The Role of IL-6 in Ischemic Stroke
Abstract
1. Introduction
2. The Role and Properties of Cytokine IL-6
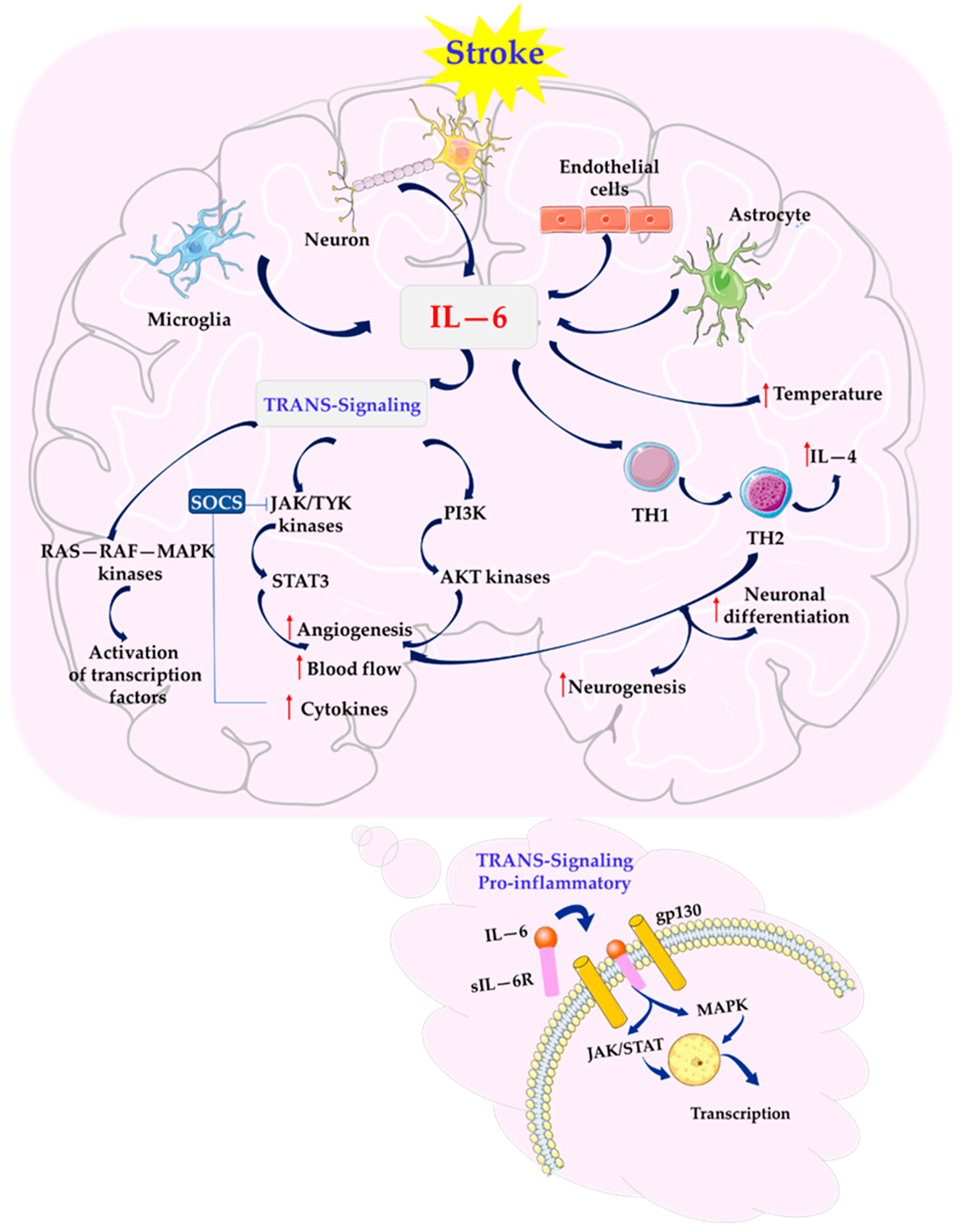
3. IL-6 as a Mediator of Inflammatory Response in Stroke
4. IL-6 and Cognitive Functions in Stroke
5. Circulating IL-6 and the Risk in AIS
6. Modulation of IL-6
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pawluk, H.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Woźniak, A.; Grzechowiak, E.; Szumny, M.; Sobolewski, P.; Bieniaszewski, L.; Kozera, G. Effectof IL-6 and hsCRP serum levels on functional prognosis in stroke patients undergoing IV- thrombolysis: Retrospective analysis. Clin. Interv. Aging 2020, 6, 1295–1303. [Google Scholar] [CrossRef]
- Pawluk, H.; Kołodziejska, R.; Grześk, G.; Kozakiewicz, M.; Woźniak, A.; Pawluk, M.; Kosinska, A.; Grześk, M.; Wojtasik, J.; Kozera, G. Selected mediators of inflammation in patients with acute ischemic stroke. Int. J. Mol. Sci. 2022, 23, 10614. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Kołodziejska, R.; Grześk, G.; Woźniak, A.; Kozakiewicz, M.; Kosinska, A.; Pawluk, M.; Grzechowiak, E.; Wojtasik, J.; Kozera, G. Increased oxidative stress markers in Acute Ischemic Stroke patients treated with thrombolytics. Int. J. Mol. Sci. 2022, 23, 15625. [Google Scholar] [CrossRef]
- Žitňanová, I.; Šiarnik, P.; Kollár, B.; Chomová, M.; Pazderová, P.; Andrezálová, L.; Ježovičová, M.; Koňariková, K.; Laubertová, L.; Krivošíková, Z.; et al. Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke oxidative. Oxid. Med. Cell. Longev. 2016, 2016, 9761697. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord.-Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Zheng, Z.; Yenari, M.A. Post-ischemic inflammation: Molecular mechanisms and therapeutic implications. Neurol. Res. 2004, 26, 884–892. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.C.; Li, Z.G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Sharma, P.; Naga, P.; Medhi, B.; Hari, D.; Reddy, K. The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. 2024, 78, 105–119. [Google Scholar] [CrossRef]
- Ozaki, E.; Campbell, M.; Doyle, S.L. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives. J. Inflamm. Res. 2015, 8, 15–27. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128–139. [Google Scholar] [CrossRef]
- Yang, C.-S.; Kim, J.-J.; Kim, T.S.; Lee, P.Y.; Kim, S.Y.; Lee, H.-M.; Shin, D.-M.; Nguyen, L.T.; Lee, M.-S.; Jin, H.S.; et al. Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome. Nat. Commun. 2015, 6, 6115–6126. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.; Palaiopanos, K.; Björkbacka, H.; Peters, A.; de Lemos, J.A.; Seshadri, S.; Dichgans, M.; Georgakis, M.K. Circulating interleukin-6 levels and incident Ischemic Stroke: A systematic review and meta-analysis of prospective studies. Neurology 2022, 98, e1002–e1012. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Malik, R.; Gill, D.; Franceschini, N.; Sudlow, C.L.M.; Dichgans, M. Interleukin-6 signaling effects on ischemic stroke and other cardiovascular outcomes: A mendelian randomization study. Circ. Genom. Precis. Med. 2020, 13, e002872. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Malik, R.; Li, X.; Gill, D.; Levin, M.G.; Vy, H.M.T.; Judy, R.; Ritchie, M.; Verma, S.S.; Nadkarni, G.N.; et al. Genetically downregulated interleukin-6 signaling is associated with a favorable cardiometabolic profile: A phenome-wide association study. Circulation 2021, 143, 1177–1180. [Google Scholar] [CrossRef]
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.O.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589. [Google Scholar] [CrossRef]
- Jenny, N.S.; Callas, P.W.; Judd, S.E.; McClure, L.A.; Kissela, B.; Zakai, N.A.; Cushman, M. Inflammatory cytokines and ischemic stroke risk: The REGARDS cohort. Neurology 2019, 92, e2375–e2384. [Google Scholar] [CrossRef]
- Patterson, C.C.; Smith, A.E.; Yarnell, J.W.; Rumley, A.; Ben-Shlomo, Y.; Lowe, G.D. The associations of interleukin-6 (IL-6) and downstream inflammatory markers with risk of cardiovascular disease: The Caerphilly Study. Atherosclerosis 2010, 209, 551–557. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Libby, P.; Rocha, V.Z. All roads lead to IL-6: A central hub of cardiometabolic signaling. Int. J. Cardiol. 2018, 259, 213–215. [Google Scholar] [CrossRef]
- Loppnow, H.; Libby, P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J. Clin. Investig. 1990, 85, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Rayasam, A.; Hsu, M.; Kijak, J.A.; Kissel, L.; Hernandez, G.; Sandor, M.; Fabry, Z. Immune responses in stroke: How the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology 2018, 154, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, Y.; Shu, H.; Xie, B.; Tao, Y.; Yuan, Y.; Shang, Y.; Yuan, S.; Zhang, J. Hyperforin promotes post-stroke neuroangiogenesis via astrocytic IL-6-mediated negative immune regulation in the ischemic brain. Front. Cell. Neurosci. 2019, 13, 201. [Google Scholar] [CrossRef]
- Fang, J.; Wang, Z.; Miao, C.-Y. Angiogenesis after ischemic stroke. Acta Pharmacol. Sin. 2023, 44, 1305–1321. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Y.; Zhong, Y.; Ye, Y.; Hu, X.; Gu, L.; Xiong, X. Inflammation-mediated angiogenesis in ischemic stroke. Front. Cell. Neurosci. 2021, 15, 652647. [Google Scholar] [CrossRef]
- Swartz, K.R.; Liu, F.; Sewell, D.; Schochet, T.; Campbell, I.; Sandor, M.; Fabry, Z. Interleukin-6 promotes post-traumatic healing in the Central Nervous System. Brain Res. 2001, 896, 86–95. [Google Scholar] [CrossRef]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the regulation of neuronal development, survival and function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef]
- Chiba, T.; Umegaki, K. Pivotal roles of monocytes/macrophages in stroke. Mediat. Inflamm. 2013, 27, 1–10. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, S.; Li, Y.; Sun, Y.; Xiong, X.; Hu, X.; Chen, J.; Qiu, S. Interleukins and Ischemic Stroke. Front. Immunol. 2022, 13, 828447. [Google Scholar] [CrossRef]
- Kamtchum-Tatuene, J.; Saba, L.; Heldner, M.R.; Rundek, T.; Kakkos, S.K.; Chaturvedi, S.; Poorthuis, M.H.F.; de Borst, G.J.; Topakian, R.; Polak, J.F.; et al. Interleukin-6 predicts Carotid Plaque Severity, vulnerability, and progression. Circ. Res. 2022, 131, e22–e33. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.J.; Chow, D.; Brevnova, E.E.; Garcia, K.C. Hexameric Structure and Assembly of the Interleukin-6/IL-6 α-Receptor/gp130 Complex. Science 2003, 300, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, L.; Simats, A.; García-Berrocoso, T.; Montaner, J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther. Adv. Neurol. Disord. 2018, 1, 1–24. [Google Scholar] [CrossRef]
- Monsour, M.; Croci, D.M.; Agazzi, S. The role of IL-6 in TBI and PTSD, a potential therapeutic target? Clin. Neurol. Neurosurg. 2022, 218, 107280. [Google Scholar] [CrossRef]
- Suzuki, S.; Tanaka, K.; Suzuki, N. Ambivalent aspects of interleukin-6 in Cerebral Ischemia: Inflammatory versus neurotrophic aspects. J. Cereb. Blood Flow Metab. 2009, 29, 464–479. [Google Scholar] [CrossRef]
- Schofer, N.; Ludwig, S.; Rübsamen, N.; Schnabel, R.; Lackner, K.J.; Ruprecht, H.J.; Bickel, C.; Landmesser, U.; Blankenberg, S.; Zeller, T. Prognostic impact of interleukin-1 receptor antagonist in patients with documented coronary artery disease. Int. J. Cardiol. 2018, 257, 24–29. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Meng, X.; Lin, J.; Liu, L.; Wang, C.; Wang, A.; Wang, Y.; Wang, Y. High-sensitive C-reactive protein predicts recurrent stroke and poor functional outcome: Subanalysis of the clopidogrel in high-risk patients with acute nondisabling cerebrovascular events trial. Stroke 2016, 47, 2025–2030. [Google Scholar] [CrossRef]
- Kjaergaard, A.D.; Bojesen, S.E.; Johansen, J.S.; Nordestgaard, B.G. Elevated plasma YKL-40 levels and ischemic stroke in the general population. Ann. Neurol. 2010, 68, 672–680. [Google Scholar] [CrossRef]
- Park, H.Y.; Jun, C.D.; Jeon, S.J.; Choi, S.-S.; Kim, H.-R.; Choi, D.-B.; Kwak, S.; Lee, H.-S.; Cheong, J.S.; So, H.-S.; et al. Serum YKL-40 levels correlate with infarct volume, stroke severity, and functional outcome in acute ischemic stroke patients. PLoS ONE 2012, 7, e51722. [Google Scholar] [CrossRef]
- Hartman, J.; Frishman, W.H. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol. Rev. 2014, 22, 147–151. [Google Scholar] [CrossRef]
- Schieffer, B.; Selle, T.; Hilfiker, A.; Hilfiker-Kleiner, D.; Grote, K.; Tietge, U.J.; Traut-Wein, C.; Luchtefeld, M.; Schmittkamp, C.; Heeneman, S.; et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation 2004, 110, 3493–3500. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Shon, Y.M.; Yang, D.W.; Kim, B.S.; Cho, A.H. Coexisting carotid atherosclerosis in patients with intracranial small- or large-vessel disease. J. Clin. Neurol. 2012, 8, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.; Pan, Y.; Wang, M.; Meng, X.; Li, H.; Wang, Y.; Zhao, X.; Qin, H.; Liu, L.; et al. Residual inflammatory risk predicts poor prognosis in Acute Ischemic Stroke or Transient Ischemic Attack Patients. Stroke 2021, 52, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Mei, P.; Tung, T.-H.; Wu, G.; Wang, F.; Wang, E.; Ni, H.; Zhu, X.; He, Z.; et al. High Interleukin-6 levels are associated with Large-Artery Atherosclerotic Stroke. Neurologist 2023, 28, 277–280. [Google Scholar] [CrossRef]
- Li, J.; Lin, J.; Pan, Y.; Wang, M.; Meng, X.; Li, H.; Wang, Y.; Zhao, X.; Qin, H.; Liu, L.; et al. Interleukin-6 and YKL-40 predicted recurrent stroke after ischemic stroke or TIA: Analysis of 6 inflammation biomarkers in a prospective cohort study. J. Neuroinflamm. 2022, 19, 131. [Google Scholar] [CrossRef]
- Lockard, G.M.; Alayli, A.; Monsour, M.; Gordon, J.; Schimmel, S.; Elsayed, B.; Borlongan, C.V. Probing Interleukin-6 in stroke pathology and neural stem cell transplantation. Int. J. Mol. Sci. 2022, 23, 15453. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 signalling in health and disease. F1000Research 2020, 9, F1000 Faculty Rev-1013. [Google Scholar] [CrossRef]
- Hodes, G.E.; Menard, C.; Russo, S.J. Integrating Interleukin-6 into depression diagnosis and treatment. Neurobiol. Stress 2016, 4, 15–22. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Schuett, H.; Oestreich, R.; Waetzig, G.H.; Annema, W.; Luchtefeld, M.; Hillmer, A.; Bavendiek, U.; von Felden, J.; Divchev, D.; Kempf, T.; et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 281–920. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Tieu, B.C.; Ray, S.; Iii, A.R.; Cui, R.; Tilton, R.G.; Brasier, A.R. Roles of IL-6-gp130 signaling in vascular inflammation. Curr. Cardiol. Rev. 2008, 4, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Monsour, M.; Croci, D.M.; Agazzi, S.; Borlongan, C.V. Contemplating IL-6, a double-edged sword cytokine: Which side to use for stroke pathology? CNS Neurosci. Ther. 2023, 29, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, L.; Wallen, H.; Aspberg, S.; de Faire, U.; Gigante, B. IL6 trans-signaling associates with ischemic stroke but not with atrial fibrillation. BMC Neurol. 2021, 21, 306. [Google Scholar] [CrossRef]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 trans-signalling: Past, present and future prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef]
- Yan, M.; Sun, Z.; Zhang, S.; Yang, G.; Jiang, X.; Wang, G.; Li, R.; Wang, Q.; Tian, X. SOCS modulates JAK-STAT pathway as a novel target to mediate the occurrence of neuroinflammation: Molecular details and treatment options. Brain Res. Bull. 2024, 213, 110988. [Google Scholar] [CrossRef]
- Hirano, T.; Ishihara, K.; Hibi, M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 2000, 19, 2548–2556. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Chowdhury, P.; Bhowmik, P.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Targeting cytokine-mediated inflammation in brain disorders: Developing new treatment strategies. Pharmaceuticals 2025, 18, 104. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Yu, Y.; Yuan, Y.; Zhang, Y.-J.; Fan, X.-P.; Yuan, S.-Y.; Zhang, J.-C.; Yao, S.-L. Enriched housing promotes post-stroke functional recovery through astrocytic HMGB1-IL-6-mediated angiogenesis. Cell Death Discov. 2017, 3, 17054. [Google Scholar] [CrossRef]
- Gertz, K.; Kronenberg, G.; Kalin, R.E.; Baldinger, T.; Werner, C.; Balkaya, M.; Eom, G.D.; Hellmann-Regen, J.; Kröber, J.; Miller, K.R.; et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain 2012, 135, 1964–1980. [Google Scholar] [CrossRef]
- Kong, L.; Li, W.; Chang, E.; Wang, W.; Shen, N.; Xu, X.; Wang, X.; Zhang, Y.; Wen Sun, W.; Hu, W.; et al. mtDNA-STING axis mediates microglial polarization via IRF3/NF-κB signaling after Ischemic Stroke. Front. Immunol. 2022, 13, 860977. [Google Scholar] [CrossRef]
- He, T.; Yang, G.-Y.; Zhang, Z. Crosstalk of astrocytes and other cells during Ischemic Stroke. Life 2022, 12, 910. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Rothwell, P.M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009, 8, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shin, K.Y.; Chang, K.-A. Potential biomarkers for post-stroke cognitive impairment: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 602. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y.; Yang, T.; He, X.; Yang, Y.; Chen, J.; Han, L. Blood biomarkers for post-stroke cognitive impairment: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2024, 33, 107632. [Google Scholar] [CrossRef]
- Kulesh, A.; Drobakha, V.; Kuklina, E.; Nekrasova, I.; Shestakov, V. Cytokine response, tract-specific fractional anisotropy, and brain morphometry in post-stroke cognitive impairment. J. Stroke Cerebrovasc. Dis. 2018, 27, 1752–1759. [Google Scholar] [CrossRef]
- Singh-Manoux, A.; Dugravot, A.; Brunner, E.; Kumari, M.; Shipley, M.; Elbaz, A.; Kivimaki, M. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 2014, 83, 486–493. [Google Scholar] [CrossRef]
- Fromme, S.E.; Joergens, S.; Schwarte, K.; Hohoff, C.; Dietrich, D.E.; Baune, B.T. The association between cytokines and cognitive function in patients with major depressive disorder and controls. J. Affect. Disord. 2025, 373, 374–382. [Google Scholar] [CrossRef]
- Khan, H.; Naseem, T.; Kaushik, P.; Narang, J.; Khan, R.; Panwar, S.; Parvez, S. Decoding paradoxical links of cytokine markers in cognition: Cross talk between physiology, inflammaging, and Alzheimer’s disease- related cognitive decline. Ageing Res. Rev. 2024, 101, 102535. [Google Scholar] [CrossRef]
- Palta, P.; Xue, Q.L.; Deal, J.A.; Fried, L.P.; Walston, J.D.; Carlson, M.C. Interleukin-6 and C-reactive protein levels and 9-year cognitive decline in community-dwelling older women: The women’s health and aging Study II. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 873–878. [Google Scholar] [CrossRef][Green Version]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Ibhiedu, J.O.; Egbunu, E.O.; Ndam, A.R.; Ogala, F.; Ologunde, T.; Peterson, J.C.; Boluwatife, A.I.; et al. Stroke and cognitive impairment: Understanding the connection and managing symptoms. Ann. Med. Surg. 2023, 85, 6057–6066. [Google Scholar] [CrossRef]
- Dik, M.G.; Jonker, C.; Hack, C.E.; Smit, J.H.; Comijs, H.C.; Eikelenboom, P. Serum inflammatory proteins and cognitive decline in older persons. Neurology 2005, 64, 1371–1377. [Google Scholar] [CrossRef]
- Loga-Andrijić, N.; Petrović, N.T.; Filipović-Danić, S.; Marjanovic, S. The significance of interleukin-6 and tumor necrosis factor-alpha levels in cognitive impairment among first-ever acute ischaemic stroke patients. Psychiatr. Danub. 2021, 33, 37–42. [Google Scholar]
- Sandvig, H.V.; Aam, S.; Alme, K.N.; Askim, T.; Beyer, M.K.; Ellekjær, H.; Ihle-Hansen, H.; Lydersen, S.; Mollnes, T.E.; Munthe-Kaas, R.; et al. Plasma inflammatory biomarkers are associated with poststroke cognitive impairment: The Nor-COAST study. Stroke 2023, 54, 1303–1311. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Pan, Y.; Wang, M.; Lin, J.; Meng, X.; Liao, X.; Wang, Y. Interleukin-6 as predictor of one-year cognitive function after Ischemic Stroke or TIA. Neuropsychiatr. Dis. Treat. 2022, 18, 391–399. [Google Scholar] [CrossRef]
- Kowalska, K.; Klimiec, E.; Weglarczyk, K.; Pera, J.; Slowik, A.; Siedlar, M.; Dziedzic, T. Reduced ex vivo release of pro-inflammatory cytokines and elevated plasma Interleukin-6 are inflammatory signatures of post-stroke delirium. J. Neuroinflamm. 2018, 15, 111. [Google Scholar] [CrossRef]
- Shi, Q.; Li, R.; Qu, Z.; Lang, Y.; Sheng, G.; Ning, J.; Zhang, W. Longitudinal change of six common inflammatory cytokines and their relationship to anxiety, depression, and cognitive impairment in acute ischemic stroke patients. Braz. J. Med. Biol. Res. 2023, 56, e13025. [Google Scholar] [CrossRef]
- Li, R.; Fan, W.; Li, D.; Liu, X. Correlation of common inflammatory cytokines with cognition impairment, anxiety, and depression in acute ischemic stroke patients. Braz. J. Med. Biol. Res. 2022, 55, e11517. [Google Scholar] [CrossRef]
- Zhang, X.; Bi, X. Post-stroke cognitive impairment: A review focusing on molecular biomarkers. J. Mol. Neurosci. 2020, 70, 1244–1254. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, Z.; Huang, P.; Xiang, B.; Li, X.; Dong, H.; Zhang, G.; Wu, Y.; Li, P. L-6/STAT3 induced neuron apoptosis in hypoxia by downregulating ATF6 expression. Front. Physiol. 2021, 12, 729925. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-S.; Yuan, M.; Han, Y.; Shen, X.-Y.; Gao, Z.-K.; Bi, X. Therapeutic potential of cytokines in demyelinating lesions after stroke. J. Mol. Neurosci. 2021, 71, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, R.M.; Elsaid, A.F. Infarction size, interleukin-6, and their interaction are predictors of short-term stroke outcome in young Egyptian adults. J. Stroke Cerebrovasc. Dis. 2016, 25, 2475–2481. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L. Anti-inflammatory effects of vinpocetine in atherosclerosis and ischemic stroke: A review of the literature. Molecules 2014, 20, 335–347. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Narasimhalu, K.; Lee, J.; Leong, Y.L.; Ma, L.; De Silva, D.A.; Wong, M.C.; Chang, H.M.; Chen, C. Inflammatory markers and their association with post stroke cognitive decline. Int. J. Stroke 2015, 10, 513–518. [Google Scholar] [CrossRef]
- Grigolashvili, M.A.; Mustafina, R.M. The role of the inflammatory process in the development of post-stroke cognitive impairment. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2021, 12, 16–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.; Wang, J.; Xi, X.; Cefalo, P.; Wood, L.J.; Luo, X.; Wang, Q.M. Multiplex array analysis of serum cytokines offers minimal predictive value for cognitive function in the subacute phase after stroke. Front. Neurol. 2022, 13, 886018. [Google Scholar] [CrossRef]
- Loga-Andrijić, N.; Petrović, N.T.; Filipović-Danić, S.; Marjanović, S.; Mitrović, V.; Loga-Zec, S. Analysis of serum inflammatory markers in Cognitive Impairment among Acute Ischaemic Stroke patients. Psychiatr. Danub. 2021, 33, 1287–1293. [Google Scholar]
- Hall, C.; Nguyen, D.T.; Mendoza, K.; Tan, C.; Chauhan, A. Inhibition of IL-6 trans-signaling promotes post-stroke functional recovery in a sex and dose-dependent manner. J Neuroinflamm. 2025, 22, 52. [Google Scholar] [CrossRef]
- Shaafi, S.; Sharifipour, E.; Rahmanifar, R.; Hejazi, S.S.; Andalib, S.; Nikanfar, M.; Baradarn, B.; Mehdizadeh, R. Interleukin-6, a reliable prognostic factor for ischemic stroke. Iran. J. Neurol. 2014, 13, 70–76. [Google Scholar] [PubMed]
- Waje-Andreassen, U.; Kråkenes, J.; Ulvestad, E.; Thomassen, L.; Myhr, K.-M.; Aarseth, J.; Vedeler, C.A. IL-6: An early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005, 111, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Sciacca, R.D.; Raimondo, D.D.; Serio, A.; D’Aguanno, G.; La Placa, S.; Pecoraro, R.; Arnao, V.; Marino, L.; Monaco, S.; et al. Plasma levels of inflammatory and thrombotic/fibrinolytic markers in acute ischemic strokes. Relationship with TOAST subtype, outcome and infarct site. J. Neuroimmunol. 2009, 215, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Licata, A.; Tuttolomondo, S.; Corrao, D.; Di Raimondo, D.; Fernandez, P.; Caruso, C.; Avellone, G.; Pinto, A. Immunoinflammatory activation during the acute phase of lacunar and non-lacunar ischemic stroke: Association with time of onset and diabetic state. Int. J. Immunopathol. Pharmacol. 2006, 19, 639–646. [Google Scholar] [CrossRef]
- Choudhary, S.; Chowdhur, D.; Mishra, T.K.; Agarwal, S. Temporal profile of serum levels of IL-6 in acute ischemic stroke and its relationship with stroke severity and outcome in Indian population. Int. J. Integr. Med. Sci. 2018, 5, 555–560. [Google Scholar] [CrossRef][Green Version]
- Park, S.Y.; Kim, J.; Kim, O.J.; Kim, J.-K.; Song, J.; Shin, D.-A.; Oh, S.-H. Predictive value of circulating interleukin-6 and heart-type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: Multiple blood markers profiling study. Crit. Care. 2013, 17, R45. [Google Scholar] [CrossRef]
- Whiteley, W.; Chong, W.L.; Sengupta, A.; Sandercock, P. Blood markers for the prognosis of ischemic stroke: A systematic review. Stroke 2009, 40, 380–389. [Google Scholar] [CrossRef]
- Cojocaru, I.M.; Cojocaru, M.; Tănăsescu, R.; Iliescu, I.; Dumitrescu, L.; Silosi, I. Expression of IL-6 activity in patients with acute ischemic stroke. Rom. J. Intern. Med. 2009, 47, 393–396. [Google Scholar]
- Worthmann, H.; Tryc, A.B.; Goldbecker, A. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc. Dis. 2010, 30, 85–92. [Google Scholar] [CrossRef]
- Nayak, A.R.; Kashyap, S.R.; Kabra, D.; Hemant, J.; Taori, G.M.; Daginawala, H.F. Time course of inflammatory cytokines in acute ischemic stroke patients and their relation to inter-alfa trypsin inhibitor heavy chain 4 and outcome. Ann. Indian Acad. Neurol. 2012, 15, 181–185. [Google Scholar] [CrossRef]
- Puz, P.; Lasek-Bal, A.; Kazibutowska, Z. Evaluation of the serum concentration of selected inflammatory cytokines in patients with carotid artery stenosis. Chir. Pol. 2014, 16, 57–65. [Google Scholar]
- Wytrykowska, A.; Prosba-Mackiewicz, M.; Nyka, W.M. IL-1β, TNF-α, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J. Oral Sci. 2016, 58, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, Z.-X.; Jiang, Y.-Y.; Jiang, Y.; Meng, X.; Li, H.; Zhao, X.-Q.; Wang, Y.-L.; Liu, L.-P.; Wang, Y.-J.; et al. Inflammatory marker profiles and in-hospital neurological deterioration in patients with acute minor ischemic stroke. CNS Neurosci. Ther. 2024, 30, e14648. [Google Scholar] [CrossRef] [PubMed]
- Kes, V.B.; Simundic, A.-M.; Nikolac, N.; Topic, E.; Demarin, V. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin. Biochem. 2008, 41, 1330–1334. [Google Scholar] [CrossRef]
- Akyol, A.; Ozkul, A.; Yenisey, C.; Kiylioglu, N. The relationship between protein C, protein S and cytokines in acute ischemic stroke. Neuroimmunomodulation 2006, 13, 187–193. [Google Scholar] [CrossRef]
- Coveney, S.; Murph, S.; Belton, O.; Cassidy, T.; Crowe, M.; Dolan, E.; de Gaetano, M.; Harbison, J.; Horgan, G.; Marnane, M.; et al. Inflammatory cytokines, high-sensitivity C-reactive protein, and risk of one-year vascular events, death, and poor functional outcome after stroke and transient ischemic attack. Int. J. Stroke 2021, 17, 163–171. [Google Scholar] [CrossRef]
- Alfieri, D.F.; Lehmann, M.F.; Flauzino, T.; Martins de Araújo, M.C.; Pivoto, N.; Tirolla, R.M.; Colado Simão, A.; Maes, M.; Vissoci Reiche, E.M. Immune-inflammatory, metabolic, oxidative, and nitrosative stress biomarkers predict Acute Ischemic Stroke and Short-Term Outcome. Neurotox. Res. 2020, 38, 330–343. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, T. IL-6 and stroke recurrence in ischemic stroke. Biomark. Med. 2024, 18, 739–747. [Google Scholar] [CrossRef]
- Ou, M.; Liu, S.; Ma, X.; Xing, X.; He, W.; Gao, H. IL-6 promoter polymorphism increased risks of recurrent stroke in the young patients with moderate internal carotid artery stenosis. J. Cell. Biochem. 2018, 119, 2886–2890. [Google Scholar] [CrossRef]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Li, L.; Miyamoto, N.; Montaner, J.; et al. C-Reactive protein, interleukin-6, and vascular recurrence according to stroke subtype: An individual participant data meta-analysis. Neurology 2024, 102, e208016. [Google Scholar] [CrossRef]
- McCabe, J.J.; Walsh, C.; Gorey, S.; Arnold, M.; DeMarchis, G.M.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Katan, M.; et al. Interleukin-6, C-Reactive protein, and recurrence after stroke: A time-course analysis of individual-participant data. Stroke 2024, 55, 2825–2834. [Google Scholar] [CrossRef] [PubMed]
- Esenwa, C.C.; Elkind, M.S. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat. Rev. Neurol. 2016, 12, 594–604. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, K.; Shi, M.; Guo, D.; Yang, P.; Bu, X.; Chen, J.; Wang, A.; Xu, T.; He, J.; et al. Associations of rheumatoid factor, rheumatoid arthritis, and interleukin-6 inhibitor with prognosis of ischemic stroke: A prospective multicenter cohort study and Mendelian randomization analysis. Transl. Stroke Res. 2024, 15, 750–760. [Google Scholar] [CrossRef]
- Gu, H.-Q.; Yang, K.-X.; Li, J.-J.; Lin, J.-X.; Jing, J.; Xiong, Y.-Y.; Zhao, X.-Q.; Wang, Y.-L.; Liu, L.-P.; Meng, X.; et al. Mediation effect of stroke recurrence in the association between post-stroke interleukin-6 and functional disability. CNS Neurosci. Ther. 2023, 29, 3579–3587. [Google Scholar] [CrossRef]
- Staszewski, J.; Skrobowska, E.; Piusińska-Macoch, R.; Brodacki, B.; Stępień, A. IL-1α and IL-6 predict vascular events or death in patients with cerebral small vessel disease—Data from the SHEF-CSVD study. Adv. Med. Sci. 2019, 64, 258–266. [Google Scholar] [CrossRef]
- Lu, H.; Li, S.; Zhong, X.; Huang, S.; Jiao, X.; He, G.; Jiang, B.; Liu, Y.; Gao, Z.; Wei, J.; et al. Immediate outcome prognostic value of plasma factors in patients with acute ischemic stroke after intravenous thrombolytic treatment. BMC Neurol. 2022, 22, 359. [Google Scholar] [CrossRef]
- Yuan, S.; Lin, A.; He, Q.-Q.; Burgess, S.; Larsson, S.C. Circulating interleukins in relation to coronary artery disease, atrial fibrillation and ischemic stroke and its subtypes: A two-sample Mendelian randomization study. Int. J. Cardiol. 2020, 313, 99–104. [Google Scholar] [CrossRef]
- Boehme, A.K.; McClure, L.A.; Zhang, Y.; Luna, J.M.; Del Brutto, O.H.; Benavente, O.R.; Elkind, M.S.V. Inflammatory markers and outcomes after lacunar stroke: Levels of inflammatory markers in treatment of stroke study. Stroke 2016, 47, 659–667. [Google Scholar] [CrossRef]
- Mukaz, D.K.; Zakai, N.A.; Cruz-Flores, S.; McCullough, L.D.; Cushman, M. Identifying genetic and biological determinants of race-ethnic disparities in stroke in the United States. Stroke 2020, 51, 3417–3424. [Google Scholar] [CrossRef]
- Tian, Y.; Jing, J.; Wang, H.; Wang, A.; Zhang, Y.; Jiang, Y.; Lin, J.; Zhao, X.; Li, H.; Wang, Y.; et al. Association of polyvascular disease and elevated interleukin-6 with outcomes in Acute Ischemic Stroke or Transient Ischemic attack. Front. Neurol. 2021, 12, 661779. [Google Scholar] [CrossRef]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Da Silva, C.C.; Buisson, M.; Amaz, C.; Derex, L.; Ong, E.; Berthezene, Y.; Eker, O.F.; et al. Association of interleukin-6 levels and futile reperfusion after mechanical thrombectomy. Neurology 2021, 96, e752–e757. [Google Scholar] [CrossRef] [PubMed]
- Mechtouff, L.; Bochaton, T.; Paccalet, A.; Da Silva, C.C.; Buisson, M.; Amaz, C.; Derex, L.; Ong, E.; Berthezene, Y.; Dufay, N.; et al. A lower admission level of interleukin-6 is associated with first-pass effect in ischemic stroke patients. J. Neurointerv. Surg. 2022, 14, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Emam, W.A.; Ibrahim, N.E.; El-Senosy, F.M.; Elsheikh, A.A.; Elsayed, A.E.; Attar, R.E.; Elhadad, S.M.; Alhabibi, A.M.; Mohammed, A.R. Prognostic utility of MicroRNA-221 and interleukin-6 in cerebral ischemic stroke. Egypt J. Immunol. 2023, 30, 11–20. [Google Scholar] [PubMed]
- Purroy, F.; Farré-Rodriguez, J.; Mauri-Capdevila, G.; Vicente-Pascual, M.; Farré, J. Basal IL-6 and S100b levels are associated with infarct volume. Acta Neurol. Scand. 2021, 144, 517–523. [Google Scholar] [CrossRef]
- Jia, W.-L.; Jiang, Y.-Y.; Jiang, Y.; Meng, X.; Li, H.; Zhao, X.-Q.; Wang, Y.-L.; Wang, Y.-J.; Gu, H.-Q.; Li, Z.-X. Associations between admission levels of multiple biomarkers and subsequent worse outcomes in acute ischemic stroke patients. J. Cereb. Blood Flow. Metab. 2024, 44, 742–756. [Google Scholar] [CrossRef]
- Smith, C.J.; Emsley, H.C.A.; Gavin, C.M.; Georgiou, R.F.; Vail, A.; Barberan, E.M.; del Zoppo, G.J.; Hallenbeck, J.M.; Rothwell, N.J.; Hopkins, S.J.; et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004, 4, 2. [Google Scholar] [CrossRef]
- Shenhar-Tsarfaty, S.; Ben, E.; Assayag, I.; Bova, L.; Shopin, M.; Fried, S.; Berliner, I.; Shapira, N.M. Bornstein Interleukin-6 as an early predictor for one-year survival following an ischaemic stroke/transient ischaemic attack. Int. J. Stroke 2010, 5, 16–20. [Google Scholar] [CrossRef]
- Meisinger, C.; Freuer, D.; Schmitz, T.; Ertl, M.; Zickler, P.; Naumann, M.; Linseisen, J. Inflammation biomarkers in acute ischemic stroke according to different etiologies. Eur. J. Neurol. 2024, 31, e16006. [Google Scholar] [CrossRef]
- Orion, D.; Schwammenthal, Y.; Reshef, T.; Schwartz, R.; Tsabari, R.; Merzeliak, O.; Chapman, J.; Mekori, Y.A.; Tanne, D. Interleukin-6 and soluble intercellular adhesion molecule-1 in acute brain ischaemia. Eur. J. Neurol. 2008, 15, 323–328. [Google Scholar] [CrossRef]
- Bustamante, A.; Sobrino, T.; Giralt, D.; García-Berrocoso, T.; Llombart, V.; Ugarriza, I.; Espadaler, M.; Rodríguez, N.; Sudlow, C.; Castellanos, M.; et al. Prognostic value of blood interleukin-6 in the prediction of functional outcome after stroke: A systematic review and meta-analysis. J. Neuroimmunol. 2014, 274, 215–224. [Google Scholar] [CrossRef]
- Martínez-Sánchez, P.; Gutiérrez-Fernández, M.; Fuentes, B.; Masjuán, J.; de Leciñana Cases, M.A.; Novillo-López, M.E.; Díez-Tejedor, E. Biochemical and inflammatory biomarkers in ischemic stroke: Translational study between humans and two experimental rat models. J. Transl. Med. 2014, 3, 220–229. [Google Scholar] [CrossRef]
- Hotter, B.; Hoffmann, S.; Ulm, L.; Meisel, C.; Fiebach, J.B.; Meisel, A. IL-6 Plasma levels correlate with cerebral perfusion deficits and infarct sizes in stroke patients without associated infections. Front. Neurol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Rodríguez-Yáñez, M.; Castillo, J. Role of inflammatory markers in brain ischemia. Curr. Opin. Neurol. 2008, 21, 353–357. [Google Scholar] [CrossRef]
- Beridze, M.; Sanikidze, T.; Shakarishvilil, R.; Intskirveli, N.; Bornstein, N.M. Selected acute phase CSF factors in ischemic stroke: Findings and prognostic value. BMC Neurol. 2011, 11, 41–48. [Google Scholar] [CrossRef]
- Băcilă, C.I.; Vlădoiu, M.G.; Văleanu, M.; Mogą, D.F.C.; Pumnea, P.M. The role of IL-6 and TNF-alpha biomarkers in predicting disability outcomes in Acute Ischemic Stroke patients. Life 2025, 15, 47. [Google Scholar] [CrossRef]
- Hervella, P.; Rodríguez-Castro, E.; Rodríguez-Yáñez, M.; Arias, S.; Santamaría-Cadavid, M.; López-Dequidt, I.; Estany-Gestal, A.; Maqueda, E.; López-Loureiro, I.; Sobrino, T.; et al. Intra- and extra-hospital improvement in ischemic stroke patients: Influence of reperfusion therapy and molecular mechanisms. Sci. Rep. 2020, 10, 3513. [Google Scholar] [CrossRef]
- Dargazanli, C.; Blaquière, M.; Moynier, M.; de Bock, F.; Labreuche, J.; Schiphorst, A.T.; Derraz, I.; Radu, R.A.; Gascou, G.; Lefevre, P.H.; et al. Inflammation biomarkers in the intracranial blood are associated with outcome in patients with ischemic stroke. J. Neurointerv. Surg. 2024, 17, 159–166. [Google Scholar] [CrossRef]
- Kwan, J.; Horsfield, G.; Timothy Bryant, T.; Gawne-Cain, M.; Durward, G.; Byrne, C.D.; Englyst, N.A. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp. Gerontol. 2013, 48, 960–965. [Google Scholar] [CrossRef]
- Dusanovic Pjevic, M.; Vojvodic, L.; Grk, M.; Todorovic, J.; Maksimovic, N.; Rasic, M.; Perovic, D.; Damnjanovic, T.; Trickovic, J.; Kacar, K.; et al. Association of IL-6 rs1800795, but not TNF-α rs1800629, and IL-1β rs16944 polymorphisms’ genotypes with recovery of ischemic stroke patients following thrombolysis. Neurol. Res. 2024, 46, 157–164. [Google Scholar] [CrossRef]
- Huang, X.; Ye, Q.; Zhu, Z.; Chen, W.; Chen, Y.; Li, J.; Sun, J.; Ye, Z. Association between a functional interleukin 6 receptor genetic variant and the risk and functional outcome of large artery atherosclerotic stroke. Int. J. Neurosci. 2020, 130, 355–362. [Google Scholar] [CrossRef]
- Rosário, M.; Fonseca, A.C. Update on biomarkers associated with Large-Artery Atherosclerosis stroke. Biomolecules 2023, 13, 1251. [Google Scholar] [CrossRef] [PubMed]
- Klimiec-Moskal, E.; Piechota, M.; Pera, J.; Weglarczyk, K.; Slowik, A.; Siedlar, M.; Dziedzic, T. The specific ex vivo released cytokine profile is associated with ischemic stroke outcome and improves its prediction. J Neuroinflamm. 2020, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Sotgiu, S.; Zanda, B.; Marchetti, B.; Fois, M.L.; Arru, G.; Pes, G.M.; Salaris, F.S.; Arru, A.; Pirisi, A.; Rosati, G. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur. J. Neurol. 2006, 13, 505–513. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Su, Y.; Sun, Y.; Zhang, Y.; Chen, H. Association of inflammatory and platelet volume markers with clinical outcome in patients with anterior circulation ischaemic stroke after endovascular thrombectomy. Neurol. Res. 2021, 43, 503–510. [Google Scholar] [CrossRef]
- Bahorik, A.L.; Hoang, T.D.; Jacobs, D.R.; Levine, D.A.; Yaffe, K. Association of changes in C-reactive protein level trajectories through early adulthood with cognitive function at midlife the CARDIA study. Neurology 2024, 103, e209526. [Google Scholar] [CrossRef]
- Kelly, P.J.; Murphy, S.; Coveney, S.; Purroy, F.; Lemmens, R.; Tsivgoulis, G.; Price, C. Anti-inflammatory approaches to ischaemic stroke prevention. J. Neurol. Neurosurg. Psychiatry 2018, 89, 211–218. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Hudobenko, J. Ischemic Stroke Damage is Reduced by Inhibition of IL-6 Signaling with Tocilizumab. Master’s Thesis, The University of Texas MD Anderson Cancer Center, Houston, TX, USA, 2018. [Google Scholar]
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320. [Google Scholar] [CrossRef]
- Yamashita, T.; Sawamoto, K.; Suzuki, S.; Suzuki, N.; Adachi, K.; Kawase, T.; Mihara, M.; Ohsugi, Y.; Abe, K.; Okano, H. Blockade of interleukin-6 signaling aggravates ischemic cerebral damage in mice: Possible involvement of Stat3 activation in the protection of neurons. J. Neurochem. 2005, 94, 459–468. [Google Scholar] [CrossRef]
- Ziegler, L.; Frumento, P.; Wallen, H.; de Faire, U.; Gigante, B. The predictive role of interleukin 6 trans-signalling in middle-aged men and women at low-intermediate risk of cardiovascular events. Eur. J. Prev. Cardiol. 2020, 27, 122–129. [Google Scholar] [CrossRef]
- Taei, A.A.; Dargahi, L.; Khodabakhsh, P.; Kadivar, M.; Farahmandfar, M. Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke. CNS Neurosci. Ther. 2022, 28, 1425–1438. [Google Scholar] [CrossRef]
- Sakata, H.; Narasimhan, P.; Niizuma, K.; Maier, C.M.; Wakai, T.; Chan, P.H. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain 2012, 135, 3298–3310. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gu, L.; Wu, K.; Wang, K.; Ru, J.; Yang, S.; Wang, Z.; Zhuge, Q.; Huang, L.; Huang, S. VX-765 enhances autophagy of human umbilical cord mesenchymal stem cells against stroke-induced apoptosis and inflammatory responses via AMPK/mTOR signaling pathway. CNS Neurosci. Ther. 2020, 26, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Neal, E.G.; Acosta, S.A.; Kaneko, Y.; Ji, X.; Borlongan, C.V. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1750–1758. [Google Scholar] [CrossRef]
- Zarriello, S.; Neal, E.G.; Kaneko, Y.; Borlongan, C.V. T-regulatory cells confer increased myelination and stem cell activity after stroke-induced white matter injury. J. Clin. Med. 2019, 8, 537. [Google Scholar] [CrossRef]
- Huang, L.; Wong, S.; Snyder, E.Y.; Hamblin, M.H.; Lee, J.-P. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res. Ther. 2014, 5, 129. [Google Scholar] [CrossRef]
- Chi, L.; Huang, Y.; Mao, Y.; Wu, K.; Zhang, L.; Nan, G. Tail vein infusion of adipose-derived mesenchymal stem cell alleviated inflammatory response and improved Blood Brain Barrier condition by suppressing endoplasmic reticulum stress in a Middle Cerebral Artery Occlusion rat model. Med. Sci. Monit. 2018, 24, 3946–3957. [Google Scholar] [CrossRef]
- Kaneko, Y.; Lee, J.Y.; Tajiri, N.; Tuazon, J.P.; Lippert, T.; Russo, E.; Yu, S.J.; Bonsack, B.; Corey, S.; Coats, A.B.; et al. Translating intracarotid artery transplantation of bone marrow-derived NCS-01 cells for ischemic stroke: Behavioral and histological readouts and mechanistic insights into stem cell therapy. Stem Cells Transl. Med. 2020, 9, 203–220. [Google Scholar] [CrossRef]
| Collection Time | Average Concentration | Control | Results | Conclusions | Reference |
|---|---|---|---|---|---|
| from 5 to 7 days | Peak plasma IL-6 30.5 pg/mL (n = 37) | – | Peak plasma IL-6 concentration above the median sample (30.5 pg/mL) at 5 to 7 days correlated significantly with cerebral infarct volume on computed tomography (r = 0.62, p = 0.006), mRS at 3 months (r = 0.62, p = 0.002), mRS at 12 months (r = 0.77, p < 0.001), stroke severity (r = 0.66, p < 0.001), Barthel index (BI) at 3 months (r = −0.74, p < 0.001), BI at 12 months (r = -0.77, p < 0.001) and was associated with increased mortality at 12 months (p = 0.0011). | Peak plasma IL-6 concentration in the first week of ischemic stroke significantly correlated with infarct volume, stroke severity, and clinical outcome at 3 months and mortality at 12 months. | [126] |
| at admission | 6.4 ± 2.6 pg/mL (n = 11) | 2.8 ± 1.5 pg/mL (n = 9) p = 0.002 | Significant correlation of IL-6 with larger stroke volume (p = 0.012, r = 0.72,) and poorer prognosis at 1 year, as measured by ESS (p = 0.014, r = −0.71) and BI (p = 0.006, r = −0.80). | In acute ischemic stroke, IL-6 can be an early indicator of prognosis. | [92] |
| 24–72 h | 10.5 (6–27.25) pg/mL (n = 60) | 9 (2.90–18) pg/mL (n = 123) p = 0.002 | No differences in IL-6 levels at 24–72 h after stroke onset in non-lacunar versus lacunar strokes. | Higher IL-6 values were demonstrated compared to healthy individuals. | [94] |
| <20 h | 9.7 (7.9–15.7) pg/mL (n = 50) | – | The level IL-6 was significantly higher in patients with better outcome (median 12.6 (7.6–21.4)) when compared with those with worse outcome (median 8.492.4–13.70 p < 0.0001). There was a significant inverse correlation between IL-6 and TNF-α levels in the entire cohort of patients (r = 0.78, p = 0.002) | Serum IL-6 concentration was inversely correlated with final neurological impairment and infarct size (p < 0.001). In patients with ischemic brain injury, IL-6 is associated with neuroprotection but not neurotoxicity. | [143] |
| 50 h | 13.78 ± 19.46 pg/mL (n = 60) | 4.38 ± 15.88 pg/mL (n = 30) p = 0.002 | The levels of serum IL-6 were significantly higher in stroke patients. IL-6 showed negative correlation with protein S (r = –0.504, p = 0.000) and CNS scores (r = –0.451, p = 0.000). | IL-6 was correlated with the worse clinical outcome. | [105] |
| at admission | 8 (4–21) pg/mL (n = 68) | 2 (2–4) pg/mL (n = 71) p = 0.035 | IL-6 was higher in stroke patients than in controls. Higher IL-6 concentration was significantly associated with greater neurological deficit (NIHSS ≤ 16 (n = 30), 7 (4–11) pg/mL, NIHSS 17–22 (n = 24), 7 (5–16) pg/mL; NIHSS ≥ 23 (n = 17), 22 (10–71) pg/mL; p = 0.007) at admission and greater degree of patient disability assessed with BI (BI ≤ 29 (n = 28), 13 (6–28) pg/mL; BI 30–59 (n = 15), 8 (5–20) pg/mL; BI ≥ 60 (n = 28), 6 (4–12) pg/mL; p = 0.030) and mRS (mRS = 0–2 (n = 28), 6 (4–12) pg/mL; mRS = 3–4 (n = 14), 8 (5–11) pg/mL; mRS = 5–6 (n = 29), 13 (6–36) pg/mL; p = 0.019). | IL-6 levels measured 12 h after the onset of cerebral ischemia were higher in stroke patients compared to the control group, and this increase was associated with more severe stroke and worse stroke outcomes. | [104] |
| within <36 h of symptom onset | 5.14 ± 4.98 pg/mL (mean ± SD) [3.18 (1.97–6.22) median (25–75%)] (n = 113) | – | The proportion of patients with severe stroke (NIHSS > 10) at admission rose in a graded fashion from 0% in patients with IL-6 in the bottom tertile (≤2.26 pg/mL) to 46% in those in the top tertile (>4.63 pg/mL). Similar trends were observed at day 1–2, day 4–5, and at discharge (p < 0.01). Disability during follow-up (mRS ≥ 2) rose from 13% for IL-6 in the bottom tertile to 48% in the top tertile (p = 0.01). Association with poor physical function (Stroke Impact Scale (SIS ≤ 85)) revealed a comparable trend rising from 32% at the bottom tertile to 63% at the top tertile (p = 0.04). | High plasma IL-6 levels are associated with stroke severity and poorer functional outcomes. | [129] |
| at admission | 10 (6–28) pg/mL (n = 107) | 8 (3.1–12) pg/mL (n = 102) p < 0.001 | Significant association between IL-6 demographic variables (age), diagnostic subtype (lacunar (5 (2–8) pg/mL) or cardioembolic (12 (6.5–18) pg/mL; p = 0.003), and SSS score (b1= −0.068; p < 0.0001). | Significant association between IL-6 and severity of neurological deficit at admission, diagnostic subtype. | [93] |
| 24 h | 26.5 +/−2.3 pg/mL (range 6.4–161.3) (n = 46) | 3.9 +/−1.5 pg/mL (range 2.3–5.9) (n = 98; p < 0.0001 | The concentration of IL-6 in the blood of patients with acute ischemic stroke was significantly higher than in healthy controls. | There is an association between inflammation and circulating IL-6 levels in patients with acute hypoxic–ischemic injury, therefore IL-6 might be used as a warning signal, the concentration of which increases at an early inflammatory state. | [98] |
| 24 h | <6.47 pg/mL (n = 200) lower NIHSS ≥6.47 pg/mL (n = 50) higher NIHSS | – | Plasma IL-6 concentration correlated with a more severe stroke as demonstrated by NIHSS and mRS (r = 0.318, p < 0.001 and r = 0.302, p < 0.001, respectively). IL-6 concentration was found to be the optimal predictor, with a cut point of 6.47, χ2 (I, n= 250) = 20.5, p < 0·001. The value of IL-6 above 6.47 during the acute phase predicted a subsequent lack of survival (p= 0.006, odds ratio 8.0). | IL-6 shows potential as an early signal of survival after acute ischemic stroke and suggests a clear cut-off point for high-risk patients who might benefit from closer clinical surveillance and/or therapeutic interventions. | [127] |
| 2–6 h, 6 h, 24 h, 3 days, 7 days | Figure 1a in [99] ng/L (n = 69) | – | IL-6 showed different time courses depending on stroke outcome. The IL-6 levels were significantly increased in the patients with severe strokes compared to the mild/moderate stroke group as early as 6 h after stroke onset. The levels stayed elevated only until 24 h after symptom onset (6 h p = 0.022, 12 h p = 0.011, 24 h p = 0.006). IL-6 at 12 h, 24 h, and 3 days was independently related to the NIHSS. In the first days after stroke, IL-6 significantly correlated with CRP and MMP-9 at different time points, while only at 6 h after stroke onset, IL-6 correlated with MCP-1. | The data indicate significant differences in the early time course of several potential markers for the complex network of inflammation and brain damage after ischemic stroke, depending on the outcome. | [99] |
| 72 h 6 months | No infection 1.4 ± 0.3 ng/mL (n = 37) Infection 1.6 ± 0.3 ng/mL (n = 45) | – | Plasma IL-6 at baseline was associated with decreased survival at 2 years (OR = 9.2, [95% CI 1.0, 85.1], p = 0.031) and this association was independent of SAI, severity of stroke, age and sex. | IL-6 is associated with SAI, after adjusting for recognized risk factors, and IL-6 is associated with mortality in the first two years post stroke. | [138] |
| at admission | 4.6 (0.9 to 13.0) pg/mL (n = 175) | – | Strong association between IL-6 and poor stroke outcomes (favorable outcome (n = 111), 2.2 (0.6 to 5.5) versus poor outcome (n = 64), 12.1 (6.7 to 48.0); p < 0.001). The logistic regression analysis of individual blood markers after adjusting for age and initial NIHSS score revealed that plasma logIL-6 (adjusted OR: 1.75, 95% CI: 1.25 to 2.25, p = 0.001) reached a statistical significance. The statistical significance of logIL-6 remained after further adjustment for 72 h infarct volume and Afib (logIL-6, OR: 1.74, 95% CI: 1.24 to 2.44, p = 0.003), time of stroke onset (logIL-6, OR: 1.74, 95% CI: 1.24 to 2.44, p = 0.001) and previous statin use (logIL-6, OR: 1.81, 95% CI: 1.27 to 2.57, p = 0.001). | Circulating IL-6 levels have clinical value in predicting stroke outcomes. | [96] |
| 1 day | 42.92 ± 72.2 pg/mL | – | Statistically significant correlation between IL-6 and NIHSS and mRS of patients from the moment of admission to the end of the observation period (p < 0.001, r = 0.6). Statistically significant correlation between IL-6 and infarct size in brain magnetic resonance imaging (MRI) (p < 0.001, r = 0.7). | IL-6 contributes to determination of severity of ischemic stroke. | [91] |
| 5 days | 56.91 ± 82.63 pg/mL | ||||
| The samples were drawn on the day of randomization, but before the initiation of antiplatelet therapy | Age: <50 1.8 pg/mL (1.4–3.4) Age: 50–65 2.4 pg/mL (1.6–3.7) Age:>65–75 2.2 pg/mL (1.6–3.6) Age:>75 2.5 pg/mL (1.7–3.5) (n = 1244) | – | In unadjusted analyses, IL-6 was significantly related to the risk of having a recurrent ischemic stroke (adjusted HR per SD, 1.1; 95% confidence interval [CI], 1.0–1.2; p < 0.01. IL-6 was also associated with an increase in risk of major vascular events (adjusted HR per SD, 1.1; 95% CI, 1.02–1.19; p = 0.008). In categorical analyses, there was evidence that the treatment effect of dual antiplatelet therapy depended on IL-6 levels (p for interaction = 0.04 for major vascular events). | Among recent lacunar stroke patients, IL-6 concentration predict risk of recurrent vascular events, and they are associated with the effect of antiplatelet therapies. | [118] |
| 48 h | 15.82 ± 16.64 pg/mL (n = 42) | 6.64 ± 2.5 (n = 34) p = 0.000035 | Patients with early ischemic stroke had significantly higher serum IL-6 levels than controls. | Ischemic stroke is associated with higher serum IL-6 levels. | [102] |
| at admission | 21.91 ±30.75 pg/mL (n = 33) | 1.04 ± 1.12 pg/mL (n = 60) | Baseline levels of IL-6 and stroke severity: The higher baseline IL-6 level showed a significant positive correlation with the NIHSS score at day 1(r2 = 0.18; p = 0.01), on day 7 following admission (r2 = 0.20; p = 0.01) and with the infarct volume on DWI (r2 = 0.17; p = 0.01). IL-6 was found to correlate with worse long-term outcomes at both 1 month (r2 = 0.19; p = 0.01) and 3 months (r2 = 0.15; p = 0.04), as measured by MRS. The baseline IL-6 levels was independent predictors of short term outcome as assessed by NIHSS at day 7 (p = 0.04); also for long term outcome as measured by MRS at one month (p = 0.02) and three months (p = 0.04). Baseline levels of IL-6 were higher (p = 0.04) in those patients who died than those who survived. | Serum IL-6 concentrations were significantly higher in patients with ischemic stroke than in the healthy control group. A correlation was observed between the clinical and radiological severity of stroke and the level of IL-6, and the poor outcome could be predicted (the higher baseline IL-6 may predict mortality in ischemic stroke). This suggests that the IL-6 serum may be a potentially useful inflammatory biomarker for the prognosis of acute ischemic stroke. | [95] |
| 27 days | 12.80 ±21.18 pg/mL (n = 33) | ||||
| 1 month | 6.18 ±14.21 pg/mL (n = 33) | ||||
| 3 months | 2.75 ± 2.54 pg/mL (n = 33) | ||||
| 24 h | 2.61 (1.60–4.73) pg/mL (n = 7053) | – | Stroke recurrence was observed in 458 (6.5%) patients, and functional disability was seen in 1708 (24.2%) patients at the 90-day follow-up. Per stand deviation (4.26 pg/mL) increase in IL-6 concentration was associated with a significantly 19% increased risk of stroke recurrence (OR, 1.19; 95% CI, 1.09–1.29) and a significantly 22% increased risk of disability (OR, 1.22; 95% CI, 1.15–1.30) at 90 days. The mediated proportion of the association between IL-6 and functional disability by stroke recurrence was 18.72% (95% CI, 9.26–28.18%) in the adjusted model. | Stroke recurrence mediates less than 20% of the association between IL-6 and functional outcome at 90 days among patients with acute ischemic stroke. | [114] |
| – | Lacunar stroke 6.6 (7.5) pg/mL (n = 52) | – | Baseline IL-6 was significantly associated with risk of main outcome in them ultivariable analysis, after adjustment for age, sex, and small vessel disease score (1.4 (1.02–2.2); p = 0.04). IL-6 was significantly related to along-term risk of vascular events or death in patients with cerebral small vessel disease (CSVD). | It demonstrated the important prognostic role of Il-6 in persons with different clinical manifestations of CSVD. The strongest association occurred between IL-6 and recurrent stroke, other vascular events and death. | [115] |
| – | 4.5 (3.1) ng/mL (n = 557) | cohort random sample 3.7 (2.6) ng/mL (n = 951) p < 0.001 | IL-6 was associated with risk factors for stroke. After adjusting for different factors (age, sex, race) the hazard ratio (HR; 95% CI) for incident stroke for the highest versus lowest quartile of IL-6 was 2.4 (1.6–3.4). IL-6 mediated the black-white disparity in stroke risk, but mediation was mediated by associations of IL-6 with stroke risk factors. | IL-6 was strongly associated with stroke risk factors and a substantially increased risk of incident stroke, independent of racial disparity. | [16] |
| at admission | Intra-hospital improvement h/extra-hospital improvement i | – | Th high levels of IL-6 on admission had a negative correlation with extrahospital improvement (β: −0.305, p < 0.0001), while high levels of IL6 at 24 hours correlates with a negative intrahospital improvement and a positive extrahospital improvement (β:−0.121, p < 0.0001 vs. β: 0.131, p < 0.0001). | High IL-6 level at 24 hours was associated with a worse intra-hospital improvement and with better extra-hospital improvement. The variations in IL6 level in the first 24 hours clearly showed a relationship between the molecular components of the ischemic cascade and the clinical outcome of patients. | [136] |
| 21.1 ± 15.8/22.4 ± 15.3 (NO n = 1176/1326) 21.3 ± 15.2/20.1 ± 15.3 (YES n = 3119/2527) p = 0.844/0.043 | |||||
| 24 h | 62.2 ± 47.5/20.1 ± 25.0 (NO n = 1176/1326) 51.1 ± 46.3/96.5 ± 30.8 (YES n = 3119/2527) p = 0.006/<0.0001 | ||||
| 24 h | 18.8 (4.0) pg/mL (n = 176) | 6.7 (4.6) pg/mL (n = 176) p < 0.001 | The results showed that the best predictors of IS were SBP, glucose, NOx, hydroperoxides, IL-6, and WBC (all positively associated), 25(OH)D (negatively associated), and sex (male) (x2 = 159.64, df = 8, p < 0.001, Nagelkerke = 0.787) and that 89.4% of all cases were correctly classified with sensitivity of 86.2% and specificity of 93.0%. IL-6 is significantly higher in patients with an endpoint mRS ≥ 3 (27.5 (4.1) pg/mL) vs. those with an endpoint mRS < 3 (8.7(7.2) pg/mL; p < 0.001). | The cumulative effects of immune-inflammatory, metabolic, oxidative, and nitrosative stress (IMO&NS) biomarkers are associated with AIS and predict a poor outcome at 3-month follow-up. | [107] |
| <7 days | Figure 2a,b in [106] | – | Baseline IL-6 was associated with poor functional outcome (odds ratio [OR] per 1-unit increase 1.02, CI 1.01–1.04, p = 0.002). After adjustment for age, sex, qualifying event, baseline mRS, hypertension, anti-platelet and statins on discharge, the association remained unchanged (adjusted OR 1.02, CI 1.01–1.04, p = 0.004). A relationship between IL-6 and mortality after 1 year was observed. When the highest quartile was compared to the lowest quartile IL-6, the HR was 2.30 (CI 1.10–4.84, p = 0.03) | Baseline inflammatory cytokines independently predicted late recurrence, supporting a rationale for randomized trials of anti-inflammatory agents for prevention after stroke and suggesting that targeted therapy to high-risk patients with high baseline inflammation may be beneficial. | [106] |
| at admission | a 1.3 [0.3–3.6] pg/mL | – | High IL-6 levels at 6, 24, and 48 h, in combination with a higher age, a pre-stroke mRS score > 2, a past history of hypertension or diabetes, current smoking, a higher baseline National Institute of Health Stroke Scale Score, the absence of associated intravenous thrombolysis, an intracranial internal carotid artery or a tandem occlusion and an increased infarct growth were associated with futile reperfusion. | IL-6 is a marker of futile reperfusion in the setting of MT. | [121] |
| b 1.3 [0.7–3.4] pg/mL | |||||
| c 2.0 [0.7–4.5] pg/mL | |||||
| 6 h | a 3.1 [2.0–6.0] pg/mL | ||||
| b 2.1 [1.1–4.4] pg/mL | |||||
| c 3.3 [2.2–6.2] pg/mL | |||||
| 24 h | a 5.0 [3.3–7.3] pg/mL | ||||
| b 2.7 [1.7–5.5] pg/mL | |||||
| c 4.3 [2.6–9.0] pg/mL | |||||
| 48 h | a 5.2 [2.9–15.9] pg/mL | ||||
| b 2.5 [1.2–5.2] pg/mL | |||||
| c 5.3 [2.5–8.2] pg/mL | |||||
| 3 months | a 1.2 [0.3–2.0] pg/mL | ||||
| b 0.6 [0.3–1.1] pg/mL | |||||
| c 1.0 [0.4–2.6] pg/mL | |||||
| 24 h | (4.3; 8; 14.9) pg/mL (n = 332) | – | In linear regression analysis that was adjusted for etiology, IL-6 was found to be a biomarker independently associated with infarct volume (Atheromatous, (n = 59), 8.9 (4.9–14.6); Cardioembolic (n = 126), 10.9 (6.1–23.4); Small vessel (n = 63), 4.8 (2.8–9.7); Indeterminate, (n = 77), 8.1 (5.4–14.7); Unusual, (n = 7), 4.5 (1.9–10.0); p < 0.001). | IL-6 levels may be independently associated with infarct volume. The results may have clinical significance because IL-6 levels in blood may indicate the extension of brain infarction without the need to use neuroimaging techniques. | [124] |
| 27–96 h | IL-6: Q1: ≤1.6 ng/L (n = 2896) Q2: 1.6–2.6 ng/L (n = 1872) Q3: 2.6–5.1 ng/mL (n = 1934) Q4: >5.1 ng/mL (n = 3051) | – | The highest quartiles of IL-6 (adjusted HR, 1.36; 95% CI 1.13–1.64; p = 0.001) were associated with increased risk of recurrent stroke; and the highest quartiles of IL-6 (adjusted OR 1.93; 95% CI 1.64–2.27; p < 0.0001), were correlated with increased risk of poor functional outcome. | In patients with ischemic stroke, an association was found between IL-6 and recurrent stroke, composite vascular events, and poor functional outcome. | [45] |
| Oset, 24 h, 72 h | poor outcome (mRS ≥ 2)/ good outcome after 90 days 8.72 (IQR 3.04–26.89) pg/mL/ 3.72 (IQR, 1.88–8.58) pg/mL p = 0.012 | – | The level of IL-6 was significantly increased in the NIHSS > 5 group at admission (p < 0.001) compared to the NIHSS ≤ 5 group. IL-6 was notably higher in the poor outcome group (p = 0.012). IL-6 was an independent predictor of risk for AIS patients with an adjusted OR of 1.152 (p = 0.028). Patients with a NIHSS score of less than 5 exhibited lower IL-6 levels, indicating that increased levels of IL-6 correlated with AIS severity after intravenous thrombolytic treatment (IVT). | IL-6 might be useful plasma marker to predict the prognosis in AIS patients at 90 days after IVT. | [116] |
| 4.5 h | 6.37 (4.35; 7.43) pg/mL (n = 125) | 3.59(3.42; 4.04) (n = 28; p < 0.001) | Correlations were found between IL-6 serum concentration measured during the onset and on the 1st day of ischemic stroke and the NIHSS results assessed on admission (R = 0.43, p < 0.01 and R = 0.4, p < 0.01, respectively) and at discharge (R = 0.61, p < 0.01 and R = 0.52, p < 0.01, respectively. Moreover, an association was observed between IL-6 serum levels determined in <4.5 h and on the 1st day of ischemic stroke, and results evaluated according to the mRS scale on admission (R = 0.52, p < 0.01 and R = 0.44, p < 0.01, respectively), at discharge (R = 0.61, p < 0.01 and R = 0.47, p < 0.01, respectively), after 3 months (R = 0.68, p < 0.01 and R = 0.3, p < 0.01, respectively), and 1 year since the stroke (R = 0.73, p < 0.01 and R = 0.50, p < 0.01, respectively) and IL-6 concentration measured 7 days after discharge (R = 0.28, p = 0.04). The significant statistical connection between IL-6 levels during onset and TNF-α levels assessed in <4.5 h and after 24 h since the stroke (p < 0.01) were detected. The IL-6 (5.59 pg/mL vs. 7.29 pg/mL) with p = 0.016 assessed in time < 4.5 h was statistically lower in patients suffering from the LACI (lacunar cerebral infarct) ischemic stroke in comparison to the patients with PACI (partial anterior circulation infarct) stroke. In the case of IL-6 level measured on the 7th day (>4 pg/mL), an increase in mortality was observed. | IL-6 levels were higher compared to the control group and in the subgroup of patients with an unfavorable functional outcome (mRS: 3–6 points). A positive correlation was found between IL-6 and TNF-α levels in patients with AIS within <4.5 h and on the day of stroke. Higher IL-6 levels in the acute phase of stroke and on the first and seventh day were associated with a worse early and late prognosis in patients treated with intravenous thrombolysis. An association between IL-6 levels in subacute AIS and the severity of neurological deficit was shown. IL-6 may be a prognostic factor in thrombolytic treatment of AIS. | [2] |
| 24 h | 7.09 (6.48; 9.30) pg/L (n = 125) | ||||
| 7 days | 6.54 (4.44; 7.58) pg/mL (n = 125) | ||||
| at admission | FPE <3.0 pg/mL (n = 43) Figure 2 in [122] | Non-FPE (n = 108) p < 0.05 Figure 2 in [122]. | FPE was found to be associated with low IL-6 levels and platelet count, an older age, a lack of hypertension, a lack of tandem occlusion, a shorter thrombus length, and a reduced procedural time. Multivariate analysis showed that low IL-6 admission level was associated with FPE (OR 0.66, 95% CI 0.46 to 0.94). Optimal cut-off of IL-6 level for distinguishing FPE from non-FPE was 3.0 pg/mL (sensitivity 92.3%, specificity 42.3%). | A lower admission level of IL-6 is associated with FPE. | [122] |
| - | 310.7 ± 110.6 pg/mL (155–522) (n = 45) | 34.2 ± 14.3 pg/mL (13–62) (n = 45) p < 0.0001 | The patient subgroups had significantly different levels of IL-6 as the severe cases had the highest level and mild ones had the lowest level (p < 0.001). There was a significant negative correlation between serum miR 221 with NIHSS score and IL-6 while there was a significant positive correlation with the grade of weakness (r = −0.75, p < 0.0001, r = −0.74, p < 0.0001 and r = 0.79, p < 0.001, respectively). The ROC curve with AUC of 0.99 for serum miR-221 combined with IL-6, showed both higher sensitivity and specificity of 96.4% and 89%, respectively. | Serum level of miR-221 may be considered a sensitive and specific marker for diagnosis and for assessing the severity of ischemic stroke especially if combined with IL-6. Such markers could be recommended as diagnostic tools and early predictive markers for the severity of the ischemic stroke disease. | [123] |
| <24 h | Table 1 in [137]. | – | IL-6 were significantly correlated (p < 0.05) with disability assessed by mRS score. Higher intracranial levels of IL-6 were associated with a lower likelihood (mRS score at 90 days > 2) of favorable outcome (adjusted OR per SD increase, 0.51; 95% CI 0.27 to 0.95). | The correlation between IL-6 and mRS score outcome support an avenue for add-on and localized immune modulatory strategies in AIS. | [137] |
| - | LAA 3.7 (2.2–8.2) pg/mL; (n = 272) d/3.1(1.9–5.7); (n = 1467) e CE 6.0(3.0–12.1) pg/mL; (n = 85) d/4.3 (2.1–7.7); (n = 369) e SAO 2.5 (1.6–4.9) pg/mL; (n = 173) d/2.2 (1.4–3.7); (n = 1397) e | – | At 3 months poststroke, 1026 (14.8%) patients experienced worse outcomes. Compared with patients with good outcomes, patients with poorer outcomes had significantly higher levels of inflammatory biomarkers, including IL-6 (p < 0.05). The highest quartile of IL-6 (1.43 [1.16–1.76]) was associated with an increased risk of worse outcomes at 3 months. | Significant associations have been found between elevated levels of inflammatory markers, including IL-6, and increased risk of poorer outcomes in patients with acute ischemic stroke. Targeting the inflammatory response, through novel anti-inflammatory therapies, may hold promise for improving neurological outcomes in stroke patients. | [113] |
| 24 h | Mean ± S 3.8 ± 4.0 pg/mL Median (IQR) 2.4(1.5–4.2) pg/mL; (n = 3910) f Mean ± S 5.7 ± 5.7 pg/mL Median (IQR) 2.8(1.9–8.1) pg/mL; (n = 121) g | – | Patients with ND had higher levels of IL-6 (median, 2.4 vs. 2.8 pg/mL) than patients without ND. IL-6 levels (adjusted OR, 1.43 [95% CI, 1.24–1.66]) remained independent predictor of in-hospital ND. The above relationship was also observed in long-term poor outcomes, including unfavorable functional outcomes (defined as mRS 2–5) and death. | Elevated IL-6 was an independent predictor for ND in minor AIS patients with LAA and SVO subtypes. IL-6 had the most notable incremental predictive value of in-hospital ND in addition to conventional predictors, both collectively and in the LAA subgroup. | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawluk, H.; Woźniak, A.; Tafelska-Kaczmarek, A.; Kosinska, A.; Pawluk, M.; Sergot, K.; Grochowalska, R.; Kołodziejska, R. The Role of IL-6 in Ischemic Stroke. Biomolecules 2025, 15, 470. https://doi.org/10.3390/biom15040470
Pawluk H, Woźniak A, Tafelska-Kaczmarek A, Kosinska A, Pawluk M, Sergot K, Grochowalska R, Kołodziejska R. The Role of IL-6 in Ischemic Stroke. Biomolecules. 2025; 15(4):470. https://doi.org/10.3390/biom15040470
Chicago/Turabian StylePawluk, Hanna, Alina Woźniak, Agnieszka Tafelska-Kaczmarek, Agnieszka Kosinska, Mateusz Pawluk, Krzysztof Sergot, Renata Grochowalska, and Renata Kołodziejska. 2025. "The Role of IL-6 in Ischemic Stroke" Biomolecules 15, no. 4: 470. https://doi.org/10.3390/biom15040470
APA StylePawluk, H., Woźniak, A., Tafelska-Kaczmarek, A., Kosinska, A., Pawluk, M., Sergot, K., Grochowalska, R., & Kołodziejska, R. (2025). The Role of IL-6 in Ischemic Stroke. Biomolecules, 15(4), 470. https://doi.org/10.3390/biom15040470







