Exploring Chronic Pain, Immune Dysfunction and Lifestyle: A Focus on T Cell Exhaustion and Senescence
Abstract
1. Linking Chronic Pain to Immune Dysfunction and Lifestyle Factors
2. Immune Exhaustion and Senescence
3. Role of Lifestyle Factors in Immune Exhaustion and Senescence
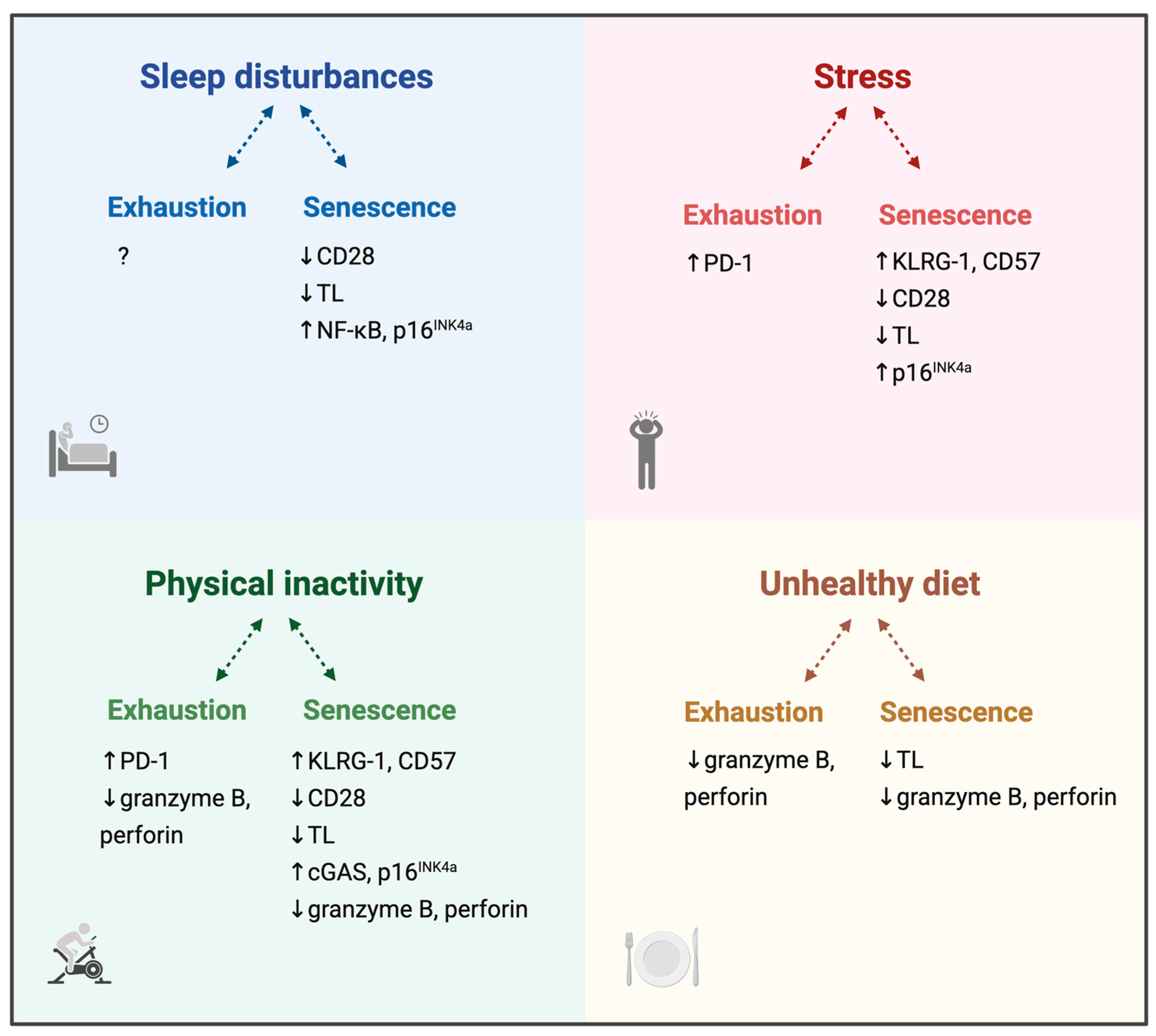
3.1. Sleep and T Cell Dysregulation
3.2. Stress and T Cell Dysregulation
3.3. Physical Activity and T Cell Dysregulation
3.4. Diet and T Cell Dysregulation
3.5. Lifestyle Factors, Immune Exhaustion and Immune Senescence
4. Immune Exhaustion and Senescence in Chronic Pain Conditions
4.1. Primary Chronic Pain Conditions
4.2. Secondary Chronic Pain Conditions
4.3. Potential Treatments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| BTLA | B- and T-lymphocyte attenuator |
| CD27/28/57 | Cluster of differentiation 27/28/57 |
| cGAS | Cyclic GMP-AMP synthase |
| COVID | Coronavirus disease |
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen 4 |
| CWP | Chronic widespread pain |
| Eomes | Eomesodermin |
| FM | Fibromyalgia |
| Gal-9 | Galectin-9 |
| HPA axis | Hypothalamic–pituitary–adrenal axis |
| ICIs | Immune checkpoint inhibitors |
| IFN-γ | Interferon gamma |
| IL-2/6/10 | Interleukin 2/6/10 |
| KLRG-1 | Killer cell lectin-like receptor G1 |
| LAG-3 | Lymphocyte-activation gene 3 |
| Long COVID | Long Coronavirus disease |
| MHC | Major histocompatibility complex |
| MM | Macrophagic myofasciitis |
| ME/CFS | Myalgic encephalomyelitis/chronic fatigue syndrome |
| NF-κB | Nuclear factor kappa B |
| NK cell | Natural killer cell |
| OA | Osteoarthritis |
| PBMCs | Peripheral blood mononuclear cells |
| PD-L1 | Programmed Death-Ligand 1 |
| PD-1 | Programmed cell death protein 1 |
| PHN | Postherpetic neuralgia |
| p16INK4a | Cyclin-dependent kinase inhibitor 2A |
| qPCR | Quantitative polymerase chain reaction |
| RA | Rheumatoid arthritis |
| SASP | Senescence-associated secretory phenotype |
| SCD | Sickle cell disease |
| T-bet | T-box expressed in T cells |
| TCF-1 | T cell factor 1 |
| TIGIT | T-cell immunoreceptor with Ig and ITIM domains |
| Tim-3 | T-cell immunoglobulin and mucin domain 3 |
| TL | Telomere length |
| TNF-α | Tumour necrosis factor alpha |
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). PAIN 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Dahlhamer, J.; Lucas, J.; Zelaya, C.; Nahin, R.; Mackey, S.; DeBar, L.; Kerns, R.; Von Korff, M.; Porter, L.; Helmick, C. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Abraham, J.; Ali, M.K.; Alvarado, M.; Atkinson, C.; Baddour, L.M.; Bartels, D.H.; Benjamin, E.J.; Bhalla, K.; Birbeck, G.; et al. The State of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef]
- Polli, A.; Nijs, J.; Thienpont, B. Epigenetics as the molecular substrate of multimodal lifestyle approaches for patients with persistent pain. Braz. J. Phys. Ther. 2024, 29, 101170. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Ji, R.R.; Chamessian, A.; Zhang, Y.Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef]
- Sawicki, C.M.; Humeidan, M.L.; Sheridan, J.F. Neuroimmune Interactions in Pain and Stress: An Interdisciplinary Approach. Neuroscientist 2021, 27, 113–128. [Google Scholar] [CrossRef]
- Laumet, G.; Ma, J.; Robison, A.J.; Kumari, S.; Heijnen, C.J.; Kavelaars, A. T Cells as an Emerging Target for Chronic Pain Therapy. Front. Mol. Neurosci. 2019, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.N.; Henson, S.M. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 2011, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Ray, D.; Yung, R. Immune senescence, epigenetics and autoimmunity. Clin. Immunol. 2018, 196, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Weyh, C.; Krüger, K.; Strasser, B. Physical Activity and Diet Shape the Immune System during Aging. Nutrients 2020, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflugers Arch. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Raza, M.L. The stress-immune system axis: Exploring the interplay between stress and immunity. Prog. Brain Res. 2025, 291, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S3–S23. [Google Scholar] [CrossRef]
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923. [Google Scholar] [CrossRef]
- DeMaio, A.; Mehrotra, S.; Sambamurti, K.; Husain, S. The role of the adaptive immune system and T cell dysfunction in neurodegenerative diseases. J. Neuroinflammation 2022, 19, 251. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and senescence: Two crucial dysfunctional states of T cells in the tumor microenvironment. Cell Mol. Immunol. 2020, 17, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Okoye, I.; Xu, L.; Motamedi, M.; Parashar, P.; Walker, J.W.; Elahi, S. Galectin-9 expression defines exhausted T cells and impaired cytotoxic NK cells in patients with virus-associated solid tumors. J. Immunother. Cancer 2020, 8, e001849. [Google Scholar] [CrossRef]
- Zhang, J.; Lyu, T.; Cao, Y.; Feng, H. Role of TCF-1 in differentiation, exhaustion, and memory of CD8(+) T cells: A review. FASEB J. 2021, 35, e21549. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Tabana, Y.; Moon, T.C.; Siraki, A.; Elahi, S.; Barakat, K. Reversing T-cell exhaustion in immunotherapy: A review on current approaches and limitations. Expert. Opin. Ther. Targets 2021, 25, 347–363. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Adams, P.D. Healing and hurting: Molecular mechanisms, functions, and pathologies of cellular senescence. Mol. Cell 2009, 36, 2–14. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Jin, P.; Duan, X.; Li, L.; Zhou, P.; Zou, C.G.; Xie, K. Cellular senescence in cancer: Molecular mechanisms and therapeutic targets. MedComm 2024, 5, e542. [Google Scholar] [CrossRef]
- Yang, H.; Wang, H.; Ren, J.; Chen, Q.; Chen, Z.J. cGAS is essential for cellular senescence. Proc. Natl. Acad. Sci. USA 2017, 114, E4612–E4620. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal 2022, 20, 44. [Google Scholar] [CrossRef]
- Carroll, J.E.; Irwin, M.R.; Levine, M.; Seeman, T.E.; Absher, D.; Assimes, T.; Horvath, S. Epigenetic Aging and Immune Senescence in Women With Insomnia Symptoms: Findings From the Women’s Health Initiative Study. Biol. Psychiatry 2017, 81, 136–144. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Almendros, I.; Díaz-García, E.; Toledano, V.; Casitas, R.; Galera, R.; López-Collazo, E.; Farre, R.; Gozal, D.; García-Rio, F. Differential effect of intermittent hypoxia and sleep fragmentation on PD-1/PD-L1 upregulation. Sleep 2020, 43, zsz285. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Nakadai, A.; Qu, T.; Matsushima, H.; Katsumata, M.; Shimizu, T.; Inagaki, H.; Hirata, Y.; Hirata, K.; et al. Healthy lifestyles are associated with higher levels of perforin, granulysin and granzymes A/B-expressing cells in peripheral blood lymphocytes. Prev. Med. 2007, 44, 117–123. [Google Scholar] [CrossRef]
- Aho, V.; Ollila, H.M.; Rantanen, V.; Kronholm, E.; Surakka, I.; van Leeuwen, W.M.; Lehto, M.; Matikainen, S.; Ripatti, S.; Härmä, M.; et al. Partial sleep restriction activates immune response-related gene expression pathways: Experimental and epidemiological studies in humans. PLoS ONE 2013, 8, e77184. [Google Scholar] [CrossRef]
- Prather, A.A.; Marsland, A.L.; Hall, M.; Neumann, S.A.; Muldoon, M.F.; Manuck, S.B. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol. Psychol. 2009, 82, 12–17. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Ribeiro, D.; Cho, H.J.; Olmstead, R.; Breen, E.C.; Martinez-Maza, O.; Cole, S. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry 2008, 64, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Cole, S.W.; Seeman, T.E.; Breen, E.C.; Witarama, T.; Arevalo, J.M.G.; Ma, J.; Irwin, M.R. Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav. Immun. 2016, 51, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef]
- Carroll, J.E.; Olmstead, R.; Cole, S.W.; Breen, E.C.; Arevalo, J.M.; Irwin, M.R. Remission of insomnia in older adults treated with cognitive behavioral therapy for insomnia (CBT-I) reduces p16(INK4a) gene expression in peripheral blood: Secondary outcome analysis from a randomized clinical trial. Geroscience 2023, 45, 2325–2335. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Krauss, J.; Blackburn, E.H.; Epel, E.S. Determinants of telomere attrition over 1 year in healthy older women: Stress and health behaviors matter. Mol. Psychiatry 2015, 20, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Prather, A.A.; Puterman, E.; Lin, J.; O’Donovan, A.; Krauss, J.; Tomiyama, A.J.; Epel, E.S.; Blackburn, E.H. Shorter leukocyte telomere length in midlife women with poor sleep quality. J. Aging Res. 2011, 2011, 721390. [Google Scholar] [CrossRef]
- Prather, A.A.; Gurfein, B.; Moran, P.; Daubenmier, J.; Acree, M.; Bacchetti, P.; Sinclair, E.; Lin, J.; Blackburn, E.; Hecht, F.M.; et al. Tired telomeres: Poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun. 2015, 47, 155–162. [Google Scholar] [CrossRef]
- Cribbet, M.R.; Carlisle, M.; Cawthon, R.M.; Uchino, B.N.; Williams, P.G.; Smith, T.W.; Gunn, H.E.; Light, K.C. Cellular aging and restorative processes: Subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep 2014, 37, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Schernhammer, E.; Qi, L.; Gao, X.; De Vivo, I.; Han, J. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS ONE 2011, 6, e23462. [Google Scholar] [CrossRef]
- Jackowska, M.; Hamer, M.; Carvalho, L.A.; Erusalimsky, J.D.; Butcher, L.; Steptoe, A. Short sleep duration is associated with shorter telomere length in healthy men: Findings from the Whitehall II cohort study. PLoS ONE 2012, 7, e47292. [Google Scholar] [CrossRef]
- Grieshober, L.; Wactawski-Wende, J.; Hageman Blair, R.; Mu, L.; Liu, J.; Nie, J.; Carty, C.L.; Hale, L.; Kroenke, C.H.; LaCroix, A.Z.; et al. A Cross-Sectional Analysis of Telomere Length and Sleep in the Women’s Health Initiative. Am. J. Epidemiol. 2019, 188, 1616–1626. [Google Scholar] [CrossRef]
- Révész, D.; Milaneschi, Y.; Terpstra, E.M.; Penninx, B.W. Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology 2016, 67, 153–162. [Google Scholar] [CrossRef]
- Tempaku, P.F.; Mazzotti, D.R.; Hirotsu, C.; Andersen, M.L.; Xavier, G.; Maurya, P.K.; Rizzo, L.B.; Brietzke, E.; Belangero, S.I.; Bittencourt, L.; et al. The effect of the severity of obstructive sleep apnea syndrome on telomere length. Oncotarget 2016, 7, 69216–69224. [Google Scholar] [CrossRef]
- Hu, J.; Lu, J.; Lu, Q.; Weng, W.; Guan, Z.; Wang, Z. Mendelian randomization and colocalization analyses reveal an association between short sleep duration or morning chronotype and altered leukocyte telomere length. Commun. Biol. 2023, 6, 1014. [Google Scholar] [CrossRef] [PubMed]
- James, S.; McLanahan, S.; Brooks-Gunn, J.; Mitchell, C.; Schneper, L.; Wagner, B.; Notterman, D.A. Sleep Duration and Telomere Length in Children. J. Pediatr. 2017, 187, 247–252.e241. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Irwin, M.R.; Seeman, T.E.; Diez-Roux, A.V.; Prather, A.A.; Olmstead, R.; Epel, E.; Lin, J.; Redline, S. Obstructive sleep apnea, nighttime arousals, and leukocyte telomere length: The Multi-Ethnic Study of Atherosclerosis. Sleep 2019, 42, zsz089. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.M.; Baik, I.; Thomas, R.J.; Shin, C. The association between leukocyte telomere lengths and sleep instability based on cardiopulmonary coupling analysis. Sleep Breath. 2015, 19, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Puterman, E.; Epel, E.S.; Lin, J.; Blackburn, E.H.; Gross, J.J.; Whooley, M.A.; Cohen, B.E. Multisystem resiliency moderates the major depression-telomere length association: Findings from the Heart and Soul Study. Brain Behav. Immun. 2013, 33, 65–73. [Google Scholar] [CrossRef]
- Wynchank, D.; Bijlenga, D.; Penninx, B.W.; Lamers, F.; Beekman, A.T.; Kooij, J.J.S.; Verhoeven, J.E. Delayed sleep-onset and biological age: Late sleep-onset is associated with shorter telomere length. Sleep 2019, 42, zsz139. [Google Scholar] [CrossRef]
- Zgheib, N.K.; Sleiman, F.; Nasreddine, L.; Nasrallah, M.; Nakhoul, N.; Isma’eel, H.; Tamim, H. Short Telomere Length is Associated with Aging, Central Obesity, Poor Sleep and Hypertension in Lebanese Individuals. Aging Dis. 2018, 9, 77–89. [Google Scholar] [CrossRef]
- Tempaku, P.; Hirotsu, C.; Mazzotti, D.; Xavier, G.; Maurya, P.; Brietzke, E.; Belangero, S.; Poyares, D.; Bittencourt, L.; Tufik, S. Long Sleep Duration, Insomnia, and Insomnia With Short Objective Sleep Duration Are Independently Associated With Short Telomere Length. J. Clin. Sleep. Med. 2018, 14, 2037–2045. [Google Scholar] [CrossRef]
- Garland, S.N.; Palmer, C.; Donelson, M.; Gehrman, P.; Johnson, F.B.; Mao, J.J. A nested case-controlled comparison of telomere length and psychological functioning in breast cancer survivors with and without insomnia symptoms. Rejuvenation Res. 2014, 17, 453–457. [Google Scholar] [CrossRef]
- Carroll, J.E.; Esquivel, S.; Goldberg, A.; Seeman, T.E.; Effros, R.B.; Dock, J.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Insomnia and Telomere Length in Older Adults. Sleep 2016, 39, 559–564. [Google Scholar] [CrossRef]
- Barragán, R.; Ortega-Azorín, C.; Sorlí, J.V.; Asensio, E.M.; Coltell, O.; St-Onge, M.P.; Portolés, O.; Corella, D. Effect of Physical Activity, Smoking, and Sleep on Telomere Length: A Systematic Review of Observational and Intervention Studies. J. Clin. Med. 2021, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Freier, E.; Weber, C.S.; Nowottne, U.; Horn, C.; Bartels, K.; Meyer, S.; Hildebrandt, Y.; Luetkens, T.; Cao, Y.; Pabst, C.; et al. Decrease of CD4(+)FOXP3(+) T regulatory cells in the peripheral blood of human subjects undergoing a mental stressor. Psychoneuroendocrinology 2010, 35, 663–673. [Google Scholar] [CrossRef]
- Bosch, J.A.; Fischer, J.E.; Fischer, J.C. Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain Behav. Immun. 2009, 23, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, Y.; Deng, X.; Du, J. Causal Relationship Between Post-Traumatic Stress Disorder and Immune Cell Traits: A Mendelian Randomization Study. Brain Behav. 2024, 14, e70073. [Google Scholar] [CrossRef]
- Maydych, V.; Claus, M.; Dychus, N.; Ebel, M.; Damaschke, J.; Diestel, S.; Wolf, O.T.; Kleinsorge, T.; Watzl, C. Impact of chronic and acute academic stress on lymphocyte subsets and monocyte function. PLoS ONE 2017, 12, e0188108. [Google Scholar] [CrossRef] [PubMed]
- Klopack, E.T.; Crimmins, E.M.; Cole, S.W.; Seeman, T.E.; Carroll, J.E. Social stressors associated with age-related T lymphocyte percentages in older US adults: Evidence from the US Health and Retirement Study. Proc. Natl. Acad. Sci. USA 2022, 119, e2202780119. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Upton, J.; Phillips, A.C.; Hampson, P.; Lord, J.M. Depressive symptoms post hip fracture in older adults are associated with phenotypic and functional alterations in T cells. Immun. Ageing 2014, 11, 25. [Google Scholar] [CrossRef]
- Reed, R.G.; Presnell, S.R.; Al-Attar, A.; Lutz, C.T.; Segerstrom, S.C. Perceived stress, cytomegalovirus titers, and late-differentiated T and NK cells: Between-, within-person associations in a longitudinal study of older adults. Brain Behav. Immun. 2019, 80, 266–274. [Google Scholar] [CrossRef]
- Amati, M.; Tomasetti, M.; Ciuccarelli, M.; Mariotti, L.; Tarquini, L.M.; Bracci, M.; Baldassari, M.; Balducci, C.; Alleva, R.; Borghi, B.; et al. Relationship of job satisfaction, psychological distress and stress-related biological parameters among healthy nurses: A longitudinal study. J. Occup. Health 2010, 52, 31–38. [Google Scholar] [CrossRef]
- Aquino-Acevedo, A.N.; Knochenhauer, H.; Castillo-Ocampo, Y.; Ortiz-León, M.; Rivera-López, Y.A.; Morales-López, C.; Cruz-Robles, M.E.; Hernández-Cordero, E.R.; Russell, S.; Whitaker, R.; et al. Stress hormones are associated with inflammatory cytokines and attenuation of T-cell function in the ascites from patients with high grade serous ovarian cancer. Brain Behav. Immun. Health 2022, 26, 100558. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Z.; Young, M.R.I. Reduced Expression of Immune Mediators by T-Cell Subpopulations of Combat-Exposed Veterans With Post-Traumatic Stress Disorder. Front. Psychiatry 2019, 10, 693. [Google Scholar] [CrossRef]
- Damjanovic, A.K.; Yang, Y.; Glaser, R.; Kiecolt-Glaser, J.K.; Nguyen, H.; Laskowski, B.; Zou, Y.; Beversdorf, D.Q.; Weng, N.P. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J. Immunol. 2007, 179, 4249–4254. [Google Scholar] [CrossRef]
- von Känel, R.; Hepp, U.; Kraemer, B.; Traber, R.; Keel, M.; Mica, L.; Schnyder, U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007, 41, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Atanackovic, D.; Schnee, B.; Schuch, G.; Faltz, C.; Schulze, J.; Weber, C.S.; Schafhausen, P.; Bartels, K.; Bokemeyer, C.; Brunner-Weinzierl, M.C.; et al. Acute psychological stress alerts the adaptive immune response: Stress-induced mobilization of effector T cells. J. Neuroimmunol. 2006, 176, 141–152. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Chan, J.M.; Epel, E.; Kemp, C.; Weidner, G.; Marlin, R.; Frenda, S.J.; Magbanua, M.J.M.; Daubenmier, J.; et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013, 14, 1112–1120. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Puterman, E.; Lin, J.; Blackburn, E.; O’Donovan, A.; Adler, N.; Epel, E. The power of exercise: Buffering the effect of chronic stress on telomere length. PLoS ONE 2010, 5, e10837. [Google Scholar] [CrossRef] [PubMed]
- Parks, C.G.; Miller, D.B.; McCanlies, E.C.; Cawthon, R.M.; Andrew, M.E.; DeRoo, L.A.; Sandler, D.P. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Schaakxs, R.; Wielaard, I.; Verhoeven, J.E.; Beekman, A.T.; Penninx, B.W.; Comijs, H.C. Early and recent psychosocial stress and telomere length in older adults. Int. Psychogeriatr. 2016, 28, 405–413. [Google Scholar] [CrossRef]
- Rentscher, K.E.; Carroll, J.E.; Repetti, R.L.; Cole, S.W.; Reynolds, B.M.; Robles, T.F. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16(INK4a). Psychoneuroendocrinology 2019, 102, 139–148. [Google Scholar] [CrossRef]
- Martino, P.; Perez-Alarcón, M.; Deconinck, L.; De Raedt, R.; Vanderhasselt, M.A.; Kozusznik, M.W.; Kooy, F.; Hidalgo, V.; Venero, C.; Salvador, A.; et al. Stress and telomere length in leukocytes: Investigating the role of GABRA6 gene polymorphism and cortisol. Psychoneuroendocrinology 2025, 173, 107358. [Google Scholar] [CrossRef]
- Englund, D.A.; Sakamoto, A.E.; Fritsche, C.M.; Heeren, A.A.; Zhang, X.; Kotajarvi, B.R.; Lecy, D.R.; Yousefzadeh, M.J.; Schafer, M.J.; White, T.A.; et al. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 2021, 20, e13415. [Google Scholar] [CrossRef]
- Cao Dinh, H.; Bautmans, I.; Beyer, I.; Onyema, O.O.; Liberman, K.; De Dobbeleer, L.; Renmans, W.; Vander Meeren, S.; Jochmans, K.; Delaere, A.; et al. Six weeks of strength endurance training decreases circulating senescence-prone T-lymphocytes in cytomegalovirus seropositive but not seronegative older women. Immun. Ageing 2019, 16, 17. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Rama, L.; Chupel, M.U.; Rosado, F.; Dos Santos, J.V.; Simpson, R.; Martinho, A.; Paiva, A.; Teixeira, A.M. Effects of lifelong training on senescence and mobilization of T lymphocytes in response to acute exercise. Exerc. Immunol. Rev. 2018, 24, 72–84. [Google Scholar]
- Spielmann, G.; McFarlin, B.K.; O’Connor, D.P.; Smith, P.J.; Pircher, H.; Simpson, R.J. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav. Immun. 2011, 25, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Du, L.J.; Song, Y.H.; Tang, L.X. Effect of acute exercise on gene expression in peripheral blood mononuclear cells of puberty children. Sci. Rep. 2024, 14, 27977. [Google Scholar] [CrossRef]
- Despeghel, M.; Reichel, T.; Zander, J.; Krüger, K.; Weyh, C. Effects of a 6 Week Low-Dose Combined Resistance and Endurance Training on T Cells and Systemic Inflammation in the Elderly. Cells 2021, 10, 843. [Google Scholar] [CrossRef]
- Duggal, N.A.; Pollock, R.D.; Lazarus, N.R.; Harridge, S.; Lord, J.M. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell 2018, 17, e12750. [Google Scholar] [CrossRef]
- Minuzzi, L.G.; Rama, L.; Bishop, N.C.; Rosado, F.; Martinho, A.; Paiva, A.; Teixeira, A.M. Lifelong training improves anti-inflammatory environment and maintains the number of regulatory T cells in masters athletes. Eur. J. Appl. Physiol. 2017, 117, 1131–1140. [Google Scholar] [CrossRef]
- Simpson, R.J.; Cosgrove, C.; Ingram, L.A.; Florida-James, G.D.; Whyte, G.P.; Pircher, H.; Guy, K. Senescent T-lymphocytes are mobilised into the peripheral blood compartment in young and older humans after exhaustive exercise. Brain Behav. Immun. 2008, 22, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, S.; Kohno, H.; Kimura, K.; Komura, T.; Asai, H.; Inai, R.; Oka, K.; Kurokawa, Y.; Shephard, R. Physical activity and immune senescence in men. Med. Sci. Sports Exerc. 1995, 27, 1516–1526. [Google Scholar] [CrossRef]
- Tylutka, A.; Morawin, B.; Gramacki, A.; Zembron-Lacny, A. Lifestyle exercise attenuates immunosenescence; flow cytometry analysis. BMC Geriatr. 2021, 21, 200. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, L.F.; Hunkin, J.L.; Kato, B.S.; Richards, J.B.; Gardner, J.P.; Surdulescu, G.L.; Kimura, M.; Lu, X.; Spector, T.D.; Aviv, A. The association between physical activity in leisure time and leukocyte telomere length. Arch. Intern. Med. 2008, 168, 154–158. [Google Scholar] [CrossRef]
- Dankel, S.J.; Loenneke, J.P.; Loprinzi, P.D. The impact of overweight/obesity duration and physical activity on telomere length: An application of the WATCH paradigm. Obes. Res. Clin. Pract. 2017, 11, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Prescott, J.; Kraft, P.; Han, J.; Giovannucci, E.; Hankinson, S.E.; De Vivo, I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am. J. Epidemiol. 2012, 175, 414–422. [Google Scholar] [CrossRef]
- Fretts, A.M.; Mete, M.; Howard, B.V.; Best, L.G.; Siscovick, D.S.; Eilat-Adar, S.; Zhao, J. Physical activity and telomere length in American Indians: The Strong Heart Study. Eur. J. Epidemiol. 2018, 33, 497–500. [Google Scholar] [CrossRef]
- Kim, J.H.; Ko, J.H.; Lee, D.C.; Lim, I.; Bang, H. Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause 2012, 19, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Latifovic, L.; Peacock, S.D.; Massey, T.E.; King, W.D. The Influence of Alcohol Consumption, Cigarette Smoking, and Physical Activity on Leukocyte Telomere Length. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 374–380. [Google Scholar] [CrossRef]
- Saßenroth, D.; Meyer, A.; Salewsky, B.; Kroh, M.; Norman, K.; Steinhagen-Thiessen, E.; Demuth, I. Sports and Exercise at Different Ages and Leukocyte Telomere Length in Later Life--Data from the Berlin Aging Study II (BASE-II). PLoS ONE 2015, 10, e0142131. [Google Scholar] [CrossRef]
- Shadyab, A.H.; LaMonte, M.J.; Kooperberg, C.; Reiner, A.P.; Carty, C.L.; Manini, T.M.; Hou, L.; Di, C.; Macera, C.A.; Gallo, L.C.; et al. Leisure-time physical activity and leukocyte telomere length among older women. Exp. Gerontol. 2017, 95, 141–147. [Google Scholar] [CrossRef]
- Tucker, L.A. Physical activity and telomere length in U.S. men and women: An NHANES investigation. Prev. Med. 2017, 100, 145–151. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Seals, D.R.; Pierce, G.L. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech. Ageing Dev. 2010, 131, 165–167. [Google Scholar] [CrossRef]
- Cassidy, A.; De Vivo, I.; Liu, Y.; Han, J.; Prescott, J.; Hunter, D.J.; Rimm, E.B. Associations between diet, lifestyle factors, and telomere length in women. Am. J. Clin. Nutr. 2010, 91, 1273–1280. [Google Scholar] [CrossRef]
- von Känel, R.; Bruwer, E.J.; Hamer, M.; de Ridder, J.H.; Malan, L. Association between objectively measured physical activity, chronic stress and leukocyte telomere length. J. Sports Med. Phys. Fitness 2017, 57, 1349–1358. [Google Scholar] [CrossRef]
- Tiainen, A.M.; Männistö, S.; Blomstedt, P.A.; Moltchanova, E.; Perälä, M.M.; Kaartinen, N.E.; Kajantie, E.; Kananen, L.; Hovatta, I.; Eriksson, J.G. Leukocyte telomere length and its relation to food and nutrient intake in an elderly population. Eur. J. Clin. Nutr. 2012, 66, 1290–1294. [Google Scholar] [CrossRef]
- Kado, T.; Nawaz, A.; Takikawa, A.; Usui, I.; Tobe, K. Linkage of CD8(+) T cell exhaustion with high-fat diet-induced tumourigenesis. Sci. Rep. 2019, 9, 12284. [Google Scholar] [CrossRef]
- Messaoudi, I.; Warner, J.; Fischer, M.; Park, B.; Hill, B.; Mattison, J.; Lane, M.A.; Roth, G.S.; Ingram, D.K.; Picker, L.J.; et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc. Natl. Acad. Sci. USA 2006, 103, 19448–19453. [Google Scholar] [CrossRef] [PubMed]
- Mascarucci, P.; Taub, D.; Saccani, S.; Paloma, M.A.; Dawson, H.; Roth, G.S.; Lane, M.A.; Ingram, D.K. Cytokine responses in young and old rhesus monkeys: Effect of caloric restriction. J. Interferon Cytokine Res. 2002, 22, 565–571. [Google Scholar] [CrossRef]
- Gürel, S.; Pak, E.N.; Tek, N.A. Aging Processes Are Affected by Energy Balance: Focused on the Effects of Nutrition and Physical Activity on Telomere Length. Curr. Nutr. Rep. 2024, 13, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Jun, N.R.; Yoon, D.; Shin, C.; Baik, I. Association between dietary patterns in the remote past and telomere length. Eur. J. Clin. Nutr. 2015, 69, 1048–1052. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, L.; Cui, X.; Feng, L.; Zhao, X.; He, S.; Ping, F.; Li, W.; Li, Y. Influence of diet on leukocyte telomere length, markers of inflammation and oxidative stress in individuals with varied glucose tolerance: A Chinese population study. Nutr. J. 2016, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Crous-Bou, M.; Giovannucci, E.; De Vivo, I. Coffee Consumption Is Positively Associated with Longer Leukocyte Telomere Length in the Nurses’ Health Study. J. Nutr. 2016, 146, 1373–1378. [Google Scholar] [CrossRef]
- Chang, X.; Dorajoo, R.; Sun, Y.; Wang, L.; Ong, C.N.; Liu, J.; Khor, C.C.; Yuan, J.M.; Koh, W.P.; Friedlander, Y.; et al. Effect of plasma polyunsaturated fatty acid levels on leukocyte telomere lengths in the Singaporean Chinese population. Nutr. J. 2020, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed]
- Mathot, E.; Liberman, K.; Cao Dinh, H.; Njemini, R.; Bautmans, I. Systematic review on the effects of physical exercise on cellular immunosenescence-related markers—An update. Exp. Gerontol. 2021, 149, 111318. [Google Scholar] [CrossRef]
- Yi, H.S.; Kim, S.Y.; Kim, J.T.; Lee, Y.S.; Moon, J.S.; Kim, M.; Kang, Y.E.; Joung, K.H.; Lee, J.H.; Kim, H.J.; et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019, 10, 249. [Google Scholar] [CrossRef]
- Valentine, Y.; Nikolajczyk, B.S. T cells in obesity-associated inflammation: The devil is in the details. Immunol. Rev. 2024, 324, 25–41. [Google Scholar] [CrossRef]
- Shirakawa, K.; Sano, M. T Cell Immunosenescence in Aging, Obesity, and Cardiovascular Disease. Cells 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef]
- Van Campenhout, J.; Buntinx, Y.; Xiong, H.Y.; Wyns, A.; Polli, A.; Nijs, J.; Aerts, J.L.; Laeremans, T.; Hendrix, J. Unravelling the Connection Between Energy Metabolism and Immune Senescence/Exhaustion in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomolecules 2025, 15, 357. [Google Scholar] [CrossRef]
- Masson, J.D.; Badran, G.; Gherardi, R.K.; Authier, F.J.; Crépeaux, G. Widespread Myalgia and Chronic Fatigue: Phagocytes from Macrophagic Myofasciitis Patients Exposed to Aluminum Oxyhydroxide-Adjuvanted Vaccine Exhibit Specific Inflammatory, Autophagic, and Mitochondrial Responses. Toxics 2024, 12, 491. [Google Scholar] [CrossRef]
- Saito, S.; Shahbaz, S.; Osman, M.; Redmond, D.; Bozorgmehr, N.; Rosychuk, R.J.; Lam, G.; Sligl, W.; Cohen Tervaert, J.W.; Elahi, S. Diverse immunological dysregulation, chronic inflammation, and impaired erythropoiesis in long COVID patients with chronic fatigue syndrome. J. Autoimmun. 2024, 147, 103267. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Bote, M.E.; García, J.J.; Hinchado, M.D.; Ortega, E. Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation 2012, 19, 343–351. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Becerril, E.; Perez, M.; Leff, P.; Anton, B.; Estrada, S.; Estrada, I.; Sarasa, M.; Serrano, E.; Pavon, L. Proinflammatory cytokine levels in fibromyalgia patients are independent of body mass index. BMC Res. Notes 2010, 3, 156. [Google Scholar] [CrossRef]
- Bazzichi, L.; Rossi, A.; Massimetti, G.; Giannaccini, G.; Giuliano, T.; De Feo, F.; Ciapparelli, A.; Dell’Osso, L.; Bombardieri, S. Cytokine patterns in fibromyalgia and their correlation with clinical manifestations. Clin. Exp. Rheumatol. 2007, 25, 225–230. [Google Scholar]
- Ranzolin, A.; Duarte, A.L.; Bredemeier, M.; da Costa Neto, C.A.; Ascoli, B.M.; Wollenhaupt-Aguiar, B.; Kapczinski, F.; Xavier, R.M. Evaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia—A controlled cross-sectional study. Cytokine 2016, 84, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Pernambuco, A.P.; Schetino, L.P.; Alvim, C.C.; Murad, C.M.; Viana, R.S.; Carvalho, L.S.; Reis, D. Increased levels of IL-17A in patients with fibromyalgia. Clin. Exp. Rheumatol. 2013, 31 (Suppl. S79), S60–S63. [Google Scholar] [PubMed]
- Ernberg, M.; Christidis, N.; Ghafouri, B.; Bileviciute-Ljungar, I.; Löfgren, M.; Bjersing, J.; Palstam, A.; Larsson, A.; Mannerkorpi, K.; Gerdle, B.; et al. Plasma Cytokine Levels in Fibromyalgia and Their Response to 15 Weeks of Progressive Resistance Exercise or Relaxation Therapy. Mediators Inflamm. 2018, 2018, 3985154. [Google Scholar] [CrossRef] [PubMed]
- Topal, G.; Donmez, A.; Doğan, B.S.; Kucur, M.; Cengiz, D.T.; Berkoz, F.B.; Erdogan, N. Asymmetric dimethylarginine (ADMA) levels are increased in patients with fibromyalgia: Correlation with tumor necrosis factor-α (TNF-α) and 8-iso-prostaglandin F(2α) (8-iso-PGF(2α)). Clin. Biochem. 2011, 44, 364–367. [Google Scholar] [CrossRef]
- Tsilioni, I.; Russell, I.J.; Stewart, J.M.; Gleason, R.M.; Theoharides, T.C. Neuropeptides CRH, SP, HK-1, and Inflammatory Cytokines IL-6 and TNF Are Increased in Serum of Patients with Fibromyalgia Syndrome, Implicating Mast Cells. J. Pharmacol. Exp. Ther. 2016, 356, 664–672. [Google Scholar] [CrossRef]
- Wang, H.; Moser, M.; Schiltenwolf, M.; Buchner, M. Circulating cytokine levels compared to pain in patients with fibromyalgia—A prospective longitudinal study over 6 months. J. Rheumatol. 2008, 35, 1366–1370. [Google Scholar]
- Ghizal, F.; Das, S.K.; Verma, N.; Mahdi, A.A. Evaluating relationship in cytokines level, Fibromyalgia Impact Questionnaire and Body Mass Index in women with Fibromyalgia syndrome. J. Back. Musculoskelet. Rehabil. 2016, 29, 145–149. [Google Scholar] [CrossRef]
- Mendieta, D.; De la Cruz-Aguilera, D.L.; Barrera-Villalpando, M.I.; Becerril-Villanueva, E.; Arreola, R.; Hernández-Ferreira, E.; Pérez-Tapia, S.M.; Pérez-Sánchez, G.; Garcés-Alvarez, M.E.; Aguirre-Cruz, L.; et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J. Neuroimmunol. 2016, 290, 22–25. [Google Scholar] [CrossRef]
- Wallace, D.J.; Linker-Israeli, M.; Hallegua, D.; Silverman, S.; Silver, D.; Weisman, M.H. Cytokines play an aetiopathogenetic role in fibromyalgia: A hypothesis and pilot study. Rheumatology 2001, 40, 743–749. [Google Scholar] [CrossRef]
- Montesino-Goicolea, S.; Sinha, P.; Huo, Z.; Rani, A.; Foster, T.C.; Cruz-Almeida, Y. Enrichment of genomic pathways based on differential DNA methylation profiles associated with chronic musculoskeletal pain in older adults: An exploratory study. Mol. Pain. 2020, 16, 1744806920966902. [Google Scholar] [CrossRef]
- Chae, S.C.; Shim, S.C.; Chung, H.T. Association of TBX21 polymorphisms in a Korean population with rheumatoid arthritis. Exp. Mol. Med. 2009, 41, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Koetz, K.; Bryl, E.; Spickschen, K.; O’Fallon, W.M.; Goronzy, J.J.; Weyand, C.M. T cell homeostasis in patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9203–9208. [Google Scholar] [CrossRef]
- Schönland, S.O.; Lopez, C.; Widmann, T.; Zimmer, J.; Bryl, E.; Goronzy, J.J.; Weyand, C.M. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc. Natl. Acad. Sci. USA 2003, 100, 13471–13476. [Google Scholar] [CrossRef]
- Pedersen-Lane, J.H.; Zurier, R.B.; Lawrence, D.A. Analysis of the thiol status of peripheral blood leukocytes in rheumatoid arthritis patients. J. Leukoc. Biol. 2007, 81, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Fasth, A.E.; Cao, D.; van Vollenhoven, R.; Trollmo, C.; Malmström, V. CD28nullCD4+ T cells—characterization of an effector memory T-cell population in patients with rheumatoid arthritis. Scand. J. Immunol. 2004, 60, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Chalan, P.; van den Berg, A.; Kroesen, B.J.; Brouwer, L.; Boots, A. Rheumatoid Arthritis, Immunosenescence and the Hallmarks of Aging. Curr. Aging Sci. 2015, 8, 131–146. [Google Scholar] [CrossRef]
- Li, S.; Wan, J.; Anderson, W.; Sun, H.; Zhang, H.; Peng, X.; Yu, Z.; Wang, T.; Yan, X.; Smith, W. Downregulation of IL-10 secretion by Treg cells in osteoarthritis is associated with a reduction in Tim-3 expression. Biomed. Pharmacother. 2016, 79, 159–165. [Google Scholar] [CrossRef]
- Montesino-Goicolea, S.; Meng, L.; Rani, A.; Huo, Z.; Foster, T.C.; Fillingim, R.B.; Cruz-Almeida, Y. Enrichment of genomic pathways based on differential DNA methylation profiles associated with knee osteoarthritis pain. Neurobiol. Pain. 2022, 12, 100107. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Meng, J.; Jiang, H.; Zhao, J.; Qian, H.; Chen, T. Epigenetic Modification Mediates the Increase of LAG-3(+) T Cells in Chronic Osteomyelitis. Inflammation 2017, 40, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Surendar, J.; Hackenberg, R.K.; Schmitt-Sánchez, F.; Ossendorff, R.; Welle, K.; Stoffel-Wagner, B.; Sage, P.T.; Burger, C.; Wirtz, D.C.; Strauss, A.C.; et al. Osteomyelitis is associated with increased anti-inflammatory response and immune exhaustion. Front. Immunol. 2024, 15, 1396592. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; McGuire, H.M.; Shinko, D.; Fazekas de St Groth, B.; Russo, M.A.; Bailey, D.; Santarelli, D.M.; Wynne, K.; Austin, P.J. T lymphocyte and monocyte subsets are dysregulated in type 1 diabetes patients with peripheral neuropathic pain. Brain Behav. Immun. Health 2021, 15, 100283. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Guo, X.; Luo, Y.; Wang, G.; Zhong, L.; Zhu, J.; Li, Y.; Zeng, X.; Feng, Z. Dynamic Immune Landscape and VZV-Specific T Cell Responses in Patients With Herpes Zoster and Postherpetic Neuralgia. Front. Immunol. 2022, 13, 887892. [Google Scholar] [CrossRef]
- Li, W.; Pucka, A.Q.; Houran, L.; Huang, X.; Debats, C.; Reyes, B.A.; O’Brien, A.R.; Yu, Q.; Wang, Y. Soluble immune checkpoints are dysregulated in patients with sickle cell disease and correlate with inflammatory mediators, autoantibodies, immune cell profiles, and clinical outcomes. medRxiv 2025. [Google Scholar] [CrossRef]
- Brandow, A.M.; Liem, R.I. Advances in the diagnosis and treatment of sickle cell disease. J. Hematol. Oncol. 2022, 15, 20. [Google Scholar] [CrossRef]
- Wanderley, C.W.S.; Maganin, A.G.M.; Adjafre, B.; Mendes, A.S.; Silva, C.E.A.; Quadros, A.U.; Luiz, J.P.M.; Silva, C.M.S.; Silva, N.R.; Oliveira, F.F.B.; et al. PD-1/PD-L1 Inhibition Enhances Chemotherapy-Induced Neuropathic Pain by Suppressing Neuroimmune Antinociceptive Signaling. Cancer Immunol. Res. 2022, 10, 1299–1308. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, T.; Ma, L.; Zhao, W.; Huang, S.; Wang, K.; Shu, S.; Chen, X. PD-L1/PD-1 pathway: A potential neuroimmune target for pain relief. Cell Biosci. 2024, 14, 51. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Mannarino, M.; Cherif, H.; Ghazizadeh, S.; Martinez, O.W.; Sheng, K.; Cousineau, E.; Lee, S.; Millecamps, M.; Gao, C.; Gilbert, A.; et al. Senolytic treatment for low back pain. Sci. Adv. 2025, 11, eadr1719. [Google Scholar] [CrossRef]
- Techameena, P.; Feng, X.; Zhang, K.; Hadjab, S. The single-cell transcriptomic atlas iPain identifies senescence of nociceptors as a therapeutical target for chronic pain treatment. Nat. Commun. 2024, 15, 8585. [Google Scholar] [CrossRef]
- Du, J.; Cheng, N.; Deng, Y.; Xiang, P.; Liang, J.; Zhang, Z.; Hei, Z.; Li, X. Astrocyte senescence-like response related to peripheral nerve injury-induced neuropathic pain. Cell Mol. Biol. Lett. 2023, 28, 65. [Google Scholar] [CrossRef]
- Borgonetti, V.; Galeotti, N. Rosmarinic Acid Reduces Microglia Senescence: A Novel Therapeutic Approach for the Management of Neuropathic Pain Symptoms. Biomedicines 2022, 10, 1468. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.H.; Kim, C.; Laberge, R.M.; Demaria, M.; Rathod, S.; Vasserot, A.P.; Chung, J.W.; Kim, D.H.; Poon, Y.; David, N.; et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 2017, 23, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.M.; Ghosh, M.; Lee, D.S.; Son, Y.O. Senolytic therapeutics: An emerging treatment modality for osteoarthritis. Ageing Res. Rev. 2024, 96, 102275. [Google Scholar] [CrossRef] [PubMed]
| T Cell Exhaustion | T Cell Senescence | |
|---|---|---|
| Trigger | Persistent antigenic stimulation | Ageing, cellular stress |
| Inhibitory receptors | ⇑ PD-1, CTLA-4, BTLA, Tim-3, TIGIT, Gal-9, LAG-3 | ⇑ CD57, KLRG-1, Tim-3, TIGIT |
| Stimulatory receptors | - | ⇓ CD27, CD28 |
| Effector proteins, chemokines, and signalling molecules | ⇓ granzyme B, perforin | SASP ⇓ granzyme B, perforin ⇑ cGAS, NF-κB, p16INK4a |
| Cytokines | ⇓ TNF-α, IFN-γ, IL-2 ⇑ IL-10 | ⇑ TNF-α, IFN-γ, IL-6, IL-10 |
| Transcription factors | T-bet, Eomes, TCF-1 | - |
| Telomere length | - | ⇓ |
| Reversible? | Yes | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buntinx, Y.; Hendrix, J.; Wyns, A.; Van Campenhout, J.; Xiong, H.-Y.; Laeremans, T.; Cuesta-Sancho, S.; Aerts, J.L.; Nijs, J.; Polli, A. Exploring Chronic Pain, Immune Dysfunction and Lifestyle: A Focus on T Cell Exhaustion and Senescence. Biomolecules 2025, 15, 1601. https://doi.org/10.3390/biom15111601
Buntinx Y, Hendrix J, Wyns A, Van Campenhout J, Xiong H-Y, Laeremans T, Cuesta-Sancho S, Aerts JL, Nijs J, Polli A. Exploring Chronic Pain, Immune Dysfunction and Lifestyle: A Focus on T Cell Exhaustion and Senescence. Biomolecules. 2025; 15(11):1601. https://doi.org/10.3390/biom15111601
Chicago/Turabian StyleBuntinx, Yanthe, Jolien Hendrix, Arne Wyns, Jente Van Campenhout, Huan-Yu Xiong, Thessa Laeremans, Sara Cuesta-Sancho, Joeri L. Aerts, Jo Nijs, and Andrea Polli. 2025. "Exploring Chronic Pain, Immune Dysfunction and Lifestyle: A Focus on T Cell Exhaustion and Senescence" Biomolecules 15, no. 11: 1601. https://doi.org/10.3390/biom15111601
APA StyleBuntinx, Y., Hendrix, J., Wyns, A., Van Campenhout, J., Xiong, H.-Y., Laeremans, T., Cuesta-Sancho, S., Aerts, J. L., Nijs, J., & Polli, A. (2025). Exploring Chronic Pain, Immune Dysfunction and Lifestyle: A Focus on T Cell Exhaustion and Senescence. Biomolecules, 15(11), 1601. https://doi.org/10.3390/biom15111601









