High-Sensitivity C-Reactive Protein Levels in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), Metabolic Alcohol-Associated Liver Disease (MetALD), and Alcoholic Liver Disease (ALD) with Metabolic Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participations
2.2. Definition of SLD and Its Subtypes
2.3. Hs-CRP
2.4. Statistical Approach
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Im, H.J.; Ahn, Y.C.; Wang, J.H.; Lee, M.M.; Son, C.G. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101526. [Google Scholar] [CrossRef]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Dodge, J.L.; Terrault, N.A. National prevalence estimates for steatotic liver disease and subclassifications using consensus nomenclature. Hepatology 2024, 79, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, M.; Youn, J.; Singh, S.; Ahn, S.H. Liver Diseases in South Korea: A Pulse Check of the Public’s Knowledge, Awareness, and Behaviors. Yonsei Med. J. 2022, 63, 1088–1098. [Google Scholar] [CrossRef]
- Song, K.; Yang, J.; Lee, H.S.; Kim, S.J.; Lee, M.; Suh, J.; Kwon, A.; Kim, H.S.; Chae, H.W. Changes in the Prevalences of Obesity, Abdominal Obesity, and Non-Alcoholic Fatty Liver Disease among Korean Children during the COVID-19 Outbreak. Yonsei Med. J. 2023, 64, 269–277. [Google Scholar] [CrossRef]
- Lee, N.H.; Jeong, S.J.; Wang, J.H.; Choi, Y.J.; Oh, H.M.; Cho, J.H.; Ahn, Y.C.; Son, C.G. The Clinical Diagnosis-Based Nationwide Epidemiology of Metabolic Dysfunction-Associated Liver Disease in Korea. J. Clin. Med. 2023, 12, 7634. [Google Scholar] [CrossRef]
- Han, E.; Han, K.D.; Lee, Y.H.; Kim, K.S.; Hong, S.; Park, J.H.; Park, C.Y. Fatty Liver & Diabetes Statistics in Korea: Nationwide Data 2009 to 2017. Diabetes Metab. J. 2023, 47, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Chan, W.K.; Chuah, K.H.; Rajaram, R.B.; Lim, L.L.; Ratnasingam, J.; Vethakkan, S.R. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD): A State-of-the-Art Review. J. Obes. Metab. Syndr. 2023, 32, 197–213. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Obes. Facts 2024, 17, 374–444. [Google Scholar] [CrossRef]
- Israelsen, M.; Torp, N.; Johansen, S.; Hansen, C.D.; Hansen, E.D.; Thorhauge, K.; Hansen, J.K.; Villesen, I.; Bech, K.; Wernberg, C.; et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: An analysis of data from a prospective cohort study. Lancet Gastroenterol. Hepatol. 2024, 9, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Kim, S.; Cho, S.H.; Kim, J.; Lee, Y.B.; Jin, S.M.; Hur, K.Y.; Kim, G.; Kim, J.H. Metabolic Dysfunction-Associated Steatotic Liver Disease and All-Cause and Cause-Specific Mortality. Diabetes Metab. J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.C. No More NAFLD: The Term Is Now MASLD. Endocrinol. Metab. 2024, 39, 92–94. [Google Scholar] [CrossRef]
- Kalligeros, M.; Vassilopoulos, A.; Vassilopoulos, S.; Victor, D.W.; Mylonakis, E.; Noureddin, M. Prevalence of Steatotic Liver Disease (MASLD, MetALD, and ALD) in the United States: NHANES 2017-2020. Clin. Gastroenterol. Hepatol. 2024, 22, 1330–1332.e4. [Google Scholar] [CrossRef]
- He, L.; Zheng, W.; Qiu, K.; Kong, W.; Zeng, T. Changing from NAFLD to MASLD: The new definition can more accurately identify individuals at higher risk for diabetes. J. Hepatol. 2023, 80, e85–e87. [Google Scholar] [CrossRef]
- Chung, G.E.; Yu, S.J.; Yoo, J.J.; Cho, Y.; Lee, K.N.; Shin, D.W.; Kim, Y.J.; Yoon, J.H.; Han, K.; Cho, E.J. Differential risk of 23 site-specific incident cancers and cancer-related mortality among patients with metabolic dysfunction-associated fatty liver disease: A population-based cohort study with 9.7 million Korean subjects. Cancer Commun. 2023, 43, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, M.Y.; Kim, S.H.; Zheng, M.H.; Targher, G.; Byrne, C.D.; Sung, K.C. Comparison of cardiovascular mortality between MAFLD and NAFLD: A cohort study. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 947–955. [Google Scholar] [CrossRef]
- Chun, H.S.; Lee, M.; Lee, J.S.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Lee, Y.H.; Kim, J.H.; et al. Metabolic dysfunction associated fatty liver disease identifies subjects with cardiovascular risk better than non-alcoholic fatty liver disease. Liver Int. 2023, 43, 608–625. [Google Scholar] [CrossRef]
- Corrao, S.; Calvo, L.; Granà, W.; Scibetta, S.; Mirarchi, L.; Amodeo, S.; Falcone, F.; Argano, C. Metabolic dysfunction-associated steatotic liver disease: A pathophysiology and clinical framework to face the present and the future. Nutr. Metab. Cardiovasc. Dis. 2024, in press. [Google Scholar] [CrossRef]
- Mladenic, K.; Lenartic, M.; Marinovic, S.; Polic, B.; Wensveen, F.M. The “Domino effect” in MASLD: The inflammatory cascade of steatohepatitis. Eur. J. Immunol. 2024, 54, e2149641. [Google Scholar] [CrossRef]
- Nemer, M.; Osman, F.; Said, A. Dietary macro and micronutrients associated with MASLD: Analysis of a national US cohort database. Ann. Hepatol. 2024, 29, 101491. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Choi, J.; Lee, B.; Kim, S.G.; Kim, Y.S.; Yoo, J.-J. Association between Inflammatory Biomarkers and Nutritional Status in Fatty Liver. Clin. Nutr. Res. 2020, 9, 182–194. [Google Scholar] [CrossRef]
- Duan, Y.; Pan, X.; Luo, J.; Xiao, X.; Li, J.; Bestman, P.L.; Luo, M. Association of Inflammatory Cytokines With Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2022, 13, 880298. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, D.N.; Pathak, A.; Agrawal, A. IL-6 regulates induction of C-reactive protein gene expression by activating STAT3 isoforms. Mol. Immunol. 2022, 146, 50–56. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, M.; Samols, D.; Kushner, I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J. Biol. Chem. 1996, 271, 9503–9509. [Google Scholar] [CrossRef]
- Ding, Z.; Wei, Y.; Peng, J.; Wang, S.; Chen, G.; Sun, J. The Potential Role of C-Reactive Protein in Metabolic-Dysfunction-Associated Fatty Liver Disease and Aging. Biomedicines 2023, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Coste, S.C.; Orășan, O.H.; Cozma, A.; Negrean, V.; Sitar-Tăut, A.V.; Filip, G.A.; Hangan, A.C.; Lucaciu, R.L.; Iancu, M.; Procopciuc, L.M. Metabolic Dysfunction-Associated Steatotic Liver Disease: The Associations between Inflammatory Markers, TLR4, and Cytokines IL-17A/F, and Their Connections to the Degree of Steatosis and the Risk of Fibrosis. Biomedicines 2024, 12, 2144. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Castillo, E.; Gonzalez-Pacheco, H.; Saenz-San Martin, A.; Mendez-Ocampo, P.; Gutierrez-Moctezuma, I.; Masso, F.; Sierra-Lara, D.; Springall, R.; Rodriguez, E.; Arias-Mendoza, A.; et al. C-Reactive Protein: The Quintessential Marker of Systemic Inflammation in Coronary Artery Disease-Advancing toward Precision Medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef]
- Ridker, P.M. Cardiology Patient Page. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation 2003, 108, e81–e85. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Huang, C.-C.; Chan, W.-L.; Chen, J.-W.; Leu, H.-B. The severity of non-alcoholic fatty liver disease correlates with high sensitivity C-reactive protein value and is independently associated with increased cardiovascular risk in healthy population. Clin. Biochem. 2010, 43, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zheng, Z.; Zhang, Y. Association of Hematological Biomarkers of Inflammation with 10-Year Major Adverse Cardiovascular Events and All-Cause Mortality in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: The ARIC Study. J. Inflamm. Res. 2024, 17, 4247–4256. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Gui, Z.; Liu, L.; Wang, N.; Shen, J. Hs-CRP and HOMA-IR: Include them in the MASLD definition, or treat them as mediators between MASLD and atherosclerotic cardiovascular disease? J. Hepatol. 2024, 81. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, C.; Huang, Y.Z.; Zhang, L.L. Nonalcoholic fatty liver disease shows significant sex dimorphism. World J. Clin. Cases 2022, 10, 1457–1472. [Google Scholar] [CrossRef] [PubMed]
- Koceva, A.; Herman, R.; Janez, A.; Rakusa, M.; Jensterle, M. Sex- and Gender-Related Differences in Obesity: From Pathophysiological Mechanisms to Clinical Implications. Int. J. Mol. Sci. 2024, 25, 7342. [Google Scholar] [CrossRef]
- Cherubini, A.; Della Torre, S.; Pelusi, S.; Valenti, L. Sexual dimorphism of metabolic dysfunction-associated steatotic liver disease. Trends Mol. Med. 2024. [Google Scholar] [CrossRef]
- Joo, S.K.; Kim, W. Sex differences in metabolic dysfunction-associated steatotic liver disease: A narrative review. Ewha Med. J. 2024, 47, e17. [Google Scholar] [CrossRef]
- Tapper, E.B.; Krajewski, K.; Lai, M.; Challies, T.; Kane, R.; Afdhal, N.; Lau, D. Simple non-invasive biomarkers of advanced fibrosis in the evaluation of non-alcoholic fatty liver disease. Gastroenterol. Rep. 2014, 2, 276–280. [Google Scholar] [CrossRef]
- Bambha, K.; Belt, P.; Abraham, M.; Wilson, L.A.; Pabst, M.; Ferrell, L.; Unalp-Arida, A.; Bass, N.; Nonalcoholic Steatohepatitis Clinical Research Network Research Group. Ethnicity and nonalcoholic fatty liver disease. Hepatology 2012, 55, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Sakhuja, P.; Malhotra, V.; Gondal, R.; Sarin, S.K. Independent predictors of steatohepatitis and fibrosis in Asian Indian patients with non-alcoholic steatohepatitis. Dig. Dis. Sci. 2008, 53, 1967–1976. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, M.; Hu, Z.; Hultstrom, M.; Lai, E. Sex-specific prevalence of fatty liver disease and associated metabolic factors in Wuhan, south central China. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Cheng, S.; Heart-Liver Axis Research, Collaboration. Sex differences in prevalence and prognosis of steatotic liver disease phenotypes: Biological sex matters. J. Hepatol. 2024, 80, e68–e69. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Kim, Y.; Kweon, S.; Kim, S.; Yun, S.; Park, S.; Lee, Y.K.; Kim, Y.; Park, O.; Jeong, E.K. Korea National Health and Nutrition Examination Survey, 20th anniversary: Accomplishments and future directions. Epidemiol. Health 2021, 43, e2021025. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Miwa, T.; Tajirika, S.; Imamura, N.; Adachi, M.; Horita, R.; Hanai, T.; Ng, C.H.; Siddiqui, M.S.; Fukao, T.; Shimizu, M.; et al. Usefulness of health checkup-based indices in identifying metabolic dysfunction-associated steatotic liver disease. JGH Open 2024, 8, e13110. [Google Scholar] [CrossRef]
- Mahachai, N.; Washirasaksiri, C.; Ariyakunaphan, P.; Kositamongkol, C.; Sitasuwan, T.; Tinmanee, R.; Auesomwang, C.; Sayabovorn, N.; Chaisathaphol, T.; Phisalprapa, P.; et al. Clinical Predictive Score for Identifying Metabolic Dysfunction-Associated Steatotic Liver Disease in Individuals with Prediabetes Using Transient Elastography. J. Clin. Med. 2023, 12, 7617. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Kite, C.; Lagojda, L.; Dallaway, A.; Chatha, K.K.; Chaggar, S.S.; Dalamaga, M.; Kassi, E.; Kyrou, I.; Randeva, H.S. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review From NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 2024, 13, 510–531. [Google Scholar] [CrossRef]
- Mantovani, A.; Morieri, M.L.; Aldigeri, R.; Palmisano, L.; Masulli, M.; Bonomo, K.; Baroni, M.G.; Cossu, E.; Cimini, F.A.; Cavallo, G.; et al. MASLD, hepatic steatosis and fibrosis are associated with the prevalence of chronic kidney disease and retinopathy in adults with type 1 diabetes mellitus. Diabetes Metab. 2024, 50, 101497. [Google Scholar] [CrossRef] [PubMed]
- Banait, T.; Wanjari, A.; Danade, V.; Banait, S.; Jain, J. Role of High-Sensitivity C-reactive Protein (Hs-CRP) in Non-communicable Diseases: A Review. Cureus 2022, 14, e30225. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.; Min, J.; Kang, D.W.; Kim, J.Y.; Yang, H.I.; Park, J.; Lee, M.K.; Lee, M.Y.; Park, I.; et al. Development of the Korean Global Physical Activity Questionnaire: Reliability and validity study. Glob. Health Promot. 2020, 27, 44–55. [Google Scholar] [CrossRef]
- Jayedi, A.; Rahimi, K.; Bautista, L.E.; Nazarzadeh, M.; Zargar, M.S.; Shab-Bidar, S. Inflammation markers and risk of developing hypertension: A meta-analysis of cohort studies. Heart 2019, 105, 686–692. [Google Scholar] [CrossRef]
- Yang, X.; Tao, S.; Peng, J.; Zhao, J.; Li, S.; Wu, N.; Wen, Y.; Xue, Q.; Yang, C.X.; Pan, X.F. High-sensitivity C-reactive protein and risk of type 2 diabetes: A nationwide cohort study and updated meta-analysis. Diabetes Metab. Res. Rev. 2021, 37, e3446. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef]
- Mogna-Peláez, P.; Riezu-Boj, J.I.; Milagro, F.I.; Herrero, J.I.; Elorz, M.; Benito-Boillos, A.; Tobaruela-Resola, A.L.; Tur, J.A.; Martínez, J.A.; Abete, I.; et al. Inflammatory markers as diagnostic and precision nutrition tools for metabolic dysfunction-associated steatotic liver disease: Results from the Fatty Liver in Obesity trial. Clin. Nutr. 2024, 43, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, S.; Tian, C.; Wang, Q.; Yang, Z.; Che, W.; Li, Y.; Luo, Y. Association of systemic immune biomarkers with metabolic dysfunction-associated steatotic liver disease: A cross-sectional study of NHANES 2007-2018. Front. Nutr. 2024, 11, 1415484. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, B.J.; Hur, W.; Lee, M.; Han, S.H. Associations of Insulin Resistance and High-Sensitivity C-Reactive Protein with Metabolic Abnormalities in Korean Patients with Type 2 Diabetes Mellitus: A Preliminary Study. Metabolites 2024, 14, 371. [Google Scholar] [CrossRef]
- Kumar, R.; Porwal, Y.C.; Dev, N.; Kumar, P.; Chakravarthy, S.; Kumawat, A. Association of high-sensitivity C-reactive protein (hs-CRP) with non-alcoholic fatty liver disease (NAFLD) in Asian Indians: A cross-sectional study. J. Fam. Med. Prim. Care 2020, 9, 390–394. [Google Scholar] [CrossRef]
- Jamialahmadi, T.; Bo, S.; Abbasifard, M.; Sathyapalan, T.; Jangjoo, A.; Moallem, S.A.; Almahmeed, W.; Ashari, S.; Johnston, T.P.; Sahebkar, A. Association of C-reactive protein with histological, elastographic, and sonographic indices of non-alcoholic fatty liver disease in individuals with severe obesity. J. Health Popul. Nutr. 2023, 42, 30. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Arab, J.P.; Wong, V.W. MASLD: A disease in flux. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Yu, L.; Gao, F.; Li, Y.; Su, D.; Han, L.; Li, Y.; Zhang, X.; Feng, Z. Role of pattern recognition receptors in the development of MASLD and potential therapeutic applications. Biomed. Pharmacother. 2024, 175, 116724. [Google Scholar] [CrossRef] [PubMed]
- Okekunle, A.P.; Youn, J.; Song, S.; Chung, G.E.; Yang, S.Y.; Kim, Y.S.; Lee, J.E. Predicted pro-inflammatory hs-CRP score and non-alcoholic fatty liver disease. Gastroenterol. Rep. 2023, 11, goad059. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, K.K.; Hwang, I.C. Association between the Severity of Nonalcoholic Fatty Liver Disease and High Sensitivity C-reactive Protein in Adults. J. Obes. Metab. Syndr. 2012, 21, 166–174. [Google Scholar] [CrossRef]
- Wang, L.R.; Liu, W.Y.; Wu, S.J.; Zhu, G.Q.; Lin, Y.Q.; Braddock, M.; Zhang, D.C.; Zheng, M.H. Parabolic relationship between sex-specific serum high sensitive C reactive protein and non-alcoholic fatty liver disease in Chinese adults: A large population-based study. Oncotarget 2016, 7, 14241–14250. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Lonardo, A.; Trande, P. Are there any sex differences in fatty liver? A study of glucose metabolism and body fat distribution. J. Gastroenterol. Hepatol. 2000, 15, 775–782. [Google Scholar] [CrossRef]
- Ciardullo, S.; Oltolini, A.; Cannistraci, R.; Muraca, E.; Perseghin, G. Sex-related association of nonalcoholic fatty liver disease and liver fibrosis with body fat distribution in the general US population. Am. J. Clin. Nutr. 2022, 115, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Vachher, M.; Arora, T.; Kumar, B.; Burman, A. Visceral fat: A key mediator of NAFLD development and progression. Hum. Nutr. Metab. 2023, 33, 200210. [Google Scholar] [CrossRef]
- Eng, P.C.; Forlano, R.; Tan, T.; Manousou, P.; Dhillo, W.S.; Izzi-Engbeaya, C. Non-alcoholic fatty liver disease in women—Current knowledge and emerging concepts. JHEP Rep. 2023, 5, 100835. [Google Scholar] [CrossRef]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef]
- Ahn, S.B. Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments. Clin. Mol. Hepatol. 2023, 29, S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Biciusca, T.; Stan, S.I.; Balteanu, M.A.; Cioboata, R.; Ghenea, A.E.; Danoiu, S.; Bumbea, A.M.; Biciusca, V. The Role of the Fatty Liver Index (FLI) in the Management of Non-Alcoholic Fatty Liver Disease: A Systematic Review. Diagnostics 2023, 13, 3316. [Google Scholar] [CrossRef]
- Caretta, N.; Scafa, R.; Graziani, A.; Crepaldi, M.C.; Vedovato, M.; Avogaro, A.; Ferlin, A. Noninvasive Indices of MASLD Are Associated with Hypogonadism in Male Patients With Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2024, 109, e522–e530. [Google Scholar] [CrossRef]
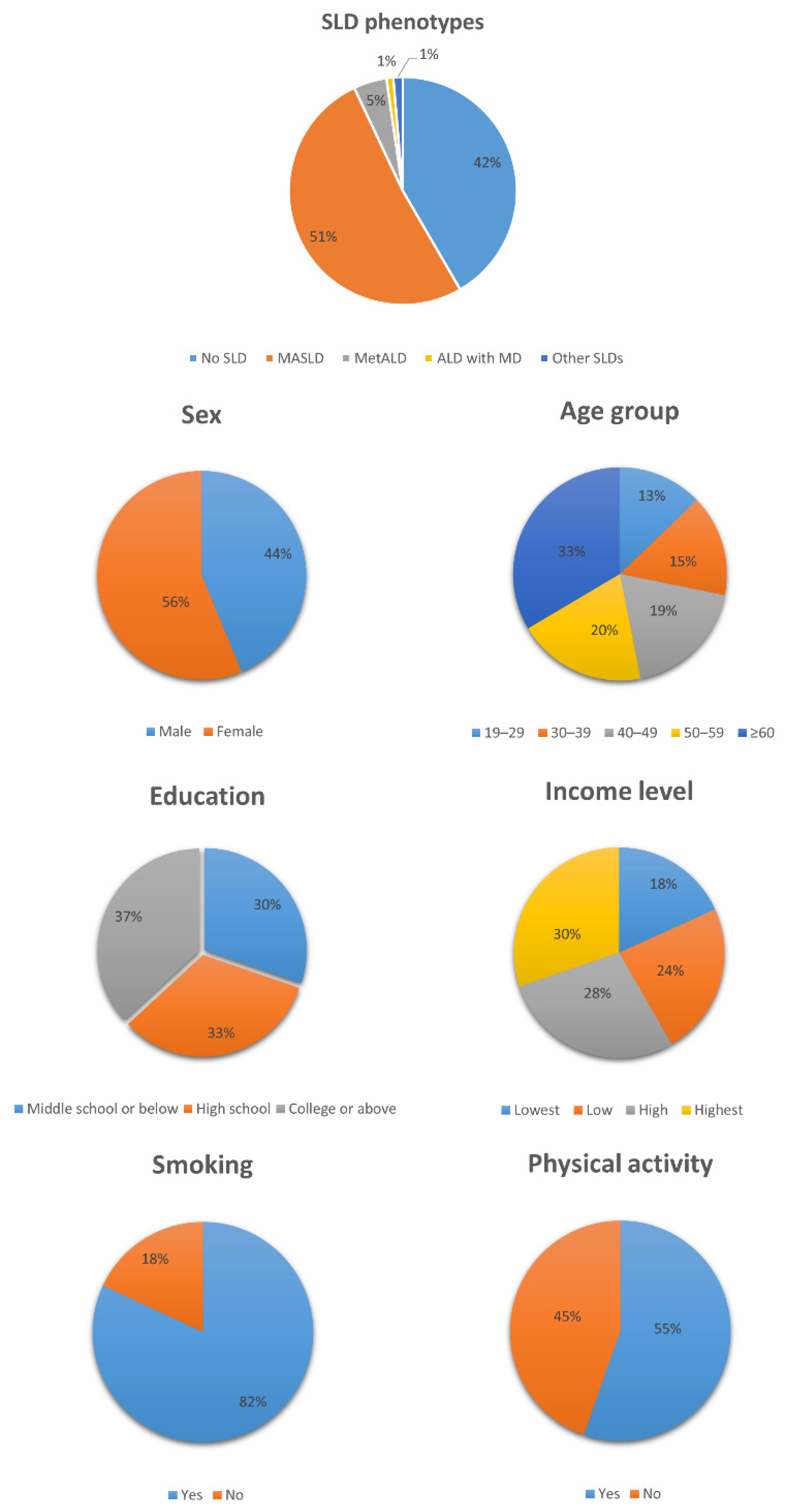
| Operationalization | |
|---|---|
| Hepatic steatosis | Hepatic steatosis index (HSI) > 31 |
| Cardiometabolic risk factors (CMRF) | |
| Overweight or obesity | Body mass index ≥ 23 kg/m2 or Waist circumference ≥ 90 cm for male or ≥85 cm for female |
| Prediabetes or Diabetes mellitus | Fasting glucose ≥ 100 mg/dL or glycated hemoglobin A1c ≥ 5.7% or use of insulin or oral hypoglycemic agents |
| Elevated blood pressure | Blood pressure ≥ 130/85 mm Hg or use of anti-hypertensive medications |
| Hypertriglyceridemia | Triglycerides ≥ 150 mg/dL or use of lipid lowering drugs |
| Low high-density lipoprotein cholesterol (HDL) | HDL < 40 mg/dL for male or <50 mg/dL for female or use of lipid lowering drugs |
| SLD subtypes | (1) no SLD: HSI ≤ 31 |
| (2) MASLD: HSI > 31 + ≥1 of CMRFs + alcohol consumption < 20 g/day (female) < 30 g/day (male) (3) MetALD: HSI > 31 + ≥1 of CMRFs + alcohol consumption 20–50 g/day (female) 30–60 g/day (male) (4) ALD with MD: HSI > 31 + ≥1 of CMRFs + alcohol consumption > 50 g/day (female) > 60 g/day (male) (5) other SLDs: HSI > 31 and does not meet criteria for (1)–(4) |
| Overall | SLD Categories | |||||
|---|---|---|---|---|---|---|
| No SLD | MASLD | MetALD | ALD with MD | Other SLDs | ||
| N = 20,141 | N = 8383 | N = 10,346 | N = 947 | N = 195 | N = 270 | |
| Sex | ||||||
| Male | 8813 (43.8%) | 3294 (39.3%) | 4542 (43.9%) | 743 (78.5%) | 147 (75.4%) | 87 (32.2%) |
| Female | 11,328 (56.2%) | 5089 (60.7%) | 5804 (56.1%) | 204 (21.5%) | 48 (24.6%) | 183 (67.8%) |
| Age | ||||||
| Mean (SD) | 50.8 (16.7) | 48.4 (17.7) | 53.6 (15.7) | 47.9 (13.6) | 42 (13.2) | 38 (12.5) |
| Education level | ||||||
| Middle school or below | 6095 (30.3%) | 2096 (25.0%) | 3710 (35.9%) | 238 (25.1%) | 33 (16.9%) | 18 (6.7%) |
| High school | 6617 (32.9%) | 2839 (33.9%) | 3238 (31.3%) | 346 (36.5%) | 86 (44.1%) | 108 (40.0%) |
| College or above | 7429 (36.9%) | 3448 (41.1%) | 3398 (32.8%) | 363 (38.3%) | 76 (39.0%) | 144 (53.3%) |
| Income level | ||||||
| Lowest | 3634 (18.0%) | 1422 (17.0%) | 2052 (19.8%) | 121 (12.8%) | 21 (10.8%) | 18 (6.7%) |
| Low | 4881 (24.2%) | 1886 (22.5%) | 2648 (25.6%) | 240 (25.3%) | 57 (29.2%) | 50 (18.5%) |
| High | 5601 (27.8%) | 2359 (28.1%) | 2827 (27.3%) | 269 (28.4%) | 60 (30.8%) | 86 (31.9%) |
| Highest | 6025 (29.9%) | 2716 (32.4%) | 2819 (27.2%) | 317 (33.5%) | 57 (29.2%) | 116 (43.0%) |
| Smoking | ||||||
| Yes | 16,502 (81.9%) | 6971 (83.2%) | 8697 (84.1%) | 518 (54.7%) | 83 (42.6%) | 233 (86.3%) |
| No | 3639 (18.1%) | 1412 (16.8%) | 1649 (15.9%) | 429 (45.3%) | 112 (57.4%) | 37 (13.7%) |
| Physical activity | ||||||
| Yes | 11,153 (55.4%) | 4465 (53.3%) | 5921 (57.2%) | 536 (56.6%) | 100 (51.3%) | 131 (48.5%) |
| No | 8988 (44.6%) | 3918 (46.7%) | 4425 (42.8%) | 411 (43.4%) | 95 (48.7%) | 139 (51.5%) |
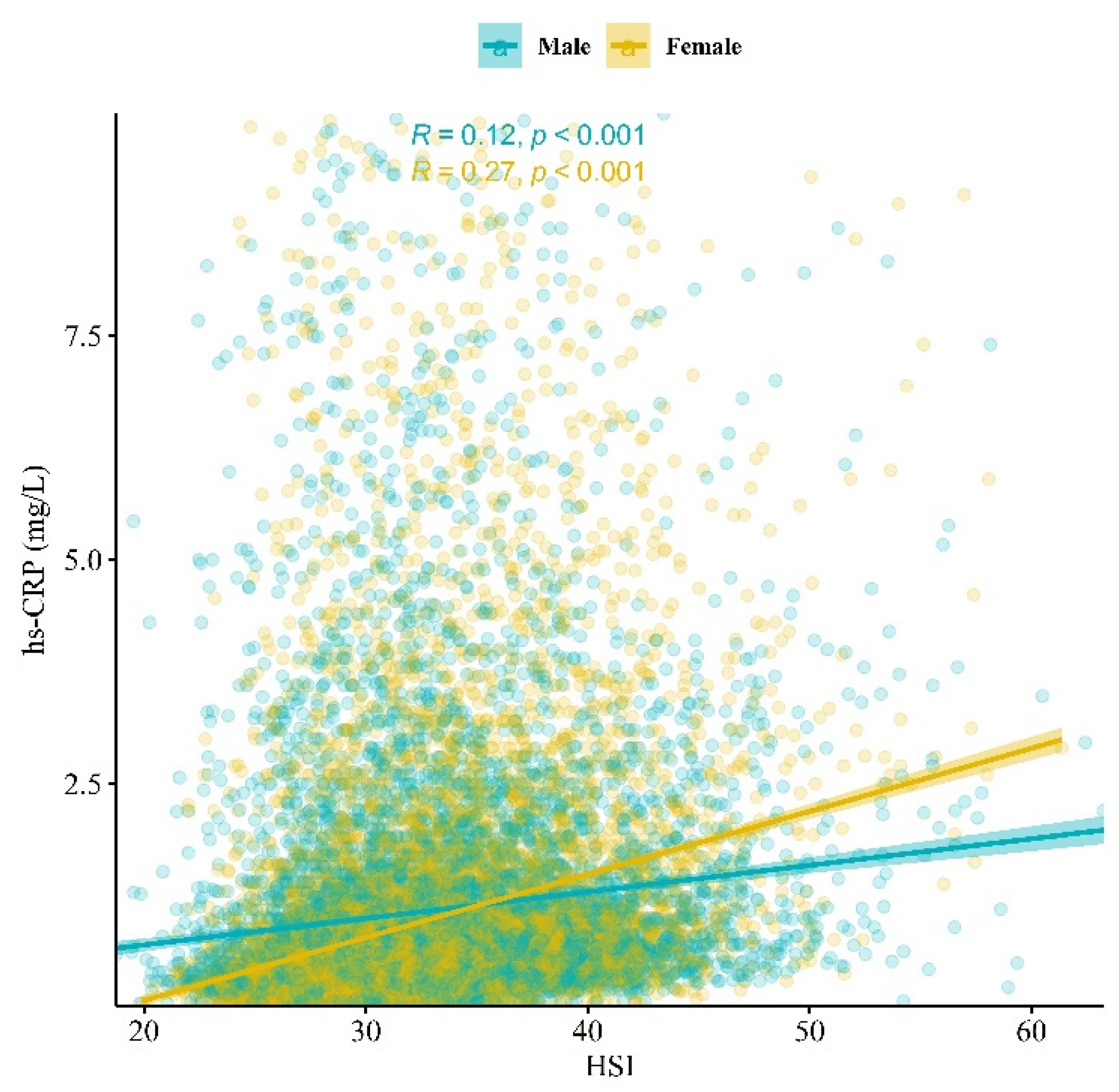
| HSI | ||||||
| Median (Q1, Q3) | 32.1 (28.9, 35.7) | 28.4 (26.7, 29.7) | 35.1 (32.9, 38.2) | 35.1 (32.8, 38.5) | 35.1 (32.6, 39.0) | 32.0 (31.4, 33.1) |
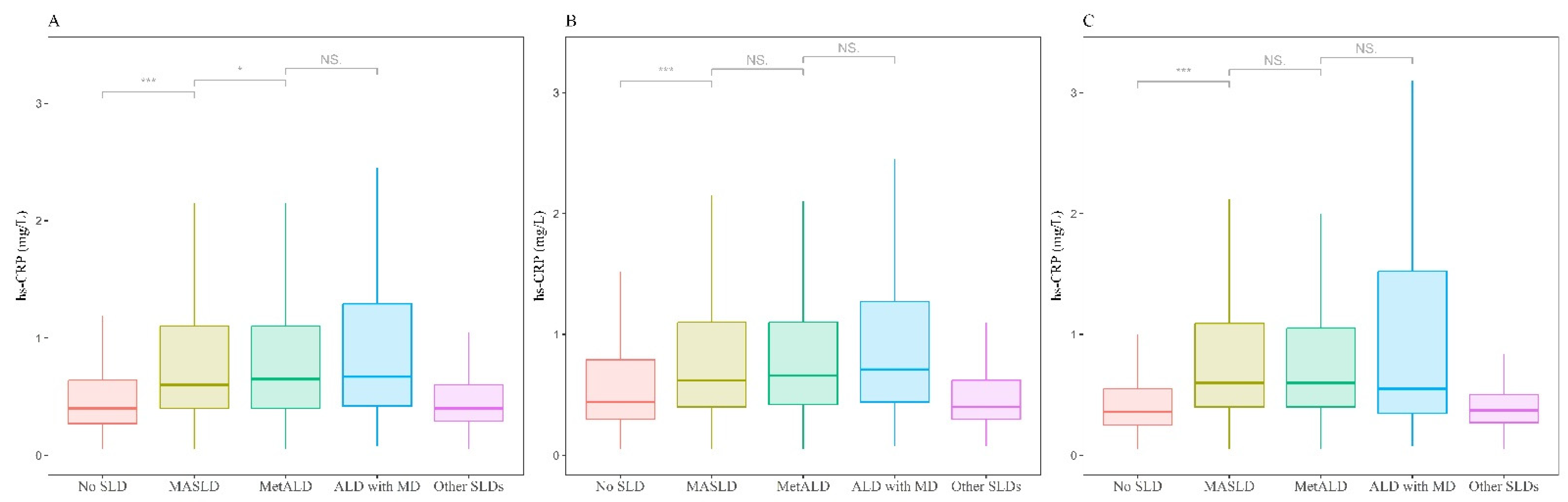
| hs-CRP (g/L) | ||||||
| Median (Q1, Q3) | 0.5 (0.3, 1.0) | 0.4 (0.3, 0.7) | 0.7 (0.4, 1.3) | 0.7 (0.4, 1.3) | 0.7 (0.4, 1.4) | 0.4 (0.3, 0.6) |
| AST (IU/L) | ||||||
| Median (Q1, Q3) | 20.0 (17.0, 25.0) | 19.0 (16.0, 23.0) | 21.0 (18.0, 26.0) | 24.0 (19.0, 30.0) | 24.0 (19.0, 31.0) | 19.0 (16.0, 24.0) |
| ALT (IU/L) | ||||||
| Median (Q1, Q3) | 17.0 (13.0, 25.0) | 13.0 (10.0, 17.0) | 21.0 (16.0, 31.0) | 25.0 (18.0, 36.0) | 25.0 (19.0, 38.0) | 21.0 (16.0, 28.0) |
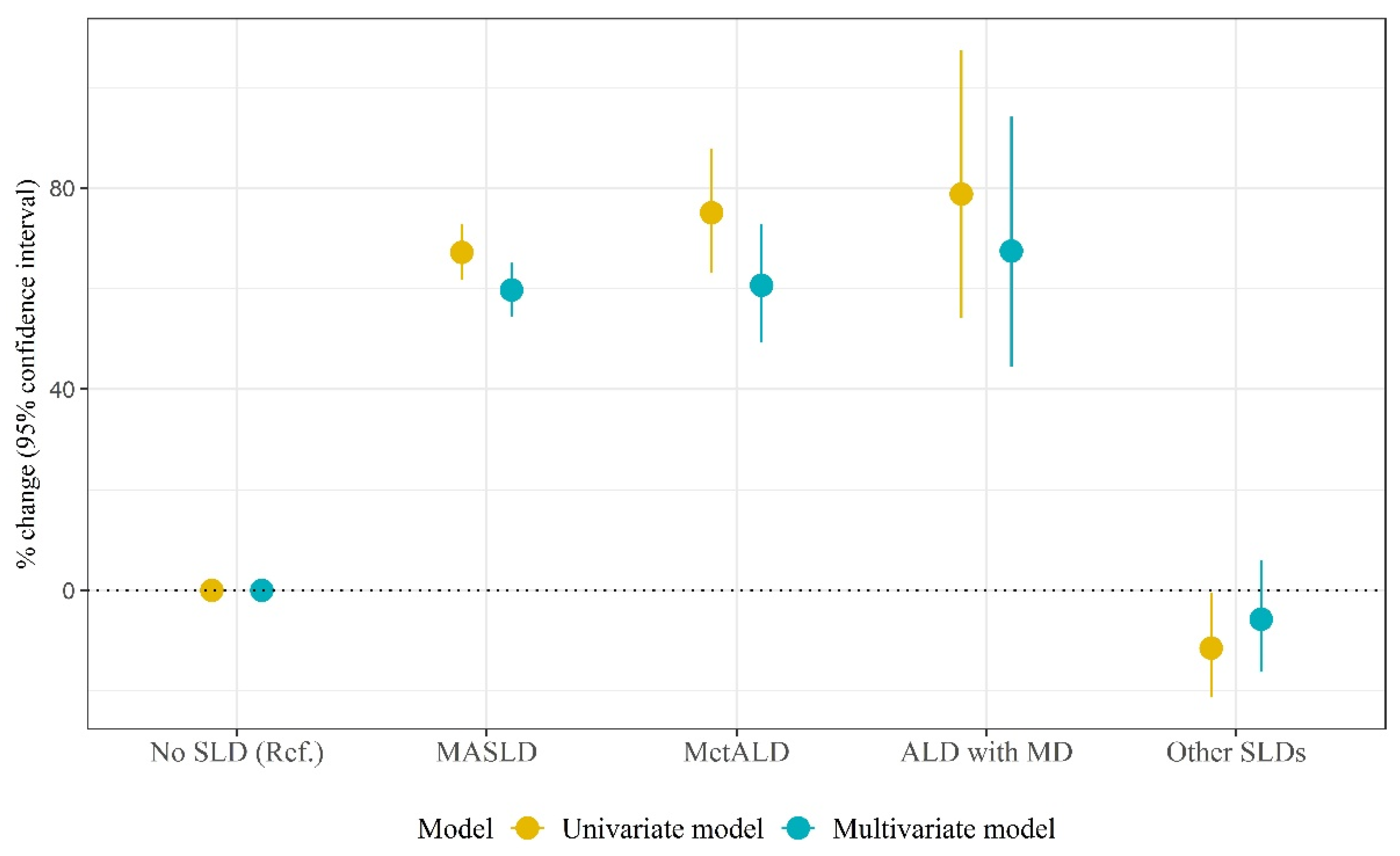
| Univariate Model | Multivariate Model | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | % Change (95% CI) | p | β | SE | % Change (95% CI) | p | |
| SLD types | ||||||||
| No SLD | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| MASLD | 0.51 | 0.02 | 67.2 (61.8, 72.8) | <0.001 | 0.47 | 0.02 | 59.7 (54.5, 65.1) | <0.001 |
| MetALD | 0.56 | 0.04 | 75.1 (63.1, 87.9) | <0.001 | 0.47 | 0.04 | 60.6 (49.3, 72.8) | <0.001 |
| ALD with MD | 0.58 | 0.08 | 78.8 (54.2, 107.3) | <0.001 | 0.52 | 0.08 | 67.5 (44.4, 94.2) | <0.001 |
| Other SLDs | −0.12 | 0.06 | −11.5 (−21.3, −0.5) | 0.041 | −0.06 | 0.06 | −5.8 (−16.2, 6.0) | 0.323 |
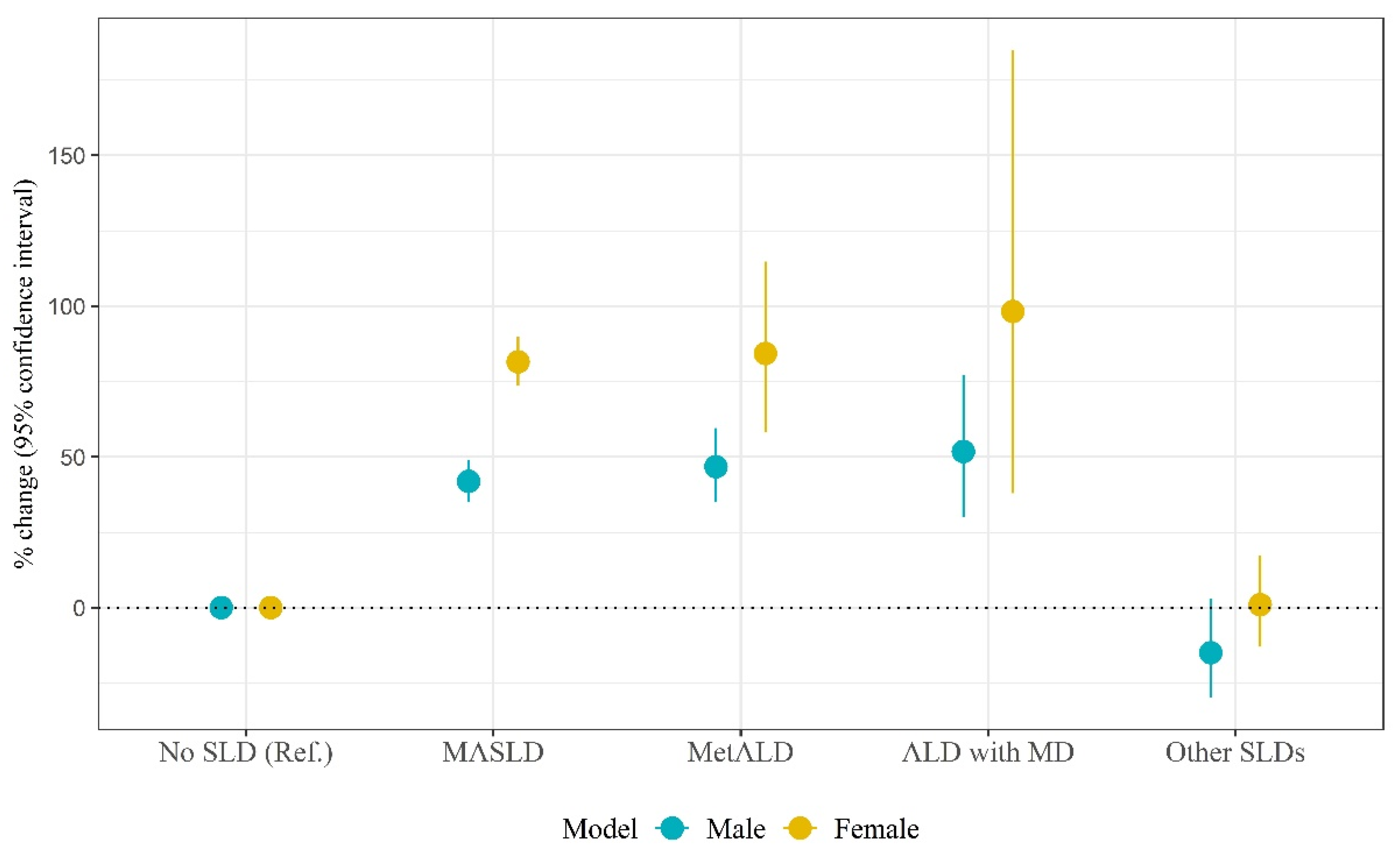
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| β | SE | % Change (95% CI) | p | β | SE | % Change (95% CI) | p | |
| SLD types | ||||||||
| No SLD | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. | ||
| MASLD | 0.35 | 0.03 | 41.9 (35.1, 49.1) | <0.001 | 0.60 | 0.02 | 81.5 (73.6, 89.8) | <0.001 |
| MetALD | 0.38 | 0.04 | 46.8 (35.0, 59.6) | <0.001 | 0.61 | 0.08 | 84.3 (58.1, 114.8) | <0.001 |
| ALD with MD | 0.42 | 0.08 | 51.8 (30.0, 77.2) | <0.001 | 0.68 | 0.18 | 98.2 (38.0, 184.8) | <0.001 |
| Other SLDs | −0.16 | 0.10 | −14.9 (−29.8, 3.0) | 0.097 | 0.01 | 0.08 | 1.0 (−13.0, 17.3) | 0.893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, S.-U.; Yoon, J.-H. High-Sensitivity C-Reactive Protein Levels in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), Metabolic Alcohol-Associated Liver Disease (MetALD), and Alcoholic Liver Disease (ALD) with Metabolic Dysfunction. Biomolecules 2024, 14, 1468. https://doi.org/10.3390/biom14111468
Baek S-U, Yoon J-H. High-Sensitivity C-Reactive Protein Levels in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), Metabolic Alcohol-Associated Liver Disease (MetALD), and Alcoholic Liver Disease (ALD) with Metabolic Dysfunction. Biomolecules. 2024; 14(11):1468. https://doi.org/10.3390/biom14111468
Chicago/Turabian StyleBaek, Seong-Uk, and Jin-Ha Yoon. 2024. "High-Sensitivity C-Reactive Protein Levels in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), Metabolic Alcohol-Associated Liver Disease (MetALD), and Alcoholic Liver Disease (ALD) with Metabolic Dysfunction" Biomolecules 14, no. 11: 1468. https://doi.org/10.3390/biom14111468
APA StyleBaek, S.-U., & Yoon, J.-H. (2024). High-Sensitivity C-Reactive Protein Levels in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD), Metabolic Alcohol-Associated Liver Disease (MetALD), and Alcoholic Liver Disease (ALD) with Metabolic Dysfunction. Biomolecules, 14(11), 1468. https://doi.org/10.3390/biom14111468






