The Role of Circulating Biomarkers in Patients with Coronary Microvascular Disease
Abstract
1. Introduction
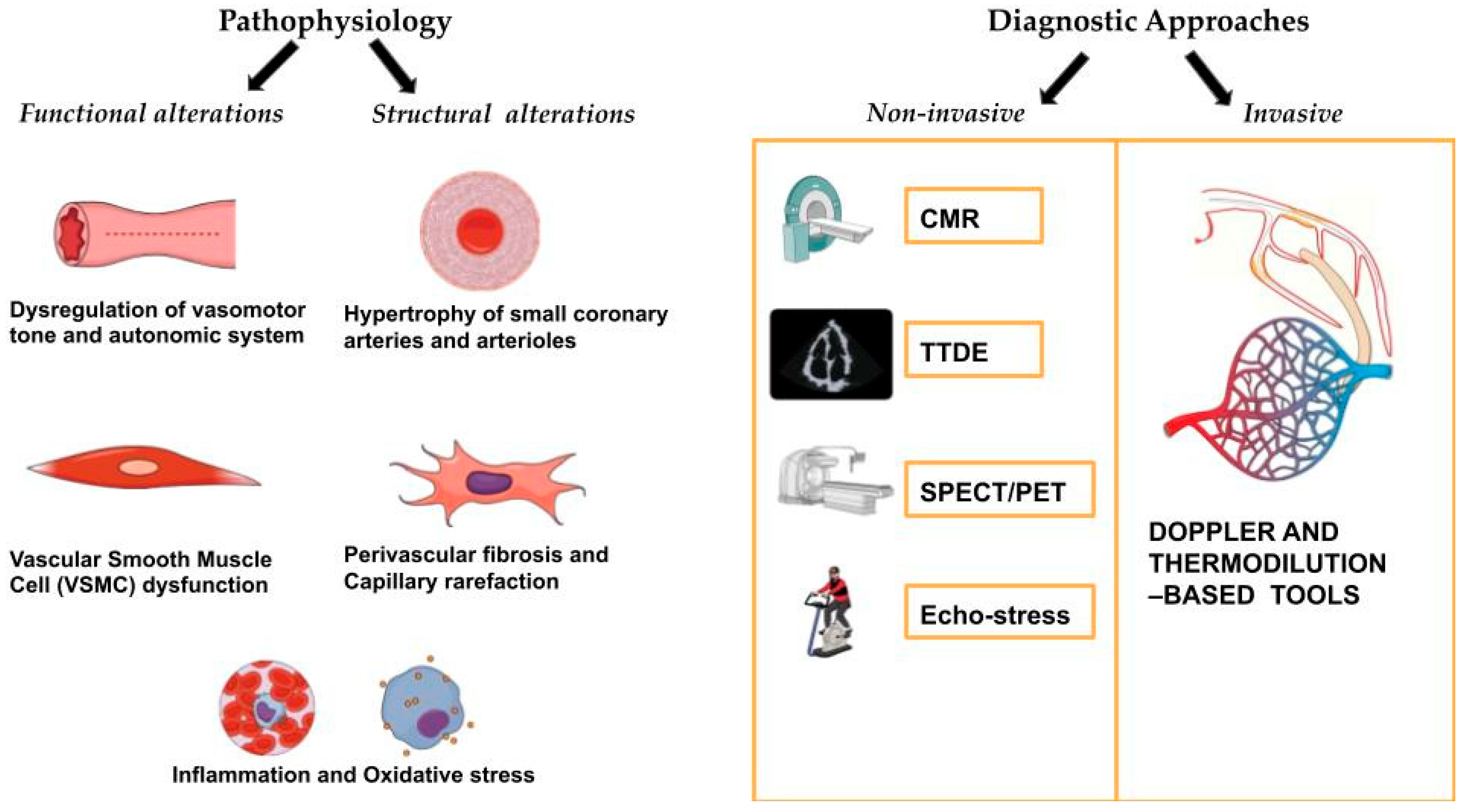
2. Pathophysiology of Coronary Microvascular Disease
2.1. Functional Alterations
2.2. Structural Alterations
3. Diagnostic Approaches for Coronary Microvascular Disease
3.1. Invasive Diagnostic Techniques
3.2. Non-Invasive Diagnostic Techniques
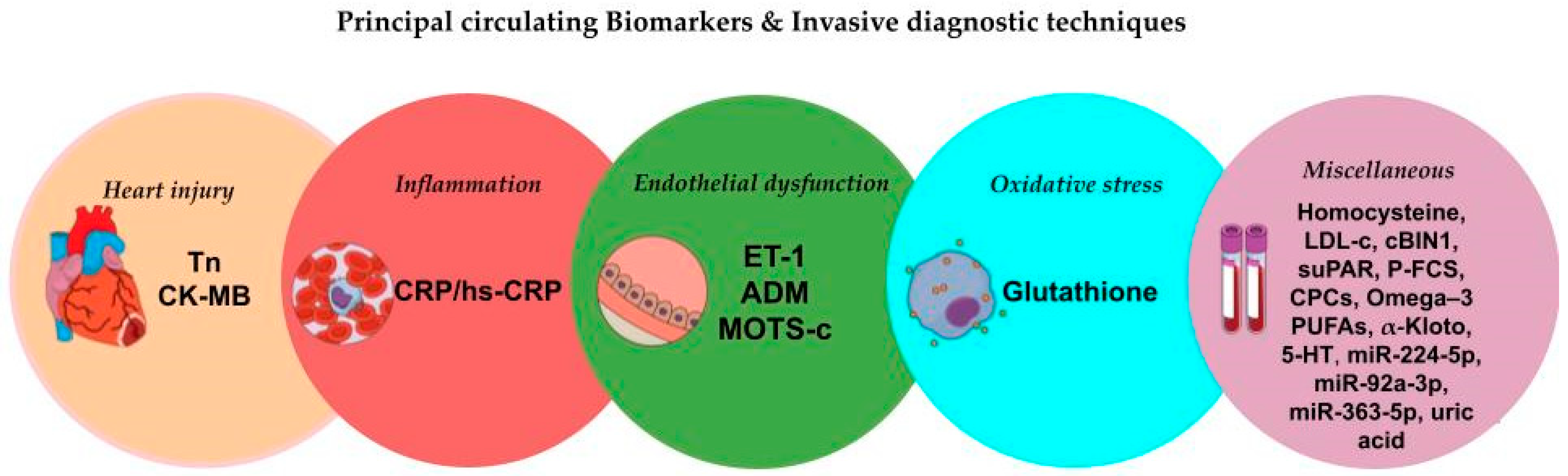
4. Circulating Biomarkers in Coronary Microvascular Dysfunction
4.1. Traditional Biomarkers of Heart Injury
4.2. Biomarkers of Inflammation
4.3. Biomarkers of Oxidative Stress
4.4. Biomarkers of Endothelial Dysfunction
4.5. Miscellaneous Biomarkers
| Authors | Year | Biomarkers Evaluated | N. Subjects | Main Findings | Invasive Measures |
|---|---|---|---|---|---|
| Takashio et al. [40] | 2013 | cTnT, BNP, hs-CRP, IL-6, TNF-α | 90 patients 47 controls | cTnT levels were higher in patients with CMD and increased LVEDP | CFR |
| Kitabata et al. [42] | 2013 | CK-MB | 24 patients | CK-MB levels were higher in patients with a higher MVRI | MVRI |
| Herrmann et al. [43] | 2001 | CK, cTnT | 55 patients | rCVR after successful coronary stenting was independently associated with elevation of cTnT and CK | CFVR |
| Park et al. [44] | 2014 | cTnI | 19 patients | cTnI was correlated with IMR but not with CFR values | CFR, IMR |
| Ong et al. [49] | 2015 | sCD40L, hs-CRP | 62 patients | Patients with Ach-induced coronary spasm and no significant coronary stenoses had elevated levels of sCD40L and hs-CRP | ACh provocation test |
| Teragawa et al. [50] | 2004 | CRP | 46 patients | Increased CRP levels had reduced CBF response to acetylcholine | ACh provocation test |
| Hung et al. [51] | 2005 | hs-CRP | 428 patients | hs-CRP was associated with coronary vasospastic angina | Ergonovine provocation test |
| Long et al. [52] | 2017 | hs-CRP | 20 patients 20 controls | hs-CRP was positively correlated with IMR | CFR, IMR |
| Dhawan et al. [57] | 2011 | Cystine/Glutathione, hs-CRP | 47 patients | Lower plasma glutathione levels and higher cystine/glutathione ratios were associated with impaired coronary microvascular function and greater plaque necrotic core content | CFVR, hMR, IVUS |
| Theuerle et al. [58] | 2019 | ET-1, ADM | 32 patients | Elevated ET-1 levels were associated with increased IMR. ADM levels were correlated with CFR. | IMR, CFR |
| Qin et al. [59] | 2018 | MOTS-c | 20 patients 20 controls | MOTS-c levels were positively correlated with both microvascular and epicardial endothelial function | ACh provocation test |
| Ahmad et al. [60] | 2020 | Homocysteine | 1418 patients | Elevated homocysteine levels positively correlate with CMD and early atherosclerosis | ACh provocation test |
| Mangiacapra et al. [61] | 2012 | Total cholesterol, LDL-c, HDL-c, TG | 95 patients | Significant correlation between IMR and total cholesterol and LDL-c | IMR |
| Pacheco et al. [62] | 2021 | cBIN1 | 95 patients 50 controls | Higher cBIN1 score was associated with vasoconstriction to Ach | CFR, ACh provocation test |
| Mekonnen et al. [63] | 2015 | suPAR | 66 patients | Higher suPAR levels correlated with lower CFR | CFR |
| Kang et al. [64] | 2021 | P-FCS, D-dimer | 116 patients | P-FCS significantly increased the risk of CMD post-procedurally | IMR |
| Mekonnen et al. [65] | 2016 | CPCs | 123 patients | Lower CFR was associated with higher levels of CPCs cells | CFR |
| Muroya et al. [66] | 2018 | EPA/AA, DGLA | 108 patients | Lower levels of the EPA/AA were significantly correlated with increased hMVRI | hMVRI |
| Akhiyat et al. [67] | 2024 | α-Klotho | 98 patients | Patients with CMD had significantly lower circulating α-Klotho levels | CFR |
| Odaka et al. [69] | 2016 | Serotonin | 198 patients | Plasma serotonin concentration was found to be significantly higher in patients with CMD | ACh provocation test |
| James et al. [73] | 2022 | miR-224-5p | 120 patients | miR-224-5p was found to be significantly elevated in the low-CFR group | CFR |
| Kuwahata et al. [74] | 2010 | UA | 194 patients | Elevated UA levels were associated with impaired CBF | ACh and papaverin provocation test |
| Prasad et al. [75] | 2017 | UA | 229 patients | Elevated UA levels were associated with impaired CBF | ACh provocation test |
| Keeley et al. [76] | 2022 | Resolvins, Maresin 1, EPA, DHA and 18-HEPE | 31 patients 12 controls | Women with CMD had significantly lower levels of resolvin D1 and maresin 1 but higher levels of DHA and 18-HEPE compared to the control group | CFR |
| Ito et al. [77] | 2024 | MDA-LDL | 95 patients | Elevated levels of MDA-LDL were significantly associated with CMD in patients without CAD | CFR, IMR |
5. Therapeutic Implications of Biomarkers in Coronary Microvascular Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crea, F.; Montone, R.A.; Rinaldi, R. Pathophysiology of Coronary Microvascular Dysfunction. Circ. J. 2022, 86, 1319–1328. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: A scientific statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Myftari, V.; Colombo, L.; Tomarelli, E.; Piccialuti, A.; Di Pietro, G.; Birtolo, L.I.; Maestrini, V.; et al. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Focus on Coronary Microvascular Dysfunction and Genetic Susceptibility. J. Clin. Med. 2023, 12, 3586. [Google Scholar] [CrossRef]
- Indolfi, C.; Porto, I.; Muscoli, S.; Fedele, F.; Mancone, M. La patologia microvascolare coronarica: Meccanismi e contesti clinici. G. Ital. Cardiol. 2022, 23, 397–407. [Google Scholar] [CrossRef]
- La Vecchia, G.; Fumarulo, I.; Caffè, A.; Chiatto, M.; Montone, R.A.; Aspromonte, N. Microvascular Dysfunction across the Spectrum of Heart Failure Pathology: Pathophysiology, Clinical Features and Therapeutic Implications. Int. J. Mol. Sci. 2024, 25, 7628. [Google Scholar] [CrossRef]
- Padro, T.; Manfrini, O.; Bugiardini, R.; Canty, J.; Cenko, E.; De Luca, G.; Duncker, D.J.; Eringa, E.C.; Koller, A.; Tousoulis, D.; et al. ESC Working Group on Coronary Pathophysiology and Microcirculation position paper on ‘coronary microvascular dysfunction in cardiovascular disease’. Cardiovasc. Res. 2020, 116, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, M.; Foà, A.; Paolisso, P.; Bergamaschi, L.; Gallinoro, E.; Polimeni, A.; Scarsini, R.; Muscoli, S.; Amicone, S.; De Vita, A.; et al. Coronary microvascular dysfunction beyond the spectrum of chronic coronary syndromes. Prog. Cardiovasc. Dis. 2024, 87, 73–82. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Indolfi, C.; Polimeni, A.; Mongiardo, A.; De Rosa, S.; Spaccarotella, C. Old unsolved problems: When and how to treat silent ischaemia. Eur. Heart J. Suppl. 2020, 22, L82–L85. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, Z.; Song, Z.; Li, Z.; Xiao, Y.; Zhang, Y.; Wen, T.; Pan, G.; Xu, H.; Sheng, X.; et al. Inflammation and coronary microvascular disease: Relationship, mechanism and treatment. Front. Cardiovasc. Med. 2024, 11, 1280734. [Google Scholar] [CrossRef] [PubMed]
- Rocco, E.; Grimaldi, M.C.; Maino, A.; Cappannoli, L.; Pedicino, D.; Liuzzo, G.; Biasucci, L.M. Advances and Challenges in Biomarkers Use for Coronary Microvascular Dysfunction: From Bench to Clinical Practice. J. Clin. Med. 2022, 11, 2055. [Google Scholar] [CrossRef]
- Scarsini, R.; Campo, G.; Di Serafino, L.; Zanon, S.; Rubino, F.; Monizzi, G.; Biscaglia, S.; Ancona, M.; Polimeni, A.; Niccoli, G.; et al. #FullPhysiology: A systematic step-by-step guide to implement intracoronary physiology in daily practice. Minerva Cardiol. Angiol. 2023, 71, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Montone, R.A.; Meucci, M.C.; De Vita, A.; Lanza, G.A.; Niccoli, G. Coronary provocative tests in the catheterization laboratory: Pathophysiological bases, methodological considerations and clinical implications. Atherosclerosis 2021, 318, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic Dikic, A.; Dedic, S.; Jovanovic, I.; Boskovic, N.; Giga, V.; Nedeljkovic, I.; Tesic, M.; Aleksandric, S.; Cortigiani, L.; Ciampi, Q.; et al. Noninvasive evaluation of dynamic microvascular dysfunction in ischemia and no obstructive coronary artery disease patients with suspected vasospasm. J. Cardiovasc. Med. 2024, 25, 123–131. [Google Scholar]
- Vijayan, S.; Barmby, D.S.; Pearson, I.R.; Davies, A.G.; Wheatcroft, S.B.; Sivananthan, M. Assessing coronary blood flow physiology in the cardiac catheterisation laboratory. Curr. Cardiol. Rev. 2017, 13, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Nowroozpoor, A.; Gutterman, D.; Safdar, B. Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys?—A scoping review. Microvasc. Res. 2021, 134, 104123. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hanna, P.; Mori, S. Innervation of the coronary arteries and its role in controlling microvascular resistance. J. Cardiol. 2024, 84, 1–13. [Google Scholar] [CrossRef]
- Crea, F.; Camici, P.G.; Merz, C.N.B. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014, 35, 1101–1111. [Google Scholar] [CrossRef]
- Chakrala, T.; Prakash, R.; Valdes, C.; Pepine, C.J.; Keeley, E.C. Circulating Biomarkers in Coronary Microvascular Dysfunction. J. Am. Heart Assoc. 2023, 12, e029341. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.G.; Tschöpe, C.; Di Carli, M.F.; Rimoldi, O.; Van Linthout, S. Coronary microvascular dysfunction in hypertrophy and heart failure. Cardiovasc. Res. 2020, 116, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Webb, R.C. New insights into RhoA/Rho-kinase signaling: A key regulator of vascular contraction. Small GTPases 2021, 12, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Rizzoni, D.; Taddei, S.; Widmer, R.J.; Montezano, A.C.; Lüscher, T.F.; Schiffrin, E.L.; Touyz, R.M.; Paneni, F.; Lerman, A.; et al. Assessment and pathophysiology of microvascular disease: Recent progress and clinical implications. Eur. Heart J. 2021, 42, 2590–2604. [Google Scholar] [CrossRef] [PubMed]
- Demola, P.; Beneduce, A.; Masiero, G.; Serino, F.; Baldi, E.; Polimeni, A.; Attisano, T.; Contarini, M.; Castiglioni, B.; De Marco, F.; et al. Diagnosi e trattamento dei pazienti affetti da ischemia/infarto miocardico in assenza di coronaropatia ostruttiva nei laboratori di emodinamica italiani: Risultati dell’indagine nazionale SICI-GISE promossa dal Comitato GISE Young. G. Ital. Cardiol. 2023, 24, 42S–52S. [Google Scholar]
- Polimeni, A.; Campo, G. Editorial: Coronary epicardial and microvascular hemodynamics. Front. Cardiovasc. Med. 2022, 9, 969928. [Google Scholar] [CrossRef]
- De Rosa, S.; Polimeni, A.; De Velli, G.; Conte, M.; Sorrentino, S.; Spaccarotella, C.; Mongiardo, A.; Sabatino, J.; Contarini, M.; Indolfi, C. Reliability of Instantaneous Wave-Free Ratio (iFR) for the Evaluation of Left Main Coronary Artery Lesions. J. Clin. Med. 2019, 8, 1143. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.; Polimeni, A.; Petraco, R.; Davies, J.E.; Indolfi, C. Diagnostic Performance of the Instantaneous Wave-Free Ratio: Comparison With Fractional Flow Reserve. Circ. Cardiovasc. Interv. 2018, 11, e004613. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Morrone, D.; De Rosa, S.; Montone, R.A.; Polimeni, A.; Aimo, A.; Mancone, M.; Muscoli, S.; Pedrinelli, R.; Indolfi, C.; et al. The central role of invasive functional coronary assessment for patients with ischemic heart disease. Int. J. Cardiol. 2021, 331, 17–25. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Safdar, B.; Seitz, A.; Hubert, A.; Beltrame, J.F.; Prescott, E. Diagnosis of coronary microvascular dysfunction in the clinic. Cardiovasc. Res. 2020, 116, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Pompei, G.; Ganzorig, N.; Kotanidis, C.P.; Alkhalil, M.; Collet, C.; Sinha, A.; Perera, D.; Beltrame, J.; Kunadian, V. Novel diagnostic approaches and management of coronary microvascular dysfunction. Am. J. Prev. Cardiol. 2024, 19, 100712. [Google Scholar] [CrossRef]
- Goncharov, N.V.; Nadeev, A.D.; Jenkins, R.O.; Avdonin, P.V. Markers and Biomarkers of Endothelium: When Something Is Rotten in the State. Oxid. Med. Cell. Longev. 2017, 2017, 9759735. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, L.; Chen, S.; Yuan, Z.; Liu, S.; Shen, X.; Zheng, X.; Qi, X.; Lee, K.K.H.; Chan, J.Y.H.; et al. BDNF-mediated migration of cardiac microvascular endothelial cells is impaired during ageing. J. Cell. Mol. Med. 2012, 16, 1518–1526. [Google Scholar] [CrossRef]
- Bahls, M.; Könemann, S.; Markus, M.R.P.; Wenzel, K.; Friedrich, N.; Nauck, M.; Völzke, H.; Steveling, A.; Janowitz, D.; Grabe, H.-J.; et al. Brain-derived neurotrophic factor is related with adverse cardiac remodeling and high NTproBNP. Sci. Rep. 2019, 9, 15421. [Google Scholar] [CrossRef] [PubMed]
- Kytikova, O.Y.; Novgorodtseva, T.P.; Denisenko, Y.K.; Antonyuk, M.V.; Gvozdenko, T.A.; Atamas, O.V. Brain-derived neurotrophic factor and coronary artery disease. Russ. Open Med. J. 2022, 11, e0202. [Google Scholar] [CrossRef]
- Giannessi, D.; Colotti, C.; Maltinti, M.; Del Ry, S.; Prontera, C.; Turchi, S.; L’Abbate, A.; Neglia, D. Circulating heat shock proteins and inflammatory markers in patients with idiopathic left ventricular dysfunction: Their relationships with myocardial and microvascular impairment. Cell Stress Chaperones 2007, 12, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat Shock Protein 60 in Cardiovascular Physiology and Diseases. Front. Mol. Biosci. 2020, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Takashio, S.; Yamamuro, M.; Izumiya, Y.; Sugiyama, S.; Kojima, S.; Yamamoto, E.; Tsujita, K.; Tanaka, T.; Tayama, S.; Kaikita, K.; et al. Coronary Microvascular Dysfunction and Diastolic Load Correlate with Cardiac Troponin T Release Measured by a Highly Sensitive Assay in Patients with Nonischemic Heart Failure. J. Am. Coll. Cardiol. 2013, 62, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Kawasaki, D.; Oka, K.; Akahori, H.; Iwasaku, T.; Fukunaga, M.; Eguchi, A.; Sawada, H.; Masutani, M.; Lee-Kawabata, M.; et al. The Impact of Pravastatin Pre-Treatment on Periprocedural Microcirculatory Damage in Patients Undergoing Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2011, 4, 513–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitabata, H.; Kubo, T.; Ishibashi, K.; Komukai, K.; Tanimoto, T.; Ino, Y.; Kashiwagi, M.; Ozaki, Y.; Shiono, Y.; Shimamura, K.; et al. Prognostic Value of Microvascular Resistance Index Immediately After Primary Percutaneous Coronary Intervention on Left Ventricular Remodeling in Patients with Reperfused Anterior Acute ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2013, 6, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Haude, M.; Lerman, A.; Schulz, R.; Volbracht, L.; Ge, J.; Schmermund, A.; Wieneke, H.; von Birgelen, C.; Eggebrecht, H.; et al. Abnormal Coronary Flow Velocity Reserve After Coronary Intervention Is Associated with Cardiac Marker Elevation. Circulation 2001, 103, 2339–2345. [Google Scholar] [CrossRef]
- Park, K.; Kim, M.; Cho, Y.-R.; Park, J.-S.; Park, T.-H.; Kim, M.H.; Kim, Y.-D. Association Between Cardiac Troponin Level and Coronary Flow Reserve in Patients Without Coronary Artery Disease: Insight from a Thermodilution Technique Using an Intracoronary Pressure Wire. Korean Circ. J. 2014, 44, 141–147. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cook, N. Clinical Usefulness of Very High and Very Low Levels of C-Reactive Protein Across the Full Range of Framingham Risk Scores. Circulation 2004, 109, 1955–1959. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, C.G.; Nan, J.L.; Li, J.; Li, Z.C.; Zeng, H.S.; Gao, Z.; Qin, X.W.; Zhang, C.Y. Elevated circulating inflammatory markers in female patients with cardiac syndrome X. Cytokine 2007, 40, 172–176. [Google Scholar] [CrossRef]
- Recio-Mayoral, A.; Rimoldi, O.E.; Camici, P.G.; Kaski, J.C. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. J. Am. Coll. Cardiol. Imaging 2013, 6, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Vaccarino, V.; Khan, D.; Votaw, J.; Faber, T.; Veledar, E.; Jones, D.P.; Goldberg, J.; Raggi, P.; Quyyumi, A.A.; Bremner, J.D. Inflammation Is Related to Coronary Flow Reserve Detected by Positron Emission Tomography in Asymptomatic Male Twins. J. Am. Coll. Cardiol. 2011, 57, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Carro, A.; Athanasiadis, A.; Borgulya, G.; Schäufele, T.; Ratge, D.; Gaze, D.; Sechtem, U.; Kaski, J.C. Acetylcholine-induced coronary spasm in patients with unobstructed coronary arteries is associated with elevated concentrations of soluble CD40 ligand and high-sensitivity C-reactive protein. Coron. Artery Dis. 2015, 26, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Teragawa, H.; Fukuda, Y.; Matsuda, K.; Ueda, K.; Higashi, Y.; Oshima, T.; Yoshizumi, M.; Chayama, K. Relation between C-reactive protein concentrations and coronary microvascular endothelial function. Heart 2004, 90, 750–754. [Google Scholar] [CrossRef]
- Hung, M.-J.; Cherng, W.-J.; Yang, N.-I.; Cheng, C.-W.; Li, L.-F. Relation of high-sensitivity C-reactive protein level with coronary vasospastic angina pectoris in patients without hemodynamically significant coronary artery disease. Am. J. Cardiol. 2005, 96, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Huang, Z.; Zhuang, X.; Huang, Z.; Guo, Y.; Liao, X.; Luo, C. Association of inflammation and endothelial dysfunction with coronary microvascular resistance in patients with cardiac syndrome X. Arq. Bras. Cardiol. 2017, 109, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Vichova, T.; Motovska, Z. Oxidative Stress: Predictive Marker for Coronary Artery Disease. Exp. Clin. Cardiol. 2013, 18, e88–e91. [Google Scholar]
- Sahin, D.Y.; Elbasan, Z.; Gür, M.; Türkoglu, C.; Özaltun, B.; Sümbül, Z.; Çaylı, M. Relationship between Oxidative Stress Markers and Cardiac Syndrome X. J. Clin. Exp. Investig. 2012, 3, 174–180. [Google Scholar] [CrossRef]
- Nishihira, K.; Yamashita, A.; Imamura, T.; Hatakeyama, K.; Sato, Y.; Nakamura, H.; Yodoi, J.; Ogawa, H.; Kitamura, K.; Asada, Y. Thioredoxin in Coronary Culprit Lesions: Possible Relationship to Oxidative Stress and Intraplaque Hemorrhage. Atherosclerosis 2008, 201, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; AlBadri, A.; Wei, J.; Mehta, P.K.; Maughan, J.; Gadh, A.; Thomson, L.; Jones, D.P.; Quyyumi, A.A.; Pepine, C.J.; et al. Oxidative Stress Is Associated with Diastolic Dysfunction in Women with Ischemia with No Obstructive Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015602. [Google Scholar] [CrossRef]
- Dhawan, S.S.; Eshtehardi, P.; McDaniel, M.C.; Fike, L.V.; Jones, D.P.; Quyyumi, A.A.; Samady, H. The role of plasma aminothiols in the prediction of coronary microvascular dysfunction and plaque vulnerability. Atherosclerosis 2011, 219, 266–272. [Google Scholar] [CrossRef]
- Theuerle, J.; Farouque, O.; Vasanthakumar, S.; Patel, S.K.; Burrell, L.M.; Clark, D.J.; Al-Fiadh, A.H. Plasma endothelin-1 and adrenomedullin are associated with coronary artery function and cardiovascular outcomes in humans. Int. J. Cardiol. 2019, 291, 168–172. [Google Scholar] [CrossRef]
- Qin, Q.; Delrio, S.; Wan, J.; Widmer, R.J.; Cohen, P.; Lerman, L.O.; Lerman, A. Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int. J. Cardiol. 2018, 254, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Corban, M.T.; Toya, T.; Sara, J.D.; Lerman, B.; Park, J.Y.; Lerman, L.O.; Lerman, A. Coronary microvascular endothelial dysfunction in patients with angina and nonobstructive coronary artery disease is associated with elevated serum homocysteine levels. J. Am. Heart Assoc. 2020, 9, e017746. [Google Scholar] [CrossRef]
- Mangiacapra, F.; De Bruyne, B.; Peace, A.J.; Melikian, N.; Wijns, W.; Barbato, E. High cholesterol levels are associated with coronary microvascular dysfunction. J. Cardiovasc. Med. 2012, 13, 439–442. [Google Scholar] [CrossRef]
- Pacheco, C.; Wei, J.; Shufelt, C.; Hitzeman, T.C.; Cook-Wiens, G.; Pepine, C.J.; Handberg, E.; Anderson, R.D.; Petersen, J.; Hong, T.; et al. Association of coronary microvascular dysfunction and cardiac bridge integrator 1, a cardiomyocyte dysfunction biomarker. Clin. Cardiol. 2021, 44, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, G.; Corban, M.T.; Hung, O.Y.; Eshtehardi, P.; Eapen, D.J.; Al-Kassem, H.; Rasoul-Arzrumly, E.; Gogas, B.D.; McDaniel, M.C.; Pielak, T.; et al. Plasma soluble urokinase-type plasminogen activator receptor level is independently associated with coronary microvascular function in patients with non-obstructive coronary artery disease. Atherosclerosis 2015, 239, 55–60. [Google Scholar] [CrossRef]
- Kang, M.G.; Koo, B.-K.; Tantry, U.S.; Kim, K.; Ahn, J.-H.; Park, H.W.; Park, J.R.; Hwang, S.-J.; Hwang, J.-Y.; Gurbel, P.A.; et al. Association between thrombogenicity indices and coronary microvascular dysfunction in patients with acute myocardial infarction. JACC Basic Transl. Sci. 2021, 6, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, G.; Hayek, S.S.; Mehta, P.K.; Li, Q.; Mahar, E.; Mou, L.; Kenkre, T.S.; Petersen, J.W.; Azarbal, B.; Samuels, B.; et al. Circulating progenitor cells and coronary microvascular dysfunction: Results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation—Coronary Vascular Dysfunction Study (WISE-CVD). Atherosclerosis 2016, 253, 111–117. [Google Scholar] [CrossRef]
- Muroya, T.; Kawano, H.; Koga, S.; Ikeda, S.; Yamamoto, F.; Miwa, T.; Kohno, Y.; Maemura, K. Lower circulating omega-3 polyunsaturated fatty acids are associated with coronary microvascular dysfunction evaluated by hyperemic microvascular resistance in patients with stable coronary artery disease. Int. Heart J. 2018, 59, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Akhiyat, N.; Ozcan, I.; Gulati, R.; Prasad, A.; Tchkonia, T.; Kirkland, J.L.; Lewis, B.; Lerman, L.O.; Lerman, A. Patients with Coronary Microvascular Dysfunction Have Less Circulating α-Klotho. J. Am. Heart Assoc. 2024, 13, e031972. [Google Scholar] [CrossRef]
- McFadden, E.P.; Clarke, J.G.; Davies, G.J.; Kaski, J.C.; Haider, A.W.; Maseri, A. Effect of Intracoronary Serotonin on Coronary Vessels in Patients with Stable Angina and Patients with Variant Angina. N. Engl. J. Med. 1991, 324, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Odaka, Y.; Takahashi, J.; Tsuburaya, R.; Nishimiya, K.; Hao, K.; Matsumoto, Y.; Ito, K.; Sakata, Y.; Miyata, S.; Manita, D.; et al. Plasma concentration of serotonin is a novel biomarker for coronary microvascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur. Heart J. 2017, 38, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Iaconetti, C.; Gareri, C.; Polimeni, A.; Indolfi, C. Non-Coding RNAs: The “Dark Matter” of Cardiovascular Pathophysiology. Int. J. Mol. Sci. 2013, 14, 19987–20018. [Google Scholar] [CrossRef]
- Matute-Blanco, L.; Fernández-Rodríguez, D.; Casanova-Sandoval, J.; Belmonte, T.; Benítez, I.D.; Rivera, K.; Garcia-Guimaraes, M.; Cortés Villar, C.; Peral Disdier, V.; Millán Segovia, R.; et al. Study protocol for the epigenetic characterization of angor pectoris according to the affected coronary compartment: Global and comprehensive assessment of the relationship between invasive coronary physiology and microRNAs. PLoS ONE 2023, 18, e0283097. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, M.; Ciccarelli, M.; Varzideh, F.; Kansakar, U.; Guerra, G.; Cerasuolo, F.A.; Buonaiuto, A.; Fiordelisi, A.; Venga, E.; Esposito, M.; et al. Non-Coding RNAs: The “Dark Matter” of Cardiovascular Pathophysiology. Cardiovasc. Diabetol. 2024, 23, 268. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Bryl-Gorecka, P.; Olde, B.; Gidlof, O.; Torngren, K.; Erlinge, D. Increased expression of miR-224-5p in circulating extracellular vesicles of patients with reduced coronary flow reserve. BMC Cardiovasc. Disord. 2022, 22, 321. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, S.; Hamasaki, S.; Ishida, S.; Kataoka, T.; Yoshikawa, A.; Orihara, K.; Ogawa, M.; Oketani, N.; Saihara, K.; Okui, H.; et al. Effect of uric acid on coronary microvascular endothelial function in women: Association with eGFR and ADMA. J. Atheroscler. Thromb. 2010, 17, 259–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasad, M.; Matteson, E.L.; Herrmann, J.; Gulati, R.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Uric Acid Is Associated with Inflammation, Coronary Microvascular Dysfunction, and Adverse Outcomes in Postmenopausal Women. Hypertension 2017, 69, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Keeley, E.C.; Li, H.J.; Cogle, C.R.; Handberg, E.M.; Merz, C.N.B.; Pepine, C.J. Specialized Proresolving Mediators in Symptomatic Women with Coronary Microvascular Dysfunction (from the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD [WARRIOR] Trial). Am. J. Cardiol. 2022, 162, 1–5. [Google Scholar] [CrossRef]
- Ito, T.; Yokoi, M.; Kitada, S.; Kawada, Y.; Mizoguchi, T.; Kikuchi, S.; Goto, T.; Seo, Y. Increased circulating levels of malondialdehyde-modified low-density lipoprotein in patients with coronary microvascular dysfunction. Am. J. Cardiol. 2021, 158, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sorop, O.; van de Wouw, J.; Chandler, S.; Ohanyan, V.; Tune, J.D.; Chilian, W.M.; Merkus, D.; Bender, S.B.; Duncker, D.J. Experimental animal models of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Kayikcioglu, M. Benefits of statin treatment in cardiac syndrome-X. Eur. Heart J. 2003, 24, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.; Cressman, M.; Glynn, R.J. Efficacy of rosuvastatin among men and women with moderate chronic kidney disease and elevated high-sensitivity C-reactive protein: A secondary analysis from the JUPITER trial. J. Am. Coll. Cardiol. 2010, 55, 1266–1273. [Google Scholar] [CrossRef]
- Pauly, D.F.; Johnson, B.D.; Anderson, R.D.; Handberg, E.M.; Smith, K.M.; Cooper-DeHoff, R.M.; Sopko, G.; Sharaf, B.M.; Kelsey, S.F.; Bairey Merz, C.N.; et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2011, 162, 678–684. [Google Scholar]
- Pizzi, C.; Manfrini, O.; Fontana, F.; Bugiardini, R. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac Syndrome X: Role of superoxide dismutase activity. Circulation 2004, 109, 53–58. [Google Scholar] [CrossRef]
- Kalinowski, L.; Dobrucki, L.W.; Szczepanska-Konkel, M.; Jankowski, M.; Martyniec, L.; Angielski, S.; Malinski, T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: A novel mechanism for antihypertensive action. Circulation 2003, 107, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Bugiardini, R.; Borghi, A.; Pozzati, A.; Ottani, F.; Morgagni, G.L.; Puddu, P. The paradox of nitrates in patients with angina pectoris and angiographically normal coronary arteries. Am. J. Cardiol. 1993, 72, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Denardo, S.J.; Wen, X.; Handberg, E.M.; Bairey Merz, C.N.; Sopko, G.S.; Cooper-Dehoff, R.M.; Pepine, C.J. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: A Women’s Ischemia Syndrome Evaluation (WISE) ancillary study. Clin. Cardiol. 2011, 34, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Mohri, M.; Shimokawa, H. Rho-kinase inhibitors for ischemic heart disease. Nihon Rinsho 2003, 61 (Suppl. 5), 874–879. [Google Scholar]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Handberg, E.M.; Shufelt, C.L.; Mehta, P.; Minissian, M.B.; Wei, J.; Thomson, L.E.J.; Berman, D.S.; Shaw, L.J.; Petersen, J.W.; et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): Impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016, 37, 1504–1513. [Google Scholar] [CrossRef]
- Villano, A.; Di Franco, A.; Nerla, R.; Sestito, A.; Tarzia, P.; Lamendola, P.; Di Monaco, A.; Sarullo, F.M.; Lanza, G.A.; Crea, F. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am. J. Cardiol. 2013, 112, 8–13. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Greasley, P.J.; Ahlström, C.; Althage, M.; Dwyer, J.P.; Law, G.; Wijkmark, E.; Lin, M.; Mercier, A.K.; Sunnåker, M.; et al. Efficacy and safety of zibotentan and dapagliflozin in patients with chronic kidney disease: Study design and baseline characteristics of the ZENITH-CKD trial. Nephrol. Dial. Transplant. 2024, 39, 414–425. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarta, R.; Martino, G.; Romano, L.R.; Lopes, G.; Greco, F.F.; Spaccarotella, C.A.M.; Indolfi, C.; Curcio, A.; Polimeni, A. The Role of Circulating Biomarkers in Patients with Coronary Microvascular Disease. Biomolecules 2025, 15, 177. https://doi.org/10.3390/biom15020177
Quarta R, Martino G, Romano LR, Lopes G, Greco FF, Spaccarotella CAM, Indolfi C, Curcio A, Polimeni A. The Role of Circulating Biomarkers in Patients with Coronary Microvascular Disease. Biomolecules. 2025; 15(2):177. https://doi.org/10.3390/biom15020177
Chicago/Turabian StyleQuarta, Rossella, Giovanni Martino, Letizia Rosa Romano, Giovanni Lopes, Francesco Fabio Greco, Carmen Anna Maria Spaccarotella, Ciro Indolfi, Antonio Curcio, and Alberto Polimeni. 2025. "The Role of Circulating Biomarkers in Patients with Coronary Microvascular Disease" Biomolecules 15, no. 2: 177. https://doi.org/10.3390/biom15020177
APA StyleQuarta, R., Martino, G., Romano, L. R., Lopes, G., Greco, F. F., Spaccarotella, C. A. M., Indolfi, C., Curcio, A., & Polimeni, A. (2025). The Role of Circulating Biomarkers in Patients with Coronary Microvascular Disease. Biomolecules, 15(2), 177. https://doi.org/10.3390/biom15020177






