High Dose C6 Ceramide-Induced Response in Embryonic Hippocampal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Cell Viability
2.3. Flow Cytometry Analysis for Measurement of Cell Cycle and Apoptosis
2.4. Mitochondrial Activity and Biogenesis
2.5. Cell Morphology
2.6. Immunocytochemistry
2.7. Ultrafast Liquid Chromatography–Tandem Mass Spectrometry
2.8. Fluorescence
2.9. Reverse Transcription Quantitative PCR (RTqPCR)
2.10. Statistical Analysis
3. Results
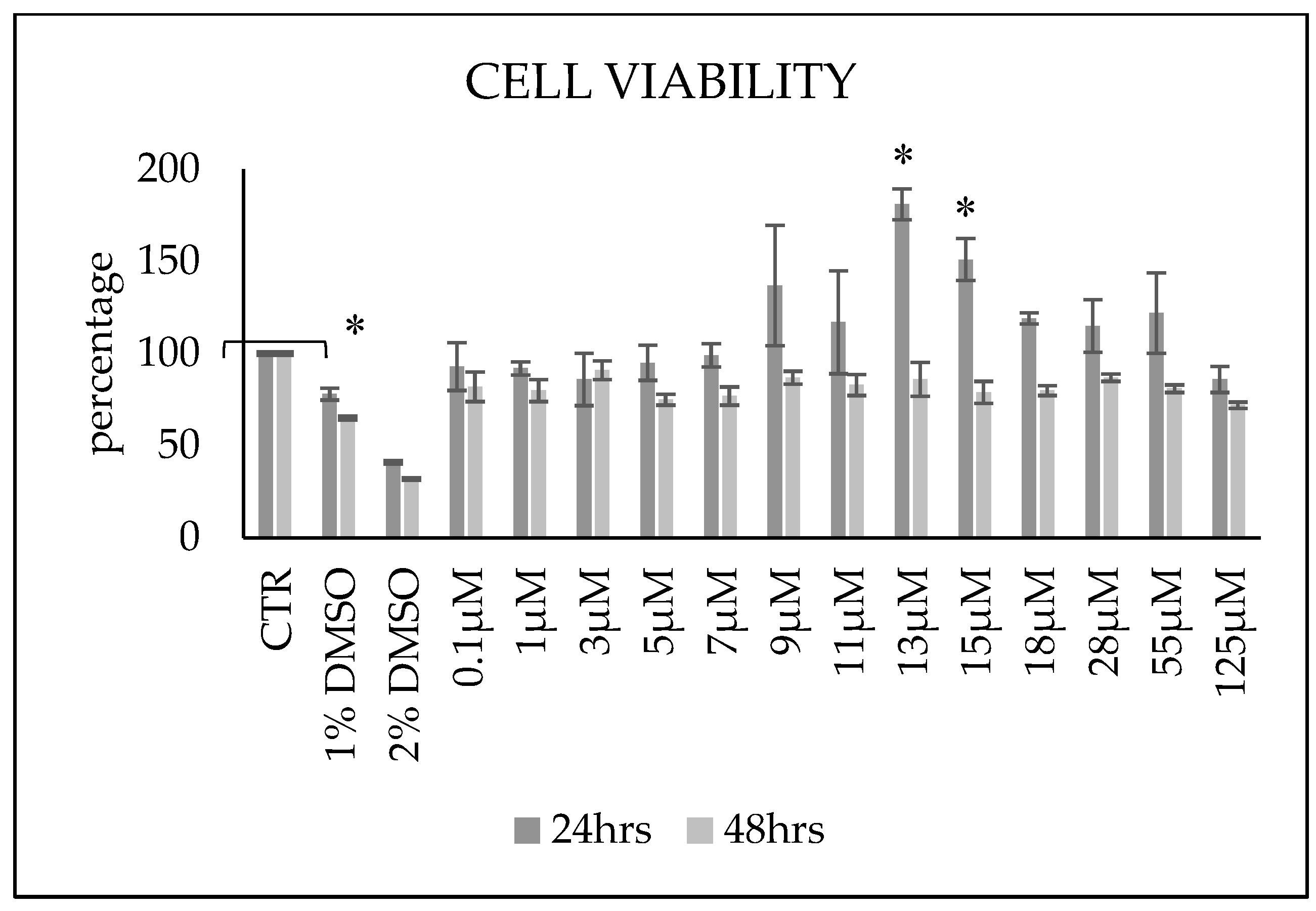
3.1. Ceramide Treatment Influences Cell Viability and Does Not Affect the Cell Cycle Progression and Apoptotic Levels of Cells
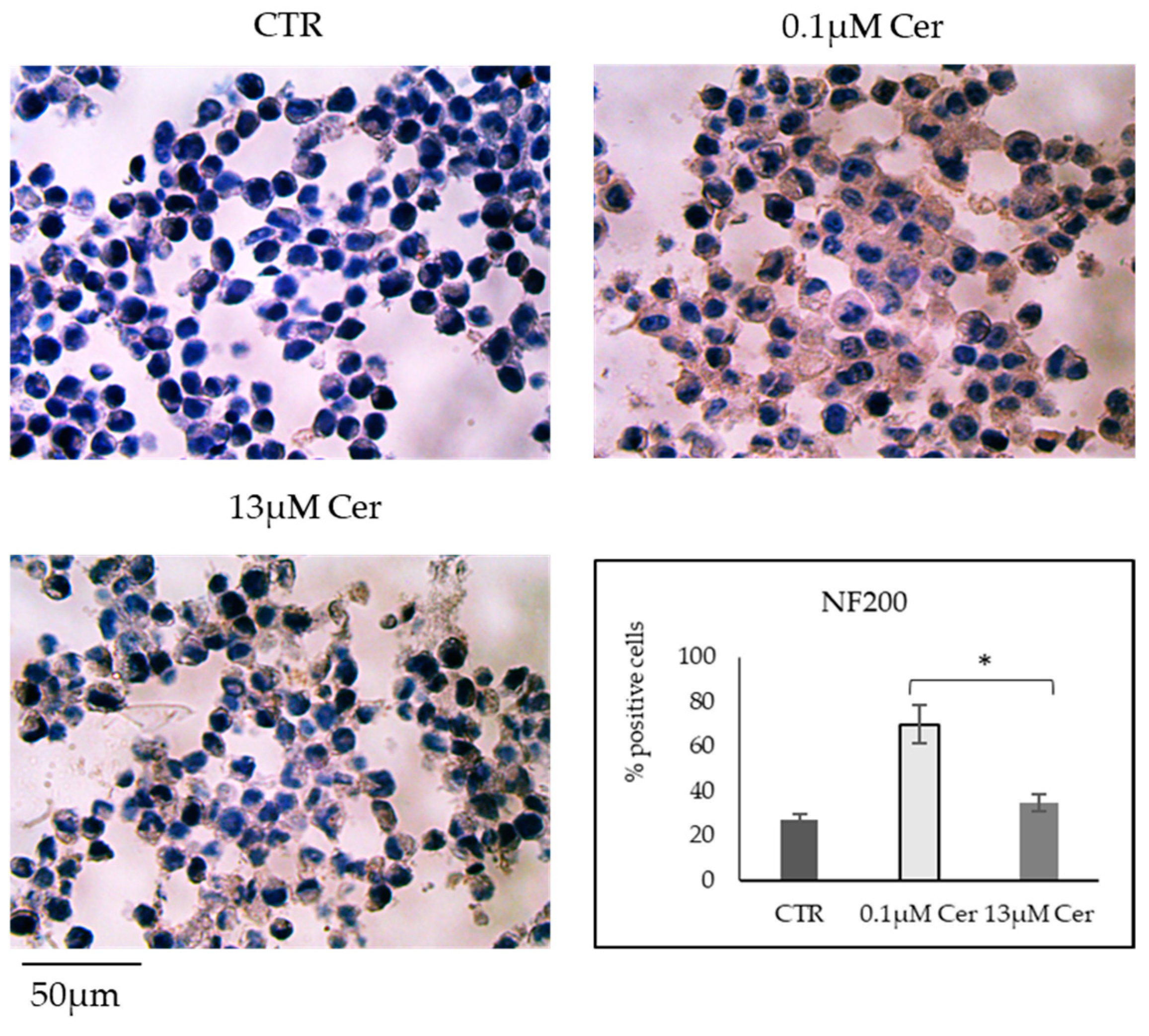
3.2. High Concentrations of Ceramide Increase the Length of Neurites
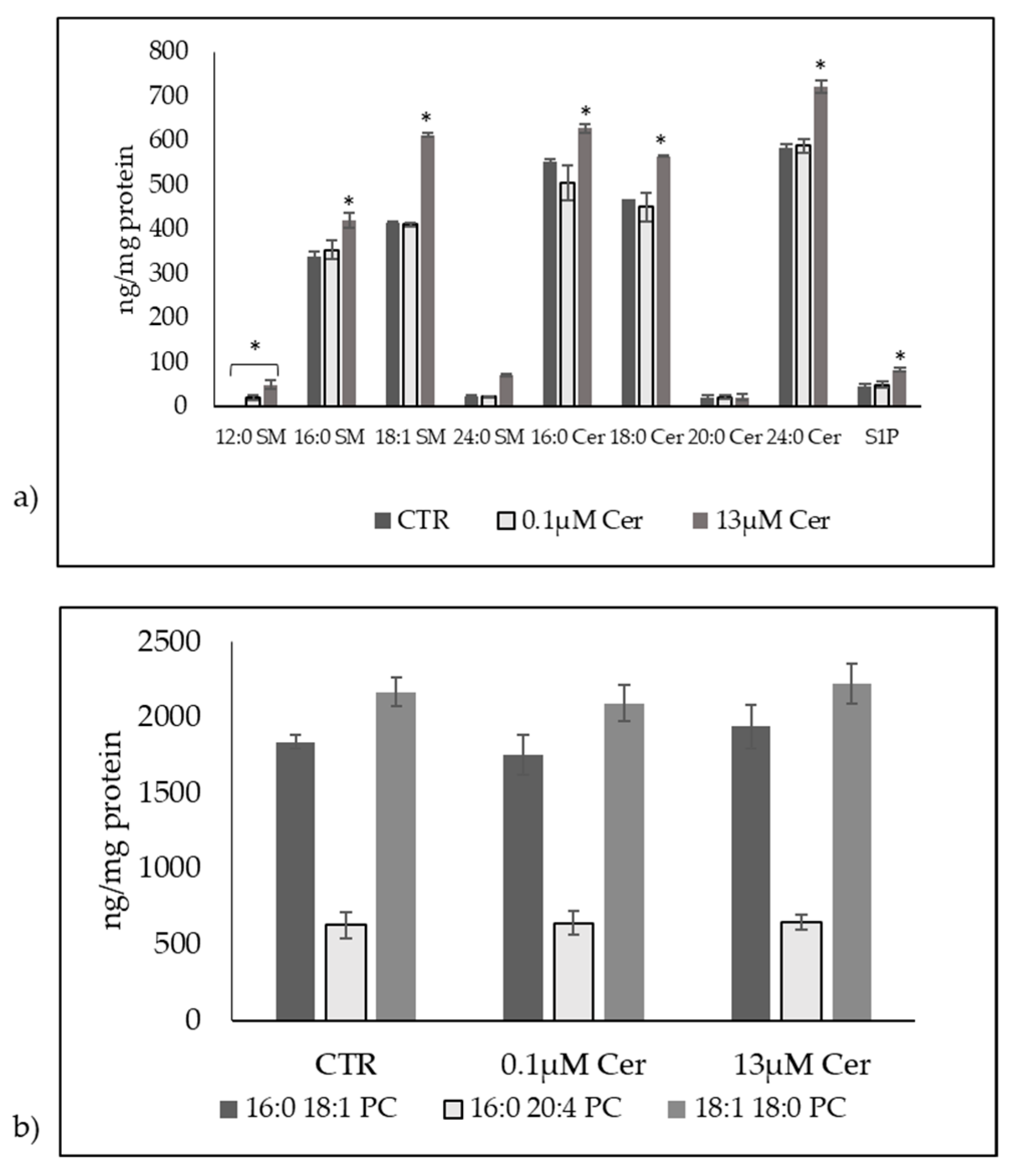
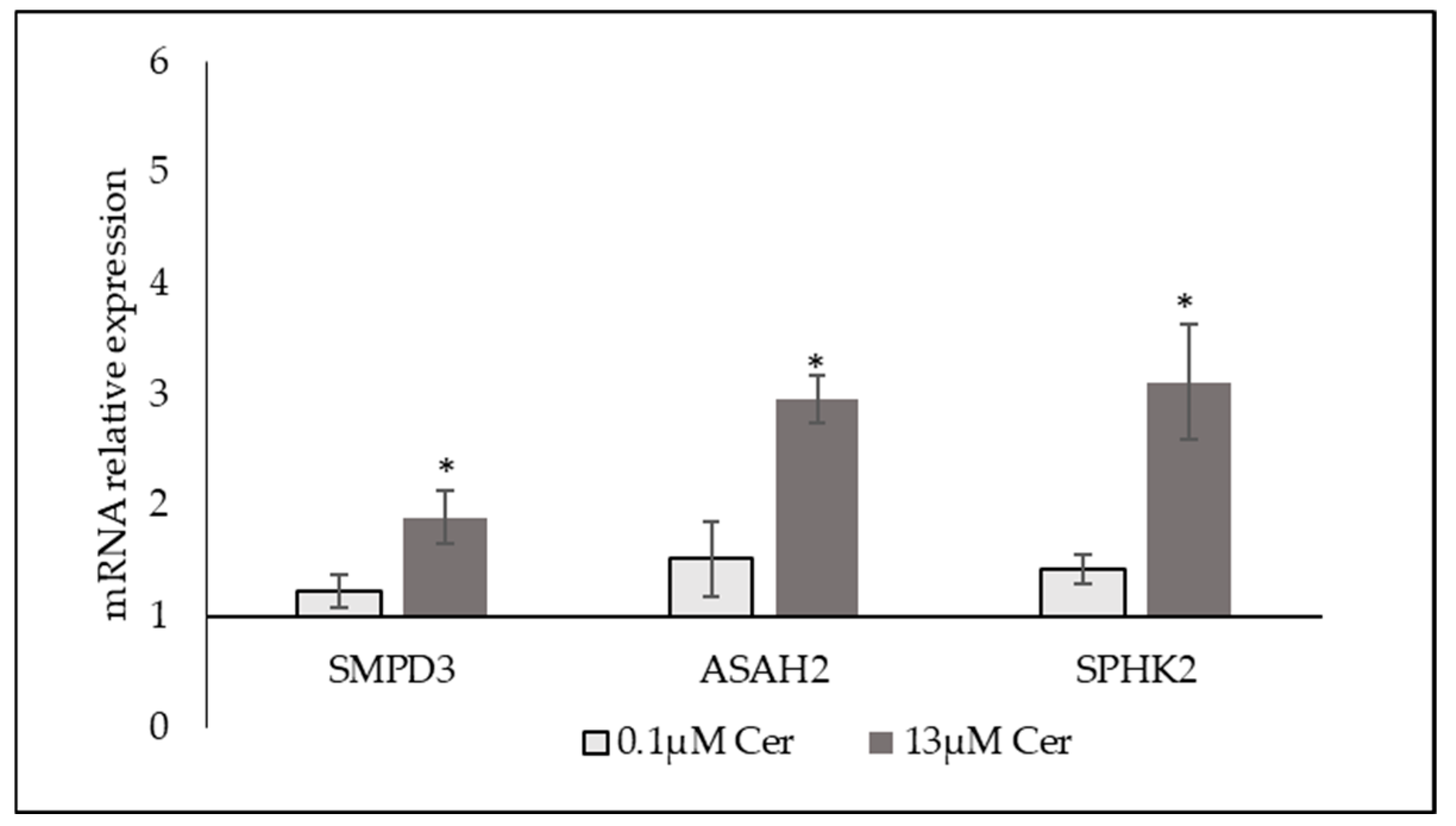
3.3. High Level of C6:0 Cer Influences Sphingolipid Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaurasia, B.; Summers, S.A. Ceramides in Metabolism: Key Lipotoxic Players. Annu. Rev. Physiol. 2021, 83, 303–330. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, F.; Domenis, R.; Dalla, E.; Cataldi, S.; Conte, C.; Mandarano, M.; Sidoni, A.; Cifù, A.; Beccari, T.; Mirarchi, A.; et al. Ceramide releases exosomes with a specific miRNA signature for cell differentiation. Sci. Rep. 2023, 13, 10993. [Google Scholar] [CrossRef]
- Kong, X.; Patel, N.A.; Chalfant, C.E.; Cooper, D.R. Ceramide synthesis regulates biogenesis and packaging of exosomal MALAT1 from adipose derived stem cells, increases dermal fibroblast migration and mitochondrial function. Cell Commun. Signal. 2023, 21, 221. [Google Scholar] [CrossRef] [PubMed]
- Ogretmen, B.; Pettus, B.J.; Rossi, M.J.; Wood, R.; Usta, J.; Szulc, Z.; Bielawska, A.; Obeid, L.M.; Hannun, Y.A. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adenocarcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J. Biol. Chem. 2002, 277, 12960–12969. [Google Scholar] [CrossRef] [PubMed]
- DiPasquale, M.; Deering, T.G.; Desai, D.; Sharma, A.K.; Amin, S.; Fox, T.E.; Kester, M.; Katsaras, J.; Marquardt, D.; Heberle, F.A. Influence of ceramide on lipid domain stability studied with small-angle neutron scattering: The role of acyl chain length and unsaturation. Chem. Phys. Lipids 2022, 245, 105205. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Kimchi, E.T.; Kaifi, J.T.; Qi, X.; Manjunath, Y.; Liu, X.; Deering, T.; Avella, D.M.; Fox, T.; et al. Nanoliposome C6 Ceramide Increases the Anti-Tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology 2018, 154, 1024–1036.e9. [Google Scholar] [CrossRef]
- Wilhelm, R.; Eckes, T.; Imre, G.; Kippenberger, S.; Meissner, M.; Thomas, D.; Trautmann, S.; Merlio, J.P.; Chevret, E.; Kaufmann, R.; et al. C6 Ceramide (d18:1/6:0) as a Novel Treatment of Cutaneous T Cell Lymphoma. Cancers 2021, 13, 270. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, F.; Zhang, Y.; Lin, Z.; Chen, J.L.; Diao, H. C6 Ceramide Inhibits Canine Mammary Cancer Growth and Metastasis by Targeting EGR3 through JAK1/STAT3 Signaling. Animals 2024, 14, 422. [Google Scholar] [CrossRef]
- Fujiwara, K.; Yazama, H.; Donishi, R.; Koyama, S.; Fukuhara, T.; Kitatani, K.; Kataoka, H.; Takeuchi, H. C6 Ceramide Inhibits the Motility of Anaplastic Thyroid Carcinoma Cells. Yonago Acta Med. 2020, 63, 95–98. [Google Scholar] [CrossRef]
- Thim, E.A.; Fox, T.; Deering, T.; Vass, L.R.; Sheybani, N.D.; Kester, M.; Price, R.J. Solid tumor treatment via augmentation of bioactive C6 ceramide levels with thermally ablative focused ultrasound. Drug Deliv. Transl. Res. 2023, 13, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.N.S.; Deol, K.K.; Lange, M.; Olzmann, J.A. A genome-wide CRISPR screen implicates plasma membrane asymmetry in exogenous C6 Ceramide toxicity. Biol. Open. 2022, 11, bio059695. [Google Scholar] [CrossRef]
- Baloglu, M.; Sezer, C.V.; Karadağ, G.; Yayla, E.; Kutlu, H.M. Exogenous Ceramide Treatment Induce Death and Cytotoxicity in Glioma Cells. Turk. Neurosurg. 2024, 34, 907–911. [Google Scholar]
- Dufau, C.; Genais, M.; Mucher, E.; Jung, B.; Garcia, V.; Montfort, A.; Tosolini, M.; Clarke, C.J.; Medin, J.A.; Levade, T.; et al. Ceramide metabolism alterations contribute to Tumor Necrosis Factor-induced melanoma dedifferentiation and predict resistance to immune checkpoint inhibitors in advanced melanoma patients. Front. Immunol. 2024, 15, 1421432. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.; Geiger, N.; Schlegel, J.; Diesendorf, V.; Kersting, L.; Fink, J.; Stelz, L.; Schneider-Schaulies, S.; Sauer, M.; Bodem, J.; et al. Azido-Ceramides, a Tool to Analyse SARS-CoV-2 Replication and Inhibition-SARS-CoV-2 Is Inhibited by Ceramides. Int. J. Mol. Sci. 2023, 24, 7281. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Liu, F.; Hu, J.; Liu, F.; Zou, J.; Wang, X. Ephrin B2 (EFNB2) potentially protects against intervertebral disc degeneration through inhibiting nucleus pulposus cell apoptosis. Arch. Biochem. Biophys. 2024, 756, 109990. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, S.; Liu, Q.; Qian, Q.; Fu, A.; Chen, L.; Zhang, L.; Suzaki, T.; Yu, Z.; Dou, X. Ectopic accumulation of ceramide in cardiomyocytes modulates alcoholic cardiomyopathy via the TLR4-dependent pathway. Alcohol Clin. Exp Res. 2022, 46, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, S.; Ding, Y.; Flores, J.J.; Zhang, X.; Jia, X.; Hu, X.; Peng, J.; Zuo, G.; Zhang, J.H.; et al. Secukinumab attenuates neuroinflammation and neurobehavior defect via PKCβ/ERK/NF-κB pathway in a rat model of GMH. Exp. Neurol. 2023, 360, 114276. [Google Scholar] [CrossRef]
- Zanieri, F.; Levi, A.; Montefusco, D.; Longato, L.; De Chiara, F.; Frenguelli, L.; Omenetti, S.; Andreola, F.; Luong, T.V.; Massey, V.; et al. Exogenous Liposomal Ceramide-C6 Ameliorates Lipidomic Profile, Energy Homeostasis, and Anti-Oxidant Systems in NASH. Cells 2020, 9, 1237. [Google Scholar] [CrossRef]
- Albi, E.; Arcuri, C.; Kobayashi, T.; Tomishige, N.; Cas, M.D.; Paroni, R.; Signorelli, P.; Cerquiglini, L.; Troiani, S.; Gizzi, C.; et al. Sphingomyelin in Human Breast Milk might be Essential for the Hippocampus Maturation. Front. Biosci. 2022, 27, 247. [Google Scholar] [CrossRef]
- Conte, C.; Cataldi, S.; Arcuri, C.; Mirarchi, A.; Lazzarini, A.; Garcia-Gil, M.; Beccari, T.; Curcio, F.; Albi, E. Vitamin D3 Enriches Ceramide Content in Exosomes Released by Embryonic Hippocampal Cells. Int. J. Mol. Sci. 2021, 22, 9287. [Google Scholar] [CrossRef]
- Tomishige, N.; Murate, M.; Didier, P.; Richert, L.; Mely, Y.; Kobayashi, T. The use of pore-forming toxins to image lipids and lipid domains. Methods Enzymol. 2021, 649, 503–542. [Google Scholar] [PubMed]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087361. [Google Scholar] [CrossRef] [PubMed]
- Shmagel, K.; Saidakova, E.; Korolevskaya, L.; Vlasova, V.; Younes, S.A. Activated/Cycling Treg Deficiency and Mitochondrial Alterations in Immunological Non-Responders to Antiretroviral Therapy. Front. Biosci. 2024, 29, 429. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Arcuri, C.; Hunot, S.; Mecca, C.; Codini, M.; Laurenti, M.E.; Ferri, I.; Loreti, E.; Garcia-Gil, M.; Traina, G.; et al. Effect of Vitamin D in HN9.10e Embryonic Hippocampal Cells and in Hippocampus from MPTP-Induced Parkinson’s Disease Mouse Model. Front. Cell Neurosci. 2018, 12, 31. [Google Scholar] [CrossRef]
- Jayadev, S.; Barrett, J.C.; Murphy, E. Elevated ceramide is downstream of altered calcium homeostasis in low serum-induced apoptosis. Am. J. Physiol. Cell Physiol. 2000, 279, C1640–C1647. [Google Scholar] [CrossRef]
- O’Hare Doig, R.L.; Bartlett, C.A.; Smith, N.M.; Hodgetts, S.I.; Dunlop, S.A.; Hool, L.; Fitzgerald, M. Specific combinations of ion channel inhibitors reduce excessive Ca2+ influx as a consequence of oxidative stress and increase neuronal and glial cell viability in vitro. Neuroscience 2016, 339, 450–462. [Google Scholar] [CrossRef]
- Zhu, Y.; Hui, Q.; Zhang, Z.; Fu, H.; Qin, Y.; Zhao, Q.; Li, Q.; Zhang, J.; Guo, L.; He, W.; et al. Advancements in the study of synaptic plasticity and mitochondrial autophagy relationship. J. Neurosci. Res. 2024, 102, e25309. [Google Scholar] [CrossRef]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef]
- Harada, A.J.; Foley, M.; Moskowitz, M.A.; Waeber, C. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J. Neurochem. 2004, 88, 1026–1039. [Google Scholar] [CrossRef]
- Taylor, O.; DeGroff, N.; El-Hodiri, H.; Gao, C.; Fischer, A.J. Sphingosine-1-phosphate signaling regulates the ability of Müller glia to become neurogenic, proliferating progenitor-like cells. bioRxiv 2025, 606815. [Google Scholar]
- Miranda, G.E.; Abrahan, C.E.; Politi, L.E.; Rotstein, N.P. Sphingosine-1-phosphate is a key regulator of proliferation and differentiation in retina photoreceptors. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4416–4428. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorani, F.; Mandarano, M.; Cataldi, S.; Mirarchi, A.; Bruscoli, S.; Ragonese, F.; Fioretti, B.; Kobayashi, T.; Tomishige, N.; Beccari, T.; et al. High Dose C6 Ceramide-Induced Response in Embryonic Hippocampal Cells. Biomolecules 2025, 15, 430. https://doi.org/10.3390/biom15030430
Fiorani F, Mandarano M, Cataldi S, Mirarchi A, Bruscoli S, Ragonese F, Fioretti B, Kobayashi T, Tomishige N, Beccari T, et al. High Dose C6 Ceramide-Induced Response in Embryonic Hippocampal Cells. Biomolecules. 2025; 15(3):430. https://doi.org/10.3390/biom15030430
Chicago/Turabian StyleFiorani, Federico, Martina Mandarano, Samuela Cataldi, Alessandra Mirarchi, Stefano Bruscoli, Francesco Ragonese, Bernard Fioretti, Toshihide Kobayashi, Nario Tomishige, Tommaso Beccari, and et al. 2025. "High Dose C6 Ceramide-Induced Response in Embryonic Hippocampal Cells" Biomolecules 15, no. 3: 430. https://doi.org/10.3390/biom15030430
APA StyleFiorani, F., Mandarano, M., Cataldi, S., Mirarchi, A., Bruscoli, S., Ragonese, F., Fioretti, B., Kobayashi, T., Tomishige, N., Beccari, T., Floridi, C., Arcuri, C., & Albi, E. (2025). High Dose C6 Ceramide-Induced Response in Embryonic Hippocampal Cells. Biomolecules, 15(3), 430. https://doi.org/10.3390/biom15030430







