Inositol and PIP2/PIP3 Ratio: At the Crossroad of the Biodynamic Interface Between Cells and Their Microenvironment
Abstract
1. Inositol(s), Inositol Phosphates, and Phosphoinositides
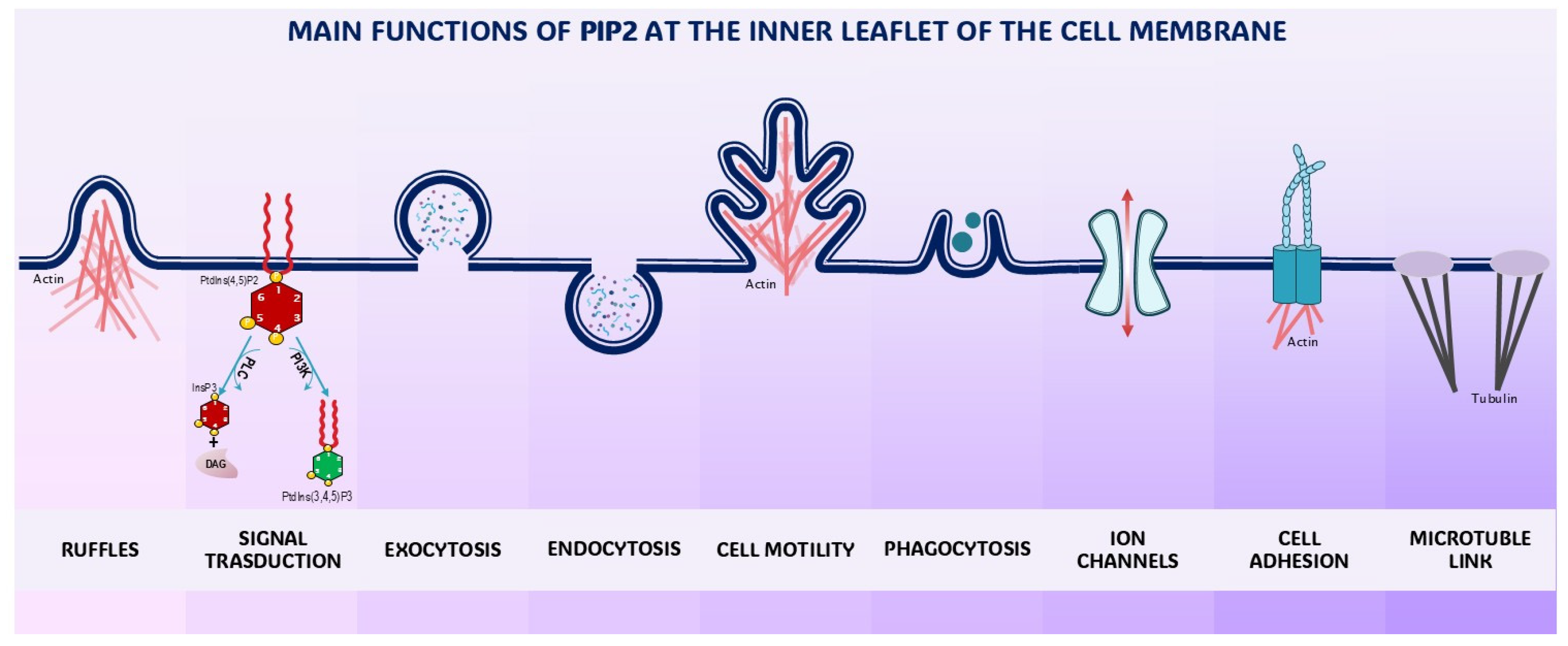
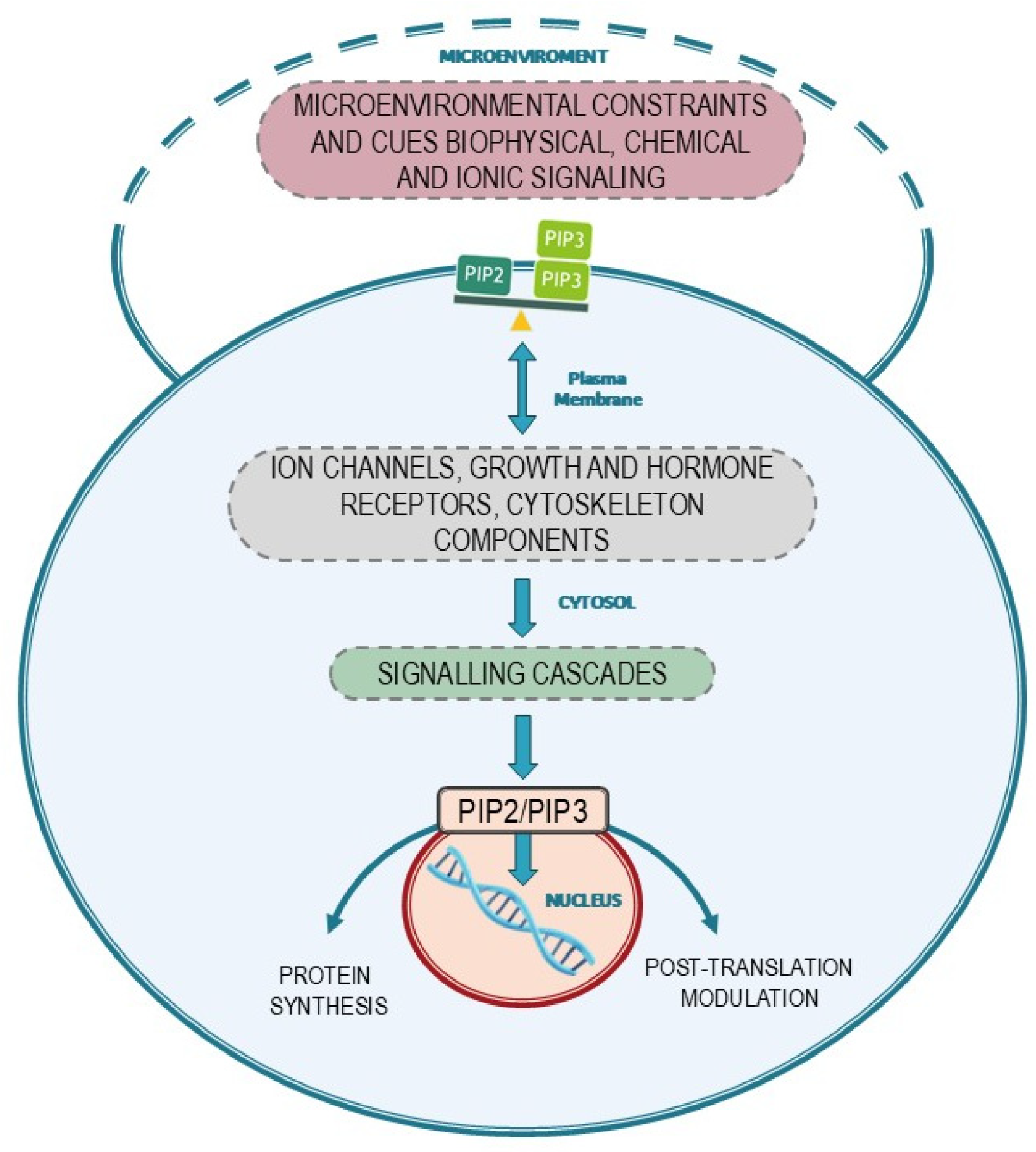
2. Phosphoinositides as Gates of the Membrane Biodynamic Interface
3. Synthesis and Distribution of PIP2 and PIP3
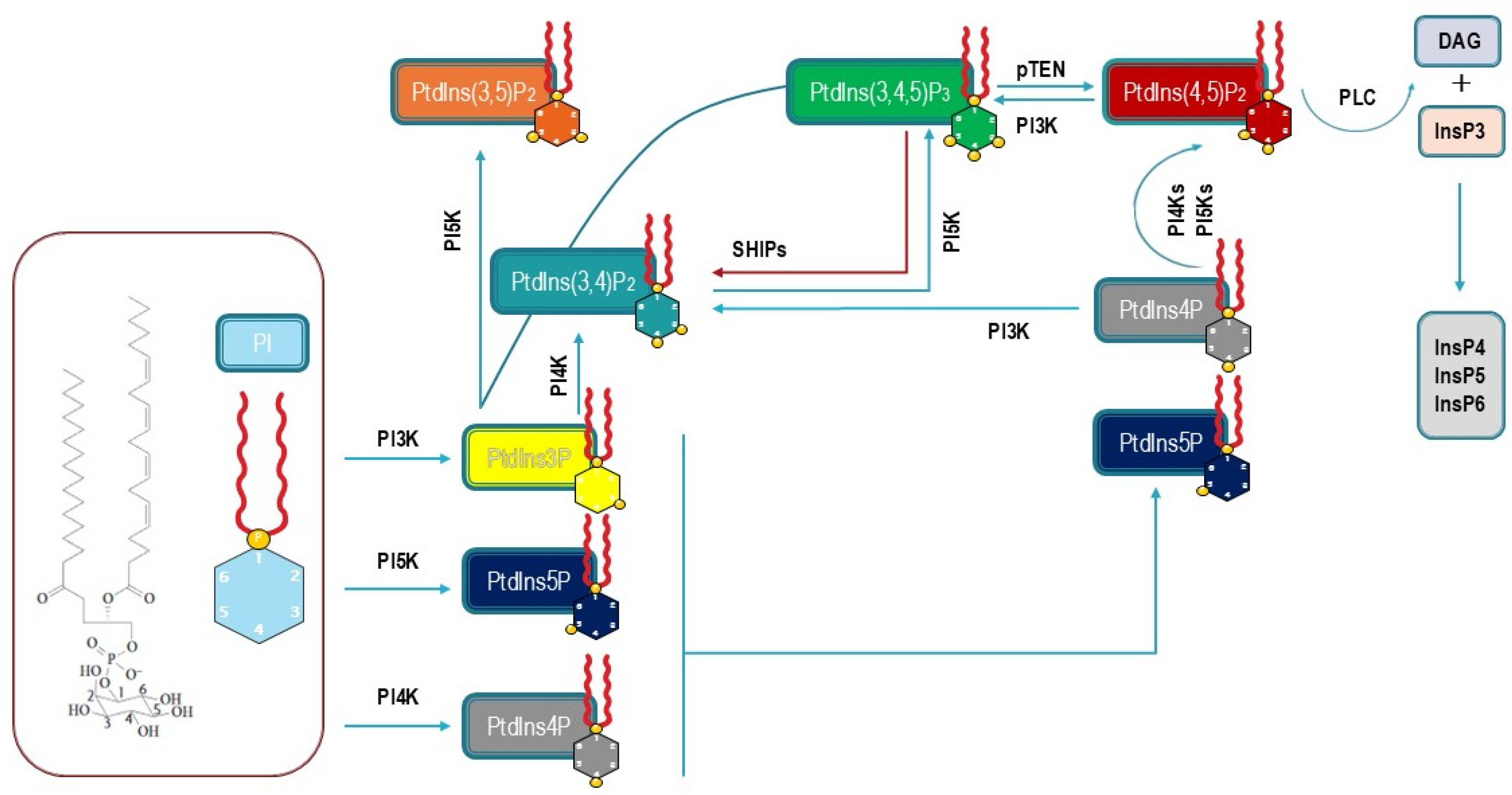
3.1. Synthesis of PIP2
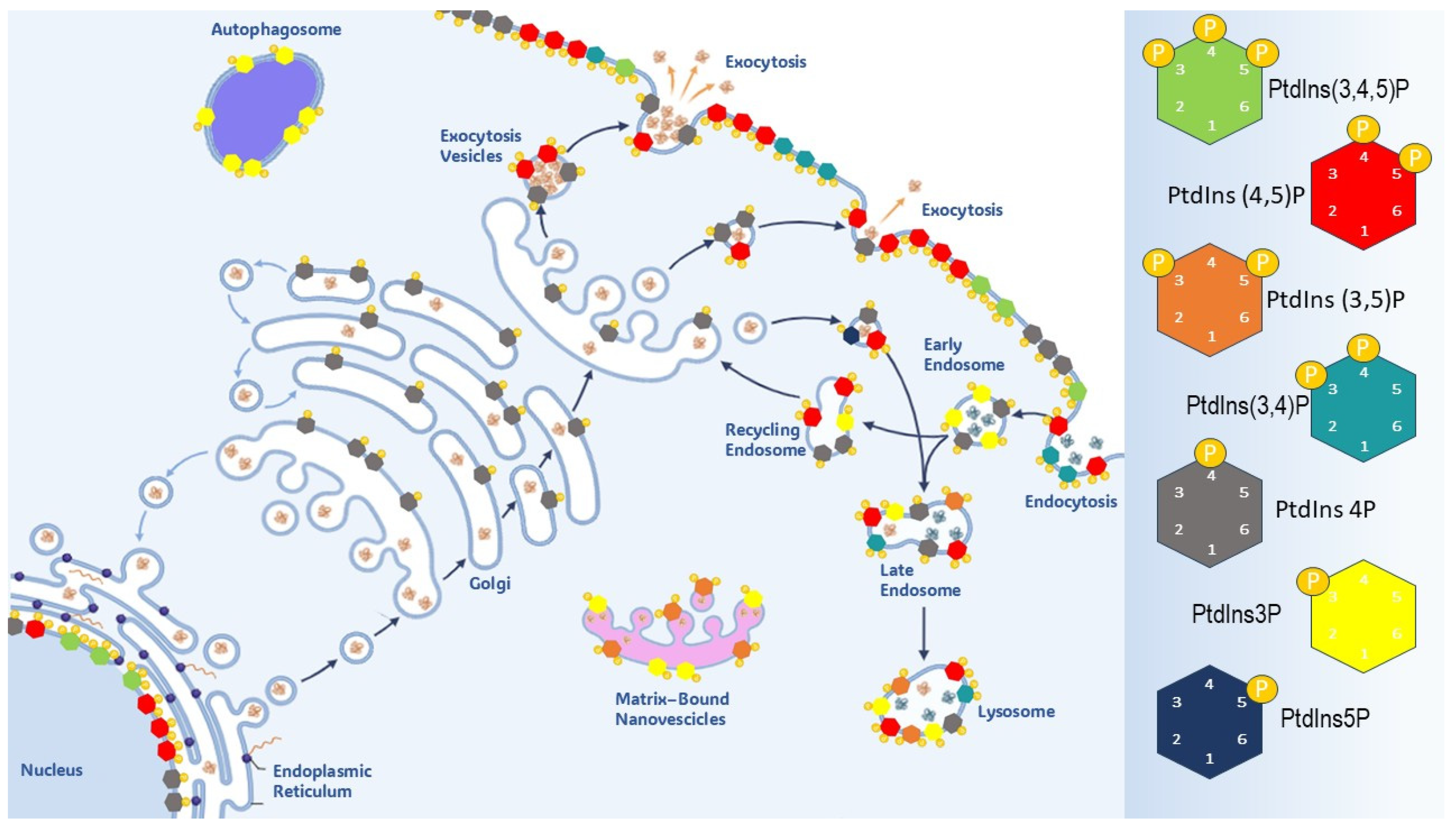
3.2. Intracellular Localization
3.3. The Pivotal Role of PI3K
4. Phosphoinositides: The Cytoskeleton, Ion Channels, and Mechanotransduction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shewan, A.; Eastburn, D.J.; Mostov, K. Phosphoinositides in Cell Architecture. Cold Spring Harb. Perspect. Biol. 2011, 3, a004796. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sengupta, N.; Sohn, M.; Mandal, A.; Pemberton, J.G.; Choi, U.; Balla, T. Metabolic routing maintains the unique fatty acid composition of phosphoinositides. EMBO Rep. 2022, 23, e54532. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Hipps, P.P.; Holland, W.H.; Sherman, W.R. Interconversion of myo- and scyllo-inositol with simultaneous formation of neo-inositol by an NADP+ dependent epimerase from bovine brain. Biochem. Biophys. Res. Commun. 1977, 77, 340–346. [Google Scholar] [CrossRef]
- Sun, T.; Heimark, D.B.; Nguygen, T.; Nadler, J.L.; Larner, J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem. Biophys. Res. Commun. 2002, 293, 1092–1098. [Google Scholar] [CrossRef]
- Su, X.B.; Ko, A.A.; Saiardi, A. Regulations of myo-inositol homeostasis: Mechanisms, implications, and perspectives. Adv. Biol. Regul. 2023, 87, 100921. [Google Scholar] [CrossRef] [PubMed]
- York, J.D. Regulation of nuclear processes by inositol polyphosphates. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2006, 1761, 552–559. [Google Scholar] [CrossRef]
- Shears, S.B. Diphosphoinositol Polyphosphates: Metabolic Messengers? Mol. Pharmacol. 2009, 76, 236–252. [Google Scholar] [CrossRef]
- Michell, R.H. Inositol derivatives: Evolution and functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 151–161. [Google Scholar] [CrossRef]
- Roberts, M.F. Inositol in Bacteria and Archaea. In Biology of Inositols and Phosphoinositides; Springer: New York, NY, USA, 2006; pp. 103–133. [Google Scholar] [CrossRef]
- Deranieh, R.M.; Greenberg, M.L. Cellular consequences of inositol depletion. Biochem. Soc. Trans. 2009, 37, 1099–1103. [Google Scholar] [CrossRef]
- Arora, M.; Giuliani, A.; Curtin, P. Biodynamic Interfaces Are Essential for Human–Environment Interactions. BioEssays 2020, 42, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Martino, A.; Giuliani, A.; Todde, V.; Bizzarri, M.; Rizzi, A. Metabolic networks classification and knowledge discovery by information granulation. Comput. Biol. Chem. 2020, 84, 107187. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Palombo, A.; Cucina, A. Theoretical aspects of Systems Biology. Prog. Biophys. Mol. Biol. 2013, 112, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A. General System Theory: Essential Concepts & Applications; CRC Press: Boca Raton, FL, USA, 1986; Volume 10. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Wilson, J., Hunt, T., Eds.; W.W. Norton & Company: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Küchler, A.; Yoshimoto, M.; Luginbühl, S.; Mavelli, F.; Walde, P. Enzymatic reactions in confined environments. Nat. Nanotechnol. 2016, 11, 409–420. [Google Scholar] [CrossRef]
- Leonard, T.A.; Loose, M.; Martens, S. The membrane surface as a platform that organizes cellular and biochemical processes. Dev. Cell 2023, 58, 1315–1332. [Google Scholar] [CrossRef]
- Marat, A.L.; Haucke, V. Phosphatidylinositol 3-phosphates—At the interface between cell signalling and membrane traffic. EMBO J. 2016, 35, 561–579. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny Lipids with Giant Impact on Cell Regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef]
- Hokin, M.R.; Hokin, L.E. Enzyme Secretion and the Incorporation of P32 into Phospholipides of Pancreas Slices. J. Biol. Chem. 1953, 203, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Shears, S.B. The versatility of inositol phosphates as cellular signals. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 1998, 1436, 49–67. [Google Scholar] [CrossRef]
- Kim, S.; Bhandari, R.; Brearley, C.A.; Saiardi, A. The inositol phosphate signalling network in physiology and disease. Trends Biochem. Sci. 2024, 49, 969–985. [Google Scholar] [CrossRef]
- Wilson, M.S.C.; Livermore, T.M.; Saiardi, A. Inositol pyrophosphates: Between signalling and metabolism. Biochem. J. 2013, 452, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, T.; Itoh, T.; Fukami, K. Regulation of phosphatidylinositol 4,5-bisphosphate levels and its roles in cytoskeletal re-organization and malignant transformation. Chem. Phys. Lipids 1999, 98, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.L.; Aregullin, M.A.; Jesch, S.A.; Henry, S.A. Inositol Induces a Profound Alteration in the Pattern and Rate of Synthesis and Turnover of Membrane Lipids in Saccharomyces cerevisiae. J. Biol. Chem. 2006, 281, 22773–22785. [Google Scholar] [CrossRef] [PubMed]
- Minini, M.; Senni, A.; He, X.; Proietti, S.; Liguoro, D.; Catizone, A.; Giuliani, A.; Mancini, R.; Fuso, A.; Cucina, A.; et al. miR-125a-5p impairs the metastatic potential in breast cancer via IP6K1 targeting. Cancer Lett. 2021, 520, 48–56. [Google Scholar] [CrossRef]
- Czech, M.P. PIP2 and PIP3. Cell 2000, 100, 603–606. [Google Scholar] [CrossRef]
- Tóth, D.J.; Tóth, J.T.; Damouni, A.; Hunyady, L.; Várnai, P. Effect of hormone-induced plasma membrane phosphatidylinositol 4,5-bisphosphate depletion on receptor endocytosis suggests the importance of local regulation in phosphoinositide signaling. Sci. Rep. 2024, 14, 291. [Google Scholar] [CrossRef]
- Lanier, L.M.; Gertler, F.B. Actin cytoskeleton: Thinking globally, actin’ locally. Curr. Biol. 2000, 10, R655–R657. [Google Scholar] [CrossRef]
- Prever, L.; Squillero, G.; Hirsch, E.; Gulluni, F. Linking phosphoinositide function to mitosis. Cell Rep. 2024, 43, 114273. [Google Scholar] [CrossRef]
- Hille, B.; Dickson, E.J.; Kruse, M.; Vivas, O.; Suh, B.-C. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2015, 1851, 844–856. [Google Scholar] [CrossRef]
- Rusinova, R.; Hobart, E.A.; Koeppe, R.E.; Andersen, O.S. Phosphoinositides alter lipid bilayer properties. J. Gen. Physiol. 2013, 141, 673–690. [Google Scholar] [CrossRef]
- Suh, B.-C.; Hille, B. PIP2Is a Necessary Cofactor for Ion Channel Function: How and Why? Annu. Rev. Biophys. 2008, 37, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Senju, Y.; Lappalainen, P. Regulation of actin dynamics by PI(4,5)P2 in cell migration and endocytosis. Curr. Opin. Cell Biol. 2019, 56, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gross, C. Defective phosphoinositide metabolism in autism. J. Neurosci. Res. 2016, 95, 1161–1173. [Google Scholar] [CrossRef]
- Tariq, K.; Luikart, B.W. Striking a balance: PIP2 and PIP3 signaling in neuronal health and disease. Explor. Neuroprot. Ther. 2021, 1, 86. [Google Scholar] [CrossRef] [PubMed]
- Reversi, A.; Loeser, E.; Subramanian, D.; Schultz, C.; De Renzis, S. Plasma membrane phosphoinositide balance regulates cell shape during Drosophila embryo morphogenesis. J. Cell Biol. 2014, 205, 395–408. [Google Scholar] [CrossRef]
- Clarke, R.J.; Hossain, K.R.; Cao, K. Physiological roles of transverse lipid asymmetry of animal membranes. Biochim. Biophys. Acta BBA—Biomembr. 2020, 1862, 183382. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Sheetz, M.P. When PIP2 Meets p53: Nuclear Phosphoinositide Signaling in the DNA Damage Response. Front. Cell Dev. Biol. 2022, 10, 903994. [Google Scholar] [CrossRef]
- Divecha, N.; Letcher, A.J.; Banfic, H.H.; Rhee, S.G.; Irvine, R.F. Changes in the components of a nuclear inositide cycle during differentiation in murine erythroleukaemia cells. Biochem. J. 1995, 312, 63–67. [Google Scholar] [CrossRef]
- Choi, S.; Chen, M.; Cryns, V.L.; Anderson, R.A. A nuclear phosphoinositide kinase complex regulates p53. Nat. Cell Biol. 2019, 21, 462–475. [Google Scholar] [CrossRef]
- Berridge, M.J. The Inositol Trisphosphate/Calcium Signaling Pathway in Health and Disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef]
- Putta, P.; Rankenberg, J.; Korver, R.A.; van Wijk, R.; Munnik, T.; Testerink, C.; Kooijman, E.E. Phosphatidic acid binding proteins display differential binding as a function of membrane curvature stress and chemical properties. Biochim. Biophys. Acta BBA—Biomembr. 2016, 1858, 2709–2716. [Google Scholar] [CrossRef]
- Claing, A. Endocytosis of G protein-coupled receptors: Roles of G protein-coupled receptor kinases and ß-arrestin proteins. Prog. Neurobiol. 2002, 66, 61–79. [Google Scholar] [CrossRef]
- Tahirovic, S.; Bradke, F. Neuronal Polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a001644. [Google Scholar] [CrossRef]
- Gassama-Diagne, A.; Yu, W.; ter Beest, M.; Martin-Belmonte, F.; Kierbel, A.; Engel, J.; Mostov, K. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 2006, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Richards, D.A. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol. Open 2012, 1, 857–862. [Google Scholar] [CrossRef]
- Goebbels, S.; Oltrogge, J.H.; Kemper, R.; Heilmann, I.; Bormuth, I.; Wolfer, S.; Wichert, S.P.; Mobius, W.; Liu, X.; Lappe-Siefke, C.; et al. Elevated Phosphatidylinositol 3,4,5-Trisphosphate in Glia Triggers Cell-Autonomous Membrane Wrapping and Myelination. J. Neurosci. 2010, 30, 8953–8964. [Google Scholar] [CrossRef]
- Li, Z.; Venable, R.M.; Rogers, L.A.; Murray, D.; Pastor, R.W. Molecular Dynamics Simulations of PIP2 and PIP3 in Lipid Bilayers: Determination of Ring Orientation, and the Effects of Surface Roughness on a Poisson-Boltzmann Description. Biophys. J. 2009, 97, 155–163. [Google Scholar] [CrossRef]
- Tan, X.; Thapa, N.; Choi, S.; Anderson, R.A. Emerging roles of PtdIns(4,5)P2—Beyond the plasma membrane. J. Cell Sci. 2015, 128, 4047–4056. [Google Scholar] [CrossRef]
- Wada, T.; Sasaoka, T.; Funaki, M.; Hori, H.; Murakami, S.; Ishiki, M.; Haruta, T.; Asano, T.; Ogawa, W.; Ishihara, H.; et al. Overexpression of SH2-Containing Inositol Phosphatase 2 Results in Negative Regulation of Insulin-Induced Metabolic Actions in 3T3-L1 Adipocytes via Its 5′-Phosphatase Catalytic Activity. Mol. Cell. Biol. 2001, 21, 1633–1646. [Google Scholar] [CrossRef]
- Clément, S.; Krause, U.; Desmedt, F.; Tanti, J.-F.; Behrends, J.; Pesesse, X.; Sasaki, T.; Penninger, J.; Doherty, M.; Malaisse, W.; et al. The lipid phosphatase SHIP2 controls insulin sensitivity. Nature 2001, 409, 92–97. [Google Scholar] [CrossRef]
- Langille, S.E.; Patki, V.; Klarlund, J.K.; Buxton, J.M.; Holik, J.J.; Chawla, A.; Corvera, S.; Czech, M.P. ADP-ribosylation Factor 6 as a Target of Guanine Nucleotide Exchange Factor GRP1. J. Biol. Chem. 1999, 274, 27099–27104. [Google Scholar] [CrossRef] [PubMed]
- Siddhanta, U.; McIlroy, J.; Shah, A.; Zhang, Y.; Backer, J.M. Distinct Roles for the p110α and hVPS34 Phosphatidylinositol 3′-Kinases in Vesicular Trafficking, Regulation of the Actin Cytoskeleton, and Mitogenesis. J. Cell Biol. 1998, 143, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Saarikangas, J.; Zhao, H.; Lappalainen, P. Regulation of the Actin Cytoskeleton-Plasma Membrane Interplay by Phosphoinositides. Physiol. Rev. 2010, 90, 259–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Herzmark, P.; Weiner, O.D.; Srinivasan, S.; Servant, G.; Bourne, H.R. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 2002, 4, 513–518. [Google Scholar] [CrossRef]
- Chen, Y.; Thelin, W.R.; Yang, B.; Milgram, S.L.; Jacobson, K. Transient anchorage of cross-linked glycosyl-phosphatidylinositol–anchored proteins depends on cholesterol, Src family kinases, caveolin, and phosphoinositides. J. Cell Biol. 2006, 175, 169–178. [Google Scholar] [CrossRef]
- Bretscher, M.S.; Aguado-Velasco, C. EGF induces recycling membrane to form ruffles. Curr. Biol. 1998, 8, 721-S1–724-S4. [Google Scholar] [CrossRef]
- Dowler, S.; Currie, R.A.; Campbell, D.G.; Deak, M.; Kular, G.; Downes, C.P.; Alessi, D.R. Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 2000, 351, 19–31. [Google Scholar] [CrossRef]
- Lawlor, M.A.; Alessi, D.R. PKB/Akt. J. Cell Sci. 2001, 114, 2903–2910. [Google Scholar] [CrossRef]
- Lang, F.; Cohen, P. Regulation and Physiological Roles of Serum- and Glucocorticoid-Induced Protein Kinase Isoforms. Sci. STKE 2001, 2001, re17. [Google Scholar] [CrossRef]
- Tall, E.G.; Spector, I.; Pentyala, S.N.; Bitter, I.; Rebecchi, M.J. Dynamics of phosphatidylinositol 4,5-bisphosphate in actin-rich structures. Curr. Biol. 2000, 10, 743–746. [Google Scholar] [CrossRef]
- Ekblad, L. Localization of phosphatidylinositol 4-kinase isoenzymes in rat liver plasma membrane domains. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2001, 1531, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.A.; Kular, G.; Fleming, I.N.; Downes, C.P.; Lucocq, J.M. Subcellular localization of phosphatidylinositol 4,5-bisphosphate using the pleckstrin homology domain of phospholipase C δ1. Biochem. J. 2002, 363, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Toker, A. The synthesis and cellular roles of phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Cell Biol. 1998, 10, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Nogami, M.; Yokozeki, T.; Yamazaki, M.; Nakamura, H.; Watanabe, H.; Kawamoto, K.; Nakayama, K.; Morris, A.J.; Frohman, M.A.; et al. Phosphatidylinositol 4-Phosphate 5-Kinase α Is a Downstream Effector of the Small G Protein ARF6 in Membrane Ruffle Formation. Cell 1999, 99, 521–532. [Google Scholar] [CrossRef]
- Ikeda, Y.; Lala, D.S.; Luo, X.; Kim, E.; Moisan, M.P.; Parker, K.L. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol. Endocrinol. 1993, 7, 852–860. [Google Scholar] [CrossRef][Green Version]
- Blind, R.D.; Suzawa, M.; Ingraham, H.A. Direct Modification and Activation of a Nuclear Receptor–PIP2 Complex by the Inositol Lipid Kinase IPMK. Sci. Signal. 2012, 5, ra44. [Google Scholar] [CrossRef]
- Vidalle, M.C.; Sheth, B.; Fazio, A.; Marvi, M.V.; Leto, S.; Koufi, F.-D.; Neri, I.; Casalin, I.; Ramazzotti, G.; Follo, M.Y.; et al. Nuclear Phosphoinositides as Key Determinants of Nuclear Functions. Biomolecules 2023, 13, 1049. [Google Scholar] [CrossRef]
- Czech, M.P. Dynamics of Phosphoinositides in Membrane Retrieval and Insertion. Annu. Rev. Physiol. 2003, 65, 791–815. [Google Scholar] [CrossRef]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Overduin, M.; Kervin, T.A. The phosphoinositide code is read by a plethora of protein domains. Expert Rev. Proteom. 2021, 18, 483–502. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K. Class II phosphatidylinositol 3-kinase isoforms in vesicular trafficking. Biochem. Soc. Trans. 2021, 49, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E.; Williams, R.L. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 2015, 40, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Foukas, L.C.; Claret, M.; Pearce, W.; Okkenhaug, K.; Meek, S.; Peskett, E.; Sancho, S.; Smith, A.J.H.; Withers, D.J.; Vanhaesebroeck, B. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature 2006, 441, 366–370. [Google Scholar] [CrossRef]
- Okkenhaug, K. Signaling by the Phosphoinositide 3-Kinase Family in Immune Cells. Annu. Rev. Immunol. 2013, 31, 675–704. [Google Scholar] [CrossRef]
- Samuels, Y.; Waldman, T. Oncogenic Mutations of PIK3CA in Human Cancers. In Phosphoinositide 3-Kinase in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2010; pp. 21–41. [Google Scholar] [CrossRef]
- Laketa, V.; Zarbakhsh, S.; Traynor-Kaplan, A.; MacNamara, A.; Subramanian, D.; Putyrski, M.; Mueller, R.; Nadler, A.; Mentel, M.; Saez-Rodriguez, J.; et al. PIP3 Induces the Recycling of Receptor Tyrosine Kinases. Sci. Signal. 2014, 7, ra5. [Google Scholar] [CrossRef]
- Posor, Y.; Eichhorn-Gruenig, M.; Puchkov, D.; Schöneberg, J.; Ullrich, A.; Lampe, A.; Müller, R.; Zarbakhsh, S.; Gulluni, F.; Hirsch, E.; et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 2013, 499, 233–237. [Google Scholar] [CrossRef]
- Sopasakis, V.R.; Liu, P.; Suzuki, R.; Kondo, T.; Winnay, J.; Tran, T.T.; Asano, T.; Smyth, G.; Sajan, M.P.; Farese, R.V.; et al. Specific Roles of the p110α Isoform of Phosphatidylinsositol 3-Kinase in Hepatic Insulin Signaling and Metabolic Regulation. Cell Metab. 2010, 11, 220–230. [Google Scholar] [CrossRef]
- Guillermet-Guibert, J.; Bjorklof, K.; Salpekar, A.; Gonella, C.; Ramadani, F.; Bilancio, A.; Meek, S.; Smith, A.J.H.; Okkenhaug, K.; Vanhaesebroeck, B. The p110β isoform of phosphoinositide 3-kinase signals downstream of G protein-coupled receptors and is functionally redundant with p110γ. Proc. Natl. Acad. Sci. USA 2008, 105, 8292–8297. [Google Scholar] [CrossRef]
- Yin, H.L.; Janmey, P.A. Phosphoinositide Regulation of the Actin Cytoskeleton. Annu. Rev. Physiol. 2003, 65, 761–789. [Google Scholar] [CrossRef]
- Lemmon, M.A. Phosphoinositide Recognition Domains. Traffic 2003, 4, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Kervin, T.A.; Overduin, M. Membranes are functionalized by a proteolipid code. BMC Biol. 2024, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Lillemeier, B.F.; Pfeiffer, J.R.; Surviladze, Z.; Wilson, B.S.; Davis, M.M. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc. Natl. Acad. Sci. USA 2006, 103, 18992–18997. [Google Scholar] [CrossRef]
- Hyvönen, M.; Macias, M.J.; Nilges, M.; Oschkinat, H.; Saraste, M.; Wilmanns, M. Structure of the binding site for inositol phosphates in a PH domain. EMBO J. 1995, 14, 4676–4685. [Google Scholar] [CrossRef]
- Prestwich, G.D. Phosphoinositide Signaling. Chem. Biol. 2004, 11, 619–637. [Google Scholar] [CrossRef]
- Posor, Y.; Jang, W.; Haucke, V. Phosphoinositides as membrane organizers. Nat. Rev. Mol. Cell Biol. 2022, 23, 797–816. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Ball, R. Regulation of Cardiac Na+,Ca2+ Exchange and K ATP Potassium Channels by PIP2. Science 1996, 273, 956–959. [Google Scholar] [CrossRef]
- Borreguero-Muñoz, N.; Fletcher, G.C.; Aguilar-Aragon, M.; Elbediwy, A.; Vincent-Mistiaen, Z.I.; Thompson, B.J. The Hippo pathway integrates PI3K–Akt signals with mechanical and polarity cues to control tissue growth. PLoS Biol. 2019, 17, e3000509. [Google Scholar] [CrossRef]
- Davis, M.J.; Wu, X.; Nurkiewicz, T.R.; Kawasaki, J.; Gui, P.; Hill, M.A.; Wilson, E. Regulation of Ion Channels by Integrins. Cell Biochem. Biophys. 2002, 36, 41–66. [Google Scholar] [CrossRef]
- Legate, K.R.; Takahashi, S.; Bonakdar, N.; Fabry, B.; Boettiger, D.; Zent, R.; Fässler, R. Integrin adhesion and force coupling are independently regulated by localized PtdIns(4,5)2 synthesis. EMBO J. 2011, 30, 4539–4553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McNamee, H.P.; Ingber, D.E.; Schwartz, M.A. Adhesion to fibronectin stimulates inositol li-pid synthesis and enhances PDGF-induced inositol lipid breakdown. J. Cell Biol. 1993, 121, 673–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsson, C. Protein kinase C and the regulation of the actin cytoskeleton. Cell. Signal. 2006, 18, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Niggli, V. Regulation of protein activities by phosphoinositide phosphates. Annu. Rev. Cell Dev. Biol. 2005, 21, 57–79. [Google Scholar] [CrossRef]
- Levina, A.; Fleming, K.D.; Burke, J.E.; Leonard, T.A. Activation of the essential kinase PDK1 by phosphoinositide-driven trans-autophosphorylation. Nat. Commun. 2022, 13, 1874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krajnik, A.; Brazzo, J.A.; Vaidyanathan, K.; Das, T.; Redondo-Muñoz, J.; Bae, Y. Phosphoinositide Signaling and Mechanotransduction in Cardiovascular Biology and Disease. Front. Cell Dev. Biol. 2020, 8, 595849. [Google Scholar] [CrossRef]
- Monti, N.; Dinicola, S.; Querqui, A.; Fabrizi, G.; Fedeli, V.; Gesualdi, L.; Catizone, A.; Unfer, V.; Bizzarri, M. Myo-Inositol Reverses TGF-β1-Induced EMT in MCF-10A Non-Tumorigenic Breast Cells. Cancers 2023, 15, 2317. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Feng, S.; Nasuhoglu, C. The Complex and Intriguing Lives of PIP2 with Ion Channels and Transporters. Sci. STKE 2001, 2001, re19. [Google Scholar] [CrossRef]
- Tong, Q.; Gamper, N.; Medina, J.L.; Shapiro, M.S.; Stockand, J.D. Direct Activation of the Epithelial Na+ Channel by Phosphatidylinositol 3,4,5-Trisphosphate and Phosphatidylinositol 3,4-Bisphosphate Produced by Phosphoinositide 3-OH Kinase. J. Biol. Chem. 2004, 279, 22654–22663. [Google Scholar] [CrossRef]
- Harraz, O.F.; Hill-Eubanks, D.; Nelson, M.T. PIP2: A critical regulator of vascular ion channels hiding in plain sight. Proc. Natl. Acad. Sci. USA 2020, 117, 20378–20389. [Google Scholar] [CrossRef]
- Tóth, B.I.; Konrad, M.; Ghosh, D.; Mohr, F.; Halaszovich, C.R.; Leitner, M.G.; Vriens, J.; Oberwinkler, J.; Voets, T. Regulation of the transient receptor potential channel TRPM3 by phosphoinositides. J. Gen. Physiol. 2015, 146, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2009, 1793, 933–940. [Google Scholar] [CrossRef]
- Senning, E.N.; Collins, M.D.; Stratiievska, A.; Ufret-Vincenty, C.A.; Gordon, S.E. Regulation of TRPV1 Ion Channel by Phosphoinositide (4,5)-Bisphosphate. J. Biol. Chem. 2014, 289, 10999–11006. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.; Hille, B. Regulation of KCNQ channels by manipulation of phosphoinositides. J. Physiol. 2007, 582, 911–916. [Google Scholar] [CrossRef]
- He, L.; Ahmad, M.; Perrimon, N. Mechanosensitive channels and their functions in stem cell differentiation. Exp. Cell Res. 2019, 374, 259–265. [Google Scholar] [CrossRef]
- Logothetis, D.E.; Petrou, V.I.; Zhang, M.; Mahajan, R.; Meng, X.-Y.; Adney, S.K.; Cui, M.; Baki, L. Phosphoinositide Control of Membrane Protein Function: A Frontier Led by Studies on Ion Channels. Annu. Rev. Physiol. 2015, 77, 81–104. [Google Scholar] [CrossRef]
- Suh, B.-C.; Hille, B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005, 15, 370–378. [Google Scholar] [CrossRef]
- Altamura, C.; Greco, M.R.; Carratù, M.R.; Cardone, R.A.; Desaphy, J.-F. Emerging Roles for Ion Channels in Ovarian Cancer: Pathomechanisms and Pharmacological Treatment. Cancers 2021, 13, 668. [Google Scholar] [CrossRef]
- Warren, P.B.; ten Wolde, P.R. Chemical Models of Genetic Toggle Switches. J. Phys. Chem. B 2005, 109, 6812–6823. [Google Scholar] [CrossRef]
- Xu, C.; Tobi, D.; Bahar, I. Allosteric Changes in Protein Structure Computed by a Simple Mechanical Model: Hemoglobin T↔R2 Transition. J. Mol. Biol. 2003, 333, 153–168. [Google Scholar] [CrossRef]
- Shin, J.; Ming, G.; Song, H. Molecular Toggle Switch of Histone Demethylase LSD1. Mol. Cell 2015, 57, 949–950. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lentini, G.; Querqui, A.; Giuliani, A.; Verna, R.; Bizzarri, M. Inositol and PIP2/PIP3 Ratio: At the Crossroad of the Biodynamic Interface Between Cells and Their Microenvironment. Biomolecules 2025, 15, 451. https://doi.org/10.3390/biom15030451
Lentini G, Querqui A, Giuliani A, Verna R, Bizzarri M. Inositol and PIP2/PIP3 Ratio: At the Crossroad of the Biodynamic Interface Between Cells and Their Microenvironment. Biomolecules. 2025; 15(3):451. https://doi.org/10.3390/biom15030451
Chicago/Turabian StyleLentini, Guglielmo, Alessandro Querqui, Alessandro Giuliani, Roberto Verna, and Mariano Bizzarri. 2025. "Inositol and PIP2/PIP3 Ratio: At the Crossroad of the Biodynamic Interface Between Cells and Their Microenvironment" Biomolecules 15, no. 3: 451. https://doi.org/10.3390/biom15030451
APA StyleLentini, G., Querqui, A., Giuliani, A., Verna, R., & Bizzarri, M. (2025). Inositol and PIP2/PIP3 Ratio: At the Crossroad of the Biodynamic Interface Between Cells and Their Microenvironment. Biomolecules, 15(3), 451. https://doi.org/10.3390/biom15030451








