CXCL12 as a Potential Hub Gene for N-Acetylcysteine Treatment of T1DM Liver Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishment of the Beagle Dog T1DM Model
2.2. MRNA Transcriptome Sequencing Analysis and Acquisition of Differentially Expressed Genes (DEGs)
2.3. Weighted Gene Co-Expression Network Analysis (WGCNA) and Identification of Hub Modules
2.4. Acquisition of Intersection Genes and Hub Genes
2.5. Functional Enrichment Analysis
2.6. Western Blotting
2.7. RNA Extraction and qPCR Assay
2.8. HE Stain
2.9. Transmission Electron Microscopy (TEM)
2.10. Detection of Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content
2.11. Immunofluorescence Assay
2.12. Immunohistochemistry
2.13. Statistical Analysis
3. Results
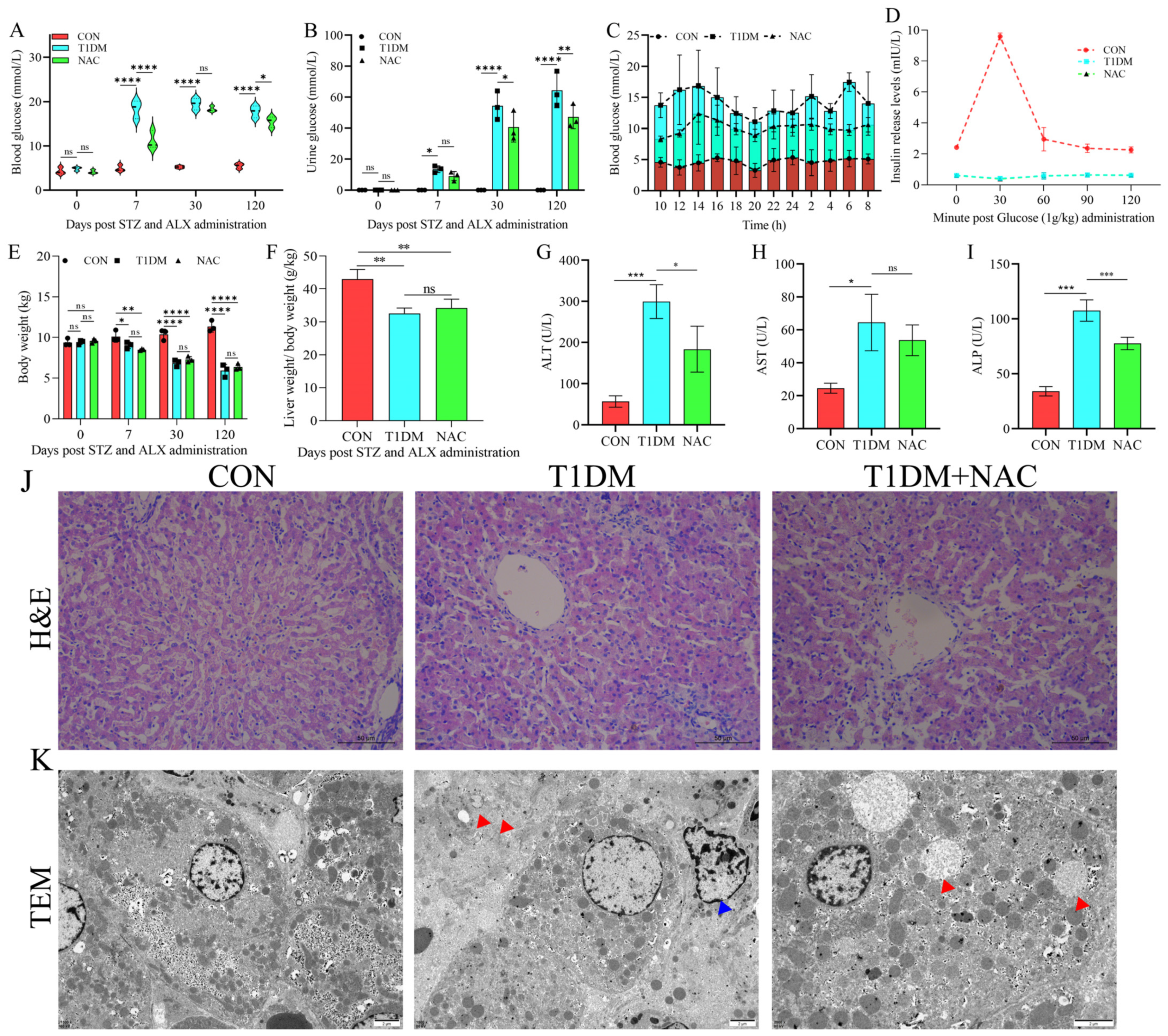
3.1. NAC Can Regulate T1DM Liver Disease and Reduce Liver Damage
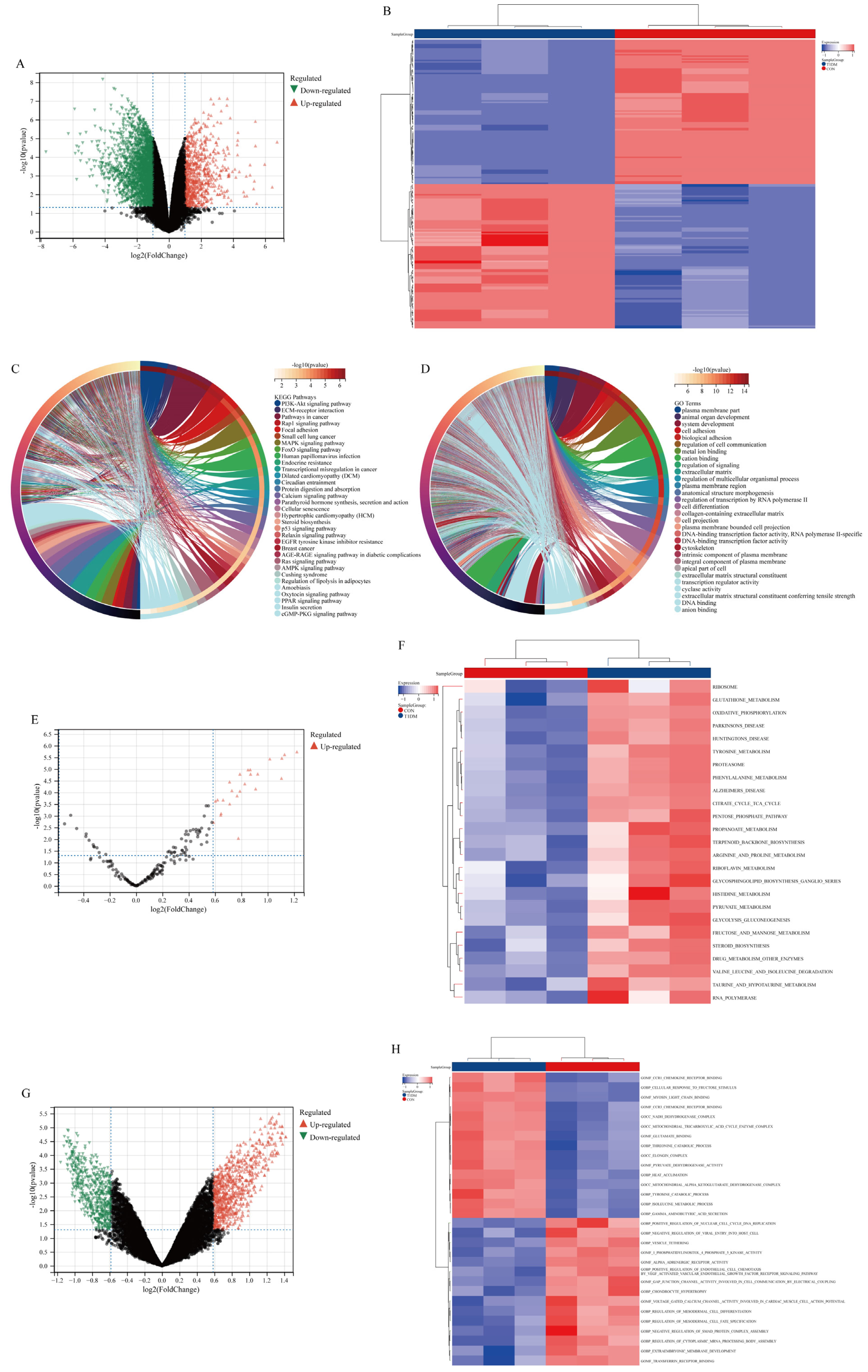
3.2. DEGs and Functional Enrichment Analysis in Liver Disease of T1DM
3.3. Construction of the WGCNA Network
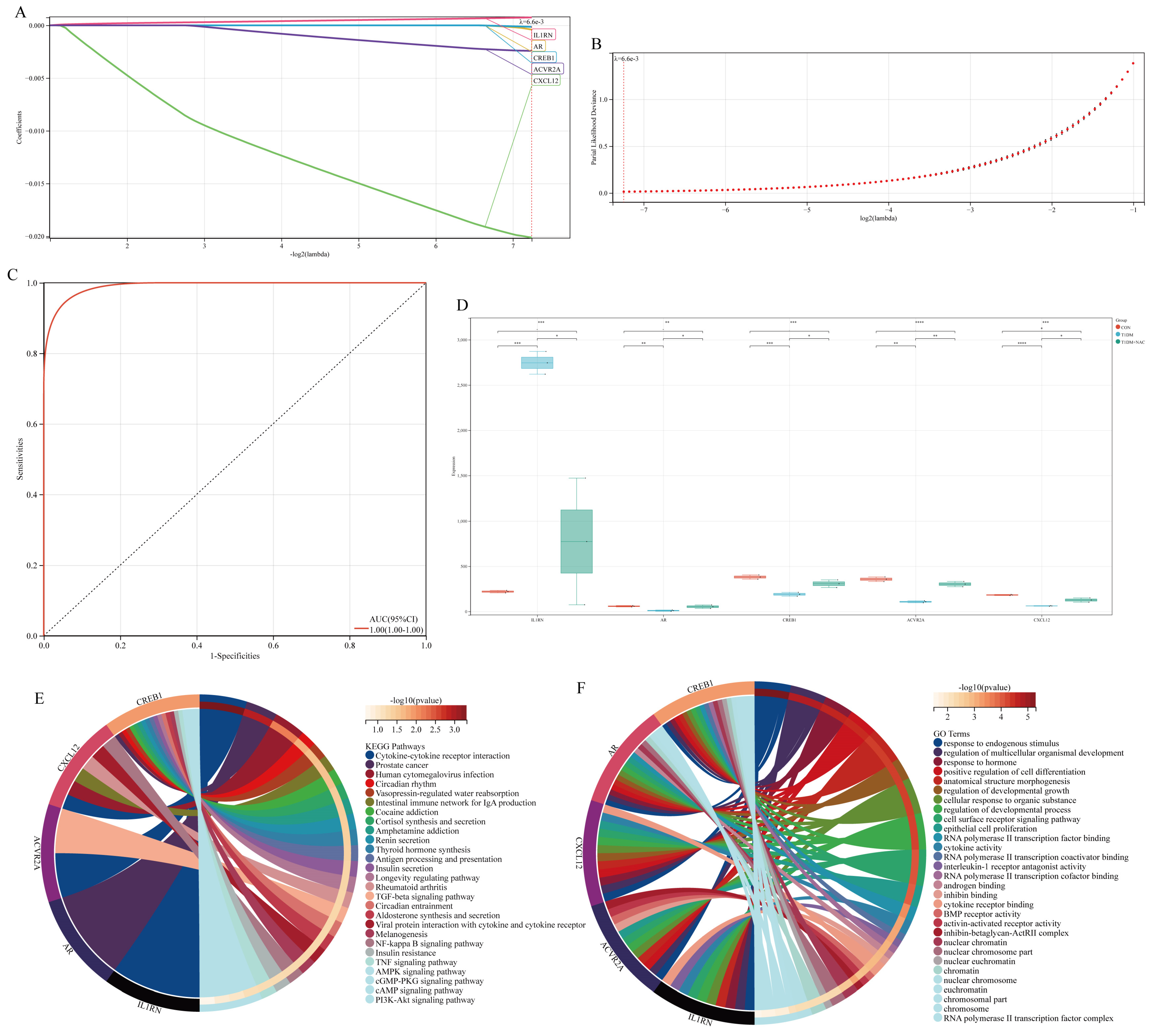
3.4. Acquisition and Functional Analysis of Potential Hub Genes
3.5. Acquisition of Hub Genes
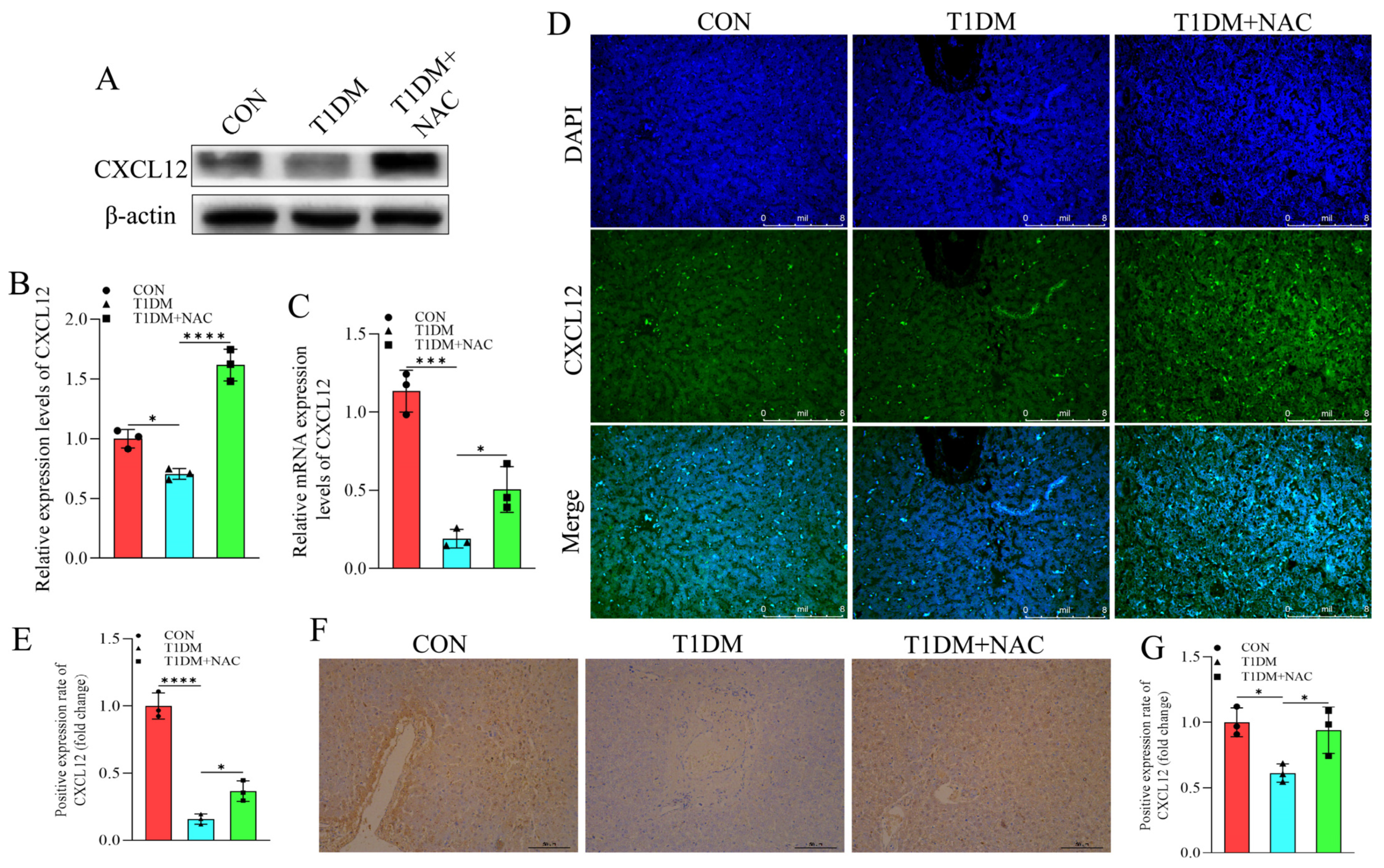
3.6. NAC Reverse the Inhibition of CXCL12 Caused by T1DM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef]
- Subramanian, S.; Khan, F.; Hirsch, I.B. New advances in type 1 diabetes. BMJ 2024, 384, e075681. [Google Scholar] [CrossRef] [PubMed]
- Tuo, L.; Yan, L.; Liu, Y.; Yang, X. Type 1 diabetes mellitus and non-alcoholic fatty liver disease: A two-sample Mendelian randomization study. Front. Endocrinol. 2024, 15, 1315046. [Google Scholar] [CrossRef]
- Jensen, A.S.H.; Sørensen, M.W.; Burisch, J.; Bergquist, A.; Ytting, H.; Gluud, L.L.; Albrechtsen, N.J.W. Autoimmune liver diseases and diabetes: A propensity score matched analysis and a proportional meta-analysis. Liver Int. 2023, 43, 2479–2491. [Google Scholar] [CrossRef]
- de Vries, M.; El-Morabit, F.; van Erpecum, K.J.; Westerink, J.; Bac, S.T.; Kaasjager, H.K.; de Valk, H.W. Non-alcoholic fatty liver disease: Identical etiologic factors in patients with type 1 and type 2 diabetes. Eur. J. Intern. Med. 2022, 100, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.; Zohar, Y.; Shehadeh, N.; Saadi, T. Glycogenic hepatopathy. Hepatob. Pancreatic. Dis. Int. 2018, 17, 113–118. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Prevalence of elevated liver stiffness in patients with type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pr. 2022, 190, 109981. [Google Scholar] [CrossRef]
- Lombardo, F.; Passanisi, S.; Gasbarro, A.; Tuccari, G.; Ieni, A.; Salzano, G. Hepatomegaly and type 1 diabetes: A clinical case of Mauriac’s syndrome. Ital. J. Pediatr. 2019, 45, 3. [Google Scholar] [CrossRef]
- Yan, J.; Xie, J.; Xu, S.; Guo, Y.; Ji, K.; Li, C.; Gao, H.; Zhao, L. Fibroblast growth factor 21 protects the liver from apoptosis in a type 1 diabetes mouse model via regulating L-lactate homeostasis. Biomed. Pharmacother. 2023, 168, 115737. [Google Scholar] [CrossRef] [PubMed]
- Piperno, E.; Berssenbruegge, D. Reversal of experimental paracetamol toxicosis with N-acetylcysteine. Lancet 1976, 308, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; Heard, K.J.; Reynolds, K.M.; Albert, D. Oral and intravenous acetylcysteine for treatment of acetaminophen toxicity: A systematic review and meta-analysis. West. J. Emerg. Med. 2013, 14, 218. [Google Scholar] [CrossRef]
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020, 34, 13185. [Google Scholar] [CrossRef]
- Venketaraman, V.; Dayaram, Y.K.; Amin, A.G.; Ngo, R.; Green, R.M.; Talaue, M.T.; Mann, J.; Connell, N.D. Role of glutathione in macrophage control of mycobacteria. Infect. Immun. 2003, 71, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.; Nicoletti, F.; Calverley, P.M. The multifaceted therapeutic role of N-acetylcysteine (NAC) in disorders characterized by oxidative stress. Curr. Neuropharmacol. 2021, 19, 1202. [Google Scholar]
- Smaga, I.; Frankowska, M.; Filip, M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 2021, 178, 2569–2594. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Li, Q.; Wang, X.; Yang, X.; Wang, D. N-acetylcysteine attenuates cardiopulmonary bypass-induced lung injury in dogs. J. Cardiothorac. Surg. 2013, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Ding, Q.; Sun, B.; Wang, M.; Li, T.; Li, H.; Han, Q.; Liao, J.; Tang, Z. N-acetylcysteine alleviates oxidative stress and apoptosis and prevents skeletal muscle atrophy in type 1 diabetes mellitus through the NRF2/HO-1 pathway. Life Sci. 2023, 329, 121975. [Google Scholar] [CrossRef]
- Saha, D.; Paul, S.; Gaharwar, U.; Priya, A.; Neog, A.; Singh, A.; Bk, B. Cdk5-Mediated Brain Unfolded Protein Response Upregulation Associated with Cognitive Impairments in Type 2 Diabetes and Ameliorative Action of NAC. ACS Chem. Neurosci. 2023, 14, 2761–2774. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Martinez-Rachadell, L.; Navarrete, M.; Pose-Utrilla, J.; Davila, J.C.; Pignatelli, J.; Diaz-Pacheco, S.; Guerra-Cantera, S.; Viedma-Moreno, E.; Palenzuela, R.; et al. Insulin regulates neurovascular coupling through astrocytes. Proc. Natl. Acad. Sci. USA 2022, 119, e2090440177. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.E.; Ollivier, F.J. Matrix metalloproteinase inhibition in corneal ulceration. Vet. Clin. N. Am.-Small Anim. Pr. 2004, 34, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liao, J.; Chen, W.; Zhang, K.; Li, H.; Ma, F.; Zhang, H.; Han, Q.; Guo, J.; Li, Y. NAC alleviative ferroptosis in diabetic nephropathy via maintaining mitochondrial redox homeostasis through activating SIRT3-SOD2/Gpx4 pathway. Free Radic. Biol. Med. 2022, 187, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, H.; Huo, H.; Han, Q.; Liao, J.; Zhang, H.; Li, Y.; Pan, J.; Hu, L.; Guo, J. N-acetyl-L-cysteine alleviates FUNDC1-mediated mitophagy by regulating mitochondrial dynamics in type 1 diabetic nephropathy canine. Life Sci. 2023, 313, 121278. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pang, X.; Zhang, X.; Qiu, W.; Zhang, X.; Wang, R.; Xie, W.; Bai, Y.; Zhou, S.; Liao, J. N-acetylcysteine combined with insulin attenuates myocardial injury in canines with type 1 diabetes mellitus by modulating TNF-α-mediated apoptotic pathways and affecting linear ubiquitination. Transl. Res. 2023, 262, 1–11. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, K.; Hou, L.; Liao, J.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; Hu, L.; Pan, J. Endoplasmic reticulum stress contributes to pyroptosis through NF-κB/NLRP3 pathway in diabetic nephropathy. Life Sci. 2023, 322, 121656. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.; Wu, H.; Ma, F.; Li, X.; Liao, J.; Hu, L.; Han, Q.; Li, Y.; Pan, J.; Zhang, H. N-acetyl-L-cysteine ameliorates hepatocyte pyroptosis of dog type 1 diabetes mellitus via suppression of NLRP3/NF-κB pathway. Life Sci. 2022, 306, 120802. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.I.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. TIG 1997, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM. Org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Han, M.; Li, J.; Wu, Y.; Tang, Z. Potential immune-related therapeutic mechanisms of multiple traditional Chinese medicines on type 2 diabetic nephropathy based on bioinformatics, network pharmacology and molecular docking. Int. Immunopharmacol. 2024, 133, 112044. [Google Scholar] [CrossRef] [PubMed]
- Rot, A.; Von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef]
- Coppieters, K.T.; Dotta, F.; Amirian, N.; Campbell, P.D.; Kay, T.W.; Atkinson, M.A.; Roep, B.O.; von Herrath, M.G. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 2012, 209, 51–60. [Google Scholar] [CrossRef]
- Eisenbarth, G.S. Banting Lecture 2009: An unfinished journey: Molecular pathogenesis to prevention of type 1a diabetes. Diabetes 2020, 59, 759. [Google Scholar] [CrossRef]
- Vidaković, M.; Grdović, N.; Dinić, S.; Mihailović, M.; Uskoković, A.; Jovanović, J.A. The importance of the CXCL12/CXCR4 axis in therapeutic approaches to diabetes mellitus attenuation. Front. Immunol. 2015, 6, 403. [Google Scholar] [CrossRef]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gürtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef] [PubMed]
- Alagpulinsa, D.A.; Cao, J.J.; Sobell, D.; Poznansky, M.C. Harnessing CXCL12 signaling to protect and preserve functional β-cell mass and for cell replacement in type 1 diabetes. Pharmacol. Ther. 2019, 193, 63–74. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, Y.; Zhong, Y.; Du, S.; Hou, X.; Li, W.; Li, H.; Wang, S.; Wang, C.; Yan, J.; et al. LNP-RNA-engineered adipose stem cells for accelerated diabetic wound healing. Nat. Commun. 2024, 15, 739. [Google Scholar] [CrossRef] [PubMed]
- Pillon, N.J.; Smith, J.A.; Alm, P.S.; Chibalin, A.V.; Alhusen, J.; Arner, E.; Carninci, P.; Fritz, T.; Otten, J.; Olsson, T. Distinctive exercise-induced inflammatory response and exerkine induction in skeletal muscle of people with type 2 diabetes. Sci. Adv. 2022, 8, eabo3192. [Google Scholar] [CrossRef] [PubMed]
- Citro, A.; Pellegrini, S.; Dugnani, E.; Eulberg, D.; Klussmann, S.; Piemonti, L. CCL2/MCP-1 and CXCL12/SDF-1 blockade by L-aptamers improve pancreatic islet engraftment and survival in mouse. Am. J. Transplant. 2019, 19, 3131–3138. [Google Scholar] [CrossRef]
- Bernat-Peguera, A.; Simón-Extremera, P.; Da Silva-Diz, V.; De Munain, M.L.; Díaz-Gil, L.; Penin, R.M.; González-Suárez, E.; Sidelnikova, D.P.; Bermejo, O.; Viñals, J.M. PDGFR-induced autocrine SDF-1 signaling in cancer cells promotes metastasis in advanced skin carcinoma. Oncogene 2019, 38, 5021–5037. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Boscaro, E.; Albiero, M.; Menegazzo, L.; Frison, V.; De Kreutzenberg, S.; Agostini, C.; Tiengo, A.; Avogaro, A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: Possible role of stromal-derived factor-1α. Diabetes Care 2010, 33, 1607–1609. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Thal, M.; Verma, S.; Hoxha, E.; Lambers, E.; Ramirez, V.; Qin, G.; Losordo, D.; Kishore, R. Interleukin-10 deficiency impairs bone marrow–derived endothelial progenitor cell survival and function in ischemic myocardium. Circ. Res. 2011, 109, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Chen, Q.; Lan, X.; Xu, H.; Yang, H.; Lin, C.; Xue, Q.; Xie, B. Preventing CXCL12 elevation helps to reduce acute exacerbation of COPD in individuals co-existing type-2 diabetes: A bioinformatics and clinical pharmacology study. Int. Immunopharmacol. 2024, 132, 111894. [Google Scholar] [CrossRef] [PubMed]
- Sharp, C.D.; Huang, M.; Glawe, J.; Patrick, D.R.; Pardue, S.; Barlow, S.C.; Kevil, C.G. Stromal Cell–Derived Factor-1/CXCL12 Stimulates Chemorepulsion of NOD/LtJ T-Cell Adhesion to Islet Microvascular Endothelium. Diabetes 2008, 57, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Chen, H.; Wang, Y.; Huang, L.; Huang, K.; Xiao, G.; Han, C.; Hu, J.; Lin, D.; Wan, X. Cancer-associated fibroblasts undergoing neoadjuvant chemotherapy suppress rectal cancer revealed by single-cell and spatial transcriptomics. Cell Rep. Med. 2023, 4, 101231. [Google Scholar] [CrossRef] [PubMed]
- Chirillo, R.; Aversa, I.; Di Vito, A.; Salatino, A.; Battaglia, A.M.; Sacco, A.; Di Sanzo, M.A.; Faniello, M.C.; Quaresima, B.; Palmieri, C. FtH-mediated ROS dysregulation promotes CXCL12/CXCR4 axis activation and EMT-like trans-differentiation in erythroleukemia K562 cells. Front. Oncol. 2020, 10, 698. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Kaili, D.; Freeman, J.; Lei, C.Y.; Geng, B.C.; Tan, T.; He, J.F.; Shi, Z.; Ma, J.J.; Luo, Y.H.; et al. Diabetes inhibits corneal epithelial cell migration and tight junction formation in mice and human via increasing ROS and impairing Akt signaling. Acta Pharmacol. Sin. 2019, 40, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, H.; Hou, X.; Song, L.; Feng, M. N-acetyl-L-cysteine ameliorates gestational diabetes mellitus by inhibiting oxidative stress. Biotechnol. Appl. Biochem. 2023, 70, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Liggett, J.R.; Kang, J.; Ranjit, S.; Rodriguez, O.; Loh, K.; Patil, D.; Cui, Y.; Duttargi, A.; Nguyen, S.; He, B.; et al. Oral N-acetylcysteine decreases IFN-gamma production and ameliorates ischemia-reperfusion injury in steatotic livers. Front. Immunol. 2022, 13, 898799. [Google Scholar] [CrossRef] [PubMed]
- Medved, I.; Brown, M.J.; Bjorksten, A.R.; Mckenna, M.J. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J. Appl. Physiol. 2004, 96, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Terrill, J.R.; Grounds, M.D.; Arthur, P.G. Taurine deficiency, synthesis and transport in the mdx mouse model for Duchenne Muscular Dystrophy. Int. J. Biochem. Cell Biol. 2015, 66, 141–148. [Google Scholar] [CrossRef]
- Yiu, D.C.; Lin, H.; Wong, V.W.; Wong, G.L.; Liu, K.; Yip, T.C. Dipeptidyl peptidase-4 inhibitors are associated with improved survival of patients with diabetes mellitus and hepatocellular carcinoma receiving immunotherapy: Letter to the editor on “Statin and aspirin for chemoprevention of hepatocellular carcinoma: Time to use or wait further?”. Clin. Mol. Hepatol. 2024, 30, 970–973. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Han, M.; Guo, S.; Tang, Z. CXCL12 as a Potential Hub Gene for N-Acetylcysteine Treatment of T1DM Liver Disease. Biomolecules 2025, 15, 176. https://doi.org/10.3390/biom15020176
Zhao M, Han M, Guo S, Tang Z. CXCL12 as a Potential Hub Gene for N-Acetylcysteine Treatment of T1DM Liver Disease. Biomolecules. 2025; 15(2):176. https://doi.org/10.3390/biom15020176
Chicago/Turabian StyleZhao, Menglong, Mingzheng Han, Shuaihao Guo, and Zhaoxin Tang. 2025. "CXCL12 as a Potential Hub Gene for N-Acetylcysteine Treatment of T1DM Liver Disease" Biomolecules 15, no. 2: 176. https://doi.org/10.3390/biom15020176
APA StyleZhao, M., Han, M., Guo, S., & Tang, Z. (2025). CXCL12 as a Potential Hub Gene for N-Acetylcysteine Treatment of T1DM Liver Disease. Biomolecules, 15(2), 176. https://doi.org/10.3390/biom15020176



