Revolutionizing Implantation Studies: Uterine-Specific Models and Advanced Technologies
Abstract
1. Introduction
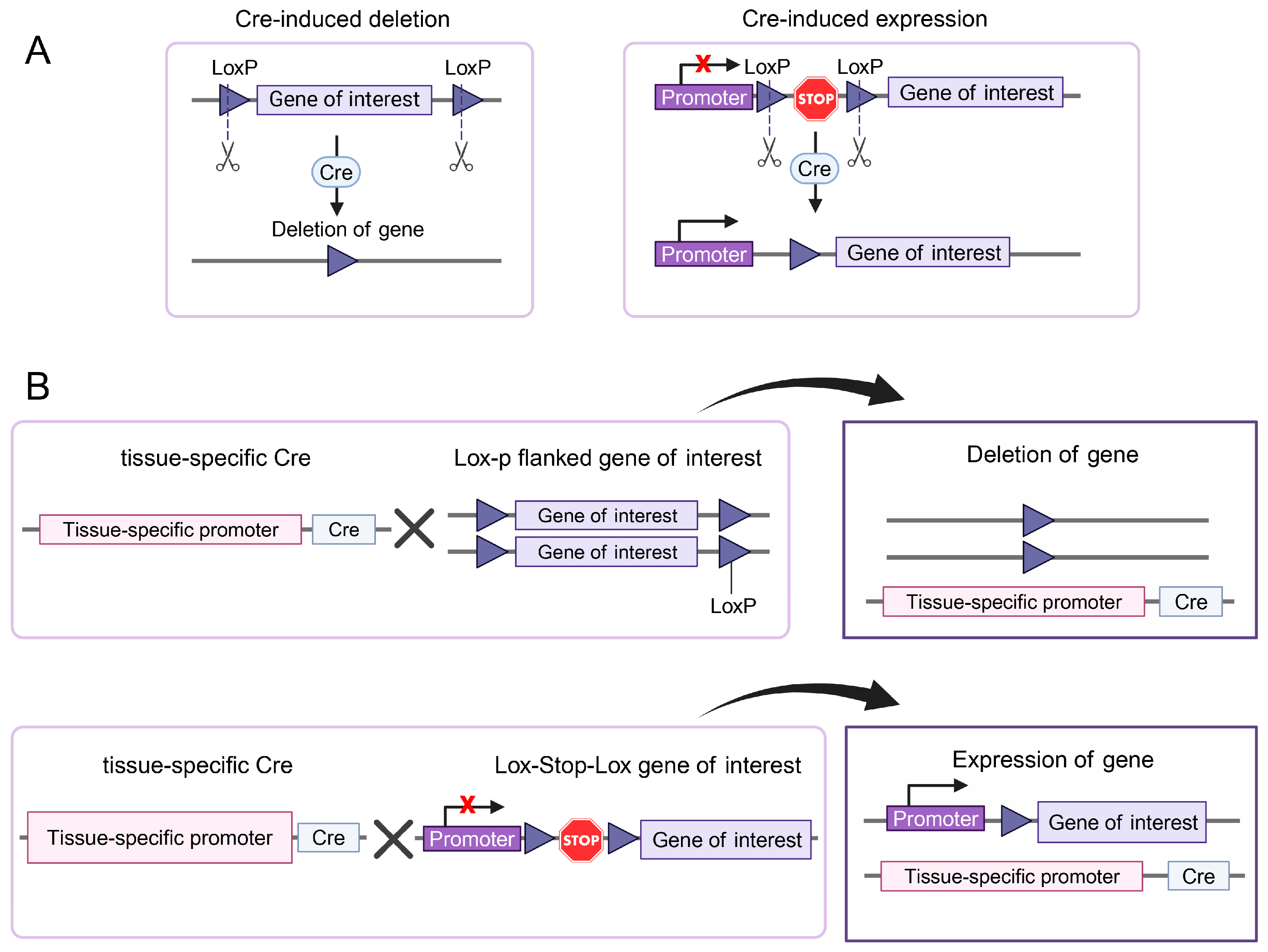
2. Genetically Engineered Mice
2.1. Tissue-Specific Gene Ablation
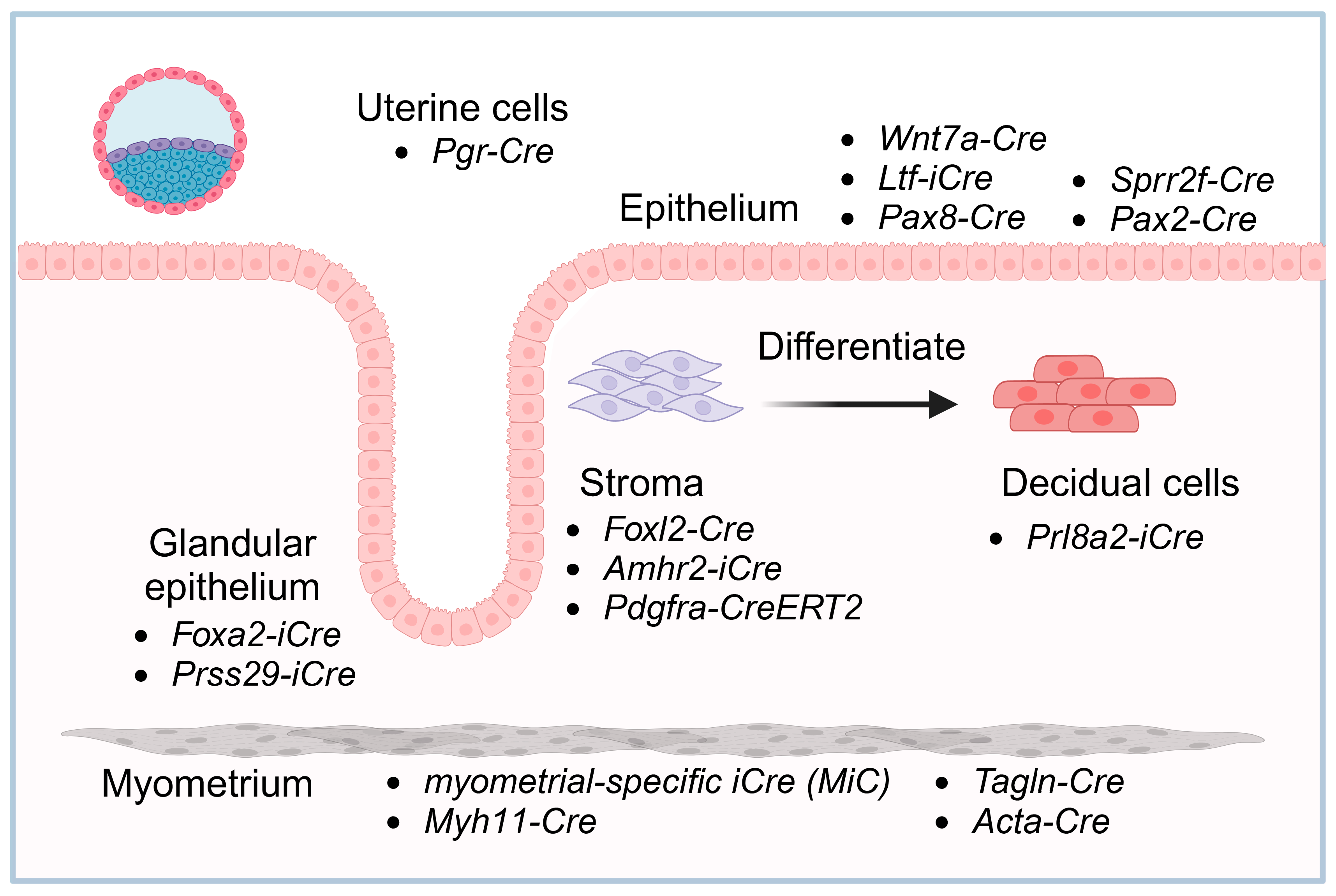
2.2. Uterine Cell-Specific Pgr-Cre Mouse Strain
2.3. Uterine Epithelium
2.3.1. Wnt7a-Cre
2.3.2. Ltf-iCre
2.3.3. Uterine Glandular Epithelium-Specific Strains
2.3.4. Other Epithelium-Specific Cre Lines
2.4. Stroma
2.5. Decidual Cells
2.6. Myometrium
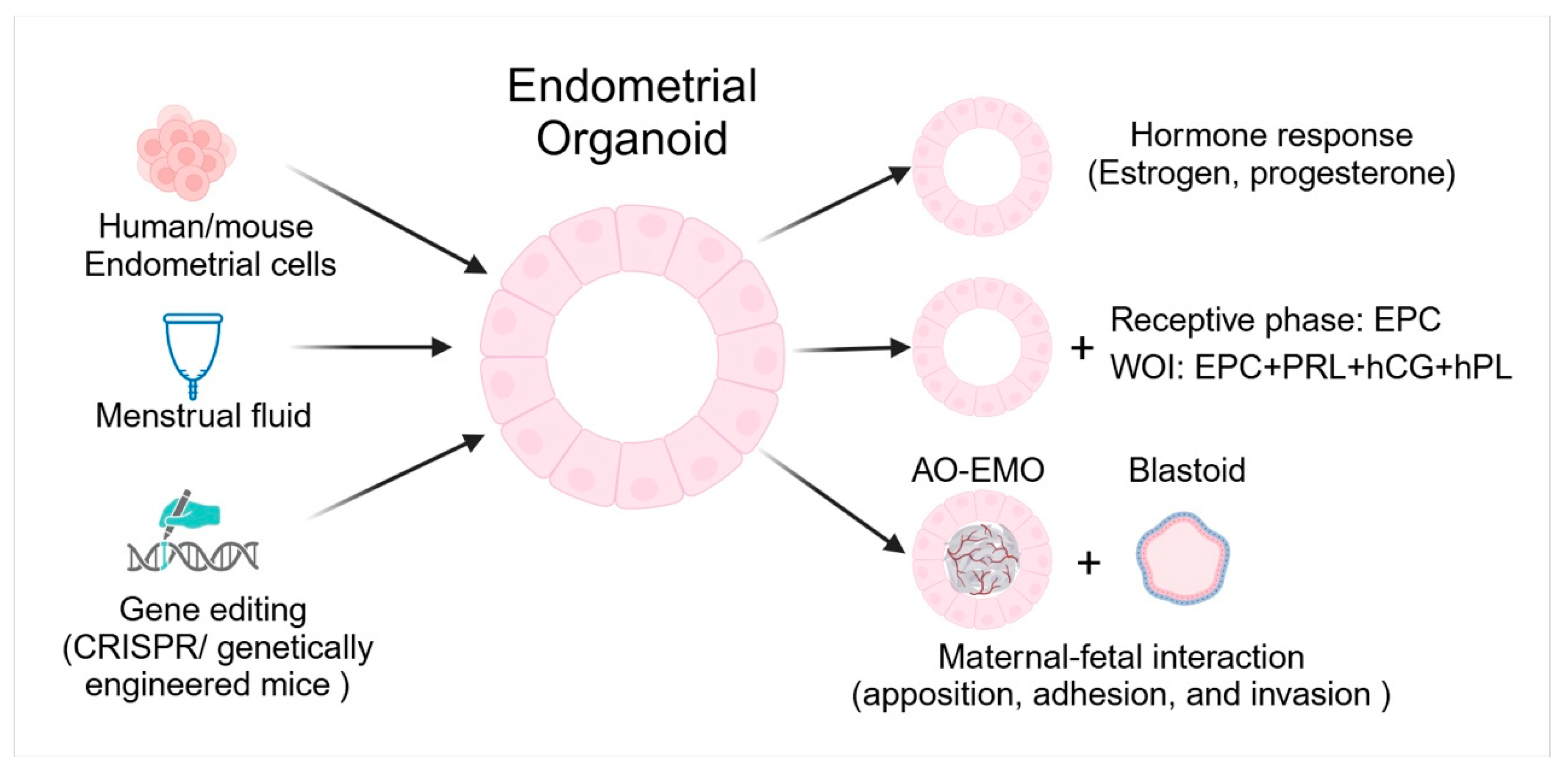
3. Endometrial Organoids
3.1. Respond to Hormones
3.2. Implantation
3.3. Maternal-Fetal Interactions
3.4. Genetic Manipulation
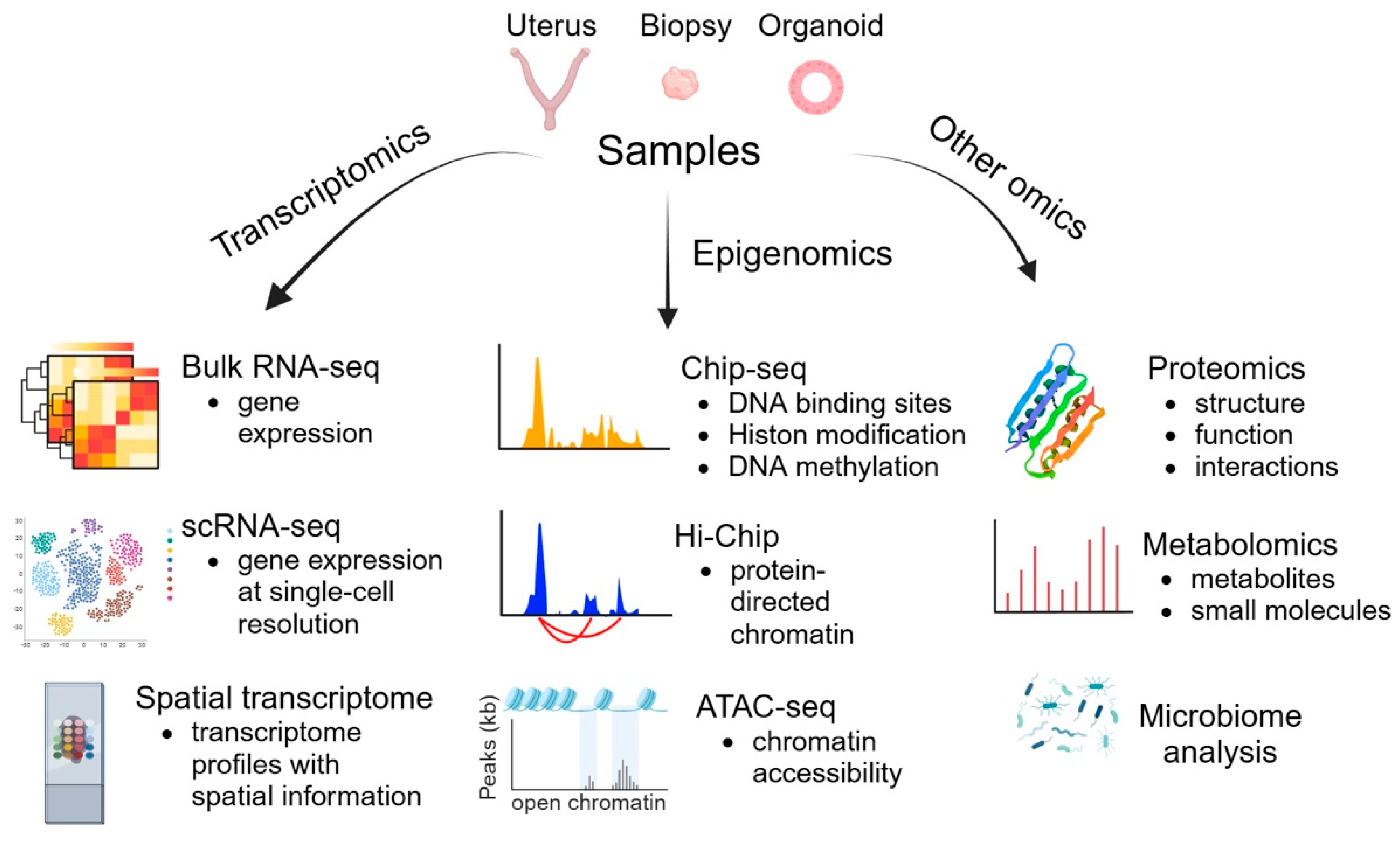
4. Omics Technologies
4.1. Transcriptomics
4.1.1. scRNA-seq
4.1.2. Spatial Transcriptomics
4.2. Epigenomics
4.3. Other Omics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular Signaling Regulating Endometrium-Blastocyst Crosstalk. Int. J. Mol. Sci. 2019, 21, 23. [Google Scholar] [CrossRef]
- Blanco-Breindel, M.F.; Singh, M.; Kahn, J. Endometrial Receptivity. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Akaeda, S.; Aikawa, S.; Hirota, Y. Spatial and molecular anatomy of the endometrium during embryo implantation: A current overview of key regulators of blastocyst invasion. FEBS J. 2024, 291, 4206–4221. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.G.; Song, H.; Das, S.K.; Paria, B.C.; Dey, S.K. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc. Natl. Acad. Sci. USA 2003, 100, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A.Z.; Karli, P.; Gulumser, C. Does high estrogen level negatively affect pregnancy success in frozen embryo transfer? Arch. Med. Sci. 2022, 18, 647–651. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Ghaffari, H.O.; Marmazi, F.; Keikhah, N. Overexpression of Endometrial Estrogen Receptor-Alpha in The Window of Implantation in Women with Unexplained Infertility. Int. J. Fertil. Steril. 2018, 12, 37–42. [Google Scholar] [CrossRef]
- Gebril, M.; Hirota, Y.; Aikawa, S.; Fukui, Y.; Kaku, T.; Matsuo, M.; Hirata, T.; Akaeda, S.; Hiraoka, T.; Shimizu-Hirota, R.; et al. Uterine Epithelial Progesterone Receptor Governs Uterine Receptivity Through Epithelial Cell Differentiation. Endocrinology 2020, 161, bqaa195. [Google Scholar] [CrossRef] [PubMed]
- Hantak, A.M.; Bagchi, I.C.; Bagchi, M.K. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 2014, 58, 139–146. [Google Scholar] [CrossRef]
- Ng, S.W.; Norwitz, G.A.; Pavlicev, M.; Tilburgs, T.; Simon, C.; Norwitz, E.R. Endometrial Decidualization: The Primary Driver of Pregnancy Health. Int. J. Mol. Sci. 2020, 21, 4092. [Google Scholar] [CrossRef]
- Okada, H.; Tsuzuki, T.; Murata, H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018, 17, 220–227. [Google Scholar] [CrossRef]
- Ramathal, C.Y.; Bagchi, I.C.; Taylor, R.N.; Bagchi, M.K. Endometrial decidualization: Of mice and men. Semin. Reprod. Med. 2010, 28, 17–26. [Google Scholar] [CrossRef]
- Ticconi, C.; Di Simone, N.; Campagnolo, L.; Fazleabas, A. Clinical consequences of defective decidualization. Tissue Cell 2021, 72, 101586. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, T.M.; Makwana, K.; Tryfonos, M.; Lucas, E.S. Organoids to model the endometrium: Implantation and beyond. Reprod. Fertil. 2021, 2, R85–R101. [Google Scholar] [CrossRef] [PubMed]
- Aplin, J.D.; Stevens, A. Use of ’omics for endometrial timing: The cycle moves on. Hum. Reprod. 2022, 37, 644–650. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Namiki, T.; Ito, J.; Kashiwazaki, N. Molecular mechanisms of embryonic implantation in mammals: Lessons from the gene manipulation of mice. Reprod. Med. Biol. 2018, 17, 331–342. [Google Scholar] [CrossRef]
- Chemerinski, A.; Liu, C.; Morelli, S.S.; Babwah, A.V.; Douglas, N.C. Mouse Cre drivers: Tools for studying disorders of the human female neuroendocrine-reproductive axisdagger. Biol. Reprod. 2022, 106, 835–853. [Google Scholar] [CrossRef] [PubMed]
- Benson, G.V.; Lim, H.; Paria, B.C.; Satokata, I.; Dey, S.K.; Maas, R.L. Mechanisms of reduced fertility in Hoxa-10 mutant mice: Uterine homeosis and loss of maternal Hoxa-10 expression. Development 1996, 122, 2687–2696. [Google Scholar] [CrossRef]
- Hsieh-Li, H.M.; Witte, D.P.; Weinstein, M.; Branford, W.; Li, H.; Small, K.; Potter, S.S. Hoxa 11 structure, extensive antisense transcription, and function in male and female fertility. Development 1995, 121, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Kaspar, P.; Brunet, L.J.; Bhatt, H.; Gadi, I.; Kontgen, F.; Abbondanzo, S.J. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 1992, 359, 76–79. [Google Scholar] [CrossRef]
- Bilinski, P.; Roopenian, D.; Gossler, A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998, 12, 2234–2243. [Google Scholar] [CrossRef]
- Robb, L.; Li, R.; Hartley, L.; Nandurkar, H.H.; Koentgen, F.; Begley, C.G. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 1998, 4, 303–308. [Google Scholar] [CrossRef]
- Lubahn, D.B.; Moyer, J.S.; Golding, T.S.; Couse, J.F.; Korach, K.S.; Smithies, O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc. Natl. Acad. Sci. USA 1993, 90, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Robker, R.L.; Hennebold, J.D.; Russell, D.L. Coordination of Ovulation and Oocyte Maturation: A Good Egg at the Right Time. Endocrinology 2018, 159, 3209–3218. [Google Scholar] [CrossRef]
- Takeda, K.; Noguchi, K.; Shi, W.; Tanaka, T.; Matsumoto, M.; Yoshida, N.; Kishimoto, T.; Akira, S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 1997, 94, 3801–3804. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; Lloyd, K.C.K. Generating mouse models for biomedical research: Technological advances. Dis. Model. Mech. 2019, 12, dmm029462. [Google Scholar] [CrossRef]
- Mareckova, M.; Garcia-Alonso, L.; Moullet, M.; Lorenzi, V.; Petryszak, R.; Sancho-Serra, C.; Oszlanczi, A.; Icoresi Mazzeo, C.; Wong, F.C.K.; Kelava, I.; et al. An integrated single-cell reference atlas of the human endometrium. Nat. Genet. 2024, 56, 1925–1937. [Google Scholar] [CrossRef]
- Winkler, I.; Tolkachov, A.; Lammers, F.; Lacour, P.; Daugelaite, K.; Schneider, N.; Koch, M.L.; Panten, J.; Grunschlager, F.; Poth, T.; et al. The cycling and aging mouse female reproductive tract at single-cell resolution. Cell 2024, 187, 981–998 e925. [Google Scholar] [CrossRef] [PubMed]
- Branda, C.S.; Dymecki, S.M. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev. Cell 2004, 6, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar] [CrossRef]
- Mukherjee, A.; Soyal, S.M.; Wheeler, D.A.; Fernandez-Valdivia, R.; Nguyen, J.; DeMayo, F.J.; Lydon, J.P. Targeting iCre expression to murine progesterone receptor cell-lineages using bacterial artificial chromosome transgenesis. Genesis 2006, 44, 601–610. [Google Scholar] [CrossRef]
- Namiki, T.; Kamoshita, M.; Kageyama, A.; Terakawa, J.; Ito, J.; Kashiwazaki, N. Utility of progesterone receptor-ires-Cre to generate conditional knockout mice for uterine study. Anim. Sci. J. 2021, 92, e13615. [Google Scholar] [CrossRef]
- Winuthayanon, W.; Hewitt, S.C.; Orvis, G.D.; Behringer, R.R.; Korach, K.S. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc. Natl. Acad. Sci. USA 2010, 107, 19272–19277. [Google Scholar] [CrossRef]
- Daikoku, T.; Ogawa, Y.; Terakawa, J.; Ogawa, A.; DeFalco, T.; Dey, S.K. Lactoferrin-iCre: A new mouse line to study uterine epithelial gene function. Endocrinology 2014, 155, 2718–2724. [Google Scholar] [CrossRef]
- Uetzmann, L.; Burtscher, I.; Lickert, H. A mouse line expressing Foxa2-driven Cre recombinase in node, notochord, floorplate, and endoderm. Genesis 2008, 46, 515–522. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Allen, C.C.; Davis, D.J.; Spencer, T.E. Prss29 Cre recombinase mice are useful to study adult uterine gland function. Genesis 2022, 60, e23493. [Google Scholar] [CrossRef]
- Bouchard, M.; Souabni, A.; Busslinger, M. Tissue-specific expression of cre recombinase from the Pax8 locus. Genesis 2004, 38, 105–109. [Google Scholar] [CrossRef]
- Contreras, C.M.; Akbay, E.A.; Gallardo, T.D.; Haynie, J.M.; Sharma, S.; Tagao, O.; Bardeesy, N.; Takahashi, M.; Settleman, J.; Wong, K.K.; et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis. Model. Mech. 2010, 3, 181–193. [Google Scholar] [CrossRef]
- Jamin, S.P.; Arango, N.A.; Mishina, Y.; Hanks, M.C.; Behringer, R.R. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat. Genet. 2002, 32, 408–410. [Google Scholar] [CrossRef]
- Cen, C.; Chen, M.; Zhou, J.; Zhang, L.; Duo, S.; Jiang, L.; Hou, X.; Gao, F. Inactivation of Wt1 causes pre-granulosa cell to steroidogenic cell transformation and defect of ovary developmentdagger. Biol. Reprod. 2020, 103, 60–69. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Gorre, N.; Risal, S.; Shen, Y.; Liu, K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 2014, 23, 920–928. [Google Scholar] [CrossRef]
- Chung, M.I.; Bujnis, M.; Barkauskas, C.E.; Kobayashi, Y.; Hogan, B.L.M. Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation. Development 2018, 145, dev163014. [Google Scholar] [CrossRef]
- Dickson, M.J.; Oh, Y.; Gruzdev, A.; Li, R.; Balaguer, N.; Kelleher, A.M.; Spencer, T.E.; Wu, S.P.; DeMayo, F.J. Inserting Cre recombinase into the Prolactin 8a2 gene for decidua-specific recombination in mice. Genesis 2022, 60, e23473. [Google Scholar] [CrossRef]
- Holtwick, R.; Gotthardt, M.; Skryabin, B.; Steinmetz, M.; Potthast, R.; Zetsche, B.; Hammer, R.E.; Herz, J.; Kuhn, M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc. Natl. Acad. Sci. USA 2002, 99, 7142–7147. [Google Scholar] [CrossRef]
- Wendling, O.; Bornert, J.M.; Chambon, P.; Metzger, D. Efficient temporally-controlled targeted mutagenesis in smooth muscle cells of the adult mouse. Genesis 2009, 47, 14–18. [Google Scholar] [CrossRef]
- Xin, H.B.; Deng, K.Y.; Rishniw, M.; Ji, G.; Kotlikoff, M.I. Smooth muscle expression of Cre recombinase and eGFP in transgenic mice. Physiol. Genom. 2002, 10, 211–215. [Google Scholar] [CrossRef]
- Cloud, A.S.; Koohestani, F.; McWilliams, M.M.; Ganeshkumar, S.; Gunewardena, S.; Graham, A.; Nothnick, W.B.; Chennathukuzhi, V.M. Loss of the repressor REST affects progesterone receptor function and promotes uterine leiomyoma pathogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2205524119. [Google Scholar] [CrossRef]
- Ismail, P.M.; Li, J.; DeMayo, F.J.; O’Malley, B.W.; Lydon, J.P. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol. Endocrinol. 2002, 16, 2475–2489. [Google Scholar] [CrossRef]
- Medina-Laver, Y.; Rodriguez-Varela, C.; Salsano, S.; Labarta, E.; Dominguez, F. What Do We Know about Classical and Non-Classical Progesterone Receptors in the Human Female Reproductive Tract? A Review. Int. J. Mol. Sci. 2021, 22, 11278. [Google Scholar] [CrossRef]
- Sun, X.; Bartos, A.; Whitsett, J.A.; Dey, S.K. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Mol. Endocrinol. 2013, 27, 1492–1501. [Google Scholar] [CrossRef]
- Lee, K.; Jeong, J.; Kwak, I.; Yu, C.T.; Lanske, B.; Soegiarto, D.W.; Toftgard, R.; Tsai, M.J.; Tsai, S.; Lydon, J.P.; et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat. Genet. 2006, 38, 1204–1209. [Google Scholar] [CrossRef]
- Daikoku, T.; Cha, J.; Sun, X.; Tranguch, S.; Xie, H.; Fujita, T.; Hirota, Y.; Lydon, J.; DeMayo, F.; Maxson, R.; et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev. Cell 2011, 21, 1014–1025. [Google Scholar] [CrossRef]
- Franco, H.L.; Dai, D.; Lee, K.Y.; Rubel, C.A.; Roop, D.; Boerboom, D.; Jeong, J.W.; Lydon, J.P.; Bagchi, I.C.; Bagchi, M.K.; et al. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J. 2011, 25, 1176–1187. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.S.; Franco, H.L.; Broaddus, R.R.; Taketo, M.M.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 2009, 28, 31–40. [Google Scholar] [CrossRef]
- Yuan, J.; Cha, J.; Deng, W.; Bartos, A.; Sun, X.; Ho, H.H.; Borg, J.P.; Yamaguchi, T.P.; Yang, Y.; Dey, S.K. Planar cell polarity signaling in the uterus directs appropriate positioning of the crypt for embryo implantation. Proc. Natl. Acad. Sci. USA 2016, 113, E8079–E8088. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, D.K.; Franco, H.L.; Lydon, J.P.; Jeong, J.W. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol. Reprod. 2010, 82, 706–713. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, J.W.; Wang, J.; Ma, L.; Martin, J.F.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. Bmp2 is critical for the murine uterine decidual response. Mol. Cell. Biol. 2007, 27, 5468–5478. [Google Scholar] [CrossRef]
- Hawkins, S.M.; Andreu-Vieyra, C.V.; Kim, T.H.; Jeong, J.W.; Hodgson, M.C.; Chen, R.; Creighton, C.J.; Lydon, J.P.; Gunaratne, P.H.; DeMayo, F.J.; et al. Dysregulation of uterine signaling pathways in progesterone receptor-Cre knockout of dicer. Mol. Endocrinol. 2012, 26, 1552–1566. [Google Scholar] [CrossRef]
- Kurihara, I.; Lee, D.K.; Petit, F.G.; Jeong, J.; Lee, K.; Lydon, J.P.; DeMayo, F.J.; Tsai, M.J.; Tsai, S.Y. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007, 3, e102. [Google Scholar] [CrossRef]
- Sun, X.; Terakawa, J.; Clevers, H.; Barker, N.; Daikoku, T.; Dey, S.K. Ovarian LGR5 is critical for successful pregnancy. FASEB J. 2014, 28, 2380–2389. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Yoo, J.Y.; Teasley, H.E.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Arora, R.; Jeong, J.W. Endometrial epithelial ARID1A is critical for uterine gland function in early pregnancy establishment. FASEB J. 2021, 35, e21209. [Google Scholar] [CrossRef]
- Matsuo, M.; Yuan, J.; Kim, Y.S.; Dewar, A.; Fujita, H.; Dey, S.K.; Sun, X. Targeted depletion of uterine glandular Foxa2 induces embryonic diapause in mice. Elife 2022, 11, e78277. [Google Scholar] [CrossRef]
- Parr, B.A.; McMahon, A.P. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 1998, 395, 707–710. [Google Scholar] [CrossRef]
- Dunlap, K.A.; Filant, J.; Hayashi, K.; Rucker, E.B., 3rd; Song, G.; Deng, J.M.; Behringer, R.R.; DeMayo, F.J.; Lydon, J.; Jeong, J.W.; et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol. Reprod. 2011, 85, 386–396. [Google Scholar] [CrossRef]
- Stewart, C.A.; Wang, Y.; Bonilla-Claudio, M.; Martin, J.F.; Gonzalez, G.; Taketo, M.M.; Behringer, R.R. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Mullerian duct and postnatal uterine development. Mol. Endocrinol. 2013, 27, 1442–1454. [Google Scholar] [CrossRef]
- Chi, R.A.; Xu, X.; Li, J.L.; Xu, X.; Hu, G.; Brown, P.; Willson, C.; Kirsanov, O.; Geyer, C.; Huang, C.L.; et al. WNK1 is required during male pachynema to sustain fertility. iScience 2023, 26, 107616. [Google Scholar] [CrossRef]
- Cheng, J.; Rosario, G.; Cohen, T.V.; Hu, J.; Stewart, C.L. Tissue-Specific Ablation of the LIF Receptor in the Murine Uterine Epithelium Results in Implantation Failure. Endocrinology 2017, 158, 1916–1928. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Peng, W.; Pru, J.K.; Pru, C.A.; DeMayo, F.J.; Spencer, T.E. Forkhead box a2 (FOXA2) is essential for uterine function and fertility. Proc. Natl. Acad. Sci. USA 2017, 114, E1018–E1026. [Google Scholar] [CrossRef]
- Yuan, J.; Deng, W.; Cha, J.; Sun, X.; Borg, J.P.; Dey, S.K. Tridimensional visualization reveals direct communication between the embryo and glands critical for implantation. Nat. Commun. 2018, 9, 603. [Google Scholar] [CrossRef]
- Cha, J.; Dey, S.K. Hunting for Fox(A2): Dual roles in female fertility. Proc. Natl. Acad. Sci. USA 2017, 114, 1226–1228. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Milano-Foster, J.; Behura, S.K.; Spencer, T.E. Uterine glands coordinate on-time embryo implantation and impact endometrial decidualization for pregnancy success. Nat. Commun. 2018, 9, 2435. [Google Scholar] [CrossRef]
- Rizo, J.A.; Davenport, K.M.; Winuthayanon, W.; Spencer, T.E.; Kelleher, A.M. Estrogen receptor alpha regulates uterine epithelial lineage specification and homeostasis. iScience 2023, 26, 107568. [Google Scholar] [CrossRef]
- Fu, D.J.; De Micheli, A.J.; Bidarimath, M.; Ellenson, L.H.; Cosgrove, B.D.; Flesken-Nikitin, A.; Nikitin, A.Y. Cells expressing PAX8 are the main source of homeostatic regeneration of adult mouse endometrial epithelium and give rise to serous endometrial carcinoma. Dis. Model. Mech. 2020, 13, dmm047035. [Google Scholar] [CrossRef]
- Suryo Rahmanto, Y.; Shen, W.; Shi, X.; Chen, X.; Yu, Y.; Yu, Z.C.; Miyamoto, T.; Lee, M.H.; Singh, V.; Asaka, R.; et al. Inactivation of Arid1a in the endometrium is associated with endometrioid tumorigenesis through transcriptional reprogramming. Nat. Commun. 2020, 11, 2717. [Google Scholar] [CrossRef]
- Russo, A.; Czarnecki, A.A.; Dean, M.; Modi, D.A.; Lantvit, D.D.; Hardy, L.; Baligod, S.; Davis, D.A.; Wei, J.J.; Burdette, J.E. PTEN loss in the fallopian tube induces hyperplasia and ovarian tumor formation. Oncogene 2018, 37, 1976–1990. [Google Scholar] [CrossRef]
- Granger, K.; Fitch, S.; Shen, M.; Lloyd, J.; Bhurke, A.; Hancock, J.; Ye, X.; Arora, R. Murine uterine gland branching is necessary for gland function in implantation. Mol. Hum. Reprod. 2024, 30, gaae020. [Google Scholar] [CrossRef]
- Mullen, R.D.; Ontiveros, A.E.; Moses, M.M.; Behringer, R.R. AMH and AMHR2 mutations: A spectrum of reproductive phenotypes across vertebrate species. Dev. Biol. 2019, 455, 1–9. [Google Scholar] [CrossRef]
- Huang, C.C.; Orvis, G.D.; Wang, Y.; Behringer, R.R. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS ONE 2012, 7, e44285. [Google Scholar] [CrossRef]
- McCallum, M.L.; Pru, C.A.; Niikura, Y.; Yee, S.P.; Lydon, J.P.; Peluso, J.J.; Pru, J.K. Conditional Ablation of Progesterone Receptor Membrane Component 1 Results in Subfertility in the Female and Development of Endometrial Cysts. Endocrinology 2016, 157, 3309–3319. [Google Scholar] [CrossRef]
- Robker, R.L.; Watson, L.N.; Robertson, S.A.; Dunning, K.R.; McLaughlin, E.A.; Russell, D.L. Identification of sites of STAT3 action in the female reproductive tract through conditional gene deletion. PLoS ONE 2014, 9, e101182. [Google Scholar] [CrossRef]
- Ghosh, A.; Syed, S.M.; Kumar, M.; Carpenter, T.J.; Teixeira, J.M.; Houairia, N.; Negi, S.; Tanwar, P.S. In Vivo Cell Fate Tracing Provides No Evidence for Mesenchymal to Epithelial Transition in Adult Fallopian Tube and Uterus. Cell Rep. 2020, 31, 107631. [Google Scholar] [CrossRef]
- Dickson, M.J.; Gruzdev, A.; DeMayo, F.J. iCre recombinase expressed in the anti-Mullerian hormone receptor 2 gene causes global genetic modification in the mousedagger. Biol. Reprod. 2023, 108, 575–583. [Google Scholar] [CrossRef]
- Bellessort, B.; Bachelot, A.; Heude, E.; Alfama, G.; Fontaine, A.; Le Cardinal, M.; Treier, M.; Levi, G. Role of Foxl2 in uterine maturation and function. Hum. Mol. Genet. 2015, 24, 3092–3103. [Google Scholar] [CrossRef]
- Zhang, H.; Risal, S.; Gorre, N.; Busayavalasa, K.; Li, X.; Shen, Y.; Bosbach, B.; Brannstrom, M.; Liu, K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr. Biol. 2014, 24, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Yu, G.; Feng, I.; Dean, J. Chromatin remodeling of prostaglandin signaling in smooth muscle enables mouse embryo passage through the female reproductive tract. Dev. Cell 2023, 58, 1716–1732 e1718. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Mustea, A.; Tempfer, C. Tamoxifen and Endometrial Cancer: A Janus-Headed Drug. Cancers 2020, 12, 2535. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.E.; Kaplan, J.R.; Fontenot, M.B.; Williams, J.K.; Cline, J.M. Endometrial profile of tamoxifen and low-dose estradiol combination therapy. Clin. Cancer Res. 2010, 16, 946–956. [Google Scholar] [CrossRef]
- Peri, L.E.; Koh, B.H.; Ward, G.K.; Bayguinov, Y.; Hwang, S.J.; Gould, T.W.; Mullan, C.J.; Sanders, K.M.; Ward, S.M. A novel class of interstitial cells in the mouse and monkey female reproductive tracts. Biol. Reprod. 2015, 92, 102. [Google Scholar] [CrossRef]
- Kirkwood, P.M.; Gibson, D.A.; Shaw, I.; Dobie, R.; Kelepouri, O.; Henderson, N.C.; Saunders, P.T.K. Single-cell RNA sequencing and lineage tracing confirm mesenchyme to epithelial transformation (MET) contributes to repair of the endometrium at menstruation. Elife 2022, 11, e77663. [Google Scholar] [CrossRef]
- Wattez, J.S.; Qiao, L.; Lee, S.; Natale, D.R.C.; Shao, J. The platelet-derived growth factor receptor alpha promoter-directed expression of cre recombinase in mouse placenta. Dev. Dyn. 2019, 248, 363–374. [Google Scholar] [CrossRef]
- Herring, B.P.; Hoggatt, A.M.; Burlak, C.; Offermanns, S. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc. Cell 2014, 6, 21. [Google Scholar] [CrossRef]
- Wu, S.P.; Wang, T.; Yao, Z.C.; Peavey, M.C.; Li, X.; Zhou, L.; Larina, I.V.; DeMayo, F.J. Myometrial progesterone receptor determines a transcription program for uterine remodeling and contractions during pregnancy. PNAS Nexus 2022, 1, pgac155. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Cindrova-Davies, T.; Zhao, X.; Elder, K.; Jones, C.J.P.; Moffett, A.; Burton, G.J.; Turco, M.Y. Menstrual flow as a non-invasive source of endometrial organoids. Commun. Biol. 2021, 4, 651. [Google Scholar] [CrossRef] [PubMed]
- Filby, C.E.; Wyatt, K.A.; Mortlock, S.; Cousins, F.L.; McKinnon, B.; Tyson, K.E.; Montgomery, G.W.; Gargett, C.E. Comparison of Organoids from Menstrual Fluid and Hormone-Treated Endometrium: Novel Tools for Gynecological Research. J. Pers. Med. 2021, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell. Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef]
- Zhou, W.; Barton, S.; Cui, J.; Santos, L.L.; Yang, G.; Stern, C.; Kieu, V.; Teh, W.T.; Ang, C.; Lucky, T.; et al. Infertile human endometrial organoid apical protein secretions are dysregulated and impair trophoblast progenitor cell adhesion. Front. Endocrinol. 2022, 13, 1067648. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, R.; Yang, C.; Song, J.; Liu, P.; Li, Y.; Liu, B.; Li, T.; Yin, C.; Lu, M.; et al. Human Receptive Endometrial Organoid for Deciphering the Implantation Window; eLife Sciences Publications, Ltd.: Cambridge, UK, 2024. [Google Scholar] [CrossRef]
- Kagawa, H.; Javali, A.; Khoei, H.H.; Sommer, T.M.; Sestini, G.; Novatchkova, M.; Scholte Op Reimer, Y.; Castel, G.; Bruneau, A.; Maenhoudt, N.; et al. Human blastoids model blastocyst development and implantation. Nature 2022, 601, 600–605. [Google Scholar] [CrossRef]
- Corujo-Simon, E.; Bates, L.E.; Yanagida, A.; Jones, K.; Clark, S.; von Meyenn, F.; Reik, W.; Nichols, J. Human trophectoderm becomes multi-layered by internalization at the polar region. Dev. Cell 2024, 59, 2497–2505 e2494. [Google Scholar] [CrossRef]
- Ahmad, V.; Yeddula, S.G.R.; Telugu, B.; Spencer, T.E.; Kelleher, A.M. Development of Polarity-Reversed Endometrial Epithelial Organoids. Reproduction 2024, 167, e230478. [Google Scholar] [CrossRef]
- Kleinova, M.; Varga, I.; Cehakova, M.; Valent, M.; Klein, M. Exploring the black box of human reproduction: Endometrial organoids and assembloids-generation, implantation modeling, and future clinical perspectives. Front. Cell. Dev. Biol. 2024, 12, 1482054. [Google Scholar] [CrossRef]
- Shibata, S.; Endo, S.; Nagai, L.A.E.; Kobayashi, E.H.; Oike, A.; Kobayashi, N.; Kitamura, A.; Hori, T.; Nashimoto, Y.; Nakato, R.; et al. Modeling embryo-endometrial interface recapitulating human embryo implantation. Sci. Adv. 2024, 10, eadi4819. [Google Scholar] [CrossRef] [PubMed]
- Menche, C.; Farin, H.F. Strategies for genetic manipulation of adult stem cell-derived organoids. Exp. Mol. Med. 2021, 53, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Syed, S.M.; Kumar, M.; Ghosh, A.; Tomasetig, F.; Ali, A.; Whan, R.M.; Alterman, D.; Tanwar, P.S. Endometrial Axin2(+) Cells Drive Epithelial Homeostasis, Regeneration, and Cancer following Oncogenic Transformation. Cell Stem Cell 2020, 26, 64–80 e13. [Google Scholar] [CrossRef]
- Vitorino, R. Transforming Clinical Research: The Power of High-Throughput Omics Integration. Proteomes 2024, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qin, H.; Yang, Y.; Chen, X.; Zhang, J.; Laird, S.; Wang, C.C.; Chan, T.F.; Li, T.C. A comparison of transcriptomic profiles in endometrium during window of implantation between women with unexplained recurrent implantation failure and recurrent miscarriage. Reproduction 2017, 153, 749–758. [Google Scholar] [CrossRef]
- Aljubran, F.; Nothnick, W.B. Long non-coding RNAs in endometrial physiology and pathophysiology. Mol. Cell. Endocrinol. 2021, 525, 111190. [Google Scholar] [CrossRef]
- Wang, C.; Lin, Y.; Li, S.; Guan, J. Deconvolution from bulk gene expression by leveraging sample-wise and gene-wise similarities and single-cell RNA-Seq data. BMC Genom. 2024, 25, 875. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef]
- Walker, E.R.; McGrane, M.; Aplin, J.D.; Brison, D.R.; Ruane, P.T. A systematic review of transcriptomic studies of the human endometrium reveals inconsistently reported differentially expressed genes. Reprod. Fertil. 2023, 4, e220115. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Gu, Z.; Wu, J.; Jia, S.; Shi, J.; Dai, Y.; Wu, Y.; Yan, H.; Zhang, J.; et al. Single-cell and spatial transcriptomic profiling revealed niche interactions sustaining growth of endometriotic lesions. Cell Genom. 2025, 5, 100737. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.Z.; Wang, Y.; Zhou, W.J.; Liang, Z.; Shi, J.W.; Yang, H.L.; Xie, F.; Chen, W.D.; Zhu, R.; Zhang, C.; et al. Single-cell transcriptome profiling of the human endometrium of patients with recurrent implantation failure. Theranostics 2022, 12, 6527–6547. [Google Scholar] [CrossRef] [PubMed]
- Krjutskov, K.; Katayama, S.; Saare, M.; Vera-Rodriguez, M.; Lubenets, D.; Samuel, K.; Laisk-Podar, T.; Teder, H.; Einarsdottir, E.; Salumets, A.; et al. Single-cell transcriptome analysis of endometrial tissue. Hum. Reprod. 2016, 31, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Bunis, D.G.; Wang, W.; Vallve-Juanico, J.; Houshdaran, S.; Sen, S.; Ben Soltane, I.; Kosti, I.; Vo, K.C.; Irwin, J.C.; Giudice, L.C.; et al. Whole-Tissue Deconvolution and scRNAseq Analysis Identify Altered Endometrial Cellular Compositions and Functionality Associated With Endometriosis. Front. Immunol. 2021, 12, 788315. [Google Scholar] [CrossRef]
- Koot, Y.E.; Teklenburg, G.; Salker, M.S.; Brosens, J.J.; Macklon, N.S. Molecular aspects of implantation failure. Biochim. Biophys. Acta 2012, 1822, 1943–1950. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Q.Y.; Liu, J.L. Deciphering mouse uterine receptivity for embryo implantation at single-cell resolution. Cell Prolif. 2021, 54, e13128. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.P.; Liu, J.L. Cell-Cell Communication at the Embryo Implantation Site of Mouse Uterus Revealed by Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 5177. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, D.; Yeung, W.S.B.; Lee, K.F. Single-Cell RNA-Sequencing Reveals Interactions between Endometrial Stromal Cells, Epithelial Cells, and Lymphocytes during Mouse Embryo Implantation. Int. J. Mol. Sci. 2022, 24, 213. [Google Scholar] [CrossRef]
- Cable, D.M.; Murray, E.; Zou, L.S.; Goeva, A.; Macosko, E.Z.; Chen, F.; Irizarry, R.A. Robust decomposition of cell type mixtures in spatial transcriptomics. Nat. Biotechnol. 2022, 40, 517–526. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Y.; Cheng, Y.; Wang, J.; Zhang, B.; Zhai, Y.; Zhu, K.; Liu, Y.; Shang, Y.; Xiao, X.; et al. Time-series single-cell transcriptomic profiling of luteal-phase endometrium uncovers dynamic characteristics and its dysregulation in recurrent implantation failures. Nat. Commun. 2025, 16, 137. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, T.Y.; Xu, X.; Emery, O.M.; Yi, M.; Wu, S.P.; DeMayo, F.J. Spatial transcriptomic profiles of mouse uterine microenvironments at pregnancy day 7.5dagger. Biol. Reprod. 2022, 107, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ong, J.; Meng, F.; Zhang, F.; Shen, H.; Kitt, K.; Liu, T.; Tao, W.; Du, P. Spatiotemporal insight into early pregnancy governed by immune-featured stromal cells. Cell 2023, 186, 4271–4288 e4224. [Google Scholar] [CrossRef] [PubMed]
- Retis-Resendiz, A.M.; Gonzalez-Garcia, I.N.; Leon-Juarez, M.; Camacho-Arroyo, I.; Cerbon, M.; Vazquez-Martinez, E.R. The role of epigenetic mechanisms in the regulation of gene expression in the cyclical endometrium. Clin. Epigenetics 2021, 13, 116. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Munro, S.K.; Farquhar, C.M.; Mitchell, M.D.; Ponnampalam, A.P. Epigenetic regulation of endometrium during the menstrual cycle. Mol. Hum. Reprod. 2010, 16, 297–310. [Google Scholar] [CrossRef]
- Monteiro, J.B.; Colon-Diaz, M.; Garcia, M.; Gutierrez, S.; Colon, M.; Seto, E.; Laboy, J.; Flores, I. Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod. Sci. 2014, 21, 305–318. [Google Scholar] [CrossRef]
- Samadieh, Y.; Favaedi, R.; Ramezanali, F.; Afsharian, P.; Aflatoonian, R.; Shahhoseini, M. Epigenetic Dynamics of HOXA10 Gene in Infertile Women With Endometriosis. Reprod. Sci. 2019, 26, 88–96. [Google Scholar] [CrossRef]
- Taylor, H.S.; Arici, A.; Olive, D.; Igarashi, P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Investig. 1998, 101, 1379–1384. [Google Scholar] [CrossRef]
- Skene, P.J.; Henikoff, S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife 2017, 6, e21856. [Google Scholar] [CrossRef]
- Mumbach, M.R.; Rubin, A.J.; Flynn, R.A.; Dai, C.; Khavari, P.A.; Greenleaf, W.J.; Chang, H.Y. HiChIP: Efficient and sensitive analysis of protein-directed genome architecture. Nat. Methods 2016, 13, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Mumbach, M.R.; Satpathy, A.T.; Boyle, E.A.; Dai, C.; Gowen, B.G.; Cho, S.W.; Nguyen, M.L.; Rubin, A.J.; Granja, J.M.; Kazane, K.R.; et al. Enhancer connectome in primary human cells identifies target genes of disease-associated DNA elements. Nat. Genet. 2017, 49, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Barral, A.; Dejardin, J. The chromatin signatures of enhancers and their dynamic regulation. Nucleus 2023, 14, 2160551. [Google Scholar] [CrossRef]
- Vrljicak, P.; Lucas, E.S.; Lansdowne, L.; Lucciola, R.; Muter, J.; Dyer, N.P.; Brosens, J.J.; Ott, S. Analysis of chromatin accessibility in decidualizing human endometrial stromal cells. FASEB J. 2018, 32, 2467–2477. [Google Scholar] [CrossRef]
- Vrljicak, P.; Lucas, E.S.; Tryfonos, M.; Muter, J.; Ott, S.; Brosens, J.J. Dynamic chromatin remodeling in cycling human endometrium at single-cell level. Cell. Rep. 2023, 42, 113525. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sarmiento, J.A.; Cosma, M.P.; Lakadamyali, M. Dissecting gene activation and chromatin remodeling dynamics in single human cells undergoing reprogramming. Cell. Rep. 2024, 43, 114170. [Google Scholar] [CrossRef]
- Ye, L.; Dimitriadis, E. Endometrial Receptivity-Lessons from “Omics”. Biomolecules 2025, 15, 106. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ma, J.; Diao, L.; Chen, J.; Cheng, Y.; Yang, J.; Li, L. Endometrial proteomic profile of patients with repeated implantation failure. Front. Endocrinol. 2023, 14, 1144393. [Google Scholar] [CrossRef]
- Guo, X.; Li, T.C.; Chen, X. The endometrial proteomic profile around the time of embryo implantationdagger. Biol. Reprod. 2021, 104, 11–26. [Google Scholar] [CrossRef]
- Molina, N.M.; Jurado-Fasoli, L.; Sola-Leyva, A.; Sevilla-Lorente, R.; Canha-Gouveia, A.; Ruiz-Duran, S.; Fontes, J.; Aguilera, C.M.; Altmae, S. Endometrial whole metabolome profile at the receptive phase: Influence of Mediterranean Diet and infertility. Front. Endocrinol. 2023, 14, 1120988. [Google Scholar] [CrossRef]
- Mancini, V.; Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A.; Picton, H.M.; Pensabene, V. Metabolomic Analysis Evidences That Uterine Epithelial Cells Enhance Blastocyst Development in a Microfluidic Device. Cells 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Reschini, M.; Benaglia, L.; Ceriotti, F.; Borroni, R.; Ferrari, S.; Castiglioni, M.; Guarneri, D.; Porcaro, L.; Vigano, P.; Somigliana, E.; et al. Endometrial microbiome: Sampling, assessment, and possible impact on embryo implantation. Sci. Rep. 2022, 12, 8467. [Google Scholar] [CrossRef]
- Lozano, F.M.; Lledo, B.; Morales, R.; Cascales, A.; Hortal, M.; Bernabeu, A.; Bernabeu, R. Characterization of the Endometrial Microbiome in Patients with Recurrent Implantation Failure. Microorganisms 2023, 11, 741. [Google Scholar] [CrossRef] [PubMed]
| Tissue | Strain | Modifications | Advantages | Considered |
|---|---|---|---|---|
| Uterine | Pgr-Cre [30] | Knock-in | Targets uterine Pgr-expressing cells; widely used for uterine studies. | Also active in ovaries, pituitary, and mammary gland; may cause unintended gene deletions; activity begins postnatally, complicating early development studies. |
| Pgr-BAC-iCre [31] | Transgenic | Enable to breeding of homozygous mice. | Similar off-target expression concerns as Pgr-Cre. | |
| Pgr-IRES-Cre [32] | Transgenic | Enable to breeding of homozygous mice. | Similar off-target expression concerns as Pgr-Cre. | |
| Epithelium | Wnt7a-Cre [33] | Transgenic | Targets uterine epithelium from early stages; used for Müllerian duct studies. | Off-target activity in oviduct epithelium, ovarian germ cells, and other tissues. |
| Ltf-iCre [34] | Knock-in | Restricts Cre activity to post-pubertal uterine epithelium; useful for adult uterine studies. | Expression is estrogen-dependent, complicating hormone-related studies. | |
| Foxa2-Cre [35] | Knock-in | Effective for studying glandular epithelium. | Off-target activity in multiple tissues (liver, pancreas, lung, etc.); limits uterine specificity. | |
| Prss29-Cre [36] | Knock-in | Targets uterine glandular epithelium exclusively after implantation. | Limited to glandular function post-implantation, restricting broader uterine studies. | |
| Pax8-Cre [37] | Knock-in | Targets Müllerian epithelium of the uterus and oviducts. | Potential off-target effects. | |
| Sprr2f-Cre [38] | Transgenic | Targets endometrial epithelium. | Cre activity detected in the cerebellum and kidney. | |
| Stroma | Amhr2-IRES-Cre(Bhr) [39] | Transgenic | Commonly used for reproductive tract studies; targets both ovarian and uterine stromal cells. | Variability in recombination efficiency; off-target effects in non-uterine tissues; early embryonic Cre activity causes global recombination. |
| Foxl2-Cre [40] | Knock-in | Useful for stroma-specific studies; allows early uterine gene deletion. | Active in ovary, which complicates uterine-specific studies. | |
| Foxl2-CreERT2 [41] | Knock-in | Temporal control of gene recombination; Enables precise timing for gene deletion in stroma. | Active in ovary; Tamoxifen can have estrogenic effects on the endometrium, potentially confounding results. | |
| Pdgfrα-CreERT2 [42] | Knock-in | Temporal control of gene recombination; high specificity for stromal cells. | Requires tamoxifen induction, which has estrogenic effects; not suitable for early-stage gene deletions. | |
| Decidua | Prl8a2-iCre [43] | Knock-in | Targets decidual cells exclusively; avoids effects on implantation and ovarian function. | Limited to decidual cell studies; may not be useful for genes involved before GD 4.5. |
| Myometrium | Tagln-Cre [44] | Transgenic | High specificity for myometrial cells; no activity in non-smooth muscle cells. | Not specific to uterine smooth muscle cells. |
| Acta2-CreERT2 [45] | Transgenic | Temporal control of gene recombination. | Cre is not active in all uterine smooth muscle cells are targeted. | |
| Myh11-Cre [46] | Transgenic | Targets myometrial cells and enables study of labor mechanisms. | Not specific to uterine smooth muscle cells. | |
| MiC (Myometrial-specific M-iCre) [47] | Knock-in | Highly specific for uterine myometrium; avoids non-reproductive off-target effects. | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-Y.; DeMayo, F.J. Revolutionizing Implantation Studies: Uterine-Specific Models and Advanced Technologies. Biomolecules 2025, 15, 450. https://doi.org/10.3390/biom15030450
Li S-Y, DeMayo FJ. Revolutionizing Implantation Studies: Uterine-Specific Models and Advanced Technologies. Biomolecules. 2025; 15(3):450. https://doi.org/10.3390/biom15030450
Chicago/Turabian StyleLi, Shu-Yun, and Francesco John DeMayo. 2025. "Revolutionizing Implantation Studies: Uterine-Specific Models and Advanced Technologies" Biomolecules 15, no. 3: 450. https://doi.org/10.3390/biom15030450
APA StyleLi, S.-Y., & DeMayo, F. J. (2025). Revolutionizing Implantation Studies: Uterine-Specific Models and Advanced Technologies. Biomolecules, 15(3), 450. https://doi.org/10.3390/biom15030450






