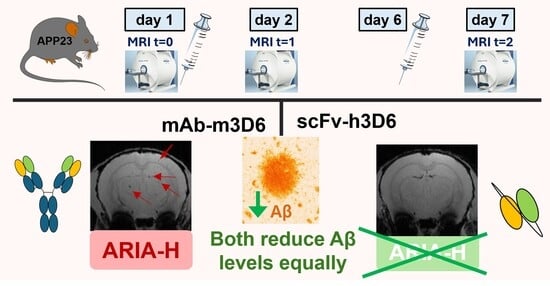
ScFv-h3D6 Prevents Bapineuzumab-Induced Hemorrhagic Events in the APP23 Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. ScFv-h3D6 Production
2.2. mAb-m3D6 Production
2.3. Animals
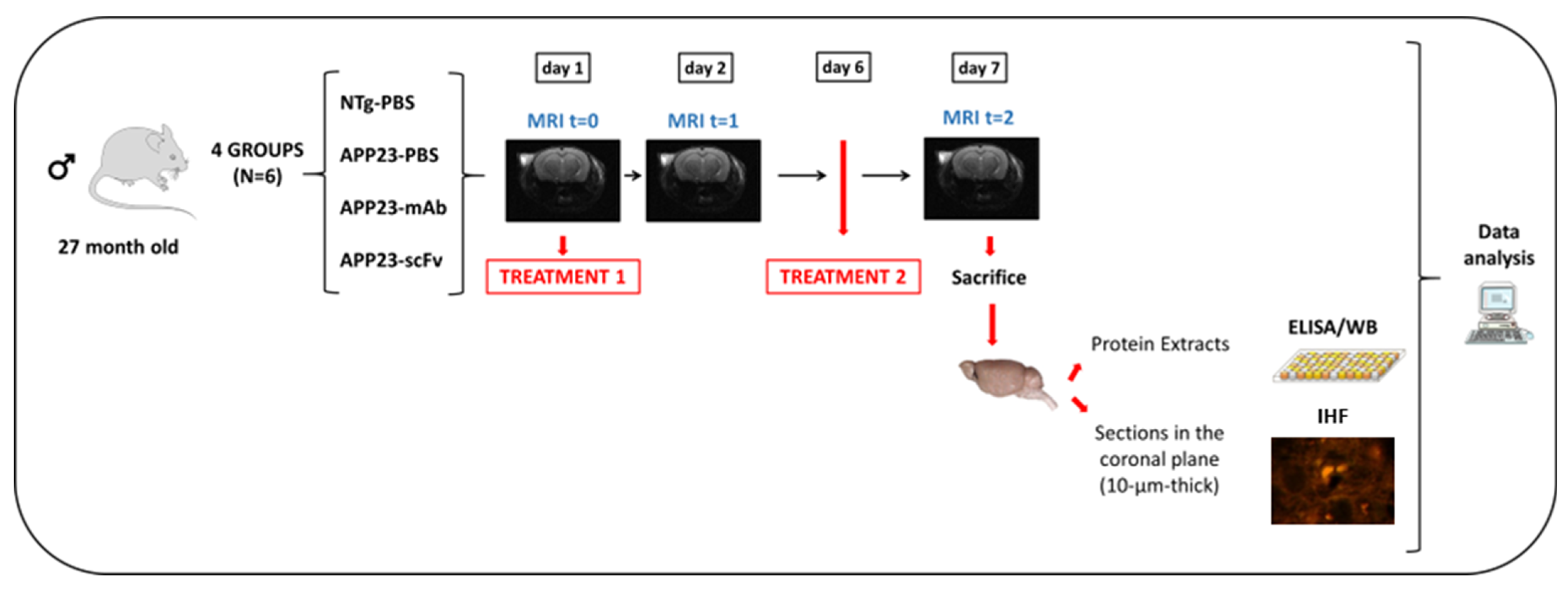
2.4. Experimental Design and Statistics
2.5. Magnetic Resonance Imaging (MRI)
2.5.1. Volume Measurements
2.5.2. Imaging ARIA-H
2.5.3. Imaging ARIA-E
2.5.4. Axonal and Myelin Damage
2.6. Sample Collection and Processing
2.7. Immunofluorescence
2.8. Image Capture and Processing
2.9. Enzyme-Linked Immunosorbent Assays (ELISAs)
3. Results
3.1. Hemorrhagic Events Induced by mAb-m3D6 Are Prevented by scFv-h3D6
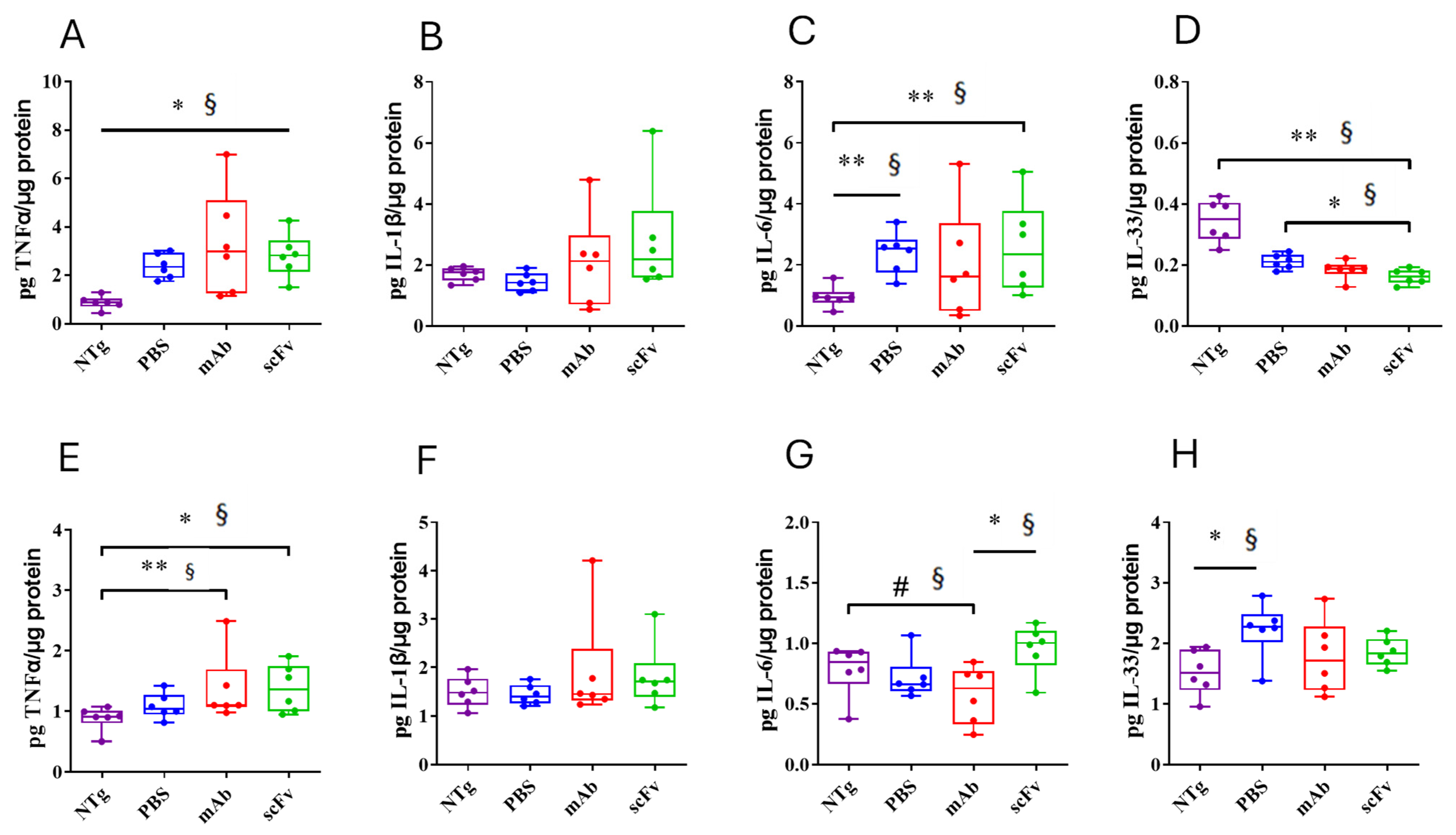
3.2. Both, mAb-m3D6 and scFv-h3D6 Do Not Trigger a General Inflammatory Response
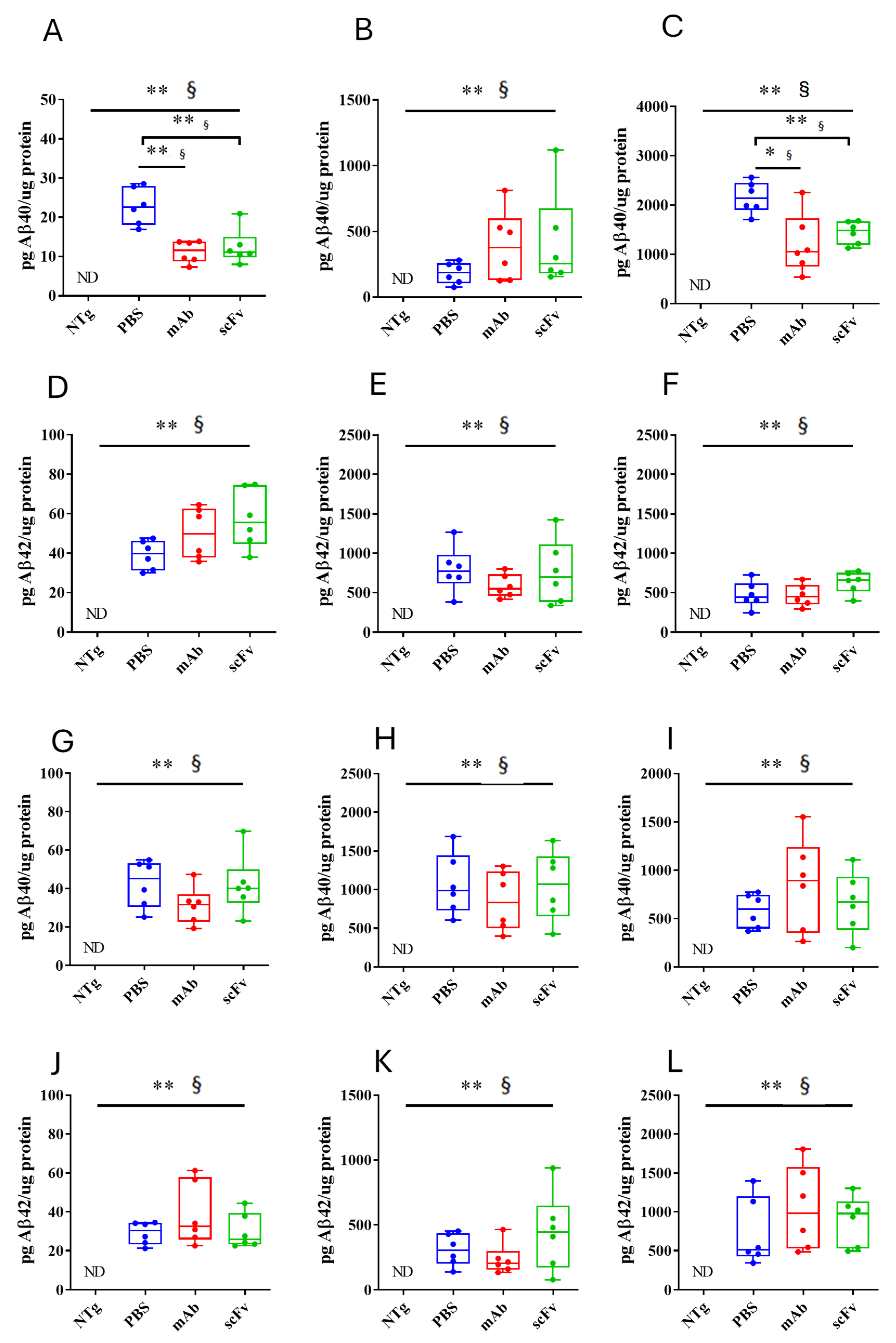
3.3. Both mAb-m3D6 and scFv-h3D6 Reduce Aβ Levels
3.4. Reduction in Aβ Is Not Enough to Recover from Neurodegeneration in Late Stages of AD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | β-amyloid |
| AD | Alzheimer’s disease |
| ADC | Apparent diffusion coefficient |
| ARIA | Amyloid-related imaging abnormalities dichroism |
| ARIA-E | Vasogenic edema ARIA |
| ARIA-H | Hemorrhagic ARIA |
| BBB | Blood–brain barrier |
| CAA | Cerebral amyloid angiopathy |
| CC | Corpus callosum |
| DTI | Diffusion tensor imaging |
| Fc | Fragment crystallizable |
| FA | Fractional anisotropy |
| mAbs | Monoclonal antibodies |
| MRI | Magnetic resonance imaging |
| MTX | Matrix size |
| NTg | Non-transgenic mice |
| RARE | Multislice rapid acquisition with relaxation enhancement sequence |
| ROIs | Regions of interest |
| scFv | Single-chain variable fragment |
| T2w | T2-weighted |
| TEeff | Effective echo time |
| TR | Repetition time |
| VOI | Volume of interest |
| λ|| | Axial diffusivity |
| λ⊥ | Radial diffusivity |
References
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 31 March 2025).
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–665. [Google Scholar] [CrossRef]
- Villegas, S.; Roda, A.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L. Amyloid-beta peptide and tau protein crosstalk in Alzheimer ’s disease. Neural Regen. Res. 2022, 8, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C.; et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef]
- Orgogozo, J.-M.; Gilman, S.; Dartigues, J.-F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Black, R.; Thal, L.J.; Fox, N.C.; Daniels, M.; McLennan, G.; Tompkins, C.; Leibman, C.; Pomfret, M.; Grundman, M. Long-Term Follow-Up of Patients Immunized with AN1792: Reduced Functional Decline in Antibody Responders. Curr. Alzheimer Res. 2009, 6, 144–151. [Google Scholar] [CrossRef]
- Nicoll, J.A.; Wilkinson, D.; Holmes, C.; Steart, P.; Markham, H.; Weller, R.O. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: A case report. Nat. Med. 2003, 9, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-X.; Qiang, W.; Yau, W.-M.; Schwieters, C.D.; Meredith, S.C.; Tycko, R. Molecular structure of β-amyloid fibrils in alzheimer’s disease brain tissue. Cell 2013, 154, 1257. [Google Scholar] [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-β(1–42) by cryo–electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef]
- Black, R.S.; Sperling, R.A.; Safirstein, B.; Motter, R.N.; Pallay, A.; Nichols, A.; Grundman, M. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2010, 24, 198–203. [Google Scholar] [CrossRef]
- Salloway, S.; Sperling, R.; Gilman, S.; Fox, N.C.; Blennow, K.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Doody, R.; van Dyck, C.H.; et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009, 73, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Jack, C.R., Jr.; Black, S.E.; Frosch, M.P.; Greenberg, S.M.; Hyman, B.T.; Scheltens, P.; Carrillo, M.C.; Thies, W.; Bednar, M.M.; et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s Dement. 2011, 7, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Sperling, R.; Fox, N.C.; Blennow, K.; Klunk, W.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; Ferris, S.; et al. Two Phase 3 Trials of Bapineuzumab in Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Jack, C.R., Jr.; Aisen, P.S. Testing the right target and right drug at the right stage. Sci. Transl. Med. 2011, 3, 111. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef]
- Tamura, Y.; Hamajima, K.; Matsui, K.; Yanoma, S.; Narita, M.; Tajima, N.; Xin, K.-Q.; Klinman, D.; Okuda, K. The F(ab′)2 fragment of an Aβ-specific monoclonal antibody reduces Aβ deposits in the brain. Neurobiol. Dis. 2005, 20, 541–549. [Google Scholar] [CrossRef]
- Bacskai, B.J.; Kajdasz, S.T.; McLellan, M.E.; Games, D.; Seubert, P.; Schenk, D.; Hyman, B.T. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. J. Neurosci. 2002, 22, 7873–7878. [Google Scholar] [CrossRef]
- Delnomdedieu, M.; Duvvuri, S.; Li, D.J.; Atassi, N.; Lu, M.; Brashear, H.R.; Liu, E.; Ness, S.; Kupiec, J.W. First-In-Human safety and long-term exposure data for AAB-003 (PF-05236812) and biomarkers after intravenous infusions of escalating doses in patients with mild to moderate Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8, 12. [Google Scholar] [CrossRef]
- Castellano, J.M.; Kim, J.; Stewart, F.R.; Jiang, H.; DeMattos, R.B.; Patterson, B.W.; Fagan, A.M.; Morris, J.C.; Mawuenyega, K.G.; Cruchaga, C.; et al. Human ApoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 2011, 3, 89ra57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manoutcharian, K.; Gevorkian, G. Recombinant Antibody Fragments for Neurological Disorders: An Update. Curr. Neuropharmacol. 2024, 22, 2157–2167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roda, A.R.; Montoliu-Gaya, L.; Serra-Mir, G.; Villegas, S. Both amyloid-β peptide and tau protein are affected by an anti-amyloid-β antibody fragment in elderly 3xtg-ad mice. Int. J. Mol. Sci. 2020, 21, 6630. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.R.; Esquerda-Canals, G.; Martí-Clúa, J.; Villegas, S. Cognitive impairment in the 3xtg-AD mouse model of Alzheimer’s disease is affected by aβ-immunotherapy and cognitive stimulation. Pharmaceutics 2020, 12, 944. [Google Scholar] [CrossRef] [PubMed]
- Güell-Bosch, J.; Lope-Piedrafita, S.; Esquerda-Canals, G.; Montoliu-Gaya, L.; Villegas, S. Progression of Alzheimer’s disease and effect of scFv-h3D6 immunotherapy in the 3xTg-AD mouse model: An in vivo longitudinal study using Magnetic Resonance Imaging and Spectroscopy. NMR Biomed. 2020, 33, e4263. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Roda, A.R.; Martí-Clúa, J.; Montoliu-Gaya, L.; Rivera-Hernández, G.; Villegas, S. Treatment with scFv-h3D6 Prevented Neuronal Loss and Improved Spatial Memory in Young 3xTg-AD Mice by Reducing the Intracellular Amyloid-β Burden. J. Alzheimer’s Dis. 2019, 70, 1069–1091. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Esquerda-Canals, G.; Bronsoms, S.; Villegas, S. Production of an anti-Aβ antibody fragment in Pichia pastoris and in vitro and in vivo validation of its therapeutic effect. PLoS ONE 2017, 12, e0181480. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Marti, J.; Rivera-Hernández, G.; Giménez-Llort, L.; Villegas, S. Loss of deep cerebellar nuclei neurons in the 3xTg-AD mice and protection by an anti-amyloid β antibody fragment. mAbs 2013, 5, 660–664. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Rivera-Hernández, G.; Marín-Argany, M.; Sánchez-Quesada, J.L.; Villegas, S. Early intervention in the 3xTg-AD mice with an amyloid β-antibody fragment ameliorates first hallmarks of alzheimer disease. mAbs 2013, 5, 665–864. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Martí-Clúa, J.; Villegas, S. Pharmacokinetic parameters and mechanism of action of an efficient anti-Aβ single chain antibody fragment. PLoS ONE 2019, 14, e0217793. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Güell-Bosch, J.; Esquerda-Canals, G.; Roda, A.R.; Serra-Mir, G.; Lope-Piedrafita, S.; Sánchez-Quesada, J.L.; Villegas, S. Differential effects of apoE and apoJ mimetic peptides on the action of an anti-Aβ scFv in 3xTg-AD mice. Biochem. Pharmacol. 2018, 155, 380–392. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Mulder, S.D.; Veerhuis, R.; Villegas, S. Effects of an Aβ-antibody fragment on Aβ aggregation and astrocytic uptake are modulated by apolipoprotein E and J mimetic peptides. PLoS ONE 2017, 12, e0188191. [Google Scholar] [CrossRef]
- Montoliu-Gaya, L.; Mulder, S.D.; Herrebout, M.A.; Baayen, J.C.; Villegas, S.; Veerhuis, R. Aβ-oligomer uptake and the resulting inflammatory response in adult human astrocytes are precluded by an anti-Aβ single chain variable fragment in combination with an apoE mimetic peptide. Mol. Cell. Neurosci. 2018, 89, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Güell-Bosch, J. Diagnòstic i Monitorització de la Malaltia d’Alzheimer Mitjançant Tècniques de Ressonància Magnètica In Vivo en el Model 3xtg-ad: Avaluació de L’eficàcia del Tractament Longitudinal amb dos Fragments d’Anticòs Contra el Pèptid aβ. UAB. 2017. Available online: https://www.educacion.gob.es/teseo/mostrarSeleccion.do (accessed on 1 June 2023).
- Jullienne, A.; Quan, R.; Szu, J.I.; Trinh, M.V.; Behringer, E.J.; Obenaus, A. Progressive Vascular Abnormalities in the Aging 3xTg-AD Mouse Model of Alzheimer’s Disease. Biomedicines 2022, 10, 1967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beckmann, N.; Doelemeyer, A.; Zurbruegg, S.; Bigot, K.; Theil, D.; Frieauff, W.; Kolly, C.; Moulin, P.; Neddermann, D.; Kreutzer, R.; et al. Longitudinal noninvasive magnetic resonance imaging of brain microhemorrhages in BACE inhibitor-treated APP transgenic mice. Neurobiol. Aging 2016, 45, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Wendt, H.W. Dealing with a common problem in Social science: A simplified rank-biserial coefficient of correlation based on the U statistic. Eur. J. Soc. Psychol. 1972, 2, 463–465. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, Compact, 5th ed.; Academic Press: Cambridge, MA, USA, 2019; Available online: https://www.elsevier.com/books/paxinos-and-franklins-the-mouse-brain-in-stereotaxic-coordinates-compact/franklin/978-0-12-816159-3 (accessed on 1 June 2023).
- Marazuela, P.; Paez-Montserrat, B.; Bonaterra-Pastra, A.; Solé, M.; Hernández-Guillamon, M. Impact of Cerebral Amyloid Angiopathy in Two Transgenic Mouse Models of Cerebral β-Amyloidosis: A Neuropathological Study. Int. J. Mol. Sci. 2022, 23, 4972. [Google Scholar] [CrossRef]
- Basser, P.J.; Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Ser. B 1996, 111, 209–219. [Google Scholar] [CrossRef]
- Aung, W.Y.; Mar, S.; Benzinger, T.L. Diffusion tensor MRI as a biomarker in axonal and myelin damage. Imaging Med. 2013, 5, 427–440. [Google Scholar] [CrossRef]
- Beckmann, N.; Gérard, C.; Abramowski, D.; Cannet, C.; Staufenbiel, M. Noninvasive magnetic resonance imaging detection of cerebral amyloid angiopathy-related microvascular alterations using superparamagnetic iron oxide particles in APP transgenic mouse models of Alzheimer’s disease: Application to passive Aβ immunotherapy. J. Neurosci. 2011, 31, 1023–1031. [Google Scholar] [CrossRef]
- Czirr, E.; Wyss-Coray, T. The immunology of neurodegeneration. J. Clin. Investig. 2012, 122, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.K.Y.; Hung, K.-W.; Yuen, M.Y.F.; Zhou, X.; Mak, D.S.Y.; Chan, I.C.W.; Cheung, T.H.; Zhang, B.; Fu, W.-Y.; Liew, F.Y.; et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. USA. 2016, 113, E2705-13. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Frisardi, V.; Imbimbo, B.P.; D‘oNofrio, G.; Pietrarossa, G.; Seripa, D.; Pilotto, A.; Solfrizzi, V. Bapineuzumab: Anti-β-amyloid monoclonal antibodies for the treatment of Alzheimers disease. Immunotherapy 2010, 2, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Syvänen, S.; Hultqvist, G.; Gustavsson, T.; Gumucio, A.; Laudon, H.; Söderberg, L.; Ingelsson, M.; Lannfelt, L.; Sehlin, D. Efficient clearance of Aβ protofibrils in AβPP-transgenic mice treated with a brain-penetrating bifunctional antibody. Alzheimers Res. Ther. 2018, 10, 49. [Google Scholar] [CrossRef]
- Scahill, R.I.; Frost, C.; Jenkins, R.; Whitwell, J.L.; Rossor, M.N.; Fox, N.C. A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch. Neurol. 2003, 60, 989–994. [Google Scholar] [CrossRef]
- Esquerda-Canals, G.; Martí-Clúa, J.; Roda, A.R.; Villegas, S. An Intracellular Amyloid-β/AβPP Epitope Correlates with Neurodegeneration in those Neuronal Populations Early Involved in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 59, 1079–1096. [Google Scholar] [CrossRef]
- Hampel, H.; Teipel, S.J.; Alexander, G.E.; Pogarell, O.; Rapoport, S.I.; Möller, H.-J. In vivo imaging of region and cell type specific neocortical neurodegeneration in Alzheimer’s disease: Perspectives of MRI derived corpus callosum measurement for mapping disease progression and effects of therapy. Evidence from studies with MRI, EEG and PET. J. Neural Transm. 2002, 109, 837–855. [Google Scholar] [CrossRef]
- Soldan, A.; Pettigrew, C.; Zhu, Y.; Wang, M.-C.; Moghekar, A.; Gottesman, R.F.; Singh, B.; Martinez, O.; Fletcher, E.; DeCarli, C.; et al. White matter hyperintensities and CSF Alzheimer disease biomarkers in preclinical Alzheimer disease. Neurology 2020, 94, e950–e960. [Google Scholar] [CrossRef]
- Agosta, F.; Scola, E.; Canu, E.; Marcone, A.; Magnani, G.; Sarro, L.; Copetti, M.; Caso, F.; Cerami, C.; Comi, G.; et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb. Cortex 2012, 22, 2705–2714. [Google Scholar] [CrossRef]
- Xie, M.; Tobin, J.E.; Budde, M.D.; Chen, C.-I.; Trinkaus, K.; Cross, A.H.; McDaniel, D.P.; Song, S.-K.; Armstrong, R.C. Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J. Neuropathol. Exp. Neurol. 2010, 69, 704–716. [Google Scholar] [CrossRef]
- Falangola, M.F.; Nie, X.; Ward, R.; McKinnon, E.T.; Dhiman, S.; Nietert, P.J.; Helpern, J.A.; Jensen, J.H. Diffusion MRI Detects Early Brain Microstructure Abnormalities in 2-month-old 3xTg-AD Mice. NMR Biomed. 2020, 33, e4346. [Google Scholar] [CrossRef]
- Gold, B.T.; Powell, D.K.; Andersen, A.H.; Smith, C.D. Alterations in multiple measures of white matter integrity in normal women at high risk for Alzheimer’s disease. Neuroimage 2010, 52, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Chebrolu, H.; Andersen, A.H.; Powell, D.A.; Lovell, M.A.; Xiong, S.; Gold, B.T. White matter diffusion alterations in normal women at risk of Alzheimer’s disease. Neurobiol. Aging 2010, 31, 1122–1131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pizzo, M.E.; Plowey, E.D.; Khoury, N.; Kwan, W.; Abettan, J.; DeVos, S.L.; Discenza, C.B.; Earr, T.; Joy, D.; Lye-Barthel, M.; et al. Transferrin receptor-targeted anti-amyloid antibody enhances brain delivery and mitigates ARIA. Science 2025, 389, eads3204. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Song, W. Improving Alzheimer’s disease immunotherapy. Science. 2025, 389, 571–572. [Google Scholar] [CrossRef]
- Wagner, J.; Ryazanov, S.; Leonov, A.; Levin, J.; Shi, S.; Schmidt, F.; Prix, C.; Pan-Montojo, F.; Bertsch, U.; Mitteregger-Kretzschmar, G.; et al. Anle138b: A novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013, 125, 795–813. [Google Scholar] [CrossRef]
- Blockx, I.; Einstein, S.; Guns, P.-J.; Van Audekerke, J.; Guglielmetti, C.; Zago, W.; Roose, D.; Verhoye, M.; Van der Linden, A.; Bard, F. Monitoring Blood-Brain Barrier Integrity Following Amyloid-β Immunotherapy Using Gadolinium-Enhanced MRI in a PDAPP Mouse Model. J. Alzheimers Dis. 2016, 54, 723–735. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef]
- Ketter, N.; Brashear, H.R.; Bogert, J.; Di, J.; Miaux, Y.; Gass, A.; Purcell, D.D.; Barkhof, F.; Arrighi, H.M. Central Review of Amyloid-Related Imaging Abnormalities in Two Phase III Clinical Trials of Bapineuzumab in Mild-To-Moderate Alzheimer’s Disease Patients. J. Alzheimers. Dis. 2017, 57, 557–573. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 12, 1437–1443, Erratum in: Drugs 2021, 14, 1701. [Google Scholar] [CrossRef]
- Haeberlein, S.B.; von Hehn, C.; Tian, Y.; Chalkias, S.; Muralidharan, K.K.; Chen, T.; Wu, S.; Skordos, L.; Nisenbaum, L.; Rajagovindan, R.; et al. Emerge and Engage topline results: Phase 3 studies of aducanumab in early Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, e047259. [Google Scholar] [CrossRef]
- George, J. Controversial Alzheimer’s Drug Abandoned. 2024. Available online: https://www.medpagetoday.com/neurology/alzheimersdisease/108514 (accessed on 31 March 2025).
- Chowdhury, S.; Chowdhury, N.S. Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer’s disease: A systematic review. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231209839. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- FDA News. 2024. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-adults-alzheimers-disease (accessed on 31 March 2025).
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Nery, E.S.M.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef]
- Kim, B.-H.; Kim, S.; Nam, Y.; Park, Y.H.; Shin, S.M.; Moon, M. Second-generation anti-amyloid monoclonal antibodies for Alzheimer’s disease: Current landscape and future perspectives. Transl. Neurodegener. 2025, 14, 6. [Google Scholar] [CrossRef]



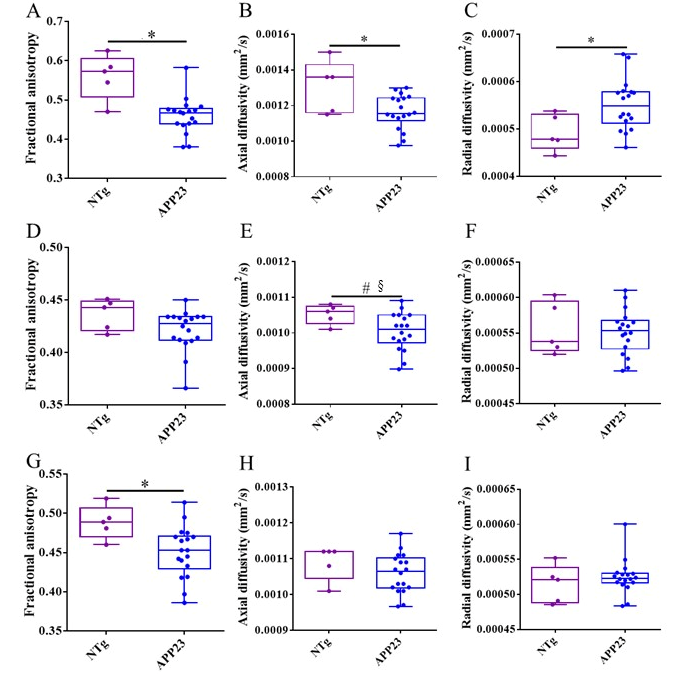
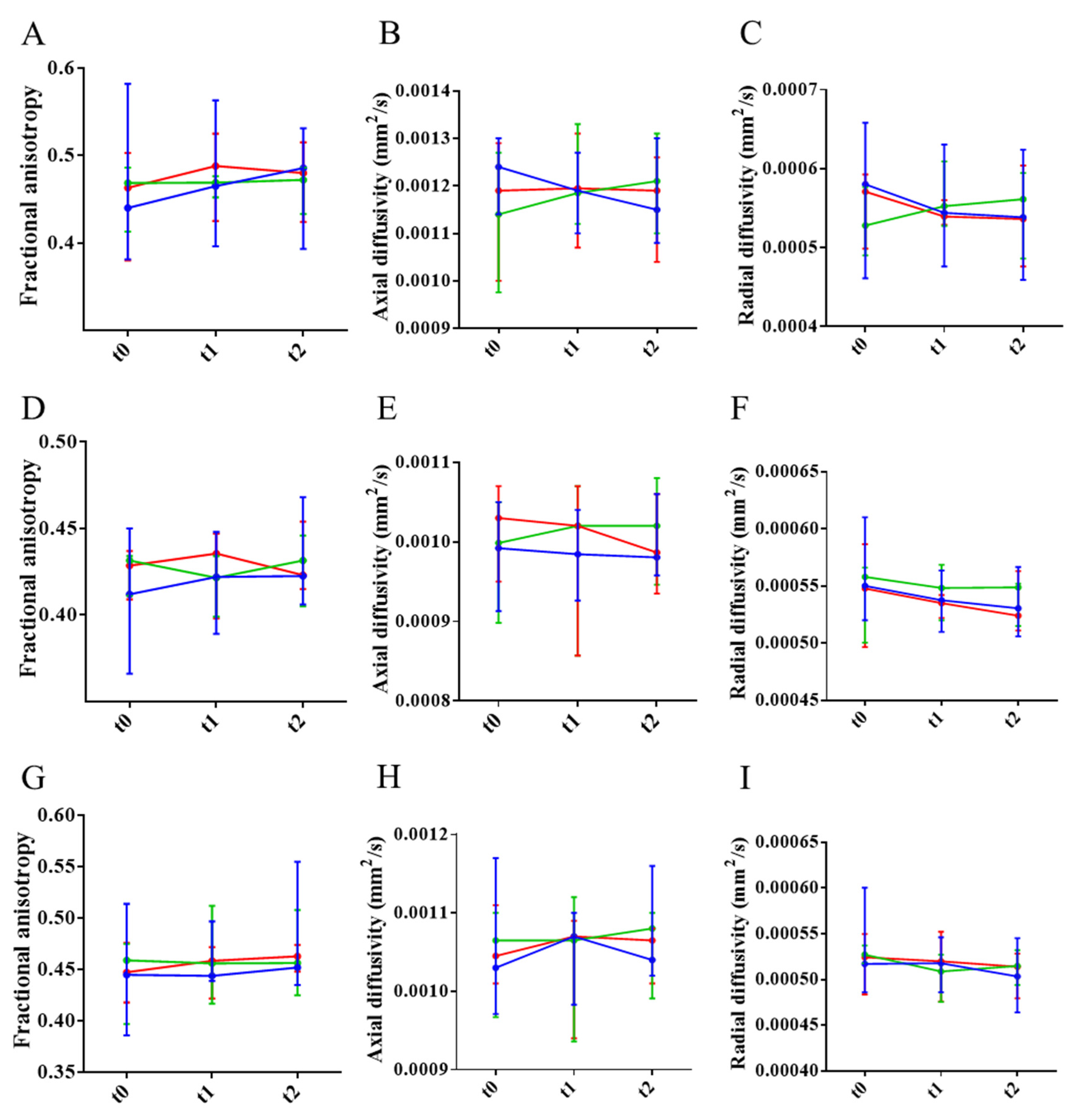
| Ntg (n = 6) | APP23 (n = 18) | APP23-PBS (n = 6) | APP23-mAb-m3D6 (n = 6) | APP23-scFv-h3D6 (n = 6) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Lesions | Number of Lesions | Number of New Lesions | Number of Lesions | Number of New Lesions | Number of Lesions | Number of New Lesions | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Total | t0 | 17.3 | ±2.6 | 24.9# | ±15.9 | 16.3 | ±8.4 | 27.3 | ±13.0 | 31.2 | ±21.0 | ||||||
| t1 | 17.0 | ±9.0 | 0.7 | ±1.2 | 31.8 | ±17.7 | 4.5 *§ | ±2.8 | 32.0 | ±20.9 | 0.8 | ±1.0 | |||||
| t2 | 17.7 | ±8.8 | 0.7 | ±0.8 | 37.2 | ±20.5 | 5.3 #§ | ±0.4 | 33.0 | ±21.8 | 1.0 | ±1.1 | |||||
| Cx | t0 | 3.5 | ±2.8 | 11.5 * | ±10.0 | 6.5 | ± 4.8 | 13.0 | ±12.1 | 15.0 | ±10.9 | ||||||
| t1 | 6.7 | ±4.8 | 0.2 | ±0.4 | 16.2 | ±14.1 | 3.2 *§ | ±2.8 | 15.0 | ±10.9 | 0.0 | ±0.0 | |||||
| t2 | 7.2 | ±4.6 | 0.5 | ±0.6 | 19.0 | ±16.7 | 2.8 | ±3.4 | 15.7 | ±12.0 | 0.7 | ±1.2 | |||||
| Thal | t0 | 10.3 | ±3.2 | 10.2 | ±6.5 | 8.2 | ±4.9 | 9.2 | ±4.5 | 13.2 | ±9.0 | ||||||
| t1 | 8.5 | ±5.4 | 0.3 | ±0.8 | 10.3 | ±5.4 | 1.2 | ±0.10 | 13.8 | ±9.3 | 0.7 | ±0.8 | |||||
| t2 | 8.5 | ±5.4 | 0.0 | ±0.0 | 12.2 | ±7.2 | 1.8 # | ±2.5 | 13.8 | ±9.3 | 0.0 | ±0.0 | |||||
| Hc | t0 | 1.0 | ±1.1 | 2.2 | ±2.0 | 1.7 | ±1.8 | 3.2 | ±2.7 | 1.7 | ±1.4 | ||||||
| t1 | 1.7 | ±1.8 | 0.0 | ±0.0 | 3.3 | ±2.8 | 0.2 | ±0.4 | 1.7 | ±1.4 | 0.0 | ±0.0 | |||||
| t2 | 1.7 | ±1.8 | 0.0 | ±0.0 | 3.7 | ±3.0 | 0.3 | ±0.5 | 2.0 | ±0.9 | 0.3 | ±0.5 | |||||
| Oth | t0 | 2.5 | ±3.1 | 1.1 | ±1.9 | 0.0 | ±0.0 | 2.0 | ±3.0 | 1.3 | ±1.0 | ||||||
| t1 | 0.2 | ±0.4 | 0.2 | ±0.4 | 2.0 | ±3.0 | 0.0 | ±0.0 | 1.5 *§ | ±1.0 | 0.2 | ±0.4 | |||||
| t2 | 0.3 | ±0.8 | 0.2 | ±0.4 | 2.3 | ± 3.3 | 0.3 | ±0.5 | 1.5 | ±1.0 | 0.0 | ±0.0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lope-Piedrafita, S.; Serra-Mir, G.; Melón, P.; Bonaterra, A.; Hernández-Guillamon, M.; Villegas, S. ScFv-h3D6 Prevents Bapineuzumab-Induced Hemorrhagic Events in the APP23 Mouse Model of Alzheimer’s Disease. Biomolecules 2025, 15, 1602. https://doi.org/10.3390/biom15111602
Lope-Piedrafita S, Serra-Mir G, Melón P, Bonaterra A, Hernández-Guillamon M, Villegas S. ScFv-h3D6 Prevents Bapineuzumab-Induced Hemorrhagic Events in the APP23 Mouse Model of Alzheimer’s Disease. Biomolecules. 2025; 15(11):1602. https://doi.org/10.3390/biom15111602
Chicago/Turabian StyleLope-Piedrafita, Silvia, Gabriel Serra-Mir, Paula Melón, Anna Bonaterra, Mar Hernández-Guillamon, and Sandra Villegas. 2025. "ScFv-h3D6 Prevents Bapineuzumab-Induced Hemorrhagic Events in the APP23 Mouse Model of Alzheimer’s Disease" Biomolecules 15, no. 11: 1602. https://doi.org/10.3390/biom15111602
APA StyleLope-Piedrafita, S., Serra-Mir, G., Melón, P., Bonaterra, A., Hernández-Guillamon, M., & Villegas, S. (2025). ScFv-h3D6 Prevents Bapineuzumab-Induced Hemorrhagic Events in the APP23 Mouse Model of Alzheimer’s Disease. Biomolecules, 15(11), 1602. https://doi.org/10.3390/biom15111602