Structural and Metabolic Changes in Pregnant Rat Uterine and Adipose Tissue Induced by a High-Fat High-Sugar Diet
Abstract
1. Introduction
2. Materials and Methods
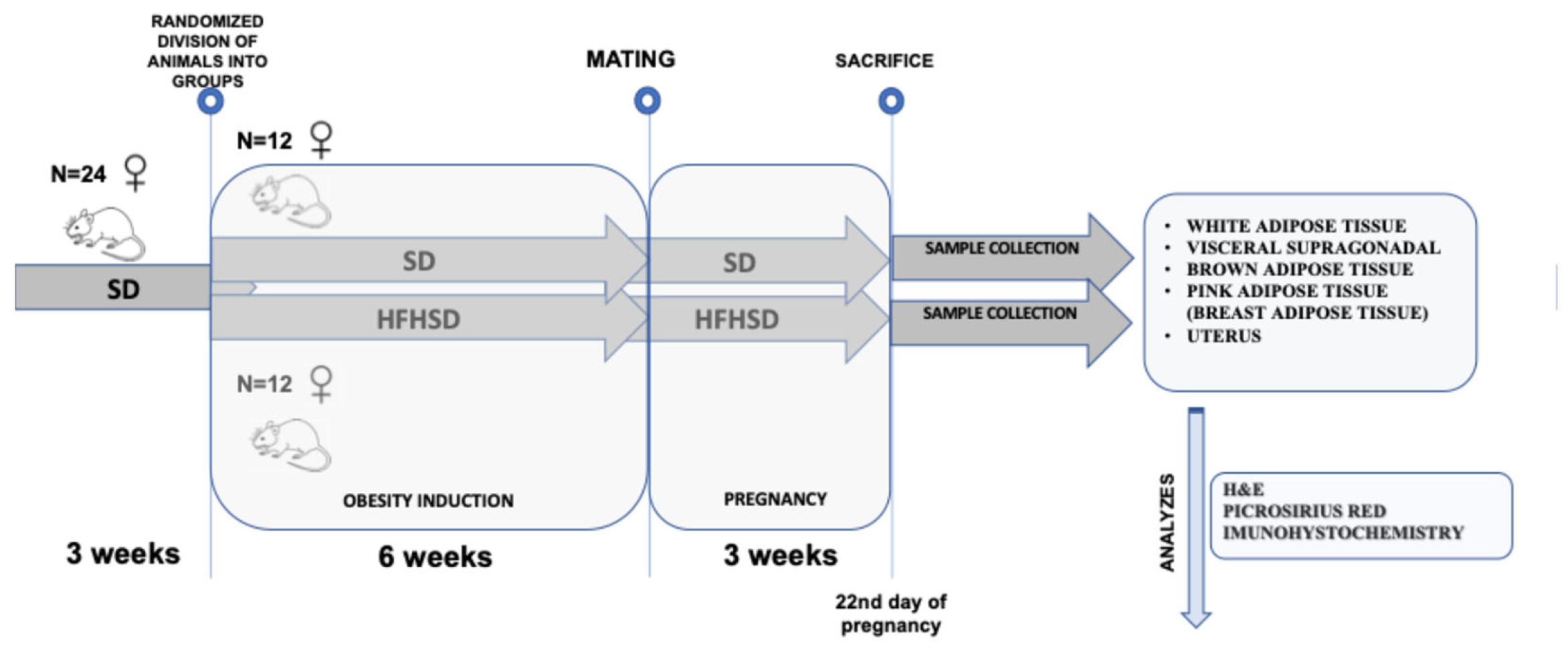
2.1. Animal Model and Study Design
2.2. Sample Preparation
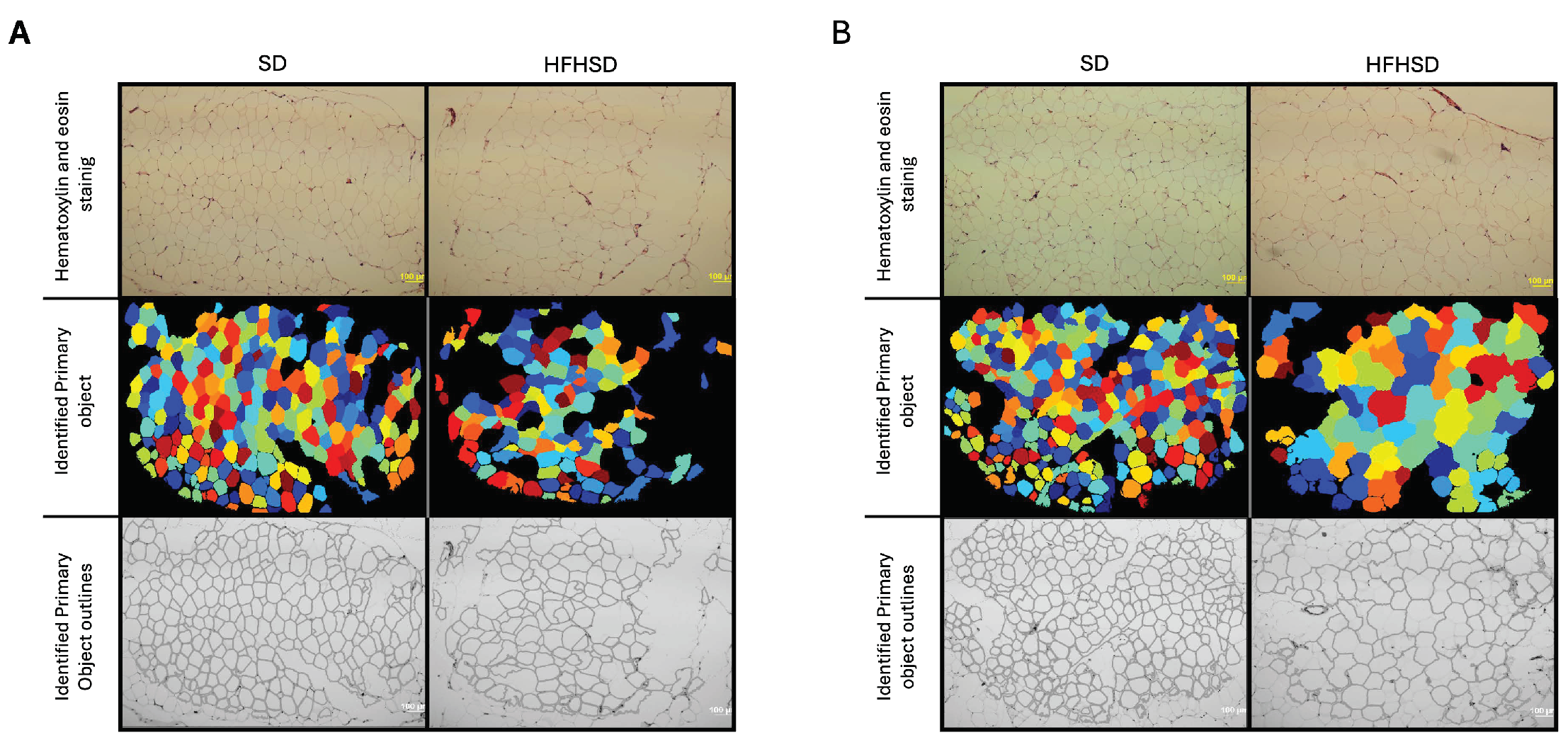
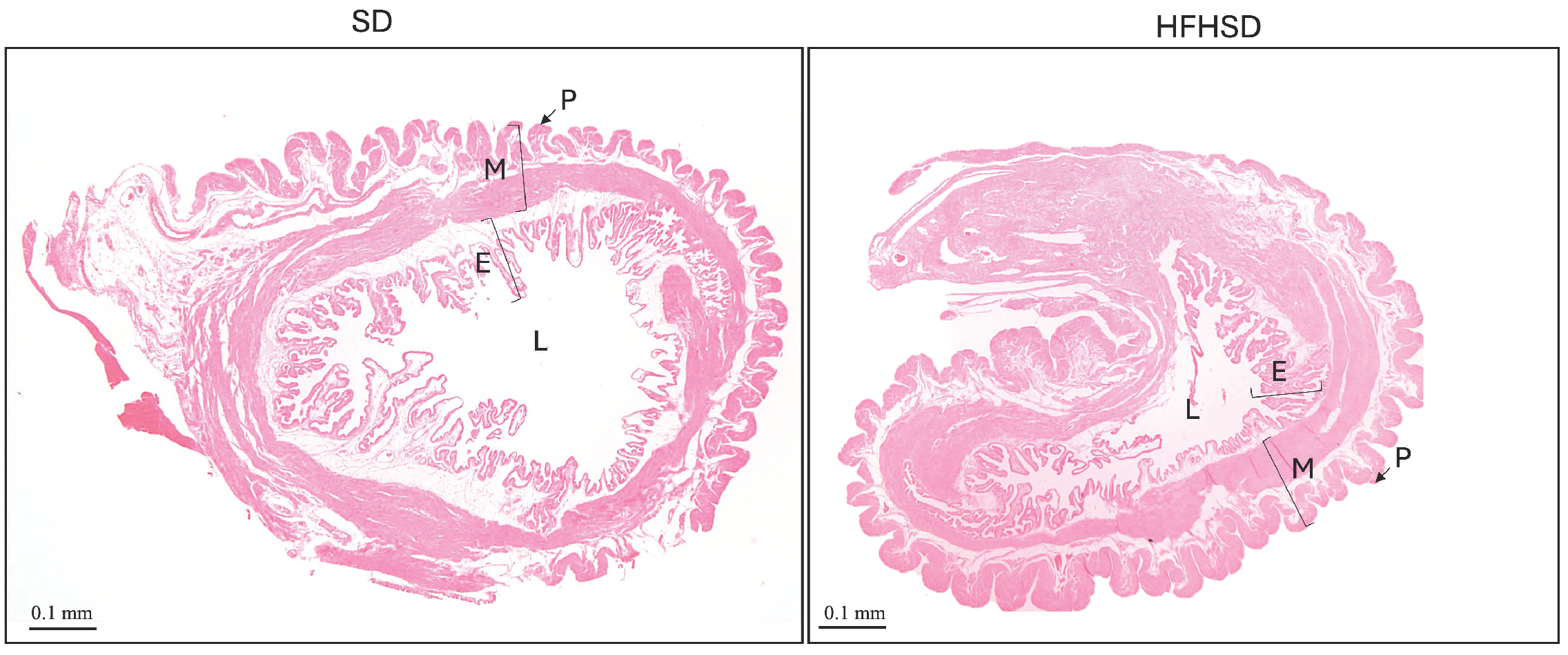
2.3. Hematoxylin and Eosin Staining of Adipose Tissue and Uterus
2.4. PicroSirius Red Staining of Uterus
2.5. Periodic Acid Schiff (PAS) Staining
2.6. Uterus Immunohistochemistry
2.7. Statistical Analysis
3. Results
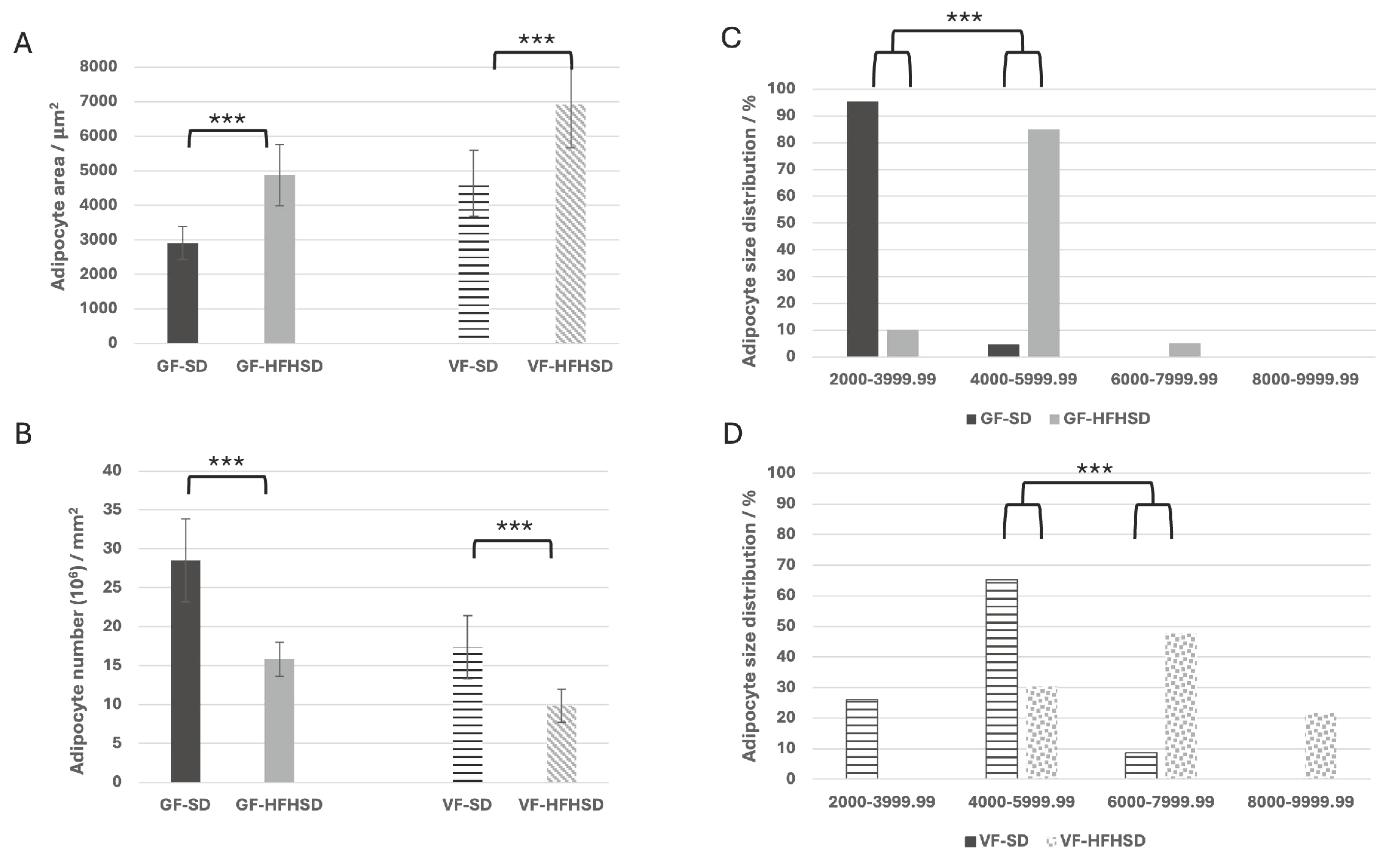
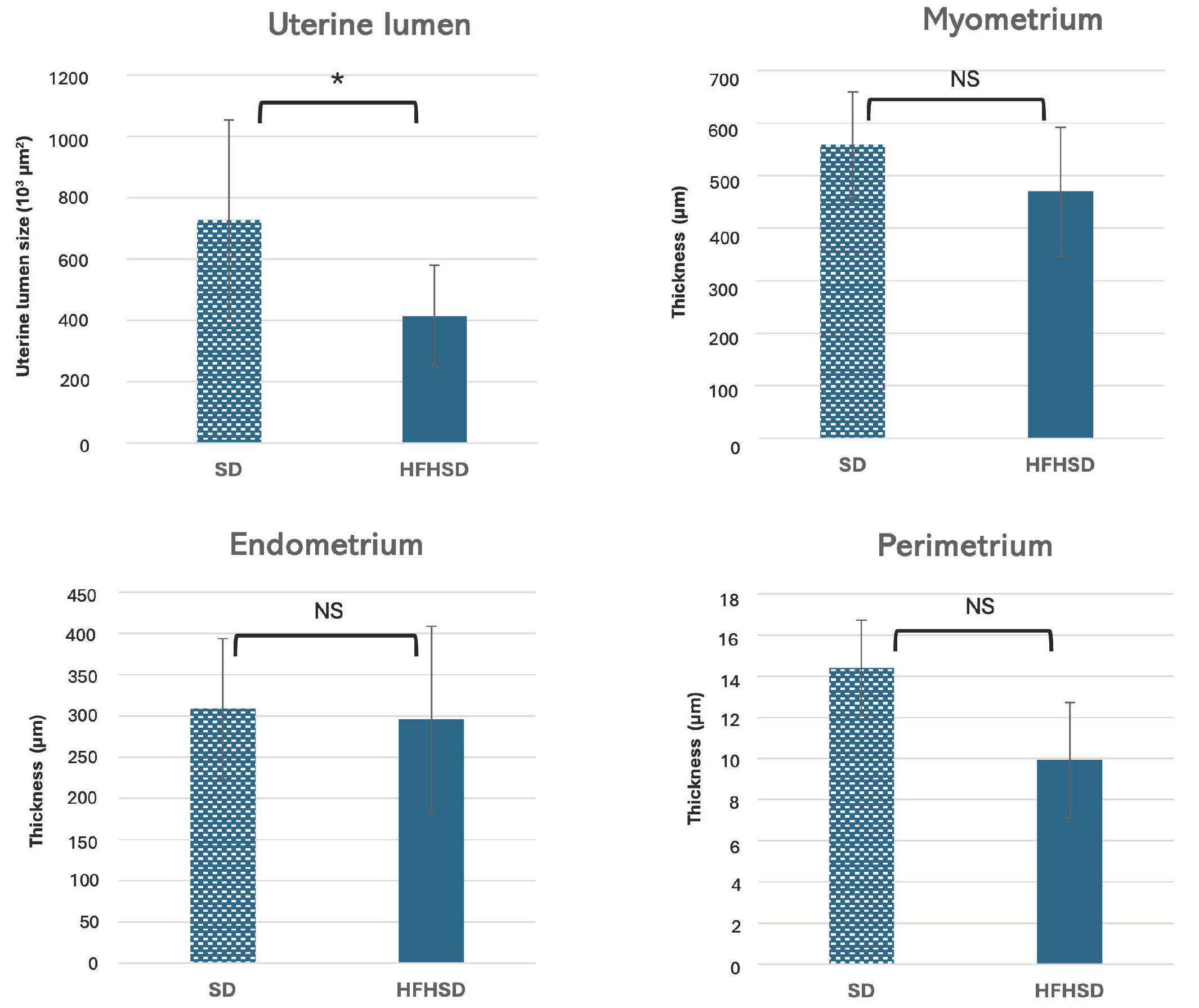
3.1. Morphological Changes of Adipose Tissue and Uterus
3.2. Glycogen Deposition and Collagen in Uterus
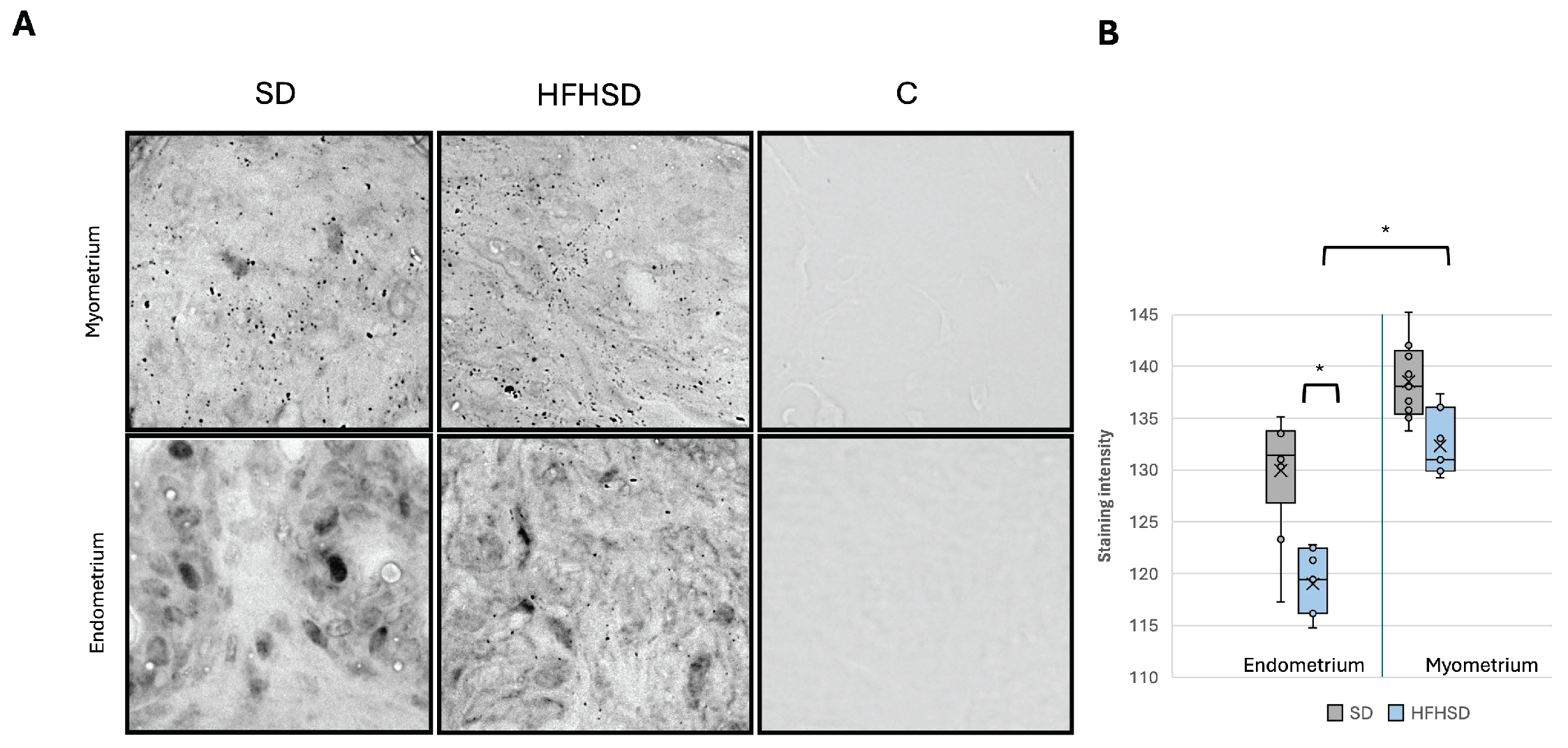
3.3. Insulin and Leptin Receptors Expression in Uterus
3.4. Uncoupling Protein 1 and 3 Expression in Uterus
4. Discussion
4.1. The Strengths and Limitations of the Study
4.2. The Potential Clinical Implication and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GF | Gonadal fat |
| HFHSD | High-fat high-sugar diet |
| IR | Insulin receptor |
| ObR | Leptin receptor |
| SD | Standard diet |
| UCP1 | Uncoupling protein 1 |
| UCP3 | Uncoupling protein 3 |
| VF | Visceral fat |
References
- Lee, Y.-A.; Song, S.-W.; Kim, S.-H.; Kim, H.-N. Associations between Dietary Patterns and Metabolic Syndrome: Findings of the Korean National Health and Nutrition Examination Survey. Nutrients 2023, 15, 2676. [Google Scholar] [CrossRef] [PubMed]
- Saghafi-Asl, M.; Mirmajidi, S.; Asghari Jafarabadi, M.; Vahid, F.; Shivappa, N.; Hébert, J.R.; Ebrahimzadeh Attari, V. The Association of Dietary Patterns with Dietary Inflammatory Index, Systemic Inflammation, and Insulin Resistance, in Apparently Healthy Individuals with Obesity. Sci. Rep. 2021, 11, 7515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, B.; Shen, C.; Sahakian, B.J.; Li, Z.; Zhang, W.; Zhao, Y.; Li, Y.; Feng, J.; Cheng, W. Associations of Dietary Patterns with Brain Health from Behavioral, Neuroimaging, Biochemical and Genetic Analyses. Nat. Ment. Health 2024, 2, 535–552. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Bennett, S.; Zou, J.; Xu, J.; Zhang, L. The Effects of Different Dietary Patterns on Bone Health. Nutrients 2024, 16, 2289. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Mercuri, N.D.; Cox, B.J. The Need for More Research into Reproductive Health and Disease. eLife 2022, 11, e75061. [Google Scholar] [CrossRef]
- Hormonal Biophysiology of the Uterus—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/32406025/ (accessed on 18 January 2025).
- Krzyżanowski, J.; Paszkowski, T.; Woźniak, S. The Role of Nutrition in Pathogenesis of Uterine Fibroids. Nutrients 2023, 15, 4984. [Google Scholar] [CrossRef]
- Peral-Sanchez, I.; Hojeij, B.; Ojeda, D.A.; Steegers-Theunissen, R.P.M.; Willaime-Morawek, S. Epigenetics in the Uterine Environment: How Maternal Diet and ART May Influence the Epigenome in the Offspring with Long-Term Health Consequences. Genes 2021, 13, 31. [Google Scholar] [CrossRef]
- Šišljagić, D.; Blažetić, S.; Heffer, M.; Vranješ Delać, M.; Muller, A. The Interplay of Uterine Health and Obesity: A Comprehensive Review. Biomedicines 2024, 12, 2801. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Holdsworth-Carson, S.J.; Menkhorst, E.; Maybin, J.A.; King, A.; Girling, J.E. Cyclic Processes in the Uterine Tubes, Endometrium, Myometrium, and Cervix: Pathways and Perturbations. Mol. Hum. Reprod. 2023, 29, gaad012. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef]
- Gáspár, R.; Hajagos-Tóth, J.; Schaffer, A.; Kothencz, A.; Siska-Szabó, L.; Ducza, E.; Csányi, A.; Tábi, T.; Bagaméry, F.; Szökő, É.; et al. High Fat High Sucrose Diet Modifies Uterine Contractility and Cervical Resistance in Pregnant Rats: The Roles of Sex Hormones, Adipokines and Cytokines. Life 2022, 12, 794. [Google Scholar] [CrossRef]
- Ivić, V.; Zjalić, M.; Blažetić, S.; Fenrich, M.; Labak, I.; Scitovski, R.; Szűcs, K.F.; Ducza, E.; Tábi, T.; Bagamery, F.; et al. Elderly Rats Fed with a High-Fat High-Sucrose Diet Developed Sex-Dependent Metabolic Syndrome Regardless of Long-Term Metformin and Liraglutide Treatment. Front. Endocrinol. 2023, 14, 1181064. [Google Scholar] [CrossRef]
- Gasner, A.; Aatsha, P.A. Physiology, Uterus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Monaco-Brown, M.; Lawrence, D.A. Obesity and Maternal-Placental-Fetal Immunology and Health. Front. Pediatr. 2022, 10, 859885. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of Implantation: Strategies for Successful Pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Burns, G.W.; Behura, S.; Wu, G.; Spencer, T.E. Uterine Glands Impact Uterine Receptivity, Luminal Fluid Homeostasis and Blastocyst Implantation. Sci. Rep. 2016, 6, 38078. [Google Scholar] [CrossRef] [PubMed]
- Pathare, A.D.S.; Loid, M.; Saare, M.; Gidlöf, S.B.; Zamani Esteki, M.; Acharya, G.; Peters, M.; Salumets, A. Endometrial Receptivity in Women of Advanced Age: An Underrated Factor in Infertility. Hum. Reprod. Update 2023, 29, 773–793. [Google Scholar] [CrossRef]
- Ferrer, M.J.; Abruzzese, G.A.; Heber, M.F.; Ferreira, S.R.; Campo Verde Arbocco, F.; Motta, A.B. Intrauterine Androgen Exposure Impairs Gonadal Adipose Tissue Functions of Adult Female Rats. Theriogenology 2023, 198, 131–140. [Google Scholar] [CrossRef]
- Detsrisuwan, J.; Luewan, S.; Sirichotiyakul, S.; Tongsong, T. Maternal Visceral Fat in Prediction of Gestational Diabetes Mellitus. J. Clin. Med. 2024, 13, 493. [Google Scholar] [CrossRef]
- Li, Q.; Spalding, K.L. Profiling Hypertrophic Adipocytes in Humans, from Transcriptomics to Diagnostics. eBioMedicine 2022, 81, 104105. [Google Scholar] [CrossRef]
- Ye, R.Z.; Montastier, E.; Frisch, F.; Noll, C.; Allard-Chamard, H.; Gévry, N.; Tchernof, A.; Carpentier, A.C. Adipocyte Hypertrophy Associates with in Vivo Postprandial Fatty Acid Metabolism and Adipose Single-Cell Transcriptional Dynamics. iScience 2024, 27, 108692. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 522637. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.T.; Czech, M.P.; Corvera, S. What Causes the Insulin Resistance Underlying Obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Dhokte, S.; Czaja, K. Visceral Adipose Tissue: The Hidden Culprit for Type 2 Diabetes. Nutrients 2024, 16, 1015. [Google Scholar] [CrossRef]
- Sun, K.; Xie, Y.; Zhao, N.; Li, Z. A Case-Control Study of the Relationship between Visceral Fat and Development of Uterine Fibroids. Exp. Ther. Med. 2019, 18, 404–410. [Google Scholar] [CrossRef]
- Li, Q.; Spalding, K.L. The Regulation of Adipocyte Growth in White Adipose Tissue. Front. Cell Dev. Biol. 2022, 10, 1003219. [Google Scholar] [CrossRef]
- Svensson, H.; Wetterling, L.; Bosaeus, M.; Odén, B.; Odén, A.; Jennische, E.; Edén, S.; Holmäng, A.; Lönn, M. Body Fat Mass and the Proportion of Very Large Adipocytes in Pregnant Women Are Associated with Gestational Insulin Resistance. Int. J. Obes. 2016, 40, 646–653. [Google Scholar] [CrossRef]
- Nicholson, L.; Lindsay, L.; Murphy, C.R. Change in Distribution of Cytoskeleton-Associated Proteins, Lasp-1 and Palladin, during Uterine Receptivity in the Rat Endometrium. Reprod. Fertil. Dev. 2018, 30, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.T.; Greenway, F.; Tucker, T.R.; Alexander, M.; Jackson, L.K.; Hepford, S.A.; Loveridge, B.; Lakey, J.R.T. A Receptor Story: Insulin Resistance Pathophysiology and Physiologic Insulin Resensitization’s Role as a Treatment Modality. Int. J. Mol. Sci. 2023, 24, 10927. [Google Scholar] [CrossRef] [PubMed]
- Flannery, C.A.; Choe, G.H.; Cooke, K.M.; Fleming, A.G.; Radford, C.C.; Kodaman, P.H.; Jurczak, M.J.; Kibbey, R.G.; Taylor, H.S. Insulin Regulates Glycogen Synthesis in Human Endometrial Glands Through Increased GYS2. J. Clin. Endocrinol. Metab. 2018, 103, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Azaïs, H.; Leroy, A.; Ghesquiere, L.; Deruelle, P.; Hanssens, S. Effects of Adipokines and Obesity on Uterine Contractility. Cytokine Growth Factor Rev. 2017, 34, 59–66. [Google Scholar] [CrossRef]
- Hajagos-Tóth, J.; Ducza, E.; Samavati, R.; Vari, S.G.; Gaspar, R. Obesity in Pregnancy: A Novel Concept on the Roles of Adipokines in Uterine Contractility. Croat. Med. J. 2017, 58, 96–104. [Google Scholar] [CrossRef]
- Srinivasan, G.; Parida, S.; Pavithra, S.; Panigrahi, M.; Sahoo, M.; Singh, T.U.; Madhu, C.L.; Manickam, K.; Shyamkumar, T.S.; Kumar, D.; et al. Leptin Receptor Stimulation in Late Pregnant Mouse Uterine Tissue Inhibits Spontaneous Contractions by Increasing NO and cGMP. Cytokine 2021, 137, 155341. [Google Scholar] [CrossRef] [PubMed]
- Benomar, Y.; Roy, A.-F.; Aubourg, A.; Djiane, J.; Taouis, M. Cross Down-Regulation of Leptin and Insulin Receptor Expression and Signalling in a Human Neuronal Cell Line. Biochem. J. 2005, 388, 929–939. [Google Scholar] [CrossRef]
- Chan, C.B.; Harper, M.-E. Uncoupling Proteins: Role in Insulin Resistance and Insulin Insufficiency. Curr. Diabetes Rev. 2006, 2, 271–283. [Google Scholar] [CrossRef]
- Kleinridders, A.; Ferris, H.A.; Tovar, S. Editorial: Crosstalk of Mitochondria With Brain Insulin and Leptin Signaling. Front. Endocrinol. 2018, 9, 761. [Google Scholar] [CrossRef]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef]
- JCI—Overexpression of Uncoupling Protein 3 in Skeletal Muscle Protects Against Fat-Induced Insulin Resistance. Available online: https://www.jci.org/articles/view/13579 (accessed on 22 January 2025).
- Fisler, J.S.; Warden, C.H. Uncoupling Proteins, Dietary Fat and the Metabolic Syndrome. Nutr. Metab. 2006, 3, 38. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamada, T. UCP1 Dependent and Independent Thermogenesis in Brown and Beige Adipocytes. Front. Endocrinol. 2020, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Kalinovich, A.V.; de Jong, J.M.A.; Cannon, B.; Nedergaard, J. UCP1 in Adipose Tissues: Two Steps to Full Browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šišljagić, D.; Blažetić, S.; Zjalić, M.; Labak, I.; Ivić, V.; Szűcs, K.F.; Gáspár, R.; Ducza, E.; Vari, S.G.; Muller, A.; et al. Structural and Metabolic Changes in Pregnant Rat Uterine and Adipose Tissue Induced by a High-Fat High-Sugar Diet. Biomolecules 2025, 15, 446. https://doi.org/10.3390/biom15030446
Šišljagić D, Blažetić S, Zjalić M, Labak I, Ivić V, Szűcs KF, Gáspár R, Ducza E, Vari SG, Muller A, et al. Structural and Metabolic Changes in Pregnant Rat Uterine and Adipose Tissue Induced by a High-Fat High-Sugar Diet. Biomolecules. 2025; 15(3):446. https://doi.org/10.3390/biom15030446
Chicago/Turabian StyleŠišljagić, Dina, Senka Blažetić, Milorad Zjalić, Irena Labak, Vedrana Ivić, Kálmán Ferenc Szűcs, Róbert Gáspár, Eszter Ducza, Sandor G. Vari, Andrijana Muller, and et al. 2025. "Structural and Metabolic Changes in Pregnant Rat Uterine and Adipose Tissue Induced by a High-Fat High-Sugar Diet" Biomolecules 15, no. 3: 446. https://doi.org/10.3390/biom15030446
APA StyleŠišljagić, D., Blažetić, S., Zjalić, M., Labak, I., Ivić, V., Szűcs, K. F., Gáspár, R., Ducza, E., Vari, S. G., Muller, A., & Heffer, M. (2025). Structural and Metabolic Changes in Pregnant Rat Uterine and Adipose Tissue Induced by a High-Fat High-Sugar Diet. Biomolecules, 15(3), 446. https://doi.org/10.3390/biom15030446





