Enhancing Antimicrobial Peptides from Frog Skin: A Rational Approach
Abstract
1. Introduction
2. Materials and Methods
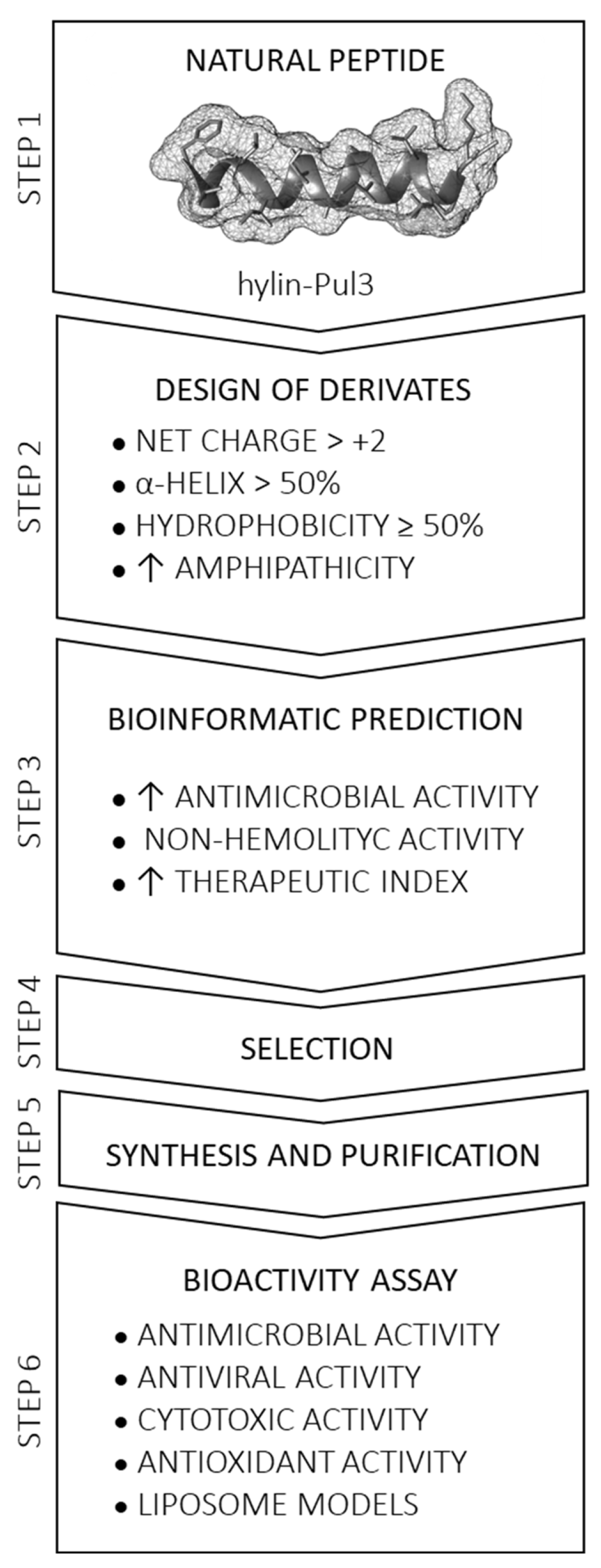
2.1. Rational Design of Derivative Peptides and Bioinformatic Analysis
2.2. Synthesis and Purification of Derivative Peptides
2.2.1. Solid-Phase Peptide Synthesis (SPPS)
2.2.2. Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) and Characterization with Mass Spectrometry Analysis
2.3. Biological Activity
2.3.1. Antibacterial Assays
2.3.2. Synergistic Checkerboard Assay
2.3.3. Antiviral Activity Assays
2.3.4. Hemolysis Test
2.3.5. In Vitro Cytotoxicity Against Human Fibroblasts
Cell Lines and Cell Culture
Cell Viability and Proliferation
2.3.6. In Vitro Cytotoxicity Against Vero Cells
2.4. In Vitro Antioxidant Assays
2.4.1. DPPH Radical-Scavenging Activity
2.4.2. ABTS Assay
2.5. Interaction of Peptides with Lipid Membranes
2.5.1. Monolayers at the Air/Water Interface
2.5.2. Large Unilamellar Vesicles (LUVs)
2.5.3. Fluorescence Excitation Spectra of 5,6-Carboxyfluorescein (CF)
2.5.4. Dynamic Light Scattering (DLS)
3. Results
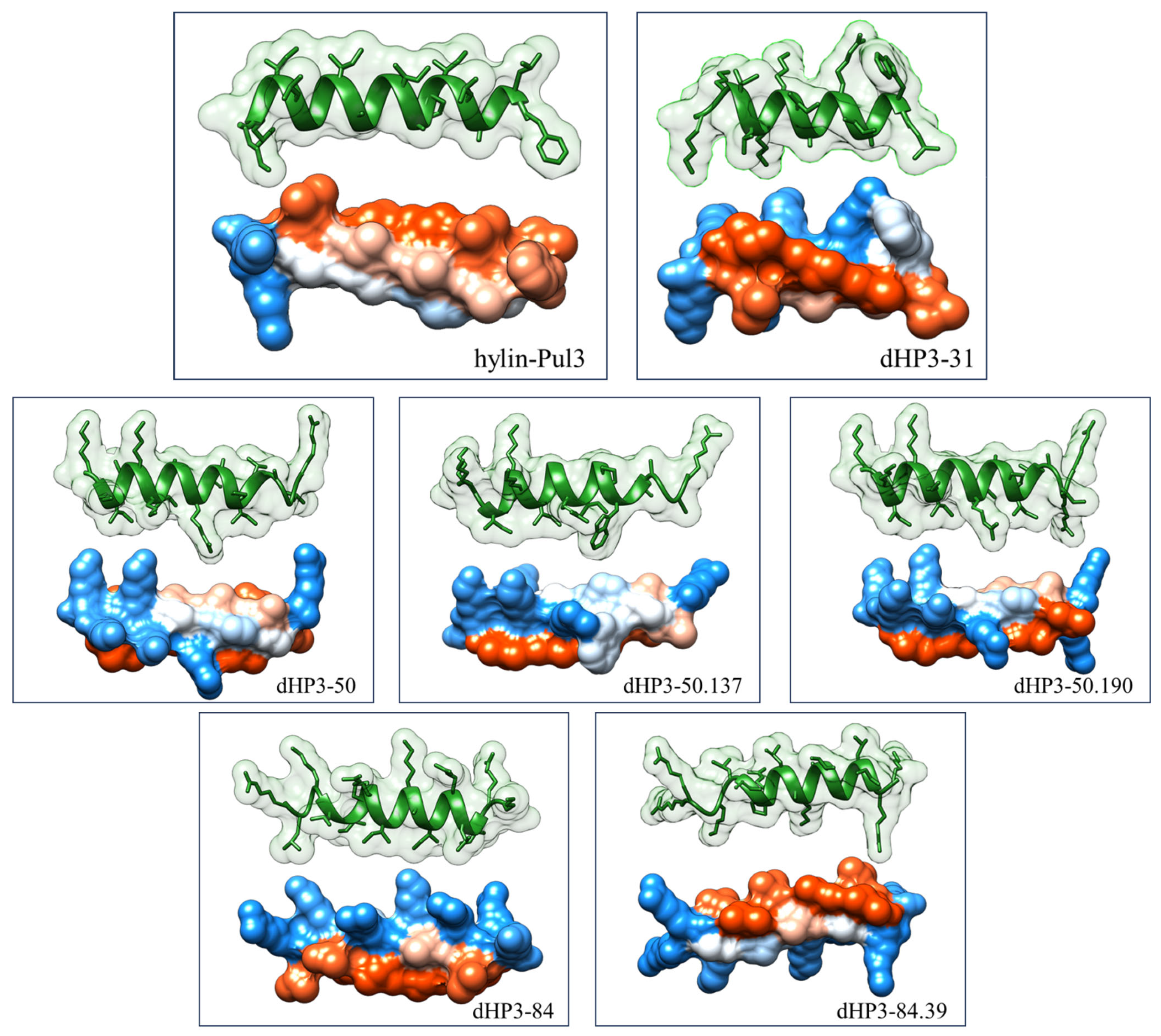
3.1. Selection of Peptides and Sequence Analysis
3.2. Synthesis of Peptides
3.3. Improved Biological Activity
3.4. Hemolytic Activity
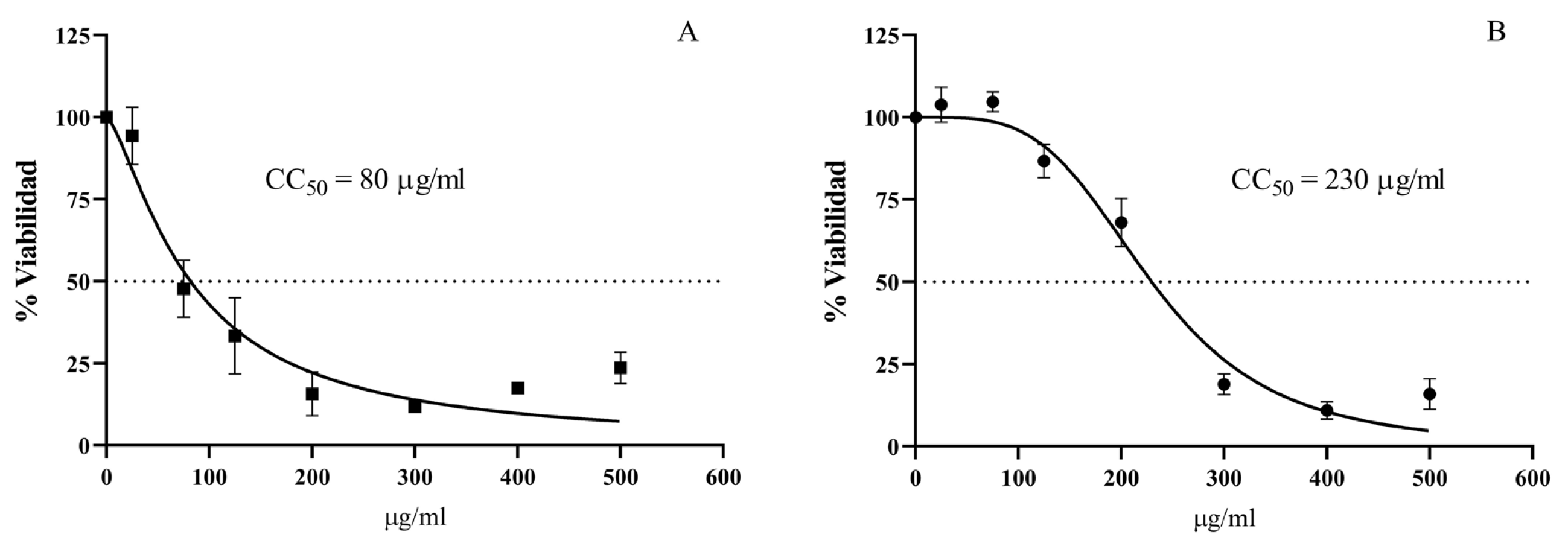
3.5. Cytotoxic Activity
3.6. In Vitro Antioxidant Assays
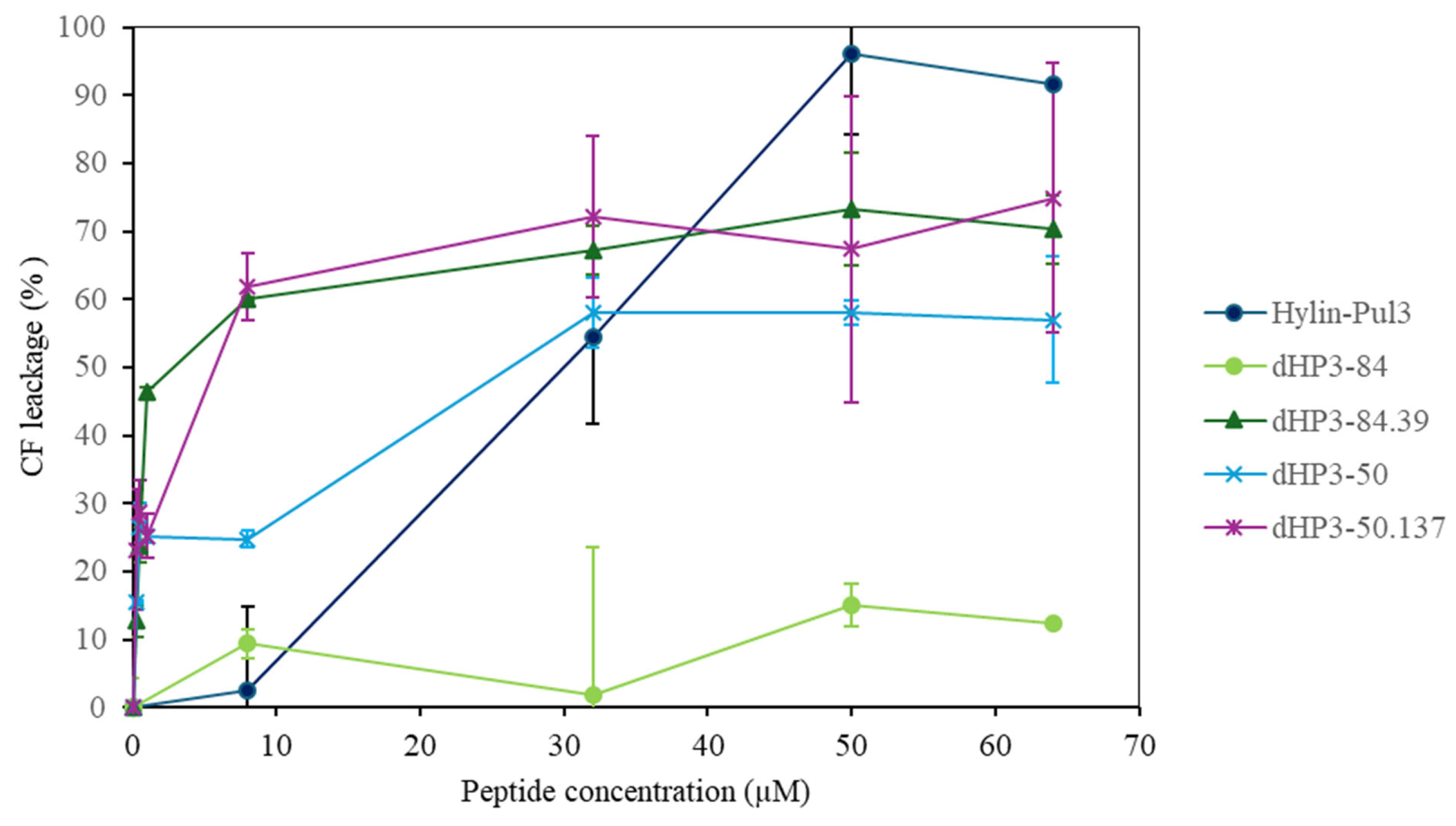
3.7. Interaction with Lipid Membranes
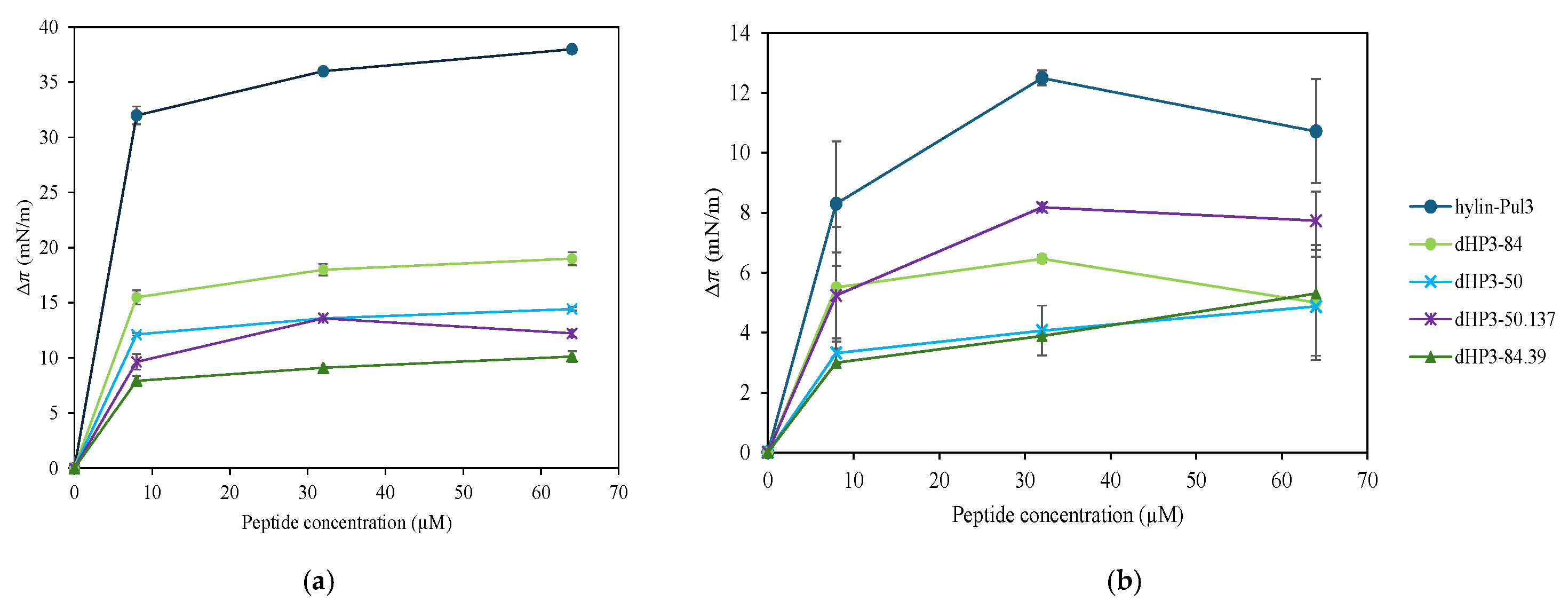
3.7.1. Monolayers at the Air/Water Interface
3.7.2. Peptide Activity on Large Unilamellar Vesicles (LUVs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024.
- Liu, Y.; Shi, J.; Tong, Z.; Jia, Y.; Yang, B.; Wang, Z. The revitalization of antimicrobial peptides in the resistance era. Pharmacol Res. 2021, 163, 105276. [Google Scholar] [CrossRef]
- Rodríguez, A.A.; Otero-González, A.; Ghattas, M.; Ständker, L. Discovery, optimization, and clinical application of natural antimicrobial peptides. Biomedicines 2021, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, B.G.; Albericio, F. The pharmaceutical industry in 2023: An analysis of FDA drug approvals from the perspective of molecules. Molecules 2024, 29, 585. [Google Scholar] [CrossRef]
- Corrêa, J.A.F.; Evangelista, A.G.; de Melo Nazareth, T.; Luciano, F.B. Fundamentals on the molecular mechanism of action of antimicrobial peptides. Materialia 2019, 8, 100494. [Google Scholar] [CrossRef]
- Conlon, J.M.; Sonnevend, A. Clinical applications of amphibian antimicrobial peptides. Hamdan Med. J. 2011, 4, 62–72. [Google Scholar] [CrossRef]
- Wang, G. Bioinformatic analysis of 1000 amphibian antimicrobial peptides uncovers multiple length-dependent correlations for peptide design and prediction. Antibiotics 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, S.; Fang, J.; Zheng, S.; Wang, Z.; Jiao, Y.; Xia, P.; Wu, H.; Ma, Z.; Hao, L. Peptides isolated from amphibian skin secretions with emphasis on antimicrobial peptides. Toxins 2022, 14, 722. [Google Scholar] [CrossRef]
- Cresti, L.; Cappello, G.; Pini, A. Antimicrobial peptides towards clinical application—A long history to be concluded. Int. J. Mol. Sci. 2024, 25, 4870. [Google Scholar] [CrossRef]
- Tossi, A.; Sandri, L.; Giangaspero, A. Amphipathic, α-helical antimicrobial peptides. Pept. Sci. 2000, 55, 4–30. [Google Scholar] [CrossRef]
- Zou, R.; Zhu, X.; Tu, Y.; Wu, J.; Landry, M.P. Activity of antimicrobial peptide aggregates decreases with increased cell membrane embedding free energy cost. Biochemistry 2018, 57, 2606–2610. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.; Cândido, E.S.; Franco, O.L. Computer-aided design of antimicrobial peptides: Are we generating effective drug candidates? Front. Microbiol. 2020, 10, 3097. [Google Scholar] [CrossRef]
- Azuma, E.; Choda, N.; Odaki, M.; Yano, Y.; Matsuzaki, K. Improvement of therapeutic index by the combination of enhanced peptide cationicity and proline introduction. ACS Infect. Dis. 2020, 6, 2271–2278. [Google Scholar] [CrossRef]
- Bui Thi Phuong, H.; Doan Ngan, H.; Le Huy, B.; Vu Dinh, H.; Luong Xuan, H. The amphipathic design in helical antimicrobial peptides. ChemMedChem 2024, 19, e202300480. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.; Vasil, M.L.; Hodges, R.S. Rational design of α-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Huang, J.; Lu, J.; Lu, M.; Huang, Q.; Li, G. The Design and Construction of K11: A Novel α-Helical Antimicrobial Peptide. Int. J. Microbiol. 2012, 2012, 764834. [Google Scholar]
- Plisson, F.; Ramírez-Sánchez, O.; Martínez-Hernández, C. Machine learning-guided discovery and design of non-hemolytic peptides. Sci. Rep. 2020, 10, 16581. [Google Scholar] [CrossRef] [PubMed]
- Aronica, P.G.; Reid, L.M.; Desai, N.; Li, J.; Fox, S.J.; Yadahalli, S.; Essex, J.W.; Verma, C.S. Computational methods and tools in antimicrobial peptide research. J. Chem. Inf. Model. 2021, 61, 3172–3196. [Google Scholar] [CrossRef]
- Torres, M.D.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Aguilar, S.; Brunetti, A.E.; Garay, A.V.; Santos, L.C.; Perez, L.O.; Moreira, D.; Cancelarich, N.L.; Barbosa, E.A.; Basso, N.G.; de Freitas, S.M. Structure and function of cationic hylin bioactive peptides from the tree frog Boana pulchella in interaction with lipid membranes. Peptides 2023, 159, 170900. [Google Scholar] [CrossRef]
- Chan, W.; White, P. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; OUP Oxford: Oxford, UK, 1999; Volume 222. [Google Scholar]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.e.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Guermeur, Y. Combinaison de Classifieurs Statistiques: Application à la Prédiction de la Structure Secondaire des Protéines. Ph.D. Thesis, Universite Paris, Paris, France, 1997. [Google Scholar]
- Gautier, R.; Douguet, D.; Antonny, B.; Drin, G. HELIQUEST: A web server to screen sequences with specific α-helical properties. Bioinformatics 2008, 24, 2101–2102. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef]
- Waghu, F.H.; Idicula-Thomas, S. Collection of antimicrobial peptides database and its derivatives: Applications and beyond. Protein Sci. 2020, 29, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of antimicrobial/cytotoxic activity and structure of peptides as a resource for development of new therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef]
- Timmons, P.B.; Hewage, C.M. HAPPENN is a novel tool for hemolytic activity prediction for therapeutic peptides which employs neural networks. Sci. Rep. 2020, 10, 10869. [Google Scholar] [CrossRef]
- Win, T.S.; Malik, A.A.; Prachayasittikul, V.; S Wikberg, J.E.; Nantasenamat, C.; Shoombuatong, W. HemoPred: A web server for predicting the hemolytic activity of peptides. Future Med. Chem. 2017, 9, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Marani, M.M.; Dourado, F.v.S.; Quelemes, P.V.; de Araujo, A.R.; Perfeito, M.r.L.G.; Barbosa, E.A.; Véras, L.M.C.; Coelho, A.L.R.; Andrade, E.B.; Eaton, P. Characterization and biological activities of ocellatin peptides from the skin secretion of the frog Leptodactylus pustulatus. J. Nat. Prod. 2015, 78, 1495–1504. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Torres, C.V.; Domínguez, M.J.; Carbonari, J.L.; Sabini, M.C.; Sabini, L.I.; Zanon, S.M. Study of antiviral and virucidal activities of aqueous extract of Baccharis articulata against Herpes suis virus. Nat. Prod. Commun. 2011, 6, 1934578X1100600717. [Google Scholar] [CrossRef]
- Cancelarich, N.L.; Wilke, N.; Fanani, M.a.L.; Moreira, D.C.; Perez, L.O.; Alves Barbosa, E.; Placido, A.; Socodato, R.; Portugal, C.C.; Relvas, J.B. Somuncurins: Bioactive peptides from the skin of the endangered endemic Patagonian frog Pleurodema somuncurense. J. Nat. Prod. 2020, 83, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Escobar, F.M.; Cariddi, L.N.; Sabini, M.C.; Reinoso, E.; Sutil, S.B.; Torres, C.V.; Zanon, S.M.; Sabini, L.I. Lack of cytotoxic and genotoxic effects of Minthostachys verticillata essential oil: Studies in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 3062–3067. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Travkova, O.G.; Moehwald, H.; Brezesinski, G. The interaction of antimicrobial peptides with membranes. Adv. Colloid Interface Sci. 2017, 247, 521–532. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R.M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]
- Weinstein, J.; Yoshikami, S.; Henkart, P.; Blumenthal, R.; Hagins, W. Liposome-cell interaction: Transfer and intracellular release of a trapped fluorescent marker. Science 1977, 195, 489–492. [Google Scholar] [CrossRef]
- Gagnon, M.-C.; Strandberg, E.; Grau-Campistany, A.; Wadhwani, P.; Reichert, J.; Bürck, J.; Rabanal, F.; Auger, M.; Paquin, J.-F.; Ulrich, A.S. Influence of the length and charge on the activity of α-helical amphipathic antimicrobial peptides. Biochemistry 2017, 56, 1680–1695. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Zelezetsky, I.; Tossi, A. Alpha-helical antimicrobial peptides—Using a sequence template to guide structure–activity relationship studies. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1436–1449. [Google Scholar] [CrossRef]
- Dennison, S.R.; Wallace, J.; Harris, F.; Phoenix, D.A. Amphiphilic α-helical antimicrobial peptides and their structure/function relationships. Protein Pept. Lett. 2005, 12, 31–39. [Google Scholar] [CrossRef]
- Ramos-Martín, F.; D’Amelio, N. Biomembrane lipids: When physics and chemistry join to shape biological activity. Biochimie 2022, 203, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the activity of Trp-rich antimicrobial peptides by Arg/Lys substitutions and changing the length of cationic residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Strömstedt, A.A.; Ringstad, L.; Schmidtchen, A.; Malmsten, M. Interaction between amphiphilic peptides and phospholipid membranes. Curr. Opin. Colloid Interface Sci. 2010, 15, 467–478. [Google Scholar] [CrossRef]
- Wang, C.-K.; Shih, L.-Y.; Chang, K.Y. Large-scale analysis of antimicrobial activities in relation to amphipathicity and charge reveals novel characterization of antimicrobial peptides. Molecules 2017, 22, 2037. [Google Scholar] [CrossRef]
- Jiang, Z.; Vasil, A.I.; Gera, L.; Vasil, M.L.; Hodges, R.S. Rational Design of α-Helical Antimicrobial Peptides to Target Gram-negative Pathogens, Acinetobacter baumannii and Pseudomonas aeruginosa: Utilization of Charge, ‘Specificity Determinants’, Total Hydrophobicity, Hydrophobe Type and Location as Design Parameters to Improve the Therapeutic Ratio. Chem. Biol. Drug Des. 2011, 77, 225–240. [Google Scholar]
- Ferreira, A.R.; Teixeira, C.; Sousa, C.F.; Bessa, L.J.; Gomes, P.; Gameiro, P. How insertion of a single tryptophan in the N-terminus of a cecropin A-melittin hybrid peptide changes its antimicrobial and biophysical profile. Membranes 2021, 11, 48. [Google Scholar] [CrossRef]
- Torcato, I.M.; Huang, Y.-H.; Franquelim, H.G.; Gaspar, D.; Craik, D.J.; Castanho, M.A.; Henriques, S.T. Design and characterization of novel antimicrobial peptides, R-BP100 and RW-BP100, with activity against Gram-negative and Gram-positive bacteria. BBA-Biomembranes 2013, 1828, 944–955. [Google Scholar] [CrossRef]
- Gong, H.; Liao, M.; Hu, X.; Fa, K.; Phanphak, S.; Ciumac, D.; Hollowell, P.; Shen, K.; Clifton, L.A.; Campana, M. Aggregated amphiphilic antimicrobial peptides embedded in bacterial membranes. ACS Appl. Mater. Interfaces 2020, 12, 44420–44432. [Google Scholar] [CrossRef]
- Medvedeva, A.; Teimouri, H.; Kolomeisky, A.B. Differences in relevant physicochemical properties correlate with synergistic activity of antimicrobial peptides. Biophys. J. 2024, 123, 41a–42a. [Google Scholar] [CrossRef]
- Al Tall, Y.; Abualhaijaa, A.; Alsaggar, M.; Almaaytah, A.; Masadeh, M.; Alzoubi, K.H. Design and characterization of a new hybrid peptide from LL-37 and BMAP-27. Infect. Drug Resist. 2019, 12, 1035–1045. [Google Scholar] [CrossRef]
- Chianese, A.; Giugliano, R.; Palma, F.; Nastri, B.M.; Monti, A.; Doti, N.; Zannella, C.; Galdiero, M.; De Filippis, A. The antiherpetic and anti-inflammatory activity of the frog-derived peptide Hylin-a1. J. Appl. Microbiol. 2024, 135, lxae165. [Google Scholar] [CrossRef] [PubMed]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The broad-spectrum antiviral potential of the amphibian peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef]
- Marcocci, M.; Amatore, D.; Villa, S.; Casciaro, B.; Aimola, P.; Franci, G.; Grieco, P.; Galdiero, M.; Palamara, A.; Mangoni, M. The amphibian antimicrobial peptide temporin b inhibits in vitro herpes simplex virus 1 infection. Antimicrob. Agents Chemother. 2018, 62, e02367-17. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Lebeau, L.; Chessa, C.; Damour, A.; Ladram, A.; Oury, B.; Boutolleau, D.; Bodet, C.; Lévêque, N. Comparison of anti-viral activity of frog skin anti-microbial peptides temporin-sha and [K3] SHa to LL-37 and temporin-Tb against herpes simplex virus type 1. Viruses 2019, 11, 77. [Google Scholar] [CrossRef]
- Urmi, U.L.; Vijay, A.K.; Kuppusamy, R.; Islam, S.; Willcox, M.D. A review of the antiviral activity of cationic antimicrobial peptides. Peptides 2023, 166, 171024. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, T.; Chen, L.; Zhou, J.; Wang, C. Analogs of the cathelicidin-derived antimicrobial peptide PMAP-23 exhibit improved stability and antibacterial activity. Probiotics Antimicrob. Proteins 2021, 13, 273–286. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Almeida, L.H.; de Oliveira, C.F.R.; de Souza Rodrigues, M.; Neto, S.M.; de Araújo Boleti, A.P.; Taveira, G.B.; de Oliveira Mello, É.; Gomes, V.M.; Dos Santos, E.L.; Crusca Jr, E. Adepamycin: Design, synthesis and biological properties of a new peptide with antimicrobial properties. Arch. Biochem. Biophys. 2020, 691, 108487. [Google Scholar]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, T.; Shang, L.; Yang, Y.; Li, Z.; Zhu, J.; Shan, A. Design of Trp-rich dodecapeptides with broad-spectrum antimicrobial potency and membrane-disruptive mechanism. J. Med. Chem. 2019, 62, 6941–6957. [Google Scholar] [CrossRef]
- Yang, N.; Rekdal, Ø.; Stensen, W.; Svendsen, J. Enhanced antitumor activity and selectivity of lactoferrin-derived peptides. Int. J. Pept. 2002, 60, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Q.; Lu, Q. Purification, structural analysis, and stability of antioxidant peptides from purple wheat bran. BMC Chem. 2020, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.A.; Oliveira, A.; Plácido, A.; Socodato, R.; Portugal, C.C.; Mafud, A.C.; Ombredane, A.S.; Moreira, D.C.; Vale, N.; Bessa, L.J. Structure and function of a novel antioxidant peptide from the skin of tropical frogs. Free Radic. Biol. Med. 2018, 115, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Int. Food Res. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Kong, B.; Xiong, Y.L. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem. 2006, 54, 6059–6068. [Google Scholar] [CrossRef]
- Aguilera-Puga, M.d.C.; Cancelarich, N.L.; Marani, M.M.; de la Fuente-Nunez, C.; Plisson, F. Accelerating the discovery and design of antimicrobial peptides with artificial intelligence. In Computational Drug Discovery and Design; Springer: New York, NY, USA, 2023; pp. 329–352. [Google Scholar]
- Torres, M.D.T.; de la Fuente-Nunez, C. Reprogramming biological peptides to combat infectious diseases. Chem. Commun. 2019, 55, 15020–15032. [Google Scholar] [CrossRef]


| Name | Antimicrobial Activity (MIC, µM) | Synergistic Activity | Hemolytic Activity (2%) (µM) | ||||
|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | K. pneumoniae | A. baumannii | S. mutans | E. coli (ATCC 25922) | ||
| ATCC 25922 | ATCC 25923 | ATCC 13883 | ATCC 13304 | ATCC 25175 | FIC Index | ||
| hylin-Pul3 | 32.0 ± 0.0 | 7.3 ± 1.6 | >64 | 32.0 ± 0.0 | 4.6 ± 1.6 | 10 | |
| dHP3-31 | 16.0 ± 0.0 | 37.3 ± 13.1 | >64 | 9.3 ± 3.2 | 18.7 ± 6.5 | >115 | |
| dHP3-50 | 8.0 ± 0.0 | 18.6 ± 6.5 | 64.0 ± 0.0 | 6.6 ± 2 | 8.0 ± 0.0 | >97 | |
| dHP3-50.137 | 4.0 ± 0.0 | 64.0 ± 0.0 | 53.3 ± 16.5 | 8.0 ± 0.0 | 32.0 ± 0.0 | 0.5 (Synergy) | 221 |
| dHP3-50.190 | 9.3 ± 3.2 | 29.3 ± 6.5 | >64 | 16.0 ± 0.0 | 32.0 ± 0.0 | >93 | |
| dHP3-84 | 8.0 ± 0.0 | 9.3 ± 3.3 | 18.6 ± 6.5 | 8.0 ± 0.0 | 7.3 ± 1.6 | 0.5 (Synergy) | 39 |
| dHP3-84.39 | 6.6 ± 4.8 | 26.6 ± 8.3 | 64.0 ± 0.0 | 16.0 ± 0.0 | >64 | 376 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, S.; Moreira, D.; Pereira Lourenço, A.L.; Wilke, N.; Crosio, M.A.; Vasconcelos, A.; Barbosa, E.A.; Bispo, E.C.I.; Saldanha-Araujo, F.; Ramada, M.H.S.; et al. Enhancing Antimicrobial Peptides from Frog Skin: A Rational Approach. Biomolecules 2025, 15, 449. https://doi.org/10.3390/biom15030449
Aguilar S, Moreira D, Pereira Lourenço AL, Wilke N, Crosio MA, Vasconcelos A, Barbosa EA, Bispo ECI, Saldanha-Araujo F, Ramada MHS, et al. Enhancing Antimicrobial Peptides from Frog Skin: A Rational Approach. Biomolecules. 2025; 15(3):449. https://doi.org/10.3390/biom15030449
Chicago/Turabian StyleAguilar, Silvana, Daniel Moreira, Ana Laura Pereira Lourenço, Natalia Wilke, Matías A. Crosio, Andreanne Vasconcelos, Eder Alves Barbosa, Elizabete C. I. Bispo, Felipe Saldanha-Araujo, Marcelo H. S. Ramada, and et al. 2025. "Enhancing Antimicrobial Peptides from Frog Skin: A Rational Approach" Biomolecules 15, no. 3: 449. https://doi.org/10.3390/biom15030449
APA StyleAguilar, S., Moreira, D., Pereira Lourenço, A. L., Wilke, N., Crosio, M. A., Vasconcelos, A., Barbosa, E. A., Bispo, E. C. I., Saldanha-Araujo, F., Ramada, M. H. S., Escobar, F. M., Torres, C. V., Leite, J. R. S. A., & Marani, M. M. (2025). Enhancing Antimicrobial Peptides from Frog Skin: A Rational Approach. Biomolecules, 15(3), 449. https://doi.org/10.3390/biom15030449












