The Type I Interferon Axis in Systemic Autoimmune Diseases: From Molecular Pathways to Targeted Therapy
Abstract
1. Introduction
2. Type I Interferon Biology and Signaling Architecture
2.1. Molecular Diversity and Subtype Characteristics
2.2. Upstream Sensing Pathways: TLRs, RLRs, and cGAS–STING
2.3. Canonical IFNAR–JAK–STAT Signaling Cascade
2.4. Noncanonical Signaling Routes and Crosstalk
2.5. Regulatory Checkpoints of IFN-I Activity
3. Functional Landscape of Type I Interferon Across the Immune Systems
3.1. B Cells
3.2. T Cells
3.3. DCs
3.4. Monocytes, Macrophages, and NK Cells
4. Type I Interferon in Systemic Lupus Erythematosus
4.1. Biomarker Landscape: Blood and Tissue Interferon Signatures
4.2. Genetics, Pathways, and Epigenomic Imprinting of the IFN Program
4.3. Cellular Circuits Executing the IFN Program
4.3.1. DCs
4.3.2. T Cells
4.3.3. B Cells
4.3.4. NK Cells, Neutrophils, Monocytes and Macrophages
5. Type I Interferon in Rheumatoid Arthritis
5.1. Interferon Signatures in Rheumatoid Arthritis: Biomarkers of Therapeutic Response and Subtype Bias
5.2. Determinants of IFN-I Activity in RA—IGS Dynamics, Susceptibility Loci, Epigenetic Remodeling, and Nucleic-Acid Triggers
5.3. Cellular and Tissue Drivers of Type I Interferon Signaling in Rheumatoid Arthritis
6. Type I Interferon in Vasculitis
7. Therapeutic Implications and Stratification Strategies
7.1. Upstream Strategies to Limit Type I Interferon Production: Receptor Blockade, pDC Targeting, and Sensor/Transcriptional Modulation
7.2. Neutralizing IFN-α and Blocking IFNAR
7.3. Downstream Attenuation of IFN-I Signaling
| Class | Agent | Target | Outcome | Safety | Refs. |
|---|---|---|---|---|---|
| Upstream modulators | Hydroxychloroquine (HCQ) | Endosomal pH increase; TLR7/9 inhibition in pDCs; autophagy/HDAC10 linkage in SLE | First-line in SLE; flares/severity reduced; used in RA combinations | Ocular toxicity | [370,371,372,376,417] |
| Litifilimab; anti-CD123 | pDC targeting; IFN production reduced | IFN signature reduced in cutaneous lupus lesions | Infection risk | [224,373,418] | |
| Clobenpropit | CXCR4 agonism; IRF7 phosphorylation reduced; IFN production lowered | IFN and inflammatory cytokines reduced in lupus model | Preclinical | [375] | |
| Enpatoran; Afimetoran | Selective oral TLR7/8 inhibitors | Rapid suppression of IFN-I gene signature with early clinical signals in CLE/SLE | Phase 2 programs ongoing; safety profile still being defined | [377,378,379] | |
| Cenerimod | Selective S1P receptor modulator | IFN-associated proteins and IFN-1/IFN-γ/plasma-cell signatures reduced; larger effect at 4 mg | Dose-related lymphopenia | [380,381] | |
| IFN-I–directed agents | Sifalimumab; Rontalizumab | Anti–IFN-α mAbs | Sifalimumab: disease activity and IFN signature reduced; Rontalizumab: primary endpoint not met overall (signal in low IFN-signature subgroup) | Infections | [383,384,385] |
| Anifrolumab | IFNAR1 blockade; ISGs reduced | Multidomain clinical improvement; regulatory approval in SLE | Herpes zoster and other infections | [19,169,266,388,389,390] | |
| QX006N | IFNAR1 SD3 binding; receptor complex formation prevented | In clinical development | — | [391] | |
| Downstream modulators | Baricitinib | JAK1/2 inhibition | Improves cutaneous/articular disease; efficacy not strictly tied to IFN-signature reduction | Infections | [395,396] |
| Filgotinib; Upadacitinib | JAK1 inhibition | Filgotinib: skin disease improvement; Upadacitinib: phase III ongoing | Infections | [397,398,399] | |
| Ruxolitinib | JAK2 inhibition | ISG expression and JAK–STAT activity reduced; signal in IFN-driven states | Infections | [402] | |
| Deucravacitinib | Selective allosteric TYK2 inhibitor | Higher SRI-4 and secondary responses vs. placebo | Infections | [401] | |
| Belimumab | BAFF neutralization | Established efficacy in SLE; added to active LN increases remission and reduces relapse | infections | [407,408,409] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| ABC | age-associated/atypical B cell |
| ACOD1 | aconitate decarboxylase 1 |
| APC | antigen-presenting cell |
| ATP | adenosine triphosphate |
| BAFF | B-cell activating factor |
| BCR | B cell receptor |
| BRD | bromodomain-containing protein |
| CARDs | caspase activation and recruitment domains |
| CBP | CREB-binding protein |
| CCL | C-C motif chemokine ligand |
| cDNs | cyclic dinucleotides |
| cfDNA | cell-free DNA |
| cGAMP | cyclic GMP–AMP |
| cGAS | cyclic GMP–AMP synthase |
| CLE | cutaneous lupus erythematosus |
| CNS | central nervous system |
| CpG | cytosine–phosphate–guanine |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| DAI | DNA-dependent activators of interferon regulatory factor |
| DAMPs | damage-associated molecular patterns |
| DC | dendritic cells |
| DNA | deoxyribonucleic acid |
| dsDNA | double-stranded DNA |
| ER | endoplasmic reticulum |
| ERGIC | ER–Golgi intermediate compartment |
| GAS | gamma-activated sequence |
| GC | germinal center |
| GCA | giant cell arteritis |
| GCN5 | general control nonderepressible 5 |
| GEF | guanine-nucleotide-exchange factors |
| GTP | guanosine triphosphate |
| HAT | histone acetyltransferase |
| HCQ | hydroxychloroquine |
| HDAC | histone deacetylase |
| iE-DAP | γ-D-glutamyl–meso-diaminopimelic acid |
| IFIT | interferon-induced proteins with tetratricopeptide repeat |
| IFN-I | type I interferon |
| IFNAR | interferon-α/β receptor |
| IGS | interferon gene signature |
| IKK | inhibitor of κB kinase |
| IKKε- | IκB kinase epsilon |
| IL | interleukin |
| IRAK | IL-1 receptor associated kinase |
| IRS | insulin receptor substrate |
| IRF | IFN regulatory factor |
| ISG | interferon-stimulated gene |
| ISRE | interferon-stimulated response element |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LCMV | lymphocytic choriomeningitis virus |
| LDG | low-density granulocyte |
| LGP2 | laboratory of genetics and physiology 2 |
| LN | lupus nephritis |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MAPKAPK | MAPK-activated protein kinase |
| MAVS | mitochondrial antiviral signaling protein |
| MDA5 | melanoma differentiation–associated gene 5 |
| MDP | muramyl dipeptide |
| MNK1 | MAPK-interacting protein kinase 1 |
| MSK1 | mitogen- and stress-activated kinase 1 |
| MHC | major histocompatibility complex |
| mTOR | mechanistic target of rapamycin |
| MyD88 | myeloid differentiation primary response 88 |
| MX1 | myxovirus resistance 1 |
| NEMO | NF-κB essential modulator |
| NETs | neutrophil extracellular traps |
| NK | natural killer |
| NKLAM | natural killer lytic-associated molecule |
| NLRs | NOD–like receptors |
| NOD | nucleotide-binding oligomerization domain |
| OAS1 | 2′–5′-oligoadenylate synthetase 1 |
| pDC | plasmacytoid dendritic cell |
| PAMPs | pathogen-associated molecular patterns |
| PDK | 3-phosphoinositide–dependent protein kinase |
| PIAS1 | protein inhibitor of activated STAT1 |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PIP3 | phosphatidylinositol 3,4,5-trisphosphate |
| PI3K | including phosphoinositide 3-kinase |
| PLZF | promyelocytic leukemia zinc finger |
| PRMT1 | protein arginine methyltransferase 1 |
| PRRs | pattern-recognition receptors |
| P-TEFb | positive transcription elongation factor b |
| PTPN | protein tyrosine phosphatase non-receptor type |
| RA | rheumatoid arthritis |
| RANK | receptor activator of nuclear factor κB |
| RANKL | receptor activator of NF-κB ligand |
| RBP | RNA-binding protein |
| RICK | receptor-interacting serine/threonine kinase |
| RIG-I | retinoic acid–inducible gene I |
| RLRs | RIG-I–like receptors |
| RNA | ribonucleic acid |
| RSAD2 | Radical S-adenosyl methionine domain-containing protein 2 |
| S1P | sphingosine-1-phosphate |
| S1P1 | S1P receptor 1 |
| SHP | Src homology region 2 domain-containing phosphatase |
| SLE | systemic lupus erythematosus |
| SLIM | STAT-interacting LIM protein |
| SMURF | SMAD-specific E3 ubiquitin protein ligase |
| SNP | single-nucleotide polymorphism |
| SOCS | suppressor of cytokine signaling |
| STAT | signal transducer and activator of transcription |
| TAK | Takayasu arteritis |
| TBK1 | TANK-binding kinase 1 |
| Tfh | follicular helper T-cell |
| TGF-β | transforming growth factor-β |
| TIR | Toll/interleukin-1 receptor |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| Tph | T peripheral helper |
| TRAF | TNF receptor–associated factor |
| TRAM | TRIF-related adaptor molecule |
| Treg | regulatory T-cell |
| TRIF | TIR domain-containing adaptor protein-inducing IFN-β |
| TSLP | thymic stromal lymphopoietin |
| TYK2 | tyrosine kinase 2 |
| USP18 | ubiquitin-specific protease 18 |
References
- Piperakis, A.; Galani, I.E.; Andreakos, E. Type III interferons in innate and adaptive immunity in the respiratory tract. Curr. Opin. Immunol. 2024, 87, 102430. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Gresser, I.; Tovey, M.G.; Maury, C.; Chouroulinkov, I. Lethality of interferon preparations for newborn mice. Nature 1975, 258, 76–78. [Google Scholar] [CrossRef]
- Skurkovich, S.V.; Klinova, E.G.; Eremkina, E.I.; Levina, N.V. Immunosuppressive effect of an anti-interferon serum. Nature 1974, 247, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Skurkovich, S.; Skurkovich, B.; Bellanti, J.A. A unifying model of the immunoregulatory role of the interferon system: Can interferon produce disease in humans? Clin. Immunol. Immunopathol. 1987, 43, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Skurkovich, S.; Skurkovich, B. Anticytokine therapy, especially anti-interferon-gamma, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann. N. Y. Acad. Sci. 2005, 1051, 684–700. [Google Scholar] [CrossRef]
- Steinberg, A.D.; Baron, S.; Talal, N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc. Natl. Acad. Sci. USA 1969, 63, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Jahn, B.; Rohwer, P.; Zacher, J.; Winchester, R.J.; Kalden, J.R. Differential expression of Ia antigens by rheumatoid synovial lining cells. J. Clin. Investig. 1987, 80, 595–604. [Google Scholar] [CrossRef]
- van Nieuwland, M.; Esen, I.; Reitsema, R.D.; Abdulahad, W.H.; van Sleen, Y.; Jiemy, W.F.; Sandovici, M.; Brouwer, E.; van Bon, L. Evidence for increased interferon type I activity in CD8+ T cells in giant cell arteritis patients. Front. Immunol. 2023, 14, 1197293. [Google Scholar] [CrossRef]
- Rönnblom, L.E.; Alm, G.V.; Oberg, K.E. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 1990, 227, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Vivaldo, J.F.; Fernandez-Ruiz, R.; Paredes, J.L.; Appenzeller, S.; Niewold, T.B. Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Immunol. 2020, 67, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Ballina-García, F.J.; Suárez, A. Heterogeneity of the Type I Interferon Signature in Rheumatoid Arthritis: A Potential Limitation for Its Use As a Clinical Biomarker. Front. Immunol. 2017, 8, 2007. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Régnier, P.; Maciejewski-Duval, A.; Le Joncour, A.; Darasse-Jèze, G.; Rosenzwajg, M.; Klatzmann, D.; Cacoub, P.; Saadoun, D. Interferon signature in giant cell arteritis aortitis. J. Autoimmun. 2022, 127, 102796. [Google Scholar] [CrossRef]
- Brilland, B.; Despré, M.; Khatri, R.; Quéméneur, T.; Vandenbussche, C.; Merillon, N.; Boizard-Moracchini, A.; Roy, M.; Preisser, L.; Riou, J.; et al. A stronger type I interferon signature distinguishes ANCA-associated vasculitis phenotypes and predicts kidney prognosis. Kidney Int. 2025, in press. [CrossRef]
- Nezos, A.; Gravani, F.; Tassidou, A.; Kapsogeorgou, E.K.; Voulgarelis, M.; Koutsilieris, M.; Crow, M.K.; Mavragani, C.P. Type I and II interferon signatures in Sjogren’s syndrome pathogenesis: Contributions in distinct clinical phenotypes and Sjogren’s related lymphomagenesis. J. Autoimmun. 2015, 63, 47–58. [Google Scholar] [CrossRef]
- Hinchcliff, M.; Khanna, D.; De Lorenzis, E.; Di Donato, S.; Carriero, A.; Ross, R.L.; Huang, S.; Aren, K.A.; Bernstein, E.J.; Carns, M.; et al. Serum type I interferon score as a disease activity biomarker in patients with diffuse cutaneous systemic sclerosis: A retrospective cohort study. Lancet Rheumatol. 2025, 7, e403–e414. [Google Scholar] [CrossRef]
- Qian, J.; Li, R.; Chen, Z.; Cao, Z.; Lu, L.; Fu, Q. Type I interferon score is associated with the severity and poor prognosis in anti-MDA5 antibody-positive dermatomyositis patients. Front. Immunol. 2023, 14, 1151695. [Google Scholar] [CrossRef]
- Kalunian, K.C.; Furie, R.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Tanaka, Y.; Winthrop, K.; Hupka, I.; Zhang, L.J.; Werther, S.; et al. A Randomized, Placebo-Controlled Phase III Extension Trial of the Long-Term Safety and Tolerability of Anifrolumab in Active Systemic Lupus Erythematosus. Arthritis Rheumatol. 2023, 75, 253–265. [Google Scholar] [CrossRef]
- Blockmans, D.; Penn, S.K.; Setty, A.R.; Schmidt, W.A.; Rubbert-Roth, A.; Hauge, E.M.; Keen, H.I.; Ishii, T.; Khalidi, N.; Dejaco, C.; et al. A Phase 3 Trial of Upadacitinib for Giant-Cell Arteritis. N. Engl. J. Med. 2025, 392, 2013–2024. [Google Scholar] [CrossRef]
- Bonelli, M.; Kerschbaumer, A.; Kastrati, K.; Ghoreschi, K.; Gadina, M.; Heinz, L.X.; Smolen, J.S.; Aletaha, D.; O’Shea, J.; Laurence, A. Selectivity, efficacy and safety of JAKinibs: New evidence for a still evolving story. Ann. Rheum. Dis. 2024, 83, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. Overview of the biology of type I interferons. Arthritis Res. Ther. 2010, 12 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. Type I interferons: Diversity of sources, production pathways and effects on immune responses. Curr. Opin. Virol. 2011, 1, 463–475. [Google Scholar] [CrossRef]
- Perez-Shibayama, C.; Islander, U.; Lütge, M.; Cheng, H.W.; Onder, L.; Ring, S.S.; De Martin, A.; Novkovic, M.; Colston, J.; Gil-Cruz, C.; et al. Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8(+) T cells. Sci. Immunol. 2020, 5, eabb7066. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Chen, O.; Ji, R.R. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci. 2020, 43, 822–838. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Marchetti, M.; Monier, M.N.; Fradagrada, A.; Mitchell, K.; Baychelier, F.; Eid, P.; Johannes, L.; Lamaze, C. Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors. Mol. Biol. Cell. 2006, 17, 2896–2909. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, K.I.; Miyauchi, S.; Stoner, S.A.; Fan, J.B.; Zhang, D.E. Negative regulation of type I IFN signaling. J. Leukoc. Biol. 2018, 103, 1099–1116. [Google Scholar] [CrossRef]
- Meyer, O. Interferons and autoimmune disorders. Jt. Bone Spine 2009, 76, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; Schneider, W.M.; Hoffmann, H.H.; Yarden, G.; Busetto, A.G.; Manor, O.; Sharma, N.; Rice, C.M.; Schreiber, G. Multifaceted activities of type I interferon are revealed by a receptor antagonist. Sci. Signal. 2014, 7, ra50. [Google Scholar] [CrossRef]
- Henig, N.; Avidan, N.; Mandel, I.; Staun-Ram, E.; Ginzburg, E.; Paperna, T.; Pinter, R.Y.; Miller, A. Interferon-beta induces distinct gene expression response patterns in human monocytes versus T cells. PLoS ONE 2013, 8, e62366. [Google Scholar] [CrossRef]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Stros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef]
- Kang, D.C.; Gopalkrishnan, R.V.; Wu, Q.; Jankowsky, E.; Pyle, A.M.; Fisher, P.B. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 2002, 99, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Elkon, K.B.; Fitzgerald, K.A. Importance of Nucleic Acid Recognition in Inflammation and Autoimmunity. Annu. Rev. Med. 2016, 67, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Yarilina, A.; Park-Min, K.H.; Antoniv, T.; Hu, X.; Ivashkiv, L.B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 2008, 9, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Matsuo, K.; Suzuki, H.; Suzuki, T.; Sato, K.; Yokochi, T.; Oda, H.; Nakamura, K.; Ida, N.; et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 2002, 416, 744–749. [Google Scholar] [CrossRef]
- Sakaniwa, K.; Fujimura, A.; Shibata, T.; Shigematsu, H.; Ekimoto, T.; Yamamoto, M.; Ikeguchi, M.; Miyake, K.; Ohto, U.; Shimizu, T. TLR3 forms a laterally aligned multimeric complex along double-stranded RNA for efficient signal transduction. Nat. Commun. 2023, 14, 164. [Google Scholar] [CrossRef]
- Schoenemeyer, A.; Barnes, B.J.; Mancl, M.E.; Latz, E.; Goutagny, N.; Pitha, P.M.; Fitzgerald, K.A.; Golenbock, D.T. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 2005, 280, 17005–17012. [Google Scholar] [CrossRef]
- Moynagh, P.N. TLR signalling and activation of IRFs: Revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005, 26, 469–476. [Google Scholar] [CrossRef]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef]
- Motshwene, P.G.; Moncrieffe, M.C.; Grossmann, J.G.; Kao, C.; Ayaluru, M.; Sandercock, A.M.; Robinson, C.V.; Latz, E.; Gay, N.J. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J. Biol. Chem. 2009, 284, 25404–25411. [Google Scholar] [CrossRef] [PubMed]
- Bonham, K.S.; Orzalli, M.H.; Hayashi, K.; Wolf, A.I.; Glanemann, C.; Weninger, W.; Iwasaki, A.; Knipe, D.M.; Kagan, J.C. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 2014, 156, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef]
- Iguchi, Y.; Takahashi, Y.; Li, J.; Araki, K.; Amakusa, Y.; Kawakami, Y.; Kobayashi, K.; Yokoi, S.; Katsuno, M. IκB kinase phosphorylates cytoplasmic TDP-43 and promotes its proteasome degradation. J. Cell Biol. 2024, 223, e202302048. [Google Scholar] [CrossRef]
- McWhirter, S.M.; Fitzgerald, K.A.; Rosains, J.; Rowe, D.C.; Golenbock, D.T.; Maniatis, T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 2004, 101, 233–238. [Google Scholar] [CrossRef]
- Sharma, S.; tenOever, B.R.; Grandvaux, N.; Zhou, G.P.; Lin, R.; Hiscott, J. Triggering the interferon antiviral response through an IKK-related pathway. Science 2003, 300, 1148–1151. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Yoneyama, M.; Fujita, T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 2009, 227, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Deddouche, S.; Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 2013, 38, 855–869. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koo, J.E.; Hong, E.H.; Park, Z.Y.; Lim, K.M.; Bae, O.N.; Lee, J.Y. A Src-family-tyrosine kinase, Lyn, is required for efficient IFN-β expression in pattern recognition receptor, RIG-I, signal pathway by interacting with IPS-1. Cytokine 2015, 72, 63–70. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Sakai, K.; Matsumoto, M.; Seya, T. DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to up-regulate IFN-beta-inducing potential. Eur. J. Immunol. 2010, 40, 940–948. [Google Scholar] [CrossRef]
- Chamaillard, M.; Hashimoto, M.; Horie, Y.; Masumoto, J.; Qiu, S.; Saab, L.; Ogura, Y.; Kawasaki, A.; Fukase, K.; Kusumoto, S.; et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 2003, 4, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kimbrel, E.A.; Kenan, D.J.; McDonnell, D.P. Direct interactions between corepressors and coactivators permit the integration of nuclear receptor-mediated repression and activation. Mol. Endocrinol. 2002, 16, 1482–1491. [Google Scholar] [CrossRef]
- Leung, T.H.; Hoffmann, A.; Baltimore, D. One nucleotide in a kappaB site can determine cofactor specificity for NF-kappaB dimers. Cell 2004, 118, 453–464. [Google Scholar] [CrossRef]
- Gallucci, S.; Meka, S.; Gamero, A.M. Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine 2021, 146, 155633. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Cassol, E. Role of cellular metabolism in regulating type I interferon responses: Implications for tumour immunology and treatment. Cancer Lett. 2017, 409, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Tsai, M.H.; Pai, L.M.; Lee, C.K. Fine-Tuning of Type I Interferon Response by STAT3. Front. Immunol. 2019, 10, 1448. [Google Scholar] [CrossRef]
- Cheon, H.; Stark, G.R. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9373–9378. [Google Scholar] [CrossRef]
- Fritsch, R.; de Krijger, I.; Fritsch, K.; George, R.; Reason, B.; Kumar, M.S.; Diefenbacher, M.; Stamp, G.; Downward, J. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 2013, 153, 1050–1063. [Google Scholar] [CrossRef]
- Tian, L.Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef] [PubMed]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Feller, S.M. Crk family adaptors-signalling complex formation and biological roles. Oncogene 2001, 20, 6348–6371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Batra, S.; Sassano, A.; Majchrzak, B.; Levy, D.E.; Gaestel, M.; Fish, E.N.; Davis, R.J.; Platanias, L.C. Activation of mitogen-activated protein kinase kinase (MKK) 3 and MKK6 by type I interferons. J. Biol. Chem. 2005, 280, 10001–10010. [Google Scholar] [CrossRef]
- Uddin, S.; Majchrzak, B.; Woodson, J.; Arunkumar, P.; Alsayed, Y.; Pine, R.; Young, P.R.; Fish, E.N.; Platanias, L.C. Activation of the p38 mitogen-activated protein kinase by type I interferons. J. Biol. Chem. 1999, 274, 30127–30131. [Google Scholar] [CrossRef]
- Soloaga, A.; Thomson, S.; Wiggin, G.R.; Rampersaud, N.; Dyson, M.H.; Hazzalin, C.A.; Mahadevan, L.C.; Arthur, J.S. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003, 22, 2788–2797. [Google Scholar] [CrossRef]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Lukhele, S.; Boukhaled, G.M.; Brooks, D.G. Type I interferon signaling, regulation and gene stimulation in chronic virus infection. Semin. Immunol. 2019, 43, 101277. [Google Scholar] [CrossRef]
- Ostuni, R.; Piccolo, V.; Barozzi, I.; Polletti, S.; Termanini, A.; Bonifacio, S.; Curina, A.; Prosperini, E.; Ghisletti, S.; Natoli, G. Latent enhancers activated by stimulation in differentiated cells. Cell 2013, 152, 157–171. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, K.; Giannopoulou, E.; Qiao, Y.; Kang, K.; Kim, G.; Park-Min, K.H.; Ivashkiv, L.B. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 2017, 18, 1104–1116. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Fenner, J.E.; Starr, R.; Cornish, A.L.; Zhang, J.G.; Metcalf, D.; Schreiber, R.D.; Sheehan, K.; Hilton, D.J.; Alexander, W.S.; Hertzog, P.J. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat. Immunol. 2006, 7, 33–39. [Google Scholar] [CrossRef]
- Skaug, B.; Chen, Z.J. Emerging role of ISG15 in antiviral immunity. Cell 2010, 143, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Struckhoff, J.J.; Schneider, J.; Martinez-Sobrido, L.; Wolff, T.; García-Sastre, A.; Zhang, D.E.; Lenschow, D.J. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J. Virol. 2009, 83, 1147–1151. [Google Scholar] [CrossRef]
- Mustelin, T.; Vang, T.; Bottini, N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Hong, Y.K.; Wang, X.D.; Ling, M.Y.; Dragoi, A.M.; Chung, A.S.; Campbell, A.G.; Han, Z.Y.; Feng, G.S.; Chin, Y.E. SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei. J. Biol. Chem. 2002, 277, 47572–47580. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Sassano, A.; Deb, D.K.; Verma, A.; Majchrzak, B.; Rahman, A.; Malik, A.B.; Fish, E.N.; Platanias, L.C. Protein kinase C-δ (PKC-δ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J. Biol. Chem. 2002, 277, 14408–14416. [Google Scholar] [CrossRef]
- Tenoever, B.R.; Ng, S.L.; Chua, M.A.; McWhirter, S.M.; García-Sastre, A.; Maniatis, T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science 2007, 315, 1274–1278. [Google Scholar] [CrossRef]
- Mowen, K.A.; Tang, J.; Zhu, W.; Schurter, B.T.; Shuai, K.; Herschman, H.R.; David, M. Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell 2001, 104, 731–741. [Google Scholar] [CrossRef]
- Tang, X.; Gao, J.S.; Guan, Y.J.; McLane, K.E.; Yuan, Z.L.; Ramratnam, B.; Chin, Y.E. Acetylation-dependent signal transduction for type I interferon receptor. Cell 2007, 131, 93–105. [Google Scholar] [CrossRef]
- Paulson, M.; Press, C.; Smith, E.; Tanese, N.; Levy, D.E. IFN-Stimulated transcription through a TBP-free acetyltransferase complex escapes viral shutoff. Nat. Cell Biol. 2002, 4, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Krämer, O.H.; Knauer, S.K.; Greiner, G.; Jandt, E.; Reichardt, S.; Gührs, K.H.; Stauber, R.H.; Böhmer, F.D.; Heinzel, T. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 2009, 23, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, Z.J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 2011, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liu, B.; Li, Z.; Wu, H.; Wang, P.; Zhao, K.; Jiang, G.; Zhang, L.; Gao, C. E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1. Nat. Immunol. 2016, 17, 1342–1351. [Google Scholar] [CrossRef]
- Kumar, K.G.; Tang, W.; Ravindranath, A.K.; Clark, W.A.; Croze, E.; Fuchs, S.Y. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003, 22, 5480–5490. [Google Scholar] [CrossRef]
- Tanaka, T.; Soriano, M.A.; Grusby, M.J. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity 2005, 22, 729–736. [Google Scholar] [CrossRef]
- Yuan, C.; Qi, J.; Zhao, X.; Gao, C. Smurf1 protein negatively regulates interferon-γ signaling through promoting STAT1 protein ubiquitination and degradation. J. Biol. Chem. 2012, 287, 17006–17015. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Kornbluth, J. E3 ubiquitin ligase NKLAM ubiquitinates STAT1 and positively regulates STAT1-mediated transcriptional activity. Cell. Signal. 2016, 28, 1833–1841. [Google Scholar] [CrossRef]
- Ungureanu, D.; Vanhatupa, S.; Grönholm, J.; Palvimo, J.J.; Silvennoinen, O. SUMO-1 conjugation selectively modulates STAT1-mediated gene responses. Blood 2005, 106, 224–226. [Google Scholar] [CrossRef]
- Patel, M.C.; Debrosse, M.; Smith, M.; Dey, A.; Huynh, W.; Sarai, N.; Heightman, T.D.; Tamura, T.; Ozato, K. BRD4 coordinates recruitment of pause release factor P-TEFb and the pausing complex NELF/DSIF to regulate transcription elongation of interferon-stimulated genes. Mol. Cell. Biol. 2013, 33, 2497–2507. [Google Scholar] [CrossRef]
- Chang, H.M.; Paulson, M.; Holko, M.; Rice, C.M.; Williams, B.R.; Marié, I.; Levy, D.E. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 9578–9583. [Google Scholar] [CrossRef]
- Gao, B.; Duan, Z.; Xu, W.; Xiong, S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology 2009, 50, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.C.; Schaefer, U.; Mecklenbrauker, I.; Stienen, A.; Dewell, S.; Chen, M.S.; Rioja, I.; Parravicini, V.; Prinjha, R.K.; Chandwani, R.; et al. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 2012, 209, 661–669. [Google Scholar] [CrossRef]
- Kroetz, D.N.; Allen, R.M.; Schaller, M.A.; Cavallaro, C.; Ito, T.; Kunkel, S.L. Type I Interferon Induced Epigenetic Regulation of Macrophages Suppresses Innate and Adaptive Immunity in Acute Respiratory Viral Infection. PLoS Pathog. 2015, 11, e1005338. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.J.; Thillainadesan, G.; Yousef, A.F.; Ablack, J.N.; Mossman, K.L.; Torchia, J.; Mymryk, J.S. Adenovirus evasion of interferon-mediated innate immunity by direct antagonism of a cellular histone posttranslational modification. Cell Host Microbe 2012, 11, 597–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, D.; Roswit, W.T.; Jin, X.; Patel, A.C.; Patel, D.A.; Agapov, E.; Wang, Z.; Tidwell, R.M.; Atkinson, J.J.; et al. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nat. Immunol. 2015, 16, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y. Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef]
- Kohli, R.M.; Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, K.; Shen, Q.; Han, Y.; Gu, Y.; Li, X.; Zhao, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015, 525, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.S.; Dooley, J.; Linterman, M.A.; Pierson, W.; Ucar, O.; Kyewski, B.; Zuklys, S.; Hollander, G.A.; Matthys, P.; Gray, D.H.; et al. The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-α receptor. Nat. Immunol. 2011, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, J.; Lin, L.; Wang, C.; Liu, X.; Li, D.; Ma, F.; Wang, Z.; Cao, X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J. Immunol. 2010, 185, 6226–6233. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Y.; Han, Y.; Lin, L.; Wu, C.; Xu, S.; Jiang, Z.; Xu, J.; Liu, Q.; Cao, X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014, 344, 310–313. [Google Scholar] [CrossRef]
- Josset, L.; Tchitchek, N.; Gralinski, L.E.; Ferris, M.T.; Eisfeld, A.J.; Green, R.R.; Thomas, M.J.; Tisoncik-Go, J.; Schroth, G.P.; Kawaoka, Y.; et al. Annotation of long non-coding RNAs expressed in collaborative cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol. 2014, 11, 875–890. [Google Scholar] [CrossRef]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.F.; Gopinath, S.; Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, X.; Chen, Y.; Wei, H.; Chen, Q.; Chi, X.; Qi, B.; Zhang, L.; Zhao, Y.; Gao, G.F.; et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe 2014, 16, 616–626. [Google Scholar] [CrossRef]
- Vasconcellos, R.; Braun, D.; Coutinho, A.; Demengeot, J. Type I IFN sets the stringency of B cell repertoire selection in the bone marrow. Int. Immunol. 1999, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Murira, A.; Mindt, B.C.; Germain, A.; Tarrab, E.; Lapierre, P.; Fritz, J.H.; Lamarre, A. Type I Interferon Impairs Specific Antibody Responses Early during Establishment of LCMV Infection. Front. Immunol. 2016, 7, 564. [Google Scholar] [CrossRef]
- Peters, M.; Ambrus, J.L.; Zheleznyak, A.; Walling, D.; Hoofnagle, J.H. Effect of interferon-alpha on immunoglobulin synthesis by human B cells. J. Immunol. 1986, 137, 3153–3157. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, K.A.; Schulze, L.L.; Paap, E.M.; Müller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [CrossRef]
- Davis, A.M.; Hagan, K.A.; Matthews, L.A.; Bajwa, G.; Gill, M.A.; Gale, M., Jr.; Farrar, J.D. Blockade of virus infection by human CD4+ T cells via a cytokine relay network. J. Immunol. 2008, 180, 6923–6932. [Google Scholar] [CrossRef]
- Way, S.S.; Havenar-Daughton, C.; Kolumam, G.A.; Orgun, N.N.; Murali-Krishna, K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J. Immunol. 2007, 178, 4498–4505. [Google Scholar] [CrossRef]
- Schijns, V.E.; Haagmans, B.L.; Wierda, C.M.; Kruithof, B.; Heijnen, I.A.; Alber, G.; Horzinek, M.C. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J. Immunol. 1998, 160, 3958–3964. [Google Scholar] [CrossRef]
- Ziegler, S.F. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr. Opin. Immunol. 2010, 22, 795–799. [Google Scholar] [CrossRef]
- Hegazy, A.N.; Peine, M.; Helmstetter, C.; Panse, I.; Fröhlich, A.; Bergthaler, A.; Flatz, L.; Pinschewer, D.D.; Radbruch, A.; Löhning, M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 2010, 32, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, R.M.; Lawrence, W.D. Interferon-alpha for the hypereosinophilic syndrome. Ann. Intern. Med. 1990, 113, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Schandené, L.; Del Prete, G.F.; Cogan, E.; Stordeur, P.; Crusiaux, A.; Kennes, B.; Romagnani, S.; Goldman, M. Recombinant interferon-alpha selectively inhibits the production of interleukin-5 by human CD4+ T cells. J. Clin. Investig. 1996, 97, 309–315. [Google Scholar] [CrossRef]
- Huber, J.P.; Ramos, H.J.; Gill, M.A.; Farrar, J.D. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J. Immunol. 2010, 185, 813–817. [Google Scholar] [CrossRef]
- Gong, J.; Zhan, H.; Liang, Y.; He, Q.; Cui, D. Role of Th22 Cells in Human Viral Diseases. Front. Med. 2021, 8, 708140. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Moschen, A.R.; Geiger, S.; Krehan, I.; Kaser, A.; Tilg, H. Interferon-alpha controls IL-17 expression in vitro and in vivo. Immunobiology 2008, 213, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Axtell, R.C.; de Jong, B.A.; Boniface, K.; van der Voort, L.F.; Bhat, R.; De Sarno, P.; Naves, R.; Han, M.; Zhong, F.; Castellanos, J.G.; et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010, 16, 406–412. [Google Scholar] [CrossRef]
- Szczuciński, A.; Losy, J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol. Scand. 2007, 115, 137–146. [Google Scholar] [CrossRef]
- Ifergan, I.; Kébir, H.; Bernard, M.; Wosik, K.; Dodelet-Devillers, A.; Cayrol, R.; Arbour, N.; Prat, A. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain 2008, 131, 785–799. [Google Scholar] [CrossRef]
- Tosello Boari, J.; Acosta Rodriguez, E.V. IL-1β/CD14 pathway induces IL-17 production: Dendritic cells activated with IL-1β set Th17 cells on fire by CD14-mediated mechanisms. Immunol. Cell Biol. 2016, 94, 903–904. [Google Scholar] [CrossRef]
- Kurche, J.S.; Haluszczak, C.; McWilliams, J.A.; Sanchez, P.J.; Kedl, R.M. Type I IFN-dependent T cell activation is mediated by IFN-dependent dendritic cell OX40 ligand expression and is independent of T cell IFNR expression. J. Immunol. 2012, 188, 585–593. [Google Scholar] [CrossRef]
- Simmons, D.P.; Wearsch, P.A.; Canaday, D.H.; Meyerson, H.J.; Liu, Y.C.; Wang, Y.; Boom, W.H.; Harding, C.V. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J. Immunol. 2012, 188, 3116–3126. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.; Welch, M.; Schreiber, R.D.; de la Torre, J.C.; Oldstone, M.B. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Oldstone, M.B. Infected CD8α- dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc. Natl. Acad. Sci. USA 2012, 109, 14116–14121. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Mattei, L.M.; Iwasaki, A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 284–289. [Google Scholar] [CrossRef]
- Stifter, S.A.; Bhattacharyya, N.; Pillay, R.; Flórido, M.; Triccas, J.A.; Britton, W.J.; Feng, C.G. Functional Interplay between Type I and II Interferons Is Essential to Limit Influenza A Virus-Induced Tissue Inflammation. PLoS Pathog. 2016, 12, e1005378. [Google Scholar] [CrossRef]
- Eshleman, E.M.; Delgado, C.; Kearney, S.J.; Friedman, R.S.; Lenz, L.L. Down regulation of macrophage IFNGR1 exacerbates systemic L. monocytogenes infection. PLoS Pathog. 2017, 13, e1006388. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.W.; Ewbank, J.; Howes, A.; Moreira-Teixeira, L.; Martirosyan, A.; Ghilardi, N.; Saraiva, M.; O’Garra, A. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J. Immunol. 2014, 193, 3600–3612. [Google Scholar] [CrossRef]
- Novikov, A.; Cardone, M.; Thompson, R.; Shenderov, K.; Kirschman, K.D.; Mayer-Barber, K.D.; Myers, T.G.; Rabin, R.L.; Trinchieri, G.; Sher, A.; et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 2011, 187, 2540–2547. [Google Scholar] [CrossRef]
- Henry, T.; Kirimanjeswara, G.S.; Ruby, T.; Jones, J.W.; Peng, K.; Perret, M.; Ho, L.; Sauer, J.D.; Iwakura, Y.; Metzger, D.W.; et al. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J. Immunol. 2010, 184, 3755–3767. [Google Scholar] [CrossRef]
- Ahlenstiel, G.; Edlich, B.; Hogdal, L.J.; Rotman, Y.; Noureddin, M.; Feld, J.J.; Holz, L.E.; Titerence, R.H.; Liang, T.J.; Rehermann, B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology 2011, 141, 1231–1239.e2. [Google Scholar] [CrossRef]
- Werner, J.M.; Serti, E.; Chepa-Lotrea, X.; Stoltzfus, J.; Ahlenstiel, G.; Noureddin, M.; Feld, J.J.; Liang, T.J.; Rotman, Y.; Rehermann, B. Ribavirin improves the IFN-γ response of natural killer cells to IFN-based therapy of hepatitis C virus infection. Hepatology 2014, 60, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Gil, M.P.; Wang, X.; Louten, J.; Chu, W.M.; Biron, C.A. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 2007, 204, 2383–2396. [Google Scholar] [CrossRef]
- Lee, A.J.; Chen, B.; Chew, M.V.; Barra, N.G.; Shenouda, M.M.; Nham, T.; van Rooijen, N.; Jordana, M.; Mossman, K.L.; Schreiber, R.D.; et al. Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection. J. Exp. Med. 2017, 214, 1153–1167. [Google Scholar] [CrossRef]
- Mack, E.A.; Kallal, L.E.; Demers, D.A.; Biron, C.A. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. mBio 2011, 2, e00169-11. [Google Scholar] [CrossRef] [PubMed]
- Accapezzato, D.; Caccavale, R.; Paroli, M.P.; Gioia, C.; Nguyen, B.L.; Spadea, L.; Paroli, M. Advances in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 6578. [Google Scholar] [CrossRef]
- Gensous, N.; Lazaro, E.; Blanco, P.; Richez, C. Anifrolumab: First biologic approved in the EU not restricted to patients with a high degree of disease activity for the treatment of moderate to severe systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2024, 20, 21–30. [Google Scholar] [CrossRef]
- Crow, M.K. Pathogenesis of systemic lupus erythematosus: Risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tsao, B.P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 2010, 6, 683–692. [Google Scholar] [CrossRef]
- Weckerle, C.E.; Franek, B.S.; Kelly, J.A.; Kumabe, M.; Mikolaitis, R.A.; Green, S.L.; Utset, T.O.; Jolly, M.; James, J.A.; Harley, J.B.; et al. Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 1044–1053. [Google Scholar] [CrossRef]
- Niewold, T.B.; Swedler, W.I. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin. Rheumatol. 2005, 24, 178–181. [Google Scholar] [CrossRef]
- Niewold, T.B.; Hua, J.; Lehman, T.J.; Harley, J.B.; Crow, M.K. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007, 8, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.N.; Franek, B.S.; Kumar, A.A.; Arrington, J.; Mikolaitis, R.A.; Utset, T.O.; Jolly, M.; Crow, M.K.; Skol, A.D.; Niewold, T.B. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, R151. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Lu, R.; Zhao, Y.D.; Fife, D.A.; Robertson, J.M.; Guthridge, J.M.; Niewold, T.B.; Tsokos, G.C.; Keith, M.P.; Harley, J.B.; et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 2016, 75, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K.; Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 2003, 5, 279–287. [Google Scholar] [CrossRef]
- Catalina, M.D.; Bachali, P.; Geraci, N.S.; Grammer, A.C.; Lipsky, P.E. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun. Biol. 2019, 2, 140. [Google Scholar] [CrossRef]
- Whittall Garcia, L.P.; Gladman, D.D.; Urowitz, M.; Bonilla, D.; Schneider, R.; Touma, Z.; Wither, J. Interferon-α as a biomarker to predict renal outcomes in lupus nephritis. Lupus Sci. Med. 2024, 11, e001347. [Google Scholar] [CrossRef]
- Ko, K.; Koldobskaya, Y.; Rosenzweig, E.; Niewold, T.B. Activation of the Interferon Pathway is Dependent Upon Autoantibodies in African-American SLE Patients, but Not in European-American SLE Patients. Front. Immunol. 2013, 4, 309. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Wajda, A.; Stypinska, B.; Walczuk, E.; Rzeszotarska, E.; Walczyk, M.; Haladyj, E.; Romanowska-Prochnicka, K.; Felis-Giemza, A.; Lewandowska, A.; et al. Variety of endosomal TLRs and Interferons (IFN-α, IFN-β, IFN-γ) expression profiles in patients with SLE, SSc and MCTD. Clin. Exp. Immunol. 2021, 204, 49–63. [Google Scholar] [CrossRef]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef]
- Landolt-Marticorena, C.; Bonventi, G.; Lubovich, A.; Ferguson, C.; Unnithan, T.; Su, J.; Gladman, D.D.; Urowitz, M.; Fortin, P.R.; Wither, J. Lack of association between the interferon-alpha signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2009, 68, 1440–1446. [Google Scholar] [CrossRef]
- Petri, M.; Singh, S.; Tesfasyone, H.; Dedrick, R.; Fry, K.; Lal, P.; Williams, G.; Bauer, J.; Gregersen, P.; Behrens, T.; et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus 2009, 18, 980–989. [Google Scholar] [CrossRef]
- Bauer, J.W.; Petri, M.; Batliwalla, F.M.; Koeuth, T.; Wilson, J.; Slattery, C.; Panoskaltsis-Mortari, A.; Gregersen, P.K.; Behrens, T.W.; Baechler, E.C. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: A validation study. Arthritis Rheum. 2009, 60, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, R.; Hong, S.; Cantarel, B.; Baldwin, N.; Baisch, J.; Edens, M.; Cepika, A.M.; Acs, P.; Turner, J.; Anguiano, E.; et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell 2016, 165, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Labonte, A.C.; Kegerreis, B.; Geraci, N.S.; Bachali, P.; Madamanchi, S.; Robl, R.; Catalina, M.D.; Lipsky, P.E.; Grammer, A.C. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS ONE 2018, 13, e0208132. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Sagalovskiy, I.; Guo, Q.; Nezos, A.; Kapsogeorgou, E.K.; Lu, P.; Liang Zhou, J.; Kirou, K.A.; Seshan, S.V.; Moutsopoulos, H.M.; et al. Expression of Long Interspersed Nuclear Element 1 Retroelements and Induction of Type I Interferon in Patients With Systemic Autoimmune Disease. Arthritis Rheumatol. 2016, 68, 2686–2696. [Google Scholar] [CrossRef]
- Nzeusseu Toukap, A.; Galant, C.; Theate, I.; Maudoux, A.L.; Lories, R.J.; Houssiau, F.A.; Lauwerys, B.R. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 1579–1588. [Google Scholar] [CrossRef]
- Arazi, A.; Rao, D.A.; Berthier, C.C.; Davidson, A.; Liu, Y.; Hoover, P.J.; Chicoine, A.; Eisenhaure, T.M.; Jonsson, A.H.; Li, S.; et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019, 20, 902–914. [Google Scholar] [CrossRef]
- Der, E.; Suryawanshi, H.; Morozov, P.; Kustagi, M.; Goilav, B.; Ranabothu, S.; Izmirly, P.; Clancy, R.; Belmont, H.M.; Koenigsberg, M.; et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 2019, 20, 915–927. [Google Scholar] [CrossRef]
- Nian, Z.; Mao, Y.; Xu, Z.; Deng, M.; Xu, Y.; Xu, H.; Chen, R.; Xu, Y.; Huang, N.; Mao, F.; et al. Multi-omics analysis uncovered systemic lupus erythematosus and COVID-19 crosstalk. Mol. Med. 2024, 30, 81. [Google Scholar] [CrossRef]
- Ding, X.; Cai, M.; Wang, S.; Yang, Q.; Zheng, X.; Zuo, X.; Liu, S. Gene-based association analysis identified a novel gene associated with systemic lupus erythematosus. Ann. Hum. Genet. 2021, 85, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.A.; Niewold, T.B. Interferon regulatory factors: Critical mediators of human lupus. Transl. Res. 2015, 165, 283–295. [Google Scholar] [CrossRef]
- Niewold, T.B.; Kelly, J.A.; Flesch, M.H.; Espinoza, L.R.; Harley, J.B.; Crow, M.K. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008, 58, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.B.; Kelly, J.A.; Kariuki, S.N.; Franek, B.S.; Kumar, A.A.; Kaufman, K.M.; Thomas, K.; Walker, D.; Kamp, S.; Frost, J.M.; et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann. Rheum. Dis. 2012, 71, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Cherian, T.S.; Kariuki, S.N.; Franek, B.S.; Buyon, J.P.; Clancy, R.M.; Niewold, T.B. Brief Report: IRF5 systemic lupus erythematosus risk haplotype is associated with asymptomatic serologic autoimmunity and progression to clinical autoimmunity in mothers of children with neonatal lupus. Arthritis Rheum. 2012, 64, 3383–3387. [Google Scholar] [CrossRef]
- Muskardin, T.L.W.; Niewold, T.B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 214–228. [Google Scholar] [CrossRef]
- Kariuki, S.N.; Ghodke-Puranik, Y.; Dorschner, J.M.; Chrabot, B.S.; Kelly, J.A.; Tsao, B.P.; Kimberly, R.P.; Alarcón-Riquelme, M.E.; Jacob, C.O.; Criswell, L.A.; et al. Genetic analysis of the pathogenic molecular sub-phenotype interferon-alpha identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun. 2015, 16, 15–23. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Olferiev, M.; Crow, M.K. Systemic lupus erythematosus genetics: Insights into pathogenesis and implications for therapy. Nat. Rev. Rheumatol. 2024, 20, 635–648. [Google Scholar] [CrossRef]
- Barrat, F.J.; Meeker, T.; Gregorio, J.; Chan, J.H.; Uematsu, S.; Akira, S.; Chang, B.; Duramad, O.; Coffman, R.L. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005, 202, 1131–1139. [Google Scholar] [CrossRef]
- Hua, J.; Kirou, K.; Lee, C.; Crow, M.K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006, 54, 1906–1916. [Google Scholar] [CrossRef]
- Lövgren, T.; Eloranta, M.L.; Båve, U.; Alm, G.V.; Rönnblom, L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004, 50, 1861–1872. [Google Scholar] [CrossRef]
- Barrat, F.J.; Meeker, T.; Chan, J.H.; Guiducci, C.; Coffman, R.L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 2007, 37, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Caielli, S.; Athale, S.; Domic, B.; Murat, E.; Chandra, M.; Banchereau, R.; Baisch, J.; Phelps, K.; Clayton, S.; Gong, M.; et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 2016, 213, 697–713. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Ries, M.; Schuster, P.; Thomann, S.; Donhauser, N.; Vollmer, J.; Schmidt, B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J. Leukoc. Biol. 2013, 94, 123–135. [Google Scholar] [CrossRef]
- Sisirak, V.; Sally, B.; D’Agati, V.; Martinez-Ortiz, W.; Özçakar, Z.B.; David, J.; Rashidfarrokhi, A.; Yeste, A.; Panea, C.; Chida, A.S.; et al. Digestion of Chromatin in Apoptotic Cell Microparticles Prevents Autoimmunity. Cell 2016, 166, 88–101. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, H.M. Type I interferon pathway in pediatric systemic lupus erythematosus. World J. Pediatr. 2024, 20, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Mertens, C.; Zillinger, T.; Wenzel, J.; Bald, T.; Zahn, S.; Tüting, T.; Hartmann, G.; Barchet, W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 2013, 39, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Stetson, D.B.; Ko, J.S.; Heidmann, T.; Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 2008, 134, 587–598. [Google Scholar] [CrossRef]
- Harley, I.T.; Niewold, T.B.; Stormont, R.M.; Kaufman, K.M.; Glenn, S.B.; Franek, B.S.; Kelly, J.A.; Kilpatrick, J.R.; Hutchings, D.; Divers, J.; et al. The role of genetic variation near interferon-kappa in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 2010, 706825. [Google Scholar] [CrossRef] [PubMed]
- Stannard, J.N.; Reed, T.J.; Myers, E.; Lowe, L.; Sarkar, M.K.; Xing, X.; Gudjonsson, J.E.; Kahlenberg, J.M. Lupus Skin Is Primed for IL-6 Inflammatory Responses through a Keratinocyte-Mediated Autocrine Type I Interferon Loop. J. Investig. Dermatol. 2017, 137, 115–122. [Google Scholar] [CrossRef]
- Davison, L.M.; Jorgensen, T.N. New Treatments for Systemic Lupus Erythematosus on the Horizon: Targeting Plasmacytoid Dendritic Cells to Inhibit Cytokine Production. J. Clin. Cell. Immunol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.H.; Wang, Y.; Huang, L.; Sandoval, H.; Liu, Y.J.; Wang, J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 2006, 311, 1160–1164. [Google Scholar] [CrossRef]
- Rowland, S.L.; Riggs, J.M.; Gilfillan, S.; Bugatti, M.; Vermi, W.; Kolbeck, R.; Unanue, E.R.; Sanjuan, M.A.; Colonna, M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014, 211, 1977–1991. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Ganguly, D.; Lewis, K.L.; Couillault, C.; Tanaka, L.; Bolland, S.; D’Agati, V.; Elkon, K.B.; Reizis, B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 2014, 211, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Werth, V.P.; Merola, J.F.; Stevenson, L.; Reynolds, T.L.; Naik, H.; Wang, W.; Christmann, R.; Gardet, A.; Pellerin, A.; et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J. Clin. Investig. 2019, 129, 1359–1371. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, H.; Lee, P.Y.; Li, M.; Yang, L.; Nigrovic, P.A.; Reeves, W.H. Differential Responsiveness of Monocyte and Macrophage Subsets to Interferon. Arthritis Rheumatol. 2020, 72, 100–113. [Google Scholar] [CrossRef]
- Khiewkamrop, P.; Kaewraemruaen, C.; Manipuntee, C.; Saengruengrit, C.; Insin, N.; Leelahavanichkul, A.; Kaewduangduen, W.; Sonpoung, O.; Ariya-Anandech, K.; Hirankarn, N.; et al. Immunosuppressive Polymeric Nanoparticles Targeting Dendritic Cells Alleviate Lupus Disease in Fcgr2b-/- Mice by Mediating Antigen-Specific Immune Tolerance. Int. J. Mol. Sci. 2023, 24, 8313. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Gianello, V.; Busatto, S.; Bergese, P.; Andreoli, L.; D’Oro, U.; Zingoni, A.; Tincani, A.; Sozzani, S.; Bosisio, D. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight 2018, 3, e98204. [Google Scholar] [CrossRef]
- Wiest, M.J.; Baert, L.; Gu, C.; Gayler, K.M.; Ham, H.; Gorvel, L.; Keddis, M.T.; Griffing, L.W.; Joo, H.; Gorvel, J.P.; et al. Endosomal trafficking inhibitor EGA can control TLR7-mediated IFNα expression by human plasmacytoid dendritic cells. Front. Immunol. 2023, 14, 1202197. [Google Scholar] [CrossRef] [PubMed]
- Lorant, A.K.; Yoshida, A.E.; Gilbertson, E.A.; Chu, T.; Stefani, C.; Acharya, M.; Hamerman, J.A.; Lacy-Hulbert, A. Integrin αvβ3 Limits Cytokine Production by Plasmacytoid Dendritic Cells and Restricts TLR-Driven Autoimmunity. J. Immunol. 2024, 212, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.A.; Hoi, A.; Gamell, C.; Tai, T.Y.; Linggi, B.; Jordan, J.; Cesaroni, M.; Sato, T.; Ng, M.; Oon, S.; et al. CSL362 potently and specifically depletes pDCs invitro and ablates SLE-immune complex-induced IFN responses. iScience 2023, 26, 107173. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Oke, V.; Gunnarsson, I.; Dorschner, J.; Eketjäll, S.; Zickert, A.; Niewold, T.B.; Svenungsson, E. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res. Ther. 2019, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.K.; Gordon, M.G.; Subramaniam, M.; Kim, M.C.; Hartoularos, G.C.; Targ, S.; Sun, Y.; Ogorodnikov, A.; Bueno, R.; Lu, A.; et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science 2022, 376, eabf1970. [Google Scholar] [CrossRef]
- Dai, X.; Fan, Y.; Zhao, X. Systemic lupus erythematosus: Updated insights on the pathogenesis, diagnosis, prevention and therapeutics. Signal Transduct. Target. Ther. 2025, 10, 102. [Google Scholar] [CrossRef]
- Alunno, A.; Bartoloni, E.; Bistoni, O.; Nocentini, G.; Ronchetti, S.; Caterbi, S.; Valentini, V.; Riccardi, C.; Gerli, R. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: The old and the new. Clin. Dev. Immunol. 2012, 2012, 823085. [Google Scholar] [CrossRef]
- Xin, Y.; He, Z.; Mei, Y.; Li, X.; Zhao, Z.; Zhao, M.; Yang, M.; Wu, H. Interferon-α regulates abnormally increased expression of RSAD2 in Th17 and Tfh cells in systemic lupus erythematosus patients. Eur. J. Immunol. 2023, 53, e2350420. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Qiu, H.; Yang, H.; Deng, Y.; Zhao, M.; Luo, H.; Zhou, X.; Xie, Y.; Chan, V.; et al. The expression of Bcl-6 in circulating follicular helper-like T cells positively correlates with the disease activity in systemic lupus erythematosus. Clin. Immunol. 2016, 173, 161–170. [Google Scholar] [CrossRef]
- Dong, X.; Antao, O.Q.; Song, W.; Sanchez, G.M.; Zembrzuski, K.; Koumpouras, F.; Lemenze, A.; Craft, J.; Weinstein, J.S. Type I Interferon-Activated STAT4 Regulation of Follicular Helper T Cell-Dependent Cytokine and Immunoglobulin Production in Lupus. Arthritis Rheumatol. 2021, 73, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, H.; Li, M.; Li, Y.; Zhu, X.; Amin, S.; Alexander, M.; Diadhiou, C.; Davidson, A.; Zeng, H. mTORC2 contributes to systemic autoimmunity. Immunology 2023, 168, 554–568. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, J.; Jiang, H.; Li, W.; Sun, Y.; Shan, Y.; Wei, T.; Chi, X.; Yu, S.; Ma, X. Competitive binding of transcription factors underlies flexibility of T peripheral helper cells and T follicular helper cells in SLE. Rheumatology 2022, 61, 4547–4557. [Google Scholar] [CrossRef]
- Klarquist, J.; Cantrell, R.; Lehn, M.A.; Lampe, K.; Hennies, C.M.; Hoebe, K.; Janssen, E.M. Type I IFN Drives Experimental Systemic Lupus Erythematosus by Distinct Mechanisms in CD4 T Cells and B Cells. Immunohorizons 2020, 4, 140–152. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Shan, Y.; Nakayamada, S.; Nawata, A.; Yamagata, K.; Sonomoto, K.; Tanaka, H.; Satoh-Kanda, Y.; Nguyen, M.P.; Todoroki, Y.; Nagayasu, A.; et al. TGF-β3 in differentiation and function of Tph-like cells and its relevance to disease activity in patients with systemic lupus erythematosus. Rheumatology 2023, 62, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, S.; Seki, N.; Tsujimoto, H.; Saito, S.; Kikuchi, J.; Sugahara, K.; Yoshimoto, K.; Suzuki, K.; Kaneko, Y.; Chiba, K.; et al. Role of interferons (IFNs) in the differentiation of T peripheral helper (Tph) cells. Int. Immunol. 2022, 34, 533–544. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, S.; Xie, J.; Cui, D. mTOR signaling: A pivotal player in Treg cell dysfunction in systemic lupus erythematosus. Clin. Immunol. 2022, 245, 109153. [Google Scholar] [CrossRef] [PubMed]
- Fava, A.; Buyon, J.; Mohan, C.; Zhang, T.; Belmont, H.M.; Izmirly, P.; Clancy, R.; Trujillo, J.M.; Fine, D.; Zhang, Y.; et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 2020, 5, e138345. [Google Scholar] [CrossRef]
- Blanco, L.P.; Patino-Martinez, E.; Nakabo, S.; Zhang, M.; Pedersen, H.L.; Wang, X.; Carmona-Rivera, C.; Claybaugh, D.; Yu, Z.X.; Desta, E.; et al. Modulation of the Itaconate Pathway Attenuates Murine Lupus. Arthritis Rheumatol. 2022, 74, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Kailashiya, V.; Singh, U.; Kailashiya, J. CTLA4 Gene Polymorphism and its Association with Disease Occurrence, Clinical Manifestations, Serum Markers and Cytokine Levels in SLE Patients from North India. Indian J. Dermatol. 2022, 67, 311. [Google Scholar] [CrossRef]
- Huang, B.; Li, H.; Jiang, Q.; Li, Y.; Jiang, Z.; Cao, H.; Wang, S.; Wang, X.; Li, J.; Li, G. Elevated type I IFN signalling directly affects CD8(+) T-cell distribution and autoantigen recognition of the skeletal muscles in active JDM patients. J. Autoimmun. 2024, 146, 103232. [Google Scholar] [CrossRef]
- Keller, E.J.; Dvorina, N.; Jørgensen, T.N. Spontaneous CD4+ T Cell Activation and Differentiation in Lupus-Prone B6.Nba2 Mice Is IFNAR-Independent. Int. J. Mol. Sci. 2022, 23, 874. [Google Scholar] [CrossRef]
- Radziszewska, A.; Peckham, H.; Restuadi, R.; Kartawinata, M.; Moulding, D.; de Gruijter, N.M.; Robinson, G.A.; Butt, M.; Deakin, C.T.; Wilkinson, M.G.L.; et al. Type I interferon and mitochondrial dysfunction are associated with dysregulated cytotoxic CD8+ T cell responses in juvenile systemic lupus erythematosus. Clin. Exp. Immunol. 2025, 219, uxae127. [Google Scholar] [CrossRef]
- Soni, C.; Perez, O.A.; Voss, W.N.; Pucella, J.N.; Serpas, L.; Mehl, J.; Ching, K.L.; Goike, J.; Georgiou, G.; Ippolito, G.C.; et al. Plasmacytoid Dendritic Cells and Type I Interferon Promote Extrafollicular B Cell Responses to Extracellular Self-DNA. Immunity 2020, 52, 1022–1038.e7. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.H.; Smith, F.L.; Baumgarth, N. B Cell Activation and Response Regulation During Viral Infections. Viral Immunol. 2020, 33, 294–306. [Google Scholar] [CrossRef]
- Barnas, J.L.; Albrecht, J.; Meednu, N.; Alzamareh, D.F.; Baker, C.; McDavid, A.; Looney, R.J.; Anolik, J.H. B Cell Activation and Plasma Cell Differentiation Are Promoted by IFN-λ in Systemic Lupus Erythematosus. J. Immunol. 2021, 207, 2660–2672. [Google Scholar] [CrossRef]
- Dahlgren, M.W.; Plumb, A.W.; Niss, K.; Lahl, K.; Brunak, S.; Johansson-Lindbom, B. Type I Interferons Promote Germinal Centers Through B Cell Intrinsic Signaling and Dendritic Cell Dependent Th1 and Tfh Cell Lineages. Front. Immunol. 2022, 13, 932388. [Google Scholar] [CrossRef]
- Care, M.A.; Stephenson, S.J.; Barnes, N.A.; Fan, I.; Zougman, A.; El-Sherbiny, Y.M.; Vital, E.M.; Westhead, D.R.; Tooze, R.M.; Doody, G.M. Network Analysis Identifies Proinflammatory Plasma Cell Polarization for Secretion of ISG15 in Human Autoimmunity. J. Immunol. 2016, 197, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Quách, T.D.; Manjarrez-Orduño, N.; Adlowitz, D.G.; Silver, L.; Yang, H.; Wei, C.; Milner, E.C.; Sanz, I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J. Immunol. 2011, 186, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Ferri, D.M.; Nassar, C.; Manion, K.P.; Kim, M.; Baglaenko, Y.; Muñoz-Grajales, C.; Wither, J.E. Elevated Levels of Interferon-α Act Directly on B Cells to Breach Multiple Tolerance Mechanisms Promoting Autoantibody Production. Arthritis Rheumatol. 2023, 75, 1542–1555. [Google Scholar] [CrossRef]
- Liu, Z.; Davidson, A. IFNα Inducible Models of Murine SLE. Front. Immunol. 2013, 4, 306. [Google Scholar] [CrossRef]
- Gao, M.; Liu, S.; Chatham, W.W.; Mountz, J.D.; Hsu, H.C. IL-4-Induced Quiescence of Resting Naive B Cells Is Disrupted in Systemic Lupus Erythematosus. J. Immunol. 2022, 209, 1513–1522. [Google Scholar] [CrossRef]
- van Dooren, H.J.; Atisha-Fregoso, Y.; Dorjée, A.L.; Huizinga, T.W.J.; Mackay, M.; Aranow, C.; Toes, R.E.M.; Diamond, B.; Suurmond, J. Interferon signatures fuel B cell hyperactivity and plasmablast expansion in systemic lupus erythematosus. J. Autoimmun. 2025, 154, 103438. [Google Scholar] [CrossRef]
- Bradford, H.F.; Haljasmägi, L.; Menon, M.; McDonnell, T.C.R.; Särekannu, K.; Vanker, M.; Peterson, P.; Wincup, C.; Abida, R.; Gonzalez, R.F.; et al. Inactive disease in patients with lupus is linked to autoantibodies to type I interferons that normalize blood IFNα and B cell subsets. Cell Rep. Med. 2023, 4, 100894. [Google Scholar] [CrossRef]
- Sumikawa, M.H.; Iwata, S.; Zhang, M.; Miyata, H.; Ueno, M.; Todoroki, Y.; Nagayasu, A.; Kanda, R.; Sonomoto, K.; Torimoto, K.; et al. An enhanced mitochondrial function through glutamine metabolism in plasmablast differentiation in systemic lupus erythematosus. Rheumatology 2022, 61, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Manolakou, T.; Nikolopoulos, D.; Gkikas, D.; Filia, A.; Samiotaki, M.; Stamatakis, G.; Fanouriakis, A.; Politis, P.; Banos, A.; Alissafi, T.; et al. ATR-mediated DNA damage responses underlie aberrant B cell activity in systemic lupus erythematosus. Sci. Adv. 2022, 8, eabo5840. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef]
- Henault, J.; Riggs, J.M.; Karnell, J.L.; Liarski, V.M.; Li, J.; Shirinian, L.; Xu, L.; Casey, K.A.; Smith, M.A.; Khatry, D.B.; et al. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat. Immunol. 2016, 17, 196–203. [Google Scholar] [CrossRef]
- Hagberg, N.; Berggren, O.; Leonard, D.; Weber, G.; Bryceson, Y.T.; Alm, G.V.; Eloranta, M.L.; Rönnblom, L. IFN-α production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes is promoted by NK cells via MIP-1β and LFA-1. J. Immunol. 2011, 186, 5085–5094. [Google Scholar] [CrossRef]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Yan, M.; Gu, Y.; Sun, H.; Ge, Q. Neutrophil extracellular traps in tumor progression and immunotherapy. Front. Immunol. 2023, 14, 1135086. [Google Scholar] [CrossRef]
- Matta, B.; Barnes, B.J. Coordination between innate immune cells, type I IFNs and IRF5 drives SLE pathogenesis. Cytokine 2020, 132, 154731. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Guo, X.; Smith, M.A.; Wang, S.; Sinibaldi, D.; Sanjuan, M.A.; Wang, L.; Illei, G.G.; White, W.I. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci. Med. 2018, 5, e000286. [Google Scholar] [CrossRef]
- Seridi, L.; Cesaroni, M.; Orillion, A.; Schreiter, J.; Chevrier, M.; Marciniak, S.; Migone, T.S.; Stohl, W.; Chatham, W.W.; Furie, R.A.; et al. Novel signatures associated with systemic lupus erythematosus clinical response to IFN-α/-ω inhibition. Lupus 2021, 30, 795–806. [Google Scholar] [CrossRef]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Jog, N.R.; Wagner, C.A.; Aberle, T.; Chakravarty, E.F.; Arriens, C.; Guthridge, J.M.; James, J.A. Neutrophils isolated from systemic lupus erythematosus patients exhibit a distinct functional phenotype. Front. Immunol. 2024, 15, 1339250. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, W.; Zhang, X.; Liu, W. Insights into the pathogenic role of neutrophils in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2023, 35, 82–88. [Google Scholar] [CrossRef]
- Leitinger, N.; Schulman, I.G. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1120–1126. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Liang, R.; Olsen, N.; Zheng, S.G. Roles of IRF4 in various immune cells in systemic lupus erythematosus. Int. Immunopharmacol. 2024, 133, 112077. [Google Scholar] [CrossRef]
- Patiño-Martinez, E.; Nakabo, S.; Jiang, K.; Carmona-Rivera, C.; Tsai, W.L.; Claybaugh, D.; Yu, Z.X.; Romero, A.; Bohrnsen, E.; Schwarz, B.; et al. The Aconitate Decarboxylase 1/Itaconate Pathway Modulates Immune Dysregulation and Associates with Cardiovascular Disease Markers and Disease Activity in Systemic Lupus Erythematosus. J. Immunol. 2024, 213, 419–434. [Google Scholar] [CrossRef]
- Zyzak, J.; Mitkiewicz, M.; Leszczyńska, E.; Reniewicz, P.; Moynagh, P.N.; Siednienko, J. HSV-1/TLR9-Mediated IFNβ and TNFα Induction Is Mal-Dependent in Macrophages. J. Innate Immun. 2020, 12, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Kurasawa, K.; Hasegawa, A.; Miyao, T.; Tanaka, A.; Arai, S.; Arima, M.; Maezawa, R. Differences and similarities in cytokine profiles of macrophage activation syndrome in systemic lupus erythematosus and adult-onset Still’s disease. Clin. Exp. Med. 2023, 23, 3407–3416. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, M.; Zheng, Q. IL-10 and IFN-γ as markers for early recognition of pediatric systemic lupus erythematosus complicated with macrophage activation syndrome. Rheumatology 2025, 64, 235–241. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Wang, X.; Li, B.; Yu, H.; Quan, Y.; Jiang, Y.; You, Y.; Wang, Y.; Wen, M.; et al. Cellular spermine targets JAK signaling to restrain cytokine-mediated autoimmunity. Immunity 2024, 57, 1796–1811.e8. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Shen, H.; Zhang, Y.; Liu, C.; Li, S.; Yao, J.; Jin, Z.; Yu, H. Integrative analysis of single-cell and bulk transcriptome data reveal the significant role of macrophages in lupus nephritis. Arthritis Res. Ther. 2024, 26, 84. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, B.; Ding, H.; Li, X.; Wang, X.; Zhang, X.; Liu, Q.; Feng, Q.; Han, M.; Chen, L.; et al. The Oxysterol Receptor EBI2 Links Innate and Adaptive Immunity to Limit IFN Response and Systemic Lupus Erythematosus. Adv. Sci. 2023, 10, e2207108. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Reeves, W.H.; Lee, P.Y.; Weinstein, J.S.; Satoh, M.; Lu, L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009, 30, 455–464. [Google Scholar] [CrossRef]
- Stergioti, E.M.; Manolakou, T.; Sentis, G.; Samiotaki, M.; Kapsala, N.; Fanouriakis, A.; Boumpas, D.T.; Banos, A. Transcriptomic and proteomic profiling reveals distinct pathogenic features of peripheral non-classical monocytes in systemic lupus erythematosus. Clin. Immunol. 2023, 255, 109765. [Google Scholar] [CrossRef]
- Kuga, T.; Chiba, A.; Murayama, G.; Hosomi, K.; Nakagawa, T.; Yahagi, Y.; Noto, D.; Kusaoi, M.; Kawano, F.; Yamaji, K.; et al. Enhanced GATA4 expression in senescent systemic lupus erythematosus monocytes promotes high levels of IFNα production. Front. Immunol. 2024, 15, 1320444. [Google Scholar] [CrossRef]
- Chen, K.L.; Patel, J.; Zeidi, M.; Wysocka, M.; Bashir, M.M.; Patel, B.; Maddukuri, S.; White, B.; Werth, V.P. Myeloid Dendritic Cells Are Major Producers of IFN-β in Dermatomyositis and May Contribute to Hydroxychloroquine Refractoriness. J. Investig. Dermatol. 2021, 141, 1906–1914.e2. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Raterman, H.G.; Vosslamber, S.; de Ridder, S.; Nurmohamed, M.T.; Lems, W.F.; Boers, M.; van de Wiel, M.; Dijkmans, B.A.; Verweij, C.L.; Voskuyl, A.E. The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients. Arthritis Res. Ther. 2012, 14, R95. [Google Scholar] [CrossRef]
- Iwasaki, T.; Watanabe, R.; Ito, H.; Fujii, T.; Okuma, K.; Oku, T.; Hirayama, Y.; Ohmura, K.; Murata, K.; Murakami, K.; et al. Dynamics of Type I and Type II Interferon Signature Determines Responsiveness to Anti-TNF Therapy in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 901437. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; La, D.T.; Stohl, W.; Crow, M.K. Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-beta/alpha ratios in rheumatoid arthritis patients: A post hoc analysis of a predominantly Hispanic cohort. Arthritis Rheum. 2010, 62, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wampler Muskardin, T.; Vashisht, P.; Dorschner, J.M.; Jensen, M.A.; Chrabot, B.S.; Kern, M.; Curtis, J.R.; Danila, M.I.; Cofield, S.S.; Shadick, N.; et al. Increased pretreatment serum IFN-β/α ratio predicts non-response to tumour necrosis factor α inhibition in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.D.; Vosslamber, S.; Mantel, E.; de Ridder, S.; Wesseling, J.G.; van der Pouw Kraan, T.C.; Leurs, C.; Hegen, H.; Deisenhammer, F.; Killestein, J.; et al. Physiological evidence for diversification of IFNα- and IFNβ-mediated response programs in different autoimmune diseases. Arthritis Res. Ther. 2016, 18, 49. [Google Scholar] [CrossRef]
- Coclet-Ninin, J.; Dayer, J.M.; Burger, D. Interferon-beta not only inhibits interleukin-1beta and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur. Cytokine Netw. 1997, 8, 345–349. [Google Scholar]
- Palmer, G.; Mezin, F.; Juge-Aubry, C.E.; Plater-Zyberk, C.; Gabay, C.; Guerne, P.A. Interferon beta stimulates interleukin 1 receptor antagonist production in human articular chondrocytes and synovial fibroblasts. Ann. Rheum. Dis. 2004, 63, 43–49. [Google Scholar] [CrossRef]
- van Holten, J.; Reedquist, K.; Sattonet-Roche, P.; Smeets, T.J.; Plater-Zyberk, C.; Vervoordeldonk, M.J.; Tak, P.P. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, R239. [Google Scholar] [CrossRef] [PubMed]
- van Holten, J.; Pavelka, K.; Vencovsky, J.; Stahl, H.; Rozman, B.; Genovese, M.; Kivitz, A.J.; Alvaro, J.; Nuki, G.; Furst, D.E.; et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 2008, 226, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Orange, D.E.; Agius, P.; DiCarlo, E.F.; Robine, N.; Geiger, H.; Szymonifka, J.; McNamara, M.; Cummings, R.; Andersen, K.M.; Mirza, S.; et al. Identification of Three Rheumatoid Arthritis Disease Subtypes by Machine Learning Integration of Synovial Histologic Features and RNA Sequencing Data. Arthritis Rheumatol. 2018, 70, 690–701. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Lübbers, J.; Brink, M.; van de Stadt, L.A.; Vosslamber, S.; Wesseling, J.G.; van Schaardenburg, D.; Rantapää-Dahlqvist, S.; Verweij, C.L. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 776–780. [Google Scholar] [CrossRef]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar] [CrossRef]
- Higgs, B.W.; Liu, Z.; White, B.; Zhu, W.; White, W.I.; Morehouse, C.; Brohawn, P.; Kiener, P.A.; Richman, L.; Fiorentino, D.; et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann. Rheum. Dis. 2011, 70, 2029–2036. [Google Scholar] [CrossRef]
- Dieguez-Gonzalez, R.; Calaza, M.; Perez-Pampin, E.; de la Serna, A.R.; Fernandez-Gutierrez, B.; Castañeda, S.; Largo, R.; Joven, B.; Narvaez, J.; Navarro, F.; et al. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum. 2008, 58, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Han, T.U.; Cho, S.K.; Kim, T.; Joo, Y.B.; Bae, S.C.; Kang, C. Association of an activity-enhancing variant of IRAK1 and an MECP2-IRAK1 haplotype with increased susceptibility to rheumatoid arthritis. Arthritis Rheum. 2013, 65, 590–598. [Google Scholar] [CrossRef]
- Cooles, F.A.H.; Anderson, A.E.; Lendrem, D.W.; Norris, J.; Pratt, A.G.; Hilkens, C.M.U.; Isaacs, J.D. The interferon gene signature is increased in patients with early treatment-naive rheumatoid arthritis and predicts a poorer response to initial therapy. J. Allergy Clin. Immunol. 2018, 141, 445–448.e4. [Google Scholar] [CrossRef]
- Cooles, F.A.H.; Tarn, J.; Lendrem, D.W.; Naamane, N.; Lin, C.M.; Millar, B.; Maney, N.J.; Anderson, A.E.; Thalayasingam, N.; Diboll, J.; et al. Interferon-α-mediated therapeutic resistance in early rheumatoid arthritis implicates epigenetic reprogramming. Ann. Rheum. Dis. 2022, 81, 1214–1223. [Google Scholar] [CrossRef]
- de Jong, T.D.; Blits, M.; de Ridder, S.; Vosslamber, S.; Wolbink, G.; Nurmohamed, M.T.; Verweij, C.L. Type I interferon response gene expression in established rheumatoid arthritis is not associated with clinical parameters. Arthritis Res. Ther. 2016, 18, 290. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Gregory, B.; Cribbs, A.P.; Bhalara, S.; Li, Y.; Loreth, C.; Zhang, Y.; Guo, Y.; Lin, L.L.; Feldmann, M.; et al. Novel biomarkers of a peripheral blood interferon signature associated with drug-naïve early arthritis patients distinguish persistent from self-limiting disease course. Sci. Rep. 2020, 10, 8830. [Google Scholar] [CrossRef]
- Chalan, P.; Bijzet, J.; van den Berg, A.; Kluiver, J.; Kroesen, B.J.; Boots, A.M.; Brouwer, E. Analysis of serum immune markers in seropositive and seronegative rheumatoid arthritis and in high-risk seropositive arthralgia patients. Sci. Rep. 2016, 6, 26021. [Google Scholar] [CrossRef]
- Macías-Segura, N.; Castañeda-Delgado, J.E.; Bastian, Y.; Santiago-Algarra, D.; Castillo-Ortiz, J.D.; Alemán-Navarro, A.L.; Jaime-Sánchez, E.; Gomez-Moreno, M.; Saucedo-Toral, C.A.; Lara-Ramírez, E.E.; et al. Transcriptional signature associated with early rheumatoid arthritis and healthy individuals at high risk to develop the disease. PLoS ONE 2018, 13, e0194205. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Lundquist, A.; Alexeyenko, A.; Lejon, K.; Rantapää-Dahlqvist, S. Protein profiling and network enrichment analysis in individuals before and after the onset of rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 288. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Delgado, J.E.; Bastián-Hernandez, Y.; Macias-Segura, N.; Santiago-Algarra, D.; Castillo-Ortiz, J.D.; Alemán-Navarro, A.L.; Martínez-Tejada, P.; Enciso-Moreno, L.; Garcia-De Lira, Y.; Olguín-Calderón, D.; et al. Type I Interferon Gene Response Is Increased in Early and Established Rheumatoid Arthritis and Correlates with Autoantibody Production. Front. Immunol. 2017, 8, 285. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Briggs, T.A.; Rice, G.I.; Darmalinggam, S.; Bondet, V.; Bruce, E.; Khan, M.; Haque, S.; Chinoy, H.; Herrick, A.L.; et al. Type I interferon in patients with systemic autoimmune rheumatic disease is associated with haematological abnormalities and specific autoantibody profiles. Arthritis Res. Ther. 2019, 21, 147. [Google Scholar] [CrossRef]
- Dawidowicz, K.; Allanore, Y.; Guedj, M.; Pierlot, C.; Bombardieri, S.; Balsa, A.; Westhovens, R.; Barrera, P.; Alves, H.; Teixeira, V.H.; et al. The interferon regulatory factor 5 gene confers susceptibility to rheumatoid arthritis and influences its erosive phenotype. Ann. Rheum. Dis. 2011, 70, 117–121. [Google Scholar] [CrossRef]
- Adams, C.; Nair, N.; Plant, D.; Verstappen, S.M.M.; Quach, H.L.; Quach, D.L.; Carvidi, A.; Nititham, J.; Nakamura, M.; Graf, J.; et al. Identification of Cell-Specific Differential DNA Methylation Associated With Methotrexate Treatment Response in Rheumatoid Arthritis. Arthritis Rheumatol. 2023, 75, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Plant, D.; Verstappen, S.M.; Isaacs, J.D.; Morgan, A.W.; Hyrich, K.L.; Barton, A.; Wilson, A.G. Differential DNA methylation correlates with response to methotrexate in rheumatoid arthritis. Rheumatology 2020, 59, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Gosselt, H.R.; van Zelst, B.D.; de Rotte, M.; Hazes, J.M.W.; de Jonge, R.; Heil, S.G. Higher baseline global leukocyte DNA methylation is associated with MTX non-response in early RA patients. Arthritis Res. Ther. 2019, 21, 157. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Teng, D.; Zhou, Y.; Zhang, L.; Zhong, Z.; Yang, G.J. Epigenetic Regulation in the Pathogenesis of Rheumatoid Arthritis. Front. Immunol. 2022, 13, 859400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; Li, S.; Yang, L.; Zhu, D.; Wang, Y.; Wang, H.; Wang, T.; Shi, B.; Gai, Z.; et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 2018, 8, 14305. [Google Scholar] [CrossRef]
- van der Heijden, I.M.; Wilbrink, B.; Tchetverikov, I.; Schrijver, I.A.; Schouls, L.M.; Hazenberg, M.P.; Breedveld, F.C.; Tak, P.P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000, 43, 593–598. [Google Scholar] [CrossRef]
- Chen, T.; Rimpiläinen, M.; Luukkainen, R.; Möttönen, T.; Yli-Jama, T.; Jalava, J.; Vainio, O.; Toivanen, P. Bacterial components in the synovial tissue of patients with advanced rheumatoid arthritis or osteoarthritis: Analysis with gas chromatography-mass spectrometry and pan-bacterial polymerase chain reaction. Arthritis Rheum. 2003, 49, 328–334. [Google Scholar] [CrossRef]
- Kassiotis, G. The Immunological Conundrum of Endogenous Retroelements. Annu. Rev. Immunol. 2023, 41, 99–125. [Google Scholar] [CrossRef]
- van der Pouw Kraan, T.C.; van Baarsen, L.G.; Wijbrandts, C.A.; Voskuyl, A.E.; Rustenburg, F.; Baggen, J.M.; Dijkmans, B.A.; Tak, P.P.; Verweij, C.L. Expression of a pathogen-response program in peripheral blood cells defines a subgroup of rheumatoid arthritis patients. Genes Immun. 2008, 9, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kawane, K.; Ohtani, M.; Miwa, K.; Kizawa, T.; Kanbara, Y.; Yoshioka, Y.; Yoshikawa, H.; Nagata, S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 2006, 443, 998–1002. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yoshida, K.; Hashimoto, N.; Nakai, A.; Kaneshiro, K.; Suzuki, K.; Kawasaki, Y.; Shibanuma, N.; Hashiramoto, A. Circulating cell free DNA: A marker to predict the therapeutic response for biological DMARDs in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 722–730. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Y.; Sun, C.; Liang, H.; Dai, L.; Shen, J.; Wei, S.; Guo, S.; Leong, K.W.; Chen, Y.; et al. Identification of Specific Joint-Inflammatogenic Cell-Free DNA Molecules From Synovial Fluids of Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ye, J.; Pan, N.; Tan, C.; Herrmann, M. Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2020, 11, 578129. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, C.; Tripodo, C.; Gong, M.; Sangaletti, S.; Colombo, M.P.; Coffman, R.L.; Barrat, F.J. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 2010, 207, 2931–2942. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, X.; Zhang, S.; Deng, C.; Zhao, L.; Wang, M.; Wu, Q.; Yang, H.; Zhou, J.; Peng, L.; et al. The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2022, 24, 170. [Google Scholar] [CrossRef]
- Patterson, S.L.; Sun, S.; Rychkov, D.; Katz, P.; Tsitsiklis, A.; Nakamura, M.C.; Serpa, P.H.; Langelier, C.R.; Sirota, M. Physical Activity Associates With Lower Systemic Inflammatory Gene Expression in Rheumatoid Arthritis. J. Rheumatol. 2022, 49, 1320–1327. [Google Scholar] [CrossRef]
- Baccala, R.; Hoebe, K.; Kono, D.H.; Beutler, B.; Theofilopoulos, A.N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 2007, 13, 543–551. [Google Scholar] [CrossRef]
- Lande, R.; Giacomini, E.; Serafini, B.; Rosicarelli, B.; Sebastiani, G.D.; Minisola, G.; Tarantino, U.; Riccieri, V.; Valesini, G.; Coccia, E.M. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J. Immunol. 2004, 173, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, L.L.; Boyce, A.; Smith, L.; Padmanabha, J.; Filgueira, L.; Pietschmann, P.; Thomas, R. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res. Ther. 2005, 7, R230. [Google Scholar] [CrossRef]
- van Holten, J.; Smeets, T.J.; Blankert, P.; Tak, P.P. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: Comparison with patients with osteoarthritis and reactive arthritis. Ann. Rheum. Dis. 2005, 64, 1780–1782. [Google Scholar] [CrossRef]
- Roelofs, M.F.; Wenink, M.H.; Brentano, F.; Abdollahi-Roodsaz, S.; Oppers-Walgreen, B.; Barrera, P.; van Riel, P.L.; Joosten, L.A.; Kyburz, D.; van den Berg, W.B.; et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA). Ann. Rheum. Dis. 2009, 68, 1486–1493. [Google Scholar] [CrossRef]
- Kwok, S.K.; Lee, J.Y.; Park, S.H.; Cho, M.L.; Min, S.Y.; Park, S.H.; Kim, H.Y.; Cho, Y.G. Dysfunctional interferon-alpha production by peripheral plasmacytoid dendritic cells upon Toll-like receptor-9 stimulation in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2008, 10, R29. [Google Scholar] [CrossRef]
- Jongbloed, S.L.; Lebre, M.C.; Fraser, A.R.; Gracie, J.A.; Sturrock, R.D.; Tak, P.P.; McInnes, I.B. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R15. [Google Scholar] [CrossRef]
- Lebre, M.C.; Jongbloed, S.L.; Tas, S.W.; Smeets, T.J.; McInnes, I.B.; Tak, P.P. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am. J. Pathol. 2008, 172, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Narendra, S.C.; Chalise, J.P.; Höök, N.; Magnusson, M. Dendritic cells activated by double-stranded RNA induce arthritis via autocrine type I IFN signaling. J. Leukoc. Biol. 2014, 95, 661–666. [Google Scholar] [CrossRef]
- Nehmar, R.; Alsaleh, G.; Voisin, B.; Flacher, V.; Mariotte, A.; Saferding, V.; Puchner, A.; Niederreiter, B.; Vandamme, T.; Schabbauer, G.; et al. Therapeutic Modulation of Plasmacytoid Dendritic Cells in Experimental Arthritis. Arthritis Rheumatol. 2017, 69, 2124–2135. [Google Scholar] [CrossRef] [PubMed]
- Cooles, F.A.H.; Anderson, A.E.; Skelton, A.; Pratt, A.G.; Kurowska-Stolarska, M.S.; McInnes, I.; Hilkens, C.M.U.; Isaacs, J.D. Phenotypic and Transcriptomic Analysis of Peripheral Blood Plasmacytoid and Conventional Dendritic Cells in Early Drug Naïve Rheumatoid Arthritis. Front. Immunol. 2018, 9, 755. [Google Scholar] [CrossRef]
- Yamada, S.; Nagafuchi, Y.; Wang, M.; Ota, M.; Hatano, H.; Takeshima, Y.; Okubo, M.; Kobayashi, S.; Sugimori, Y.; Masahiro, N.; et al. Immunomics analysis of rheumatoid arthritis identified precursor dendritic cells as a key cell subset of treatment resistance. Ann. Rheum. Dis. 2023, 82, 809–819. [Google Scholar] [CrossRef]
- Németh, T.; Nagy, G.; Pap, T. Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go? Ann. Rheum. Dis. 2022, 81, 1055–1064. [Google Scholar] [CrossRef]
- Mousavi, M.J.; Karami, J.; Aslani, S.; Tahmasebi, M.N.; Vaziri, A.S.; Jamshidi, A.; Farhadi, E.; Mahmoudi, M. Transformation of fibroblast-like synoviocytes in rheumatoid arthritis; from a friend to foe. Autoimmuity Highlights 2021, 12, 3. [Google Scholar] [CrossRef]
- Carini, C.; Hunter, E.; Ramadass, A.S.; Green, J.; Akoulitchev, A.; McInnes, I.B.; Goodyear, C.S. Chromosome conformation signatures define predictive markers of inadequate response to methotrexate in early rheumatoid arthritis. J. Transl. Med. 2018, 16, 18. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Williams, R.O.; Tailor, H.; Chernajovsky, Y. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum. 1999, 42, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, J.; Wu, R. Neutrophils in Rheumatoid Arthritis Synovium: Implications on Disease Activity and Inflammation State. J. Inflamm. Res. 2025, 18, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.D.; Lübbers, J.; Turk, S.; Vosslamber, S.; Mantel, E.; Bontkes, H.J.; van der Laken, C.J.; Bijlsma, J.W.; van Schaardenburg, D.; Verweij, C.L. The type I interferon signature in leukocyte subsets from peripheral blood of patients with early arthritis: A major contribution by granulocytes. Arthritis Res. Ther. 2016, 18, 165. [Google Scholar] [CrossRef]
- Wright, H.L.; Thomas, H.B.; Moots, R.J.; Edwards, S.W. Interferon gene expression signature in rheumatoid arthritis neutrophils correlates with a good response to TNFi therapy. Rheumatology 2015, 54, 188–193. [Google Scholar] [CrossRef]
- Rönnblom, L.; Eloranta, M.L. The interferon signature in autoimmune diseases. Curr. Opin. Rheumatol. 2013, 25, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wampler Muskardin, T.L.; Fan, W.; Jin, Z.; Jensen, M.A.; Dorschner, J.M.; Ghodke-Puranik, Y.; Dicke, B.; Vsetecka, D.; Wright, K.; Mason, T.; et al. Distinct Single Cell Gene Expression in Peripheral Blood Monocytes Correlates With Tumor Necrosis Factor Inhibitor Treatment Response Groups Defined by Type I Interferon in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1384. [Google Scholar] [CrossRef]
- Obermoser, G.; Pascual, V. The interferon-alpha signature of systemic lupus erythematosus. Lupus 2010, 19, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Coutant, F. Shaping of Monocyte-Derived Dendritic Cell Development and Function by Environmental Factors in Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 13670. [Google Scholar] [CrossRef]
- Seyler, T.M.; Park, Y.W.; Takemura, S.; Bram, R.J.; Kurtin, P.J.; Goronzy, J.J.; Weyand, C.M. BLyS and APRIL in rheumatoid arthritis. J. Clin. Investig. 2005, 115, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.W.; Kolhatkar, N.S.; Rawlings, D.J. B cells take the front seat: Dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr. Opin. Immunol. 2015, 33, 70–77. [Google Scholar] [CrossRef]
- Litinskiy, M.B.; Nardelli, B.; Hilbert, D.M.; He, B.; Schaffer, A.; Casali, P.; Cerutti, A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002, 3, 822–829. [Google Scholar] [CrossRef]
- Jarry, A.; Malard, F.; Bou-Hanna, C.; Meurette, G.; Mohty, M.; Mosnier, J.F.; Laboisse, C.L.; Bossard, C. Interferon-Alpha Promotes Th1 Response and Epithelial Apoptosis via Inflammasome Activation in Human Intestinal Mucosa. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 72–81. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Baumjohann, D.; Craft, J.; Fazilleau, N.; Ma, C.S.; Tangye, S.G.; Vinuesa, C.G.; Linterman, M.A. CD4(+) T cells that help B cells—A proposal for uniform nomenclature. Trends Immunol. 2021, 42, 658–669. [Google Scholar] [CrossRef]
- Kolumam, G.A.; Thomas, S.; Thompson, L.J.; Sprent, J.; Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005, 202, 637–650. [Google Scholar] [CrossRef]
- Firestein, G.S.; Zvaifler, N.J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987, 30, 864–871. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwland, M.; Mulder, A.H.L.; Colin, E.M.; Alves, C.; van Bon, L.; Brouwer, E. Investigating interferon type I responses in patients with suspected giant cell arteritis and polymyalgia rheumatica. Clin. Exp. Immunol. 2024, 218, 308–313. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Tian, L.; Goronzy, J.J.; Weyand, C.M. Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis. Circulation 2018, 137, 1934–1948. [Google Scholar] [CrossRef] [PubMed]
- Estupiñán-Moreno, E.; Ortiz-Fernández, L.; Li, T.; Hernández-Rodríguez, J.; Ciudad, L.; Andrés-León, E.; Terron-Camero, L.C.; Prieto-González, S.; Espígol-Frigolé, G.; Cid, M.C.; et al. Methylome and transcriptome profiling of giant cell arteritis monocytes reveals novel pathways involved in disease pathogenesis and molecular response to glucocorticoids. Ann. Rheum. Dis. 2022, 81, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Berry, G.J.; Liang, D.H.; Goronzy, J.J.; Weyand, C.M. Cellular Signaling Pathways in Medium and Large Vessel Vasculitis. Front. Immunol. 2020, 11, 587089. [Google Scholar] [CrossRef]
- Corbera-Bellalta, M.; Planas-Rigol, E.; Lozano, E.; Terrades-García, N.; Alba, M.A.; Prieto-González, S.; García-Martínez, A.; Albero, R.; Enjuanes, A.; Espígol-Frigolé, G.; et al. Blocking interferon γ reduces expression of chemokines CXCL9, CXCL10 and CXCL11 and decreases macrophage infiltration in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- AbbVie Inc. Rinvoq (Upadacitinib) Receives U.S. FDA Approval for Giant Cell Arteritis [News Release]. 29 April 2025. Available online: https://news.abbvie.com/2025-04-29-RINVOQ-R-upadacitinib-Receives-U-S-FDA-Approval-for-Giant-Cell-Arteritis-GCA (accessed on 29 September 2025).
- Koster, M.J.; Crowson, C.S.; Giblon, R.E.; Jaquith, J.M.; Duarte-García, A.; Matteson, E.L.; Weyand, C.M.; Warrington, K.J. Baricitinib for relapsing giant cell arteritis: A prospective open-label 52-week pilot study. Ann. Rheum. Dis. 2022, 81, 861–867. [Google Scholar] [CrossRef]
- Batten, I.; Robinson, M.W.; White, A.; Walsh, C.; Fazekas, B.; Wyse, J.; Buettner, A.; D’Arcy, S.; Greenan, E.; Murphy, C.C.; et al. Investigation of type I interferon responses in ANCA-associated vasculitis. Sci. Rep. 2021, 11, 8272. [Google Scholar] [CrossRef] [PubMed]
- Kessler, N.; Viehmann, S.F.; Krollmann, C.; Mai, K.; Kirschner, K.M.; Luksch, H.; Kotagiri, P.; Böhner, A.M.C.; Huugen, D.; de Oliveira Mann, C.C.; et al. Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing. J. Exp. Med. 2022, 219, e20220759. [Google Scholar] [CrossRef]
- Uslu, E.; İlbay, A.; Göveç Giynaş, N.; Gaydan, A.; Ateş, E.S.; Dalva, K.; Yüksel, M.; Sezer, S.; Yayla, M.E.; Turgay, T.M.; et al. ABS0942 Type I Interferon Signature in Large Vessel Vasculitis; Present or Imaginary. Ann. Rheum. Dis. 2025, 84, 2349. [Google Scholar] [CrossRef]
- Sacre, K.; Criswell, L.A.; McCune, J.M. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, R155. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2024, 83, 15–29. [Google Scholar] [CrossRef]
- Takei, H.; Takanashi, S.; Otomo, K.; Hanaoka, H.; Kikuchi, J.; Yamaoka, K.; Yoshimoto, K.; Abe, T.; Takeuchi, T.; Kaneko, Y. Clinical and immunological effects of hydroxychloroquine in patients with active rheumatoid arthritis despite antirheumatic treatment. Mod. Rheumatol. 2023, 34, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Li, B.; Li, J.; Qiu, Y.; Zhu, Z.; Hua, H. An immunomodulatory antibody-drug conjugate targeting BDCA2 strongly suppresses plasmacytoid dendritic cell function and glucocorticoid responsive genes. Rheumatology 2024, 63, 242–250. [Google Scholar] [CrossRef]
- Yang, D.; Peng, N.; Zhang, H.; Qiu, Z.; Xu, L.; Pan, M. Cordycepin ameliorates autoimmunity by promoting STING degradation via autophagy pathway. Br. J. Pharmacol. 2025, 182, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Bekaddour, N.; Smith, N.; Caspar, B.; Grinberg, S.; Giorgiutti, S.; Rodeschini, V.; Dupuy, S.; Leboulanger, N.; Duffy, D.; Soulas-Sprauel, P.; et al. The histamine analogue clobenpropit modulates IRF7 phosphorylation and interferon production by targeting CXCR4 in systemic lupus erythematosus models. Front. Immunol. 2024, 15, 1490593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Wang, X.; Wang, B.; Zhao, Z.; Fu, J.; Wang, Y.; Zhang, X.; Zhu, P.; Jiang, M.; et al. Degradation of HDAC10 by autophagy promotes IRF3-mediated antiviral innate immune responses. Sci. Signal. 2022, 15, eabo4356. [Google Scholar] [CrossRef]
- Deshmukh, A.; Pereira, A.; Geraci, N.; Tzvetkov, E.; Przetak, M.; Catalina, M.D.; Morand, E.F.; Bender, A.T.; Vaidyanathan, B. Preclinical Evidence for the Glucocorticoid-Sparing Potential of a Dual Toll-Like Receptor 7/8 Inhibitor in Autoimmune Diseases. J. Pharmacol. Exp. Ther. 2024, 388, 751–764. [Google Scholar] [CrossRef]
- Witte, T.; Fernandez-Ruiz, R.; Abramova, N.; Weinelt, D.; Moreau, F.; Klopp-Schulze, L.; Shaw, J.; Denis, D.; Wenzel, J. Enpatoran, a first-in-class, selective, orally administered toll-like receptor 7/8 inhibitor, in systemic and cutaneous lupus erythematosus: Results from a randomised, placebo-controlled phase Ib study. Lupus Sci Med. 2025, 12, e001705. [Google Scholar] [CrossRef]
- Hosein, F.; Ignatenko, S.; Chadwick, K.D.; Zhu, L.; Baribaud, F.; Saini, J.; Bach, T.; Aras, U.; Zhang, W.; Karabeber, H.; et al. Safety, Tolerability, Efficacy, Pharmacokinetics, and Pharmacodynamics of Afimetoran, a Toll-Like Receptor 7 and 8 Inhibitor, in Patients With Cutaneous Lupus Erythematosus: A Phase 1b Randomized, Double-Blind, Placebo-Controlled Study. ACR Open Rheumatol. 2025, 7, e70059. [Google Scholar] [CrossRef]
- Suffiotti, M.; Brazauskas, P.; Keller, M.P.; Berkani, O.; Seifer, G.; Cornelisse, P.; Murphy, M.J.; Strasser, D.S. Pharmacodynamics of the S1P(1) receptor modulator cenerimod in a phase 2b randomised clinical trial in patients with moderate to severe SLE. Ann. Rheum. Dis. 2025, 84, 284–293. [Google Scholar] [CrossRef]
- Askanase, A.D.; D’Cruz, D.; Kalunian, K.; Merrill, J.T.; Navarra, S.V.; Cahuzac, C.; Cornelisse, P.; Murphy, M.J.; Strasser, D.S.; Trokan, L.; et al. Cenerimod, a sphingosine-1-phosphate receptor modulator, versus placebo in patients with moderate-to-severe systemic lupus erythematosus (CARE): An international, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Rheumatol. 2025, 7, e21–e32. [Google Scholar] [CrossRef]
- Sun, J.L.; Lyu, T.B.; Chen, Z.L.; Lian, C.F.; Liu, S.Y.; Shao, T.H.; Zhang, S.; Zhao, L.L.; Liu, J.J.; Peng, L.Y.; et al. Methylprednisolone pulse therapy promotes the differentiation of regulatory T cells by inducing the apoptosis of CD4(+) T cells in patients with systemic lupus erythematosus. Clin. Immunol. 2022, 241, 109079. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016, 75, 1909–1916. [Google Scholar] [CrossRef]
- Petri, M.; Wallace, D.J.; Spindler, A.; Chindalore, V.; Kalunian, K.; Mysler, E.; Neuwelt, C.M.; Robbie, G.; White, W.I.; Higgs, B.W.; et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: A phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013, 65, 1011–1021. [Google Scholar] [CrossRef]
- Kalunian, K.C.; Merrill, J.T.; Maciuca, R.; McBride, J.M.; Townsend, M.J.; Wei, X.; Davis, J.C., Jr.; Kennedy, W.P. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Lauwerys, B.R.; Hachulla, E.; Spertini, F.; Lazaro, E.; Jorgensen, C.; Mariette, X.; Haelterman, E.; Grouard-Vogel, G.; Fanget, B.; Dhellin, O.; et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 2013, 65, 447–456. [Google Scholar] [CrossRef]
- Higgs, B.W.; Zhu, W.; Morehouse, C.; White, W.I.; Brohawn, P.; Guo, X.; Rebelatto, M.; Le, C.; Amato, A.; Fiorentino, D.; et al. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann. Rheum. Dis. 2014, 73, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Emmas, C.; Nekeman-Nan, C.; Stirnadel-Farrant, H.; Chen, S.; Carty, L.; Waratani, M.; Seo, C.; Chen, S.; Sorrentino, A. Anifrolumab Study for Treatment Effectiveness in the Real World (ASTER) among patients with systemic lupus erythematosus: Protocol for an international observational effectiveness study. BMJ Open 2024, 14, e086055. [Google Scholar] [CrossRef] [PubMed]
- Karonitsch, T.; Yeghiazaryan, L.; Lackner, A.; Brezinsek, H.P.; Stamm, T.A.; König, F.; Aletaha, D.; Smolen, J.S. Targeting type I interferon (IFN) signalling in patients with RA with a high type I IFN gene signature. RMD Open 2022, 8, e002525. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Chen, X.; Ke, H.; Li, W.; Yin, L.; Chen, W.; Chen, T.; Wu, Y.; Qiu, J.; Feng, W. Structural basis for the recognition of IFNAR1 by the humanized therapeutic monoclonal antibody QX006N for the treatment of systemic lupus erythematosus. Int. J. Biol. Macromol. 2024, 268, 131721. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, H.; Wang, Y.; Sun, Z.; Yin, M. Discovery of WD-890: A novel allosteric TYK2 inhibitor for the treatment of multiple autoimmune diseases. Biomed. Pharmacother. 2023, 167, 115611. [Google Scholar] [CrossRef]
- Ma, X.; Nakayamada, S.; Kubo, S.; Sakata, K.; Yamagata, K.; Miyazaki, Y.; Yoshikawa, M.; Kitanaga, Y.; Zhang, M.; Tanaka, Y. Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann. Rheum. Dis. 2018, 77, 1354–1361. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Wang, C.; Qin, C. Clinical outcomes of baricitinib in patients with systemic lupus erythematosus: Pooled analysis of SLE-BRAVE-I and SLE-BRAVE-II trials. PLoS ONE 2025, 20, e0320179. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.M.; Ahmed Fatmi, S.A.; Ashraf, M.H.; Waheed, F.; Jawwad, M.; Hai, A.A.; Ahmed, S.A. Efficacy of baricitinib in the treatment of Systemic lupus erythematosus (SLE): A Systemic review and meta-analysis. Heliyon 2023, 9, e22643. [Google Scholar] [CrossRef] [PubMed]
- Werth, V.P.; Fleischmann, R.; Robern, M.; Touma, Z.; Tiamiyu, I.; Gurtovaya, O.; Pechonkina, A.; Mozaffarian, A.; Downie, B.; Matzkies, F.; et al. Filgotinib or lanraplenib in moderate to severe cutaneous lupus erythematosus: A phase 2, randomized, double-blind, placebo-controlled study. Rheumatology 2022, 61, 2413–2423. [Google Scholar] [CrossRef]
- Merrill, J.T.; Saxena, A.; Aringer, M.; Tanaka, Y.; Zeng, X.; Cheng, L.; Doan, T.T.; D’Cruz, D.; Masri, K.R.; D’Silva, K.M. Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: Results through 104 weeks in a long-term extension study. RMD Open 2025, 11, e005742. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ning, W.; Tang, H.; Mu, C.; Huang, X. Efficacy and safety study of targeted small-molecule drugs in the treatment of systemic lupus erythematosus. Arthritis Res. Ther. 2024, 26, 98. [Google Scholar] [CrossRef]
- Satoh-Kanda, Y.; Nakayamada, S.; Tanaka, Y. Fine-tuning SLE treatment: The potential of selective TYK2 inhibition. RMD Open 2024, 10, e005072. [Google Scholar] [CrossRef]
- Morand, E.; Pike, M.; Merrill, J.T.; van Vollenhoven, R.; Werth, V.P.; Hobar, C.; Delev, N.; Shah, V.; Sharkey, B.; Wegman, T.; et al. Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2023, 75, 242–252. [Google Scholar] [CrossRef]
- Wenzel, J.; van Holt, N.; Maier, J.; Vonnahme, M.; Bieber, T.; Wolf, D. JAK1/2 Inhibitor Ruxolitinib Controls a Case of Chilblain Lupus Erythematosus. J. Investig. Dermatol. 2016, 136, 1281–1283. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Kubo, S.; Yamaoka, K.; Kondo, M.; Yamagata, K.; Zhao, J.; Iwata, S.; Tanaka, Y. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann. Rheum. Dis. 2014, 73, 2192–2198. [Google Scholar] [CrossRef]
- Bonelli, M.; Dalwigk, K.; Platzer, A.; Olmos Calvo, I.; Hayer, S.; Niederreiter, B.; Holinka, J.; Sevelda, F.; Pap, T.; Steiner, G.; et al. IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes: JAKinibs suppress the interferon response in RA-FLSs. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Dörner, T.; Tanaka, Y.; Petri, M.; Smolen, J.S.; Dow, E.R.; Higgs, R.E.; Benschop, R.J.; Abel, A.; Silk, M.E.; Bono, S.d.; et al. 185 Baricitinib-associated changes in type I interferon gene signature during a 24-week phase 2 clinical SLE trial. Lupus Sci. Med. 2019, 6. [Google Scholar] [CrossRef]
- Itotagawa, E.; Tomofuji, Y.; Kato, Y.; Konaka, H.; Tsujimoto, K.; Park, J.; Nagira, D.; Hirayama, T.; Jo, T.; Hirano, T.; et al. SLE stratification based on BAFF and IFN-I bioactivity for biologics and implications of BAFF produced by glomeruli in lupus nephritis. Rheumatology 2023, 62, 1988–1997. [Google Scholar] [CrossRef]
- Li, H.; Ju, B.; Luo, J.; Zhu, L.; Zhang, J.; Hu, N.; Mo, L.; Wang, Y.; Tian, J.; Li, Q.; et al. Type I interferon-stimulated genes predict clinical response to belimumab in systemic lupus erythematosus. Eur. J. Pharmacol. 2025, 987, 177204. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, T.; Wang, P.; Chen, W.; Liu, W.; He, X.; Zhang, Y.; Jin, S.; Luo, Z.; Zhang, Z.; et al. Imatinib and M351-0056 enhance the function of VISTA and ameliorate the development of SLE via IFN-I and noncanonical NF-κB pathway. Cell Biol. Toxicol. 2023, 39, 3287–3304. [Google Scholar] [CrossRef]
- Shan, S.; Zhou, Y.; Yu, J.; Yang, Q.; Pan, D.; Wang, Y.; Li, L.; Zhu, J.; Zhang, Y.; Huang, S.; et al. Therapeutic treatment of a novel selective JAK3/JAK1/TBK1 inhibitor, CS12192, in rat and mouse models of rheumatoid arthritis. Int. Immunopharmacol. 2019, 77, 105914. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.; Ngo, D.; D’Silva, D.B.; Hansen, J.; Phillipson, L.; Jousset, H.; Novello, P.; Segal, D.; Lawlor, K.E.; Burns, C.J.; et al. Therapeutic Effects of a TANK-Binding Kinase 1 Inhibitor in Germinal Center-Driven Collagen-Induced Arthritis. Arthritis Rheumatol. 2019, 71, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Hu, Y.; Dai, J.; He, L.; He, J.; Xu, B.; Han, X.; Zhong, F.; Lan, H.; Wang, Q. CS12192, a Novel JAK3/JAK1/TBK1 Inhibitor, Synergistically Enhances the Anti-Inflammation Effect of Methotrexate in a Rat Model of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 13394. [Google Scholar] [CrossRef] [PubMed]
- Hammaker, D.; Boyle, D.L.; Firestein, G.S. Synoviocyte innate immune responses: TANK-binding kinase-1 as a potential therapeutic target in rheumatoid arthritis. Rheumatology 2012, 51, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Torigo, S.; Ihara, T.; Kamiya, H. IL-12, IFN-gamma, and TNF-alpha released from mononuclear cells inhibit the spread of varicella-zoster virus at an early stage of varicella. Microbiol. Immunol. 2000, 44, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- van Panhuys, N.; Tang, S.C.; Prout, M.; Camberis, M.; Scarlett, D.; Roberts, J.; Hu-Li, J.; Paul, W.E.; Le Gros, G. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 12423–12428. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Werth, V.P.; Furie, R.A.; Romero-Diaz, J.; Navarra, S.; Kalunian, K.; van Vollenhoven, R.F.; Nyberg, F.; Kaffenberger, B.H.; Sheikh, S.Z.; Radunovic, G.; et al. Trial of Anti-BDCA2 Antibody Litifilimab for Cutaneous Lupus Erythematosus. N. Engl. J. Med. 2022, 387, 321–331. [Google Scholar] [CrossRef]
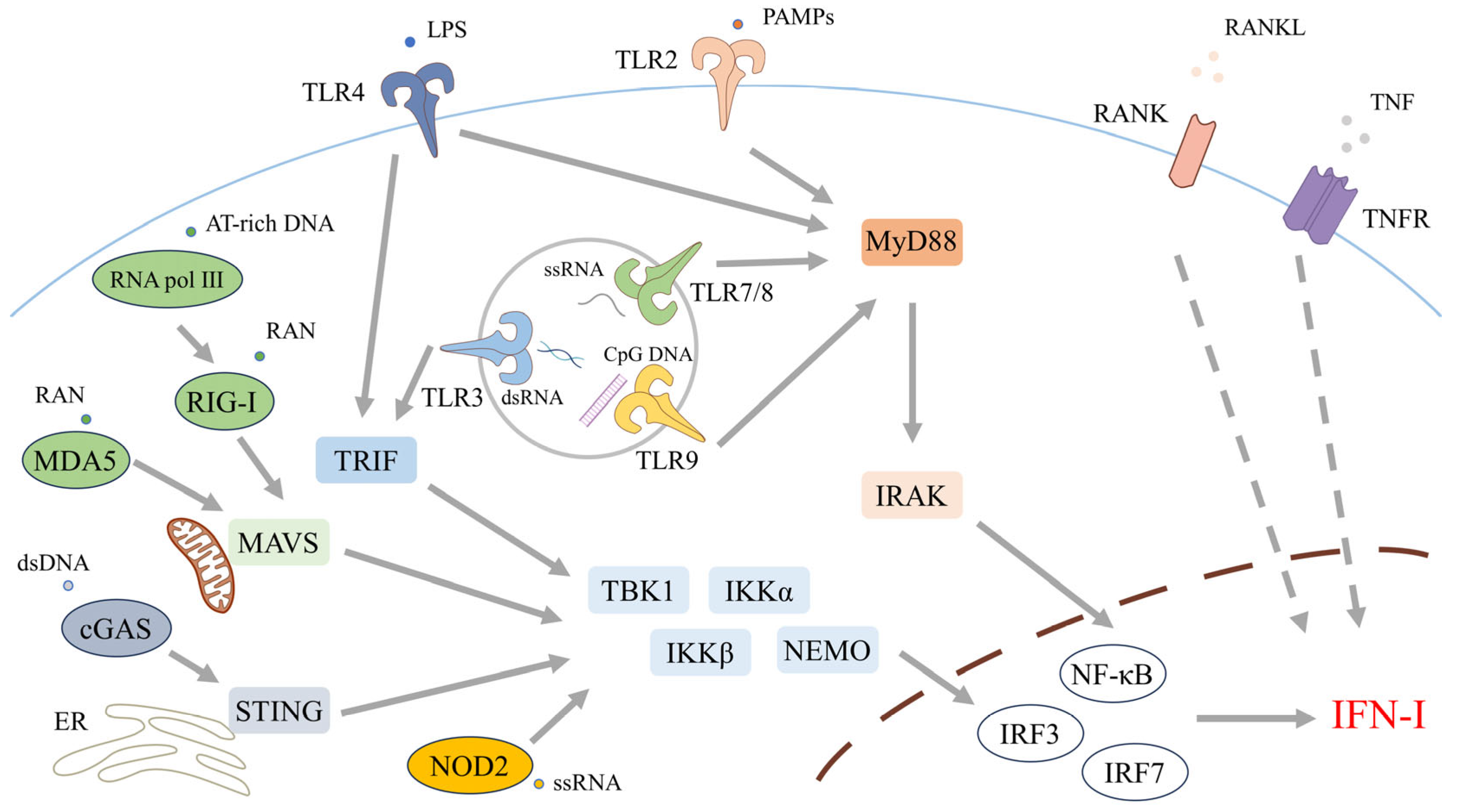
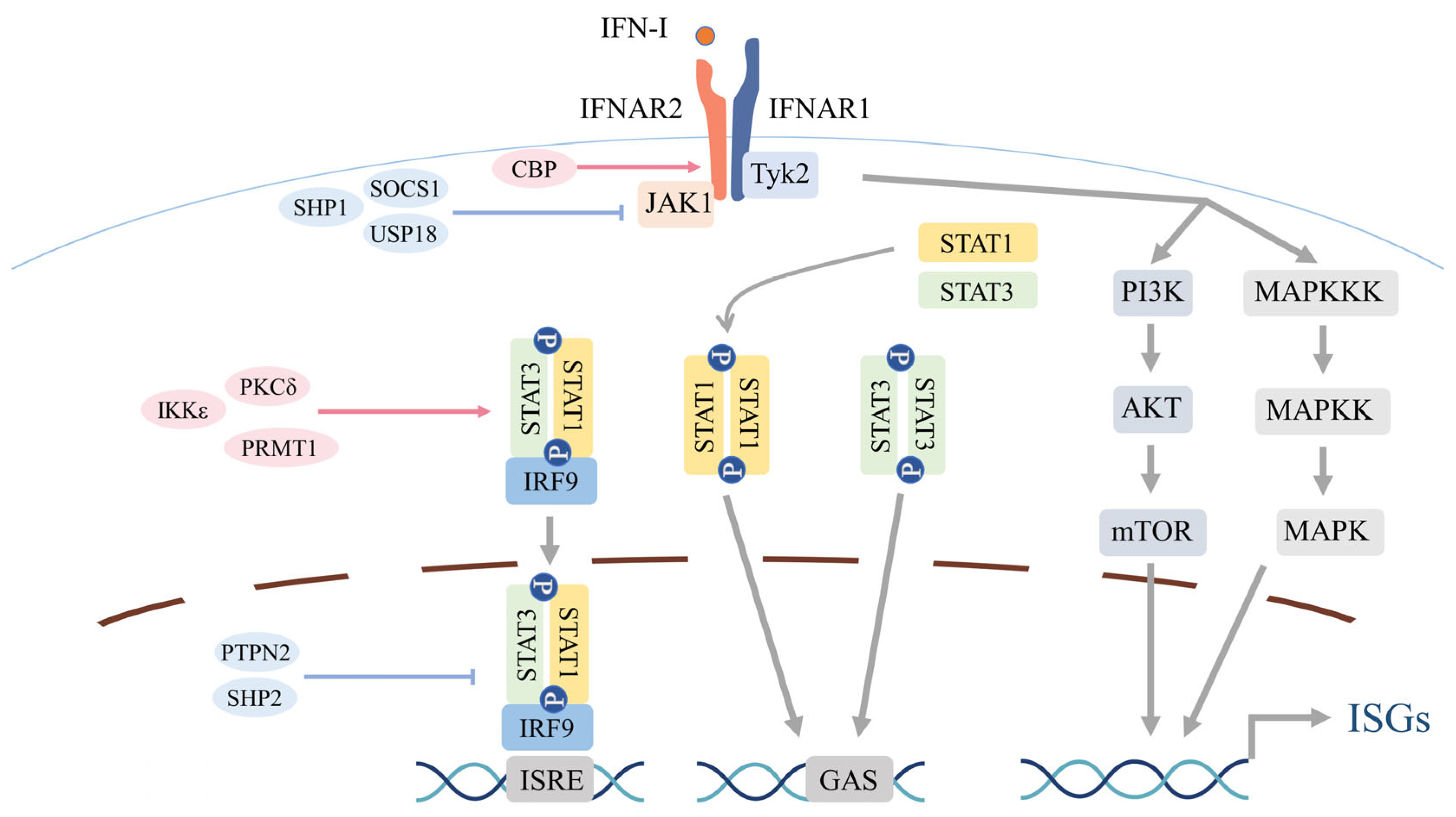
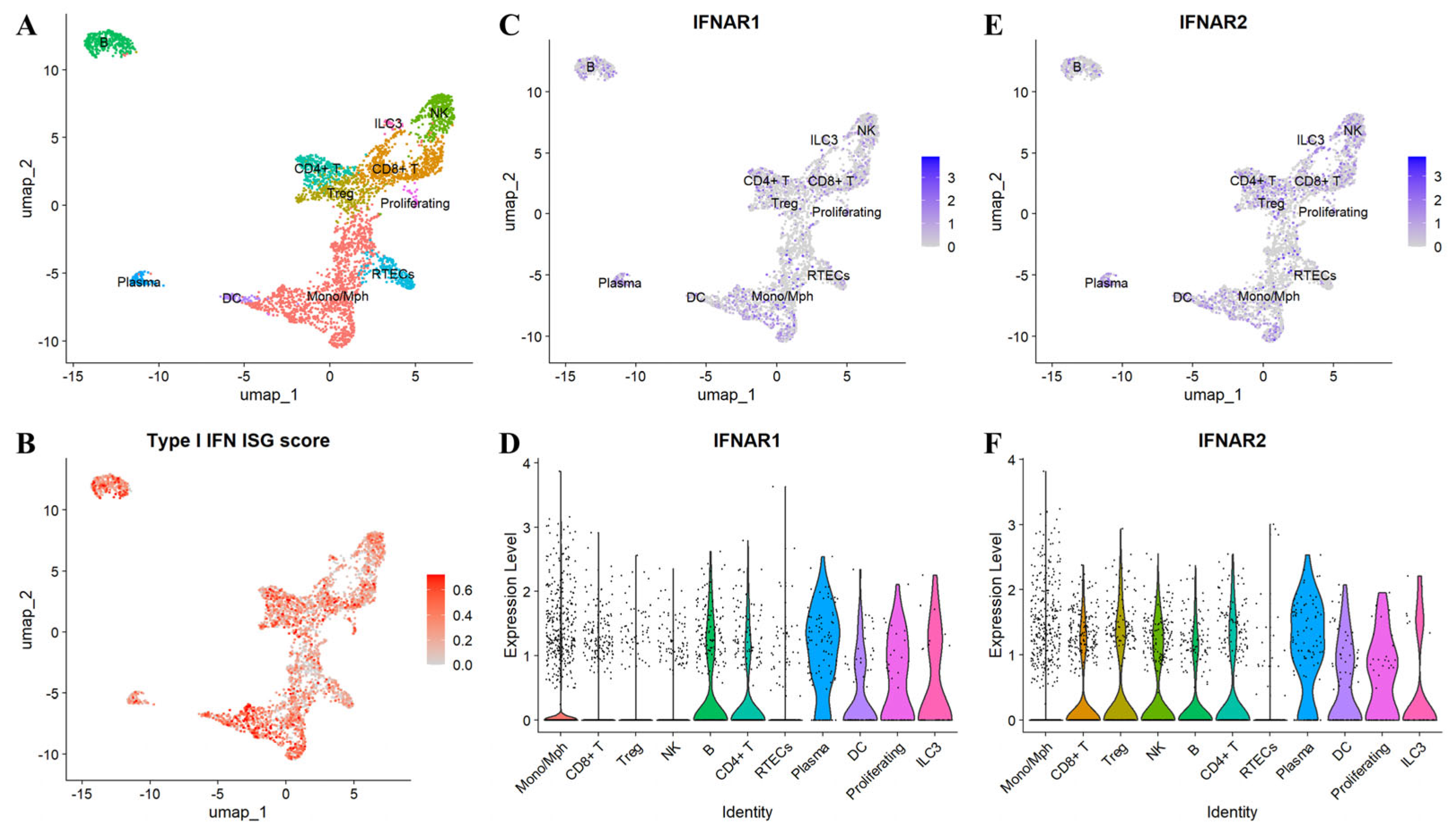
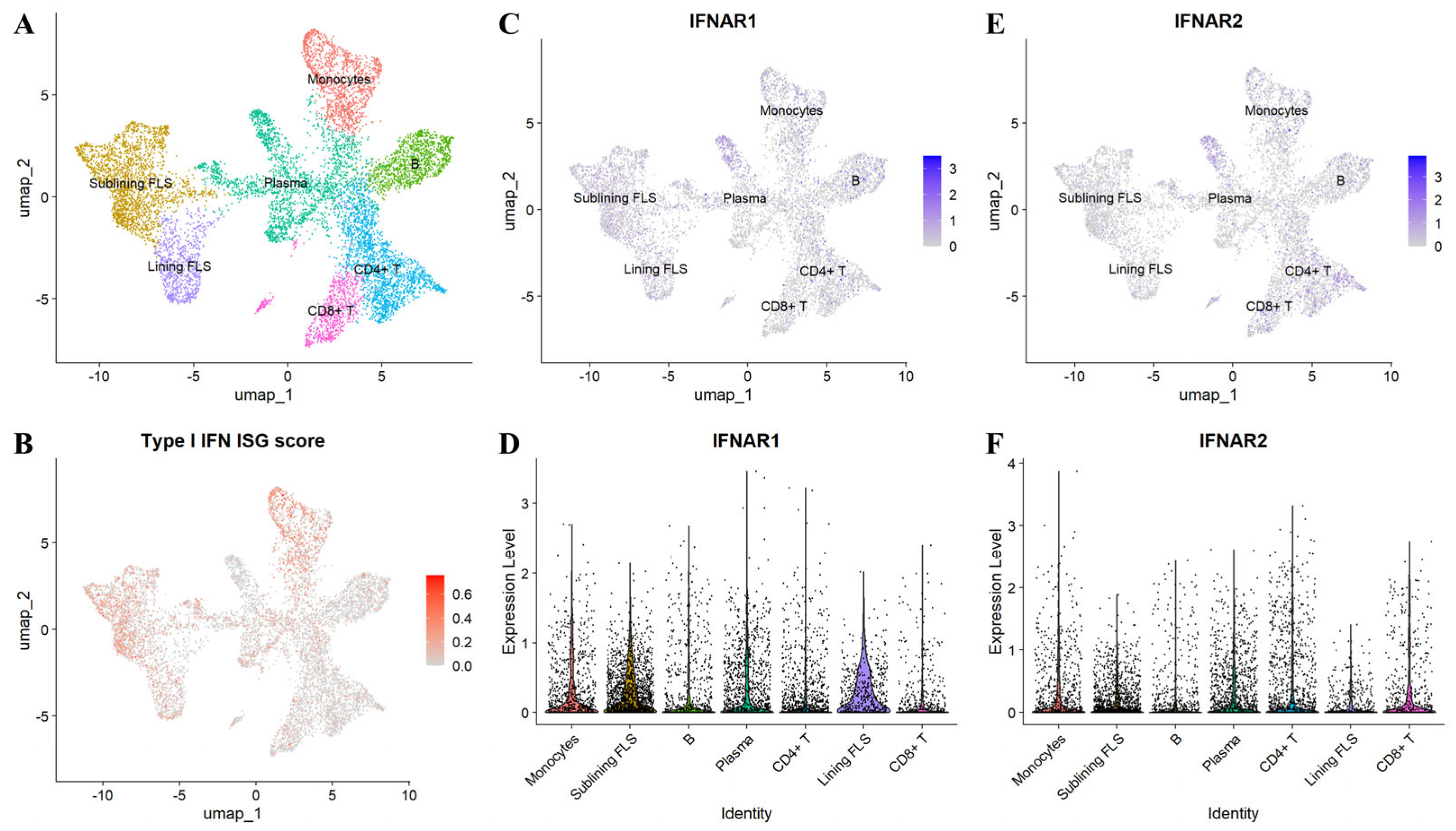
| Regulator | Level/Target | Mechanism | Net Effect | Refs. |
|---|---|---|---|---|
| SOCS family | Receptor–proximal; JAK1/TYK2; IFNAR1 | Inhibit JAK1/TYK2; block STAT recruitment/phosphorylation via IFNAR1 binding | Negative | [95,96] |
| USP18 | Receptor-proximal (IFNAR2/JAK1); ISGylation axis | Competes with JAK1 at IFNAR2 (receptor brake) and acts as the principal de-ISGylase removing ISG15 from substrates | Negative | [97,98] |
| SHP1/PTPN6, SHP2/PTPN11, PTPN2 | Receptor-proximal and nuclear JAK–STAT | Dephosphorylate receptor/JAK/STAT components—including nuclear STAT1—to terminate signaling and limit the IFN signature | Negative | [99,100] |
| PKCδ → STAT1(Ser727) | STAT1 | Phosphorylates Ser727 to boost STAT1 transcriptional output | Positive | [101] |
| IKKε → STAT1(Ser708) | STAT1 | Phosphorylates Ser708, enhancing DNA binding and ISG transcription | Positive | [102] |
| PRMT1 → STAT1 (Arg methylation) | STAT1 | Arginine methylation enhances STAT1 DNA binding and transactivation, amplifying ISG expression | Positive | [103] |
| CBP/GCN5 | Histones proximal to STAT complexes | Acetylate nearby histones in a context-dependent manner, modulating chromatin accessibility and ISG transcription | Context-dependent | [104,105] |
| STAT1 hyperacetylation | STAT1 | Impedes STAT1 phosphorylation, nuclear translocation, and DNA binding | Negative | [106] |
| HDAC3 | STAT1 | Deacetylation counterbalances inhibitory hyperacetylation, restoring STAT1 function | Positive | [106] |
| Ubiquitination | Pathway-wide (IFNAR1; STAT1/STAT4) | K48 chains drive proteasomal degradation (e.g., IFNAR1 via SCF (HOS); STATs via SLIM/Smurf1) to dampen signaling; K63 chains support signal propagation; NKLAM promotes STAT1 phosphorylation/transactivation | Context-dependent | [107,108,109,110,111,112] |
| SUMOylation (PIAS1 → STAT1 Lys703) | STAT1 | SUMOylation suppresses STAT1 activity and reduces ISG expression | Negative | [113] |
| ISGylation (ISG15 via UBE1L–UBCH8–HERC5) | Pathway-wide substrates | Covalent ISG15 conjugation that generally potentiates IFN-I signaling | Positive | [98] |
| Histone acetylation axis (BRD4–P-TEFb; HDACs incl. HDAC1–PLZF) | ISG chromatin/transcriptional elongation | Acetylation recruits BRD4–P-TEFb to promote elongation; HDAC activity remodels/limits ISG programs (HDAC1 recruits PLZF) | Context-dependent | [105,114,115,116] |
| H3K9me2 | ISG chromatin | Repressive histone mark that limits ISG induction | Negative | [117,118] |
| H2B monoubiquitination | ISG chromatin | IFN-induced H2B-ub promotes chromatin opening; PARP9–DTX3L supports H2B-ub and ISG transcription | Positive | [119,120] |
| DNA methylation/TET–5hmC axis | ISG chromatin/DNA | DNMT3A/3B install 5mC (generally repressive); TET-mediated 5hmC participates in transcriptional control; ISG-specific roles remain unresolved | Context-dependent | [121,122,123,124] |
| microRNAs (miRNAs) | IFNAR, JAK–STAT components, ISG mRNAs | Post-transcriptional silencing that can dampen or enhance IFN-I signaling (e.g., miR-29a ↓IFNAR1; miR-155 ↓SOCS1 → ↑signaling) | Context-dependent | [125,126,127] |
| long noncoding RNAs (lncRNAs) | Chromatin/RNA regulatory layer | Scaffold and cis-regulatory functions that tune IFN output (e.g., lnc-DC → STAT3 phosphorylation ↑; IFN-inducible lncRNAs; NeST/Tmevpg1 → H3K4me3 via WDR5 ↑; NRAV ↓ ISG program) | Context-dependent | [128,129,130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, R.; Watanabe, R.; Shiomi, M.; Fujita, Y.; Katsushima, M.; Fukumoto, K.; Yamada, S.; Hashimoto, M. The Type I Interferon Axis in Systemic Autoimmune Diseases: From Molecular Pathways to Targeted Therapy. Biomolecules 2025, 15, 1586. https://doi.org/10.3390/biom15111586
Ishihara R, Watanabe R, Shiomi M, Fujita Y, Katsushima M, Fukumoto K, Yamada S, Hashimoto M. The Type I Interferon Axis in Systemic Autoimmune Diseases: From Molecular Pathways to Targeted Therapy. Biomolecules. 2025; 15(11):1586. https://doi.org/10.3390/biom15111586
Chicago/Turabian StyleIshihara, Ryuhei, Ryu Watanabe, Mayu Shiomi, Yuya Fujita, Masao Katsushima, Kazuo Fukumoto, Shinsuke Yamada, and Motomu Hashimoto. 2025. "The Type I Interferon Axis in Systemic Autoimmune Diseases: From Molecular Pathways to Targeted Therapy" Biomolecules 15, no. 11: 1586. https://doi.org/10.3390/biom15111586
APA StyleIshihara, R., Watanabe, R., Shiomi, M., Fujita, Y., Katsushima, M., Fukumoto, K., Yamada, S., & Hashimoto, M. (2025). The Type I Interferon Axis in Systemic Autoimmune Diseases: From Molecular Pathways to Targeted Therapy. Biomolecules, 15(11), 1586. https://doi.org/10.3390/biom15111586






