Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis
Abstract
1. Introduction
2. Spermatogenesis
3. Redox Homeostasis and Physio-Pathological Conditions
4. Redox Homeostasis and Spermatogenesis
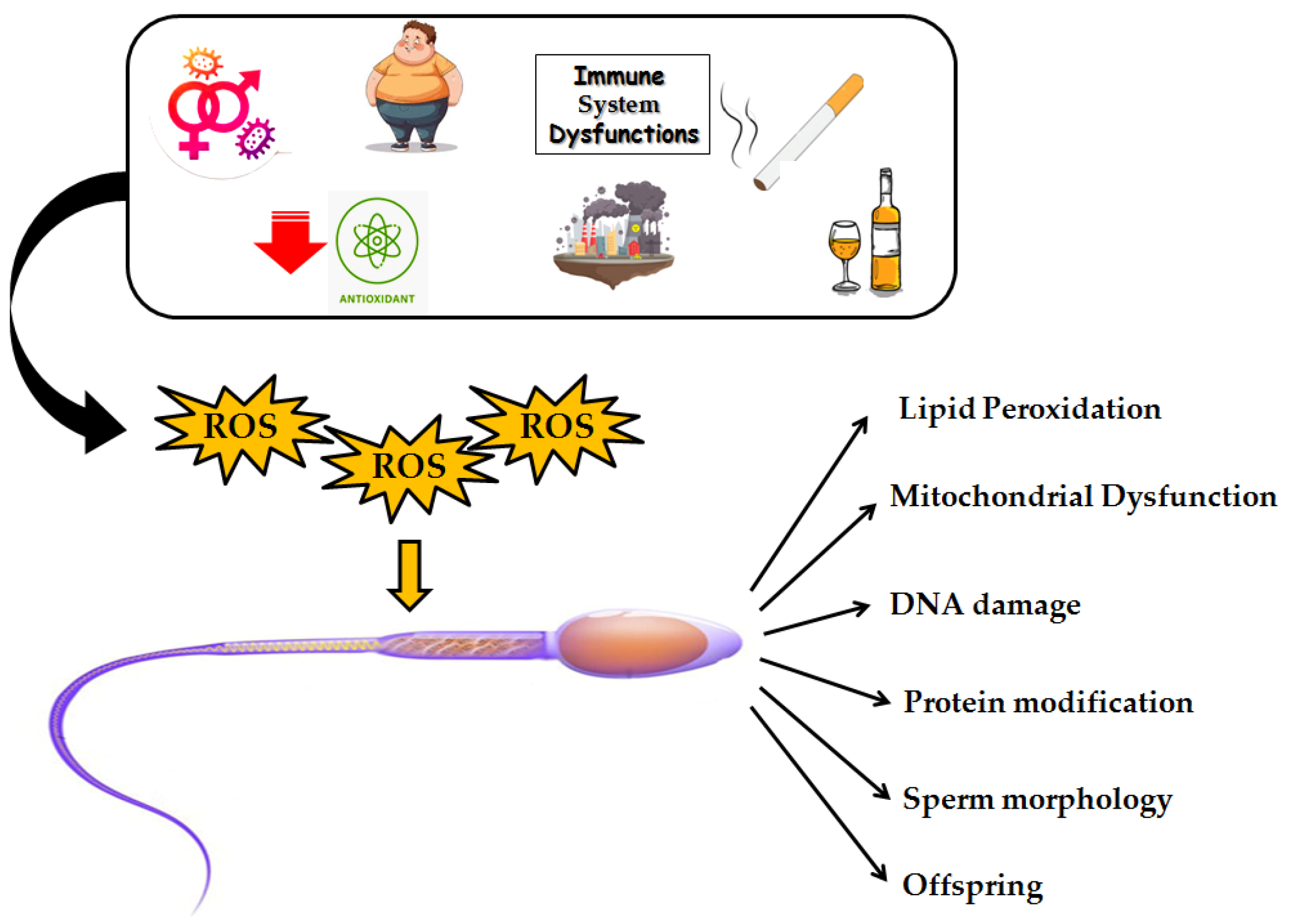
5. The Role of Oxidative Stress in Male Infertility
Testicular Cells and Oxidative Stress
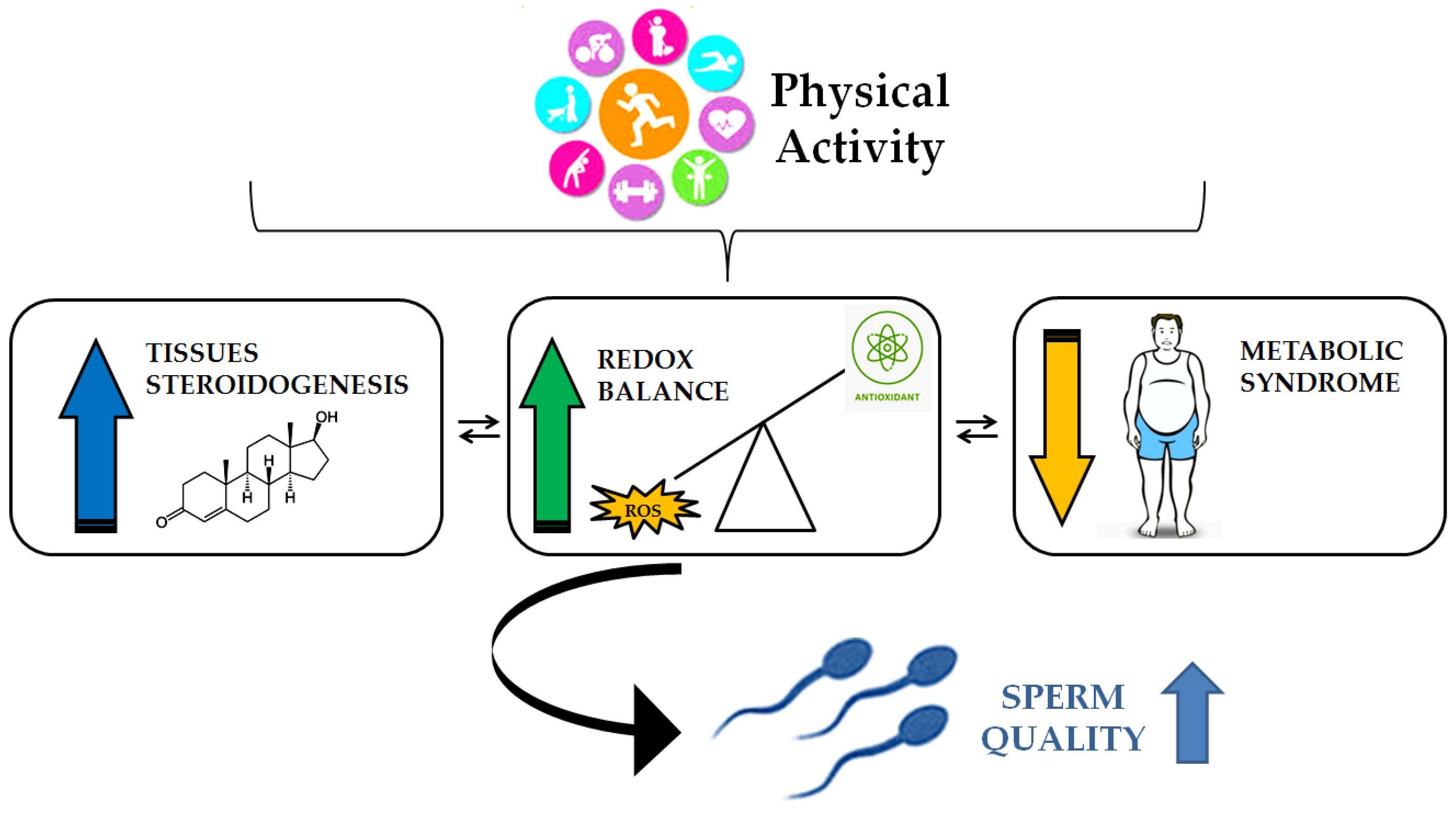
6. Spermatogenesis and Physical Activity: The Role of Oxidative Stress Control
Impact of Physical Activity on Spermatogenesis
7. Conclusions and Future Perspectives
8. Review Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cAMP | Cyclic adenosine monophosphate. |
| CAT | Catalase. |
| CO | Carbon monoxide. |
| Cx43 | Connexin-43. |
| FSH | Follicle-stimulating hormone. |
| GPx | Glutathione peroxidase. |
| GnRH | Gonadotropin-releasing hormone. |
| H2O2 | Hydrogen peroxide. |
| H2 | Hydrogen. |
| LCS | Leukocytospermia. |
| LH | Luteinizing hormone. |
| NH3 | Ammonia. |
| PKA | Protein kinase A. |
| PA | Physical activity. |
| Prdx2 | Peroxiredoxin 2. |
| PUFA | Polyunsaturated fatty acids. |
| RES | Reactive electrophile species. |
| RNS | Reactive nitrogen species. |
| RHS | Reactive halogen species. |
| REDOX | Oxidation–reduction. |
| ROS | Reactive oxygen species. |
| SSCs | Spermatogonial stem cells. |
| SOD | Superoxide dismutase. |
| SPIs | Spermiogenesis indices. |
| TBARS | Thiobarbituric acid-reactive substances. |
| TDI | Tubular differentiation. |
References
- Rivero, M.-J.; Kulkarni, N.; Thirumavalavan, N.; Ramasamy, R. Evaluation and Management of Male Genital Tract Infections in the Setting of Male Infertility: An Updated Review. Curr. Opin. Urol. 2023, 33, 180–186. [Google Scholar] [CrossRef]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A. Male Infertility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Becker, K.L. (Ed.) Principles and Practice of Endocrinology and Metabolism, 3rd ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001; ISBN 978-0-7817-1750-2. [Google Scholar]
- Champroux, A.; Torres-Carreira, J.; Gharagozloo, P.; Drevet, J.R.; Kocer, A. Mammalian Sperm Nuclear Organization: Resiliencies and Vulnerabilities. Basic Clin. Androl. 2016, 26, 17. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Men’s Health 2019, 37, 296. [Google Scholar] [CrossRef]
- Baker, M.A.; Hetherington, L.; Aitken, R.J. Identification of SRC as a Key PKA-Stimulated Tyrosine Kinase Involved in the Capacitation-Associated Hyperactivation of Murine Spermatozoa. J. Cell Sci. 2006, 119, 3182–3192. [Google Scholar] [CrossRef]
- Sharma, P.; Ghanghas, P.; Kaushal, N.; Kaur, J.; Kaur, P. Epigenetics and Oxidative Stress: A Twin-edged Sword in Spermatogenesis. Andrologia 2019, 51, e13432. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The Molecular Link between Oxidative Stress, Insulin Resistance, and Type 2 Diabetes: A Target for New Therapies against Cardiovascular Diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Adelowo, O.E.; Akindele, B.M.; Adegbola, C.A.; Oyedokun, P.A.; Akhigbe, T.M.; Akhigbe, R.E. Unraveling the Complexity of the Impact of Physical Exercise on Male Reproductive Functions: A Review of Both Sides of a Coin. Front. Physiol. 2024, 15, 1492771. [Google Scholar] [CrossRef]
- Ramírez-López, C.J.; Barros, E.; Vidigal, P.M.; Okano, D.S.; Gomes, L.L.; Carvalho, R.P.R.; De Castro, A.G.; Baracat-Pereira, M.C.; Guimarães, S.E.F.; Guimarães, J.D. Oxidative Stress Associated with Proteomic and Fatty Acid Profiles of Sperm from Nellore Bulls at Rest. Biol. Reprod. 2023, 109, 878–891. [Google Scholar] [CrossRef]
- Dunleavy, J.E.M.; O’Bryan, M.K.; Stanton, P.G.; O’Donnell, L. The Cytoskeleton in Spermatogenesis. Reproduction 2019, 157, R53–R72. [Google Scholar] [CrossRef]
- Sperry, A.O. The Dynamic Cytoskeleton of the Developing Male Germ Cell. Biol. Cell 2012, 104, 297–305. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.-E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their Role in Spermatozoa and in Male Infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Lopez, K.H. Human Reproductive Biology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-382184-3. [Google Scholar]
- O’Shaughnessy, P.J.; Mitchell, R.T.; Monteiro, A.; O’Hara, L.; Cruickshanks, L.; Der Grinten, H.C.; Brown, P.; Abel, M.; Smith, L.B. Androgen Receptor Expression Is Required to Ensure Development of Adult Leydig Cells and to Prevent Development of Steroidogenic Cells with Adrenal Characteristics in the Mouse Testis. BMC Dev. Biol. 2019, 19, 8. [Google Scholar] [CrossRef]
- Wong, W.J.; Khan, Y.S. Histology, Sertoli Cell. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Eustress and Oxidative Distress: Introductory Remarks. In Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–12. ISBN 978-0-12-818606-0. [Google Scholar]
- Sies, H. On the History of Oxidative Stress: Concept and Some Aspects of Current Development. Curr. Opin. Toxicol. 2018, 7, 122–126. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox Homeostasis: The Golden Mean of Healthy Living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.N.; Rios, N.; Trujillo, M.; Radi, R.; Denicola, A.; Alvarez, B. Detection and Quantification of Nitric Oxide–Derived Oxidants in Biological Systems. J. Biol. Chem. 2019, 294, 14776–14802. [Google Scholar] [CrossRef]
- Nakamura, T.; Lipton, S.A. Nitric Oxide-Dependent Protein Post-Translational Modifications Impair Mitochondrial Function and Metabolism to Contribute to Neurodegenerative Diseases. Antioxid. Redox Signal. 2020, 32, 817–833. [Google Scholar] [CrossRef]
- Olson, K.R. Reactive Oxygen Species or Reactive Sulfur Species: Why We Should Consider the Latter. J. Exp. Biol. 2020, 223, jeb196352. [Google Scholar] [CrossRef]
- Parvez, S.; Long, M.J.C.; Poganik, J.R.; Aye, Y. Redox Signaling by Reactive Electrophiles and Oxidants. Chem. Rev. 2018, 118, 8798–8888. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Santolini, J.; Wootton, S.A.; Jackson, A.A.; Feelisch, M. The Redox Architecture of Physiological Function. Curr. Opin. Physiol. 2019, 9, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Strategies of Antioxidant Defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Ayres, J.S. The Biology of Physiological Health. Cell 2020, 181, 250–269. [Google Scholar] [CrossRef]
- Demirovic, D.; Rattan, S.I.S. Establishing Cellular Stress Response Profiles as Biomarkers of Homeodynamics, Health and Hormesis. Exp. Gerontol. 2013, 48, 94–98. [Google Scholar] [CrossRef]
- Lloyd, D.; Aon, M.A.; Cortassa, S. Why Homeodynamics, Not Homeostasis? Sci. World J. 2001, 1, 133–145. [Google Scholar] [CrossRef]
- Selye, H. Stress and Distress. Compr. Ther. 1975, 1, 9–13. [Google Scholar]
- Lu, S.; Wei, F.; Li, G. The Evolution of the Concept of Stress and the Framework of the Stress System. CST 2021, 5, 76–85. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; Van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-W.; Bowles, J.; Koopman, P. Control of Mammalian Germ Cell Entry into Meiosis. Mol. Cell. Endocrinol. 2014, 382, 488–497. [Google Scholar] [CrossRef]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The Role of Mitochondria in Energy Production for Human Sperm Motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative Stress, Sperm Survival and Fertility Control. Mol. Cell. Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of Reactive Oxygen Species in the Spermatogenesis Regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef]
- Morimoto, H.; Iwata, K.; Ogonuki, N.; Inoue, K.; Atsuo, O.; Kanatsu-Shinohara, M.; Morimoto, T.; Yabe-Nishimura, C.; Shinohara, T. ROS Are Required for Mouse Spermatogonial Stem Cell Self-Renewal. Cell Stem Cell 2013, 12, 774–786. [Google Scholar] [CrossRef]
- Morimoto, H.; Kanatsu-Shinohara, M.; Shinohara, T. ROS-Generating Oxidase Nox3 Regulates the Self-Renewal of Mouse Spermatogonial Stem Cells. Biol. Reprod. 2015, 92, 147. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative Stress and Male Infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative Stress and Antioxidant Mechanisms in Human Body. JABB 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Shi, S.; Lan, X.; Cheng, X.; Li, L.; Zou, Y.; Jia, L.; Liu, W.; Luo, Q.; et al. LanCL2 Implicates in Testicular Redox Homeostasis and Acrosomal Maturation. Antioxidants 2024, 13, 534. [Google Scholar] [CrossRef]
- Gong, S.; Gabriel, M.C.S.; Zini, A.; Chan, P.; O’Flaherty, C. Low Amounts and High Thiol Oxidation of Peroxiredoxins in Spermatozoa from Infertile Men. J. Androl. 2012, 33, 1342–1351. [Google Scholar] [CrossRef]
- Wu, W.; Lu, J.; Tang, Q.; Zhang, S.; Yuan, B.; Li, J.; Wu, D.; Sun, H.; Lu, C.; Xia, Y.; et al. GSTM1 and GSTT1 Null Polymorphisms and Male Infertility Risk: An Updated Meta-Analysis Encompassing 6934 Subjects. Sci. Rep. 2013, 3, 2258. [Google Scholar] [CrossRef]
- Naghavi, A.; Fazeli, F.; Salimi, S.; Nemati, B.M. S25 Glutathione-S-Transferase P1 Ile105Val Polymorphism and Idiopathic Male Infertility. Eur. Urol. Suppl. 2013, 12, e1133-S25. [Google Scholar] [CrossRef]
- Faure, C.; Leveille, P.; Dupont, C.; Julia, C.; Chavatte-Palmer, P.; Alifert Group; Sutton, A.; Levy, R. Are Superoxide Dismutase 2 and Nitric Oxide Synthase Polymorphisms Associated with Idiopathic Infertility? Antioxid. Redox Signal. 2014, 21, 565–569. [Google Scholar] [CrossRef]
- Ji, G.; Gu, A.; Wang, Y.; Huang, C.; Hu, F.; Zhou, Y.; Song, L.; Wang, X. Genetic Variants in Antioxidant Genes Are Associated with Sperm DNA Damage and Risk of Male Infertility in a Chinese Population. Free Radic. Biol. Med. 2012, 52, 775–780. [Google Scholar] [CrossRef]
- Ishii, T.; Matsuki, S.; Iuchi, Y.; Okada, F.; Toyosaki, S.; Tomita, Y.; Ikeda, Y.; Fujii, J. Accelerated Impairment of Spermatogenic Cells in Sod1-Knockout Mice under Heat Stress. Free Radic. Res. 2005, 39, 697–705. [Google Scholar] [CrossRef]
- Smith, T.B.; Baker, M.A.; Connaughton, H.S.; Habenicht, U.; Aitken, R.J. Functional Deletion of Txndc2 and Txndc3 Increases the Susceptibility of Spermatozoa to Age-Related Oxidative Stress. Free Radic. Biol. Med. 2013, 65, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prabakaran, S.A. Mechanism, Measurement, and Prevention of Oxidative Stress in Male Reproductive Physiology. Indian J. Exp. Biol. 2005, 43, 963–974. [Google Scholar] [PubMed]
- Tortolero, I.; Duarte Ojeda, J.M.; Pamplona Casamayor, M.; Alvarez González, E.; Arata-Bellabarba, G.; Regadera, J.; Leiva Galvis, O. The effect of seminal leukocytes on semen quality in subfertile males with and without varicocele. Arch. Esp. Urol. 2004, 57, 921–928. [Google Scholar]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1. [Google Scholar] [CrossRef]
- Iommiello, V.M.; Albani, E.; Di Rosa, A.; Marras, A.; Menduni, F.; Morreale, G.; Levi, S.L.; Pisano, B.; Levi-Setti, P.E. Ejaculate Oxidative Stress Is Related with Sperm DNA Fragmentation and Round Cells. Int. J. Endocrinol. 2015, 2015, 321901. [Google Scholar] [CrossRef] [PubMed]
- Montano, L.; Maugeri, A.; Volpe, M.G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. IJMS 2022, 23, 1568. [Google Scholar] [CrossRef]
- Itziou, A.; Balis, V.; Lakioti, E.; Karayannis, V.; Tsanaktsidis, C. Environmental Pollution and Oxidative Stress: Health Effects During Pregnancy: A Review. Appl. Sci. 2024, 14, 9884. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E. Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 2011, 487074. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.-R.; Hu, Y.-F.; Xiong, Y.-W.; Zhu, H.-L.; Huang, Y.-C.; Wang, H. Advances in Immunology of Male Reproductive Toxicity Induced by Common Environmental Pollutants. Environ. Int. 2024, 190, 108898. [Google Scholar] [CrossRef]
- Lahimer, M.; Abou Diwan, M.; Montjean, D.; Cabry, R.; Bach, V.; Ajina, M.; Ben Ali, H.; Benkhalifa, M.; Khorsi-Cauet, H. Endocrine Disrupting Chemicals and Male Fertility: From Physiological to Molecular Effects. Front. Public Health 2023, 11, 1232646. [Google Scholar] [CrossRef]
- Krzastek, S.C.; Farhi, J.; Gray, M.; Smith, R.P. Impact of Environmental Toxin Exposure on Male Fertility Potential. Transl. Androl. Urol. 2020, 9, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Impact of Environmental Factors on Human Semen Quality and Male Fertility: A Narrative Review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Intergenerational and Transgenerational Epigenetic Inheritance in Animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally Induced Transgenerational Environmental Reprogramming of Metabolic Gene Expression in Mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Pereira, S.C.; Ferreira, R.; Monteiro, M.P.; Oliveira, P.F.; Alves, M.G. Signatures of Metabolic Diseases on Spermatogenesis and Testicular Metabolism. Nat. Rev. Urol. 2024, 21, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Stuppia, L.; Franzago, M.; Ballerini, P.; Gatta, V.; Antonucci, I. Epigenetics and Male Reproduction: The Consequences of Paternal Lifestyle on Fertility, Embryo Development, and Children Lifetime Health. Clin. Epigenet. 2015, 7, 120. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Bagchi, S.; Chhikara, B.S.; Pavlík, A.; Sláma, P.; Roychoudhury, S. Reproductive Toxicity of Combined Effects of Endocrine Disruptors on Human Reproduction. Front. Cell Dev. Biol. 2023, 11, 1162015. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, Q.; Lu, Y.; Li, M.; Zhou, Y.; Wu, P.; Li, J.; Pan, F.; Han, X.; Chen, M.; et al. Semen Quality and Sperm DNA Methylation in Relation to Long-Term Exposure to Air Pollution in Fertile Men: A Cross-Sectional Study. Environ. Pollut. 2022, 300, 118994. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, X.; Li, D.; Yang, F.; Pan, H.; Li, T.; Miao, M.; Li, R.; Yuan, W. Genome-Wide Alteration in DNA Hydroxymethylation in the Sperm from Bisphenol A-Exposed Men. PLoS ONE 2017, 12, e0178535. [Google Scholar] [CrossRef]
- Wyck, S.; Herrera, C.; Requena, C.E.; Bittner, L.; Hajkova, P.; Bollwein, H.; Santoro, R. Oxidative Stress in Sperm Affects the Epigenetic Reprogramming in Early Embryonic Development. Epigenet. Chromatin 2018, 11, 60. [Google Scholar] [CrossRef]
- Vieira De Sousa Neto, I.; Fontes, W.; Prestes, J.; De Cassia Marqueti, R. Impact of Paternal Exercise on Physiological Systems in the Offspring. Acta Physiol. 2021, 231, e13620. [Google Scholar] [CrossRef]
- Kaltsas, A.; Markou, E.; Kyrgiafini, M.-A.; Zikopoulos, A.; Symeonidis, E.N.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Oxidative-Stress-Mediated Epigenetic Dysregulation in Spermatogenesis: Implications for Male Infertility and Offspring Health. Genes 2025, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Kurkowska, W.; Bogacz, A.; Janiszewska, M.; Gabryś, E.; Tiszler, M.; Bellanti, F.; Kasperczyk, S.; Machoń-Grecka, A.; Dobrakowski, M.; Kasperczyk, A. Oxidative Stress Is Associated with Reduced Sperm Motility in Normal Semen. Am. J. Men’s Health 2020, 14, 1557988320939731. [Google Scholar] [CrossRef]
- Raj, C.J.; Aishwarya, C.V.S.; Mounika, K.V.S.S.N.; Mishra, B.; Sumithra, B.; Vishal, B.; Mandal, S.K. Deciphering the Nexus Between Oxidative Stress and Spermatogenesis: A Compendious Overview. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine; Roychoudhury, S., Kesari, K.K., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2022; Volume 1391, pp. 1–16. ISBN 978-3-031-12965-0. [Google Scholar]
- Aitken, R.J.; Roman, S.D. Antioxidant Systems and Oxidative Stress in the Testes. In Molecular Mechanisms in Spermatogenesis; Cheng, C.Y., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2009; Volume 636, pp. 154–171. ISBN 978-0-387-79990-2. [Google Scholar]
- Asadi, N. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving It: A Review. JCDR 2017, 11, IE01. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.R. Leukocytes and Oxidative Stress: Dilemma for Sperm Function and Male Fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Vahedi Raad, M.; Firouzabadi, A.M.; Tofighi Niaki, M.; Henkel, R.; Fesahat, F. The Impact of Mitochondrial Impairments on Sperm Function and Male Fertility: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef]
- Chandra, S.; Romero, M.; Shatanawi, A.; Alkilany, A.; Caldwell, R.; Caldwell, R. Oxidative Species Increase Arginase Activity in Endothelial Cells through the RhoA/Rho Kinase Pathway. Br. J. Pharmacol. 2012, 165, 506–519. [Google Scholar] [CrossRef]
- Dare, B. Role of Antioxidant in Testicular Integrity. ARRB 2014, 4, 998–1023. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress That Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; He, J.; Wu, S. Oxidative Stress and Autophagy: Unraveling the Hidden Threat to Boars’ Fertility. Antioxidants 2024, 14, 2. [Google Scholar] [CrossRef]
- Donato, F.; Rota, M.; Ceretti, E.; Viviana Viola, G.C.; Marullo, M.; Zani, D.; Lorenzetti, S.; Montano, L. Intensity and Type of Physical Activity and Semen Quality in Healthy Young Men. Fertil. Steril. 2025, 123, 88–96. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Borgmann, H.; Struck, J.; Salem, J.; Kuru, T. Antioxidant Supplementation on Male Fertility—A Systematic Review. Antioxidants 2023, 12, 836. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Washburn, R.L.; Hibler, T.; Kaur, G.; Dufour, J.M. Sertoli Cell Immune Regulation: A Double-Edged Sword. Front. Immunol. 2022, 13, 913502. [Google Scholar] [CrossRef]
- Zhang, P.; Li, F.; Zhang, L.; Lei, P.; Zheng, Y.; Zeng, W. Stage-specific Embryonic Antigen 4 Is a Membrane Marker for Enrichment of Porcine Spermatogonial Stem Cells. Andrology 2020, 8, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.A.; Khafagy, R.M. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD)-Induced Cytotoxicity Accompanied by Oxidative Stress in Rat Sertoli Cells: Possible Role of Mitochondrial Fractions of Sertoli Cells. Toxicol. Appl. Pharmacol. 2011, 252, 273–280. [Google Scholar] [CrossRef]
- Yilmaz, B.; Yildizbayrak, N.; Aydin, Y.; Erkan, M. Evidence of Acrylamide- and Glycidamide-Induced Oxidative Stress and Apoptosis in Leydig and Sertoli Cells. Hum. Exp. Toxicol. 2017, 36, 1225–1235. [Google Scholar] [CrossRef]
- Duan, P.; Quan, C.; Huang, W.-T.; Yang, K. PI3K-Akt/LKB1-AMPK-mTOR-p70S6K/4EBP1 signaling pathways participate in the regulation of testis development and spermatogenesis: An update. Zhonghua Nan Ke Xue 2016, 22, 1016–1020. [Google Scholar] [PubMed]
- Cinthya Riris, A.A.I.D.; I’tishom, R.; Khaerunnisa, S. Role of Antioxidant to Protect Leydig Cells Induced by Reactive Oxygen Species: A Literature Review. Qanun Med. 2021, 5, 49. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig Cells: Effects of Aging and Environmental Factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Cafe, S.L.; Nixon, B.; Dun, M.D.; Roman, S.D.; Bernstein, I.R.; Bromfield, E.G. Oxidative Stress Dysregulates Protein Homeostasis Within the Male Germ Line. Antioxid. Redox Signal. 2020, 32, 487–503. [Google Scholar] [CrossRef]
- Cafe, S.L.; Nixon, B.; Ecroyd, H.; Martin, J.H.; Skerrett-Byrne, D.A.; Bromfield, E.G. Proteostasis in the Male and Female Germline: A New Outlook on the Maintenance of Reproductive Health. Front. Cell Dev. Biol. 2021, 9, 660626. [Google Scholar] [CrossRef]
- Duong, L.D.; West, J.D.; Morano, K.A. Redox Regulation of Proteostasis. J. Biol. Chem. 2024, 300, 107977. [Google Scholar] [CrossRef]
- Hipp, M.S.; Hartl, F.U. Interplay of Proteostasis Capacity and Protein Aggregation: Implications for Cellular Function and Disease. J. Mol. Biol. 2024, 436, 168615. [Google Scholar] [CrossRef]
- Long, S.; Kenworthy, S. Round Cells in Diagnostic Semen Analysis: A Guide for Laboratories and Clinicians. Br. J. Biomed. Sci. 2022, 79, 10129. [Google Scholar] [CrossRef]
- Pavuluri, H.; Bakhtiary, Z.; Panner Selvam, M.K.; Hellstrom, W.J.G. Oxidative Stress-Associated Male Infertility: Current Diagnostic and Therapeutic Approaches. Medicina 2024, 60, 1008. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Bo, H.; Jiang, N.; Ji, L.L.; Zhang, Y. Mitochondrial Redox Metabolism in Aging: Effect of Exercise Interventions. J. Sport Health Sci. 2013, 2, 67–74. [Google Scholar] [CrossRef]
- Meng, Q.; Su, C.-H. The Impact of Physical Exercise on Oxidative and Nitrosative Stress: Balancing the Benefits and Risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, Oxidants, and Antioxidants Change the Shape of the Bell-Shaped Hormesis Curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Assidi, M. Infertility in Men: Advances towards a Comprehensive and Integrative Strategy for Precision Theranostics. Cells 2022, 11, 1711. [Google Scholar] [CrossRef]
- Greco, F.; Guarascio, G.; Giannetta, E.; Oranges, F.P.; Quinzi, F.; Emerenziani, G.P.; Tarsitano, M.G. The Influence of an Intense Training Regime in Professional and Non-Professional Athletes on Semen Parameters: A Systematic Review. JCM 2025, 14, 201. [Google Scholar] [CrossRef]
- Sgrò, P.; Di Luigi, L. Sport and Male Sexuality. J. Endocrinol. Invest. 2017, 40, 911–923. [Google Scholar] [CrossRef]
- Di Luigi, L.; Gentile, V.; Pigozzi, F.; Parisi, A.; Giannetti, D.; Romanelli, F. Physical Activity as a Possible Aggravating Factor for Athletes with Varicocele: Impact on the Semen Profile. Hum. Reprod. 2001, 16, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Abedpoor, N.; Taghian, F.; Hajibabaie, F. Exploring the Dynamics of Exercise Intensity on Male Fertility and Reproductive Health: Advancements and Implications for Fertility Research. Front. Reprod. Health 2024, 6, 1423916. [Google Scholar] [CrossRef]
- Minas, A.; Fernandes, A.C.C.; Maciel Júnior, V.L.; Adami, L.; Intasqui, P.; Bertolla, R.P. Influence of Physical Activity on Male Fertility. Andrologia 2022, 54, e14433. [Google Scholar] [CrossRef]
- Antinozzi, C.; Duranti, G.; Ceci, R.; Lista, M.; Sabatini, S.; Caporossi, D.; Di Luigi, L.; Sgrò, P.; Dimauro, I. Hydrogen Peroxide Stimulates Dihydrotestosterone Release in C2C12 Myotubes: A New Perspective for Exercise-Related Muscle Steroidogenesis? IJMS 2022, 23, 6566. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M. Exercise and Sex Steroid Hormones in Skeletal Muscle. J. Steroid Biochem. Mol. Biol. 2015, 145, 200–205. [Google Scholar] [CrossRef]
- Bonen, A.; Ling, W.Y.; MacIntyre, K.P.; Neil, R.; McGrail, J.C.; Belcastro, A.N. Effects of Exercise on the Serum Concentrations of FSH, LH, Progesterone, and Estradiol. Europ. J. Appl. Physiol. 1979, 42, 15–23. [Google Scholar] [CrossRef]
- Belladelli, F.; Basran, S.; Eisenberg, M.L. Male Fertility and Physical Exercise. World J. Men’s Health 2023, 41, 482. [Google Scholar] [CrossRef]
- Lavín-Pérez, A.M.; Collado-Mateo, D.; Villafaina, S.; Calle-Guisado, V. The Role of Exercise to Reduce the Impact of Diabetes in the Seminal Quality: A Systematic Review. Medicina 2021, 57, 159. [Google Scholar] [CrossRef] [PubMed]
- Lyons, H.E.; Gyawali, P.; Mathews, N.; Castleton, P.; Mutuku, S.M.; McPherson, N.O. The Influence of Lifestyle and Biological Factors on Semen Variability. J. Assist. Reprod. Genet. 2024, 41, 1097–1109. [Google Scholar] [CrossRef]
- Mehri, K.; Hamidian, G.; Babri, S.; Farajdokht, F.; Zavvari Oskuye, Z. Exercise and Insulin Glargine Administration in Mothers with Diabetes during Pregnancy Ameliorate Function of Testis in Offspring: Consequences on Apelin-13 and Its Receptor. Life Sci. 2024, 342, 122517. [Google Scholar] [CrossRef]
- Morano, S.; Gatti, A.; Mandosi, E.; Tiberti, C.; Fallarino, M.; Cipriani, R.; Buchetti, B.; Gandini, L.; Sgrò, P.; Jannini, E.A.; et al. Circulating Monocyte Oxidative Activity Is Increased in Patients with Type 2 Diabetes and Erectile Dysfunction. J. Urol. 2007, 177, 655–659. [Google Scholar] [CrossRef]
- Zampieri, N. The Effect of Aerobic Training on Serum Levels of Adiponectin, Hypothalamic-Pituitary-Gonadal Axis and Sperm Quality in Diabetic Rats. Urol. J. 2019, 16, 592–597. [Google Scholar] [CrossRef]
- Antinozzi, C.; Lista, M.; Caponecchia, L.; Salacone, P.; Minganti, C.; Battaglia, F.A.; Di Luigi, L.; Sgrò, P. Exploratory Analysis in the Differences in Blood Serum and Seminal Plasma of Adipose-Tissue Related Peptides in Obese and Non-Obese Men and Their Correlations with Semen Parameters. Front. Endocrinol. 2021, 12, 681939. [Google Scholar] [CrossRef]
- Green, D.J.; Chasland, L.C.; Yeap, B.B.; Naylor, L.H. Comparing the Impacts of Testosterone and Exercise on Lean Body Mass, Strength and Aerobic Fitness in Aging Men. Sports Med. Open 2024, 10, 30. [Google Scholar] [CrossRef]
- Di Luigi, L.; Sgrò, P.; Fierro, V.; Bianchini, S.; Battistini, G.; Magini, V.; Jannini, E.A.; Lenzi, A. Prevalence of Undiagnosed Testosterone Deficiency in Aging Athletes: Does Exercise Training Influence the Symptoms of Male Hypogonadism? J. Sex. Med. 2010, 7, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Jóźków, P.; Rossato, M. The Impact of Intense Exercise on Semen Quality. Am. J. Men’s Health 2017, 11, 654–662. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B.; Chehrazi, M. The Effects of Three Different Exercise Modalities on Markers of Male Reproduction in Healthy Subjects: A Randomized Controlled Trial. Reproduction 2017, 153, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Gebreegziabher, Y.; Marcos, E.; McKinon, W.; Rogers, G. Sperm Characteristics of Endurance Trained Cyclists. Int. J. Sports Med. 2004, 25, 247–251. [Google Scholar] [CrossRef]
- Duclos, M.; Tabarin, A. Exercise and the Hypothalamo-Pituitary-Adrenal Axis. In Frontiers of Hormone Research; Lanfranco, F., Strasburger, C.J., Eds.; S. Karger AG: Basel, Switzerland, 2016; Volume 47, pp. 12–26. ISBN 978-3-318-05868-0. [Google Scholar]
- Brownlee, K.K.; Moore, A.W.; Hackney, A.C. Relationship between Circulating Cortisol and Testosterone: Influence of Physical Exercise. J. Sports Sci. Med. 2005, 4, 76–83. [Google Scholar]
- Duclos, M.; Guinot, M.; Le Bouc, Y. Cortisol and GH: Odd and Controversial Ideas. Appl. Physiol. Nutr. Metab. 2007, 32, 895–903. [Google Scholar] [CrossRef]
- Snyder, A.C.; Hackney, A.C. The Endocrine System in Overtraining. In Endocrinology of Physical Activity and Sport; Constantini, N., Hackney, A.C., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 523–534. ISBN 978-1-62703-313-8. [Google Scholar]
- Sansone, M.; Sansone, A.; Borrione, P.; Romanelli, F.; Di Luigi, L.; Sgrò, P. Effects of Ketone Bodies on Endurance Exercise. Curr. Sports Med. Rep. 2018, 17, 444–453. [Google Scholar] [CrossRef]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus 2023, 15, e42583. [Google Scholar] [CrossRef]
- Torres-Arce, E.; Vizmanos, B.; Babio, N.; Márquez-Sandoval, F.; Salas-Huetos, A. Dietary Antioxidants in the Treatment of Male Infertility: Counteracting Oxidative Stress. Biology 2021, 10, 241. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-Induced Oxidative Stress and Dietary Antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antinozzi, C.; Di Luigi, L.; Sireno, L.; Caporossi, D.; Dimauro, I.; Sgrò, P. Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis. Biomolecules 2025, 15, 478. https://doi.org/10.3390/biom15040478
Antinozzi C, Di Luigi L, Sireno L, Caporossi D, Dimauro I, Sgrò P. Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis. Biomolecules. 2025; 15(4):478. https://doi.org/10.3390/biom15040478
Chicago/Turabian StyleAntinozzi, Cristina, Luigi Di Luigi, Laura Sireno, Daniela Caporossi, Ivan Dimauro, and Paolo Sgrò. 2025. "Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis" Biomolecules 15, no. 4: 478. https://doi.org/10.3390/biom15040478
APA StyleAntinozzi, C., Di Luigi, L., Sireno, L., Caporossi, D., Dimauro, I., & Sgrò, P. (2025). Protective Role of Physical Activity and Antioxidant Systems During Spermatogenesis. Biomolecules, 15(4), 478. https://doi.org/10.3390/biom15040478







