Herbal Medicines in the Management of Diabetes Mellitus: Plants, Bioactive Compounds, and Mechanisms of Action
Abstract
1. Introduction
2. Materials and Methods
- (i)
- Contained the antidiabetic effects of herbal medicines or plant-derived bioactive compounds;
- (ii)
- Provided insights for the mechanisms of action or therapeutic potential;
- (iii)
- Were original research, reviews, or clinical studies published in English.
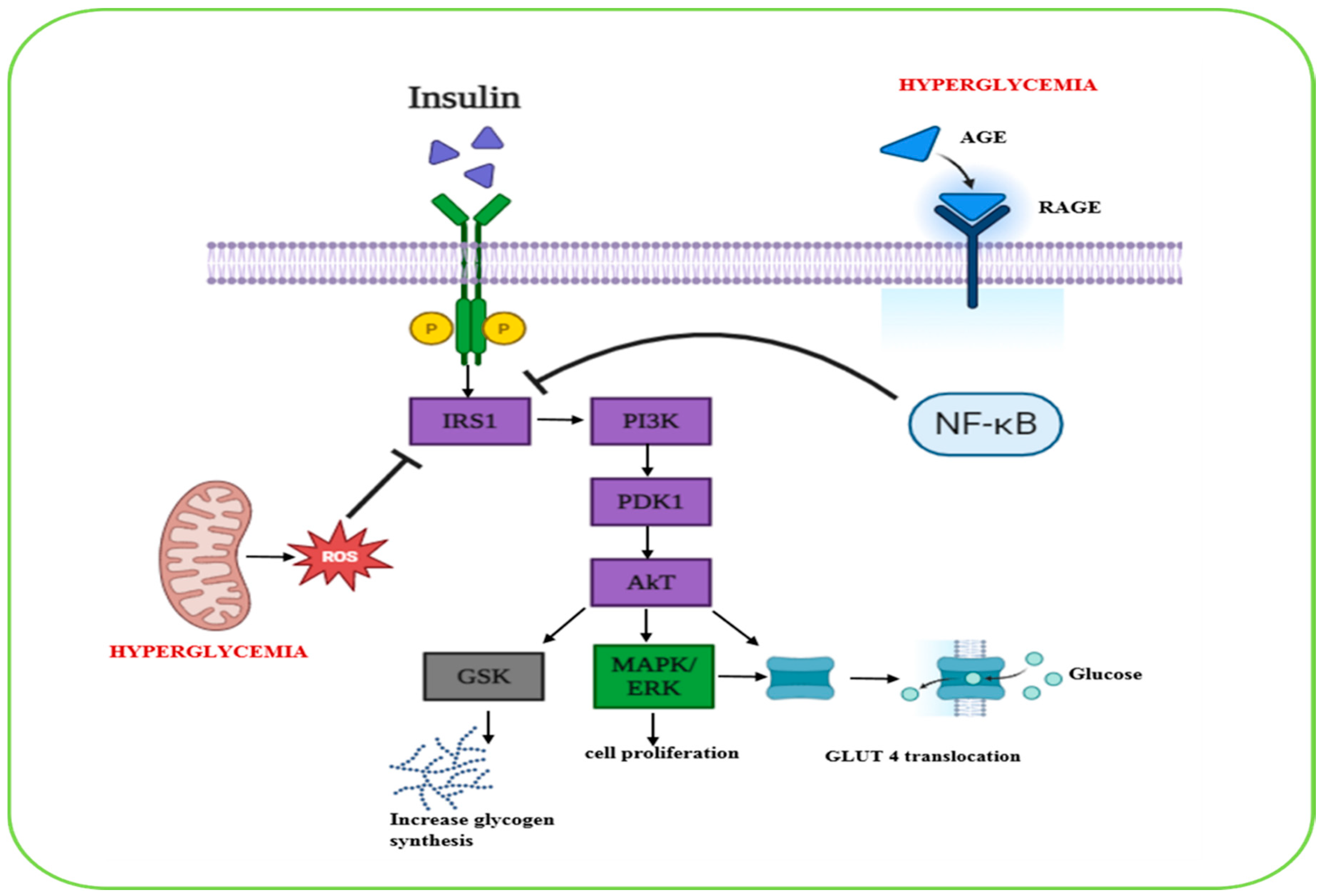
2.1. Biological Pathways Involved in Diabetes Management
2.2. Methodological Considerations and Quality Assessment
2.3. Medicinal Herbs: Past and Present Insights
2.4. Safety Considerations and Drug Interactions
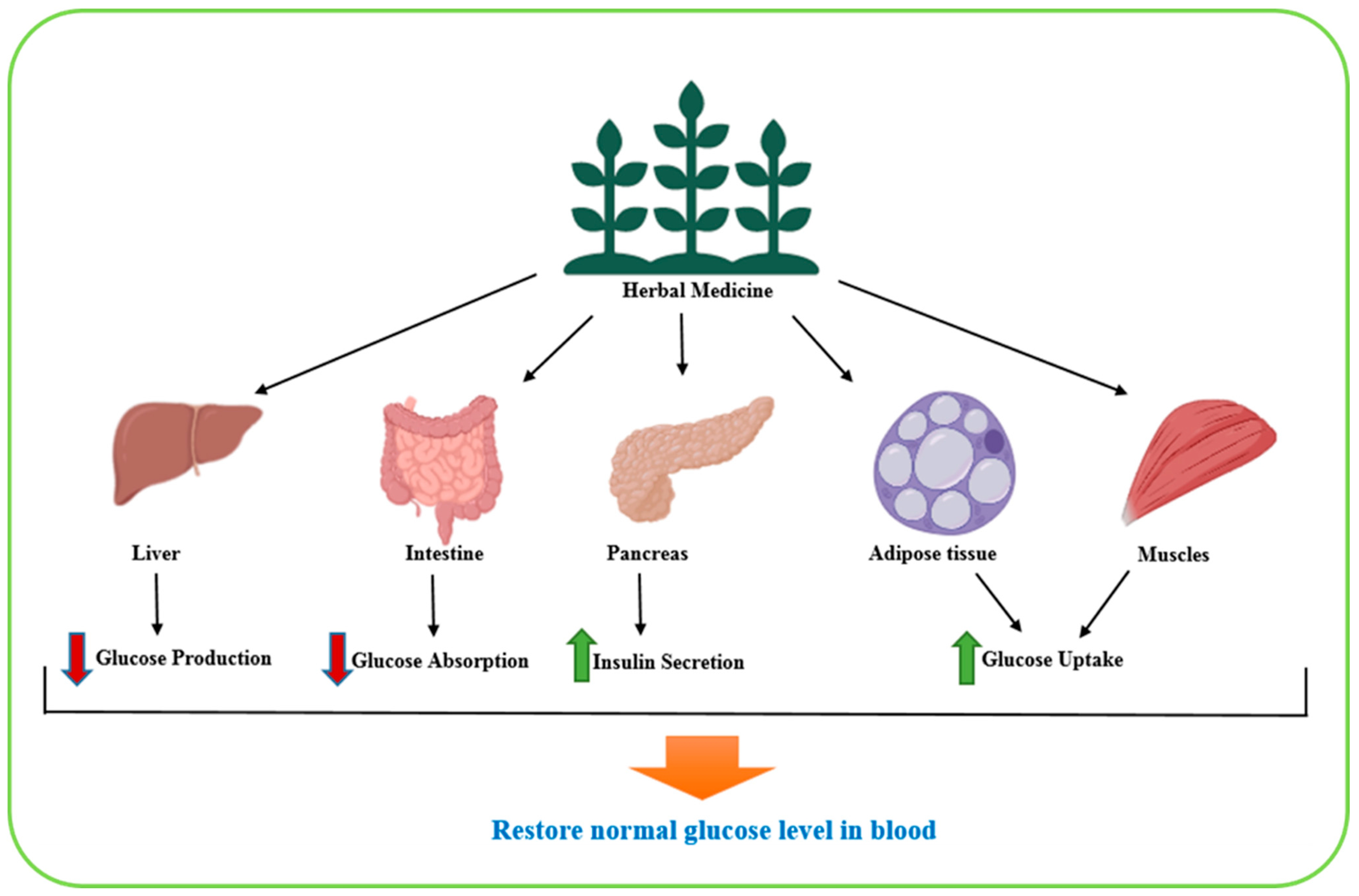
2.5. Traditional Medicine and Diabetes
2.6. Antidiabetic Medicinal Plants
2.6.1. Achyranthes aspera
2.6.2. Allium sativum
2.6.3. Aloe vera
2.6.4. Amaranthus tricolor (Lal Chaulai/Joseph’s Coat)
2.6.5. Anacardium occidentale (Cashew Tree)
2.6.6. Annona squamosa (Custard Apple/Sugar Apple)
2.6.7. Berberis vulgaris (Barberry)
2.6.8. Cinnamomum zeylanicum
2.6.9. Curcuma longa (Turmeric)
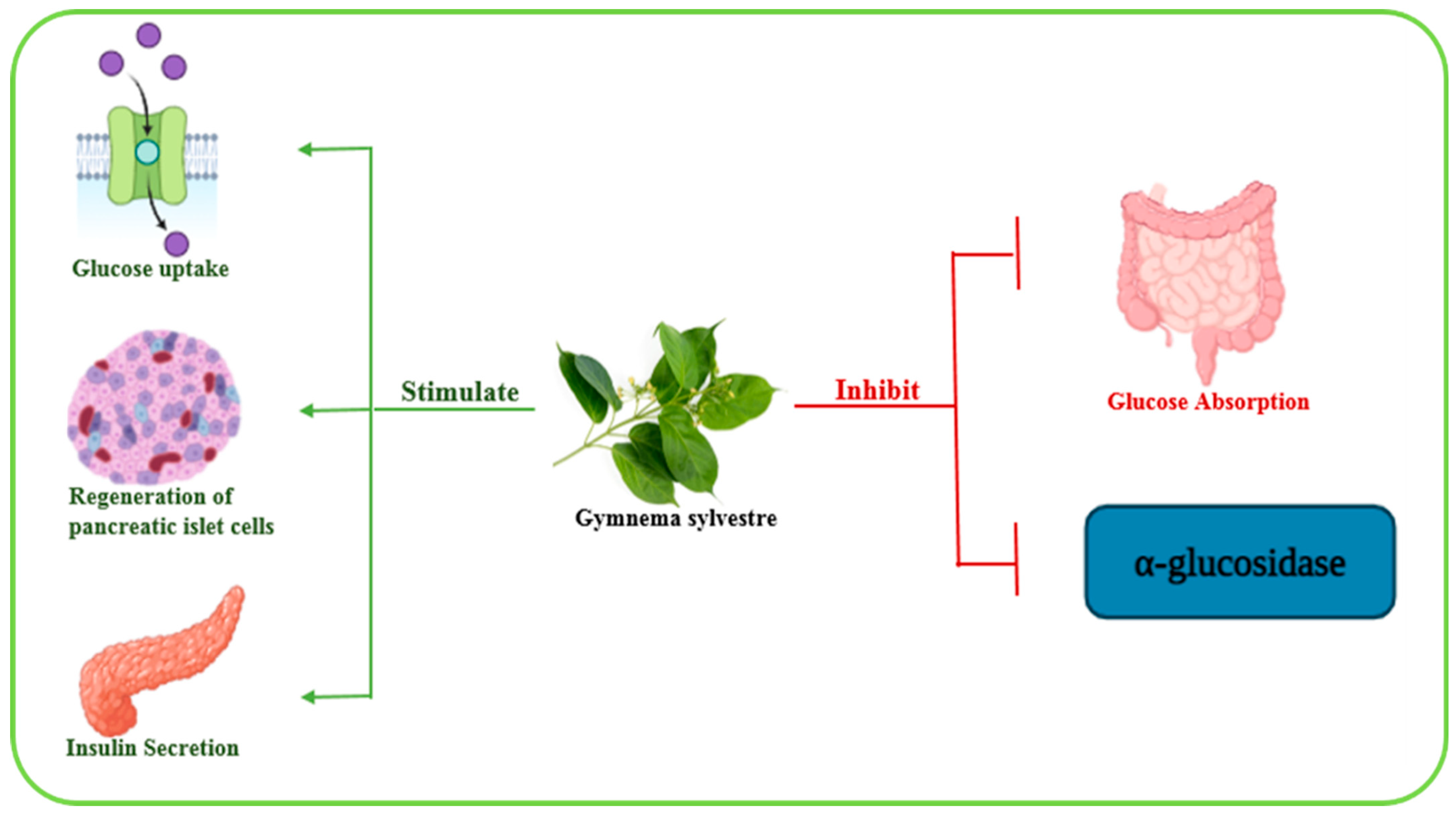
2.6.10. Gymnema sylvestre
2.6.11. Gynostemma pentaphyllum
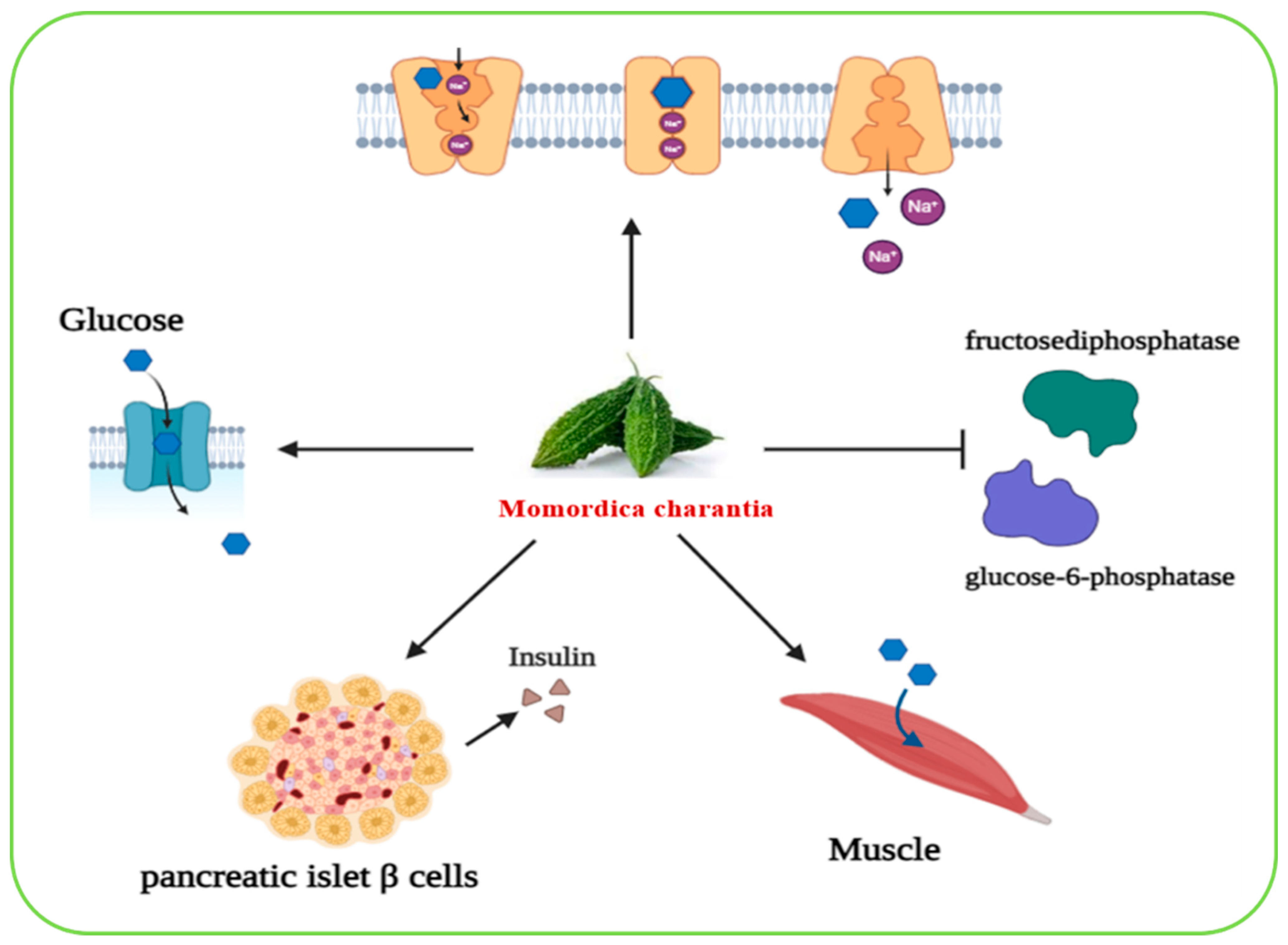
2.6.12. Momordica charantia
2.6.13. Nigella sativa (Black Seed/Black Cumin)
2.6.14. Ocimum sanctum
2.6.15. Punica granatum (Pomegranate)
2.6.16. Trigonella foenum-graecum
3. Approaches for Enhancing for Bioavailability of Phytochemicals
3.1. Diabetes and Osteoporosis
3.2. Limitations and Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes; WHO: Genave, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 14 November 2024).
- Shaikh, A.A.; Kolhatkar, M.K.; Sopane, D.K.; Thorve, A.N. Review on: Diabetes mellitus is a disease. Int. J. Res. Pharm. Sci. 2022, 13, 102–109. [Google Scholar] [CrossRef]
- Bastaki, S. A review diabetes mellitus and its treatment. Int. J. Diabetes Metab. 2005, 13, 111–134. [Google Scholar] [CrossRef]
- Dey, R.K. Diabetes Mellitus: A comprehensive review of pathophysiology, management, and emerging therapeutic approaches. Diabetes Mellitus 2023, 6, 96–100. [Google Scholar]
- Savan, C.; Viroja, D.; Kyada, A. An updated review on diabetes mellitus: Exploring its etiology, pathophysiology, complications and treatment approach. IJCAAP 2024, 9, 31–36. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Sig. Transduct. Target. Ther. 2024, 9, 262–286. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, B.A.; Adugna, A.; Yenet, A.; Yihunie Belay, W.; Yibeltal, Y.; Dagne, A.; Hibstu Teffera, Z.; Amare, G.A.; Abebaw, D.; Tewabe, H.; et al. A critical review on diabetes mellitus type 1 and type 2 management approaches: From lifestyle modification to current and novel targets and therapeutic agents. Front. Endocrinol. 2024, 15, 1440456–1440475. [Google Scholar] [CrossRef]
- Yameny, A.A. Diabetes mellitus overview. J. Biosci. Appl. Res. 2024, 10, 641–645. [Google Scholar] [CrossRef]
- Kaur, S.; Gadpayle, D.; Kumari, A.; Kaur, G.; Seen, K.; Bhardwaj, R.; Kumar, A. Antidiabetic potential of underutilized crops: Nutritional, phytochemical insights, and prospects for diabetes management. Appl. Food Res. 2025, 5, 101127. [Google Scholar] [CrossRef]
- Chanda, S.; Ramachandra, T.V. A review on some therapeutic aspects of phytochemicals present in medicinal plants. Int. J. Pharm. Life Sci. 2019, 10, 6052–6058. [Google Scholar]
- Nafiu, M.O.; Hamid, A.A.; Muritala, H.F.; Adeyemi, S.B. Preparation, Standardization, and Quality Control of Medicinal Plants in Africa. In Medicinal Plants: Ethnopharmacology, Phytochemistry and Therapeutic Applications; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 7. [Google Scholar] [CrossRef]
- Whaley, A.O.; Whaley, A.K.; Kovaleva, E.L.; Frolova, L.N.; Orlova, A.A.; Luzhanin, V.G.; Flisyiuk, E.V.; Shigarova, L.V.; Pozharitskaya, O.N.; Shikov, A.N. The Standardization of Officinal Medicinal Plants Used in the Eurasian Economic Union: Comparison with Other Pharmacopoeias. Pharm. Chem. J. 2024, 23, 349–419. [Google Scholar] [CrossRef]
- Noviana, E.; Indrayanto, G.; Rohman, A. Advances in Fingerprint Analysis for Standardization and Quality Control of Herbal Medicines. Front. Pharmacol. 2022, 13, 853023. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed. Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Overy, A.J.; Büsselberg, D. Phytochemicals and Gastrointestinal Cancer: Cellular Mechanisms and Effects to Change Cancer Progression. Biomolecules 2020, 10, 105. [Google Scholar] [CrossRef]
- Begum, V.S.M.; Tariq, N.P.M.; Hemapriya, J.; Shariq, K.M. Plants Secondary Metabolites as Medicines: A Review. IJZI 2022, 8, 490–493. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Salis, E.R.; Job, K.M.; Gubarev, M.I.; Krepkova, L.V.; Sherwin, C.M.T. Herbal Medicines: Challenges in the modern world. Part 5. Status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev. Clin. Pharmacol. 2016, 10, 327–338. [Google Scholar] [CrossRef]
- Pérez-Flores, J.G.; García-Curiel, L.; Pérez-Escalante, E.; Contreras-López, E.; Aguilar-Lira, G.Y.; Ángel-Jijón, C.; González-Olivares, L.G.; Baena-Santillán, E.S.; Ocampo-Salinas, I.O.; Guerrero-Solano, J.A.; et al. Plant Antimicrobial Compounds and Their Mechanisms of Action on Spoilage and Pathogenic Bacteria: A Bibliometric Study and Literature Review. Appl. Sci. 2025, 15, 3516. [Google Scholar] [CrossRef]
- Albahri, G.; Badran, A.; Hijazi, A.; Daou, A.; Baydoun, E.; Nasser, M.; Merah, O. The therapeutic wound healing bioactivities of various medicinal plants. Life 2023, 13, 317. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Khan, M.A.; Alalami, U.; Somvanshi, P.; Bhardwaj, T.; Pramodh, S.; Raina, R.; Shekfeh, Z.; Haque, S.; Hussain, A. Phytochemicals induce apoptosis by modulation of nitric oxide signaling pathway in cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11827–11844. [Google Scholar] [CrossRef]
- Lin, R.; Hu, X.; Chen, S.; Shi, Q.; Chen, H. Naringin induces endoplasmic reticulum stress-mediated apoptosis, inhibits β-catenin pathway and arrests cell cycle in cervical cancer cells. Acta Biochim. Pol. 2020, 67, 181–186. [Google Scholar] [CrossRef]
- Mahmoud, V.L.; Shayesteh, R.; Foong Yun Loh, T.K.; Chan, S.W.; Sethi, G.; Burgess, K.; Lee, S.H.; Wong, W.F.; Looi, C.Y. Comprehensive Review of Opportunities and Challenges of Ethnomedicinal Plants for Managing Type 2 Diabetes. Heliyon 2024, 10, e39699. [Google Scholar] [CrossRef]
- Hui, H.; Tang, G.; Go, V. Hypoglycemic Herbs and Their Action Mechanisms. Chin. Med. 2009, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The Role of Medicinal Plants in the Treatment of Diabetes: A Systematic Review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.H.; Zulkipli, I.N.; Mohd Yasin, H.; Ja’afar, F.; Ahmad, N.; Wan Ahmad, W.A.N.; Ahmad, S.R. Systematic Review of Medicinal Plants Used for Treatment of Diabetes in Human Clinical Trials: An ASEAN Perspective. Evid.-Based Complement. Altern. Med. 2021, 2021, 5570939. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, N.; Srivastava, D.; Kukreti, A.; Srivastava, S.; Arya, V. Exploring the Safety, Efficacy, and Bioactivity of Herbal Medicines: Bridging Traditional Wisdom and Modern Science in Healthcare. Future Integr. Med. 2024, 3, 35–49. [Google Scholar] [CrossRef]
- Mourya, R.; Sharma, R. Interaction Between Anti-Diabetic Drugs and Herbs: A Review. Pharmacogn. Res. 2025, 17, 385–401. [Google Scholar] [CrossRef]
- Dash, J.R.; Kar, B.; Pattnaik, G. Pharmacological Interaction Between Anti-Diabetic Drugs and Herbs: An Overview of Mechanism of Action and Clinical Implication. Plant Arch. 2020, 20, 3661–3668. [Google Scholar]
- Li, X.; Geng-Ji, J.-J.; Quan, Y.-Y.; Qi, L.-M.; Sun, Q.; Huang, Q.; Jiang, H.-M.; Sun, Z.-J.; Liu, H.-M.; Xie, X. Role of Potential Bioactive Metabolites from Traditional Chinese Medicine for Type 2 Diabetes Mellitus: An Overview. Front. Pharmacol. 2022, 13, 1023713. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Wang, H.; Kaur, J.; Nalbant, G.; Almaqhawi, A.; Kundakci, B.; Panniyammakal, J.; Heinrich, M.; Lewis, S.A.; Greenfield, S.M.; et al. Effectiveness and Safety of Ayurvedic Medicines in Type 2 Diabetes Mellitus Management: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 821810. [Google Scholar] [CrossRef]
- Abu-Odeh, A.M.; Talib, W.H. Middle East Medicinal Plants in the Treatment of Diabetes: A Review. Molecules 2021, 26, 742. [Google Scholar] [CrossRef]
- Zanzabil, K.Z.; Hossain, M.S.; Hasan, M.K. Diabetes mellitus management: An extensive review of 37 medicinal plants. Diabetology 2023, 4, 186–234. [Google Scholar] [CrossRef]
- Asiago, O.H.; Reddy, K.S. Protective effect of achyranthes aspera against high fat diet and streptozotocin induced diabetes in rats [Adventure]. J. Chem. Health Risks 2023, 13, 3124–3131. [Google Scholar]
- Vijayaraj, R.; Naresh Kumar, K.; Mani, P.; Senthil, J.; Jayaseelan, T.; Dinesh Kumar, G. Hypoglycemic and antioxidant activity of Achyranthes aspera seed extract and its effect on streptozotocin induced diabetic rats. Int. J. Biol. Pharm. Res. 2016, 7, 23–28. [Google Scholar] [CrossRef]
- Priyamvada, P.M.; Mishra, P.; Sha, A.; Mohapatra, A.K. Evaluation of antidiabetic and antioxidant activities of Achyranthes aspera leaf extracts: An in vitro study. Int. J. Pharm. Life Sci. 2021, 10, 103–110. [Google Scholar]
- Njideka, B.E.; Theophilus, A.E.N.; Ugochukwu, N.T. Use of Achyranthes aspera Linn Tea as antidiabetic and hypolipidemic herbal tea. Int. J. Health Sci. Res. 2019, 9, 32–38. [Google Scholar]
- Sanie-Jahromi, F.; Zia, Z.; Afarid, M. A review on the effect of garlic on diabetes, BDNF, and VEGF as a potential treatment for diabetic retinopathy. Chin. Med. 2023, 18, 18–31. [Google Scholar] [CrossRef]
- Xie, C.; Gao, W.; Li, X.; Luo, S.; Wu, D.; Chye, F.Y. Garlic (Allium sativum L.) polysaccharide ameliorates type 2 diabetes mellitus (T2DM) via the regulation of hepatic glycogen metabolism. NFS J. 2023, 31, 19–27. [Google Scholar] [CrossRef]
- Najafi, N.; Masoumi, S.J. The Effect of garlic (allium sativum) supplementation in patients with type 2 diabetes mellitus: A systematic review. Int. J. Nutr. Sci. 2018, 3, 7–11. [Google Scholar]
- Abdullah, H.; Miladiyah, I. Garlic (Allium sativum L.) Efficacy as an adjuvant therapy for type 2 diabetes mellitus: A scoping review. In Proceedings of the 3rd International Conference on Cardiovascular Diseases (ICCvD 2021); Nurdiyanto, H., Miladiyah, I., Jamil, N.A., Eds.; Atlantis Press International BV: Paris, France, 2023; pp. 419–434. [Google Scholar] [CrossRef]
- Harshali; Thakur, P.; Mukherjee, G. Aloe Vera as an Antidiabetic and Wound Healing Agent for Diabetic Patients. JPRI 2021, 33, 256–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Liu, D.; Zhao, T.; Tian, H. Efficacy of Aloe Vera supplementation on prediabetes and early non-treated diabetic patients: A Systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 388. [Google Scholar] [CrossRef]
- Budiastutik, I.; Subagio, H.W.; Kartasurya, M.I.; Widjanarko, B.; Kartini, A.; Soegiyanto, S.; Suhartono, S.S. The effect of aloe vera on fasting blood glucose levels in pre-diabetes and type 2 diabetes mellitus: A systematic review and meta-analysis. J. Pharm. Pharmacogn. Res. 2022, 10, 737–747. [Google Scholar] [CrossRef]
- Abo-Youssef, A.M.H.; Messiha, B.A.S. Beneficial effects of aloe vera in treatment of diabetes: Comparative in vivo and in vitro studies. Bull. Fac. Pharm. Cairo Univ. 2013, 51, 7–11. [Google Scholar] [CrossRef][Green Version]
- Fallah Huseini, H.; Kianbakht, S.; Hajiaghaee, R.; Afkhami-Ardekani, M.; Bonakdaran, A.; Hashem Dabaghian, F. Aloe vera Leaf Gel in Treatment of Advanced Type 2 Diabetes Mellitus Needing Insulin Therapy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Med. Plants 2012, 11, 19–27. [Google Scholar]
- Rahman, A.H.M.M.; Iffat Ara Gulshana, M. Taxonomy and medicinal uses on amaranthaceae family of rajshahi, Bangladesh. AEES 2014, 2, 54–59. [Google Scholar] [CrossRef]
- Aneja, S.; Vats, M.; Aggarwal, S.; Sardana, S. Phytochemistry and hepatoprotective activity of aqueous extract of Amaranthus tricolor Linn. Roots. J. Ayurveda Integr. Med. 2013, 4, 211–217. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Hosain, M.; Rahman, S.; Rahman, S.; Akter, M.; Rahman, F.; Rehana, F.; Munmun, M.; Kalpana, M. antihyperglycemic and antinociceptive activity evaluation of methanolic extract of whole plant of Amaranthus Tricolor L. (Amaranthaceae). Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 408–411. [Google Scholar] [CrossRef]
- Rahma, K.; Nurcahyanti, O. Therapeutic effect of red spinach (Amaranthus tricolor L.) extract on pancreatic MDA levels rats (Rattus norvegicus) exposed to MLD-STZ. J. Biomed. Transl. Res. 2021, 7, 129–133. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Mong, M.-C.; Wu, W.-T.; Wang, Z.-H.; Yin, M.-C. Phytochemical profiles and anti-diabetic benefits of two edible amaranthus species. CyTA J. Food 2020, 18, 94–101. [Google Scholar] [CrossRef]
- Ajao, F.O.; Iyedupe, M.O.; Akanmu, O.; Kalejaiye, N.O.; Adegoke, A.L.; Adeniji, L.A. Anti-oxidative, anti-inflammatory and anti-apoptotic efficacy of Anacardium occidentale leaf extract in diabetic rats. Int. J. Diabetes Clin. Res. 2023, 10, 177–188. [Google Scholar] [CrossRef]
- Jaiswal, Y.S.; Tatke, P.A.; Gabhe, S.Y.; Vaidya, A.B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J. Tradi. Complement. Med. 2017, 7, 421–427. [Google Scholar] [CrossRef]
- Ponce-Mora, A.; Gimeno-Mallench, L.; Lavandera, J.L.; Giebelhaus, R.T.; Domenech-Bendaña, A.; Locascio, A.; Gutierrez-Rojas, I.; Sauro, S.; De La Mata, P.; Nam, S.L.; et al. Systematic Characterization of Antioxidant Shielding Capacity Against Oxidative Stress of Aerial Part Extracts of Anacardium occidentale. Antioxidants 2025, 14, 935. [Google Scholar] [CrossRef]
- Abdullahi, S.; Olatunji, G.A. Antidiabetic activity of Anacardium occidentale in alloxan—Diabetic rats. Jnl Sci. Tech. 2010, 30, 35–41. [Google Scholar] [CrossRef]
- Olatunji, L.A.; Okwusidi, J.I.; Soladoye, A.O. Antidiabetic effect of Anacardium occidentale. Stem-bark in fructose-diabetic rats. Pharm. Biol. 2005, 43, 589–593. [Google Scholar] [CrossRef][Green Version]
- Ukwenya, V.O.; Alese, M.O.; Ogunlade, B.; Folorunso, I.M.; Omotuyi, O.I. Anacardium occidentale leaves extract and riboceine mitigate hyperglycemia through anti-oxidative effects and modulation of some selected genes associated with diabetes. J. Diabetes Metab. Disord. 2022, 22, 455–468. [Google Scholar] [CrossRef]
- Sharma, C.P.; Singh, A.; Prasad, R.K.; Mishra, D.K.; Singh, A.K.; Yadav, S. Antidiabetic and antioxidant activity of Annona squamosa bark using successive solvent extraction method. J. Complement. Herb. Res. 2024, 14, 2004–2013. Available online: https://jchr.org/index.php/JCHR/article/view/6587 (accessed on 14 October 2025).
- Almalki, G.; Alothman, N.; Mohamed, G.; Akeel, M.; El-Beltagy, A.E.-F.B.M.; Salem, E.T. Modulatory role of Annona squamosa extract against streptozotocin-induced diabetic nephropathy in male rats. Egypt. J. Basic Appl. Sci. 2024, 11, 536–556. [Google Scholar] [CrossRef]
- Sharma, A.; Chand, T.; Khardiya, M.; Yadav, K.C.; Mangal, R.; Sharma, A.K. Antidiabetic and antihyperlipidemic activity of Annona squamosa fruit peel in streptozotocin induced diabetic rats. Int. J. Pharm. Sci. Res. 2013, 4, 200–208. [Google Scholar]
- Gupta, R.K.; Kesari, A.N.; Watal, G.; Murthy, P.S.; Chandra, R.; Maithal, K.; Tandon, V. Hypoglycaemic and antidiabetic effect of aqueous extract of leaves of Annona squamosa (L.) in experimental animals. Curr. Sci. 2005, 88, 1192–1196. [Google Scholar]
- Kaleem, M.; Asif, M.; Ahmed, Q.U.; Bano, B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singap. Med. J. 2006, 47, 670–675. [Google Scholar]
- Muszalska, A.; Wiecanowska, J. Berberis vulgaris: A natural source of berberine for addressing contemporary health concerns. Herba. Pol. 2024, 70, 13–38. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, L.-H.; Zhou, Q.; Zhao, T.-Y.; Wang, H.; Gu, C.-J.; Tong, X.-L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef]
- Meliani, N.; Dib, M.E.A.; Allali, H.; Tabti, B. Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2011, 1, 468–471. [Google Scholar] [CrossRef]
- Shidfar, F.; Ebrahimi, S.S.; Hosseini, S.; Heydari, I.; Shidfar, S.; Hajhassani, G. The effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, homocysteine, glycemic control and total antioxidant capacity in type 2 diabetic patients. Iran J. Pharm. Res. 2012, 11, 643–652. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC3832145/ (accessed on 14 October 2025). [PubMed]
- Belwal, T.; Bisht, A.; Devkota, H.P.; Ullah, H.; Khan, H.; Pandey, A.; Bhatt, I.D.; Echeverría, J. Phytopharmacology and Clinical Updates of Berberis Species Against Diabetes and Other Metabolic Diseases. Front. Pharmacol. 2020, 11, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Munguia-Nolan, J.E.; García-Puga, J.A.; Robles-Zepeda, R.E.; Quintana-Zavala, M.O.; Díaz-Zavala, R.G.; Rendón-Domínguez, I.P. Efectos de Cinnamomum zeylanicum en Niveles Glucémicos en Pacientes con Diabetes Tipo 2: Ensayo Clínico Aleatorizado. Enf. Global 2024, 23, 59–82. [Google Scholar] [CrossRef]
- Senevirathne, B.S.; Jayasinghe, M.A.; Pavalakumar, D.; Siriwardhana, C.G. Ceylon cinnamon: A versatile ingredient for futuristic diabetes management. J. Future Foods 2022, 2, 125–142. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; De Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A Systemic review and meta-analysis. Diabet. Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef]
- Shihabudeen, H.M.S.; Priscilla, D.H.; Thirumurugan, K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr. Metab. 2011, 8, 46–56. [Google Scholar] [CrossRef]
- Medagama, A.B. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr. J. 2015, 14, 108–119. [Google Scholar] [CrossRef]
- Shen, Y.; Fukushima, M.; Ito, Y.; Muraki, E.; Hosono, T.; Seki, T.; Ariga, T. Verification of the antidiabetic effects of Cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci. Biotechnol. Biochem. 2010, 74, 2418–2425. [Google Scholar] [CrossRef]
- Yaghmoor, S.S.; Khoja, S.M. Effect of Cinnamon on plasma glucose concentration and the regulation of phosphofructo-1-kinase activity from the liver and small intestine of streptozotocin induced diabetic rats. J. Biol. Sci. 2010, 10, 761–766. [Google Scholar] [CrossRef]
- Taher, M.; Abdul Majid, F.A.; Sarmidi, M.R. A proanthocyanidin from Cinnamomum zeylanicum stimulates phosphorylation of insulin receptor in 3T3–L1 adipocytes. J. Teknol. 2006, 74, 53–68. [Google Scholar] [CrossRef]
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.D.S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The effects of curcumin on diabetes mellitus: A systematic review. Front. Endocrinol. 2021, 12, 669448–669459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, M.; Gao, S.-H.; Liu, J.-L. Curcumin and diabetes: A systematic review. Evid. Based Complement. Altern. Med. 2013, 1, 636053–636068. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Mancía, S.; Trujillo, J.; Chaverri, J.P. Utility of curcumin for the treatment of diabetes mellitus: Evidence from preclinical and clinical studies. J. Nutr. Intermed. Metab. 2018, 14, 29–41. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012, 35, 2121–2127. [Google Scholar] [CrossRef]
- Pathomwichaiwat, T.; Jinatongthai, P.; Prommasut, N.; Ampornwong, K.; Rattanavipanon, W.; Nathisuwan, S.; Thakkinstian, A. Effects of turmeric (curcuma longa) supplementation on glucose metabolism in diabetes mellitus and metabolic syndrome: An umbrella review and updated meta-analysis. PLoS ONE 2023, 18, 288997–289017. [Google Scholar] [CrossRef]
- Lu, W.; Khatibi, F.; Shahidi, F.; Khorsandi, K.; Hossienzadeh, R.; Asma, G.; Balck, V. An update on molecular mechanisms of curcumin effect on diabetes. Fundam. Clin. Pharmacol. 2022, 36, 653–669. [Google Scholar] [CrossRef]
- Abbas, W.; Khan, R.A.; Baig, M.T.; Shaikh, S.A.; Kumar, A. Role of Curcuma Longa in Type 2 Diabetes and Its Associated Complications. JPRI 2021, 33, 369–376. [Google Scholar] [CrossRef]
- Kanetkar, P.; Singhal, R.; Kamat, M. Gymnema sylvestre: A memoir. J. Clin. Biochem. Nutr. 2007, 41, 77–81. [Google Scholar] [CrossRef]
- Muzaffar, H.; Qamar, I.; Bashir, M.; Jabeen, F.; Irfan, S.; Anwar, H. Gymnema sylvestre Supplementation Restores Normoglycemia, Corrects Dyslipidemia, and Transcriptionally Modulates Pancreatic and Hepatic Gene Expression in Alloxan-Induced Hyperglycemic Rats. Metabolites 2023, 13, 516. [Google Scholar] [CrossRef]
- Yadav, D.; Kwak, M.; Jin, J.-O. Clinical applications of Gymnema sylvestre against type 2 diabetes mellitus and its associated abnormalities. Prog. Nutr. 2019, 21, 258–269. [Google Scholar] [CrossRef]
- Kashif, M.; Nasir, A.; Gulzaman; Rafique, M.K.; Abbas, M.; Ur Rehman, A.; Riaz, M.; Rasool, G.; Mtewa, A.G. Unlocking the anti-diabetic potential of Gymnema syvestre, Trigonella foenum-graecum, and their combination thereof: An in vivo evaluation. Food Sci. Nutr. 2023, 11, 7664–7672. [Google Scholar] [CrossRef]
- Gaonkar, V.P.; Hullatti, K. Indian Traditional medicinal plants as a source of potent Anti-diabetic agents: A Review. J. Diabetes Metab. Disord. 2020, 19, 1895–1908. [Google Scholar] [CrossRef] [PubMed]
- Huyen, V.T.T.; Phan, D.V.; Thang, P.; Ky, P.T.; Hoa, N.K.; Ostenson, C.G. Antidiabetic effects of add-on Gynostemma pentaphyllum extract therapy with sulfonylureas in type 2 diabetic patients. Evid. Based Complement. Altern. Med. 2012, 2012, 452313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, L.; Wei, S. Gynostemma pentaphyllum: A review on its traditional uses, phytochemistry and pharmacology. J. Funct. Foods 2025, 124, 106651–106674. [Google Scholar] [CrossRef]
- Song, M.; Tan, D.; Li, B.; Wang, Y.; Shi, L. Gypenoside ameliorates insulin resistance and hyperglycemia via the AMPK-mediated signaling pathways in the liver of type 2 diabetes mellitus mice. Food Sci. Hum. Wellness 2022, 11, 1347–1354. [Google Scholar] [CrossRef]
- Huyen, V.T.T.; Phan, D.V.; Thang, P.; Hoa, N.K.; Östenson, C.G. Gynostemma pentaphyllum tea improves insulin sensitivity in type 2 diabetic patients. J. Nutr. Metab. 2013, 2013, 765383. [Google Scholar] [CrossRef]
- Xie, J.-B.; Xie, P.; Guo, M.; Li, F.-F.; Xiao, M.-Y.; Qi, Y.-S.; Pei, W.-J.; Luo, H.-T.; Gu, Y.-L.; Piao, X.-L. Protective effect of heat-processed Gynostemma pentaphyllum on high fat diet-induced glucose metabolic disorders mice. Front. Pharmacol. 2023, 14, 1215150–1215164. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Leung, L.; Birtwhistle, R.; Kotecha, J.; Hannah, S.; Cuthbertson, S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (Bitter Melon): A mini review. Br. J. Nutr. 2009, 102, 1703–1708. [Google Scholar] [CrossRef]
- Richter, E.; Geetha, T.; Burnett, D.; Broderick, T.L.; Babu, J.R. The effects of Momordica charantia on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 4643. [Google Scholar] [CrossRef] [PubMed]
- Garau, C.; Cummings, E.; Phoenix, D.A.; Singh, J. Beneficial effect and mechanism of action of Momordica charantia in the treatment of diabetes mellitus: A mini review. Int. J. Diabetes Metab. 2003, 11, 46–55. [Google Scholar] [CrossRef]
- Oyelere, S.F.; Ajayi, O.H.; Ayoade, T.E.; Santana Pereira, G.B.; Dayo Owoyemi, B.C.; Ilesanmi, A.O.; Akinyemi, O.A. A detailed review on the phytochemical profiles and anti-Diabetic mechanisms of Momordica charantia. Heliyon 2022, 8, 9253–9263. [Google Scholar] [CrossRef]
- Shaukat, A.; Zaidi, A.; Anwar, H.; Kizilbash, N. Mechanism of the antidiabetic action of Nigella sativa and Thymoquinone: A review. Front. Nutr. 2023, 10, 1126272–1126299. [Google Scholar] [CrossRef]
- Maideen, N.M.P. Antidiabetic activity of nigella sativa (black seeds) and its active constituent (thymoquinone): A review of human and experimental animal studies. Chonnam Med. J. 2021, 57, 169–176. [Google Scholar] [CrossRef]
- Mashayekhi-Sardoo, H.; Sepahi, S.; Baradaran Rahimi, V.; Askari, V.R. Application of Nigella sativa as a functional food in diabetes and related complications: Insights on molecular, cellular, and metabolic effects. J. Funct. Foods 2024, 122, 106518–106550. [Google Scholar] [CrossRef]
- Hamdan, A.; Haji Idrus, R.; Mokhtar, M.H. Effects of nigella sativa on type-2 diabetes mellitus: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 4911. [Google Scholar] [CrossRef]
- El-Aarag, B.; Hussein, W.; Ibrahim, W.; Zahran, M. Thymoquinone improves anti-diabetic activity of metformin in streptozotocin-induced diabetic male rats. J. Diabetes Metab. 2017, 8, 1000780–1000787. [Google Scholar] [CrossRef]
- Afaf Jamal Ali Hmza, E.; Omar, A.; Adnan, A.; Osman, M.T. Nigella sativa oil has significant repairing ability of damaged pancreatic tissue occurs in induced type 1 diabetes mellitus. Glob. J. Pharmacol. 2013, 7, 14–19. [Google Scholar] [CrossRef]
- Choo, T.-M. Nigella sativa tea mitigates type-2 diabetes and edema: A case report. Adv. Tradit. Med. 2023, 23, 1249–1254. [Google Scholar] [CrossRef]
- Hannan, J.M.A.; Marenah, L.; Ali, L.; Rokeya, B.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Ocimum sanctum leaf extracts stimulate insulin secretion from perfused pancreas, isolated islets and clonal pancreatic β-Cells. J. Endocrinol. 2006, 189, 127–136. [Google Scholar] [CrossRef]
- Abhilash Rao, S.; Vijay, Y.; Deepthi, T.; Sri Lakshmi, C.; Vibha Rani, S.; Swetha Rani, B.; Bhuvaneswara Reddy, Y.; Ram Swaroop, P.; Sai Laxmi, V.; Nikhil Chakravarthy, K.; et al. Anti-diabetic effect of ethanolic extract of leaves of ocimum sanctum in alloxan induced diabetes in rats. Int. J. Basic Clin. Pharmacol. 2013, 2, 613. [Google Scholar] [CrossRef]
- Suanarunsawat, T.; Anantasomboon, G.; Piewbang, C. Anti-diabetic and anti-oxidative activity of fixed oil extracted from Ocimum sanctum L. leaves in diabetic rats. Exp. Ther. Med. 2016, 11, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Cheurfa, M.; Achouche, M.; Azouzi, A.; Abdalbasit, M.A. Antioxidant and anti-diabetic activity of pomegranate (Punica granatum L.) leaves extracts. Foods Raw Maters. 2020, 8, 329–336. [Google Scholar] [CrossRef]
- Gharib, E.; Kouhsari, S.M. Study of the antidiabetic activity of Punica granatum L. fruits aqueous extract on the alloxan-diabetic wistar rats. Iran. J. Pharm. Res. 2019, 18, 358–368. [Google Scholar] [PubMed]
- Tang, D.; Liu, L.; Ajiakber, D.; Ye, J.; Xu, J.; Xin, X.; Aisa, H.A. Anti-diabetic Effect of Punica granatum Flower Polyphenols Extract in Type 2 Diabetic Rats: Activation of Akt/GSK-3β and Inhibition of IRE1α-XBP1 Pathways. Front. Endocrinol. 2018, 9, 586–596. [Google Scholar] [CrossRef]
- Mabrouk Gabr, N. Effects of pomegranate (Punica granatum L.) fresh juice and peel extract on diabetic male albino rats. AMJ 2017, 46, 965–980. [Google Scholar] [CrossRef]
- Laila, O.; Murtaza, I.; Muzamil, S.; Imtiyaz Ali, S.; Abid Ali, S.; Ahamad Paray, B.; Gulnaz, A.; Vladulescu, C.; Mansoor, S. Enhancement of nutraceutical and anti-diabetic potential of fenugreek (Trigonella foenum-graecum). Sprouts with natural elicitors. Saudi Pharm. J. 2023, 31, 1–13. [Google Scholar] [CrossRef]
- Baset, M.E.; Ali, T.I.; Elshamy, H.; El Sadek, A.M.; Sami, D.; Badawy, M.; Abou-Zekry, S.; Heiba, H.; Saadeldin, M.; Abdellatif, A. Anti-Diabetic Effects of Fenugreek (Trigonella foenum-graecum): A Comparison between Oral and Intraperitoneal Administration—An Animal Study. Int. J. Funct. Nutr. 2020, 1, 2–10. [Google Scholar] [CrossRef]
- Kumar, A.; Aswal, S.; Chauhan, A.; Semwal, R.B.; Kumar, A.; Semwal, D.K. Ethnomedicinal investigation of medicinal plants of Chakrata region (Uttarakhand) used in the traditional medicine for diabetes by Jaunsari tribe. Nat. Prod. Bioprospect. 2019, 9, 175–200. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436–449. [Google Scholar] [CrossRef]
- Haxhiraj, M.; White, K.; Terry, C. The role of fenugreek in the management of type 2 diabetes. Int. J. Mol. Sci. 2024, 25, 6987. [Google Scholar] [CrossRef] [PubMed]
- Sarker, D.K.; Ray, P.; Dutta, A.K.; Rouf, R.; Uddin, S.J. Antidiabetic potential of fenugreek (Trigonella foenum-graecum): A magic herb for diabetes mellitus. Food Sci. Nutr. 2024, 12, 7108–7136. [Google Scholar] [CrossRef]
- Patel, J.; Patel, R.; Khambholja, K.; Patel, N. An overview of phytosomes as an advanced herbal drug delivery system. Asian J. Pharm. Sci. 2008, 3, 193–203. [Google Scholar]
- Epriliati, I.; Ginjom, I.R. Bioavailability of Phytochemicals. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; Rao, V., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef][Green Version]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-drug delivery systems based on natural products. Int. J. Nanomed. 2024, 19, 541–569. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Poorani, V.; Selvakumar, K.; Venkat Kumar, G. Improving bioavailability of phytochemicals through niosomes. J. Drug Deliv. Ther. 2020, 10, 119–121. [Google Scholar] [CrossRef]
- Prasad, T.N.; Arjunan, D.; Pal, R.; Bhadada, S.K. Diabetes and Osteoporosis. Indian J. Orthop. 2023, 57, 209–217. [Google Scholar] [CrossRef]
- Mohammad, O.H.; Yang, S.; Ji, W.; Ma, H.; Tao, R. Curcumin preserves bone health compromised by diabetes by inhibiting osteoporosis through regulation of the SIRT3/FoxO3a signalling pathway. Sci. Rep. 2025, 15, 29566. [Google Scholar] [CrossRef] [PubMed]




| Plant Name | Family | Used Plant Parts | Mode of Action | Ref. |
|---|---|---|---|---|
| Achyranthes aspera | Amaranthaceae | Seed, leaf |
| [34,36] |
| Allium sativum | Amaryllidaceae | Whole plant |
| [40,41] |
| Aloe vera | Liliaceal | Whole plant |
| [42,45] |
| Amaranthus tricolor | Amaranthaceae | Leaf and stem |
| [50,51] |
| Anacardium occidentale | Anacardiaceous | Leaf and stem |
| [57] |
| Annona squamosa | Annonaceae | Roots, seeds, leaves, and fruits |
| [61] |
| Berberis vulgaris | Berberidaceae | Fruit |
| [63,64,66,67] |
| Cinnamomum zeylanicum | Lauraceae | Whole plant |
| [70,71,73] |
| Curcuma longa | Zingiberaceae | Root |
| [81,82] |
| Gymnema sylvestre | Asclepiadaceae | Leaves |
| [84] |
| Gynostemma pentaphylium | Cucurbitaceae |
| [91,92] | |
| Momordica charantia | Cucurbitaceae | Fruit |
| [95,96,97] |
| Nigella sativa | Ranunculaceae | Whole plant |
| [98,99,100,101] |
| Ocimum sanctum | Lamiaceae | Leaves |
| [106] |
| Punica granatum | Lythraceae | Leave and flower |
| [109,111] |
| Trigonella foenum-graecum | Fabaceae | Seeds and leaves |
| [114,115,116,117] |
| Plant | Study Type | Model | Extract/Compound | Results | Ref |
|---|---|---|---|---|---|
| Achyranthes aspera | In vitro, in vivo | Rats | Ethanolic seed extracts | Reduced the blood glucose levels Inhibited the activities of α-amylase and α-glucosidase enzymes | [35,36] |
| Allium sativum | In vivo | Mice | Garlic polysaccharide | Reduced blood glucose; inhibited the enzyme α-glucosidase | [39,41] |
| Aleo vera | In vivo and in vitro and clinical | Rats | Aqueous crude extract (PBS-homogenized) | Increased serum insulin and decreased serum glucose | [45] |
| Amaranthus tricolor | In vivo and in vitro | Rats | 70% ethanolic extract (in vivo). Water extract (in vitro) | Reduced the pancreatic malondialdehyde (MDA) levels and blood glucose. antidiabetic, anti-lipase, anti-α-amylase, anti-α-glucosidase, and anti-acetylcholinesterase | [50,51] |
| Anacardium occidentale | In vivo | Rats | Ethanolic extract | Reduced hyperglycemia | [55] |
| Annona squamosa | In vivo | Rats | Petroleum ether, ethyl acetate and alcoholic extracts | Blood glucose levels | [60] |
| Berberis vulgaris | In vivo and clinical | Rats, human | Water extract | Reduced blood glucose. downregulating aldose reductase expression and inhibiting its enzymatic activity | [65,66] |
| Cinnamomum zeylanicum | In vivo, clinical and in vitro | Rats, human | Water extract | Decrease plasma glucose and increase plasma insulin. GLUT-4 translocation | [72,75] |
| Curcuma longa | In vivo and clinical and in vitro | Mice, human | Ethanolic extract | Reduced reactive oxygen species (ROS) and fasting glucose levels | [79] |
| Gymnema sylvestre | In vivo | Rabbits | Ethanolic extract | Regeneration of pancreatic islet cells, increased insulin secretion, inhibition of intestinal glucose absorption, inhibition of α-glucosidase enzyme, enhancement of peripheral glucose metabolism | [85] |
| Gynostemma pentaphyllum | In vivo, in vitro and clinical | Mice, human | Water extract | Lowered plasma glucose | [91] |
| Momordica charantia | In vivo and vitro | Mice | Methanolic extract | Preserved the morphology and function of pancreatic islet β-cells, which are responsible for insulin secretion | [96] |
| Nigella sativa | In vivo | Rats | Essential oil extract | Protected the pancreatic islets | [103] |
| Ocimum sanctum | In vivo | Rats | Ethanolic extract | Enhanced the insulin secretion | [106] |
| Punica granatum | In vivo | Rat | Ethanolic extract | Resulted in reduced blood glucose levels | [110] |
| Trigonella foenum-graecum ranatum | In vitro | HepG2 cells | Water extract | Increased glucose uptake through upregulation of glucose transporter-2 (GLUT-2) mRNA levels | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chahrour, J.A.; Abdel Baki, Z.; El Badan, D.; Nasser, G.; Maresca, M.; Hijazi, A. Herbal Medicines in the Management of Diabetes Mellitus: Plants, Bioactive Compounds, and Mechanisms of Action. Biomolecules 2025, 15, 1674. https://doi.org/10.3390/biom15121674
Chahrour JA, Abdel Baki Z, El Badan D, Nasser G, Maresca M, Hijazi A. Herbal Medicines in the Management of Diabetes Mellitus: Plants, Bioactive Compounds, and Mechanisms of Action. Biomolecules. 2025; 15(12):1674. https://doi.org/10.3390/biom15121674
Chicago/Turabian StyleChahrour, Jamil Atef, Zaher Abdel Baki, Dalia El Badan, Ghassan Nasser, Marc Maresca, and Akram Hijazi. 2025. "Herbal Medicines in the Management of Diabetes Mellitus: Plants, Bioactive Compounds, and Mechanisms of Action" Biomolecules 15, no. 12: 1674. https://doi.org/10.3390/biom15121674
APA StyleChahrour, J. A., Abdel Baki, Z., El Badan, D., Nasser, G., Maresca, M., & Hijazi, A. (2025). Herbal Medicines in the Management of Diabetes Mellitus: Plants, Bioactive Compounds, and Mechanisms of Action. Biomolecules, 15(12), 1674. https://doi.org/10.3390/biom15121674








