hepatitis C virus infection: Functional T-cell thresholds and relative importance of CD4 help. J. Virol. 2008, 82, 1827–1837. [Google Scholar] [CrossRef]
- Merlo, A.; Saverino, D.; Tenca, C.; Grossi, C.E.; Bruno, S.; Ciccone, E. CD85/LIR-1/ILT2 and CD152 (cytotoxic T lymphocyte antigen 4) inhibitory molecules down-regulate the cytolytic activity of human CD4+ T-cell clones specific for Mycobacterium tuberculosis. Infect. Immun. 2001, 69, 6022–6029. [Google Scholar] [CrossRef]
- Trivedi, A.; Bose, D.; Saha, P.; Roy, S.; More, M.; Skupsky, J.; Klimas, N.G.; Chatterjee, S. Prolonged Antibiotic Use in a Preclinical Model of Gulf War Chronic Multisymptom-Illness Causes Renal Fibrosis-like Pathology via Increased micro-RNA 21-Induced PTEN Inhibition That Is Correlated with Low Host Lachnospiraceae Abundance. Cells 2023, 13, 56. [Google Scholar] [CrossRef]
- Tan, L.; Chan, W.; Zhang, J.; Wang, J.; Wang, Z.; Liu, J.; Li, J.; Liu, X.; Wang, M.; Hao, L.; et al. Regulation of RIP1-Mediated necroptosis via necrostatin-1 in periodontitis. J. Periodontal Res. 2023, 58, 919–931. [Google Scholar] [PubMed]
- Meng, R.; Gu, L.; Lu, Y.; Zhao, K.; Wu, J.; Wang, H.; Han, J.; Tang, Y.; Lu, B. High mobility group box 1 enables bacterial lipids to trigger receptor-interacting protein kinase 3 (RIPK3)-mediated necroptosis and apoptosis in mice. J. Biol. Chem. 2019, 294, 8872–8884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, G.; Wu, X.; Xu, Y.; Xu, L.; Zou, Y.; Yang, X.; Pan, L.; Lei, B.; Mu, J.; et al. Fei-Yan-Qing-Hua decoction decreases hyperinflammation by inhibiting HMGB1/RAGE signaling and promotes bacterial phagocytosis in the treatment of sepsis. J. Ethnopharmacol. 2024, 321, 117553. [Google Scholar] [PubMed]
- Mao, Y.; Patial, S.; Saini, Y. Airway epithelial cell-specific deletion of HMGB1 exaggerates inflammatory responses in mice with muco-obstructive airway disease. Front. Immunol. 2022, 13, 944772. [Google Scholar]
- Jian, Z.; Ding, S.; Deng, H.; Wang, J.; Yi, W.; Wang, L.; Zhu, S.; Gu, L.; Xiong, X. Probenecid protects against oxygen-glucose deprivation injury in primary astrocytes by regulating inflammasome activity. Brain Res. 2016, 1643, 123–129. [Google Scholar]
- Xiong, X.X.; Gu, L.J.; Shen, J.; Kang, X.H.; Zheng, Y.Y.; Yue, S.B.; Zhu, S.M. Probenecid protects against transient focal cerebral ischemic injury by inhibiting HMGB1 release and attenuating AQP4 expression in mice. Neurochem. Res. 2014, 39, 216–224. [Google Scholar] [CrossRef]
- Fu, S.; Yin, R.; Zuo, S.; Liu, J.; Zhang, Y.; Guo, L.; Qiu, Y.; Ye, C.; Liu, Y.; Wu, Z.; et al. The effects of baicalin on piglets challenged with Glaesserella parasuis. Vet. Res. 2020, 51, 102. [Google Scholar] [CrossRef]
- Que, X.; Zheng, S.; Song, Q.; Pei, H.; Zhang, P. Fantastic voyage: The journey of NLRP3 inflammasome activation. Genes Dis. 2024, 11, 819–829. [Google Scholar]
- Ping, D.; Qi, J.; Li, M.; Sun, X.; Peng, Y.; Liu, C. Fuzheng Huayu recipe alleviates liver fibrosis via inhibiting NLRP3 inflammasome activation in macrophages. J. Ethnopharmacol. 2024, 318, 117001. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Liu, X.; Zhang, J.; Zhang, Y.; Wen, Y.; Yang, G. Salvianolic acid B exerts protective effects against Aβ-induced neuroinflammation through the inhibition of NLRP3 inflammasome activation and switching of M1/M2 polarization. Tissue Cell 2023, 85, 102260. [Google Scholar]
- Zhou, S.; Liu, Y.; Xue, B.; Yuan, P. Low-dose Esketamine suppresses NLRP3-mediated apoptotic and pyroptotic cell death in microglial cells to ameliorate LPS-induced depression via ablating GSK-3β. Behav. Brain Res. 2024, 459, 114782. [Google Scholar] [PubMed]
- He, L.; Zhou, W.; Wang, L.; Tang, N.; Wang, Y.; Zhong, H.; Tang, Y.; Xi, D.; He, F. Murine Cytomegalovirus Infection Induced miR-1929-3p Down-Regulation Promotes the Proliferation and Apoptosis of Vascular Smooth Muscle Cells in Mice by Targeting Endothelin A Receptor and Downstream NLRP3 Activation Pathway. Mol. Biotechnol. 2023, 65, 1954–1967. [Google Scholar] [PubMed]
- Zhou, J.; Wang, C.; Zhang, X.; Wu, Z.; Wu, Y.; Li, D.; Gao, J. Shizhifang ameliorates pyroptosis of renal tubular epithelial cells in hyperuricemia through inhibiting NLRP3 inflammasome. J. Ethnopharmacol. 2023, 317, 116777. [Google Scholar]
- Huan, P.; Sun, X.; He, Z.; Yang, S.; Wang, X.; Xie, H.; Wang, L.; He, J. Qiji Shujiang granules alleviates dopaminergic neuronal injury of parkinson’s disease by inhibiting NLRP3/Caspase-1 pathway mediated pyroptosis. Phytomed. Int. J. Phytother. Phytopharm. 2023, 120, 155019. [Google Scholar]
- Ye, Q.; Lin, B.; Xu, P.; Zhang, F.; Wang, N.; Shou, D. Yunvjian decoction attenuates lipopolysaccharide-induced periodontitis by suppressing NFκB/NLRP3/IL-1β pathway. J. Ethnopharmacol. 2024, 319, 117279. [Google Scholar]
- Barros Ferreira, L.; Ashander, L.M.; Ma, Y.; Appukuttan, B.; Williams, K.A.; Best, G.; Smith, J.R. Effects of tumor necrosis factor-α and interleukin-1β on human retinal endothelial cells. Cytokine 2024, 173, 156407. [Google Scholar]
- Fetsko, A.R.; Sebo, D.J.; Budzynski, L.B.; Scharbarth, A.; Taylor, M.R. IL-1β disrupts blood-brain barrier development by inhibiting endothelial Wnt/β-catenin signaling. bioRxiv 2023. preprint. [Google Scholar]
- Nadlonek, N.; Lee, J.H.; Reece, T.B.; Weyant, M.J.; Cleveland, J.C., Jr.; Meng, X.; Fullerton, D.A. Interleukin-1 Beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa Beta. Ann. Thorac. Surg. 2013, 96, 155–162. [Google Scholar]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar]
- Saxena, K.; Roverato, N.D.; Reithmann, M.; Mah, M.M.; Schregle, R.; Schmidtke, G.; Silbern, I.; Urlaub, H.; Aichem, A. FAT10 is phosphorylated by IKKβ to inhibit the antiviral type-I interferon response. Life Sci. Alliance 2023, 7, e202101282. [Google Scholar]
- Zhao, X.; Ali, S.; Hassan, M.F.; Bashir, M.A.; Ni, X.; Lv, C.; Yang, H.; Danzeng, B.; Quan, G. Effects of graded levels of dietary protein supplementation on milk yield, body weight gain, blood biochemical parameters, and gut microbiota in lactating ewes. Front. Vet. Sci. 2023, 10, 1223450. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, X.; Cheng, H.; Wang, X.; Liu, C.; Tan, X. Anti-triple-negative breast cancer metastasis efficacy and molecular mechanism of the STING agonist for innate immune pathway. Ann. Med. 2023, 55, 2210845. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Wu, J.; Wu, X.; Shao, Y.; Song, X.; Tu, J.; Qi, K. The two-component system histidine kinase EnvZ contributes to Avian pathogenic Escherichia coli pathogenicity by regulating biofilm formation and stress responses. Poult. Sci. 2023, 102, 102388. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, H.; Lu, S.; Wang, X.; Shi, X.; Mao, P. Autophagy-dependent ferroptosis is involved in the development of endometriosis. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2023, 39, 2242962. [Google Scholar] [CrossRef]

| Item | Control | GPS | Amo20 | Pro10 | Pro20 | Pro40 | Pro20 + Amo20 | Bai100 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | BvsA | CvsB | DvsB | EvsB | FvsB | GvsB | HvsB | ||
| TB (mg/dL) | 0.13 | 1.20 | 0.60 | 1.00 | 0.90 | 0.70 | 0.40 | 1.08 | 0.10 | 0.005 | 0.089 | 0.556 | 0.378 | 0.146 | 0.028 | 0.734 |
| TP (g/dL) | 30.99 | 30.16 | 28.40 | 28.18 | 33.48 | 30.97 | 30.84 | 29.27 | 0.48 | 0.626 | 0.311 | 0.255 | 0.065 | 0.633 | 0.689 | 0.603 |
| ALB (g/dL) | 21.73 | 16.01 | 17.76 | 17.36 | 19.67 | 18.85 | 19.41 | 17.54 | 0.46 | 0.001 | 0.248 | 0.369 | 0.024 | 0.070 | 0.034 | 0.309 |
| AST (U/L) | 124.00 | 142.00 | 113.00 | 221.00 | 268.00 | 344.00 | 258.00 | 208.00 | 17.29 | 0.621 | 0.415 | 0.039 | 0.002 | <0.001 | 0.005 | 0.077 |
| ALT (U/L) | 26.00 | 62.00 | 38.00 | 40.00 | 60.00 | 52.00 | 50.00 | 51.00 | 2.92 | <0.001 | 0.009 | 0.015 | 0.872 | 0.251 | 0.170 | 0.221 |
| ALP (U/L) | 185.00 | 97.00 | 95.00 | 99.00 | 76.00 | 96.00 | 107.00 | 106.00 | 6.80 | <0.001 | 0.881 | 0.811 | 0.078 | 0.928 | 0.360 | 0.424 |
| CHOL (mg/dL) | 1.19 | 2.00 | 1.68 | 1.74 | 1.94 | 1.85 | 1.83 | 1.51 | 0.06 | <0.001 | 0.005 | 0.015 | 0.566 | 0.149 | 0.098 | <0.001 |
| TG (mg/dL) | 1.33 | 3.10 | 2.02 | 1.54 | 3.47 | 2.23 | 1.98 | 2.69 | 0.20 | 0.015 | 0.118 | 0.030 | 0.570 | 0.203 | 0.106 | 0.544 |
| GLU (mg/dL) | 3.53 | 0.70 | 1.03 | 1.57 | 0.72 | 0.83 | 1.57 | 1.05 | 0.19 | <0.001 | 0.302 | 0.014 | 0.958 | 0.676 | 0.014 | 0.284 |
| Ca (mg/dL) | 2.11 | 1.55 | 1.99 | 1.77 | 1.54 | 1.72 | 1.79 | 1.56 | 0.05 | <0.001 | 0.002 | 0.084 | 0.956 | 0.174 | 0.065 | 0.956 |
| P (mg/dL) | 2.42 | 1.74 | 1.73 | 1.53 | 1.65 | 1.69 | 1.48 | 1.62 | 0.07 | <0.001 | 0.053 | 0.004 | 0.019 | 0.032 | 0.002 | 0.014 |
| HDL (mg/dL) | 0.81 | 0.76 | 0.77 | 0.83 | 0.89 | 0.87 | 0.87 | 0.71 | 0.02 | 0.001 | 0.985 | 0.237 | 0.606 | 0.788 | 0.153 | 0.505 |
| LDL (mg/dL) | 0.11 | 0.07 | 0.06 | 0.08 | 0.10 | 0.08 | 0.09 | 0.08 | 0.01 | 0.079 | 0.777 | 0.572 | 0.168 | 0.777 | 0.399 | 0.777 |
| LDH (U/L) | 1191.70 | 620.40 | 613.10 | 800.20 | 1094.70 | 1159.30 | 875.60 | 647.20 | 53.60 | <0.001 | 0.951 | 0.142 | 0.001 | <0.001 | 0.043 | 0.821 |
| Item | Control | GPS | Amo20 | Pro10 | Pro20 | Pro40 | Pro20 + Amo20 | Bai100 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | BvsA | CvsB | DvsB | EvsB | FvsB | GvsB | HvsB | ||
| TB (mg/dL) | 0.17 | 0.56 | 0.33 | 0.62 | 0.14 | 0.42 | 0.26 | 0.31 | 0.04 | <0.001 | 0.001 | 0.246 | <0.001 | 0.022 | <0.001 | <0.001 |
| TP (g/dL) | 32.18 | 32.15 | 31.59 | 32.63 | 31.97 | 29.72 | 29.43 | 30.09 | 0.44 | 0.989 | 0.750 | 0.786 | 0.918 | 0.179 | 0.135 | 0.252 |
| ALB (g/dL) | 22.15 | 16.44 | 17.51 | 18.62 | 18.33 | 17.67 | 20.07 | 19.99 | 0.41 | <0.001 | 0.311 | 0.048 | 0.082 | 0.245 | 0.003 | 0.003 |
| AST (U/L) | 119.00 | 135.00 | 120.00 | 159.00 | 158.00 | 218.00 | 196.00 | 154.00 | 7.72 | 0.361 | 0.402 | 0.167 | 0.184 | <0.001 | 0.002 | 0.265 |
| ALT (U/L) | 21.00 | 34.00 | 25.00 | 32.00 | 38.00 | 40.00 | 32.00 | 35.00 | 1.51 | 0.003 | 0.029 | 0.615 | 0.360 | 0.143 | 0.615 | 0.801 |
| ALP (U/L) | 183.00 | 68.00 | 77.00 | 102.00 | 100.00 | 86.00 | 103.00 | 83.00 | 7.24 | <0.001 | 0.329 | 0.002 | 0.002 | 0.053 | 0.001 | 0.099 |
| CHOL (mg/dL) | 1.21 | 1.92 | 1.57 | 1.71 | 1.88 | 1.62 | 1.55 | 1.75 | 0.05 | <0.001 | 0.018 | 0.141 | 0.754 | 0.042 | 0.014 | 0.220 |
| TG (mg/dL) | 1.32 | 1.59 | 0.90 | 0.81 | 1.20 | 0.98 | 0.96 | 1.15 | 0.07 | 0.275 | 0.011 | 0.005 | 0.125 | 0.022 | 0.019 | 0.084 |
| GLU (mg/dL) | 3.47 | 1.10 | 0.93 | 1.50 | 0.90 | 1.10 | 1.71 | 1.97 | 0.22 | 0.003 | 0.809 | 0.564 | 0.772 | 1.000 | 0.382 | 0.220 |
| Ca (mg/dL) | 2.09 | 1.47 | 1.31 | 1.97 | 1.92 | 1.68 | 1.44 | 2.05 | 0.07 | 0.002 | 0.347 | 0.007 | 0.014 | 0.206 | 0.855 | 0.003 |
| P (mg/dL) | 2.24 | 1.01 | 1.22 | 1.50 | 1.37 | 1.27 | 1.67 | 1.22 | 0.09 | <0.001 | 0.460 | 0.092 | 0.210 | 0.350 | 0.028 | 0.460 |
| HDL (mg/dL) | 0.70 | 0.74 | 0.70 | 0.76 | 0.82 | 0.78 | 0.81 | 0.84 | 0.02 | 0.968 | 0.609 | 0.480 | 0.179 | 0.330 | 0.204 | 0.111 |
| LDL (mg/dL) | 0.12 | 0.10 | 0.09 | 0.10 | 0.10 | 0.08 | 0.08 | 0.09 | 0.01 | 0.274 | 0.852 | 0.710 | 0.710 | 0.461 | 0.359 | 0.852 |
| LDH (U/L) | 1221.30 | 621.20 | 636.13 | 820.00 | 867.20 | 817.30 | 855.20 | 657.50 | 40.07 | <0.001 | 0.813 | 0.006 | 0.001 | 0.006 | 0.001 | 0.568 |
| Item | Control | GPS | Amo20 | Pro10 | Pro20 | Pro40 | Pro20 + Amo20 | Bai100 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (E) | (F) | (G) | (H) | BvsA | CvsB | DvsB | EvsB | FvsB | GvsB | HvsB | ||
| TB (mg/dL) | 0.15 | 0.57 | 0.18 | 0.25 | 0.21 | 0.41 | 0.29 | 0.22 | 0.03 | <0.001 | <0.001 | <0.001 | <0.001 | 0.045 | 0.002 | <0.001 |
| TP (g/dL) | 31.07 | 28.84 | 31.39 | 30.91 | 30.36 | 30.00 | 29.14 | 30.38 | 0.36 | 0.150 | 0.104 | 0.181 | 0.319 | 0.441 | 0.839 | 0.312 |
| ALB (g/dL) | 20.09 | 15.02 | 17.01 | 16.68 | 17.39 | 18.19 | 18.37 | 19.76 | 0.39 | <0.001 | 0.075 | 0.133 | 0.038 | 0.008 | 0.006 | <0.001 |
| AST (U/L) | 123.00 | 84.00 | 119.00 | 106.00 | 117.00 | 128.00 | 107.00 | 110.00 | 3.62 | 0.004 | 0.007 | 0.073 | 0.011 | 0.002 | 0.066 | 0.036 |
| ALT (U/L) | 21.00 | 20.00 | 13.00 | 20.00 | 16.00 | 23.00 | 12.00 | 14.00 | 0.95 | 0.622 | 0.030 | 1.000 | 0.227 | 0.274 | 0.008 | 0.061 |
| ALP (U/L) | 197.00 | 59.00 | 65.00 | 75.00 | 66.00 | 53.00 | 76.00 | 87.00 | 10.65 | <0.001 | 0.813 | 0.544 | 0.784 | 0.823 | 0.520 | 0.304 |
| CHOL (mg/dL) | 1.25 | 1.08 | 1.29 | 1.44 | 1.48 | 1.50 | 1.40 | 1.53 | 0.03 | 0.051 | 0.021 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 |
| TG (mg/dL) | 1.29 | 0.53 | 0.85 | 0.76 | 0.81 | 0.85 | 0.78 | 0.84 | 0.04 | <0.001 | <0.001 | 0.004 | 0.001 | <0.001 | 0.002 | <0.001 |
| GLU (mg/dL) | 3.44 | 1.23 | 1.30 | 1.87 | 1.43 | 1.60 | 1.33 | 1.23 | 0.16 | <0.001 | 0.776 | 0.011 | 0.377 | 0.115 | 0.656 | 1.000 |
| Ca (mg/dL) | 2.14 | 2.29 | 1.96 | 1.50 | 2.03 | 1.90 | 1.89 | 1.93 | 0.06 | 0.379 | 0.058 | <0.001 | 0.130 | 0.028 | 0.023 | 0.041 |
| P (mg/dL) | 2.31 | 1.11 | 1.18 | 1.38 | 1.09 | 1.04 | 1.26 | 2.08 | 0.11 | <0.001 | 0.778 | 0.307 | 0.959 | 0.808 | 0.566 | 0.002 |
| HDL (mg/dL) | 0.75 | 0.45 | 0.52 | 0.58 | 0.63 | 0.57 | 0.56 | 0.60 | 0.02 | <0.001 | 0.256 | 0.063 | 0.014 | 0.077 | 0.111 | 0.029 |
| LDL (mg/dL) | 0.10 | 0.15 | 0.23 | 0.22 | 0.23 | 0.22 | 0.20 | 0.21 | 0.01 | 0.073 | 0.014 | 0.023 | 0.011 | 0.018 | 0.073 | 0.058 |
| LDH (U/L) | 1435.10 | 755.10 | 1130.20 | 805.30 | 1069.60 | 1022.30 | 723.00 | 895.00 | 56.03 | <0.001 | 0.025 | 0.745 | 0.055 | 0.097 | 0.835 | 0.368 |
| Gene | Nucleotide Sequence (5′–3′) | Tm (°C) | Length (bp) | |
|---|---|---|---|---|
| NLRP3 | Forward | TTTATTTGTACCCAAGGCTGCTATC | 54.7 | 148 |
| Reverse | CAACGGACACTCGTCATCTTCA | 57.1 | ||
| ASC | Forward | CAGTTCGTGCAGAGACCACCA | 60.2 | 140 |
| Reverse | CTGCTCCAGGTCCATCACCA | 60.0 | ||
| Caspase-1 | Forward | TCCGAGGGTTGGAGCTCAAG | 59.9 | 137 |
| Reverse | CTGGCCAGGCAGCAAATTC | 57.8 | ||
| HMGB1 | Forward | ATGACAAGCAGCCCTATGAGAA | 55.9 | 82 |
| Reverse | CCTTTAGCTCTGTAGGCAGCAA | 57.1 | ||
| Fasl | Forward | GCCATCACAACCACTCCC | 56.8 | 107 |
| Reverse | CCAGAGCCACCAGAACCA | 57.9 | ||
| Bax | Forward | AGCTGCAGAGGATGATTGCTG | 57.9 | 178 |
| Reverse | CTGATCAGCTCGGGCATTTA | 54.8 | ||
| C-myc | Forward | TCGCCCAAATCCTGTACCTC | 57.2 | 193 |
| Reverse | TCTCCACAGACACCACATCAAT | 55.8 | ||
| IL-1β | Forward | TGCCACCTTTTGACAGTGATG | 55.6 | 138 |
| Reverse | TGATGTGCTGCTGCGAGATT | 57.3 | ||
| IL-18 | Forward | GGACACTTTCTTGCTTGCCA | 56.0 | 166 |
| Reverse | CAGCCTCGGGTATTCTGTTATG | 55.4 | ||
| TNF-α | Forward | GCCCCCAGTCTGTATCCTTCTA | 58.4 | 209 |
| Reverse | TTCGGAAAGCCCATTTGAGT | 54.3 | ||
| GAPDH | Forward | AAGCCCATCACCATCTTCCA | 56.4 | 88 |
| Reverse | CACCAGTAGACTCCACGACA | 56.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Luo, R.; Fu, Y.; Liu, S.; Dong, Q.; Sun, Y.; Tian, X.; Zhu, Y.; Wang, P.; Guo, L.; et al. Baicalin, Amoxicillin, and Probenecid Provide Protection in Mice Against Glaesserella parasuis Challenge. Biomolecules 2025, 15, 507. https://doi.org/10.3390/biom15040507
Li J, Luo R, Fu Y, Liu S, Dong Q, Sun Y, Tian X, Zhu Y, Wang P, Guo L, et al. Baicalin, Amoxicillin, and Probenecid Provide Protection in Mice Against Glaesserella parasuis Challenge. Biomolecules. 2025; 15(4):507. https://doi.org/10.3390/biom15040507
Chicago/Turabian StyleLi, Jingyang, Ronghui Luo, Yunjian Fu, Siyu Liu, Qiaoli Dong, Yamin Sun, Xinyue Tian, Yi Zhu, Peiyi Wang, Ling Guo, and et al. 2025. "Baicalin, Amoxicillin, and Probenecid Provide Protection in Mice Against Glaesserella parasuis Challenge" Biomolecules 15, no. 4: 507. https://doi.org/10.3390/biom15040507
APA StyleLi, J., Luo, R., Fu, Y., Liu, S., Dong, Q., Sun, Y., Tian, X., Zhu, Y., Wang, P., Guo, L., Lu, Q., Ye, C., Fu, S., & Qiu, Y. (2025). Baicalin, Amoxicillin, and Probenecid Provide Protection in Mice Against Glaesserella parasuis Challenge. Biomolecules, 15(4), 507. https://doi.org/10.3390/biom15040507







