The Role of Eosinophils, Eosinophil-Related Cytokines and AI in Predicting Immunotherapy Efficacy in NSCLC
Abstract
1. Introduction
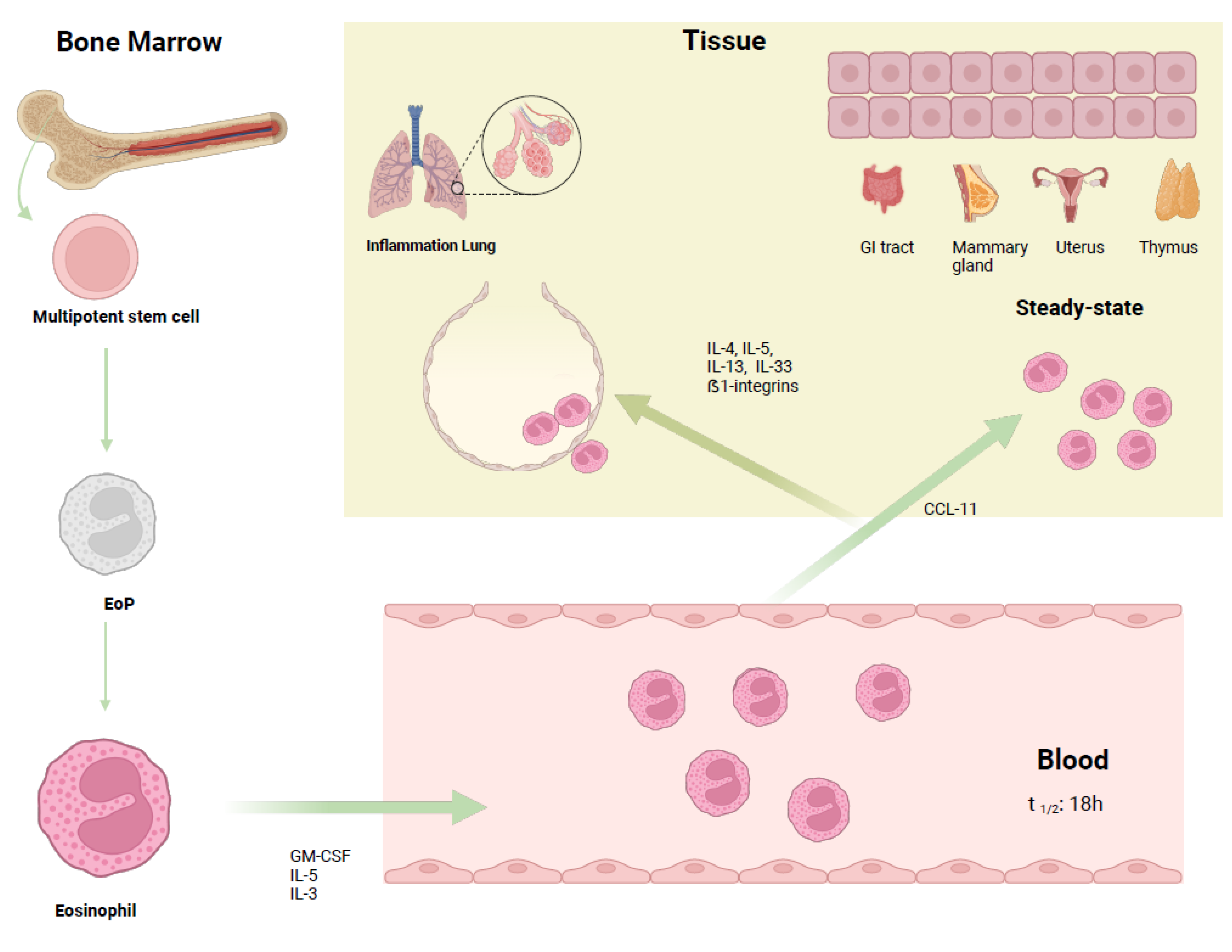
2. Biology of Eosinophils
3. Role of IL-33 and IL-31
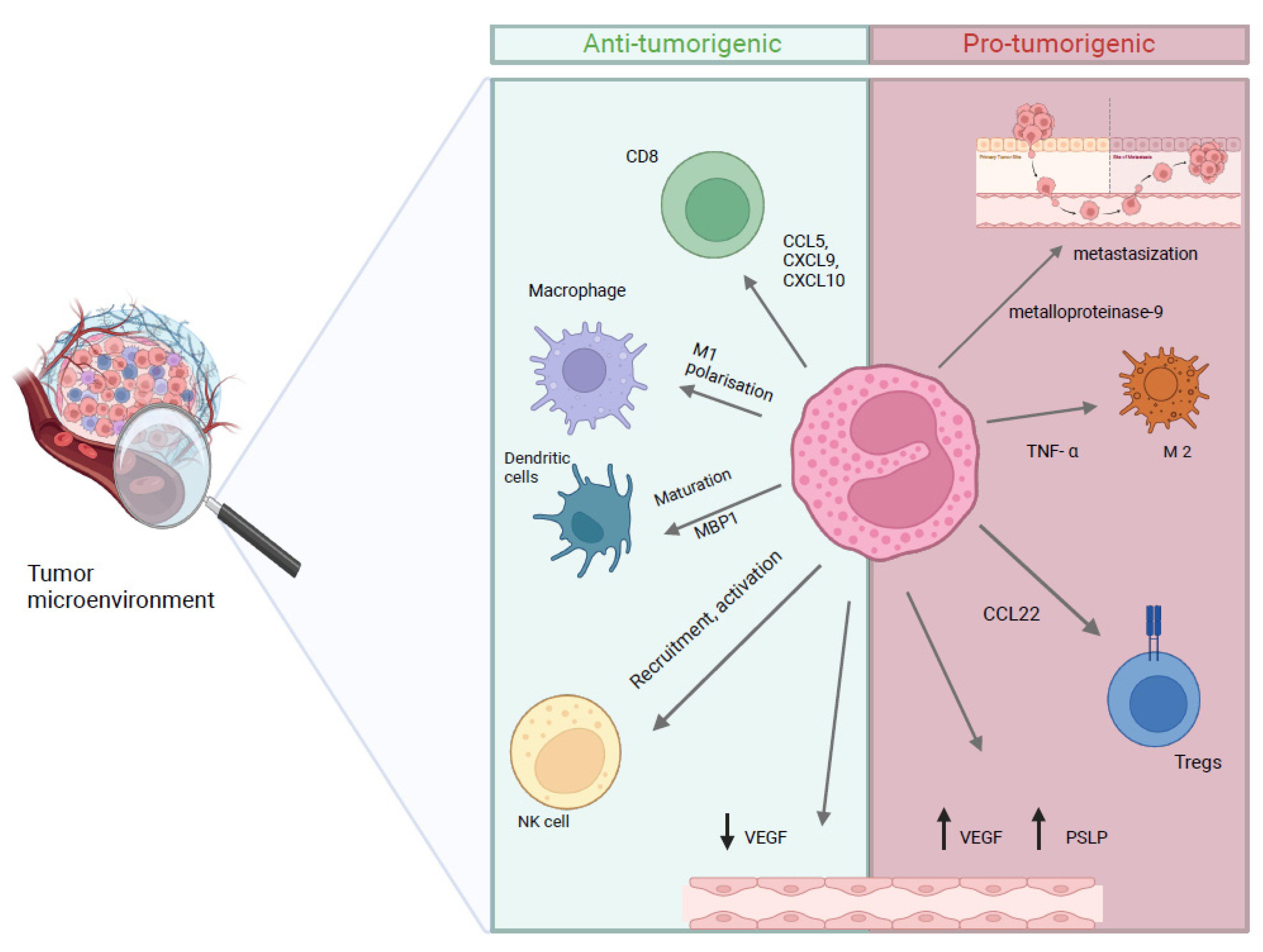
4. Role of Eosinophils in Cancer
5. Eosinophils in NSCLC
5.1. Eosinophils as Predictive Biomarkers of Clinical Efficacy from Immunotherapy
5.2. Eosinophils as a Biomarker of Immune-Related Adverse Events (irAEs)
5.3. Limitations in NSCLC
6. Perspectives: Artificial Intelligence and Biomarkers of Immunotherapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar]
- Paz-Ares, L.; Vicente, D.; Tafreshi, A.; Robinson, A.; Soto Parra, H.; Mazières, J.; Hermes, B.; Cicin, I.; Medgyasszay, B.; Rodríguez-Cid, J.; et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. 2020, 15, 1657–1669. [Google Scholar] [PubMed]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar]
- Lee, C.K.; Man, J.; Lord, S.; Links, M.; Gebski, V.; Mok, T.; Yang, J.C. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J. Thorac. Oncol. 2017, 12, 403–407. [Google Scholar] [PubMed]
- Tian, T.; Yu, M.; Li, J.; Jiang, M.; Ma, D.; Tang, S.; Lin, Z.; Chen, L.; Gong, Y.; Zhu, J.; et al. Front-Line ICI-Based Combination Therapy Post-TKI Resistance May Improve Survival in NSCLC Patients With EGFR Mutation. Front. Oncol. 2021, 11, 739090. [Google Scholar]
- Muscolino, P.; Granata, B.; Omero, F.; De Pasquale, C.; Campana, S.; Calabrò, A.; D’Anna, F.; Drommi, F.; Pezzino, G.; Cavaliere, R.; et al. Potential predictive role of gut microbiota to immunotherapy in HCC patients: A brief review. Front. Oncol. 2023, 13, 1247614. [Google Scholar]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar]
- Speranza, D.; Santarpia, M.; Luppino, F.; Omero, F.; Maiorana, E.; Cavaleri, M.; Sapuppo, E.; Cianci, V.; Pugliese, A.; Racanelli, V.; et al. Immune checkpoint inhibitors and neurotoxicity: A focus on diagnosis and management for a multidisciplinary approach. Expert. Opin. Drug Saf. 2024, 23, 1405–1418. [Google Scholar]
- Bou Zerdan, M.; Kassab, J.; Meouchy, P.; Haroun, E.; Nehme, R.; Bou Zerdan, M.; Fahed, G.; Petrosino, M.; Dutta, D.; Graziano, S. The Lung Microbiota and Lung Cancer: A Growing Relationship. Cancers 2022, 14, 4813. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer. 2017, 5, 95. [Google Scholar]
- Drommi, F.; Calabrò, A.; Vento, G.; Pezzino, G.; Cavaliere, R.; Omero, F.; Muscolino, P.; Granata, B.; D’Anna, F.; Silvestris, N.; et al. Crosstalk between ILC3s and Microbiota: Implications for Colon Cancer Development and Treatment with Immune Check Point Inhibitors. Cancers 2023, 15, 2893. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [PubMed]
- Massafra, M.; Passalacqua, M.I.; Gebbia, V.; Macrì, P.; Lazzari, C.; Gregorc, V.; Buda, C.; Altavilla, G.; Santarpia, M. Immunotherapeutic Advances for NSCLC. Biologics 2021, 15, 399–417. [Google Scholar]
- Sprangers, B.; Leaf, D.E.; Porta, C.; Soler, M.J.; Perazella, M.A. Diagnosis and management of immune checkpoint inhibitor-associated acute kidney injury. Nat. Rev. Nephrol. 2022, 18, 794–805. [Google Scholar]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar]
- Wei, Z.; Zhang, X.; Yong, T.; Bie, N.; Zhan, G.; Li, X.; Liang, Q.; Li, J.; Yu, J.; Huang, G.; et al. Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat. Commun. 2021, 12, 440. [Google Scholar]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar]
- Koh, J.; Go, H.; Keam, B.; Kim, M.Y.; Nam, S.J.; Kim, T.M.; Lee, S.H.; Min, H.S.; Kim, Y.T.; Kim, D.W.; et al. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: Comparison with histology and driver oncogenic alteration status. Mod. Pathol. 2015, 28, 1154–1166. [Google Scholar]
- Kim, M.Y.; Koh, J.; Kim, S.; Go, H.; Jeon, Y.K.; Chung, D.H. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer 2015, 88, 24–33. [Google Scholar] [PubMed]
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735. [Google Scholar]
- Zhang, Y.; Wang, L.; Li, Y.; Pan, Y.; Wang, R.; Hu, H.; Li, H.; Luo, X.; Ye, T.; Sun, Y.; et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014, 7, 567–573. [Google Scholar]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [PubMed]
- Takamori, S.; Takada, K.; Toyokawa, G.; Azuma, K.; Shimokawa, M.; Jogo, T.; Yamada, Y.; Hirai, F.; Tagawa, T.; Kawahara, A.; et al. PD-L2 Expression as a Potential Predictive Biomarker for the Response to Anti-PD-1 Drugs in Patients with Non-small Cell Lung Cancer. Anticancer. Res. 2018, 38, 5897–5901. [Google Scholar]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [PubMed]
- Aguiar, P.N., Jr.; De Mello, R.A.; Hall, P.; Tadokoro, H.; Lima Lopes, G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: Updated survival data. Immunotherapy 2017, 9, 499–506. [Google Scholar]
- Toi, Y.; Sugawara, S.; Kawashima, Y.; Aiba, T.; Kawana, S.; Saito, R.; Tsurumi, K.; Suzuki, K.; Shimizu, H.; Sugisaka, J.; et al. Association of Immune-Related Adverse Events with Clinical Benefit in Patients with Advanced Non-Small-Cell Lung Cancer Treated with Nivolumab. Oncologist 2018, 23, 1358–1365. [Google Scholar]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Akamatsu, H.; Murakami, E.; Sasaki, S.; Kanai, K.; Hayata, A.; Tokudome, N.; Akamatsu, K.; Koh, Y.; Ueda, H.; et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung. Cancer 2018, 115, 71–74. [Google Scholar] [CrossRef]
- Simon, S.C.S.; Hu, X.; Panten, J.; Grees, M.; Renders, S.; Thomas, D.; Weber, R.; Schulze, T.J.; Utikal, J.; Umansky, V. Eosinophil accumulation predicts response to melanoma treatment with immune checkpoint inhibitors. Oncoimmunology 2020, 9, 1727116. [Google Scholar] [CrossRef]
- Lou, Y.; Marin-Acevedo, J.A.; Vishnu, P.; Manochakian, R.; Dholaria, B.; Soyano, A.; Luo, Y.; Zhang, Y.; Knutson, K.L. Hypereosinophilia in a patient with metastatic non-small-cell lung cancer treated with antiprogrammed cell death 1 (anti-PD-1) therapy. Immunotherapy 2019, 11, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Buder-Bakhaya, K.; Hassel, J.C. Biomarkers for Clinical Benefit of Immune Checkpoint Inhibitor Treatment-A Review From the Melanoma Perspective and Beyond. Front. Immunol. 2018, 9, 1474. [Google Scholar] [CrossRef]
- Spagnolo, C.C.; Pepe, F.; Ciappina, G.; Nucera, F.; Ruggeri, P.; Squeri, A.; Speranza, D.; Silvestris, N.; Malapelle, U.; Santarpia, M. Circulating biomarkers as predictors of response to immune checkpoint inhibitors in NSCLC: Are we on the right path? Crit. Rev. Oncol. Hematol. 2024, 197, 104332. [Google Scholar] [CrossRef]
- Altrichter, S.; Frischbutter, S.; Fok, J.S.; Kolkhir, P.; Jiao, Q.; Skov, P.S.; Metz, M.; Church, M.K.; Maurer, M. The role of eosinophils in chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2020, 145, 1510–1516. [Google Scholar] [CrossRef]
- Muniz, V.S.; Weller, P.F.; Neves, J.S. Eosinophil crystalloid granules: Structure, function, and beyond. J. Leukoc. Biol. 2012, 92, 281–288. [Google Scholar] [CrossRef]
- Mack, E.A.; Pear, W.S. Transcription factor and cytokine regulation of eosinophil lineage commitment. Curr. Opin. Hematol. 2020, 27, 27–33. [Google Scholar] [CrossRef]
- Salter, B.M.; Ju, X.; Sehmi, R. Eosinophil Lineage-Committed Progenitors as a Therapeutic Target for Asthma. Cells 2021, 10, 412. [Google Scholar] [CrossRef]
- Dent, L.A.; Strath, M.; Mellor, A.L.; Sanderson, C.J. Eosinophilia in transgenic mice expressing interleukin 5. J. Exp. Med. 1990, 172, 1425–1431. [Google Scholar] [CrossRef]
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [PubMed]
- Verma, A.K.; Kandikattu, H.K.; Manohar, M.; Shukla, A.; Upparahalli Venkateshaiah, S.; Zhu, X.; Mishra, A. Intestinal overexpression of IL-18 promotes eosinophils-mediated allergic disorders. Immunology 2019, 157, 110–121. [Google Scholar]
- Hamann, K.J.; Barker, R.L.; Ten, R.M.; Gleich, G.J. The molecular biology of eosinophil granule proteins. Int. Arch. Allergy Appl. Immunol. 1991, 94, 202–209. [Google Scholar] [PubMed]
- Boix, E.; Torrent, M.; Sánchez, D.; Nogués, M.V. The antipathogen activities of eosinophil cationic protein. Curr. Pharm. Biotechnol. 2008, 9, 141–152. [Google Scholar] [PubMed]
- Agosti, J.M.; Altman, L.C.; Ayars, G.H.; Loegering, D.A.; Gleich, G.J.; Klebanoff, S.J. The injurious effect of eosinophil peroxidase, hydrogen peroxide, and halides on pneumocytes in vitro. J. Allergy Clin. Immunol. 1987, 79, 496–504. [Google Scholar]
- Gouon-Evans, V.; Pollard, J.W. Eotaxin is required for eosinophil homing into the stroma of the pubertal and cycling uterus. Endocrinology 2001, 142, 4515–4521. [Google Scholar]
- Gouon-Evans, V.; Rothenberg, M.E.; Pollard, J.W. Postnatal mammary gland development requires macrophages and eosinophils. Development 2000, 127, 2269–2282. [Google Scholar]
- Zimmermann, N.; Hershey, G.K.; Foster, P.S.; Rothenberg, M.E. Chemokines in asthma: Cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 2003, 111, 227–242. [Google Scholar]
- Fulkerson, P.C.; Schollaert, K.L.; Bouffi, C.; Rothenberg, M.E. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J. Immunol. 2014, 193, 4043–4052. [Google Scholar] [CrossRef]
- Lucarini, V.; Ziccheddu, G.; Macchia, I.; La Sorsa, V.; Peschiaroli, F.; Buccione, C.; Sistigu, A.; Sanchez, M.; Andreone, S.; D’Urso, M.T.; et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology 2017, 6, e1317420. [Google Scholar] [CrossRef]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 Promotes CD11b/CD18-Mediated Adhesion of Eosinophils to Cancer Cells and Synapse-Polarized Degranulation Leading to Tumor Cell Killing. Cancers 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Lefrançais, E.; Duval, A.; Mirey, E.; Roga, S.; Espinosa, E.; Cayrol, C.; Girard, J.P. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2014, 111, 15502–15507. [Google Scholar] [CrossRef] [PubMed]
- Afferni, C.; Buccione, C.; Andreone, S.; Galdiero, M.R.; Varricchi, G.; Marone, G.; Mattei, F.; Schiavoni, G. The Pleiotropic Immunomodulatory Functions of IL-33 and Its Implications in Tumor Immunity. Front. Immunol. 2018, 9, 2601. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.C.; Melo, P.H.; Piñeros, A.R.; Ferreira, R.G.; Colón, D.F.; Donate, P.B.; Castanheira, F.V.; Gozzi, A.; Czaikoski, P.G.; Niedbala, W.; et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat. Commun. 2017, 8, 14919. [Google Scholar] [CrossRef]
- Cohen, E.S.; Scott, I.C.; Majithiya, J.B.; Rapley, L.; Kemp, B.P.; England, E.; Rees, D.G.; Overed-Sayer, C.L.; Woods, J.; Bond, N.J.; et al. Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat. Commun. 2015, 6, 8327. [Google Scholar] [CrossRef]
- Lv, Y.; Tian, W.; Teng, Y.; Wang, P.; Zhao, Y.; Li, Z.; Tang, S.; Chen, W.; Xie, R.; Lü, M.; et al. Tumor-infiltrating mast cells stimulate ICOS+ regulatory T cells through an IL-33 and IL-2 axis to promote gastric cancer progression. J. Adv. Res. 2024, 57, 149–162. [Google Scholar] [CrossRef]
- Eissmann, M.F.; Dijkstra, C.; Jarnicki, A.; Phesse, T.; Brunnberg, J.; Poh, A.R.; Etemadi, N.; Tsantikos, E.; Thiem, S.; Huntington, N.D.; et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat. Commun. 2019, 10, 2735. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 10. [Google Scholar]
- Kang, M.H.; Hong, J.; Lee, J.; Cha, M.S.; Lee, S.; Kim, H.Y.; Ha, S.J.; Lim, Y.T.; Bae, Y.S. Discovery of highly immunogenic spleen-resident FCGR3+CD103+ cDC1s differentiated by IL-33-primed ST2+ basophils. Cell Mol. Immunol. 2023, 20, 820–834. [Google Scholar]
- Andreone, S.; Gambardella, A.R.; Mancini, J.; Loffredo, S.; Marcella, S.; La Sorsa, V.; Varricchi, G.; Schiavoni, G.; Mattei, F. Anti-Tumorigenic Activities of IL-33: A Mechanistic Insight. Front. Immunol. 2020, 11, 571593. [Google Scholar]
- Bonanno, A.; Gangemi, S.; La Grutta, S.; Malizia, V.; Riccobono, L.; Colombo, P.; Cibella, F.; Profita, M. 25-Hydroxyvitamin D, IL-31, and IL-33 in children with allergic disease of the airways. Mediat. Inflamm. 2014, 2014, 520241. [Google Scholar]
- Kang, M.H.; Bae, Y.S. IL-33 and IL-33-derived DC-based tumor immunotherapy. Exp. Mol. Med. 2024, 56, 1340–1347. [Google Scholar]
- Nan, Y.; Bai, Y.; Hu, X.; Zhou, K.; Wu, T.; Zhu, A.; Li, M.; Dou, Z.; Cao, Z.; Zhang, X.; et al. Targeting IL-33 reprograms the tumor microenvironment and potentiates antitumor response to anti-PD-L1 immunotherapy. J. Immunother. Cancer 2024, 12, e009236. [Google Scholar]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar]
- Park, K.; Park, J.H.; Yang, W.J.; Lee, J.J.; Song, M.J.; Kim, H.P. Transcriptional activation of the IL31 gene by NFAT and STAT6. J. Leukoc. Biol. 2012, 91, 245–257. [Google Scholar]
- Ferretti, E.; Corcione, A.; Pistoia, V. The IL-31/IL-31 receptor axis: General features and role in tumor microenvironment. J. Leukoc. Biol. 2017, 102, 711–717. [Google Scholar]
- Di Salvo, E.; Ventura-Spagnolo, E.; Casciaro, M.; Navarra, M.; Gangemi, S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediators Inflamm. 2018, 2018, 3858032. [Google Scholar]
- Kan, T.; Feldman, E.; Timaner, M.; Raviv, Z.; Shen-Orr, S.; Aronheim, A.; Shaked, Y. IL-31 induces antitumor immunity in breast carcinoma. J. Immunother. Cancer 2020, 8, e001010. [Google Scholar] [PubMed]
- Ohmatsu, H.; Sugaya, M.; Suga, H.; Morimura, S.; Miyagaki, T.; Kai, H.; Kagami, S.; Fujita, H.; Asano, Y.; Tada, Y.; et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm. Venereol. 2012, 92, 282–283. [Google Scholar] [PubMed]
- Ghilardi, N.; Li, J.; Hongo, J.A.; Yi, S.; Gurney, A.; de Sauvage, F.J. A novel type I cytokine receptor is expressed on monocytes, signals proliferation, and activates STAT-3 and STAT-5. J. Biol. Chem. 2002, 277, 16831–16836. [Google Scholar] [PubMed]
- Zhang, Q.; Putheti, P.; Zhou, Q.; Liu, Q.; Gao, W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008, 19, 347–356. [Google Scholar]
- Gangemi, S.; Franchina, T.; Minciullo, P.L.; Profita, M.; Zanghì, M.; David, A.; Kennez, I.; Adamo, V. IL-33/IL-31 axis: A new pathological mechanisms for EGFR tyrosine kinase inhibitors-associated skin toxicity. J. Cell. Biochem. 2013, 114, 2673–2676. [Google Scholar]
- Davidi, S.; Fremder, E.; Kan, T.; Raviv, Z.; Timaner, M.; Karin, N.; Hershkovitz, D.; Arohneim, A.; Shaked, Y. The antiangiogenic role of the pro-inflammatory cytokine interleukin-31. Oncotarget 2017, 8, 16430–16444. [Google Scholar]
- Liu, Q.H.; Zhang, J.W.; Xia, L.; Wise, S.G.; Hambly, B.D.; Tao, K.; Bao, S.S. Clinical implications of interleukins-31, 32, and 33 in gastric cancer. World J. Gastrointest. Oncol. 2022, 14, 1808–1822. [Google Scholar]
- Zeng, X.; Zhang, Z.; Gao, Q.Q.; Wang, Y.Y.; Yu, X.Z.; Zhou, B.; Xi, M.R. Clinical Significance of Serum Interleukin-31 and Interleukin-33 Levels in Patients of Endometrial Cancer: A Case Control Study. Dis. Markers. 2016, 2016, 9262919. [Google Scholar]
- Wang, X.; Lin, F.K.; Li, J.R.; Wang, H.S. A Comprehensive Risk Assessment Model for Ovarian Cancer Patients with Phospho-STAT3 and IL-31 as Immune Infiltration Relevant Genes. Onco-Targets Ther. 2020, 13, 5617–5628. [Google Scholar]
- Akhtar, S.; Ahmad, F.; Alam, M.; Ansari, A.W.; Uddin, S.; Steinhoff, M.; Buddenkotte, J.; Ahmad, A.; Datsi, A. Interleukin-31: The Inflammatory Cytokine Connecting Pruritus and Cancer. Front. Biosci. 2024, 29, 312. [Google Scholar]
- Jacobsen, E.A.; Helmers, R.A.; Lee, J.J.; Lee, N.A. The expanding role(s) of eosinophils in health and disease. Blood 2012, 120, 3882–3890. [Google Scholar] [CrossRef] [PubMed]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hammerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Dolitzky, A.; Shapira, G.; Grisaru-Tal, S.; Hazut, I.; Avlas, S.; Gordon, Y.; Itan, M.; Shomron, N.; Munitz, A. Transcriptional Profiling of Mouse Eosinophils Identifies Distinct Gene Signatures Following Cellular Activation. Front. Immunol. 2021, 14, 802839. [Google Scholar] [CrossRef]
- Gatault, S.; Legrand, F.; Delbeke, M.; Loiseau, S.; Capron, M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol. Immunother. 2012, 61, 1527–1534. [Google Scholar] [CrossRef]
- da Silva, J.M.; Moreira Dos Santos, T.P.; Sobral, L.M.; Queiroz-Junior, C.M.; Rachid, M.A.; Proudfoot, A.E.I.; Garlet, G.P.; Batista, A.C.; Teixeira, M.M.; Leopoldino, A.M.; et al. Relevance of CCL3/CCR5 axis in oral carcinogenesis. Oncotarget 2017, 8, 51024–51036. [Google Scholar] [PubMed]
- Huang, M.; Wang, J.; Lee, P.; Sharma, S.; Mao, J.T.; Meissner, H.; Dubinett, S.M.; Dubinett, S.M.; Uyemura, K.; Modlin, R.; et al. Human Non-Small Cell Lung Cancer Cells Express a Type 2 Cytokine Pattern. Cancer Res. 1995, 55, 3847–3853. [Google Scholar]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Mahalingam, S.; Newhouse, M.; Ramirez, R.; Rosenberg, H.F.; Foster, P.S.; Matthaei, K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 2007, 110, 1578–1586. [Google Scholar]
- McNeel, D.G.; Gardner, T.A.; Higano, C.S.; Kantoff, P.W.; Small, E.J.; Wener, M.H.; Sims, R.B.; DeVries, T.; Sheikh, N.A.; Dreicer, R. A transient increase in eosinophils is associated with prolonged survival in men with metastatic castration-resistant prostate cancer who receive sipuleucel-T. Cancer Immunol. Res. 2014, 2, 988–999. [Google Scholar] [CrossRef]
- Caliman, E.; Fancelli, S.; Ottanelli, C.; Mazzoni, F.; Paglialunga, L.; Lavacchi, D.; Michelet, M.R.G.; Giommoni, E.; Napolitano, B.; Scolari, F.; et al. Absolute eosinophil count predicts clinical outcomes and toxicity in non-small cell lung cancer patients treated with immunotherapy. Cancer Treat. Res. Commun. 2022, 32, 100603. [Google Scholar] [PubMed]
- Redman, B.G.; Flaherty, L.; Chou, T.H.; al-Katib, A.; Kraut, M.; Martino, S.; Chen, B.; Kaplan, J.; Valdivieso, M. A phase I trial of recombinant interleukin-2 combined with recombinant interferon-gamma in patients with cancer. J. Clin. Oncol. 1990, 8, 1269–1276. [Google Scholar] [PubMed]
- Shi, W.; Li, X.; Wang, Z.; Li, C.; Wang, D.; Li, C. CCL3 Promotes Cutaneous Wound Healing Through Recruiting Macrophages in Mice. Cell Transplant. 2024, 33, 9636897241264912. [Google Scholar] [PubMed]
- Xie, F.; Liu, L.B.; Shang, W.Q.; Chang, K.K.; Meng, Y.H.; Mei, J.; Yu, J.J.; Li, D.J.; Li, M.Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [PubMed]
- Zaynagetdinov, R.; Sherrill, T.P.; Gleaves, L.A.; McLoed, A.G.; Saxon, J.A.; Habermann, A.C.; Connelly, L.; Dulek, D.; Peebles, R.S., Jr.; Fingleton, B.; et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015, 75, 1624–1634. [Google Scholar]
- Schuijs, M.J.; Png, S.; Richard, A.C.; Tsyben, A.; Hamm, G.; Stockis, J.; Garcia, C.; Pinaud, S.; Nicholls, A.; Ros, X.R.; et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat. Immunol. 2020, 21, 998–1009. [Google Scholar] [PubMed]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [PubMed]
- Liu, L.; Zhang, Y.; Zheng, X.; Jin, L.; Xiang, N.; Zhang, M.; Chen, Z. Eosinophils attenuate arthritis by inducing M2 macrophage polarization via inhibiting the IκB/P38 MAPK signaling pathway. Biochem. Biophys. Res. Commun. 2019, 508, 894–901. [Google Scholar]
- Murdaca, G.; Greco, M.; Tonacci, A.; Negrini, S.; Borro, M.; Puppo, F.; Gangemi, S. IL-33/IL-31 Axis in Immune-Mediated and Allergic Diseases. Int. J. Mol. Sci. 2019, 20, 5856. [Google Scholar] [CrossRef]
- O’Flaherty, S.M.; Sutummaporn, K.; Häggtoft, W.L.; Worrall, A.P.; Rizzo, M.; Braniste, V.; Höglund, P.; Kadri, N.; Chambers, B.J. TLR-Stimulated Eosinophils Mediate Recruitment and Activation of NK Cells In Vivo. Scand. J. Immunol. 2017, 85, 417–424. [Google Scholar]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [PubMed]
- Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Wiese, M.D.; Gurney, H.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br. J. Cancer 2017, 117, 913–920. [Google Scholar] [PubMed]
- Gibney, G.T.; Weiner, L.M.; Atkins, M.B. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016, 17, e542–e551. [Google Scholar] [PubMed]
- Calabrese, L.H.; Calabrese, C.; Cappelli, L.C. Rheumatic immune-related adverse events from cancer immunotherapy. Nat. Rev. Rheumatol. 2018, 14, 569–579. [Google Scholar]
- Simon, S.C.S.; Utikal, J.; Umansky, V. Opposing roles of eosinophils in cancer. Cancer Immunol. Immunother. 2019, 68, 823–833. [Google Scholar]
- Wong, D.T.; Bowen, S.M.; Elovic, A.; Gallagher, G.T.; Weller, P.F. Eosinophil ablation and tumor development. Oral. Oncol. 1999, 35, 496–501. [Google Scholar]
- Weller, P.F.; Spencer, L.A. Functions of tissue-resident eosinophils. Nat. Rev. Immunol. 2017, 17, 746–760. [Google Scholar]
- Van Hulst, G.; Batugedara, H.M.; Jorssen, J.; Louis, R.; Bureau, F.; Desmet, C.J. Eosinophil diversity in asthma. Biochem. Pharmacol. 2020, 179, 113963. [Google Scholar]
- Bernard-Tessier, A.; Jeanville, P.; Champiat, S.; Lazarovici, J.; Voisin, A.L.; Mateus, C.; Lambotte, O.; Annereau, M.; Michot, J.M. Immune-related eosinophilia induced by anti-programmed death 1 or death-ligand 1 antibodies. Eur. J. Cancer 2017, 81, 135–137. [Google Scholar]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy 2017, 9, 115–121. [Google Scholar]
- Ye, L.; Wang, H.; Li, H.; Liu, H.; Lv, T.; Song, Y.; Zhang, F. Eosinophil peroxidase over-expression predicts the clinical outcome of patients with primary lung adenocarcinoma. J. Cancer 2019, 10, 1032–1038. [Google Scholar] [CrossRef]
- Tataroǧlu, C.; Kargi, A.; Özkal, S.; Eşrefoǧlu, N.; Akkoçlu, A. Association of macrophages, mast cells and eosinophil leukocytes with angiogenesis and tumor stage in non-small cell lung carcinomas (NSCLC). Lung Cancer 2004, 43, 47–54. [Google Scholar] [PubMed]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef]
- Chu, X.; Zhao, J.; Zhou, J.; Zhou, F.; Jiang, T.; Jiang, S.; Sun, X.; You, X.; Wu, F.; Ren, S.; et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer 2020, 150, 76–82. [Google Scholar]
- Hude, I.; Sasse, S.; Bröckelmann, P.J.; von Tresckow, B.; Momotow, J.; Engert, A.; Borchmann, S. Leucocyte and eosinophil counts predict progression-free survival in relapsed or refractory classical Hodgkin lymphoma patients treated with PD1 inhibition. Br. J. Haematol. 2018, 181, 837–840. [Google Scholar] [PubMed]
- Slungaard, A.; Ascensao, J.; Zanjani, E.; Jacob, H.S. Pulmonary carcinoma with eosinophilia. Demonstration of a tumor-derived eosinophilopoietic factor. N. Engl. J. Med. 1983, 309, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Suda, T.; Shiozaki, H.; Miura, Y.; Hitoshi, Y.; Takatsu, K.; Kasahara, T. Role of IL-5 in IL-2-induced eosinophilia. In vivo and in vitro expression of IL-5 mRNA by IL-2. J. Immunol. 2021, 45, 873–877. [Google Scholar]
- Van Gool, F.; Molofsky, A.B.; Morar, M.M.; Rosenzwajg, M.; Liang, H.E.; Klatzmann, D.; Locksley, R.M.; Bluestone, J.A. Interleukin-5—Producing group 2 innate lymphoid cells control eosinophilia induced by interleukin-2 therapy. Blood 2014, 124, 3572–3576. [Google Scholar] [CrossRef]
- Davis, B.P.; Rothenberg, M.E. Eosinophils and cancer. Cancer Immunol. Res. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Huai, Q.; Luo, C.; Song, P.; Bie, F.; Bai, G.; Li, Y.; Liu, Y.; Chen, X.; Zhou, B.; Sun, X.; et al. Peripheral blood inflammatory biomarkers dynamics reflect treatment response and predict prognosis in non-small cell lung cancer patients with neoadjuvant immunotherapy. Cancer Sci. 2023, 114, 4484–4498. [Google Scholar] [CrossRef]
- Takeuchi, E.; Kondo, K.; Okano, Y.; Ichihara, S.; Kunishige, M.; Kadota, N.; Machida, H.; Hatakeyama, N.; Naruse, K.; Ogino, H.; et al. Pretreatment eosinophil counts as a predictive biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Thorac. Cancer 2023, 30, 3042–3050. [Google Scholar]
- Takeuchi, E.; Ogino, H.; Kondo, K.; Okano, Y.; Ichihara, S.; Kunishige, M.; Kadota, N.; Machida, H.; Hatakeyama, N.; Naruse, K.; et al. An increased relative eosinophil count as a predictive dynamic biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Thorac. Cancer 2024, 3, 248–257. [Google Scholar] [CrossRef]
- Alves, A.; Dias, M.; Campainha, S.; Barroso, A. Peripheral blood eosinophilia may be a prognostic biomarker in non-small cell lung cancer patients treated with immunotherapy. J. Thorac. Dis. 2021, 13, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Sibille, A.; Henket, M.; Corhay, J.L.; Alfieri, R.; Louis, R.; Duysinx, B. White Blood Cells in Patients Treated with Programmed Cell Death-1 Inhibitors for Non-small Cell Lung Cancer. Lung 2021, 199, 549–557. [Google Scholar] [PubMed]
- Osawa, H.; Okauchi, S.; Taguchi, S.; Kagohasi, K.; Satoh, H. Immuno-checkpoint inhibitor associated hyper-eosinophilia and tumor shrinkage. Tuberk. Toraks 2018, 66, 80–83. [Google Scholar]
- Shibaki, R.; Murakami, S.; Matsumoto, Y.; Yoshida, T.; Goto, Y.; Kanda, S.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; Kusumoto, M.; et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol. Immunother. 2020, 69, 15–22. [Google Scholar]
- van Haelst Pisani, C.; Kovach, J.S.; Kita, H.; Leiferman, K.M.; Gleich, G.J.; Silver, J.E.; Dennin, R.; Abrams, J.S. Administration of interleukin-2 (IL-2) results in increased plasma concentrations of IL-5 and eosinophilia in patients with cancer. Blood 1991, 78, 1538–1544. [Google Scholar]
- Delyon, J.; Mateus, C.; Lefeuvre, D.; Lanoy, E.; Zitvogel, L.; Chaput, N.; Roy, S.; Eggermont, A.M.; Routier, E.; Robert, C. Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: An early increase in lymphocyte and eosinophil counts is associated with improved survival. Ann. Oncol. 2013, 24, 1697–1703. [Google Scholar] [CrossRef]
- Gebhardt, C.; Sevko, A.; Jiang, H.; Lichtenberger, R.; Reith, M.; Tarnanidis, K.; Holland-Letz, T.; Umansky, L.; Beckhove, P.; Sucker, A.; et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin. Cancer Res. 2015, 21, 5453–5459. [Google Scholar]
- Martens, A.; Wistuba-Hamprecht, K.; Geukes Foppen, M.; Yuan, J.; Postow, M.A.; Wong, P.; Romano, E.; Khammari, A.; Dreno, B.; Capone, M.; et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin. Cancer Res. 2016, 22, 2908–2918. [Google Scholar]
- Weide, B.; Martens, A.; Hassel, J.C.; Berking, C.; Postow, M.A.; Bisschop, K.; Simeone, E.; Mangana, J.; Schilling, B.; Di Giacomo, A.M.; et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin. Cancer Res. 2016, 22, 5487–5496. [Google Scholar] [CrossRef]
- Okauchi, S.; Shiozawa, T.; Miyazaki, K.; Nishino, K.; Sasatani, Y.; Ohara, G.; Kagohashi, K.; Sato, S.; Kodama, T.; Satoh, H.; et al. Association between peripheral eosinophils and clinical outcomes in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Pol. Arch. Intern. Med. 2021, 131, 152–160. [Google Scholar] [CrossRef]
- Takeuchi, E.; Okano, Y.; Machida, H.; Atagi, K.; Kondou, Y.; Kadota, N.; Hatakeyama, N.; Naruse, K.; Shinohara, T. Eosinophilic pleural effusion due to lung cancer has a better prognosis than non-eosinophilic malignant pleural effusion. Cancer Immunol. Immunother. 2022, 71, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, S.; Zhong, K.; Xu, F.; Huang, L.; Chen, W.; Cheng, P. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: A meta-analysis. BMC Cancer 2020, 20, 454. [Google Scholar] [CrossRef] [PubMed]
- Sakkal, S.; Miller, S.; Apostolopoulos, V.; Nurgali, K. Eosinophils in Cancer: Favourable or Unfavourable? Curr. Med. Chem. 2016, 23, 650–666. [Google Scholar] [CrossRef] [PubMed]
- Mattei, F.; Andreone, S.; Marone, G.; Gambardella, A.R.; Loffredo, S.; Varricchi, G.; Schiavoni, G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 1–28. [Google Scholar]
- Ariyasu, R.; Horiike, A.; Yoshizawa, T.; Dotsu, Y.; Koyama, J.; Saiki, M.; Sonoda, T.; Nishikawa, S.; Kitazono, S.; Nishio, N.Y.A. Adrenal insufficiency related to anti-programmed death-1 therapy. Anticancer. Res. 2017, 37, 4229–4232. [Google Scholar]
- Mirza, S.; Hill, E.; Ludlow, S.P.; Nanjappa, S. Checkpoint inhibitor associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res. 2017, 27, 271–273. [Google Scholar] [CrossRef]
- Scanvion, Q.; Béné, J.; Gautier, S.; Grandvuillemin, A.; Le Beller, C.; Chenaf, C.; Etienne, N.; Brousseau, S.; Cortot, A.B.; Mortier, L.; et al. Moderate-to-severe eosinophilia induced by treatment with immune checkpoint inhibitors: 37 cases from a national reference center for hypereosinophilic syndromes and the French pharmacovigilance database. Oncoimmunology 2020, 9, 1722022. [Google Scholar]
- Giommoni, E.; Giorgione, R.; Paderi, A.; Pellegrini, E.; Gambale, E.; Marini, A.; Antonuzzo, A.; Marconcini, R.; Roviello, G.; Matucci-Cerinic, M.; et al. Eosinophil count as predictive biomarker of immune-related adverse events (irAEs) in immune checkpoint inhibitors (ICIs) therapies in oncological patients. Immuno 2021, 1, 253–263. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Maruyama, H.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujimoto, M.; Fujisawa, Y. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn. J. Clin. Oncol. 2019, 49, 431–437. [Google Scholar] [CrossRef]
- Hu, W.T.; Zhang, Q.; Zhang, Z.; He, X.; Zhou, M.; Guo, Y.; Wang, X. Eosinophil and IFN-γ associated with immune-related adverse events as prognostic markers in patients with non-small cell lung cancer treated with immunotherapy. Front. Immunol. 2023, 14, 1112409. [Google Scholar]
- Ma, Y.; Ma, X.; Wang, J.; Wu, S.; Wang, J.; Cao, B. Absolute eosinophil count may be an optimal peripheral blood marker to identify the risk of immune-related adverse events in advanced malignant tumors treated with PD-1/PD-L1 inhibitors: A retrospective analysis. World J. Surg. Oncol. 2022, 20, 242. [Google Scholar] [PubMed]
- Pozorski, V.; Park, Y.; Mohamoud, Y.; Tesfamichael, D.; Emamekhoo, H.; Birbrair, A.; Albertini, M.R.; Ma, V.T. Neutrophil-to-eosinophil ratio as a biomarker for clinical outcomes in advanced stage melanoma patients treated with anti-PD-1 therapy. Pigment. Cell Melanoma Res. 2023, 36, 501–511. [Google Scholar] [PubMed]
- Okano, Y.; Satoh, T.; Horiguchi, K.; Toyoda, M.; Osaki, A.; Matsumoto, S.; Tomaru, T.; Nakajima, Y.; Ishii, S.; Ozawa, A.; et al. Nivolumab-induced hypophysitis in a patient with advanced malignant melanoma. Endocr. J. 2016, 63, 905–912. [Google Scholar]
- Yamada, H.; Washino, S.; Suzuki, D.; Saikawa, R.; Tonezawa, S.; Hagiwara, R.; Funazaki, S.; Yoshida, M.; Konishi, T.; Saito, K.; et al. Hypereosinophilia is a predictive biomarker of immune checkpoint inhibitor-induced hypopituitarism in patients with renal cell carcinoma. BMC Endocr. Disord. 2022, 22, 110. [Google Scholar]
- Takayasu, S.; Mizushiri, S.; Watanuki, Y.; Yamagata, S.; Usutani, M.; Nakada, Y.; Asari, Y.; Murasawa, S.; Kageyama, K.; Daimon, M. Eosinophil Counts Can Be a Predictive Marker of Immune Checkpoint Inhibitor-Induced Secondary Adrenal Insufficiency: A Retrospective Cohort Study. Sci. Rep. 2022, 12, 1294. [Google Scholar]
- Huang, S.; Chung, J.Y.; Li, C.; Wu, Y.; Qiao, G.; To, K.F.; Tang, P.M. Cellular dynamics of tumor microenvironment driving immunotherapy resistance in non-small-cell lung carcinoma. Cancer Lett. 2024, 604, 217272. [Google Scholar]
- Fang, X.; Li, D.; Wan, S.; Hu, J.; Zhang, P.; Jie, D.; Chen, L.; Jiang, G.; Song, N. Insights into the heterogeneity of the tumor microenvironment in lung adenocarcinoma and squamous carcinoma through single-cell transcriptomic analysis: Implications for distinct immunotherapy outcomes. J. Gene Med. 2024, 26, e3694. [Google Scholar]
- Lococo, F.; Ghaly, G.; Chiappetta, M.; Flamini, S.; Evangelista, J.; Bria, E.; Stefani, A.; Vita, E.; Martino, A.; Boldrini, L.; et al. Implementation of Artificial Intelligence in Personalized Prognostic Assessment of Lung Cancer: A Narrative Review. Cancers 2024, 16, 1832. [Google Scholar] [CrossRef]
- Sinha, T.; Khan, A.; Awan, M.; Bokhari, S.F.H.; Ali, K.; Amir, M.; Jadhav, A.N.; Bakht, D.; Puli, S.T.; Burhanuddin, M. Artificial Intelligence and Machine Learning in Predicting the Response to Immunotherapy in Non-small Cell Lung Carcinoma: A Systematic Review. Cureus 2024, 28, e61220. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, L.; Lu, M.; Jin, R.; Ye, H.; Ma, T. The artificial intelligence and machine learning in lung cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Stein-O’Brien, G.L.; Le, D.T.; Jaffee, E.M.; Fertig, E.J.; Zaidi, N. Converging on a cure: The roads to predictive immunotherapy. Cancer Discov. 2023, 13, 1053–1057. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef] [PubMed]
- Strickler, J.H.; Hanks, B.A.; Khasraw, M. Tumor mutational burden as a predictor of immunotherapy response: Is more always better? Clin. Cancer Res. 2021, 27, 1236–1241. [Google Scholar] [CrossRef]
- Monaco, L.; De Bernardi, E.; Bono, F.; Cortinovis, D.; Crivellaro, C.; Elisei, F.; L’Imperio, V.; Landoni, C.; Mathoux, G.; Musarra, M.; et al. The “digital biopsy” in non-small cell lung cancer (NSCLC): A pilot study to predict the PD-L1 status from radiomics features of [18F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imagin 2022, 49, 3401–3411. [Google Scholar] [CrossRef]
- Rakaee, M.; Adib, E.; Ricciuti, B.; Sholl, L.M.; Shi, W.; Alessi, J.V.; Cortellini, A.; Fulgenzi, C.A.M.; Viola, P.; Pinato, D.J.; et al. Association of Machine Learning-Based Assessment of Tumor-Infiltrating Lymphocytes on Standard Histologic Images With Outcomes of Immunotherapy in Patients With NSCLC. JAMA Oncol. 2023, 9, 51–60. [Google Scholar] [CrossRef]
- Wei, F.; Azuma, K.; Nakahara, Y.; Saito, H.; Matsuo, N.; Tagami, T.; Kouro, T.; Igarashi, Y.; Tokito, T.; Kato, T.; et al. Machine learning for prediction of immunotherapeutic outcome in non-small-cell lung cancer based on circulating cytokine signatures. J. Immunother. Cancer 2023, 11, e006788. [Google Scholar] [CrossRef]
- Carini, C.; Seyhan, A.A. Tribulations and future opportunities for artificial intelligence in precision medicine. J. Transl. Med. 2024, 22, 411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omero, F.; Speranza, D.; Murdaca, G.; Cavaleri, M.; Marafioti, M.; Cianci, V.; Berretta, M.; Casciaro, M.; Gangemi, S.; Santarpia, M. The Role of Eosinophils, Eosinophil-Related Cytokines and AI in Predicting Immunotherapy Efficacy in NSCLC. Biomolecules 2025, 15, 491. https://doi.org/10.3390/biom15040491
Omero F, Speranza D, Murdaca G, Cavaleri M, Marafioti M, Cianci V, Berretta M, Casciaro M, Gangemi S, Santarpia M. The Role of Eosinophils, Eosinophil-Related Cytokines and AI in Predicting Immunotherapy Efficacy in NSCLC. Biomolecules. 2025; 15(4):491. https://doi.org/10.3390/biom15040491
Chicago/Turabian StyleOmero, Fausto, Desirèe Speranza, Giuseppe Murdaca, Mariacarmela Cavaleri, Mariapia Marafioti, Vincenzo Cianci, Massimiliano Berretta, Marco Casciaro, Sebastiano Gangemi, and Mariacarmela Santarpia. 2025. "The Role of Eosinophils, Eosinophil-Related Cytokines and AI in Predicting Immunotherapy Efficacy in NSCLC" Biomolecules 15, no. 4: 491. https://doi.org/10.3390/biom15040491
APA StyleOmero, F., Speranza, D., Murdaca, G., Cavaleri, M., Marafioti, M., Cianci, V., Berretta, M., Casciaro, M., Gangemi, S., & Santarpia, M. (2025). The Role of Eosinophils, Eosinophil-Related Cytokines and AI in Predicting Immunotherapy Efficacy in NSCLC. Biomolecules, 15(4), 491. https://doi.org/10.3390/biom15040491








