Interactive Role of the DHPR β1a SH3 Domain in Skeletal Muscle Excitation–Contraction Coupling
Abstract
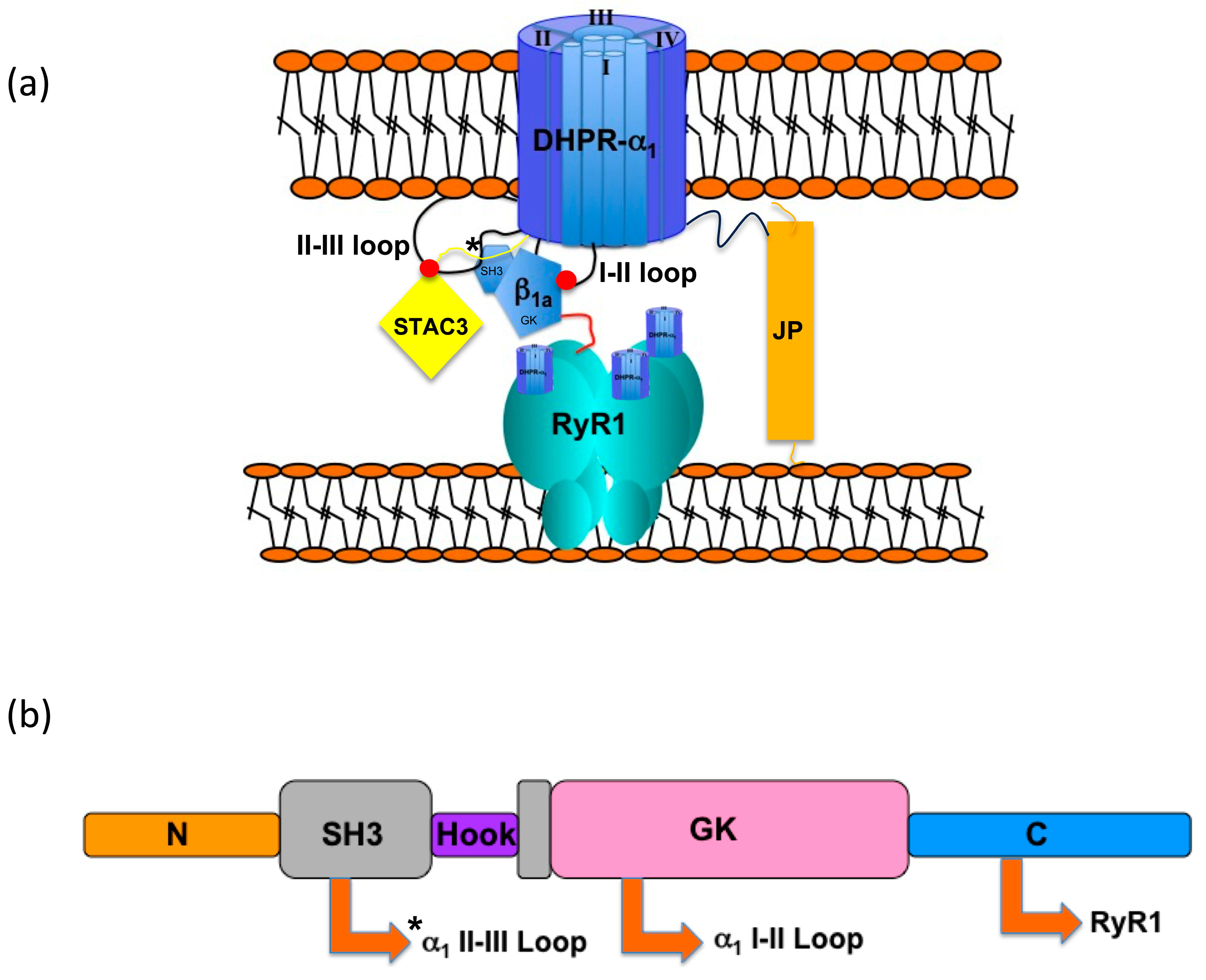
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Fluorescence Quenching Experiments
2.3. Circular Dichroism (CD)
2.4. cDNA Constructs and Virus Packaging for Myotube Studies
2.5. Isolation of Primary Myotubes, Cell Culture, and Calcium Imaging
2.6. L-Type Ca2+ Current Determinations in Cultured Myotubes
3. Results
Calcium Release and L-Type Channel Function
4. Discussion
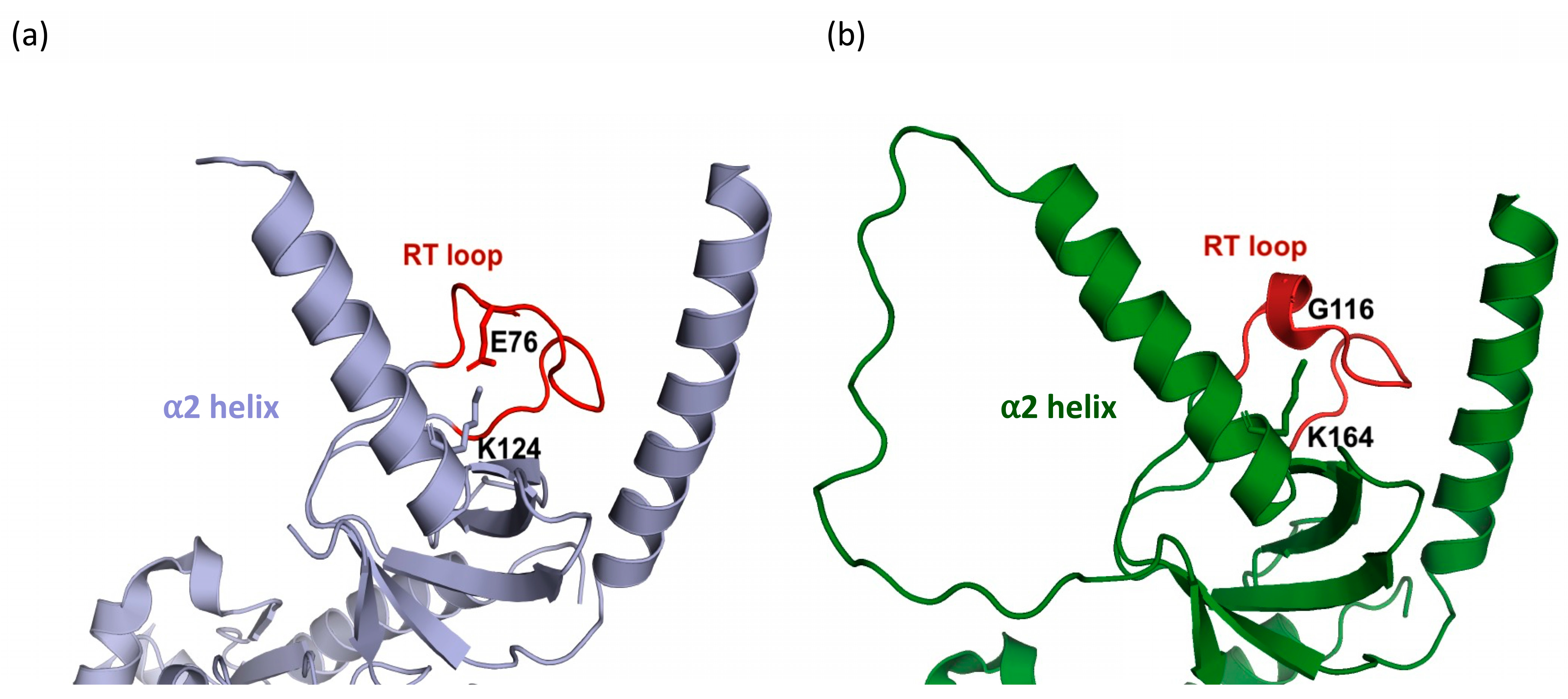
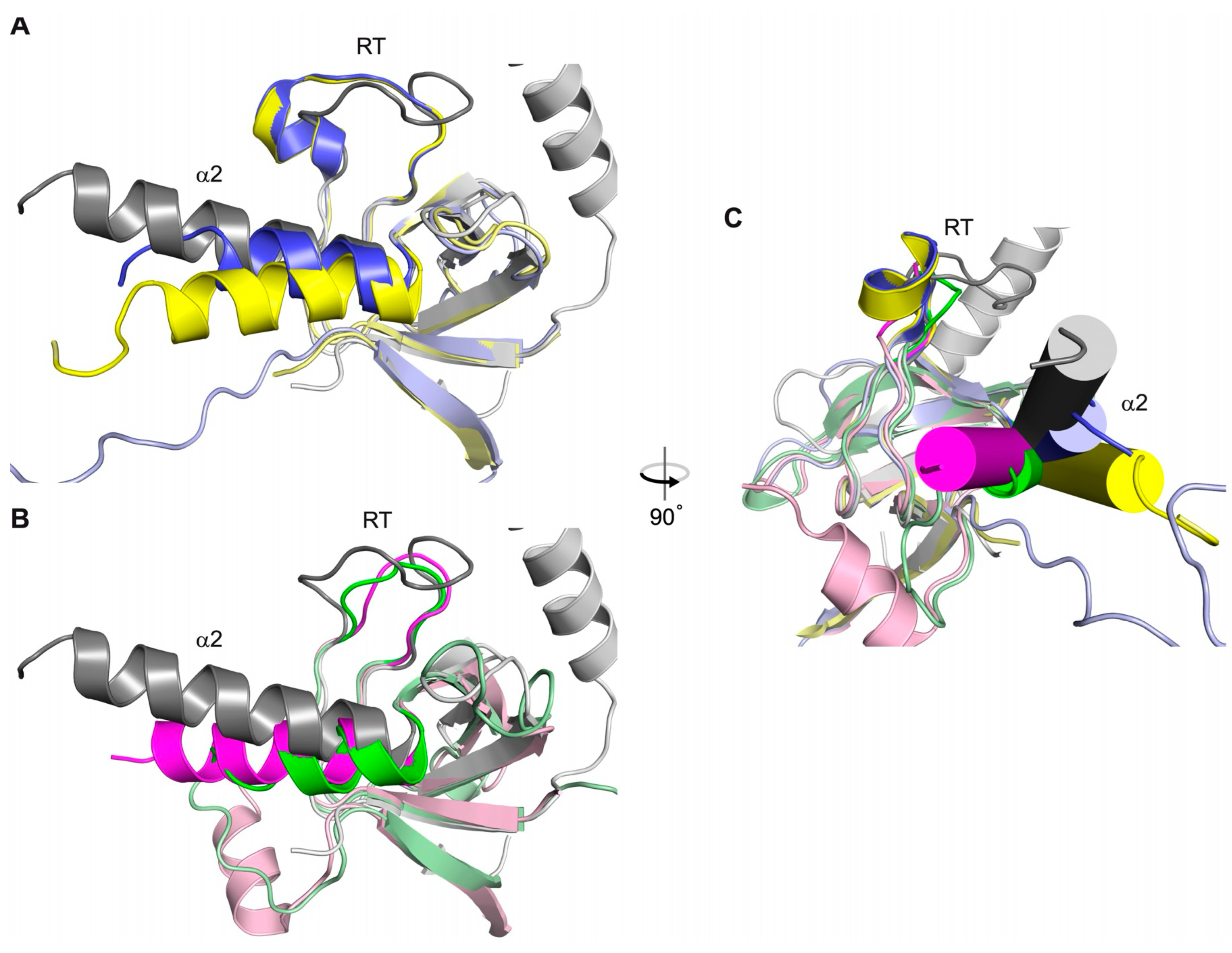
4.1. Functional Roles of the β1a SH3 Domain in Skeletal Muscle EC Coupling
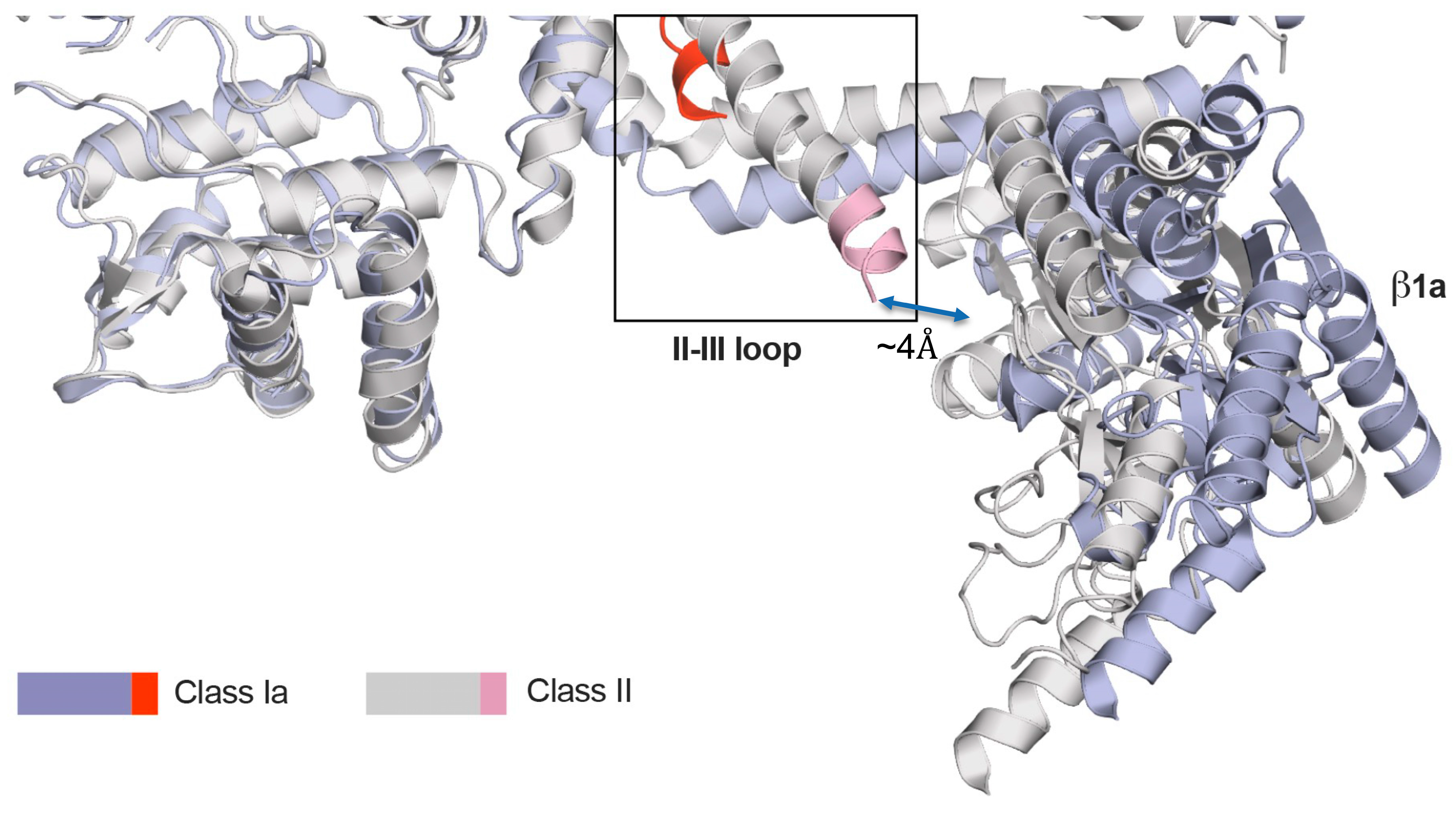
4.2. Structural Relationship Between β1a and the DHPR II-III Loop
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beam, K.G.; Bannister, R.A. Looking for Answers to Ec Coupling’s Persistent Questions. J. Gen. Physiol. 2010, 136, 7–12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perni, S.; Lavorato, M.; Beam, K.G. De Novo Reconstitution Reveals the Proteins Required for Skeletal Muscle Voltage-Induced Ca(2+) Release. Proc. Natl. Acad. Sci. USA 2017, 114, 13822–13827. [Google Scholar] [CrossRef]
- Beurg, M.; Ahern, C.A.; Vallejo, P.; Conklin, M.W.; Powers, P.A.; Gregg, R.G.; Coronado, R. Involvement of the Carboxy-Terminus Region of the Dihydropyridine Receptor Beta1a Subunit in Excitation-Contraction Coupling of Skeletal Muscle. Biophys. J. 1999, 77, 2953–2967. [Google Scholar] [CrossRef]
- Horstick, E.J.; Linsley, J.W.; Dowling, J.J.; Hauser, M.A.; McDonald, K.K.; Ashley-Koch, A.; Saint-Amant, L.; Satish, A.; Cui, W.W.; Zhou, W.; et al. Stac3 Is a Component of the Excitation-Contraction Coupling Machinery and Mutated in Native American Myopathy. Nat. Commun. 2013, 4, 1952. [Google Scholar] [CrossRef]
- Schredelseker, J.; Di Biase, V.; Obermair, G.J.; Felder, E.T.; Flucher, B.E.; Franzini-Armstrong, C.; Grabner, M. The Beta 1a Subunit Is Essential for the Assembly of Dihydropyridine-Receptor Arrays in Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2005, 102, 17219–17224. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Beam, K. Junctophilins 1, 2, and 3 All Support Voltage-Induced Ca2+ Release Despite Considerable Divergence. J. Gen. Physiol. 2022, 154, e202113024. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Panwar, P.; McFarlane, C.R.; Tuinte, W.E.; Campiglio, M.; Van Petegem, F. Structures of the Junctophilin/Voltage-Gated Calcium Channel Interface Reveal Hot Spot for Cardiomyopathy Mutations. Proc. Natl. Acad. Sci. USA 2022, 119, e2120416119. [Google Scholar] [CrossRef]
- Nakai, J.; Tanabe, T.; Konno, T.; Adams, B.; Beam, K.G. Localization in the II-III Loop of the Dihydropyridine Receptor of a Sequence Critical for Excitation-Contraction Coupling. J. Biol. Chem. 1998, 273, 24983–24986. [Google Scholar] [CrossRef]
- Grabner, M.; Dirksen, R.T.; Suda, N.; Beam, K.G. The II-III Loop of the Skeletal Muscle Dihydropyridine Receptor Is Responsible for the Bi-Directional Coupling with the Ryanodine Receptor. J. Biol. Chem. 1999, 274, 21913–21919. [Google Scholar] [CrossRef]
- Wilkens, C.M.; Kasielke, N.; Flucher, B.E.; Beam, K.G.; Grabner, M. Excitation-Contraction Coupling Is Unaffected by Drastic Alteration of the Sequence Surrounding Residues L720-L764 of the Alpha 1s II-III Loop. Proc. Natl. Acad. Sci. USA 2001, 98, 5892–5897. [Google Scholar] [CrossRef]
- Yuen, S.M.W.K.; Campiglio, M.; Tung, C.-C.; Flucher, B.E.; Van Petegem, F. Structural Insights into Binding of Stac Proteins to Voltage-Gated Calcium Channels. Proc. Natl. Acad. Sci. USA 2017, 114, E9520–E9528. [Google Scholar]
- Kugler, G.; Weiss, R.G.; Flucher, B.E.; Grabner, M. Structural Requirements of the Dihydropyridine Receptor Alpha1s II-III Loop for Skeletal-Type Excitation-Contraction Coupling. J. Biol. Chem. 2004, 279, 4721–4728. [Google Scholar] [CrossRef]
- Bichet, D.; Lecomte, C.; Sabatier, J.M.; Felix, R.; De Waard, M. Reversibility of the Ca(2+) Channel Alpha(1)-Beta Subunit Interaction. Biochem. Biophys. Res. Commun. 2000, 277, 729–735. [Google Scholar] [CrossRef]
- Gerster, U.; Neuhuber, B.; Groschner, K.; Striessnig, J.; Flucher, B.E. Current Modulation and Membrane Targeting of the Calcium Channel Alpha1c Subunit Are Independent Functions of the Beta Subunit. J. Physiol. 1999, 517 Pt 2, 353–368. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, M.H.; Zhang, Y.; He, L.L.; Yamada, Y.; Fitzmaurice, A.; Yang, J. Structural Basis of the Alpha1-Beta Subunit Interaction of Voltage-Gated Ca2+ Channels. Nature 2004, 429, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Opatowsky, Y.; Chen, C.-C.; Campbell, K.P.; Hirsch, J.A. Structural Analysis of the Voltage-Dependent Calcium Channel Beta Subunit Functional Core and Its Complex with the Alpha 1 Interaction Domain. Neuron 2004, 42, 387–399. [Google Scholar] [CrossRef]
- Van Petegem, F.; Clark, K.A.; Chatelain, F.C.; Minor, D.L., Jr. Structure of a Complex between a Voltage-Gated Calcium Channel Beta-Subunit and an Alpha-Subunit Domain. Nature 2004, 429, 671–675. [Google Scholar] [CrossRef]
- Buraei, Z.; Yang, J. The Beta-Subunit Subunit of Voltage-Gated Ca2+ Channels. Physiol. Rev. 2010, 90, 1461–1506. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Altafaj, X.; Ronjat, M.; Coronado, R. Interaction between the Dihydropyridine Receptor Ca2+ Channel Beta-Subunit and Ryanodine Receptor Type 1 Strengthens Excitation-Contraction Coupling. Proc. Natl. Acad. Sci. USA 2005, 102, 19225–19230. [Google Scholar] [CrossRef] [PubMed]
- Dayal, A.; Perni, S.; Franzini-Armstrong, C.; Beam, K.G.; Grabner, M. The Distal C Terminus of the Dihydropyridine Receptor Beta(1a) Subunit Is Essential for Tetrad Formation in Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2022, 119, e2201136119. [Google Scholar] [CrossRef]
- Sheridan, D.C.; Cheng, W.; Ahern, C.A.; Mortenson, L.; Alsammarae, D.; Vallejo, P.; Coronado, R. Truncation of the Carboxyl Terminus of the Dihydropyridine Receptor Beta1a Subunit Promotes Ca2+ Dependent Excitation-Contraction Coupling in Skeletal Myotubes. Biophys. J. 2003, 84, 220–237. [Google Scholar] [CrossRef]
- Dayal, A.; Bhat, V.; Franzini-Armstrong, C.; Grabner, M. Domain Cooperativity in the Beta1a Subunit Is Essential for Dihydropyridine Receptor Voltage Sensing in Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 7488–7493. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C. Beta Subunits of Voltage-Gated Calcium Channels. J. Bioenerg. Biomembr. 2003, 35, 599–620. [Google Scholar] [CrossRef]
- Richards, M.W.; Butcher, A.J.; Dolphin, A.C. Ca2+ Channel Beta-Subunits: Structural Insights Aid Our Understanding. Trends Pharmacol. Sci. 2004, 25, 626–632. [Google Scholar] [CrossRef]
- Van Petegem, F.; Duderstadt, K.E.; Clark, K.A.; Wang, M.; Minor, D.L. Alanine-Scanning Mutagenesis Defines a Conserved Energetic Hotspot in the Cavalpha1 Aid-Cavbeta Interaction Site That Is Critical for Channel Modulation. Structure 2008, 16, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.J.; Kühne, R.; Schneider-Mergener, J.; Oschkinat, H. Recognition of Proline-Rich Motifs by Protein-Protein-Interaction Domains. Angew. Chem. Int. Ed. Engl. 2005, 44, 2852–2869. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, A.; Soboleva, T.A.; Jans, D.A.; Board, P.G.; Baker, R.T. An Efficient System for High-Level Expression and Easy Purification of Authentic Recombinant Proteins. Protein Sci. 2004, 13, 1331–1339. [Google Scholar] [CrossRef]
- Cui, Y.; Tae, H.S.; Norris, N.C.; Karunasekara, Y.; Pouliquin, P.; Board, P.G.; Dulhunty, A.F.; Casarotto, M.G. A Dihydropyridine Receptor Alpha1s Loop Region Critical for Skeletal Muscle Contraction Is Intrinsically Unstructured and Binds to a Spry Domain of the Type 1 Ryanodine Receptor. Int. J. Biochem. Cell Biol. 2009, 41, 677–686. [Google Scholar] [CrossRef]
- Eltit, J.M.; Franzini-Armstrong, C.; Perez, C.F. Amino Acid Residues 489–503 of Dihydropyridine Receptor (Dhpr) Beta1a Subunit Are Critical for Structural Communication between the Skeletal Muscle Dhpr Complex and Type 1 Ryanodine Receptor. J. Biol. Chem. 2014, 289, 36116–36124. [Google Scholar] [CrossRef]
- Beurg, M.; Sukhareva, M.; Strube, C.; Powers, P.; Gregg, R.; Coronado, R. Recovery of Ca2+ Current, Charge Movements, and Ca2+ Transients in Myotubes Deficient in Dihydropyridine Receptor Beta 1 Subunit Transfected with Beta 1 Cdna. Biophys. J. 1997, 73, 807–818. [Google Scholar] [CrossRef][Green Version]
- Gregg, R.G.; Messing, A.; Strube, C.; Beurg, M.; Moss, R.; Behan, M.; Powers, P.A. Absence of the Beta Subunit (Cchb1) of the Skeletal Muscle Dihydropyridine Receptor Alters Expression of the Alpha 1 Subunit and Eliminates Excitation-Contraction Coupling. Proc. Natl. Acad. Sci. USA 1996, 93, 13961–13966. [Google Scholar] [CrossRef]
- Perez, C.F.; Eltit, J.M.; Lopez, J.R.; Bodnár, D.; Dulhunty, A.F.; Aditya, S.; Casarotto, M.G. Functional and Structural Characterization of a Novel Malignant Hyperthermia-Susceptible Variant of DHPR-Beta(1a) Subunit (Cacnb1). Am. J. Physiol. Cell Physiol. 2018, 314, C323–C333. [Google Scholar] [CrossRef] [PubMed]
- Eltit, J.M.; Li, H.; Ward, C.W.; Molinski, T.; Pessah, I.N.; Allen, P.D.; Lopez, J.R. Orthograde Dihydropyridine Receptor Signal Regulates Ryanodine Receptor Passive Leak. Proc. Natl. Acad. Sci. USA 2011, 108, 7046–7051. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, S.; Zou, J.; Lopez, J.R.; Yang, J.J.; Perez, C.F. Myoplasmic Resting Ca2+ Regulation by Ryanodine Receptors Is under the Control of a Novel Ca2+-Binding Region of the Receptor. Biochem. J. 2014, 460, 261–271. [Google Scholar] [CrossRef]
- Eltit, J.M.; Szpyt, J.; Li, H.; Allen, P.D.; Perez, C.F. Reduced Gain of Excitation-Contraction Coupling in Triadin-Null Myotubes Is Mediated by the Disruption of Fkbp12/Ryr1 Interaction. Cell Calcium 2011, 49, 128–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eltit, J.M.; Feng, W.; Lopez, J.R.; Padilla, I.T.; Pessah, I.N.; Molinski, T.F.; Fruen, B.R.; Allen, P.D.; Perez, C.F. Ablation of Skeletal Muscle Triadin Impairs Fkbp12/Ryr1 Channel Interactions Essential for Maintaining Resting Cytoplasmic Ca2+. J. Biol. Chem. 2010, 285, 38453–38462. [Google Scholar] [CrossRef] [PubMed]
- Norris, N.C.; Joseph, S.; Aditya, S.; Karunasekara, Y.; Board, P.G.; Dulhunty, A.F.; Casarotto, M.G. Structural and Biophysical Analyses of the Skeletal Dihydropyridine Receptor Beta Subunit Beta(1a) Reveal Critical Roles of Domain Interactions for Stability. J. Biol. Chem. 2017, 292, 8401–8411. [Google Scholar] [CrossRef]
- Fan, J.Y.; Rangasamy, D.; Luger, K.; Tremethick, D.J. H2a.Z Alters the Nucleosome Surface to Promote Hp1alpha-Mediated Chromatin Fiber Folding. Mol. Cell 2004, 16, 655–661. [Google Scholar] [CrossRef]
- Tae, H.; Norris, N.C.; Cui, Y.; Karunasekara, Y.; Board, P.G.; Dulhunty, A.F.; Casarotto, M.G. Molecular Recognition of the Disordered Dihydropyridine Receptor Ii-Iii Loop by a Conserved Spry Domain of the Type 1 Ryanodine Receptor. Clin. Exp. Pharmacol. Physiol. 2009, 36, 346–349. [Google Scholar] [CrossRef]
- Aitio, O.; Hellman, M.; Kesti, T.; Kleino, I.; Samuilova, O.; Pääkkönen, K.; Tossavainen, H.; Saksela, K.; Permi, P. Structural Basis of Pxxdy Motif Recognition in SH3 Binding. J. Mol. Biol. 2008, 382, 167–178. [Google Scholar] [CrossRef]
- Schredelseker, J.; Dayal, A.; Schwerte, T.; Franzini-Armstrong, C.; Grabner, M. Proper Restoration of Excitation-Contraction Coupling in the Dihydropyridine Receptor Beta1-Null Zebrafish Relaxed Is an Exclusive Function of the Beta1a Subunit. J. Biol. Chem. 2009, 284, 1242–1251. [Google Scholar] [CrossRef]
- Campiglio, M.; Di Biase, V.; Tuluc, P.; Flucher, B.E. Stable Incorporation Versus Dynamic Exchange of Beta Subunits in a Native Ca2+ Channel Complex. J. Cell Sci. 2013, 126, 2092–2101. [Google Scholar]
- Schartner, V.; Romero, N.B.; Donkervoort, S.; Treves, S.; Munot, P.; Pierson, T.M.; Dabaj, I.; Malfatti, E.; Zaharieva, I.T.; Zorzato, F.; et al. Dihydropyridine Receptor (Dhpr, Cacna1s) Congenital Myopathy. Acta Neuropathol. 2017, 133, 517–533. [Google Scholar] [CrossRef]
- Agrawal, V.; Kishan, K.R. Promiscuous Binding Nature of SH3 Domains to Their Target Proteins. Protein Pept. Lett. 2002, 9, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.; Sticht, H. A Proline to Glycine Mutation in the Lck SH3-Domain Affects Conformational Sampling and Increases Ligand Binding Affinity. FEBS Lett. 2007, 581, 1555–1560. [Google Scholar] [CrossRef]
- Marcette, J.; Hood, I.V.; Johnston, C.A.; Doe, C.Q.; Prehoda, K.E. Allosteric Control of Regulated Scaffolding in Membrane-Associated Guanylate Kinases. Biochemistry 2009, 48, 10014–10019. [Google Scholar] [CrossRef][Green Version]
- GGarcia, E.P.; Mehta, S.; Blair, L.A.; Wells, D.G.; Shang, J.; Fukushima, T.; Fallon, J.R.; Garner, C.C.; Marshall, J. Sap90 Binds and Clusters Kainate Receptors Causing Incomplete Desensitization. Neuron 1998, 21, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Maximov, A.; Südhof, T.C.; Bezprozvanny, I. Association of Neuronal Calcium Channels with Modular Adaptor Proteins. J. Biol. Chem. 1999, 274, 24453–24456. [Google Scholar] [CrossRef]
- Ooshio, T.; Kobayashi, R.; Ikeda, W.; Miyata, M.; Fukumoto, Y.; Matsuzawa, N.; Ogita, H.; Takai, Y. Involvement of the Interaction of Afadin with Zo-1 in the Formation of Tight Junctions in Madin-Darby Canine Kidney Cells. J. Biol. Chem. 2010, 285, 5003–5012. [Google Scholar] [CrossRef] [PubMed]
- Lye, M.F.; Fanning, A.S.; Su, Y.; Anderson, J.M.; Lavie, A. Insights into Regulated Ligand Binding Sites from the Structure of Zo-1 Src Homology 3-Guanylate Kinase Module. J. Biol. Chem. 2010, 285, 13907–13917. [Google Scholar] [CrossRef]
- McGee, A.W.; Dakoji, S.R.; Olsen, O.; Bredt, D.S.; Lim, W.A.; Prehoda, K.E. Structure of the SH3-Guanylate Kinase Module from Psd-95 Suggests a Mechanism for Regulated Assembly of Maguk Scaffolding Proteins. Mol. Cell 2001, 8, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Tavares, G.A.; Panepucci, E.H.; Brunger, A.T. Structural Characterization of the Intramolecular Interaction between the SH3 and Guanylate Kinase Domains of Psd-95. Mol. Cell 2001, 8, 1313–1325. [Google Scholar] [CrossRef]
- Morgenstern, T.J.; Nirwan, N.; Hernández-Ochoa, E.O.; Bibollet, H.; Choudhury, P.; Laloudakis, Y.D.; Colecraft, H.M. Selective Posttranslational Inhibition of Ca(V)Beta(1)-Associated Voltage-Dependent Calcium Channels with a Functionalized Nanobody. Nat. Commun. 2022, 13, 7556. [Google Scholar] [CrossRef] [PubMed]
- Mohana, M.; Perez, C.F.; Fessenden, J.D. Fluorescence Resonance Energy Transfer-Based Structural Analysis of the Dihydropyridine Receptor Alpha1s Subunit Reveals Conformational Differences Induced by Binding of the Beta1a Subunit. J. Biol. Chem. 2016, 291, 13762–13770. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Z.; Li, Z.; Qian, X.; Lu, S.; Dong, M.; Yan, N. Structure of the Voltage-Gated Calcium Channel Ca(V)1.1 at 3.6 a Resolution. Nature 2016, 537, 191–196. [Google Scholar] [CrossRef]
- Tuinte, W.E.; Török, E.; Tuluc, P.; Fattori, F.; D’Amico, A.; Campiglio, M. Stac3 Binding to Cav1.1 II-III Loop Is Nonessential but Critically Supports Skeletal Muscle Excitation-Contraction Coupling. JCI Insight 2025, 10, e191053. [Google Scholar] [CrossRef]
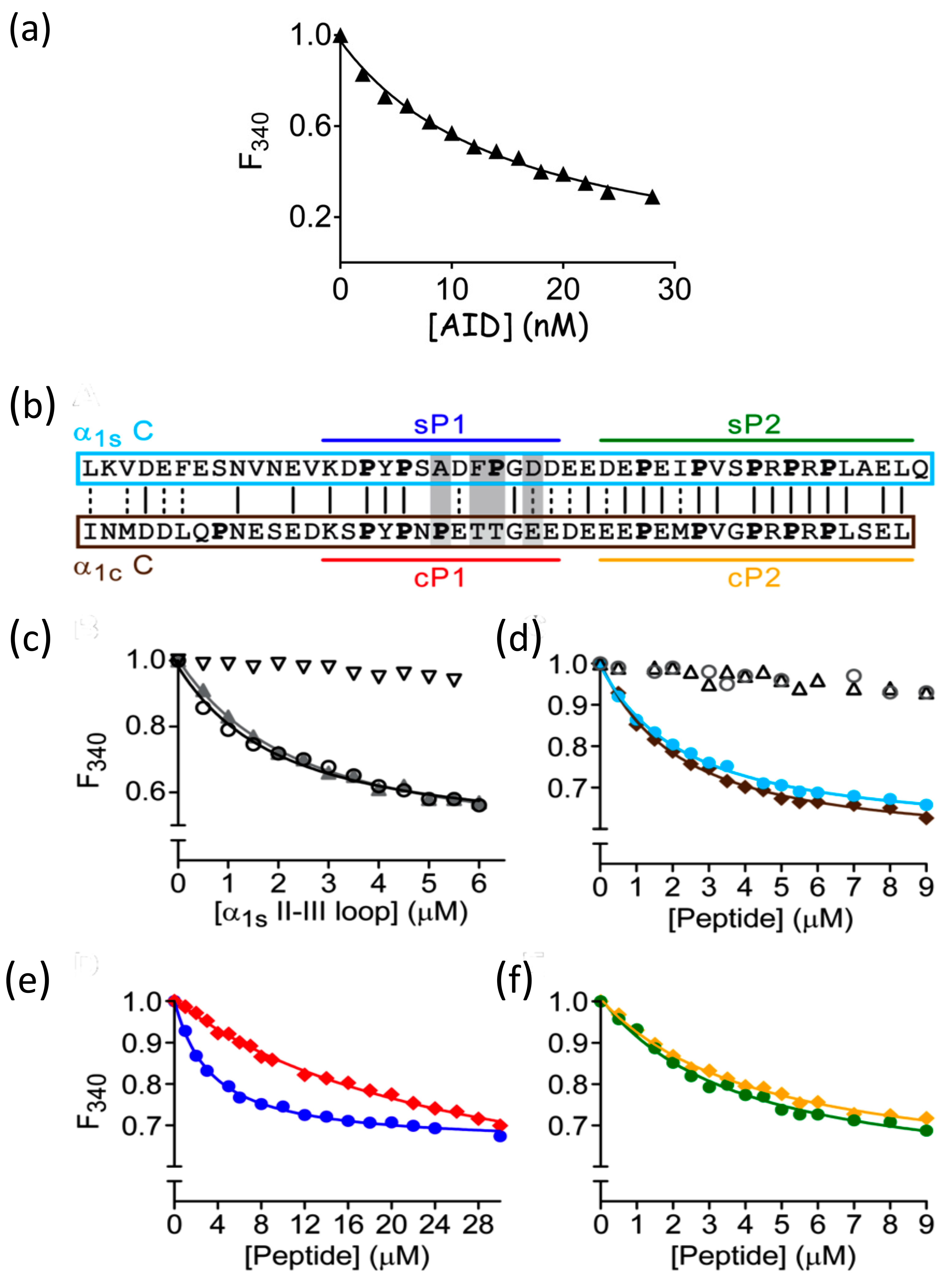

| DHPR II-III Fragment | β1a Subunit (μM) | β1a–SH3 Domain (Residues 101–272) (μM) |
|---|---|---|
| α1s DHPR II-III Loop | 2.5 ± 0.4 | 2.6 ± 0.2 |
| α1s II–III loop C region (α1s C) | 4.9 ± 0.6 | 3.6 ± 0.6 |
| α1c II–III loop C region (α1c C) | 3.5 ± 0.4 | 2.5 ± 0.2 |
| Scrambled α1s C | No binding | No binding |
| Mutated α1s C All P⟶A | No binding | No binding |
| α1s C binding peptide 1 (sP1) | 3.6 ± 0.2 | 2.6 ± 0.4 |
| sP1 A⟶P mutant | 4.5 ± 0.3 | 2.3 ± 0.2 |
| sP1 F⟶T mutant | 4.6 ± 0.4 | 4.6 ± 0.3 |
| sP1 P⟶T mutant | 22 ± 2 | 19 ± 2 |
| sP1 D⟶E mutant | 15 ± 2 | 17 ± 1 |
| α1c C binding peptide 1 (cP1) | 14 ± 2 | 19 ± 4 |
| α1s C binding peptide 2 (sP2) | 5.4 ± 0.9 | 3.8 ± 0.2 |
| α1c C binding peptide 2 (cP2) | 4.6 ± 0.3 | 3.6 ± 0.3 |
| α1s I-II Loop | 0.0152 ± 0.0018 | No binding |
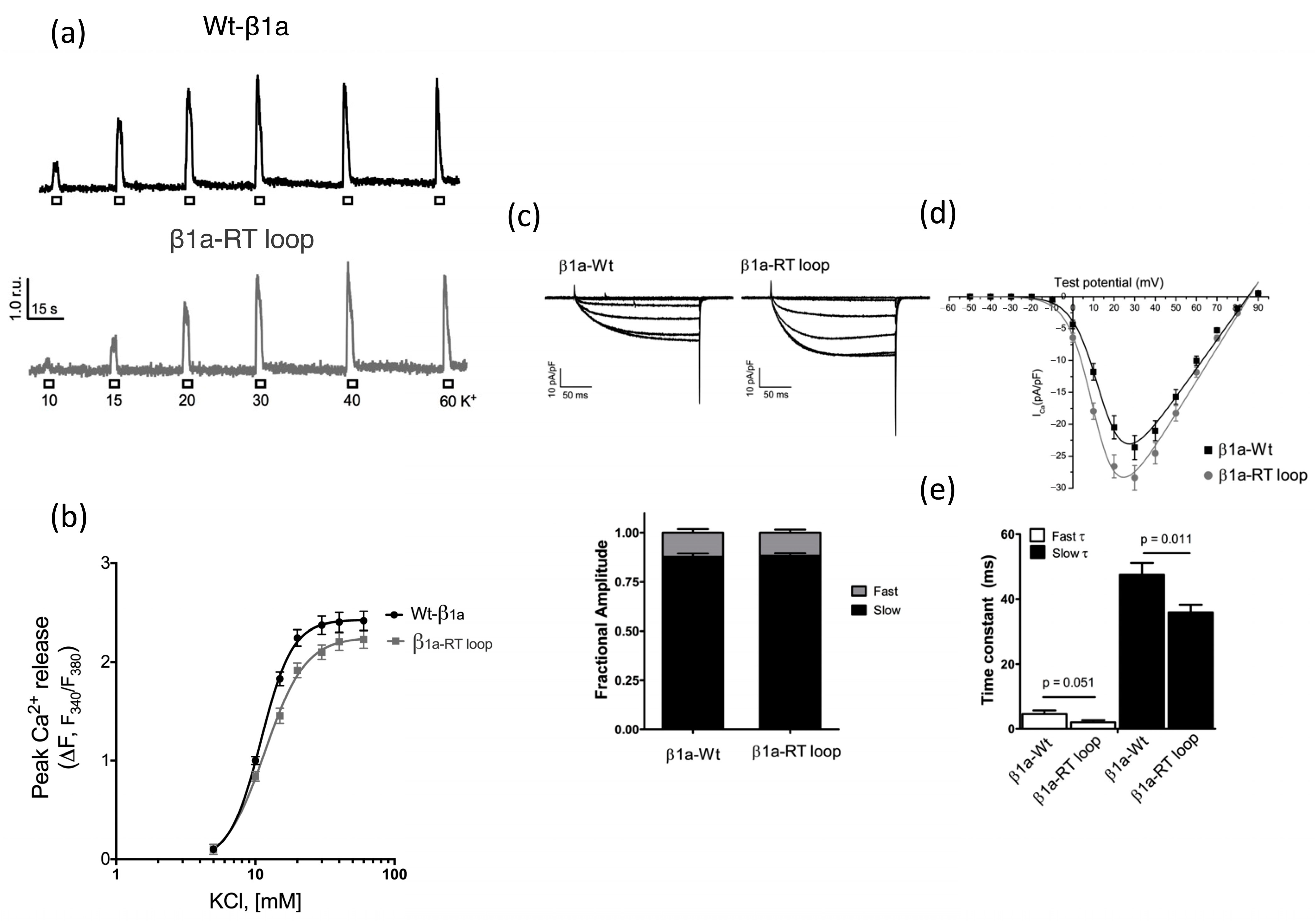
| Gmax (pS/pF) | V1/2, mV | Vr, mV | k, mV | |
|---|---|---|---|---|
| Wt-β1a (n = 22) | 453 ± 33.0 | 14.7 ± 0.88 | 84.8 ± 0.35 | 6.0 ± 0.17 |
| β1a-RT loop (n = 21) | 521 ± 36.6 | 11.2 ± 0.77 ** | 85.6 ± 0.48 | 5.8 ± 0.23 |
| β-Construct | β1a | β2a | β1a/β2a RT Loop | Β2a: HE to PG Mutant | β1a/β2a RT Loop: HE to PG Mutant |
|---|---|---|---|---|---|
| Kd (μM) | 4.9 ± 0.6 | No binding | Weak binding | 10.4 ± 0.7 | 8.7 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunasekara, Y.; Aditya, S.; Norris, N.C.; Cappello, J.; Dulhunty, A.F.; Board, P.G.; Eltit, J.M.; Perez, C.F.; Casarotto, M.G. Interactive Role of the DHPR β1a SH3 Domain in Skeletal Muscle Excitation–Contraction Coupling. Biomolecules 2025, 15, 1610. https://doi.org/10.3390/biom15111610
Karunasekara Y, Aditya S, Norris NC, Cappello J, Dulhunty AF, Board PG, Eltit JM, Perez CF, Casarotto MG. Interactive Role of the DHPR β1a SH3 Domain in Skeletal Muscle Excitation–Contraction Coupling. Biomolecules. 2025; 15(11):1610. https://doi.org/10.3390/biom15111610
Chicago/Turabian StyleKarunasekara, Yamuna, Shouvik Aditya, Nicole C. Norris, Jean Cappello, Angela F. Dulhunty, Philip G. Board, Jose M. Eltit, Claudio F. Perez, and Marco G. Casarotto. 2025. "Interactive Role of the DHPR β1a SH3 Domain in Skeletal Muscle Excitation–Contraction Coupling" Biomolecules 15, no. 11: 1610. https://doi.org/10.3390/biom15111610
APA StyleKarunasekara, Y., Aditya, S., Norris, N. C., Cappello, J., Dulhunty, A. F., Board, P. G., Eltit, J. M., Perez, C. F., & Casarotto, M. G. (2025). Interactive Role of the DHPR β1a SH3 Domain in Skeletal Muscle Excitation–Contraction Coupling. Biomolecules, 15(11), 1610. https://doi.org/10.3390/biom15111610





