Intestinal-Failure-Associated Liver Disease: Beyond Parenteral Nutrition
Abstract
1. Introduction
2. Clinical Presentation and Diagnosis of IFALD
3. PN and Its Role in IFALD Pathogenesis
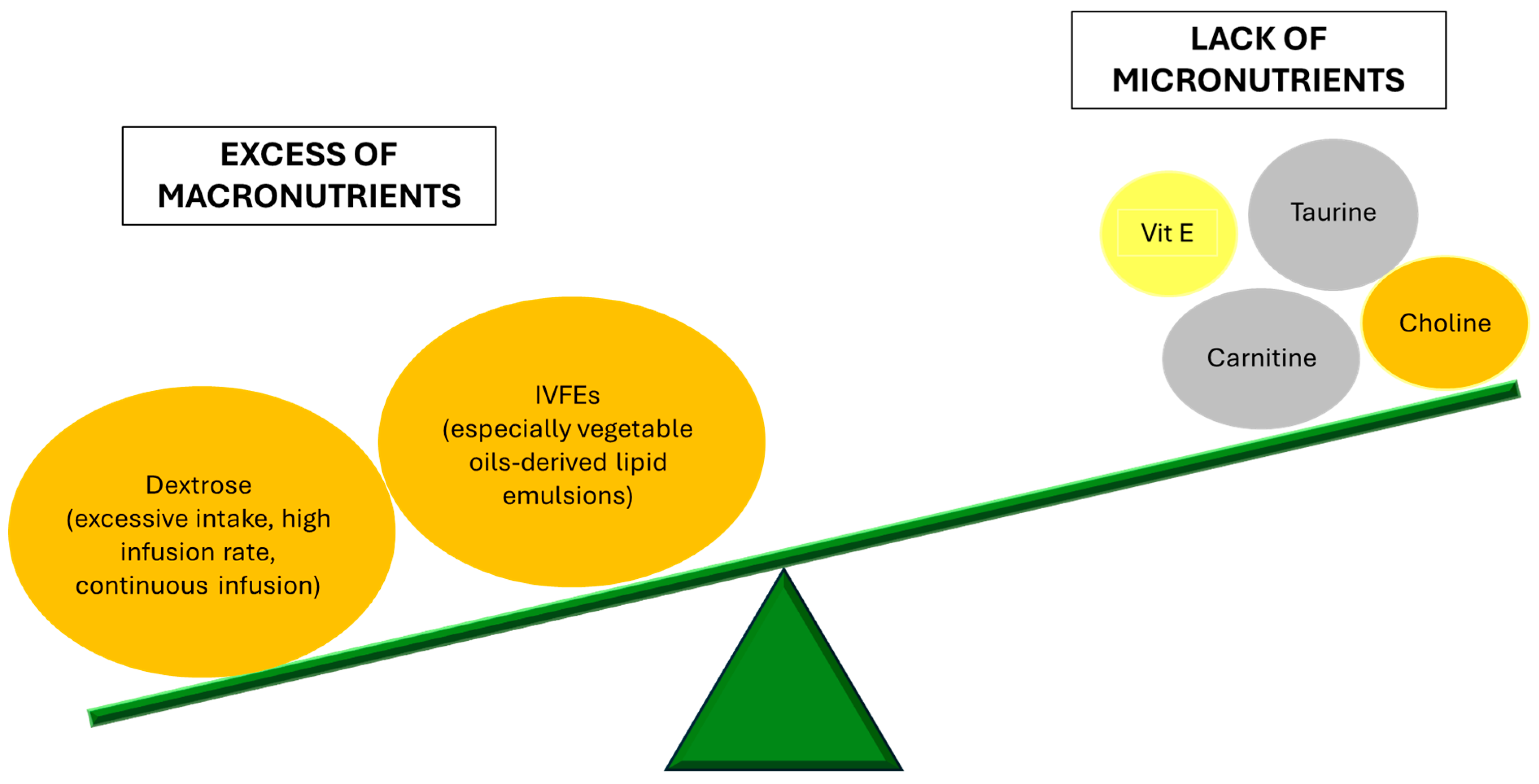
4. Beyond PN: Other Etiopathogenetic Factors
5. Therapeutic Strategies and Future Perspectives
5.1. Bile Acid Signaling Targets
5.1.1. Bile Acid Receptor Ligands
5.1.2. Bile Acid Replacement Therapy
5.2. Hormonal and Growth Factor Therapies
5.3. Microbial Modulation
5.3.1. Antibiotics
5.3.2. Probiotics
5.4. Surgical Treatments
5.4.1. Surgical Lengthening Procedures
5.4.2. Transplantation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| APRI | Aspartate-aminotransferase-to-platelet ratio index |
| AST | Aspartate aminotransferase |
| BSH | Bile salt hydrolase |
| CCK-OP | Cholecystokinin-octapeptide |
| CDCA | Chenodeoxycholic acid |
| CIF | Chronic intestinal failure |
| CRBSs | Catheter-related bloodstream infections |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| DPP4 | Dipeptidyl peptidase IV |
| FXR | Farnesoid-X receptor |
| FIB-4 | Fibrosis index based on the 4 factor |
| GLP-2 | Glucagon-like peptide-2 |
| HPN | Home parenteral nutrition |
| HIVFs | Home intravenous fluids |
| ICR | Ileocecal resection |
| IF | Intestinal failure |
| IFALD | Intestinal-failure-associated liver disease |
| ILEs | Lipid-injectable emulsions |
| IVS | Intravenous nutritional supplementation |
| IVFEs | Intravenous fat emulsions |
| LGG | Lactobacillus rhamnosus GG |
| LFTs | Liver function tests |
| LILT | Longitudinal intestinal lengthening and tailoring |
| MCTs | Medium-chain triglycerides |
| NAFLD | Nonalcoholic fatty liver disease |
| OCA | Obeticholic acid |
| PNALD | Parenteral-nutrition-associated liver disease |
| PUFA | Polyunsaturated fatty acids |
| rh-HGF | Recombinant human hepatocyte growth factor |
| SBS | Short bowel syndrome |
| SCFA | Short-chain fatty acids |
| SIBO | Small intestinal bacterial overgrowth |
| SILT | Spiral intestinal lengthening and tailoring |
| STEP | Serial transverse enteroplasty |
| TGR5 | Takeda-G-protein-coupled receptor 5 |
| TLR | Toll-like receptors |
| TNFα | Tumor necrosis factor α |
| TPN | Total parenteral nutrition |
| γGT | Gamma-glutamyl transferase |
References
- Amiot, A.; Messing, B.; Corcos, O.; Panis, Y.; Joly, F. Determinants of Home Parenteral Nutrition Dependence and Survival of 268 Patients with Non-Malignant Short Bowel Syndrome. Clin. Nutr. 2013, 32, 368–374. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN Endorsed Recommendations. Definition and Classification of Intestinal Failure in Adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Carbonnel, F.; Cosnes, J.; Chevret, S.; Beaugerie, L.; Ngô, Y.; Malafosse, M.; Parc, R.; Le Quintrec, Y.; Gendre, J.P. The Role of Anatomic Factors in Nutritional Autonomy after Extensive Small Bowel Resection. JPEN J. Parenter. Enter. Nutr. 1996, 20, 275–280. [Google Scholar] [CrossRef]
- Billiauws, L.; Maggiori, L.; Joly, F.; Panis, Y. Medical and Surgical Management of Short Bowel Syndrome. J. Visc. Surg. 2018, 155, 283–291. [Google Scholar] [CrossRef]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on Home Parenteral Nutrition for Benign Intestinal Failure: A Review of the Literature and Benchmarking with the European Prospective Survey of ESPEN. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.B.; Bone, J.N.; Van Oerle, R.; Albersheim, S.; Casey, L.; Piper, H. The Importance of the Ileocecal Valve and Colon in Achieving Intestinal Independence in Infants with Short Bowel Syndrome. J. Pediatr. Surg. 2022, 57, 117–121. [Google Scholar] [CrossRef]
- Smith, A.; Namjoshi, S.; Kerner, J.A.; Dunn, J.C.Y. Importance of Ileum and Colon in Children with Short Bowel Syndrome. J. Pediatr. Surg. 2023, 58, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Billiauws, L.; Thomas, M.; Le Beyec-Le Bihan, J.; Joly, F. Intestinal Adaptation in Short Bowel Syndrome. What Is New? Nutr. Hosp. 2018, 35, 731–737. [Google Scholar] [CrossRef]
- Pironi, L.; Cuerda, C.; Jeppesen, P.B.; Joly, F.; Jonkers, C.; Krznarić, Ž.; Lal, S.; Lamprecht, G.; Lichota, M.; Mundi, M.S.; et al. ESPEN Guideline on Chronic Intestinal Failure in Adults—Update 2023. Clin. Nutr. 2023, 42, 1940–2021. [Google Scholar] [CrossRef]
- Grainger, J.T.; Maeda, Y.; Donnelly, S.C.; Vaizey, C.J. Assessment and Management of Patients with Intestinal Failure: A Multidisciplinary Approach. Clin. Exp. Gastroenterol. 2018, 11, 233–241. [Google Scholar] [CrossRef]
- Pironi, L.; Sasdelli, A.S. Intestinal Failure-Associated Liver Disease. Clin. Liver Dis. 2019, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, L.; Schneider, S.M.; Berner, Y.N.; Cederholm, T.; Krznaric, Z.; Shenkin, A.; Stanga, Z.; Toigo, G.; Vandewoude, M.; Volkert, D.; et al. ESPEN Guidelines on Parenteral Nutrition: Geriatrics. Clin. Nutr. 2009, 28, 461–466. [Google Scholar] [CrossRef]
- Rager, R.; Finegold, M.J. Cholestasis in Immature Newborn Infants: Is Parenteral Alimentation Responsible? J. Pediatr. 1975, 86, 264–269. [Google Scholar] [CrossRef]
- Lal, S.; Pironi, L.; Wanten, G.; Arends, J.; Bozzetti, F.; Cuerda, C.; Joly, F.; Kelly, D.; Staun, M.; Szczepanek, K.; et al. Clinical Approach to the Management of Intestinal Failure Associated Liver Disease (IFALD) in Adults: A Position Paper from the Home Artificial Nutrition and Chronic Intestinal Failure Special Interest Group of ESPEN. Clin. Nutr. 2018, 37, 1794–1797. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Bozzetti, F.; Cuerda, C.; Gillanders, L.; Jeppesen, P.B.; Joly, F.; Kelly, D.; Lal, S.; Staun, M.; et al. ESPEN Guidelines on Chronic Intestinal Failure in Adults. Clin. Nutr. 2016, 35, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Naini, B.V.; Spilker, B. The Differentiation of Intestinal-Failure-Associated Liver Disease from Nonalcoholic Fatty Liver and Nonalcoholic Steatohepatitis. Semin. Liver Dis. 2017, 37, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Beath, S.V.; Kelly, D.A. Total Parenteral Nutrition-Induced Cholestasis: Prevention and Management. Clin. Liver Dis. 2016, 20, 159–176. [Google Scholar] [CrossRef]
- Cahova, M.; Bratova, M.; Wohl, P. Parenteral Nutrition-Associated Liver Disease: The Role of the Gut Microbiota. Nutrients 2017, 9, 987. [Google Scholar] [CrossRef]
- Denton, C.; Price, A.; Friend, J.; Manithody, C.; Blomenkamp, K.; Westrich, M.; Kakarla, V.; Phillips, W.; Krebs, J.; Abraham Munoz, S.; et al. Role of the Gut−Liver Axis in Driving Parenteral Nutrition-Associated Injury. Children 2018, 5, 136. [Google Scholar] [CrossRef]
- Lacaille, F.; Gupte, G.; Colomb, V.; D’Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R.; ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. Intestinal Failure-Associated Liver Disease: A Position Paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 272–283. [Google Scholar] [CrossRef]
- Pironi, L.; Labate, A.M.M.; Pertkiewicz, M.; Przedlacki, J.; Tjellesen, L.; Staun, M.; De Francesco, A.; Gallenca, P.; Guglielmi, F.W.; Van Gossum, A.; et al. Prevalence of Bone Disease in Patients on Home Parenteral Nutrition. Clin. Nutr. 2002, 21, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Fousekis, F.S.; Mitselos, I.V.; Christodoulou, D.K. New Insights into Intestinal Failure-Associated Liver Disease in Adults: A Comprehensive Review of the Literature. Saudi J. Gastroenterol. 2021, 27, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.P.; Cox, C.E.; Kleinman, L.M.; Maher, M.M.; Pittman, M.A.; Tangrea, J.A.; Brown, J.H.; Gross, E.; Beazley, R.M.; Jones, R.S. Serum Hepatic Enzyme and Bilirubin Elevations during Parenteral Nutrition. Surg. Gynecol. Obstet. 1977, 145, 573–580. [Google Scholar]
- Lee, W.S.; Chew, K.S.; Ng, R.T.; Kasmi, K.E.; Sokol, R.J. Intestinal Failure-Associated Liver Disease (IFALD): Insights into Pathogenesis and Advances in Management. Hepatol. Int. 2020, 14, 305–316. [Google Scholar] [CrossRef]
- Naini, B.V.; Lassman, C.R. Total Parenteral Nutrition Therapy and Liver Injury: A Histopathologic Study with Clinical Correlation. Hum. Pathol. 2012, 43, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.A.J.; Zabron, A.A.; Gabe, S.M. Chronic Biochemical Cholestasis in Patients Receiving Home Parenteral Nutrition: Prevalence and Predisposing Factors. Aliment. Pharmacol. Ther. 2008, 27, 552–560. [Google Scholar] [CrossRef]
- Chan, S.; McCowen, K.C.; Bistrian, B.R.; Thibault, A.; Keane-Ellison, M.; Forse, R.A.; Babineau, T.; Burke, P. Incidence, Prognosis, and Etiology of End-Stage Liver Disease in Patients Receiving Home Total Parenteral Nutrition. Surgery 1999, 126, 28–34. [Google Scholar] [CrossRef]
- Cazals-Hatem, D.; Billiauws, L.; Rautou, P.-E.; Bondjemah, V.; Poté, N.; Corcos, O.; Paradis, V.; Joly, F. Ultra-Short Bowel Is an Independent Risk Factor for Liver Fibrosis in Adults with Home Parenteral Nutrition. Liver Int. 2018, 38, 174–182. [Google Scholar] [CrossRef]
- Salvino, R.; Ghanta, R.; Seidner, D.L.; Mascha, E.; Xu, Y.; Steiger, E. Liver Failure Is Uncommon in Adults Receiving Long-Term Parenteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2006, 30, 202–208. [Google Scholar] [CrossRef]
- Cavicchi, M.; Beau, P.; Crenn, P.; Degott, C.; Messing, B. Prevalence of Liver Disease and Contributing Factors in Patients Receiving Home Parenteral Nutrition for Permanent Intestinal Failure. Ann. Intern. Med. 2000, 132, 525–532. [Google Scholar] [CrossRef]
- Luman, W.; Shaffer, J.L. Prevalence, Outcome and Associated Factors of Deranged Liver Function Tests in Patients on Home Parenteral Nutrition. Clin. Nutr. 2002, 21, 337–343. [Google Scholar] [CrossRef]
- Sorbi, D.; Boynton, J.; Lindor, K.D. The Ratio of Aspartate Aminotransferase to Alanine Aminotransferase: Potential Value in Differentiating Nonalcoholic Steatohepatitis from Alcoholic Liver Disease. Am. J. Gastroenterol. 1999, 94, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.M.; Panigrahi, M.K.; Pattnaik, K.; Bhuyan, P.; Kar, S.K.; Misra, B.; Misra, D.; Meher, C.; Agrawal, O.; Rath, J.; et al. Histological Evaluation of Non-Alcoholic Fatty Liver Disease and Its Correlation with Different Noninvasive Scoring Systems with Special Reference to Fibrosis: A Single Center Experience. J. Clin. Exp. Hepatol. 2016, 6, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Sasdelli, A.S.; Agostini, F.; Pazzeschi, C.; Guidetti, M.; Lal, S.; Pironi, L. Assessment of Intestinal Failure Associated Liver Disease According to Different Diagnostic Criteria. Clin. Nutr. 2019, 38, 1198–1205. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Rausa, E.; Invernizzi, P.; Braga, M.; Vecchi, M. Understanding Short Bowel Syndrome: Current Status and Future Perspectives. Dig. Liver Dis. 2020, 52, 253–261. [Google Scholar] [CrossRef]
- Rollins, C.J. Total Nutrient Admixtures: Stability Issues and Their Impact on Nursing Practice. J. Intraven. Nurs. 1997, 20, 299–304. [Google Scholar]
- Scolapio, J.S.; Savoy, A.D.; Kaplan, J.; Burger, C.D.; Lin, S.-C. Sleep Patterns of Cyclic Parenteral Nutrition, a Pilot Study: Are There Sleepless Nights? JPEN J. Parenter. Enter. Nutr. 2002, 26, 214–217. [Google Scholar] [CrossRef]
- Pironi, L.; Corcos, O.; Forbes, A.; Holst, M.; Joly, F.; Jonkers, C.; Klek, S.; Lal, S.; Blaser, A.R.; Rollins, K.E.; et al. Intestinal Failure in Adults: Recommendations from the ESPEN Expert Groups. Clin. Nutr. 2018, 37, 1798–1809. [Google Scholar] [CrossRef]
- Gabe, S.M.; Culkin, A. Abnormal Liver Function Tests in the Parenteral Nutrition Fed Patient. Frontline Gastroenterol. 2010, 1, 98–104. [Google Scholar] [CrossRef]
- Kang, L.-I.; Mars, W.M.; Michalopoulos, G.K. Signals and Cells Involved in Regulating Liver Regeneration. Cells 2012, 1, 1261–1292. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, B.A.; Fleming, C.R.; Ilstrup, D.; Nelson, J.; Reek, S.; Burnes, J. Plasma Carnitine Levels in Patients Receiving Home Parenteral Nutrition. Am. J. Clin. Nutr. 1986, 43, 85–91. [Google Scholar] [CrossRef]
- Ishikawa, H.; Takaki, A.; Tsuzaki, R.; Yasunaka, T.; Koike, K.; Shimomura, Y.; Seki, H.; Matsushita, H.; Miyake, Y.; Ikeda, F.; et al. L-Carnitine Prevents Progression of Non-Alcoholic Steatohepatitis in a Mouse Model with Upregulation of Mitochondrial Pathway. PLoS ONE 2014, 9, e100627. [Google Scholar] [CrossRef]
- Ohara, M.; Ogawa, K.; Suda, G.; Kimura, M.; Maehara, O.; Shimazaki, T.; Suzuki, K.; Nakamura, A.; Umemura, M.; Izumi, T.; et al. L-Carnitine Suppresses Loss of Skeletal Muscle Mass in Patients With Liver Cirrhosis. Hepatol. Commun. 2018, 2, 906–918. [Google Scholar] [CrossRef]
- Bae, J.C.; Lee, W.Y.; Yoon, K.H.; Park, J.Y.; Son, H.S.; Han, K.A.; Lee, K.W.; Woo, J.T.; Ju, Y.C.; Lee, W.J.; et al. Improvement of Nonalcoholic Fatty Liver Disease With Carnitine-Orotate Complex in Type 2 Diabetes (CORONA): A Randomized Controlled Trial. Diabetes Care 2015, 38, 1245–1252. [Google Scholar] [CrossRef]
- Geggel, H.S.; Ament, M.E.; Heckenlively, J.R.; Martin, D.A.; Kopple, J.D. Nutritional Requirement for Taurine in Patients Receiving Long-Term Parenteral Nutrition. N. Engl. J. Med. 1985, 312, 142–146. [Google Scholar] [CrossRef]
- Lourenço, R.; Camilo, M.E. Taurine: A Conditionally Essential Amino Acid in Humans? An Overview in Health and Disease. Nutr. Hosp. 2002, 17, 262–270. [Google Scholar] [PubMed]
- Belli, D.C.; Roy, C.C.; Fournier, L.A.; Tuchweber, B.; Giguère, R.; Yousef, I.M. The Effect of Taurine on the Cholestatic Potential of Sulfated Lithocholate and Its Conjugates. Liver 1991, 11, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.U.; Yu, S.; Tracy, T.F.; Aouthmany, M.M.; Llanos, A.; Brown, M.B.; Brown, M.; Shulman, R.J.; Hirschl, R.B.; Derusso, P.A.; et al. Parenteral Nutrition-Associated Cholestasis in Neonates: Multivariate Analysis of the Potential Protective Effect of Taurine. JPEN J. Parenter. Enter. Nutr. 2005, 29, 337–343. [Google Scholar] [CrossRef]
- Arrieta, F.; Balsa, J.A.; de la Puerta, C.; Botella, J.I.; Zamarrón, I.; Elías, E.; Del Río, J.I.P.; Alonso, P.; Candela, A.; Blanco-Colio, L.M.; et al. Phase IV Prospective Clinical Study to Evaluate the Effect of Taurine on Liver Function in Postsurgical Adult Patients Requiring Parenteral Nutrition. Nutr. Clin. Pract. 2014, 29, 672–680. [Google Scholar] [CrossRef]
- Vance, D.E. Role of Phosphatidylcholine Biosynthesis in the Regulation of Lipoprotein Homeostasis. Curr. Opin. Lipidol. 2008, 19, 229–234. [Google Scholar] [CrossRef]
- Buchman, A.L.; Moukarzel, A.; Jenden, D.J.; Roch, M.; Rice, K.; Ament, M.E. Low Plasma Free Choline Is Prevalent in Patients Receiving Long Term Parenteral Nutrition and Is Associated with Hepatic Aminotransferase Abnormalities. Clin. Nutr. 1993, 12, 33–37. [Google Scholar] [CrossRef]
- Buchman, A.L.; Ament, M.E.; Sohel, M.; Dubin, M.; Jenden, D.J.; Roch, M.; Pownall, H.; Farley, W.; Awal, M.; Ahn, C. Choline Deficiency Causes Reversible Hepatic Abnormalities in Patients Receiving Parenteral Nutrition: Proof of a Human Choline Requirement: A Placebo-Controlled Trial. JPEN J. Parenter. Enter. Nutr. 2001, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Dubin, M.; Jenden, D.; Moukarzel, A.; Roch, M.H.; Rice, K.; Gornbein, J.; Ament, M.E.; Eckhert, C.D. Lecithin Increases Plasma Free Choline and Decreases Hepatic Steatosis in Long-Term Total Parenteral Nutrition Patients. Gastroenterology 1992, 102, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Trotta, E.; Bortolotti, S.; Fugazzotto, G.; Gellera, C.; Montagnese, S.; Amodio, P. Familial Vitamin E Deficiency: Multiorgan Complications Support the Adverse Role of Oxidative Stress. Nutrition 2019, 63–64, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Ipsen, D.H.; Tveden-Nyborg, P.; Lykkesfeldt, J. Does Vitamin C Deficiency Promote Fatty Liver Disease Development? Nutrients 2014, 6, 5473–5499. [Google Scholar] [CrossRef]
- Ng, K.; Stoll, B.; Chacko, S.; de Pipaon, M.S.; Lauridsen, C.; Gray, M.; Squires, E.J.; Marini, J.; Zamora, I.J.; Olutoye, O.O.; et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. JPEN J. Parenter. Enter. Nutr. 2016, 40, 656–671. [Google Scholar] [CrossRef]
- Xu, Z.; Harvey, K.A.; Pavlina, T.M.; Zaloga, G.P.; Siddiqui, R.A. Tocopherol and Tocotrienol Homologs in Parenteral Lipid Emulsions. Eur. J. Lipid Sci. Technol. 2015, 117, 15–22. [Google Scholar] [CrossRef]
- Li, S.; Nussbaum, M.S.; Teague, D.; Gapen, C.L.; Dayal, R.; Fischer, J.E. Increasing Dextrose Concentrations in Total Parenteral Nutrition (TPN) Causes Alterations in Hepatic Morphology and Plasma Levels of Insulin and Glucagon in Rats. J. Surg. Res. 1988, 44, 639–648. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef]
- Eaton, R.P.; Kipnis, D.M. Effect of Glucose Feeding on Lipoprotein Synthesis in the Rat. Am. J. Physiol. 1969, 217, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Raphael, B.P.; Duggan, C. Prevention and Treatment of Intestinal Failure-Associated Liver Disease in Children. Semin. Liver Dis. 2012, 32, 341–347. [Google Scholar] [CrossRef][Green Version]
- Hwang, M.; Lee, S.W.; Park, K.C.; Sul, H.J.; Kwon, D.-S. Evaluation of a Robotic Arm-Assisted Endoscope to Facilitate Endoscopic Submucosal Dissection (with Video). Gastrointest. Endosc. 2020, 91, 699–706. [Google Scholar] [CrossRef]
- Meguid, M.M.; Akahoshi, M.P.; Jeffers, S.; Hayashi, R.J.; Hammond, W.G. Amelioration of Metabolic Complications of Conventional Total Parenteral Nutrition. A Prospective Randomized Study. Arch. Surg. 1984, 119, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Miles, J.M. Metabolic Effects of Long-Chain and Medium-Chain Triglyceride Emulsions in Humans. JPEN J. Parenter. Enter. Nutr. 1994, 18, 396–397. [Google Scholar] [CrossRef]
- Seidner, D.L.; Mascioli, E.A.; Istfan, N.W.; Porter, K.A.; Selleck, K.; Blackburn, G.L.; Bistrian, B.R. Effects of Long-Chain Triglyceride Emulsions on Reticuloendothelial System Function in Humans. JPEN J. Parenter. Enter. Nutr. 1989, 13, 614–619. [Google Scholar] [CrossRef]
- Wanten, G.J.A.; Calder, P.C. Immune Modulation by Parenteral Lipid Emulsions. Am. J. Clin. Nutr. 2007, 85, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.E.; Braun, L.P.; Mercer, L.D.; Sherrill, M.; Stevens, J.; Javid, P.J. The Effect of Lipid Restriction on the Prevention of Parenteral Nutrition-Associated Cholestasis in Surgical Infants. J. Pediatr. Surg. 2013, 48, 573–578. [Google Scholar] [CrossRef]
- Nandivada, P.; Cowan, E.; Carlson, S.J.; Chang, M.; Gura, K.M.; Puder, M. Mechanisms for the Effects of Fish Oil Lipid Emulsions in the Management of Parenteral Nutrition-Associated Liver Disease. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 153–158. [Google Scholar] [CrossRef]
- Mutanen, A.; Lohi, J.; Heikkilä, P.; Jalanko, H.; Pakarinen, M.P. Liver Inflammation Relates to Decreased Canalicular Bile Transporter Expression in Pediatric Onset Intestinal Failure. Ann. Surg. 2018, 268, 332–339. [Google Scholar] [CrossRef]
- Pálová, S.; Charvat, J.; Kvapil, M. Comparison of Soybean Oil- and Olive Oil-Based Lipid Emulsions on Hepatobiliary Function and Serum Triacylglycerols Level during Realimentation. J. Int. Med. Res. 2008, 36, 587–593. [Google Scholar] [CrossRef]
- Jia, Z.-Y.; Yang, J.; Xia, Y.; Tong, D.-N.; Zaloga, G.P.; Qin, H.-L.; OliClinomel N4 Study Group. Safety and Efficacy of an Olive Oil-Based Triple-Chamber Bag for Parenteral Nutrition: A Prospective, Randomized, Multi-Center Clinical Trial in China. Nutr. J. 2015, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, R.; Bremner, R.; Protheroe, S.; Johnson, T.; Holden, C.; Murphy, M.S. Resolution of Parenteral Nutrition-Associated Jaundice on Changing from a Soybean Oil Emulsion to a Complex Mixed-Lipid Emulsion. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Klek, S.; Chambrier, C.; Singer, P.; Rubin, M.; Bowling, T.; Staun, M.; Joly, F.; Rasmussen, H.; Strauss, B.J.; Wanten, G.; et al. Four-Week Parenteral Nutrition Using a Third Generation Lipid Emulsion (SMOFlipid)—A Double-Blind, Randomised, Multicentre Study in Adults. Clin. Nutr. 2013, 32, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.L.; White, P.Z.; Zalla, J. SMOFlipid vs Intralipid 20%: Effect of Mixed-Oil vs Soybean-Oil Emulsion on Parenteral Nutrition-Associated Cholestasis in the Neonatal Population. JPEN J. Parenter. Enter. Nutr. 2021, 45, 339–346. [Google Scholar] [CrossRef]
- Khadge, S.; Sharp, J.G.; Thiele, G.M.; McGuire, T.R.; Klassen, L.W.; Duryee, M.J.; Britton, H.C.; Dafferner, A.J.; Beck, J.; Black, P.N.; et al. Dietary Omega-3 and Omega-6 Polyunsaturated Fatty Acids Modulate Hepatic Pathology. J. Nutr. Biochem. 2018, 52, 92–102. [Google Scholar] [CrossRef]
- Yan, J.-H.; Guan, B.-J.; Gao, H.-Y.; Peng, X.-E. Omega-3 Polyunsaturated Fatty Acid Supplementation and Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Medicine 2018, 97, e12271. [Google Scholar] [CrossRef]
- Marchix, J.; Goddard, G.; Helmrath, M.A. Host-Gut Microbiota Crosstalk in Intestinal Adaptation. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 149–162. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Park, M.-Y.; Kim, S.J.; Ko, E.K.; Ahn, S.-H.; Seo, H.; Sung, M.-K. Gut Microbiota-Associated Bile Acid Deconjugation Accelerates Hepatic Steatosis in Ob/Ob Mice. J. Appl. Microbiol. 2016, 121, 800–810. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R.; Bradley, D.; Setchell, K.D.; Eagon, J.C.; Abumrad, N.; Klein, S. Weight Loss Induced by Roux-En-Y Gastric Bypass but Not Laparoscopic Adjustable Gastric Banding Increases Circulating Bile Acids. J. Clin. Endocrinol. Metab. 2013, 98, E708–E712. [Google Scholar] [CrossRef]
- Madnawat, H.; Welu, A.L.; Gilbert, E.J.; Taylor, D.B.; Jain, S.; Manithody, C.; Blomenkamp, K.; Jain, A.K. Mechanisms of Parenteral Nutrition-Associated Liver and Gut Injury. Nutr. Clin. Pract. 2020, 35, 63–71. [Google Scholar] [CrossRef]
- Matsubara, T.; Li, F.; Gonzalez, F.J. FXR Signaling in the Enterohepatic System. Mol. Cell. Endocrinol. 2013, 368, 17–29. [Google Scholar] [CrossRef]
- Wang, L.; Lee, Y.-K.; Bundman, D.; Han, Y.; Thevananther, S.; Kim, C.S.; Chua, S.S.; Wei, P.; Heyman, R.A.; Karin, M.; et al. Redundant Pathways for Negative Feedback Regulation of Bile Acid Production. Dev. Cell 2002, 2, 721–731. [Google Scholar] [CrossRef]
- Xiao, Y.-T.; Cao, Y.; Zhou, K.-J.; Lu, L.-N.; Cai, W. Altered Systemic Bile Acid Homeostasis Contributes to Liver Disease in Pediatric Patients with Intestinal Failure. Sci. Rep. 2016, 6, 39264. [Google Scholar] [CrossRef] [PubMed]
- Fligor, S.C.; Tsikis, S.T.; Hirsch, T.I.; Jain, A.; Sun, L.; Rockowitz, S.; Gura, K.M.; Puder, M. Inflammation Drives Pathogenesis of Early Intestinal Failure-Associated Liver Disease. Sci. Rep. 2024, 14, 4240. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, C.; Gratadoux, J.-J.; Bridonneau, C.; Chegdani, F.; Larroque, B.; Kapel, N.; Corcos, O.; Thomas, M.; Joly, F. Faecal D/L Lactate Ratio Is a Metabolic Signature of Microbiota Imbalance in Patients with Short Bowel Syndrome. PLoS ONE 2013, 8, e54335. [Google Scholar] [CrossRef]
- Parm, Ü.; Metsvaht, T.; Ilmoja, M.-L.; Lutsar, I. Gut Colonization by Aerobic Microorganisms Is Associated with Route and Type of Nutrition in Premature Neonates. Nutr. Res. 2015, 35, 496–503. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875.e3. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Mascianà, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased Intestinal Permeability and Tight Junction Alterations in Nonalcoholic Fatty Liver Disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Korpela, K.; Mutanen, A.; Salonen, A.; Savilahti, E.; de Vos, W.M.; Pakarinen, M.P. Intestinal Microbiota Signatures Associated With Histological Liver Steatosis in Pediatric-Onset Intestinal Failure. JPEN J. Parenter. Enter. Nutr. 2017, 41, 238–248. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, Y.; Zhou, K.; Yan, J.; Wang, P.; Yan, W.; Cai, W. FXR Agonist GW4064 Improves Liver and Intestinal Pathology and Alters Bile Acid Metabolism in Rats Undergoing Small Intestinal Resection. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G108–G115. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Chen, S.; Tian, X.; Wang, W.; Wang, Y.; Cai, W. The Farnesoid X Receptor Agonist Tropifexor Prevents Liver Damage in Parenteral Nutrition-Fed Neonatal Piglets. J. Pediatr. Gastroenterol. Nutr. 2021, 73, e11–e19. [Google Scholar] [CrossRef] [PubMed]
- Villalona, G.; Price, A.; Blomenkamp, K.; Manithody, C.; Saxena, S.; Ratchford, T.; Westrich, M.; Kakarla, V.; Pochampally, S.; Phillips, W.; et al. No Gut No Gain! Enteral Bile Acid Treatment Preserves Gut Growth but Not Parenteral Nutrition-Associated Liver Injury in a Novel Extensive Short Bowel Animal Model. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1238–1251. [Google Scholar] [CrossRef]
- Pereira-Fantini, P.M.; Lapthorne, S.; Gahan, C.G.M.; Joyce, S.A.; Charles, J.; Fuller, P.J.; Bines, J.E. Farnesoid X Receptor Agonist Treatment Alters Bile Acid Metabolism but Exacerbates Liver Damage in a Piglet Model of Short-Bowel Syndrome. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Stoll, B.; Robinson, J.; Pastor, J.J.; Marini, J.C.; Ipharraguerre, I.R.; Hartmann, B.; Holst, J.J.; Cruz, S.; Lau, P.; et al. Differential Action of TGR5 Agonists on GLP-2 Secretion and Promotion of Intestinal Adaptation in a Piglet Short Bowel Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G641–G652. [Google Scholar] [CrossRef] [PubMed]
- Roma, M.G.; Toledo, F.D.; Boaglio, A.C.; Basiglio, C.L.; Crocenzi, F.A.; Sánchez Pozzi, E.J. Ursodeoxycholic Acid in Cholestasis: Linking Action Mechanisms to Therapeutic Applications. Clin. Sci. 2011, 121, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Mouillot, T.; Beylot, M.; Drai, J.; Hillon, P.; Gelas, P.; Lauverjat, M.; Brondel, L.; Chambrier, C. Effect of Bile Acid Supplementation on Endogenous Lipid Synthesis in Patients with Short Bowel Syndrome: A Pilot Study. Clin. Nutr. 2020, 39, 928–934. [Google Scholar] [CrossRef]
- Spagnuolo, M.I.; Iorio, R.; Vegnente, A.; Guarino, A. Ursodeoxycholic Acid for Treatment of Cholestasis in Children on Long-Term Total Parenteral Nutrition: A Pilot Study. Gastroenterology 1996, 111, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Beau, P.; Labat-Labourdette, J.; Ingrand, P.; Beauchant, M. Is Ursodeoxycholic Acid an Effective Therapy for Total Parenteral Nutrition-Related Liver Disease? J. Hepatol. 1994, 20, 240–244. [Google Scholar] [CrossRef] [PubMed]
- San Luis, V.A.; Btaiche, I.F. Ursodiol in Patients with Parenteral Nutrition-Associated Cholestasis. Ann. Pharmacother. 2007, 41, 1867–1872. [Google Scholar] [CrossRef]
- Fujita, K.; Iguchi, Y.; Une, M.; Watanabe, S. Ursodeoxycholic Acid Suppresses Lipogenesis in Mouse Liver: Possible Role of the Decrease in β-Muricholic Acid, a Farnesoid X Receptor Antagonist. Lipids 2017, 52, 335–344. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Puca, P.; Lopetuso, L.R.; Petito, V.; Masi, L.; Bartocci, B.; Murgiano, M.; De Felice, M.; Petronio, L.; Gasbarrini, A.; et al. Bile Acid-Related Regulation of Mucosal Inflammation and Intestinal Motility: From Pathogenesis to Therapeutic Application in IBD and Microscopic Colitis. Nutrients 2022, 14, 2664. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Yoon, S.; Ji, S.C.; Yang, J.; Kim, Y.-K.; Lee, S.; Yu, K.-S.; Jang, I.-J.; Chung, J.-Y.; Cho, J.-Y. Ursodeoxycholic Acid Improves Liver Function via Phenylalanine/Tyrosine Pathway and Microbiome Remodelling in Patients with Liver Dysfunction. Sci. Rep. 2018, 8, 11874. [Google Scholar] [CrossRef]
- Heydorn, S.; Jeppesen, P.B.; Mortensen, P.B. Bile Acid Replacement Therapy with Cholylsarcosine for Short-Bowel Syndrome. Scand. J. Gastroenterol. 1999, 34, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Kapral, C.; Wewalka, F.; Praxmarer, V.; Lenz, K.; Hofmann, A.F. Conjugated Bile Acid Replacement Therapy in Short Bowel Syndrome Patients with a Residual Colon. Z. Gastroenterol. 2004, 42, 583–589. [Google Scholar] [CrossRef]
- Emmett, M.; Guirl, M.J.; Santa Ana, C.A.; Porter, J.L.; Neimark, S.; Hofmann, A.F.; Fordtran, J.S. Conjugated Bile Acid Replacement Therapy Reduces Urinary Oxalate Excretion in Short Bowel Syndrome. Am. J. Kidney Dis. 2003, 41, 230–237. [Google Scholar] [CrossRef]
- Gruy-Kapral, C.; Little, K.H.; Fordtran, J.S.; Meziere, T.L.; Hagey, L.R.; Hofmann, A.F. Conjugated Bile Acid Replacement Therapy for Short-Bowel Syndrome. Gastroenterology 1999, 116, 15–21. [Google Scholar] [CrossRef]
- Schneider, S.M.; Joly, F.; Gehrardt, M.-F.; Badran, A.M.; Myara, A.; Thuillier, F.; Coudray-Lucas, C.; Cynober, L.; Trivin, F.; Messing, B. Taurine Status and Response to Intravenous Taurine Supplementation in Adults with Short-Bowel Syndrome Undergoing Long-Term Parenteral Nutrition: A Pilot Study. Br. J. Nutr. 2006, 96, 365–370. [Google Scholar] [CrossRef]
- Yano, K.; Kaji, T.; Onishi, S.; Machigashira, S.; Nagai, T.; Harumatsu, T.; Yamada, K.; Yamada, W.; Muto, M.; Nakame, K.; et al. Novel Effect of Glucagon-like Peptide-2 for Hepatocellular Injury in a Parenterally Fed Rat Model of Short Bowel Syndrome. Pediatr. Surg. Int. 2019, 35, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Pertkiewicz, M.; Messing, B.; Iyer, K.; Seidner, D.L.; O’keefe, S.J.D.; Forbes, A.; Heinze, H.; Joelsson, B. Teduglutide Reduces Need for Parenteral Support among Patients with Short Bowel Syndrome with Intestinal Failure. Gastroenterology 2012, 143, 1473–1481.e3. [Google Scholar] [CrossRef] [PubMed]
- Seidner, D.L.; Gabe, S.M.; Lee, H.-M.; Olivier, C.; Jeppesen, P.B. Enteral Autonomy and Days Off Parenteral Support With Teduglutide Treatment for Short Bowel Syndrome in the STEPS Trials. JPEN J. Parenter. Enter. Nutr. 2020, 44, 697–702. [Google Scholar] [CrossRef]
- Naberhuis, J.K.; Tappenden, K.A. Teduglutide for Safe Reduction of Parenteral Nutrient and/or Fluid Requirements in Adults: A Systematic Review. JPEN J. Parenter. Enter. Nutr. 2016, 40, 1096–1105. [Google Scholar] [CrossRef]
- Iyer, K.R.; Kunecki, M.; Boullata, J.I.; Fujioka, K.; Joly, F.; Gabe, S.; Pape, U.-F.; Schneider, S.M.; Virgili Casas, M.N.; Ziegler, T.R.; et al. Independence From Parenteral Nutrition and Intravenous Fluid Support During Treatment With Teduglutide Among Patients With Intestinal Failure Associated With Short Bowel Syndrome. JPEN J. Parenter. Enter. Nutr. 2017, 41, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Kochar, B.; Long, M.D.; Shelton, E.; Young, L.; Farraye, F.A.; Yajnik, V.; Herfarth, H. Safety and Efficacy of Teduglutide (Gattex) in Patients With Crohn’s Disease and Need for Parenteral Support Due to Short Bowel Syndrome-Associated Intestinal Failure. J. Clin. Gastroenterol. 2017, 51, 508–511. [Google Scholar] [CrossRef]
- Schoeler, M.; Klag, T.; Wendler, J.; Bernhard, S.; Adolph, M.; Kirschniak, A.; Goetz, M.; Malek, N.; Wehkamp, J. GLP-2 Analog Teduglutide Significantly Reduces Need for Parenteral Nutrition and Stool Frequency in a Real-Life Setting. Therap. Adv. Gastroenterol. 2018, 11, 1756284818793343. [Google Scholar] [CrossRef]
- Naimi, R.M.; Hvistendahl, M.; Nerup, N.; Ambrus, R.; Achiam, M.P.; Svendsen, L.B.; Grønbæk, H.; Møller, H.J.; Vilstrup, H.; Steensberg, A.; et al. Effects of Glepaglutide, a Novel Long-Acting Glucagon-like Peptide-2 Analogue, on Markers of Liver Status in Patients with Short Bowel Syndrome: Findings from a Randomised Phase 2 Trial. eBioMedicine 2019, 46, 444–451. [Google Scholar] [CrossRef]
- Slim, G.M.; Lansing, M.; Wizzard, P.; Nation, P.N.; Wheeler, S.E.; Brubaker, P.L.; Jeppesen, P.B.; Wales, P.W.; Turner, J.M. Novel Long-Acting GLP-2 Analogue, FE 203799 (Apraglutide), Enhances Adaptation and Linear Intestinal Growth in a Neonatal Piglet Model of Short Bowel Syndrome with Total Resection of the Ileum. JPEN J. Parenter. Enter. Nutr. 2019, 43, 891–898. [Google Scholar] [CrossRef]
- Wismann, P.; Pedersen, S.L.; Hansen, G.; Mannerstedt, K.; Pedersen, P.J.; Jeppesen, P.B.; Vrang, N.; Fosgerau, K.; Jelsing, J. Novel GLP-1/GLP-2 Co-Agonists Display Marked Effects on Gut Volume and Improves Glycemic Control in Mice. Physiol. Behav. 2018, 192, 72–81. [Google Scholar] [CrossRef]
- Sueyoshi, R.; Miyahara, K.; Nakazawa-Tanaka, N.; Fujiwara, N.; Ochi, T.; Yamataka, A. DPP4 Inhibitor Reinforces Cell Junction Proteins in Mouse Model of Short Bowel Syndrome. Pediatr. Surg. Int. 2020, 36, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, R.; Woods Ignatoski, K.M.; Okawada, M.; Hartmann, B.; Holst, J.; Teitelbaum, D.H. Stimulation of Intestinal Growth and Function with DPP4 Inhibition in a Mouse Short Bowel Syndrome Model. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G410–G419. [Google Scholar] [CrossRef]
- Yano, K.; Sugita, K.; Muto, M.; Matsukubo, M.; Onishi, S.; Kedoin, C.; Matsui, M.; Murakami, M.; Harumatsu, T.; Yamada, K.; et al. The Preventive Effect of Recombinant Human Hepatocyte Growth Factor for Hepatic Steatosis in a Rat Model of Short Bowel Syndrome. J. Pediatr. Surg. 2022, 57, 1286–1292. [Google Scholar] [CrossRef]
- Teitelbaum, D.H.; Tracy, T.F.; Aouthmany, M.M.; Llanos, A.; Brown, M.B.; Yu, S.; Brown, M.R.; Shulman, R.J.; Hirschl, R.B.; Derusso, P.A.; et al. Use of Cholecystokinin-Octapeptide for the Prevention of Parenteral Nutrition-Associated Cholestasis. Pediatrics 2005, 115, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- De Marco, G.; Sordino, D.; Bruzzese, E.; Di Caro, S.; Mambretti, D.; Tramontano, A.; Colombo, C.; Simoni, P.; Guarino, A. Early Treatment with Ursodeoxycholic Acid for Cholestasis in Children on Parenteral Nutrition Because of Primary Intestinal Failure. Aliment. Pharmacol. Ther. 2006, 24, 387–394. [Google Scholar] [CrossRef]
- Maselli, K.M.; Gee, K.; Isani, M.; Fode, A.; Schall, K.A.; Grikscheit, T.C. Broad-Spectrum Antibiotics Alter the Microbiome, Increase Intestinal Fxr, and Decrease Hepatic Steatosis in Zebrafish Short Bowel Syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G212–G226. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lo, H.-C.; Chou, M.-C.; Tsai, H.-R. Oral Antibiotics Attenuate Bowel Segment Reversal-Induced Systemic Inflammatory Response and Body Weight Loss in Massively Bowel-Resected Rats. JPEN J. Parenter. Enter. Nutr. 2007, 31, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.R.; Thomas, S.M. Metronidazole Prevention of Serum Liver Enzyme Abnormalities during Total Parenteral Nutrition. JPEN J. Parenter. Enter. Nutr. 1985, 9, 501–503. [Google Scholar] [CrossRef]
- Capron, J.P.; Gineston, J.L.; Herve, M.A.; Braillon, A. Metronidazole in Prevention of Cholestasis Associated with Total Parenteral Nutrition. Lancet 1983, 1, 446–447. [Google Scholar] [CrossRef]
- Rangel, S.J.; Calkins, C.M.; Cowles, R.A.; Barnhart, D.C.; Huang, E.Y.; Abdullah, F.; Arca, M.J.; Teitelbaum, D.H.; 2011 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Parenteral Nutrition-Associated Cholestasis: An American Pediatric Surgical Association Outcomes and Clinical Trials Committee Systematic Review. J. Pediatr. Surg. 2012, 47, 225–240. [Google Scholar] [CrossRef]
- Sekteera, W.; Nuntnarumit, P.; Supapannachart, S. Oral Erythromycin for Treatment of Feeding Intolerance in Preterm Infants: A Preliminary Report. J. Med. Assoc. Thai. 2002, 85 (Suppl. S4), S1177–S1182. [Google Scholar] [PubMed]
- Ng, P.C.; Lee, C.H.; Wong, S.P.S.; Lam, H.S.; Liu, F.Y.B.; So, K.W.; Lee, C.Y.; Fok, T.F. High-Dose Oral Erythromycin Decreased the Incidence of Parenteral Nutrition-Associated Cholestasis in Preterm Infants. Gastroenterology 2007, 132, 1726–1739. [Google Scholar] [CrossRef]
- Ng, Y.-Y.; Su, P.-H.; Chen, J.-Y.; Quek, Y.-W.; Hu, J.-M.; Lee, I.-C.; Lee, H.-S.; Chang, H.-P. Efficacy of Intermediate-Dose Oral Erythromycin on Very Low Birth Weight Infants with Feeding Intolerance. Pediatr. Neonatol. 2012, 53, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Gokmen, T.; Oguz, S.S.; Bozdag, S.; Erdeve, O.; Uras, N.; Dilmen, U. A Controlled Trial of Erythromycin and UDCA in Premature Infants during Parenteral Nutrition in Minimizing Feeding Intolerance and Liver Function Abnormalities. J. Perinatol. 2012, 32, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.; Nusairat, L.; Al Momani, L.; Chadalavada, P.; Covut, F.; Olayan, M.; Young, M.; Romero-Marrero, C. Effects of Probiotics on Intestinal Failure-Associated Liver Disease in Adult Patients Receiving Prolonged Parenteral Support: A Tertiary Care Center Experience. Nutr. Clin. Pract. 2020, 35, 454–463. [Google Scholar] [CrossRef]
- Stoidis, C.N.; Misiakos, E.P.; Patapis, P.; Fotiadis, C.I.; Spyropoulos, B.G. Potential Benefits of Pro- and Prebiotics on Intestinal Mucosal Immunity and Intestinal Barrier in Short Bowel Syndrome. Nutr. Res. Rev. 2011, 24, 21–30. [Google Scholar] [CrossRef]
- Wu, J.; Yang, K.; Wu, W.; Tang, Q.; Zhong, Y.; Gross, G.; Lambers, T.T.; van Tol, E.A.F.; Cai, W. Soluble Mediators From Lactobacillus Rhamnosus Gorbach-Goldin Support Intestinal Barrier Function in Rats After Massive Small-Bowel Resection. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1026–1034. [Google Scholar] [CrossRef]
- Uchida, K.; Takahashi, T.; Inoue, M.; Morotomi, M.; Otake, K.; Nakazawa, M.; Tsukamoto, Y.; Miki, C.; Kusunoki, M. Immunonutritional Effects during Synbiotics Therapy in Pediatric Patients with Short Bowel Syndrome. Pediatr. Surg. Int. 2007, 23, 243–248. [Google Scholar] [CrossRef]
- Eizaguirre, I.; Urkia, N.G.; Asensio, A.B.; Zubillaga, I.; Zubillaga, P.; Vidales, C.; Garcia-Arenzana, J.M.; Aldazabal, P. Probiotic Supplementation Reduces the Risk of Bacterial Translocation in Experimental Short Bowel Syndrome. J. Pediatr. Surg. 2002, 37, 699–702. [Google Scholar] [CrossRef]
- Mezoff, E.A.; Hawkins, J.A.; Ollberding, N.J.; Karns, R.; Morrow, A.L.; Helmrath, M.A. The Human Milk Oligosaccharide 2’-Fucosyllactose Augments the Adaptive Response to Extensive Intestinal. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G427–G438. [Google Scholar] [CrossRef]
- Kanamori, Y.; Sugiyama, M.; Hashizume, K.; Yuki, N.; Morotomi, M.; Tanaka, R. Experience of Long-Term Synbiotic Therapy in Seven Short Bowel Patients with Refractory Enterocolitis. J. Pediatr. Surg. 2004, 39, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Piper, H.G.; Coughlin, L.A.; Hussain, S.; Nguyen, V.; Channabasappa, N.; Koh, A.Y. The Impact of Lactobacillus Probiotics on the Gut Microbiota in Children With Short Bowel Syndrome. J. Surg. Res. 2020, 251, 112–118. [Google Scholar] [CrossRef]
- Sentongo, T.A.; Cohran, V.; Korff, S.; Sullivan, C.; Iyer, K.; Zheng, X. Intestinal Permeability and Effects of Lactobacillus Rhamnosus Therapy in Children with Short Bowel Syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 41–47. [Google Scholar] [CrossRef]
- Degirolamo, C.; Rainaldi, S.; Bovenga, F.; Murzilli, S.; Moschetta, A. Microbiota Modification with Probiotics Induces Hepatic Bile Acid Synthesis via Downregulation of the Fxr-Fgf15 Axis in Mice. Cell Rep. 2014, 7, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, J.G.; Srugo, I.; Lurie, M.; Shaoul, R.; Coran, A.G.; Shiloni, E.; Sukhotnik, I. Effect of Probiotics on Intestinal Regrowth and Bacterial Translocation after Massive Small Bowel Resection in a Rat. J. Pediatr. Surg. 2007, 42, 1365–1371. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Li, F.; Gu, Z.; Liu, Q.; He, L.; Shao, T.; Song, Q.; Zhu, F.; Zhang, L.; et al. Probiotic Lactobacillus Rhamnosus GG Prevents Liver Fibrosis Through Inhibiting Hepatic Bile Acid Synthesis and Enhancing Bile Acid Excretion in Mice. Hepatology 2020, 71, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Reconstitution Restores Bile Acid Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef]
- Quirós-Tejeira, R.E.; Ament, M.E.; Reyen, L.; Herzog, F.; Merjanian, M.; Olivares-Serrano, N.; Vargas, J.H. Long-Term Parenteral Nutritional Support and Intestinal Adaptation in Children with Short Bowel Syndrome: A 25-Year Experience. J. Pediatr. 2004, 145, 157–163. [Google Scholar] [CrossRef]
- Reinshagen, K.; Zahn, K.; von Buch, C.; Zoeller, M.; Hagl, C.I.; Ali, M.; Waag, K.-L. The Impact of Longitudinal Intestinal Lengthening and Tailoring on Liver Function in Short Bowel Syndrome. Eur. J. Pediatr. Surg. 2008, 18, 249–253. [Google Scholar] [CrossRef]
- Andres, A.M.; Thompson, J.; Grant, W.; Botha, J.; Sunderman, B.; Antonson, D.; Langnas, A.; Sudan, D. Repeat Surgical Bowel Lengthening with the STEP Procedure. Transplantation 2008, 85, 1294–1299. [Google Scholar] [CrossRef]
- Coletta, R.; Aldeiri, B.; Morabito, A. Institutional Experience with Spiral Intestinal Lengthening and Tailoring. Eur. J. Pediatr. Surg. 2019, 29, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Yannam, G.R.; Sudan, D.L.; Grant, W.; Botha, J.; Langnas, A.; Thompson, J.S. Intestinal Lengthening in Adult Patients with Short Bowel Syndrome. J. Gastrointest. Surg. 2010, 14, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S.S.; Atkinson, J.B.; Bianchi, A.; Goulet, O.J.; Grant, D.; Langnas, A.N.; McDiarmid, S.V.; Mittal, N.; Reyes, J.; Tzakis, A.G.; et al. Indications for Pediatric Intestinal Transplantation: A Position Paper of the American Society of Transplantation. Pediatr. Transplant. 2001, 5, 80–87. [Google Scholar] [CrossRef]
- Hawksworth, J.S.; Desai, C.S.; Khan, K.M.; Kaufman, S.S.; Yazigi, N.; Girlanda, R.; Kroemer, A.; Fishbein, T.M.; Matsumoto, C.S. Visceral Transplantation in Patients with Intestinal-Failure Associated Liver Disease: Evolving Indications, Graft Selection, and Outcomes. Am. J. Transplant. 2018, 18, 1312–1320. [Google Scholar] [CrossRef]
- Sudan, D.L.; Iyer, K.R.; Deroover, A.; Chinnakotla, S.; Fox, I.J.; Shaw, B.W.; Langnas, A.N. A New Technique for Combined Liver/Small Intestinal Transplantation. Transplantation 2001, 72, 1846–1848. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elmagd, K.M.; Armanyous, S.R.; Fujiki, M.; Parekh, N.R.; Osman, M.; Scalish, M.; Newhouse, E.; Fouda, Y.; Lennon, E.; Shatnawei, A.; et al. Management of Five Hundred Patients With Gut Failure at a Single Center: Surgical Innovation Versus Transplantation With a Novel Predictive Model. Ann. Surg. 2019, 270, 656–674. [Google Scholar] [CrossRef]
- Smith, J.M.; Weaver, T.; Skeans, M.A.; Horslen, S.P.; Noreen, S.M.; Snyder, J.J.; Israni, A.K.; Kasiske, B.L. OPTN/SRTR 2017 Annual Data Report: Intestine. Am. J. Transplant. 2019, 19 (Suppl. S2), 284–322. [Google Scholar] [CrossRef]
- Kesseli, S.; Sudan, D. Small Bowel Transplantation. Surg. Clin. N. Am. 2019, 99, 103–116. [Google Scholar] [CrossRef]

| Author, Year | Drug | Mechanism of Action | Study Model | Outcomes |
|---|---|---|---|---|
| Yi Cao et al., 2019 [93] | GW4064 | FXR agonist | Animal model: SBR-ADL rats | Improved liver histology and serum liver enzymes, corrected BAs metabolism |
| Yang Liu et al., 2021 [94] | Tropifexor | FXR agonist | Animal model: neonatal piglet fed with PN | Prevented the increase of serum liver enzymes by increasing the abundance of intestinal bacteria producing bsh and CYP7A1 and altering the composition of BAs in serum, liver and intestinal content |
| Prue M Pereira-Fantini et al., 2017 [96] | OCA | FXR activation | Animal model: SBR piglets | Reduced fat malabsorption, but exacerbated liver histology |
| Gustavo Villalona et al., 2018 [95] | CDCA | FXR activation | Animal model: SBR piglets | Increased gut FXR, but not downstream FXR targets, not significant improvement in liver histology and cholestasis enzymes |
| Sen Lin et al., 2019 [97] | Ursolic acid | TGR5 agonist | Animal model: piglets receiving PN and SBR piglets | Increased GLP-2 secretion, but not intestinal adaptation after SBR. Liver outcomes not assessed. |
| Thomas Mouillot et al., 2020 [99] | UDCA | Indirect FXR activation | Human: SBS patients | Reduced hepatic cholesterol and triglucerides syntesis, decreased cholesterol and ALT serum concentrations |
| Spagnuolo M. et al., 1996 [100] | UDCA | Indirect FXR activation | Human: children on long-term TPN, with cholestasis | Normalization of biochemical markers of cholestasis within 4–8 weeks |
| De Marco G. et al., 2006 [125] | UDCA | Indirect FXR activation | Human: children on TPN, with IFALD | Decrease in serum GGT, ALT and direct bilirubin concentrations |
| Beau P. et al., 1994 [101] | UDCA | Indirect FXR activation | Human: SBS patients on TPN with cholestasis | Reduction of serum GGT and ALT, but not of ALP, bilirubin and AST. |
| Keisuke Yano et al., 2019 [111] | Different doses of GLP-2 | GLP-2 increase | Animal model: SBR rats on TPN | Lower steatosis, lobular inflammation score and NAFLD score in the low-dose GLP-2 group |
| Rahim Mohammad Naimi et al., 2019 [118] | Glepaglutide | GLP-2 analogue | Human: SBS patients | Improved liver excretory function, but increased liver stiffness, probably due to activated resident liver macrophages |
| Ryo Sueyoshi et al., 2014 [122] | MK-0626 | DPP4 inhibitor | Animal model: SBR mice | Improved intestinal adaptation. Liver outcomes not assessed |
| Keisuke Yano et al., 2022 [123] | rh-HGF | HGF agonist | Animal model: SBR rats on TPN | Reduced hepatic steatosis and inflammatory cell infiltration in the liver, higher FXR expression in the liver, lower TLR4 expression in the ileum, alteration of gut microbiota composition |
| Daniel H Teitelbaum et al., 2005 [124] | CCK-OP | CCK-OP increase | Human: neonates on TPN | No significant impact on conjugated bilirubin levels |
| Author, Year | Probiotic | Study Model | Intestinal Outcomes | Liver Outcomes |
|---|---|---|---|---|
| Mohammad Alomari et al., 2020 [135] | Unspecified probiotics | Human: patients with IF on HPN or HIVF | Not applied | Lower prevalence of IFALD in probiotic users, but not significant at the multivariate analysis |
| Jiang Wu et al., 2018 [137] | Lactobacillus rhamnosus GG | Animal model: SBR rats | Reduced bacterial translocation and intestinal permeability, lower levels of serum endotoxin and tumor necrotizing factor alpha in ileum | Not applied |
| Hannah G Piper et al., 2020 [142] | Lactobacillus rhamnosus and Lactobacillus johnsonii | Human: SBS children | No significant differences | Not applied |
| Timothy A Sentongo et al., 2008 [143] | Lactobacillus rhamnosus GG | Human: SBS children | No significant effect on intestinal permeability | Not applied |
| Jorge G Mogilner et al., 2007 [145] | Lactobacillus rhamnosus GG | Animal model: SBR rats | Reduced bacterial translocation, decrease enterocytes apoptosis and increased crypt-depth in ileum | Not applied |
| Keiichi Uchida et al., 2007 [138] | Bifidobacterium breve, Lactobacillus casei and galactooligosaccharides | Human: SBS children | Improved intestinal adaptation, potsitive gut microbiota modulation and increased SCFA levels in the feces. Increased serum pre-albumin levels and improved systemic immunonutritional status | Not applied |
| I Eizaguirre et al., 2002 [139] | Bifidobacterium lactis | Animal model: SBR rats | Reduced incidence of bacterial translocation | Not applied |
| Ethan A Mezoff et al., 2016 [140] | Human milk oligosaccharide 2′-fucosyllactose | Animal model: ICR mice | Increased weight gain and crypth depth. Increased energy availability through gut microbial modulation | Not applied |
| Yutaka Kanamori et al., 2004 [141] | Bifidobacterium breve, Lactobacillus casei, and galactooligosaccharides | Human: SBS patients | Accelerated body weight gain, positive modulation of gut microbiota, increased SCFA in the feces | Not applied |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignini, I.; Piccirilli, G.; Di Vincenzo, F.; Covello, C.; Pizzoferrato, M.; Esposto, G.; Galasso, L.; Borriello, R.; Gabrielli, M.; Ainora, M.E.; et al. Intestinal-Failure-Associated Liver Disease: Beyond Parenteral Nutrition. Biomolecules 2025, 15, 388. https://doi.org/10.3390/biom15030388
Mignini I, Piccirilli G, Di Vincenzo F, Covello C, Pizzoferrato M, Esposto G, Galasso L, Borriello R, Gabrielli M, Ainora ME, et al. Intestinal-Failure-Associated Liver Disease: Beyond Parenteral Nutrition. Biomolecules. 2025; 15(3):388. https://doi.org/10.3390/biom15030388
Chicago/Turabian StyleMignini, Irene, Giulia Piccirilli, Federica Di Vincenzo, Carlo Covello, Marco Pizzoferrato, Giorgio Esposto, Linda Galasso, Raffaele Borriello, Maurizio Gabrielli, Maria Elena Ainora, and et al. 2025. "Intestinal-Failure-Associated Liver Disease: Beyond Parenteral Nutrition" Biomolecules 15, no. 3: 388. https://doi.org/10.3390/biom15030388
APA StyleMignini, I., Piccirilli, G., Di Vincenzo, F., Covello, C., Pizzoferrato, M., Esposto, G., Galasso, L., Borriello, R., Gabrielli, M., Ainora, M. E., Gasbarrini, A., & Zocco, M. A. (2025). Intestinal-Failure-Associated Liver Disease: Beyond Parenteral Nutrition. Biomolecules, 15(3), 388. https://doi.org/10.3390/biom15030388







