Developmental Phase-Specific Molecular Signatures and Signaling Pathways in Cryptorchidism-Induced Testicular Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Cryptorchidism Model
2.2. Hematoxylin-Eosin (HE) Staining
2.3. Reproductive Assessment
2.4. Analysis of Sperm Quality
2.5. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Western Blotting
2.7. Quantification of Cytokines in Testicular Tissues
2.8. Source of Data
2.9. Differentially Expressed Genes (DEGs) and Enrichment Analysis
2.10. Identification of Key Genes
2.11. Gene Set Enrichment Analysis (GSEA) and Ingenuity Pathway Analysis (IPA)
2.12. Analysis of Correlations
2.13. Drug Prediction
2.14. Quantitative Analysis
3. Results
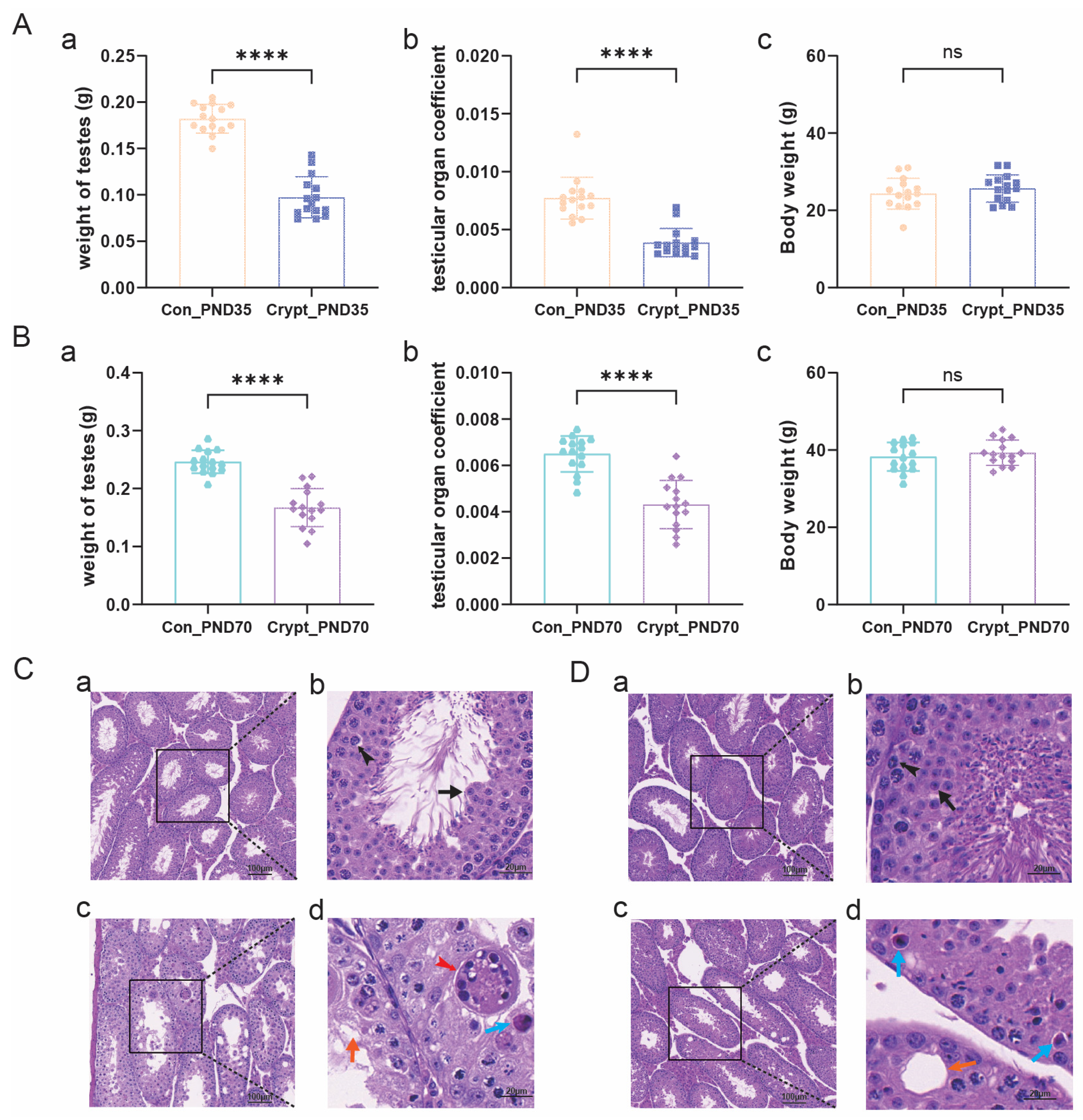
3.1. Morphological and Histopathological Alterations in Cryptorchid Testes at PND35 and PND70
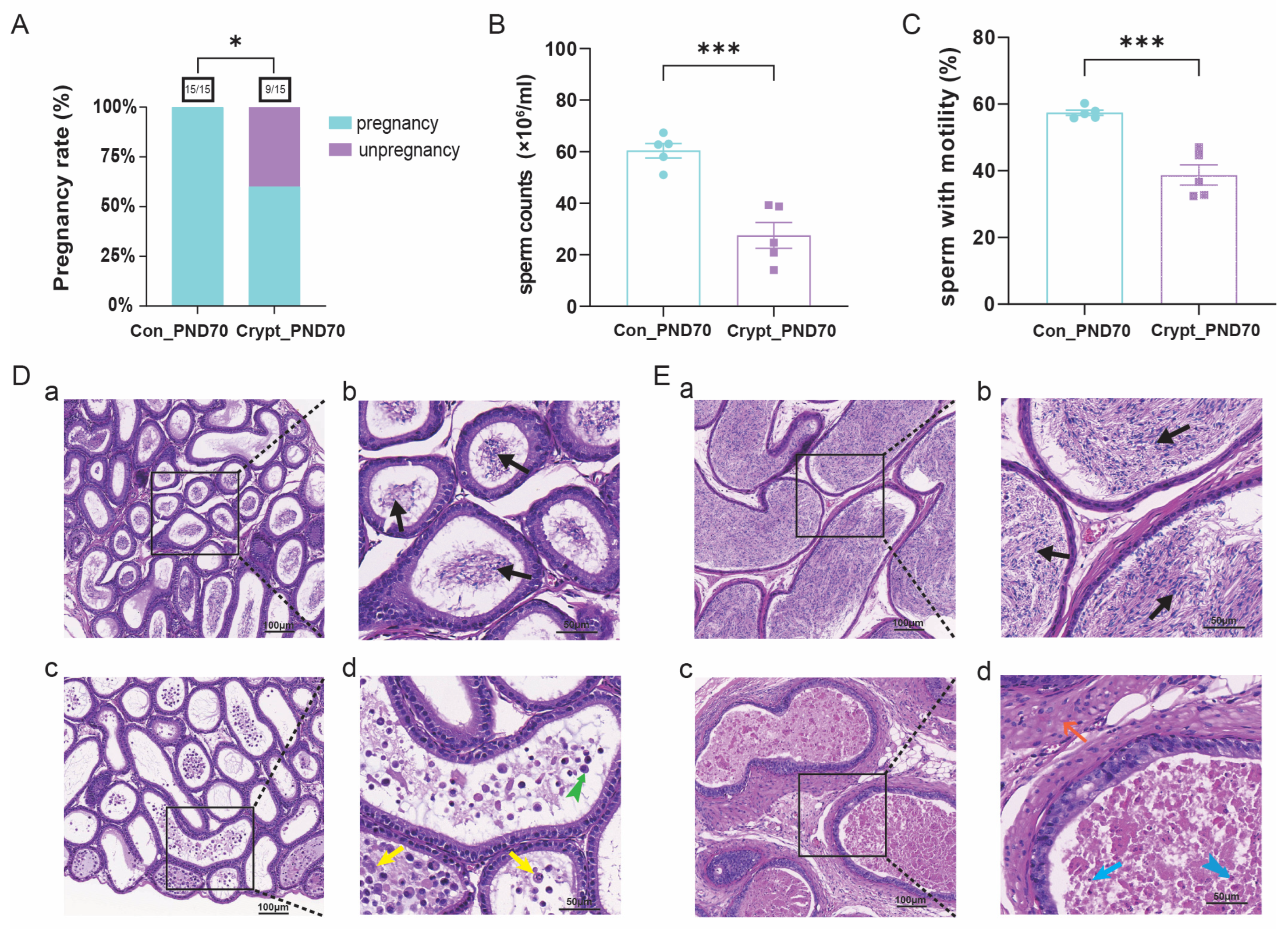
3.2. Cryptorchidism-Induced Spermatogenic Dysfunction at PND70
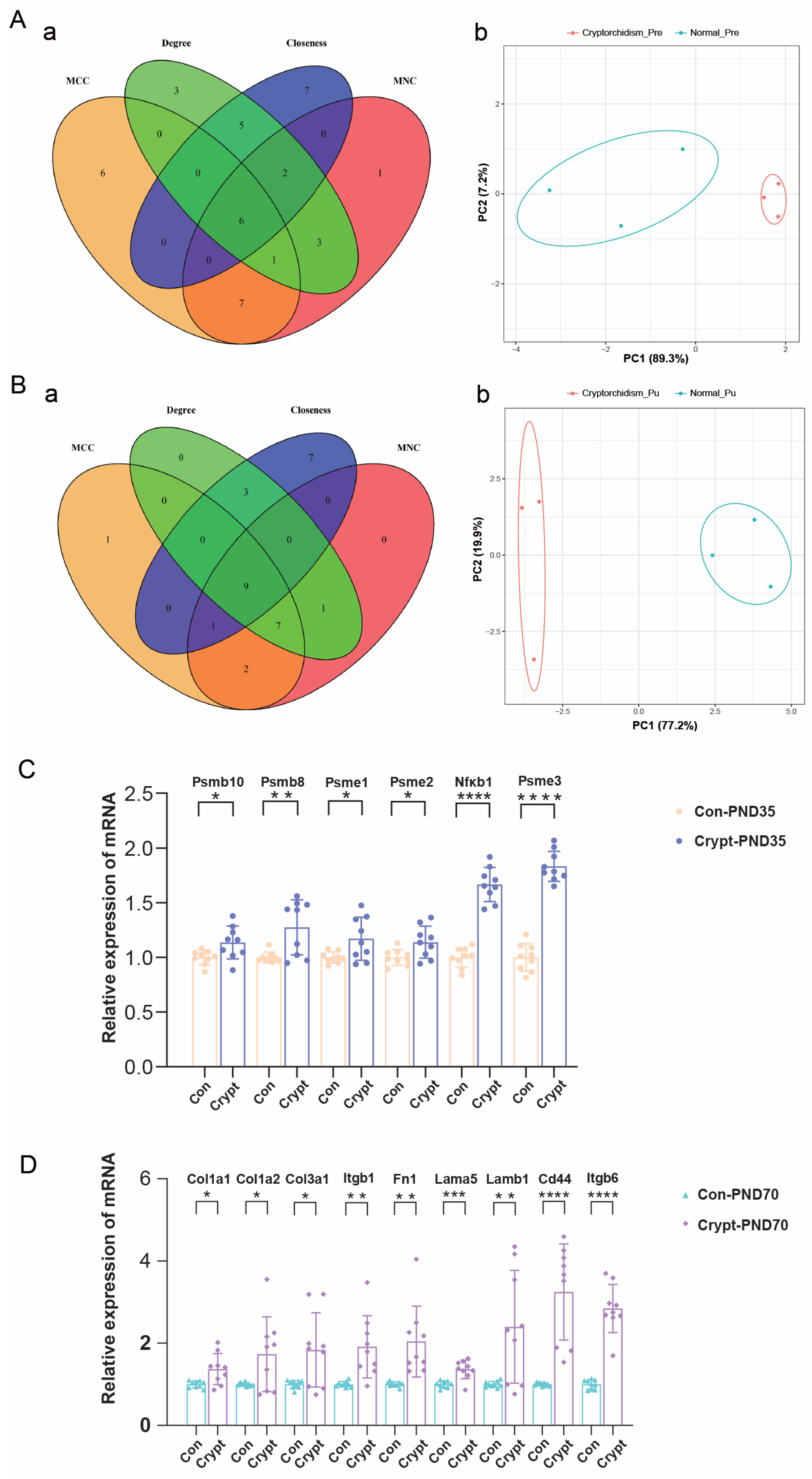
3.3. Identification of Pre-DEGs and Mat-DEGs
3.4. Phase-Specific Pathway Enrichment Analysis
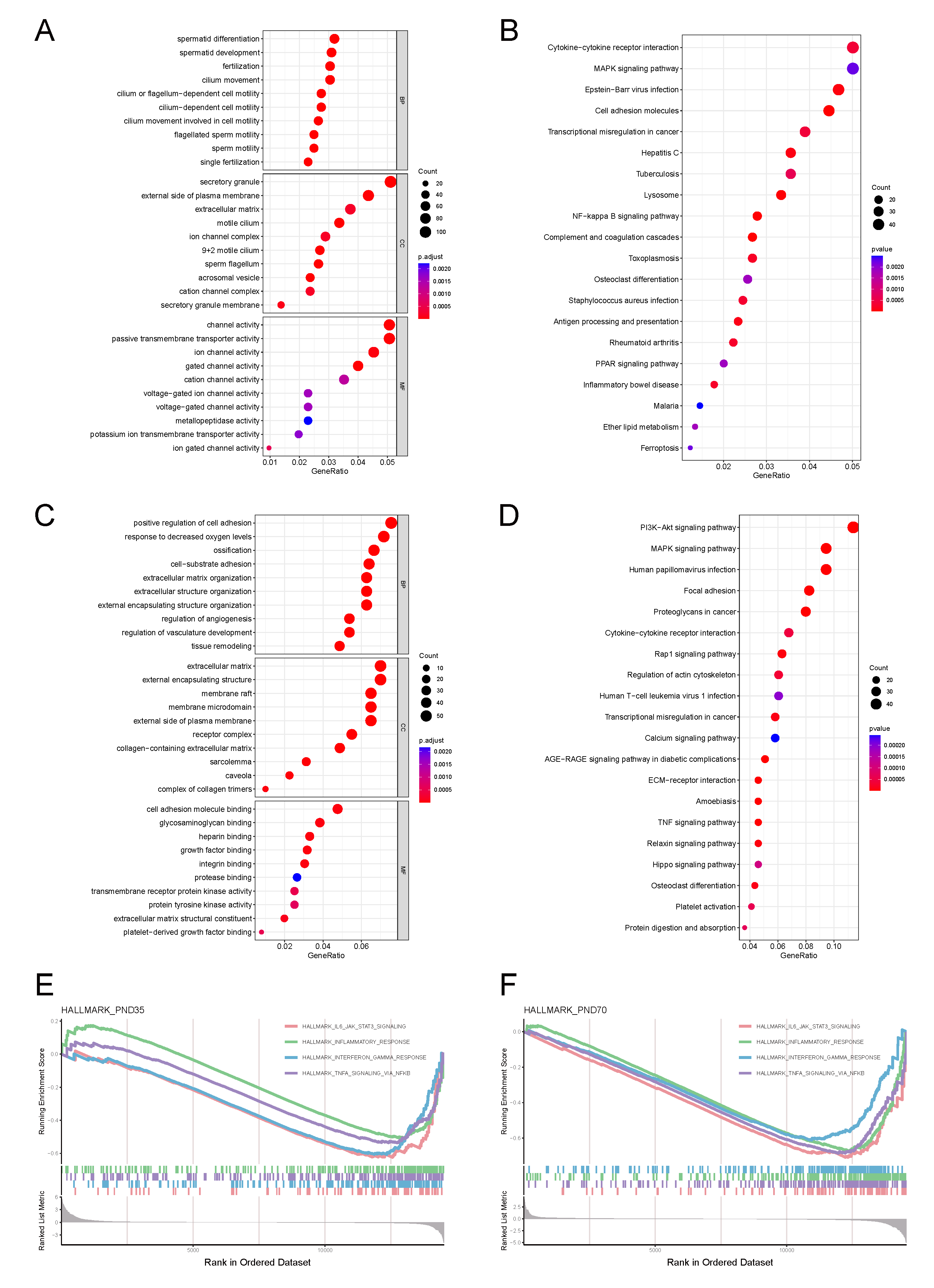
3.4.1. Prepubertal Phase Characteristics
3.4.2. Sexually Mature Phase Characteristics
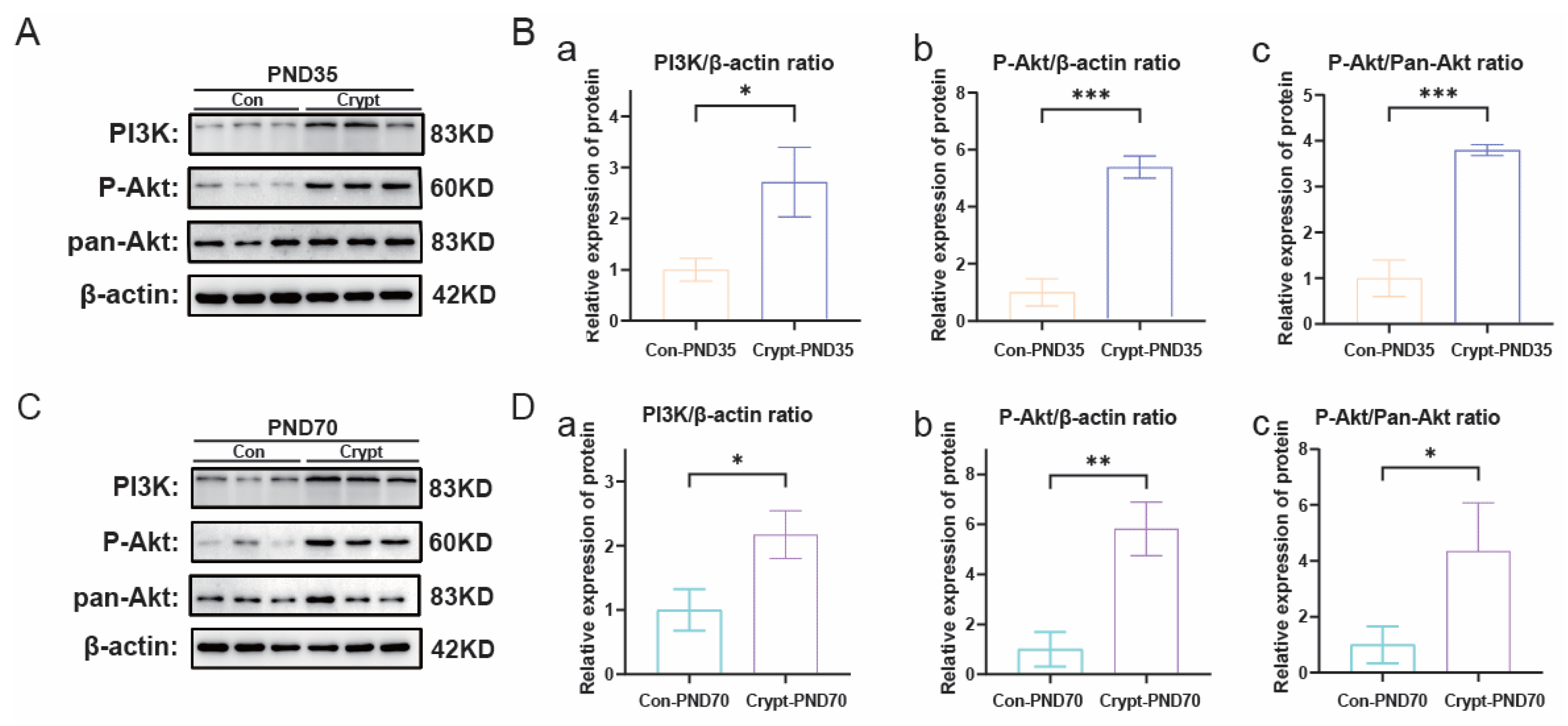
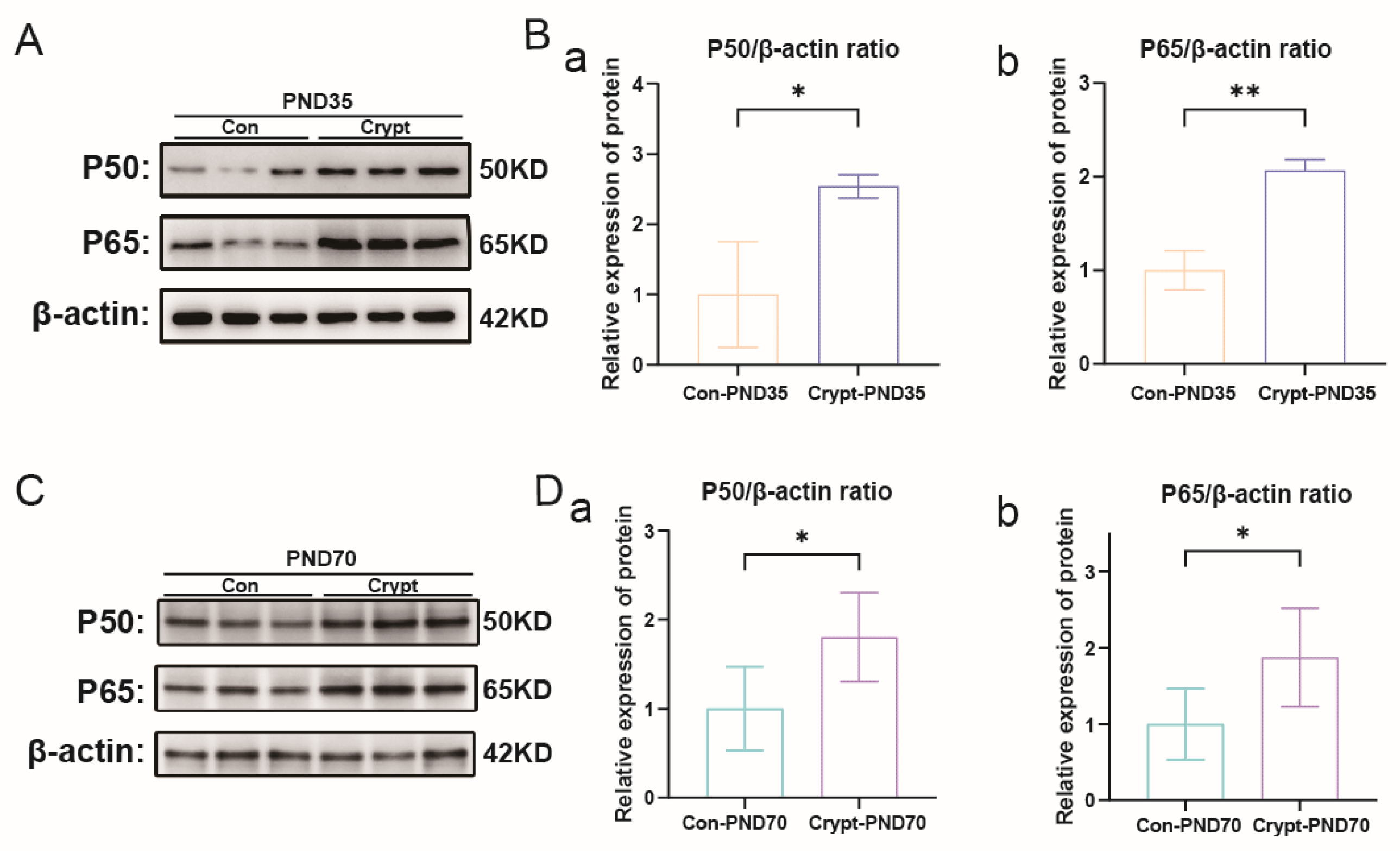
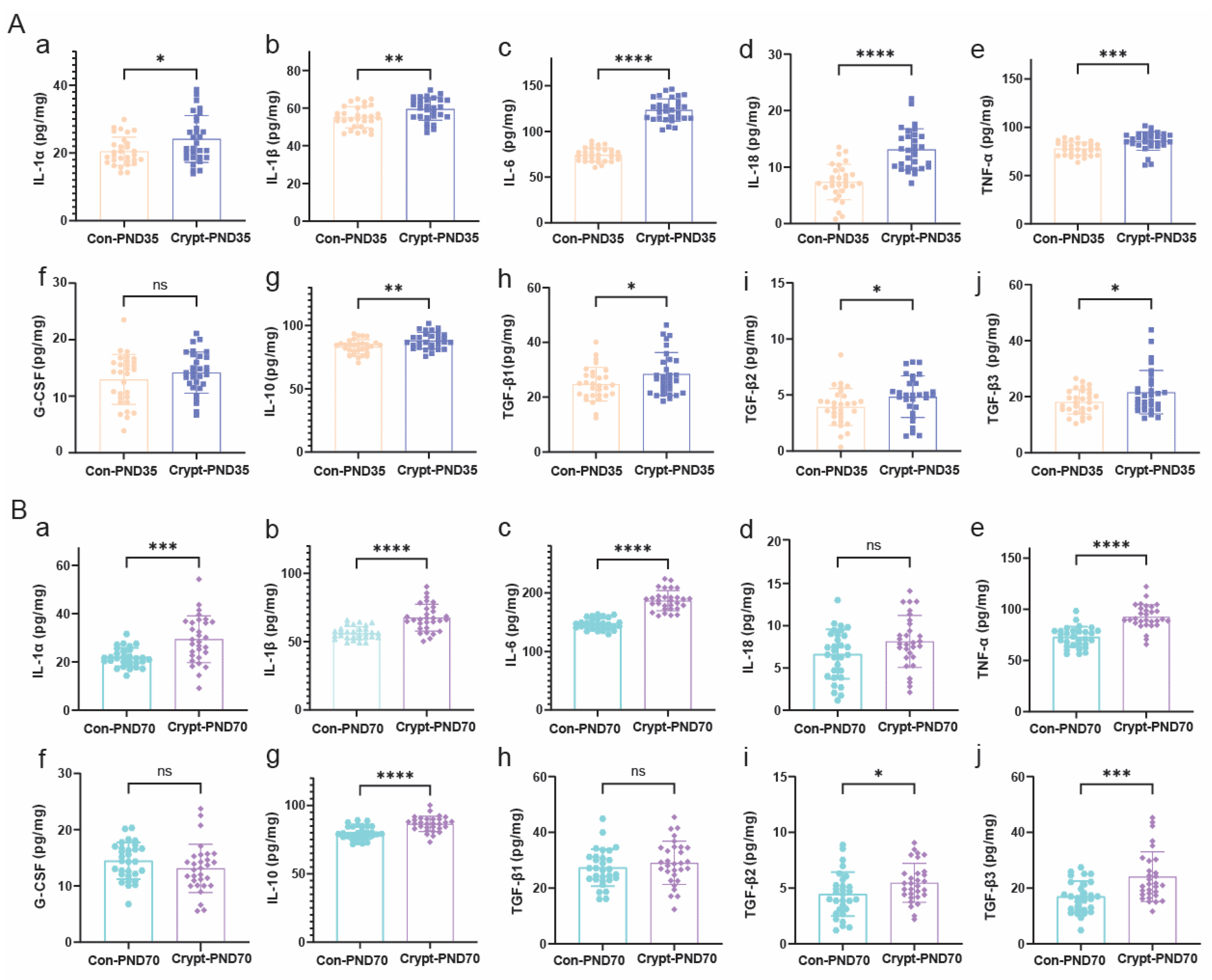
3.5. Cryptorchidism-Activated Signalling Pathways and Inflammatory Mediators
3.6. Identification and Validation of Phase-Specific Key Genes in Cryptorchidism
3.7. Functional Enrichment Analysis of Phase-Specific Key Genes
3.8. Association of Phase-Specific Key Genes with Oncogenic Pathways
3.9. Predicted Drug-Key Gene Interaction Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collet, B.; Desalegn, A.A.; Swart, K.; Naderman, M.; Iszatt, N.; Stigum, H.; Jensen, T.K.; Brouwer, A.; Eggesbø, M.; van der Burg, B. Anti-androgenic compounds in breast milk and cryptorchidism among Norwegian boys in the HUMIS birth cohort. Sci. Total Environ. 2022, 803, 149746. [Google Scholar] [CrossRef] [PubMed]
- Kargl, S.; Haid, B. Torsion of an undescended testis—A surgical pediatric emergency. J. Pediatr. Surg. 2020, 55, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Hildorf, S.; Clasen-Linde, E.; Cortes, D.; Fossum, M.; Thorup, J. Fertility Potential is Compromised in 20% to 25% of Boys with Nonsyndromic Cryptorchidism despite Orchiopexy within the First Year of Life. J. Urol. 2020, 203, 832–840. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Zhuang, Z.; Cheng, J.; Zhang, W.; Jiang, Q.; Guo, Y.; Li, R.; Lu, X.; Cui, L.; et al. Decoding the pathogenesis of spermatogenic failure in cryptorchidism through single-cell transcriptomic profiling. Cell Rep. Med. 2024, 5, 101709. [Google Scholar] [CrossRef]
- Dessanti, A.; Falchetti, D.; Alberti, D.; Milianti, S.; Iannuccelli, M.; Corasaniti, L.; Pellegrino, M.; Strusi, G.P. Two-stage orchiopexy for intra-abdominal testis with short spermatic vessels wrapped in anti-adhesion conduit. 25 years of experience. J. Pediatr. Urol. 2024, 20, 985–989. [Google Scholar] [CrossRef]
- Hildorf, S.E.; Clasen-Linde, E.; Cortes, D.; Fossum, M.; Thorup, J. The positive predictive value of using fsh and Inhibin-B serum levels to diagnose gonadotropin insufficiency in bilateral cryptorchid boys is high. J. Pediatr. Urol. 2022, 18, e1–e844. [Google Scholar] [CrossRef]
- Robinson, B.R.; Netherton, J.K.; Ogle, R.A.; Baker, M.A. Testicular heat stress, a historical perspective and two postulates for why male germ cells are heat sensitive. Biol. Rev. Camb. Philos. Soc. 2023, 98, 603–622. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Huo, Y.; Xue, H.; Wang, W.; Zhang, J.; Wang, X. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Jia, Y.; Swerdloff, R.S.; Lue, Y.; Dai-Ju, J.; Surampudi, P.; Cohen, P.; Wang, C. The IL-27 component EBI-3 and its receptor subunit IL-27Rα are essential for the cytoprotective action of humanin on male germ cells. Biol. Reprod. 2021, 104, 717–730. [Google Scholar] [CrossRef]
- Shojaeian, R.; Hiradfar, M.; Taqanaki, P.B.; Ahmadi, S.K.; Masouleh, Y.J.; Ameri, L.; Mashhadi, M.P. Investigating the effect of sudden occlusion of the testicular vessels on the testicular tissue in rat models. Ann. Med. Surg. 2023, 85, 3906–3911. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Q.; Qian, Z.; Xiao, B.; Luo, K.; Luo, N. Joint Analysis of Genome-wide DNA Methylation and Transcription Sequencing Identifies the Role of BAX Gene in Heat Stress-Induced-Sertoli Cells Apoptosis. Reprod. Sci. 2024, 31, 1311–1322. [Google Scholar] [CrossRef]
- Wang, D.; Hildorf, S.; Ntemou, E.; Dong, L.; Pors, S.E.; Mamsen, L.S.; Fedder, J.; Hoffmann, E.R.; Clasen-Linde, E.; Cortes, D.; et al. Characterization and Survival of Human Infant Testicular Cells After Direct Xenotransplantation. Front. Endocrinol. 2022, 13, 853482. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ping, H.; Li, K.; Lin, J.; Jiang, X.; Zhang, X. Environmental oestrogens disrupt testicular descent and damage male reproductive health: Mechanistic insight. J. Cell. Mol. Med. 2023, 27, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M. Undescended testis: The underlying mechanisms and the effects on germ cells that cause infertility and cancer. J. Pediatr. Surg. 2013, 48, 903–908. [Google Scholar] [CrossRef]
- Hanna, N.H.; Einhorn, L.H. Testicular cancer—discoveries and updates. N. Engl. J. Med. 2014, 371, 2005–2016. [Google Scholar] [CrossRef]
- Ornstrup, M.J.; Berglund, A.; Agerbæk, M.; Gravholt, C.H. Pre-existing morbidity pattern in a Danish testicular cancer cohort: Insights beyond the testicular dysgenesis syndrome hypothesis. Hum. Reprod. Open 2025, 2025, hoaf021. [Google Scholar] [CrossRef]
- Shiraishi, K.; Takihara, H.; Matsuyama, H. Testicular Temperature and the Effects of Orchiopexy in Infants with Cryptorchidism. J. Urol. 2021, 206, 1031–1037. [Google Scholar] [CrossRef]
- Xu, R.; McQuaid, J.W.; Paulson, V.A.; Kurtz, M.P.; Logvinenko, T.; Yu, R.N.; Lee, R.S.; Nelson, C.P. Malignancy Yield of Testis Pathology in Older Boys and Adolescents with Cryptorchidism. J. Urol. 2022, 207, 694–700. [Google Scholar] [CrossRef]
- Long, C.; Zhou, Y.; Shen, L.; Yu, Y.; Hu, D.; Liu, X.; Lin, T.; He, D.; Xu, T.; Zhang, D.; et al. Retinoic acid can improve autophagy through depression of the PI3K-Akt-mTOR signaling pathway via RARα to restore spermatogenesis in cryptorchid infertile rats. Genes Dis. 2022, 9, 1368–1377. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Wu, L.; Afedo, S.Y.; Feng, X.; Yang, J. Expression and localization of Cyclin D1/Nanog and NF-κB/Bax protein in dysplastic testicles of mice. Reprod. Toxicol. 2024, 130, 108704. [Google Scholar] [CrossRef]
- Ferragut Cardoso, A.P.; Gomide, L.M.M.; Souza, N.P.; de Jesus, C.M.N.; Arnold, L.L.; Cohen, S.M.; de Camargo, J.L.V.; e Pontes, M.G.N. Time response of rat testicular alterations induced by cryptorchidism and orchiopexy. Int. J. Exp. Pathol. 2021, 102, 57–69. [Google Scholar] [CrossRef]
- Vikraman, J.; Sarila, G.; O’conner, L.; Menheniott, T.; Hutson, J.M. BDNF is upregulated by androgen in the inguinal fat pad of immature mice and may regulate inguinoscrotal testicular descent. Pediatr. Res. 2022, 91, 846–852. [Google Scholar] [CrossRef]
- Mahmud, M.A.A.; Noguchi, M.; Domon, A.; Tochigi, Y.; Katayama, K.; Suzuki, H. Cellular Expression and Subcellular Localization of Wwox Protein During Testicular Development and Spermatogenesis in Rats. J. Histochem. Cytochem. 2021, 69, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tang, X.; Li, D.; Zhao, J.; Zhou, R.; Shu, F.; Jia, W.; Fu, W.; Xia, H.; Liu, G. Prenatal exposure to environmentally relevant levels of PBDE-99 leads to testicular dysgenesis with steroidogenesis disorders. J. Hazard. Mater. 2022, 424 Pt B, 127547. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; McKeown, M.R.; Filippakopoulos, P.; Li, Q.; Ma, L.; Agno, J.E.; Lemieux, M.E.; Picaud, S.; Yu, R.N.; Qi, J.; et al. Small-molecule inhibition of BRDT for male contraception. Cell 2012, 150, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, Y.; Zhou, Y.; Long, C.; Hong, Y.; Fu, Y.; Zhao, T.; Wang, J.; Wu, Y.; Wu, S.; et al. Bisphenol S perturbs Sertoli cell junctions in male rats via alterations in cytoskeletal organization mediated by an imbalance between mTORC1 and mTORC2. Sci. Total Environ. 2021, 762, 144059. [Google Scholar] [CrossRef]
- Tang, X.; Wu, S.; Shen, L.; Wei, Y.; Cao, X.; Wang, Y.; Long, C.; Zhou, Y.; Li, D.; Huang, F.; et al. Di-(2-ethylhexyl) phthalate (DEHP)-induced testicular toxicity through Nrf2-mediated Notch1 signaling pathway in Sprague-Dawley rats. Environ. Toxicol. 2018, 33, 720–728. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Bruggeman, J.W.; Irie, N.; Lodder, P.; van Pelt, A.M.M.; Koster, J.; Hamer, G. Tumors Widely Express Hundreds of Embryonic Germline Genes. Cancers 2020, 12, 3812. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Zhu, C.; Xia, W. The role of miR-128-3p through MAPK14 activation in the apoptosis of GC2 spermatocyte cell line following heat stress. Andrology 2021, 9, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Cen, S.; Lv, D.; Wu, J.; Zhou, X.; Yang, T.; Zhao, T.; Hou, L.; Mao, X. Exposure to elevated temperature affects the expression of PIWI-interacting RNAs and associated transcripts in mouse testes. Andrology 2023, 11, 724–737. [Google Scholar] [CrossRef]
- Bizzozzero-Hiriart, M.; Di Giorgio, N.P.; Libertun, C.; Lux-Lantos, V.A. GABAB Receptor Antagonism from Birth to Weaning Permanently Modifies Kiss1 Expression in the Hypothalamus and Gonads in Mice. Neuroendocrinology 2022, 112, 998–1026. [Google Scholar] [CrossRef]
- Adnyana, A.; Pantjoro, M.H.W.; Duarsa, G.W.K. Does bilateral undescended testis have worst testicular function than unilateral cases? A meta-analysis of adult orchidopexy patients. Arch. Ital. Urol. Androl. 2025, 97, 13918. [Google Scholar] [CrossRef]
- Cinislioglu, A.E.; Ozkaya, F.; Altay, M.S.; Aksoy, Y. The incidence of epididymal anomalies in the bilateral and unilateral cryptorchidism cases: A comparative study. J. Pediatr. Urol. 2020, 16, e1–e819. [Google Scholar] [CrossRef]
- Kowalska, M.; Tylicka, M.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Dorf, J.; Matuszczak, E. Plasma concentration of MMP-17 is elevated in boys with cryptorchidism and correlates with HSP-70. Sci. Rep. 2025, 15, 474. [Google Scholar] [CrossRef]
- Seth, A.; Bournat, J.C.; Medina-Martinez, O.; Rivera, A.; Moore, J.; Flores, H.; Rosenfeld, J.A.; Hu, L.; Jorgez, C.J. Loss of WNT4 in the gubernaculum causes unilateral cryptorchidism and fertility defects. Development 2022, 149, dev201093. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Hadziselimovic, N.; Demougin, P.; Oakeley, E. Testicular gene expression in cryptorchid boys at risk of azoospermia. Sex. Dev. 2011, 5, 49–59. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Liu, B.; Hu, D.; Shen, L.; Long, C.; Yu, Y.; Lin, T.; Liu, X.; He, D.; et al. Bioinformatic identification of key genes and molecular pathways in the spermatogenic process of cryptorchidism. Genes Dis. 2019, 6, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Leng, J.; Hou, J.-G.; Jiang, S.; Wang, Z.; Liu, Z.; Gong, X.-J.; Chen, C.; Wang, Y.-P.; Li, W. Saponins derived from the stems and leaves of Panax ginseng attenuate scrotal heat-induced spermatogenic damage via inhibiting the MAPK mediated oxidative stress and apoptosis in mice. Phytother. Res. 2021, 35, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Groettrup, M. On the Role of the Immunoproteasome in Protein Homeostasis. Cells 2021, 10, 3216. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Chama, L.L.; Ebstein, F.; Wiesrecker, B.; Wagh, P.R.; Hammer, E.; Weiss, F.U.; Junker, H.; Studencka-Turski, M.; Lerch, M.M.; Krüger, E.; et al. Immunoproteasome impairment via β5i/LMP7-deletion leads to sustained pancreatic injury from experimental pancreatitis. J. Cell. Mol. Med. 2021, 25, 6786–6799. [Google Scholar] [CrossRef]
- Kriger, D.; Podenkova, U.I.; Bakhmet, E.I.; Potapenko, E.; Ivanova, E.; Tomilin, A.N.; Tsimokha, A.S. Evidence of Immunoproteasome Expression Onset in the Formative State of Pluripotency in Mouse Cells. Cells 2024, 13, 1362. [Google Scholar] [CrossRef]
- Tang, X.; Li, D.; Zhao, T.; Zhu, S.; Gao, X.; Zhou, R.; Deng, F.; Fu, W.; Jia, W.; Liu, G. The inhibition of CFTR in the descended testis of SD rats with unilateral cryptorchidism induced by di-(2-ethylhexyl) phthalate (DEHP). Environ. Sci. Pollut. Res. Int. 2022, 29, 77047–77056. [Google Scholar] [CrossRef]
- Rana, P.S.; Ignatz-Hoover, J.J.; Guo, C.; Mosley, A.L.; Malek, E.; Federov, Y.; Adams, D.J.; Driscoll, J.J. Immunoproteasome Activation Expands the MHC Class I Immunopeptidome, Unmasks Neoantigens, and Enhances T-cell Anti-Myeloma Activity. Mol. Cancer Ther. 2024, 23, 1743–1760. [Google Scholar] [CrossRef]
- Li, M.; Tong, W.; Dai, C.; Lu, G.; Jin, D.; Deng, F. Downregulation of the immunoproteasome subunit PSMB8 attenuates sepsis-associated acute kidney injury through the NF-κB pathway. Immunobiology 2025, 230, 152862. [Google Scholar] [CrossRef]
- Zhang, F.L.; Ma, H.H.; Dong, P.Y.; Yuan, Z.N.; Zhang, S.E.; Zhao, A.H.; Liu, H.Q.; De Felici, M.; Shen, W.; Zhang, X.F. Aflatoxin B1 disrupts testicular development via the cell cycle-related Ras/PI3K/Akt signaling in mice and pig. Environ. Pollut. 2023, 329, 121729. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, Q.; Nan, J.; Chen, T.; Wang, J.; Zhang, Y.; Yuan, L. Heme oxygenase 1 (HO1) regulates autophagy and apoptosis via the PI3K/AKT/mTOR signaling pathway of yak Sertoli cells. Theriogenology 2024, 220, 96–107. [Google Scholar] [CrossRef]
- Jia, H.; Ma, T.; Jia, S.; Ouyang, Y. AKT3 and related molecules as potential biomarkers responsible for cryptorchidism and cryptorchidism-induced azoospermia. Transl. Pediatr. 2021, 10, 1805–1817. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology 2018, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Centola, C.L.; Dasso, M.E.; Riera, M.F.; Meroni, S.B.; Galardo, M.N. Role of L-glutamine in the regulation of rat Sertoli cell proliferation by FSH. Reproduction 2024, 168, e240177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qu, L.; Wan, S.; Li, Y.; Fan, D. Ginsenoside Rk1 Prevents UVB Irradiation-Mediated Oxidative Stress, Inflammatory Response, and Collagen Degradation via the PI3K/AKT/NF-κB Pathway In Vitro and In Vivo. J. Agric. Food Chem. 2022, 70, 15804–15817. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Yadav, A.; Mishra, R.K. Chronic unpredictable stress exposure disrupts testicular function by modulating germ cell-junctional dynamics and Nrf2/HO-1/IKKβ/NF-κB pathway. Reprod. Toxicol. 2025, 132, 108845. [Google Scholar] [CrossRef]
- Lee, G.E.; Jeung, D.; Chen, W.; Byun, J.; Lee, J.Y.; Kang, H.C.; Lee, H.S.; Kim, D.J.; Choi, J.S.; Lee, C.J.; et al. MEKs/ERKs-mediated FBXO1/E2Fs interaction interference modulates G(1)/S cell cycle transition and cancer cell proliferation. Arch. Pharm. Res. 2023, 46, 44–58. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Z.; Lu, N.; Wei, H.; Kang, J.; Yuan, M.; Sheng, X.; Qi, X.; Xing, K.; Guo, Y.; et al. Dihydrotestosterone through blockade of TGF-β/Smad signaling mediates the anti-fibrosis effect under hypoxia in canine Sertoli cells. J. Steroid Biochem. Mol. Biol. 2022, 216, 106041. [Google Scholar] [CrossRef]
- Hutson, J.M.; Li, R.; Southwell, B.R.; Petersen, B.L.; Thorup, J.; Cortes, D. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front. Endocrinol. 2012, 3, 176. [Google Scholar] [CrossRef]
- Soltanghoraee, H.; Pourkeramati, F.; Khoddami, M.; Amirjannati, N.; Akhondi, M.M.; Soltani, A. Prevalence of carcinoma in situ in testicular biopsies of infertile Iranian men. Andrologia 2014, 46, 726–730. [Google Scholar] [CrossRef]
- Serrano, J.J.; Medina, M. Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State. Int. J. Mol. Sci. 2025, 26, 498. [Google Scholar] [CrossRef] [PubMed]
- Safrhansova, L.; Hlozkova, K.; Starkova, J. Targeting amino acid metabolism in cancer. Int. Rev. Cell Mol. Biol. 2022, 373, 37–79. [Google Scholar] [PubMed]
- Hu, X.; Chen, L.; Liu, T.; Wan, Z.; Yu, H.; Tang, F.; Shi, J.; Chen, Z.; Wang, X.; Yang, Z. TAF1D promotes tumorigenesis and metastasis by activating PI3K/AKT/mTOR signaling in clear cell renal cell carcinoma. Cell. Signal. 2024, 124, 111425. [Google Scholar] [CrossRef] [PubMed]
- García-Andrade, F.; Vigueras-Villaseñor, R.M.; Chávez-Saldaña, M.D.; Rojas-Castañeda, J.C.; Bahena-Ocampo, I.U.; Aréchaga-Ocampo, E.; Flores-Fortis, M.; Díaz-Chávez, J.; Herrera, L.A.; Landero-Huerta, D.A. Molecular Characterization of Patients with Cryptorchidism: Preliminary Search for an Expression Profile Related to That of Testicular Germ-Cell Tumors. Diagnostics 2023, 13, 3020. [Google Scholar] [CrossRef]
- Voigt, A.L.; de Lima, E.M.L.N.; Dobrinski, I. Comparing the adult and pre-pubertal testis: Metabolic transitions and the change in the spermatogonial stem cell metabolic microenvironment. Andrology 2023, 11, 1132–1146. [Google Scholar] [CrossRef]
- Fink, C.; Baal, N.; Wilhelm, J.; Sarode, P.; Weigel, R.; Schumacher, V.; Nettersheim, D.; Schorle, H.; Schröck, C.; Bergmann, M.; et al. On the origin of germ cell neoplasia in situ: Dedifferentiation of human adult Sertoli cells in cross talk with seminoma cells in vitro. Neoplasia 2021, 23, 731–742. [Google Scholar] [CrossRef]
- Kilic, I.; Acosta, A.M.; Idrees, M.T. Evolution of Testicular Germ Cell Tumors in the Molecular Era with Histogenetic Implications. Adv. Anat. Pathol. 2024, 31, 206–214. [Google Scholar] [CrossRef]
- Guerra, F.; Quintana, S.; Giustina, S.; Mendeluk, G.; Jufe, L.; Avagnina, M.; Díaz, L.; Palaoro, L. Investigation of EGFR/pi3k/Akt signaling pathway in seminomas. Biotech. Histochem. 2021, 96, 125–137. [Google Scholar] [CrossRef]
- Bossio, S.; Perri, A.; Malivindi, R.; Giordano, F.; Rago, V.; Mirabelli, M.; Salatino, A.; Brunetti, A.; Greco, E.A.; Aversa, A. Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients 2022, 14, 2323. [Google Scholar] [CrossRef]
- Cardenas, R.P.; Zyoud, A.; McIntyre, A.; Alberio, R.; Mongan, N.P.; Allegrucci, C. NANOG controls testicular germ cell tumour stemness through regulation of MIR9-2. Stem Cell Res. Ther. 2024, 15, 128. [Google Scholar] [CrossRef]
- Zong, Y.; Huang, R.; Bitar, M.; Drakaki, A.; Zhang, L.; Lin, D.I.; Ye, H. Molecular Diversity of Embryonic-Type Neuroectodermal Tumors Arising from Testicular Germ Cell Tumors. Mod. Pathol. 2025, 38, 100702. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, J.; Tuo, Z.; Yoo, K.H.; Yu, Q.; Miyamoto, A.; Zhang, C.; Ye, X.; Wei, W.; Wu, R.; et al. Natural products and derivatives in renal, urothelial and testicular cancers: Targeting signaling pathways and therapeutic potential. Phytomedicine 2024, 127, 155503. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Chong, H.B.; Zhang, S.; Yang, T.-Y.; Lazarov, M.J.; Harry, S.; Maynard, M.; Hilbert, B.; White, R.D.; Murrey, H.E.; et al. DrugMap: A quantitative pan-cancer analysis of cysteine ligandability. Cell 2024, 187, 2536–2556.e30. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Li, W.; Kremer, D.M.; Sajjakulnukit, P.; Li, S.; Crespo, J.; Nwosu, Z.C.; Zhang, L.; Czerwonka, A.; Pawłowska, A.; et al. Cancer SLC43A2 alters T cell methionine metabolism and histone methylation. Nature 2020, 585, 277–282. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sangwan, N.; Parekh, N.V.; Selvam, M.K.P.; Gupta, S.; McCaffrey, P.; Bessoff, K.; Vala, A.; Agarwal, A.; Sabanegh, E.S.; et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur. Urol. 2021, 79, 826–836. [Google Scholar] [CrossRef]
- Zoch, A.; Konieczny, G.; Auchynnikava, T.; Stallmeyer, B.; Rotte, N.; Heep, M.; Berrens, R.V.; Schito, M.; Kabayama, Y.; Schöpp, T.; et al. C19ORF84 connects piRNA and DNA methylation machineries to defend the mammalian germ line. Mol. Cell 2024, 84, 1021–1035.e11. [Google Scholar] [CrossRef]
- Ma, Q.; Hao, S.; Hong, W.; Tergaonkar, V.; Sethi, G.; Tian, Y.; Duan, C. Versatile function of NF-ĸB in inflammation and cancer. Exp. Hematol. Oncol. 2024, 13, 68. [Google Scholar] [CrossRef]
- Yoshimori, M.; Shibayama, H.; Imadome, K.-I.; Kawano, F.; Ohashi, A.; Nishio, M.; Shimizu, N.; Kurata, M.; Fujiwara, S.; Arai, A. Antineoplastic and anti-inflammatory effects of bortezomib on systemic chronic active EBV infection. Blood Adv. 2021, 5, 1805–1815. [Google Scholar] [CrossRef]
- Fernández-Simón, E.; Lleixà, C.; Suarez-Calvet, X.; Diaz-Manera, J.; Illa, I.; Gallardo, E.; de Luna, N. Proteasome inhibitors reduce thrombospondin-1 release in human dysferlin-deficient myotubes. BMC Musculoskelet. Disord. 2020, 21, 784. [Google Scholar] [CrossRef]
- White, M.J.V.; Raczy, M.M.; Budina, E.; Yuba, E.; Solanki, A.; Shim, H.-N.; Zhang, Z.J.; Gray, L.T.; Cao, S.; Alpar, A.T.; et al. Engineering IL-10 and rapamycin to bind collagen leads to improved anti fibrotic efficacy in lung and kidney fibrosis. Sci. Rep. 2025, 15, 13279. [Google Scholar] [CrossRef]
- Feng, S.; Yan, L.; Lou, Y.; Ying, L. The protective effect of curcumin on testicular tissue in a cryptorchid rat model. J. Pediatr. Urol. 2022, 18, e1–e409. [Google Scholar] [CrossRef]
- Zeng, J.; Yuan, L.; Chen, G.; Qi, Y.; Qie, X.; Jin, Y.; Chen, Y.; Li, H. The ferroptosis of sertoli cells inducing blood-testis barrier damage is produced by oxidative stress in cryptorchidism. Free Radic. Biol. Med. 2025, 232, 97–106. [Google Scholar] [CrossRef]
- Lu, X.; Luo, Y.; Nie, X.; Zhang, B.; Wang, X.; Li, R.; Liu, G.; Zhou, Q.; Liu, Z.; Fan, L.; et al. Single-cell multi-omics analysis of human testicular germ cell tumor reveals its molecular features and microenvironment. Nat. Commun. 2023, 14, 8462. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Deng, F.; Chen, Y.; Liu, X.; Li, D.; Tang, X.; Lai, H.; Li, Q.; Fu, W.; Liu, G.; et al. Developmental Phase-Specific Molecular Signatures and Signaling Pathways in Cryptorchidism-Induced Testicular Damage. Biomolecules 2025, 15, 1584. https://doi.org/10.3390/biom15111584
Wang X, Deng F, Chen Y, Liu X, Li D, Tang X, Lai H, Li Q, Fu W, Liu G, et al. Developmental Phase-Specific Molecular Signatures and Signaling Pathways in Cryptorchidism-Induced Testicular Damage. Biomolecules. 2025; 15(11):1584. https://doi.org/10.3390/biom15111584
Chicago/Turabian StyleWang, Xinying, Fuming Deng, Yijing Chen, Xiaonan Liu, Dian Li, Xiangliang Tang, Hongkun Lai, Qianlong Li, Wen Fu, Guochang Liu, and et al. 2025. "Developmental Phase-Specific Molecular Signatures and Signaling Pathways in Cryptorchidism-Induced Testicular Damage" Biomolecules 15, no. 11: 1584. https://doi.org/10.3390/biom15111584
APA StyleWang, X., Deng, F., Chen, Y., Liu, X., Li, D., Tang, X., Lai, H., Li, Q., Fu, W., Liu, G., Chen, Z., & Zhao, T. (2025). Developmental Phase-Specific Molecular Signatures and Signaling Pathways in Cryptorchidism-Induced Testicular Damage. Biomolecules, 15(11), 1584. https://doi.org/10.3390/biom15111584






