Elevation of Plasma IL-15 and RANTES as Potential Biomarkers of Healing in Chronic Venous Ulcerations: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.1.1. Ethics Statement
2.1.2. Patient Selection
2.2. Sample Collection
2.2.1. Tissue
2.2.2. Blood and Plasma
2.2.3. Wound Measurements
2.3. RNA Sequencing and Genomic Analysis
2.3.1. Bulk RNA Sequencing
2.3.2. Gene Set Enrichment Analysis
2.4. Cytokine Analysis
2.4.1. Collection
2.4.2. Analysis of Cytokine Expression over Time and Comparison to mRNA Expression
2.4.3. Plasma Biomarker Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Patient and Wound Characteristics
3.3. Wound Biopsy Gene Expression
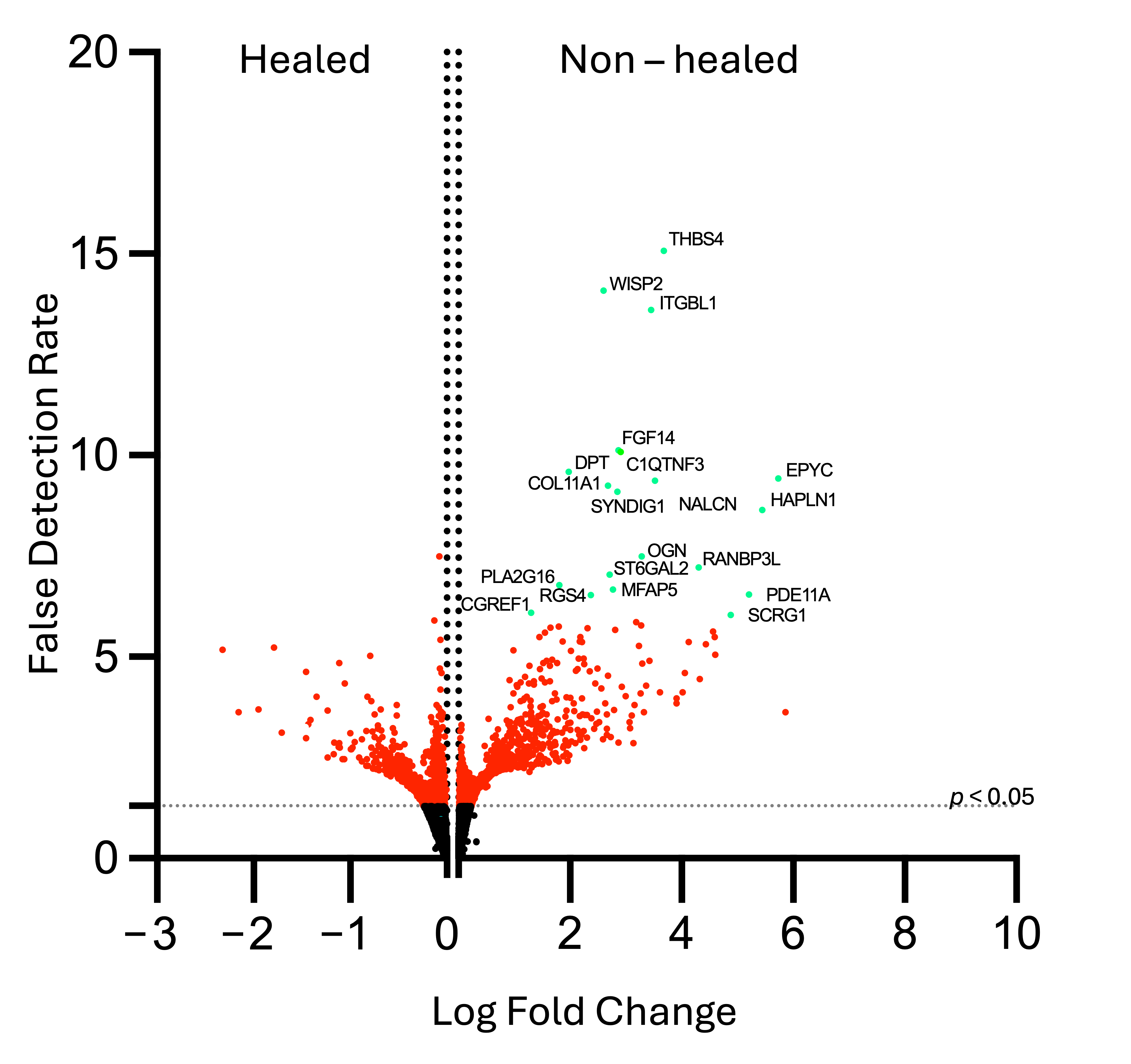
3.3.1. Most Highly Expressed Genes Are Related to Cell Signaling, Collagen Formation, and Extracellular Matrix Deposition
3.3.2. Gene Set Enrichment Analysis Reveals Pathways Enriched in Non-Healed Cohort
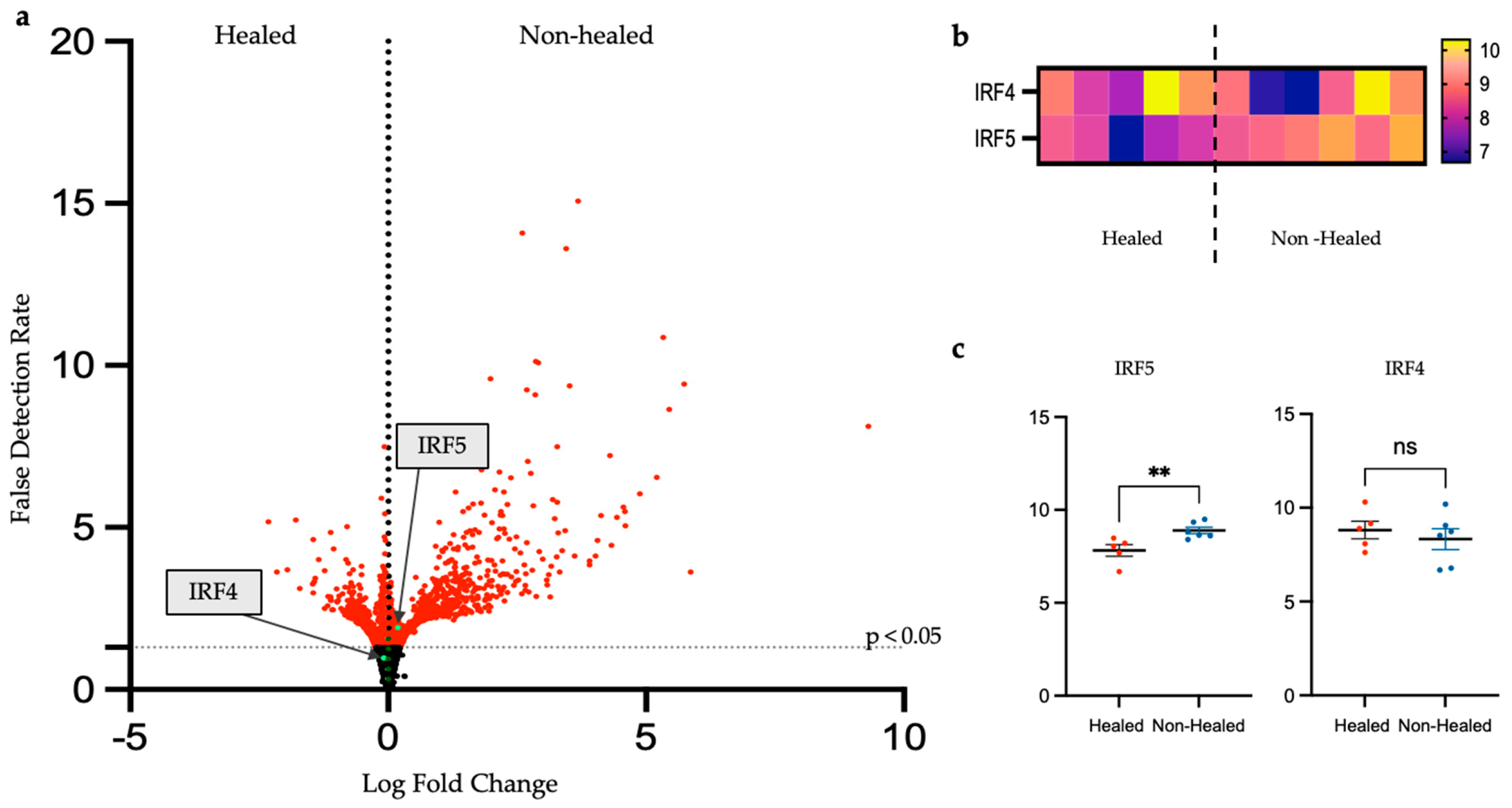
3.3.3. In-Depth Analysis of Inflammatory Gene Expression
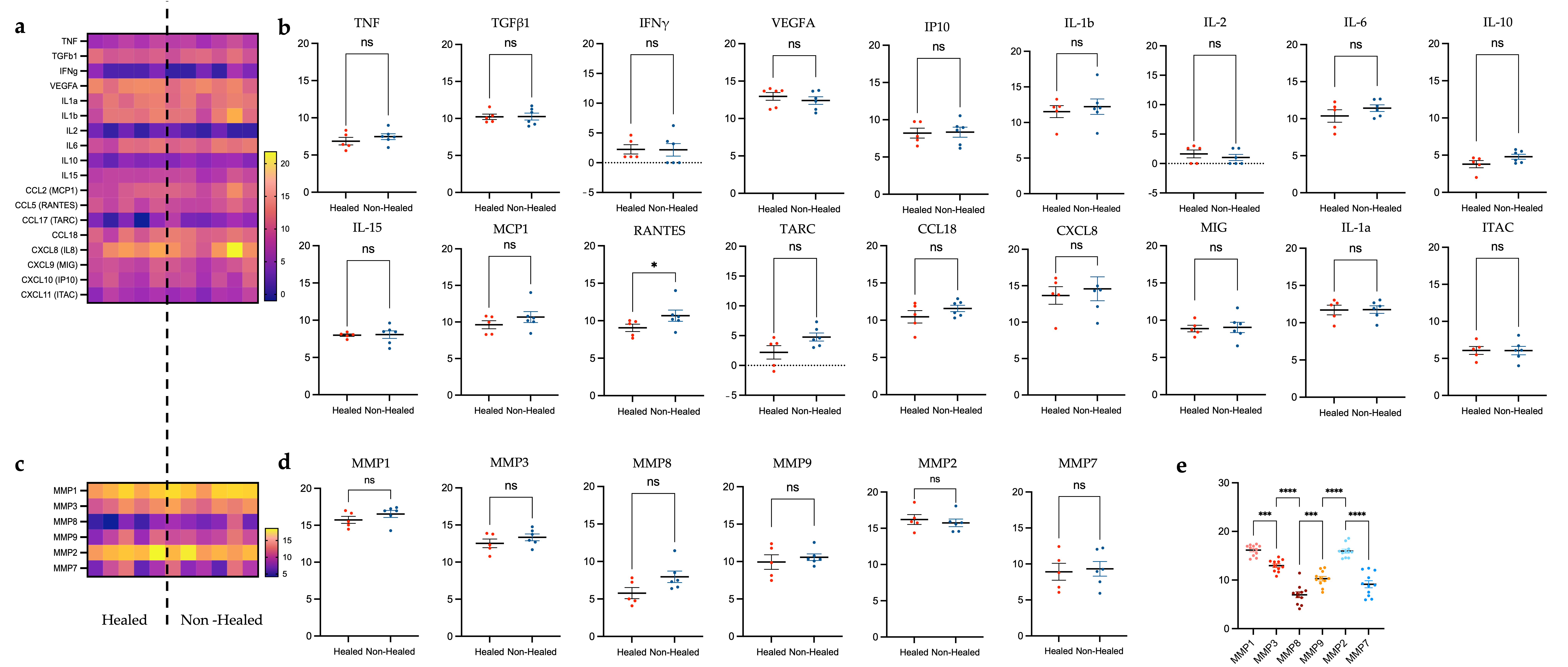
3.4. Plasma Cytokine Analysis
3.4.1. Differences in Cytokine Levels
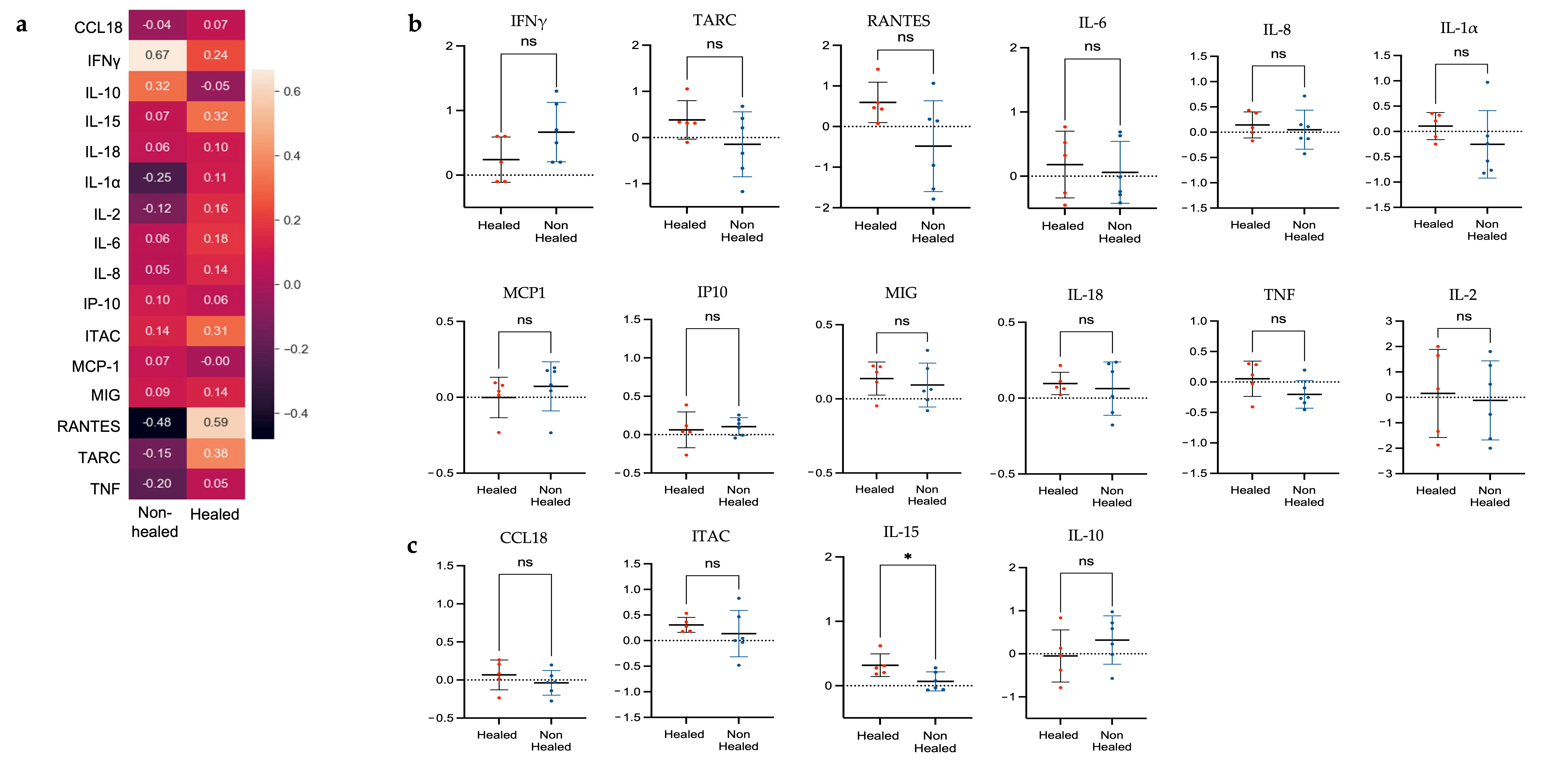
3.4.2. Correlation Analysis of Gene Expression and Cytokine Levels
3.4.3. Predictive Potential of Plasma Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates. Adv. Wound Care 2023, 12, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Stanek, A.; Mosti, G.; Nematillaevich, T.S.; Valesky, E.M.; Planinšek Ručigaj, T.; Boucelma, M.; Marakomichelakis, G.; Liew, A.; Fazeli, B.; Catalano, M.; et al. No More Venous Ulcers—What More Can We Do? J. Clin. Med. 2023, 12, 6153. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and Pathophysiology of Matrix Metalloproteases. Amino Acids 2011, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar] [PubMed]
- O’Donnell, T.F.; Passman, M.A.; Marston, W.A.; Ennis, W.J.; Dalsing, M.; Kistner, R.L.; Lurie, F.; Henke, P.K.; Gloviczki, M.L.; Eklöf, B.G.; et al. Management of Venous Leg Ulcers: Clinical Practice Guidelines of the Society for Vascular Surgery® and the American Venous Forum. J. Vasc. Surg. 2014, 60, 3S–59S. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and Management of Lower-Extremity Ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef]
- Krizanova, O.; Penesova, A.; Hokynkova, A.; Pokorna, A.; Samadian, A.; Babula, P. Chronic Venous Insufficiency and Venous Leg Ulcers: Aetiology, on the Pathophysiology-based Treatment. Int. Wound J. 2023, 21, e14405. [Google Scholar] [CrossRef]
- Ragnarson Tennvall, G.; Hjelmgren, J. Annual Costs of Treatment for Venous Leg Ulcers in Sweden and the United Kingdom. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2005, 13, 13–18. [Google Scholar] [CrossRef]
- Kantor, J.; Margolis, D.J. A Multicentre Study of Percentage Change in Venous Leg Ulcer Area as a Prognostic Index of Healing at 24 Weeks. Br. J. Dermatol. 2000, 142, 960–964. [Google Scholar] [CrossRef]
- Edwards, H.E.; Parker, C.N.; Miller, C.; Gibb, M.; Kapp, S.; Ogrin, R.; Anderson, J.; Coleman, K.; Smith, D.; Finlayson, K.J. Predicting Delayed Healing: The Diagnostic Accuracy of a Venous Leg Ulcer Risk Assessment Tool. Int. Wound J. 2017, 15, 258–265. [Google Scholar] [CrossRef]
- Goto, T.; Saligan, L.N. Wound Pain and Wound Healing Biomarkers from Wound Exudate: A Scoping Review. J. Wound Ostomy Cont. Nurs. Off. Publ. Wound Ostomy Cont. Nurses Soc. 2020, 47, 559–568. [Google Scholar] [CrossRef]
- Kirsner, R.S. The Wound Healing Society Chronic Wound Ulcer Healing Guidelines Update of the 2006 Guidelines--Blending Old with New. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2016, 24, 110–111. [Google Scholar] [CrossRef]
- Mendy, M.; Caboux, E.; Lawlor, R.T.; Wright, J.; Wild, C.P. Selected Protocols. In Common Minimum Technical Standards and Protocols for Biobanks Dedicated to Cancer Research; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Robson, M.C.; Cooper, D.M.; Aslam, R.; Gould, L.J.; Harding, K.G.; Margolis, D.J.; Ochs, D.E.; Serena, T.E.; Snyder, R.J.; Steed, D.L.; et al. Guidelines for the Treatment of Venous Ulcers. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2006, 14, 649–662. [Google Scholar] [CrossRef]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and Guidelines for Assessment of Wounds and Evaluation of Healing. Arch. Dermatol. 1994, 130, 489–493. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Burian, E.A.; Sabah, L.; Karlsmark, T.; Kirketerp-Møller, K.; Moffatt, C.J.; Thyssen, J.P.; Ågren, M.S. Cytokines and Venous Leg Ulcer Healing—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6526. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Dai, H.; Zhao, X.; Gui, C.; Gui, J. AKT3 Deficiency in M2 Macrophages Impairs Cutaneous Wound Healing by Disrupting Tissue Remodeling. Aging 2020, 12, 6928–6946. [Google Scholar] [CrossRef] [PubMed]
- Cafasso, D.E.; Bowen, D.K.; Kinkennon, S.A.; Stanbro, M.D.; Kellicut, D.C. Heterotopic Ossificans in Chronic Venous Insufficiency: A New Consideration for Clinical, Aetiology, Anatomy and Pathophysiology Staging. Phlebology 2013, 28, 361–365. [Google Scholar] [CrossRef]
- Oropallo, A.; Beneat, A.; Rao, A.; Goodman, E. Revisiting Heinz Lippman Disease as a Complication of Chronic Venous Insufficiency. J. Vasc. Surg. Cases Innov. Tech. 2023, 10, 101408. [Google Scholar] [CrossRef]
- Kan, C.; Chen, L.; Hu, Y.; Ding, N.; Lu, H.; Li, Y.; Kessler, J.A.; Kan, L. Conserved Signaling Pathways Underlying Heterotopic Ossification. Bone 2018, 109, 43–48. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Y.; Xu, Y.; Lin, W.-W.; Luo, Z.; Han, Z.; Liu, S.; Qi, B.; Sun, C.; Go, K.; et al. Single-Cell Integration Analysis of Heterotopic Ossification and Fibrocartilage Developmental Lineage: Endoplasmic Reticulum Stress Effector Xbp1 Transcriptionally Regulates the Notch Signaling Pathway to Mediate Fibrocartilage Differentiation. Oxid. Med. Cell. Longev. 2021, 2021, 7663366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, B.; Shao, J.; Song, J.; Zhang, J. Proteomic Profiling of Posterior Longitudinal Ligament of Cervical Spine. Int. J. Clin. Exp. Med. 2015, 8, 5631–5639. [Google Scholar]
- Aomatsu, E.; Takahashi, N.; Sawada, S.; Okubo, N.; Hasegawa, T.; Taira, M.; Miura, H.; Ishisaki, A.; Chosa, N. Novel SCRG1/BST1 Axis Regulates Self-Renewal, Migration, and Osteogenic Differentiation Potential in Mesenchymal Stem Cells. Sci. Rep. 2014, 4, 3652. [Google Scholar] [CrossRef]
- Gumede, D.B.; Abrahamse, H.; Houreld, N.N. Targeting Wnt/β-Catenin Signaling and Its Interplay with TGF-β and Notch Signaling Pathways for the Treatment of Chronic Wounds. Cell Commun. Signal. CCS 2024, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Maan, Z.N.; Whittam, A.J.; Siemers, F. Wnt Signaling Induces Epithelial Differentiation during Cutaneous Wound Healing. Organogenesis 2015, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.L.; Smith, A.A.; Helms, J.A. Wnt Signaling and Injury Repair. Cold Spring Harb. Perspect. Biol. 2012, 4, a008078. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.-M.; Qin, Y.-Z.; Wu, S.-W.; Ma, L.; Tan, Y.-Y.; Yong, X.; Wang, X.-L.; Wang, Z.P.; Tao, Y.-S. WISP2 Exhibits Its Potential Antitumor Activity via Targeting ERK and E-Cadherin Pathways in Esophageal Cancer Cells. J. Exp. Clin. Cancer Res. CR 2019, 38, 102. [Google Scholar] [CrossRef]
- Ferrante, C.J.; Leibovich, S.J. Regulation of Macrophage Polarization and Wound Healing. Adv. Wound Care 2012, 1, 10–16. [Google Scholar] [CrossRef]
- Wei, Z.; Yan, L.; Chen, Y.; Bao, C.; Deng, J.; Deng, J. Mangiferin Inhibits Macrophage Classical Activation via Downregulating Interferon Regulatory Factor 5 Expression. Mol. Med. Rep. 2016, 14, 1091–1098. [Google Scholar] [CrossRef]
- Juhas, U.; Ryba-Stanisławowska, M.; Szargiej, P.; Myśliwska, J. Different Pathways of Macrophage Activation and Polarization. Postepy Hig. Med. Dosw. Online 2015, 69, 496–502. [Google Scholar] [CrossRef]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 Promotes Inflammatory Macrophage Polarization and TH1-TH17 Responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef]
- Huang, S.C.-C.; Smith, A.M.; Everts, B.; Colonna, M.; Pearce, E.L.; Schilling, J.D.; Pearce, E.J. mTORC2-IRF4 Mediated Metabolic Reprograming Is Essential for Macrophage Alternative Activation. Immunity 2016, 45, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; Marikovsky, M.; Harper, J.W.; Vogt, P.; Eriksson, E.; Klagsbrun, M.; Langer, R. Temporal Study of the Activity of Matrix Metalloproteinases and Their Endogenous Inhibitors during Wound Healing. J. Cell. Biochem. 1996, 60, 379–386. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Hogaboam, C.M.; Kunkel, S.L.; Proudfoot, A.E.I.; Wallace, J.L. The Chemokine RANTES Is a Crucial Mediator of the Progression from Acute to Chronic Colitis in the Rat1. J. Immunol. 2001, 166, 552–558. [Google Scholar] [CrossRef]
- Plater-Zyberk, C.; Hoogewerf, A.J.; Proudfoot, A.E.; Power, C.A.; Wells, T.N. Effect of a CC Chemokine Receptor Antagonist on Collagen Induced Arthritis in DBA/1 Mice. Immunol. Lett. 1997, 57, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Gröne, H.J.; Weber, C.; Weber, K.S.; Gröne, E.F.; Rabelink, T.; Klier, C.M.; Wells, T.N.; Proudfood, A.E.; Schlöndorff, D.; Nelson, P.J. Met-RANTES Reduces Vascular and Tubular Damage during Acute Renal Transplant Rejection: Blocking Monocyte Arrest and Recruitment. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 1371–1383. [Google Scholar] [CrossRef]
- Li, N.; Mirzakhani, H.; Kiefer, A.; Koelle, J.; Vuorinen, T.; Rauh, M.; Yang, Z.; Krammer, S.; Xepapadaki, P.; Lewandowska-Polak, A.; et al. Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) Drives the Resolution of Allergic Asthma. iScience 2021, 24, 103163. [Google Scholar] [CrossRef]
- López-Cuevas, P.; Oates, T.C.L.; Tong, Q.; McGowan, L.M.; Cross, S.J.; Xu, C.; Zhao, Y.; Yin, Z.; Toye, A.M.; Boussahel, A.; et al. Reprogramming Macrophages with R848-Loaded Artificial Protocells to Modulate Skin and Skeletal Wound Healing. J. Cell Sci. 2024, 137, jcs262202. [Google Scholar] [CrossRef]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The Impact of Prolonged Inflammation on Wound Healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef]
- De Mesquita, C.J.G.; Leite, J.A.D.; Fechine, F.V.; De Rocha, C.J.L.; Leite, J.G.S.; Leite Filho, J.A.D.; Barbosa Filho, R.C.C. Effect of Imiquimod on Partial-Thickness Burns. Burns J. Int. Soc. Burn Inj. 2010, 36, 97–108. [Google Scholar] [CrossRef]
- Obeid, J.; Shaito, A.; El Hajj, H.; Deleuze-masquefa, C.; Bonnet, P.-A.; El-Sabban, M.; Saliba, J. Biological Applications of Imiquimod Analogues: An Update. World Acad. Sci. J. 2023, 5, 20. [Google Scholar] [CrossRef]
- Bubna, A.K. Imiquimod—Its Role in the Treatment of Cutaneous Malignancies. Indian J. Pharmacol. 2015, 47, 354–359. [Google Scholar] [CrossRef]
- Frank, S.; Kämpfer, H.; Wetzler, C.; Stallmeyer, B.; Pfeilschifter, J. Large Induction of the Chemotactic Cytokine RANTES during Cutaneous Wound Repair: A Regulatory Role for Nitric Oxide in Keratinocyte-Derived RANTES Expression. Biochem. J. 2000, 347, 265–273. [Google Scholar] [CrossRef]
- Kroeze, K.L.; Jurgens, W.J.; Doulabi, B.Z.; Van Milligen, F.J.; Scheper, R.J.; Gibbs, S. Chemokine-Mediated Migration of Skin-Derived Stem Cells: Predominant Role for CCL5/RANTES. J. Investig. Dermatol. 2009, 129, 1569–1581. [Google Scholar] [CrossRef]
- Perera, P.-Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The Role of Interleukin-15 in Inflammation and Immune Responses to Infection: Implications for Its Therapeutic Use. Microbes Infect. Inst. Pasteur 2012, 14, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Patidar, M.; Yadav, N.; Dalai, S.K. Interleukin 15: A Key Cytokine for Immunotherapy. Cytokine Growth Factor Rev. 2016, 31, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Fehniger, T.A.; Caligiuri, M.A. Interleukin 15: Biology and Relevance to Human Disease. Blood 2001, 97, 14–32. [Google Scholar] [CrossRef]
- Tang, F.; Zhao, L.; Jiang, Y.; Ba, D.; Cui, L.; He, W. Activity of Recombinant Human Interleukin-15 against Tumor Recurrence and Metastasis in Mice. Cell. Mol. Immunol. 2008, 5, 189–196. [Google Scholar] [CrossRef]
- Chamie, K.; Chang, S.S.; Kramolowsky, E.; Gonzalgo, M.L.; Agarwal, P.K.; Bassett, J.C.; Bjurlin, M.; Cher, M.L.; Clark, W.; Cowan, B.E.; et al. IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. NEJM Evid. 2022, 2, EVIDoa2200167. [Google Scholar] [CrossRef]
- Cheever, M.A. Twelve Immunotherapy Drugs That Could Cure Cancers. Immunol. Rev. 2008, 222, 357–368. [Google Scholar] [CrossRef]
- Han, K.; Zhu, X.; Liu, B.; Jeng, E.; Kong, L.; Yovandich, J.L.; Vyas, V.V.; Marcus, W.D.; Chavaillaz, P.-A.; Romero, C.A.; et al. IL-15:IL-15 Receptor Alpha Superagonist Complex: High-Level Co-Expression in Recombinant Mammalian Cells, Purification and Characterization. Cytokine 2011, 56, 804–810. [Google Scholar] [CrossRef]
- Mortier, E.; Quéméner, A.; Vusio, P.; Lorenzen, I.; Boublik, Y.; Grötzinger, J.; Plet, A.; Jacques, Y. Soluble Interleukin-15 Receptor Alpha (IL-15R Alpha)-Sushi as a Selective and Potent Agonist of IL-15 Action through IL-15R Beta/Gamma. Hyperagonist IL-15 x IL-15R Alpha Fusion Proteins. J. Biol. Chem. 2006, 281, 1612–1619. [Google Scholar] [CrossRef]
- Suzuki, A.; McCall, S.; Choi, S.S.; Sicklick, J.K.; Huang, J.; Qi, Y.; Zdanowicz, M.; Camp, T.; Li, Y.-X.; Diehl, A.M. Interleukin-15 Increases Hepatic Regenerative Activity. J. Hepatol. 2006, 45, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bai, Y.; Li, Y.; Liang, G.; Jiang, Y.; Liu, Z.; Liu, M.; Hao, J.; Zhang, X.; Hu, X.; et al. IL-15 Enhances Activation and IGF-1 Production of Dendritic Epidermal T Cells to Promote Wound Healing in Diabetic Mice. Front. Immunol. 2017, 8, 1557. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Griffiths, J.L.; Sanders, A.J.; Owen, S.; Ruge, F.; Harding, K.G.; Jiang, W.G. The Clinical Significance and Impact of Interleukin 15 on Keratinocyte Cell Growth and Migration. Int. J. Mol. Med. 2016, 38, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Komine, M.; Fujimoto, M.; Okochi, H.; Tamaki, K. Interleukin 15 Induces the Signals of Epidermal Proliferation through ERK and PI 3-Kinase in a Human Epidermal Keratinocyte Cell Line, HaCaT. Biochem. Biophys. Res. Commun. 2003, 301, 841–847. [Google Scholar] [CrossRef]
- Lindner, G.; Rückert, R.; Bulfone-Paus, S.; Paus, R. Inhibition of Chemotherapy-Induced Keratinocyte Apoptosis in Vivo by an Interleukin-15-IgG Fusion Protein. J. Investig. Dermatol. 1998, 110, 457–458. [Google Scholar] [CrossRef]
- Wong, W.; Crane, E.D.; Kuo, Y.; Kim, A.; Crane, J.D. The Exercise Cytokine Interleukin-15 Rescues Slow Wound Healing in Aged Mice. J. Biol. Chem. 2019, 294, 20024–20038. [Google Scholar] [CrossRef]
- Kagimoto, Y.; Yamada, H.; Ishikawa, T.; Maeda, N.; Goshima, F.; Nishiyama, Y.; Furue, M.; Yoshikai, Y. A Regulatory Role of Interleukin 15 in Wound Healing and Mucosal Infection in Mice. J. Leukoc. Biol. 2008, 83, 165–172. [Google Scholar] [CrossRef]




| Characteristic | Fequency | Percent | |
|---|---|---|---|
| Age | 40–49 | 2 | 18.2 |
| 50–59 | 3 | 27.3 | |
| >60 | 6 | 54.5 | |
| Sex | Male | 7 | 63.6 |
| Female | 4 | 36.4 | |
| Race | White | 4 | 36.4 |
| African American | 4 | 36.4 | |
| Asian | 1 | 9.1 | |
| Arabic | 1 | 9.1 | |
| Other | 1 | 9.1 | |
| Ethnicity | Hispanic | 1 | 9.1 |
| Non-Hispanic | 10 | 90.9 | |
| BMI | 18–24 | 1 | 9.1 |
| 25–30 | 5 | 45.5 | |
| 31–40 | 5 | 45.5 | |
| Smoking Status | Never | 4 | 36.4 |
| Former | 7 | 63.6 | |
| Comorbidities | Hypertension | 8 | 72.7 |
| Hyperlipidemia | 4 | 36.4 | |
| Coronary Artery Disease | 2 | 18.2 | |
| Pre-Diabetes | 2 | 18.2 | |
| History of VTE | 2 | 18.2 | |
| Lymphedema | 2 | 18.2 | |
| Chronic Venous Insufficiency | 11 | 100 |
| Healed | Unhealed | p-Value | |
|---|---|---|---|
| Ulcer Size (cm2) | 9.37 (±6.64) | 11.2 (±9.80) | 0.792 |
| Ulcer Age (months) | 7.8 (±4.02) | 39.5 (±69.2) | 0.365 |
| Healing Time (months) | 4.42 (±2.53) | 13.7 (±11.0) | 0.077 |
| Pathway | Gene | Log Fold Change | False Detection Rate | Adjusted p-Value |
|---|---|---|---|---|
| Cell Adhesion | WNT1-inducible Signaling Pathway Protein 2 (WISP 2) | 2.604 | 14.08 | 9.87 × 10−11 |
| Integrin Beta-like 1 Protein (ITGBL1) | 3.451 | 13.6 | 2.02 × 10−11 | |
| Cell Signaling, Growth, and Migration | Phosphodiesterase 11A (PDE11A) | 5.21 | 6.53 | 3.27 × 10−4 |
| Regulator of G-protein Signaling 4 (RGS4) | 2.377 | 6.77 | 2.26 × 10−4 | |
| Phospholipase A2, Group XVI (PLA2G16) | 1.808 | 6.54 | 3.27 × 10−4 | |
| ST6-Beta-Galactoside Alpha 2,6 Sialyltransferase (ST6GAL2) | 2.706 | 7.04 | 1.29 × 10−4 | |
| Complement C1q Tumor Necrosis Factor-related Protein 3 (C1QTNF3) | 2.866 | 10.13 | 3.28 × 10−11 | |
| Cell Growth Regulator with EF-hand Domain 1 (CGREF1) | 1.307 | 6.1 | 7.67 × 10−4 | |
| Sodium Leak Channel (NALCN) | 3.521 | 9.37 | 1.15 × 10−6 | |
| Extracellular Matrix | Thrombospondin 4 (THBS4) | 3.678 | 15.07 | 2.03 × 10−11 |
| Dermatopontin (DPT) | 1.982 | 9.59 | 8.94 × 10−7 | |
| Hyaluronan and Proteoglycan Link Protein 1 (HALPN1) | 5.447 | 8.54 | 4.95 × 10−6 | |
| Fibroblast Growth Factor 14 (FGF14) | 2.909 | 10.09 | 3.28 × 10−7 | |
| RAN Binding Protein 3-like (RANBP3L) | 4.302 | 7.22 | 9.01 × 10−5 | |
| Fibrosis and Scarring | Microfibrillar-associated protein 5 (MFAP5) | 2.77 | 6.67 | 2.55 × 10−4 |
| Chondrogenesis and Bone Formation | Osteoglycin (OGN) | 3.281 | 7.5 | 5.21 × 10−5 |
| Epiphycan (EPYC) | 5.734 | 9.42 | 1.15 × 10−6 | |
| Collagen alpha-1 (XI) (COL11A1) | 2.685 | 9.24 | 1.37 × 10−6 | |
| Stimulator of Chondrogenesis 1 (SCRG1) | 4.884 | 6.04 | 8.5 × 10−4 | |
| Neuronal Development | Synapse Differentiation Inducing 1 (SYNDIG1) | 2.851231 | 9.09283 | 1.77 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beneat, A.; Rueda, V.; Patel, H.; Brune, Z.; Sherry, B.; Shih, A.; Kaplan, S.; Rao, A.; Lee, A.; Varghese, A.; et al. Elevation of Plasma IL-15 and RANTES as Potential Biomarkers of Healing in Chronic Venous Ulcerations: A Pilot Study. Biomolecules 2025, 15, 395. https://doi.org/10.3390/biom15030395
Beneat A, Rueda V, Patel H, Brune Z, Sherry B, Shih A, Kaplan S, Rao A, Lee A, Varghese A, et al. Elevation of Plasma IL-15 and RANTES as Potential Biomarkers of Healing in Chronic Venous Ulcerations: A Pilot Study. Biomolecules. 2025; 15(3):395. https://doi.org/10.3390/biom15030395
Chicago/Turabian StyleBeneat, Amanda, Vikki Rueda, Hardik Patel, Zarina Brune, Barbara Sherry, Andrew Shih, Sally Kaplan, Amit Rao, Annette Lee, Asha Varghese, and et al. 2025. "Elevation of Plasma IL-15 and RANTES as Potential Biomarkers of Healing in Chronic Venous Ulcerations: A Pilot Study" Biomolecules 15, no. 3: 395. https://doi.org/10.3390/biom15030395
APA StyleBeneat, A., Rueda, V., Patel, H., Brune, Z., Sherry, B., Shih, A., Kaplan, S., Rao, A., Lee, A., Varghese, A., Oropallo, A., & Barnes, B. J. (2025). Elevation of Plasma IL-15 and RANTES as Potential Biomarkers of Healing in Chronic Venous Ulcerations: A Pilot Study. Biomolecules, 15(3), 395. https://doi.org/10.3390/biom15030395







