Decoding the Spectrum of Anorexia Nervosa: Clinical Impact, Molecular Insights, and Therapeutic Perspectives
Abstract
1. Introduction
2. Literature Search
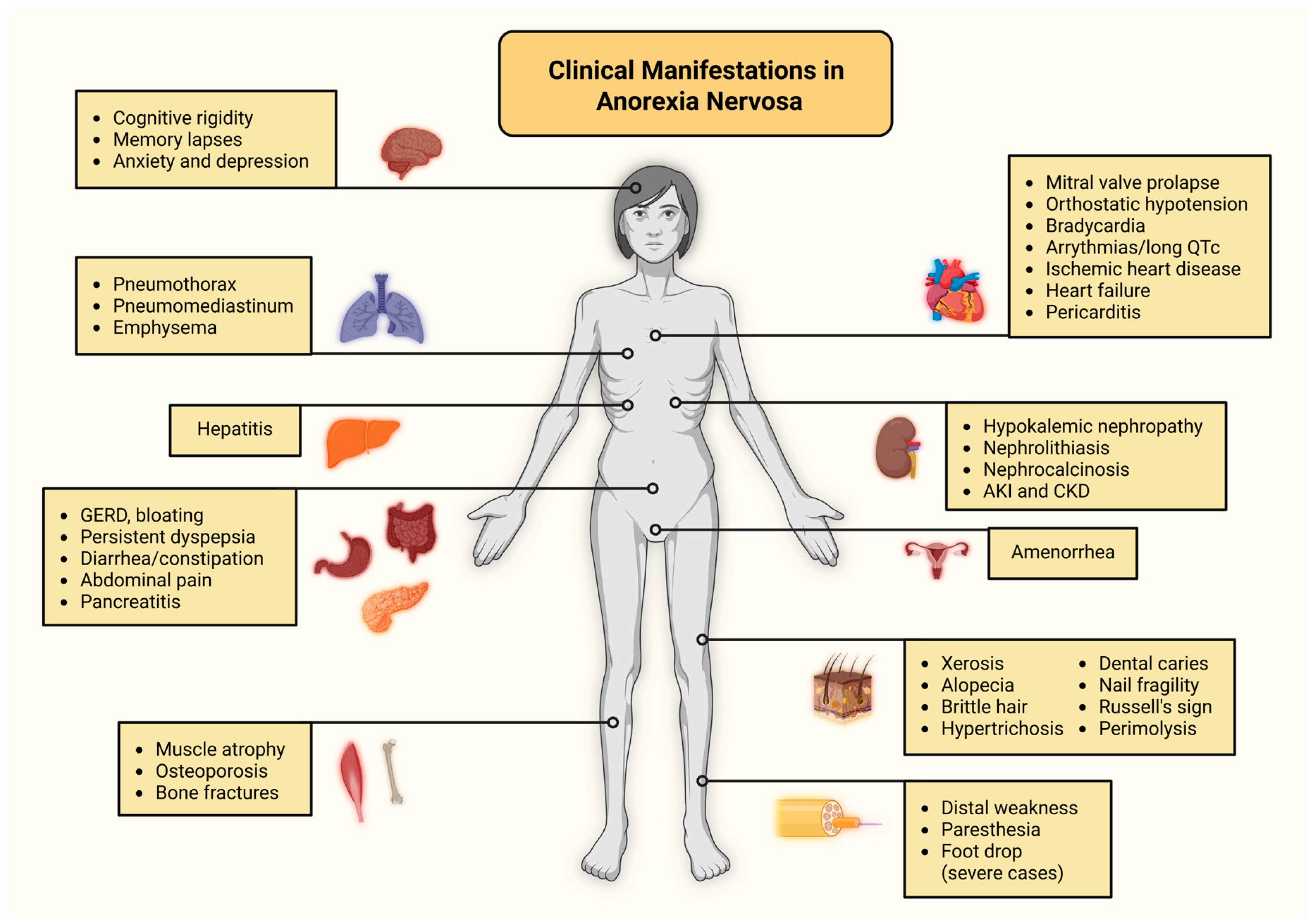
3. Neurological Manifestations in Anorexia Nervosa
4. Cardiopulmonary Disease in Anorexia Nervosa
4.1. Cardiovascular Complications
4.2. Pulmonary Complications
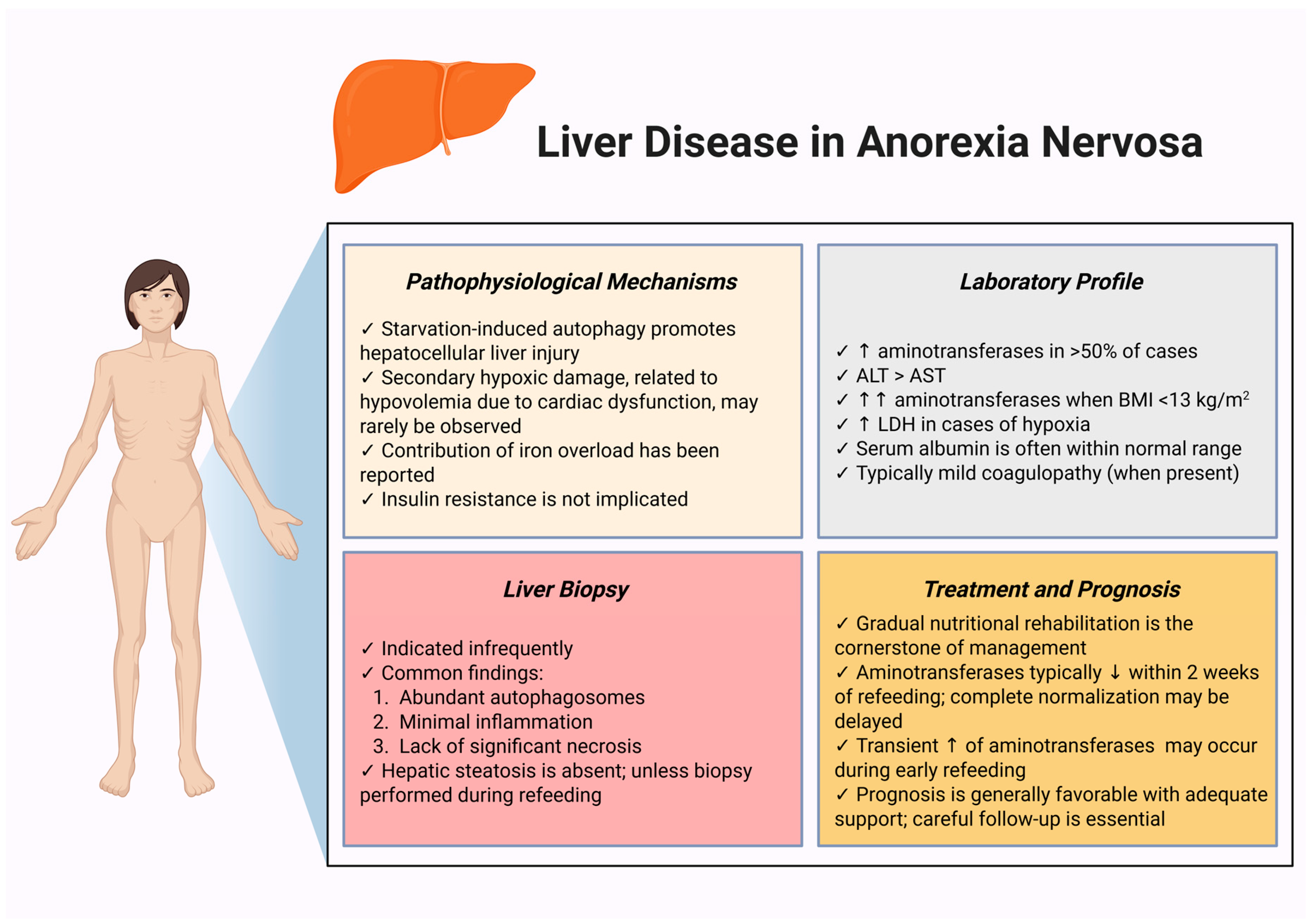
5. Liver Disease in Anorexia Nervosa
6. Gastrointestinal Disease in Anorexia Nervosa
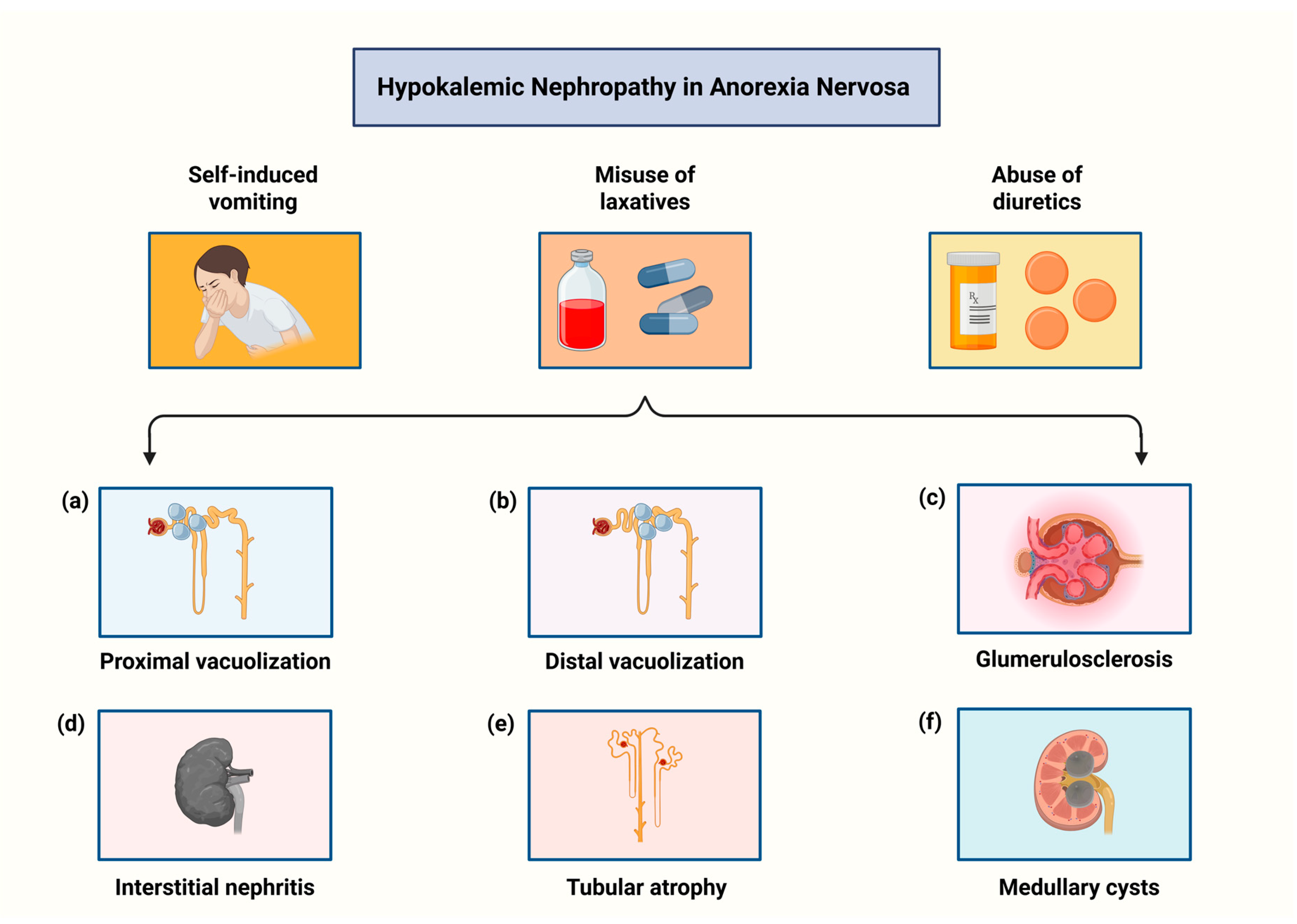
7. Kidney Disease in Anorexia Nervosa
8. Hormonal Abnormalities in Anorexia Nervosa
8.1. Hypothalamic–Pituitary–Adrenal (HPA) Axis Dysregulation
8.2. Growth Hormone–Insulin-like Growth Factor 1 (GH–IGF-1) Axis Dysregulation
8.3. Hypothalamic–Pituitary–Gonadal (HPG) Axis Dysregulation
8.4. Hypothalamic-Pituitary-Thyroid (HPT) Axis Dysregulation
8.5. Posterior Pituitary Hormone Dysregulation
8.6. Adipokine Dysregulation
9. Musculoskeletal Disease in Anorexia Nervosa
10. Cutaneous Manifestations in Anorexia Nervosa
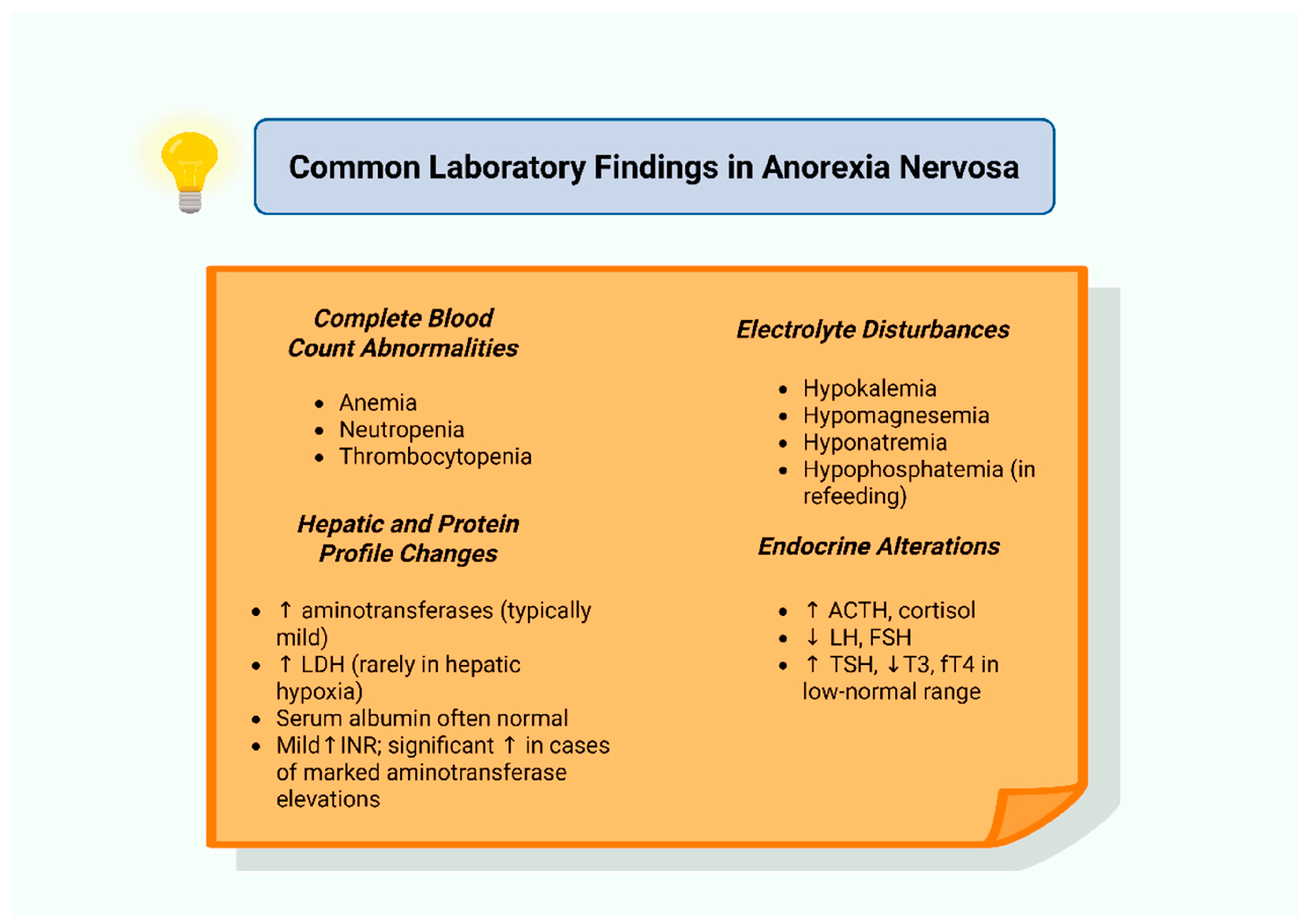
11. Hematological Alterations in Anorexia Nervosa
12. Emerging Pathophysiological Pathways in Anorexia Nervosa: The Role of Immunity and the Gut–Brain Axis
12.1. Immunological Alterations in Anorexia Nervosa
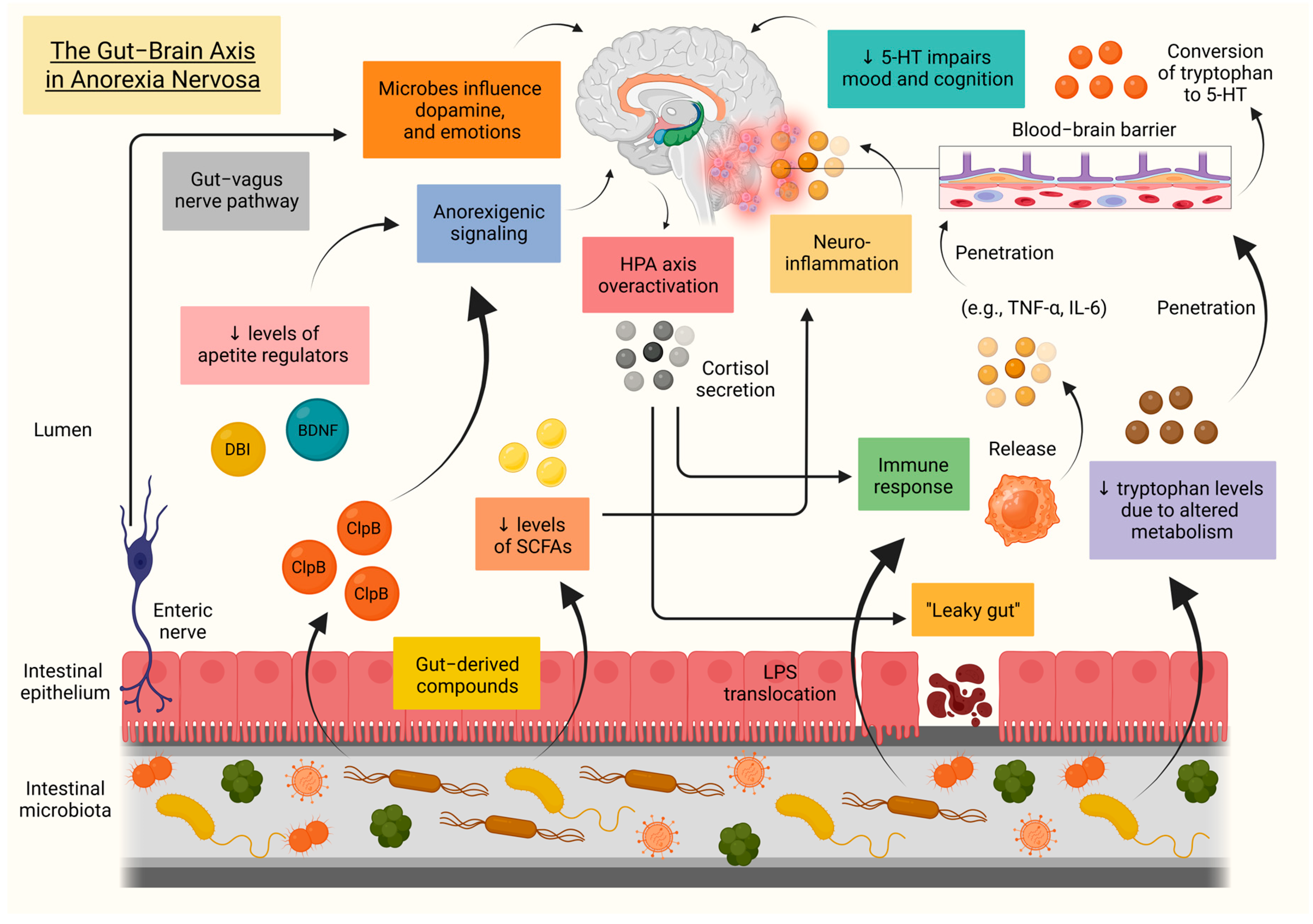
12.2. The Gut–Brain Axis in Anorexia Nervosa
13. Anorexia Nervosa Management: Current Strategies and Therapeutic Perspectives
13.1. Current Strategies
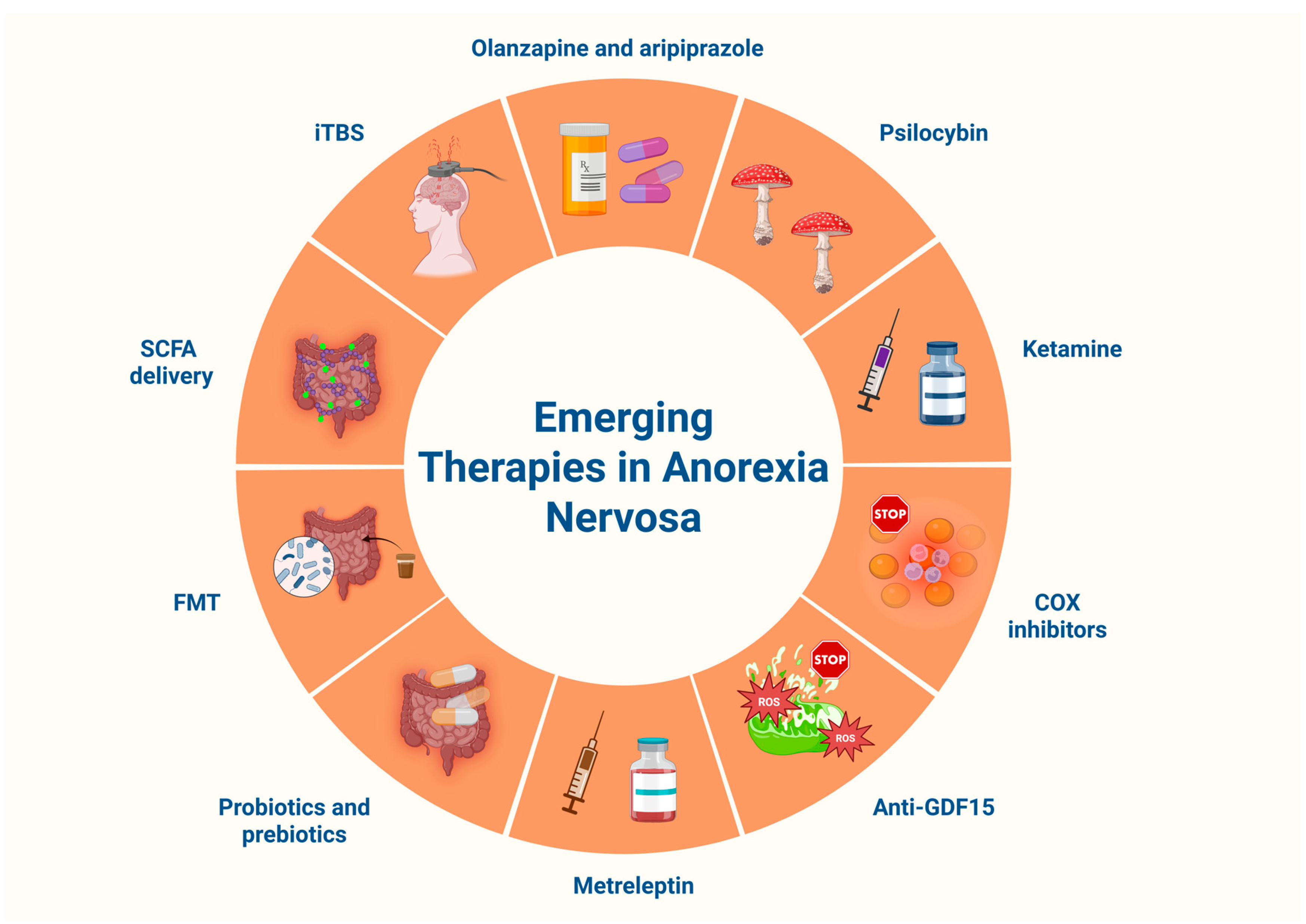
13.2. Challenges and Therapeutic Perspectives
13.2.1. Atypical Antipsychotics
13.2.2. Psychedelics, Dronabinol and Ketamine
13.2.3. Anti-Inflammatory and Mitochondria-Targeted Strategies
13.2.4. Recombinant Human Leptin Analogs
13.2.5. Gut-Directed Therapeutic Interventions
13.2.6. Neuromodulation with Intermittent Theta-Burst Stimulation (iTBS)
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Auger, N.; Potter, B.J.; Ukah, U.V.; Low, N.; Israël, M.; Steiger, H.; Healy-Profitós, J.; Paradis, G. Anorexia nervosa and the long-term risk of mortality in women. World Psychiatry 2021, 20, 448–449. [Google Scholar] [CrossRef] [PubMed]
- Søeby, M.; Gribsholt, S.B.; Clausen, L.; Richelsen, B. Overall and cause-specific mortality in anorexia nervosa; impact of psychiatric comorbidity and sex in a 40-year follow-up study. Int. J. Eat. Disord. 2024, 57, 1842–1853. [Google Scholar] [CrossRef]
- Van Eeden, A.E.; Van Hoeken, D.; Hoek, H.W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry 2021, 34, 515–524. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Sartirana, M.; El Ghoch, M.; Calugi, S. DSM-5 severity specifiers for anorexia nervosa and treatment outcomes in adult females. Eat. Behav. 2018, 31, 18–23. [Google Scholar] [CrossRef]
- Hebebrand, J.; Gradl-Dietsch, G.; Peters, T.; Correll, C.U.; Haas, V. The Diagnosis and Treatment of Anorexia Nervosa in Childhood and Adolescence. Dtsch. Arztebl. Int. 2024, 121, 164–174. [Google Scholar] [CrossRef]
- Walsh, B.T.; Hagan, K.E.; Lockwood, C. A systematic review comparing atypical anorexia nervosa and anorexia nervosa. Int. J. Eat. Disord. 2023, 56, 798–820. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.M.; Reay, W.R.; Cairns, M.J. Multiomic prioritisation of risk genes for anorexia nervosa. Psychol. Med. 2023, 53, 6754–6762. [Google Scholar] [CrossRef]
- Steiger, H.; Booij, L.; Thaler, L.; St-Hilaire, A.; Israël, M.; Casey, K.F.; Oliverio, S.; Crescenzi, O.; Lee, V.; Turecki, G.; et al. DNA methylation in people with anorexia nervosa: Epigenome-wide patterns in actively ill, long-term remitted, and healthy-eater women. World J. Biol. Psychiatry 2023, 24, 254–259. [Google Scholar] [CrossRef]
- Sirufo, M.M.; Magnanimi, L.M.; Ginaldi, L.; De Martinis, M. Anorexia nervosa and autoimmune comorbidities: A bidirectional route? CNS Neurosci. Ther. 2022, 28, 1921–1929. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The Role of the Gut Microbiome, Immunity, and Neuroinflammation in the Pathophysiology of Eating Disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.R.; Braham, S.; Dasey, L.; Reidlinger, D.P. Physicians’ perspectives on the treatment of patients with eating disorders in the acute setting. J. Eat. Disord. 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Guinhut, M.; Godart, N.; Benadjaoud, M.A.; Melchior, J.C.; Hanachi, M. Five-year mortality of severely malnourished patients with chronic anorexia nervosa admitted to a medical unit. Acta Psychiatr. Scand. 2021, 143, 130–140. [Google Scholar] [CrossRef]
- Stedal, K.; Ely, A.V.; Kurniadi, N.; Lopez, E.; Kaye, W.H.; Wierenga, C.E. A process approach to verbal memory assessment: Exploratory evidence of inefficient learning in women remitted from anorexia nervosa. J. Clin. Exp. Neuropsychol. 2018, 41, 653–663. [Google Scholar] [CrossRef]
- Nickel, K.; Joos, A.; Tebartz van Elst, L.; Matthis, J.; Holovics, L.; Endres, D.; Zeeck, A.; Hartmann, A.; Tüscher, O.; Maier, S. Recovery of cortical volume and thickness after remission from acute anorexia nervosa. Int. J. Eat. Disord. 2018, 51, 1056–1069. [Google Scholar] [CrossRef]
- De La Cruz, F.; Schumann, A.; Rieger, K.; Giuliano, M.D.; Bär, K.J. Fibre-specific white matter changes in anorexia nervosa. Psychiatry Res. Neuroimaging 2023, 336, 111736. [Google Scholar] [CrossRef] [PubMed]
- Gaiaschi, L.; Priori, E.C.; Mensi, M.M.; Verri, M.; Buonocore, D.; Parisi, S.; Hernandez, L.N.Q.; Brambilla, I.; Ferrari, B.; De Luca, F.; et al. New perspectives on the role of biological factors in anorexia nervosa: Brain volume reduction or oxidative stress, which came first? Neurobiol. Dis. 2024, 199, 106580. [Google Scholar] [CrossRef] [PubMed]
- Collantoni, E.; Madan, C.R.; Meneguzzo, P.; Chiappini, I.; Tenconi, E.; Manara, R.; Favaro, A. Cortical Complexity in Anorexia Nervosa: A Fractal Dimension Analysis. J. Clin. Med. 2020, 9, 833. [Google Scholar] [CrossRef]
- Gupta, Y.; de la Cruz, F.; Rieger, K.; di Giuliano, M.; Gaser, C.; Cole, J.; Breithaupt, L.; Holsen, L.M.; Eddy, K.T.; Thomas, J.J.; et al. Does restrictive anorexia nervosa impact brain aging? A machine learning approach to estimate age based on brain structure. Comput. Biol. Med. 2025, 194, 110484. [Google Scholar] [CrossRef]
- Mitchell, J.E.; Peterson, C.B. Anorexia Nervosa. N. Engl. J. Med. 2020, 382, 1343–1351. [Google Scholar] [CrossRef]
- Brodrick, B.B.; Adler-Neal, A.L.; Palka, J.M.; Mishra, V.; Aslan, S.; McAdams, C.J. Structural brain differences in recovering and weight-recovered adult outpatient women with anorexia nervosa. J. Eat. Disord. 2021, 9, 108. [Google Scholar] [CrossRef]
- De La Cruz, F.; Schumann, A.; Suttkus, S.; Helbing, N.; Zopf, R.; Bär, K.J. Cortical thinning and associated connectivity changes in patients with anorexia nervosa. Transl. Psychiatry 2021, 11, 95. [Google Scholar] [CrossRef]
- Walton, E.; Bernardoni, F.; Batury, V.L.; Bahnsen, K.; Larivière, S.; Abbate-Daga, G.; Andres-Perpiña, S.; Bang, L.; Bischoff-Grethe, A.; Brooks, S.J.; et al. Brain Structure in Acutely Underweight and Partially Weight-Restored Individuals with Anorexia Nervosa: A Coordinated Analysis by the ENIGMA Eating Disorders Working Group. Biol. Psychiatry 2022, 92, 730–738. [Google Scholar] [CrossRef]
- Asami, T.; Takaishi, M.; Nakamura, R.; Yoshimi, A.; Konishi, J.; Aoyama, K.; Fujita, J.; Miyazaki, H.; Aoki, Y.; Asanuma, K.; et al. Structural brain abnormalities in adolescent patients with anorexia nervosa at both the acute and weight-recovered phase. Brain Imaging Behav. 2022, 16, 1372–1380. [Google Scholar] [CrossRef]
- Fuglset, T.S. Is set-shifting and central coherence in anorexia nervosa influenced by body mass index, anxiety or depression? A systematic review. BMC Psychiatry 2021, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Fuglset, T.S. Set-shifting, central coherence and decision-making in individuals recovered from anorexia nervosa: A systematic review. J. Eat. Disord. 2019, 7, 22. [Google Scholar] [CrossRef]
- Oudman, E.; Wijnia, J.W.; Oey, M.J.; van Dam, M.J.; Postma, A. Preventing Wernicke’s encephalopathy in anorexia nervosa: A systematic review. Psychiatry Clin. Neurosci. 2018, 72, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, Z.M.; Castle, D.J.; Eikelis, N.; Phillipou, A.; Lambert, G.W.; Lambert, E.A. Autonomic nervous system function in women with anorexia nervosa. Clin. Auton. Res. 2022, 32, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Puckett, L.; Grayeb, D.; Khatri, V.; Cass, K.; Mehler, P. A Comprehensive Review of Complications and New Findings Associated with Anorexia Nervosa. J. Clin. Med. 2021, 10, 2555. [Google Scholar] [CrossRef]
- Tseng, M.M.; Chien, L.N.; Tu, C.Y.; Liu, H.Y. Mortality in anorexia nervosa and bulimia nervosa: A population-based cohort study in Taiwan, 2002–2017. Int. J. Eat. Disord. 2023, 56, 1135–1144. [Google Scholar] [CrossRef]
- Cost, J.; Krantz, M.J.; Mehler, P.S. Medical complications of anorexia nervosa. Cleve. Clin. J. Med. 2020, 87, 361–366. [Google Scholar] [CrossRef]
- Tseng, M.M.; Chiou, K.R.; Shao, J.Y.; Liu, H.Y. Incidence and Risk of Cardiovascular Outcomes in Patients with Anorexia Nervosa. JAMA Netw. Open 2024, 7, e2451094. [Google Scholar] [CrossRef] [PubMed]
- Sachs, K.V.; Harnke, B.; Mehler, P.S.; Krantz, M.J. Cardiovascular complications of anorexia nervosa: A systematic review. Int. J. Eat. Disord. 2016, 49, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Shank, C.; Ganigara, M.; Saldanha, N.; Dhar, A. Cardiac complications of malnutrition in adolescent patients: A narrative review of contemporary literature. Ann. Pediatr. Cardiol. 2021, 14, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Giovinazzo, S.; Sukkar, S.G.; Rosa, G.M.; Zappi, A.; Bezante, G.P.; Balbi, M.; Brunelli, C. Anorexia nervosa and heart disease: A systematic review. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2019, 24, 199–207. [Google Scholar] [CrossRef]
- Goldfarb, M.; De Hert, M.; Detraux, J.; Di Palo, K.; Munir, H.; Music, S.; Piña, I.; Ringen, P.A. Severe mental illness and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2022, 80, 918–933. [Google Scholar] [CrossRef]
- Cass, K.; McGuire, C.; Bjork, I.; Sobotka, N.; Walsh, K.; Mehler, P.S. Medical Complications of Anorexia Nervosa. Psychosomatics 2020, 61, 625–631. [Google Scholar] [CrossRef]
- Benayon, M.; Latchupatula, L.; Kacer, E.; Shanjer, M.; Weiss, E.; Amar, S.; Zweig, N.; Ghadim, M.; Portman, R.; Balakrishnan, N.; et al. QTc Interval Prolongation and Its Association With Electrolyte Abnormalities and Psychotropic Drug Use Among Patients With Eating Disorders. CJC Pediatr. Congenit. Heart Dis. 2023, 3, 14–21. [Google Scholar] [CrossRef]
- Mehler, P.S.; Anderson, K.; Bauschka, M.; Cost, J.; Farooq, A. Emergency room presentations of people with anorexia nervosa. J. Eat. Disord. 2023, 11, 16. [Google Scholar] [CrossRef]
- Lamzabi, I.; Syed, S.; Reddy, V.B.; Jain, R.; Harbhajanka, A.; Arunkumar, P. Myocardial changes in a patient with anorexia nervosa: A case report and review of literature. Am. J. Clin. Pathol. 2015, 143, 734–737. [Google Scholar] [CrossRef]
- Arnés-García, D.; Zamorano-García, E.; Gallego-Romero, I. Beri-beri: A rare and reversible cause of heart failure. Med. Clin. 2025, 164, 106893. [Google Scholar] [CrossRef]
- Crook, M.A. Cardiac abnormalities in the refeeding syndrome. Nutrition 2017, 35, 146–147. [Google Scholar] [CrossRef]
- O’Meara, D.; Sheward, L.; Hartman-Munick, S.; Addison, J.; Abu-El-Haija, A. Craving Answers. N. Engl. J. Med. 2022, 386, 880–886. [Google Scholar] [CrossRef]
- Jensen, V.M.; Stoving, R.K.; Andersen, P.E. Anorexia nervosa with massive pulmonary air leak and extraordinary propagation. Int. J. Eat. Disord. 2017, 50, 451–453. [Google Scholar] [CrossRef]
- Nitsch, A.; Kearns, M.; Mehler, P. Pulmonary complications of eating disorders: A literature review. J. Eat. Disord. 2023, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Cook, V.J.; Coxson, H.O.; Mason, A.G.; Bai, T.R. Bullae, bronchiectasis and nutritional emphysema in severe anorexia nervosa. Can. Respir. J. 2001, 8, 361–365. [Google Scholar] [CrossRef]
- Vootla, V.R.; Daniel, M. Abnormal Liver Function Tests in an Anorexia Nervosa Patient and an Atypical Manifestation of Refeeding Syndrome. Case Rep. Gastroenterol. 2015, 9, 261–265. [Google Scholar] [CrossRef]
- Schuster, K.; Staffeld, A.; Zimmermann, A.; Böge, N.; Lang, S.; Kuhla, A.; Frintrop, L. Starvation in Mice Induces Liver Damage Associated with Autophagy. Nutrients 2024, 16, 1191. [Google Scholar] [CrossRef] [PubMed]
- Kheloufi, M.; Boulanger, C.M.; Durand, F.; Rautou, P.E. Liver autophagy in anorexia nervosa and acute liver injury. BioMed Res. Int. 2014, 2014, 701064. [Google Scholar] [CrossRef]
- Rautou, P.E.; Cazals-Hatem, D.; Moreau, R.; Francoz, C.; Feldmann, G.; Lebrec, D.; Ogier-Denis, E.; Bedossa, P.; Valla, D.; Durand, F. Acute liver cell damage in patients with anorexia nervosa: A possible role of starvation-induced hepatocyte autophagy. Gastroenterology 2008, 135, 840–848.e3. [Google Scholar] [CrossRef]
- Yoshida, T.; Namiki, T.; Yamaga, M.; Onishi, S.; Takemoto, M. Iron overload may be critical for liver dysfunction in anorexia nervosa, and the role of haematocrit-adjusted albumin in assessing nutritional status: A case report. BMC Pediatr. 2023, 23, 547. [Google Scholar] [CrossRef]
- Fanin, A.; Miele, L.; Bertolini, E.; Giorgini, A.; Pontiroli, A.E.; Benetti, A. Liver alterations in anorexia nervosa are not caused by insulin resistance. Intern. Emerg. Med. 2020, 15, 337–339. [Google Scholar] [CrossRef]
- Rosen, E.; Sabel, A.L.; Brinton, J.T.; Catanach, B.; Gaudiani, J.L.; Mehler, P.S. Liver dysfunction in patients with severe anorexia nervosa. Int. J. Eat. Disord. 2016, 49, 151–158. [Google Scholar] [CrossRef]
- Cuntz, U.; Voderholzer, U. Liver Damage Is Related to the Degree of Being Underweight in Anorexia Nervosa and Improves Rapidly with Weight Gain. Nutrients 2022, 14, 2378. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.; Bakshi, N.; Watters, A.; Rosen, H.R.; Mehler, P.S. Hepatic Complications of Anorexia Nervosa. Dig. Dis. Sci. 2017, 62, 2977–2981. [Google Scholar] [CrossRef]
- Fong, H.F.; Divasta, A.D.; Difabio, D.; Ringelheim, J.; Jonas, M.M.; Gordon, C.M. Prevalence and predictors of abnormal liver enzymes in young women with anorexia nervosa. J. Pediatr. 2008, 153, 247–253. [Google Scholar] [CrossRef]
- Narayanan, V.; Gaudiani, J.L.; Mehler, P.S. Serum albumin levels may not correlate with weight status in severe anorexia nervosa. Eat. Disord. 2009, 17, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Arnone, S.; Santeusanio, F.; Pampanelli, S. Brief elevation of hepatic enzymes due to liver ischemia in anorexia nervosa. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2010, 15, e294–e297. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, K.; Sato, K.; Matsuoka, M.; Otani, M.; Yoshiuchi, K. Thrombocytopenia and PT-INR in patients with anorexia nervosa and severe liver dysfunction. Biopsychosoc. Med. 2023, 17, 9. [Google Scholar] [CrossRef]
- Gaudiani, J.L.; Kashuk, J.L.; Chu, E.S.; Narayanan, V.; Mehler, P.S. The use of thrombelastography to determine coagulation status in severe anorexia nervosa: A case series. Int. J. Eat. Disord. 2010, 43, 382–385. [Google Scholar] [CrossRef]
- Miyachi, Y.; Sakiko, K.; Yokoi, T.; Kaido, T. Rapidly progressing severe coagulopathy and thrombocytopenia in extreme anorexia nervosa patient with small bowel strangulation: A case report. Int. J. Surg. Case Rep. 2023, 112, 108985. [Google Scholar] [CrossRef]
- Koga, A.; Toda, K.; Tatsushima, K.; Matsuubayashi, S.; Tamura, N.; Imamura, M.; Kawai, K. Portal hypertension in prolonged anorexia nervosa with laxative abuse: A case report of three patients. Int. J. Eat. Disord. 2019, 52, 211–215. [Google Scholar] [CrossRef]
- Loisel, A.; Caunes, A.; Birsen, R.; Moro, M.R.; Boussaid, I.; Blanchet, C. When refeeding is not enough: Severe and prolonged pancytopenia in an adolescent with anorexia nervosa. Eat. Weight. Disord.-Stud. Anorex. Bulim. Obes. 2022, 27, 3797–3801. [Google Scholar] [CrossRef]
- Norris, M.L.; Harrison, M.E.; Isserlin, L.; Robinson, A.; Feder, S.; Sampson, M. Gastrointestinal complications associated with anorexia nervosa: A systematic review. Int. J. Eat. Disord. 2016, 49, 216–237. [Google Scholar] [CrossRef]
- Perkins, S.J.; Keville, S.; Schmidt, U.; Chalder, T. Eating disorders and irritable bowel syndrome: Is there a link? J. Psychosom. Res. 2005, 59, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.G.; Stephenson, K.E.; Herring, S.; Marti, J.L. Recurrent acute pancreatitis in anorexia and bulimia. JOP 2004, 5, 231–234. [Google Scholar]
- Wiklund, C.A.; Kuja-Halkola, R.; Thornton, L.M.; Hübel, C.; Leppä, V.; Bulik, C.M. Prolonged constipation and diarrhea in childhood and disordered eating in adolescence. J. Psychosom. Res. 2019, 126, 109797. [Google Scholar] [CrossRef] [PubMed]
- Riedlinger, C.; Mazurak, N.; Schäffeler, N.; Stengel, A.; Giel, K.E.; Zipfel, S.; Enck, P.; Mack, I. Gastrointestinal complaints in patients with anorexia nervosa in the timecourse of inpatient treatment. Front. Psychiatry 2022, 13, 962837. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.; Steiling, S.; Landau, D. Incidence of Impaired Kidney Function Among Adolescent Patients Hospitalized with Anorexia Nervosa. JAMA Netw. Open 2021, 4, e2134908. [Google Scholar] [CrossRef]
- Bouquegneau, A.; Dubois, B.E.; Krzesinski, J.M.; Delanaye, P. Anorexia nervosa and the kidney. Am. J. Kidney Dis. 2012, 60, 299–307. [Google Scholar] [CrossRef]
- Puckett, L. Renal and electrolyte complications in eating disorders: A comprehensive review. J. Eat. Disord. 2023, 11, 26. [Google Scholar] [CrossRef]
- Tseng, M.C.M.; Chien, L.N.; Tu, C.Y.; Zheng, C.M.; Liu, H.Y. Risk of dialysis and renal diseases in patients with anorexia nervosa in Taiwan. Int. J. Eat. Disord. 2023, 56, 991–1000. [Google Scholar] [CrossRef]
- Ohne, M.; Watanabe, Y.; Oyake, C.; Onuki, Y.; Watanabe, T.; Ikeda, H. Pediatric early-phase anorexia nervosa without hypokalemia exhibiting acute tubular injury: A case report. CEN Case Rep. 2024, 13, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Bauschka, M.; Foley, M.; Lundberg, C.; Mehler, P. A Multi-Disciplinary Approach to Managing End-Stage Renal Disease in Anorexia Nervosa: A Case Report. Clin. Med. Insights Case Rep. 2023, 16, 11795476231169385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Kawanishi, K.; Sawa, C.; Luo, P.; Chen, P.; Luo, M.; Kang, D.; Honda, K. Application of low-vacuum scanning electron microscopy and energy-dispersive X-ray spectrometry for detection of renal tubular crystals: A case of nephrocalcinosis in the setting of anorexia nervosa. BMC Nephrol. 2025, 26, 352. [Google Scholar] [CrossRef]
- Schorr, M.; Miller, K.K. The endocrine manifestations of anorexia nervosa: Mechanisms and management. Nat. Rev. Endocrinol. 2017, 13, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Shunsuke, H.; Taishi, A.; Kohei, N. A Case of Hypoglycemia Associated with Anorexia Nervosa Revealing Isolated Adrenocorticotropic Hormone (ACTH) Deficiency. Cureus 2025, 17, e79383. [Google Scholar] [CrossRef]
- Misra, M.; Klibanski, A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014, 2, 581–592. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Monteleone, P.; Marciello, F.; Pellegrino, F.; Castellini, G.; Maj, M. Differences in Cortisol Awakening Response between Binge-Purging and Restrictive Patients with Anorexia Nervosa. Eur. Eat. Disord. Rev. 2017, 25, 13–18. [Google Scholar] [CrossRef]
- Ritschel, F.; Clas, S.; Geisler, D.; Haas, V.; Seidel, M.; Steding, J.; Roessner, V.; Kirschbaum, C.; Ehrlich, S. Is hypercortisolism in anorexia nervosa detectable using hair samples? J. Psychiatr. Res. 2018, 98, 87–94. [Google Scholar] [CrossRef]
- Schmalbach, I.; Herhaus, B.; Pässler, S.; Runst, S.; Berth, H.; Wolff-Stephan, S.; Petrowski, K. Correction: Cortisol reactivity in patients with anorexia nervosa after stress induction. Transl. Psychiatry 2021, 11, 208. [Google Scholar] [CrossRef]
- Haines, M.S. Endocrine complications of anorexia nervosa. J. Eat. Disord. 2023, 11, 24. [Google Scholar] [CrossRef]
- Mattioni, J.; Duriez, P.; Aïdat, S.; Lebrun, N.; Bohlooly-Y, M.; Gorwood, P.; Viltart, O.; Tolle, V. Altered circadian pattern of activity in a chronic activity-based anorexia nervosa-like female mouse model deficient for GHSR. Psychoneuroendocrinology 2025, 177, 107453. [Google Scholar] [CrossRef]
- Modan-Moses, D.; Yaroslavsky, A.; Pinhas-Hamiel, O.; Levy-Shraga, Y.; Kochavi, B.; Iron-Segev, S.; Enoch-Levy, A.; Toledano, A.; Stein, D. Prospective Longitudinal Assessment of Linear Growth and Adult Height in Female Adolescents with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2021, 106, e1–e10. [Google Scholar] [CrossRef]
- Léger, J.; Fjellestad-Paulsen, A.; Bargiacchi, A.; Pages, J.; Chevenne, D.; Alison, M.; Alberti, C.; Guilmin-Crepon, S. One Year of GH Treatment for Growth Failure in Children with Anorexia Nervosa: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2021, 106, e2535–e2546. [Google Scholar] [CrossRef]
- Faust, J.P.; Goldschmidt, A.B.; Anderson, K.E.; Glunz, C.; Brown, M.; Loeb, K.L.; Katzman, D.K.; Le Grange, D. Resumption of menses in anorexia nervosa during a course of family-based treatment. J. Eat. Disord. 2013, 1, 12. [Google Scholar] [CrossRef]
- Galusca, B.; Gay, A.; Belleton, G.; Eisinger, M.; Massoubre, C.; Lang, F.; Grouselle, D.; Estour, B.; Germain, N. Mechanisms and predictors of menses resumption once normal weight is reached in anorexia nervosa. J. Eat. Disord. 2023, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.M.; Hoffman, E.R.; Von Holle, A.; Torgersen, L.; Stoltenberg, C.; Reichborn-Kjennerud, T. Unplanned pregnancy in women with anorexia nervosa. Obstet. Gynecol. 2010, 116, 1136–1140. [Google Scholar] [CrossRef]
- Pan, J.R.; Li, T.Y.; Tucker, D.; Chen, K.Y. Pregnancy outcomes in women with active anorexia nervosa: A systematic review. J. Eat. Disord. 2022, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Magenes, V.C.; Siccardo, F.; Hruby, C.; Basso, M.; Conte, V.; Maggioni, G.; Fabiano, V.; Russo, S.; Veggiotti, P.; et al. Thyroid dysfunction in children and adolescents affected by undernourished and overnourished eating disorders. Front. Nutr. 2023, 10, 1205331. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, N.; Bobby, Z.; Sridhar, M.G. Is euthyroid sick syndrome a defensive mechanism against oxidative stress? Med. Hypotheses 2008, 71, 404–405. [Google Scholar] [CrossRef]
- Wronski, M.L.; Tam, F.I.; Seidel, M.; Mirtschink, P.; Poitz, D.M.; Bahnsen, K.; Steinhäuser, J.L.; Bauer, M.; Roessner, V.; Ehrlich, S. Associations between pituitary-thyroid hormones and depressive symptoms in individuals with anorexia nervosa before and after weight-recovery. Psychoneuroendocrinology 2022, 137, 105630. [Google Scholar] [CrossRef]
- Nomura, R.; Miyai, K.; Kuge, R.; Okura, T.; Goto, M.; Hasegawa, Y. Free T3 to free T4 ratio less than 2.0 suggests low T3 syndrome rather than central hypothyroidism from the age of two to eighteen years. Endocr. J. 2017, 64, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mebis, L.; Eerdekens, A.; Güiza, F.; Princen, L.; Derde, S.; Vanwijngaerden, Y.M.; Vanhorebeek, I.; Darras, V.M.; Van den Berghe, G.; Langouche, L. Contribution of nutritional deficit to the pathogenesis of the nonthyroidal illness syndrome in critical illness: A rabbit model study. Endocrinology 2012, 153, 973–984. [Google Scholar] [CrossRef][Green Version]
- Evrard, F.; Pinto da Cunha, M.; Lambert, M.; Devuyst, O. Impaired osmoregulation in anorexia nervosa: A case-control study. Nephrol. Dial. Transplant. 2004, 19, 3034–3039. [Google Scholar] [CrossRef]
- Goetze, J.P.; Støving, R.K. Copeptin in anorexia nervosa. Brain Behav. 2020, 10, e01551. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.L.; Thambundit, A.; Mehler, P.S.; Mittelman, S.D. Central diabetes insipidus associated with refeeding in anorexia nervosa: A case report. Int. J. Eat. Disord. 2019, 52, 752–756. [Google Scholar] [CrossRef]
- Sato, N.; Endo, K.; Ishizaka, H.; Matsumoto, M. Serial MR intensity changes of the posterior pituitary in a patient with anorexia nervosa, high serum ADH, and oliguria. J. Comput. Assist. Tomogr. 1993, 17, 648–650. [Google Scholar] [CrossRef]
- Afinogenova, Y.; Schmelkin, C.; Plessow, F.; Thomas, J.J.; Pulumo, R.; Micali, N.; Miller, K.K.; Eddy, K.T.; Lawson, E.A. Low Fasting Oxytocin Levels Are Associated with Psychopathology in Anorexia Nervosa in Partial Recovery. J. Clin. Psychiatry 2016, 77, e1483–e1490. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Holsen, L.; Santin, M.; DeSanti, R.; Meenaghan, E.; Eddy, K.; Herzog, D.; Goldstein, J.; Klibanski, A. Correction: Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J. Clin. Psychiatry 2015, 76, 666. [Google Scholar] [CrossRef]
- Aulinas, A.; Plessow, F.; Pulumo, R.L.; Asanza, E.; Mancuso, C.J.; Slattery, M.; Tolley, C.; Thomas, J.J.; Eddy, K.T.; Miller, K.K.; et al. Disrupted Oxytocin-Appetite Signaling in Females with Anorexia Nervosa. J. Clin. Endocrinol. Metab. 2019, 104, 4931–4940. [Google Scholar] [CrossRef]
- Plessow, F.; Galbiati, F.; Eddy, K.T.; Misra, M.; Miller, K.K.; Klibanski, A.; Aulinas, A.; Lawson, E.A. Low oxytocin levels are broadly associated with more pronounced psychopathology in anorexia nervosa with primarily restricting but not binge/purge eating behavior. Front. Endocrinol. 2023, 13, 1049541. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Maguire, S.; Hunt, G.E.; Kesby, A.; Suraev, A.; Stuart, J.; Booth, J.; McGregor, I.S. Intranasal oxytocin in the treatment of anorexia nervosa: Randomized controlled trial during re-feeding. Psychoneuroendocrinology 2018, 87, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Maguire, S.; Kesby, A.; Brownlow, R.; Hunt, G.E.; Kim, M.; McAulay, C.; Grisham, J.R.; McGregor, I.S.; Suraev, A.; Kevin, R.C.; et al. A phase II randomised controlled trial of intranasal oxytocin in anorexia nervosa. Psychoneuroendocrinology 2024, 164, 107032. [Google Scholar] [CrossRef]
- Hebebrand, J.; Hildebrandt, T.; Schlögl, H.; Seitz, J.; Denecke, S.; Vieira, D.; Gradl-Dietsch, G.; Peters, T.; Antel, J.; Lau, D.; et al. The role of hypoleptinemia in the psychological and behavioral adaptation to starvation: Implications for anorexia nervosa. Neurosci. Biobehav. Rev. 2022, 141, 104807. [Google Scholar] [CrossRef]
- Hebebrand, J.; Plieger, M.; Milos, G.; Peters, T.; Hinney, A.; Antel, J. Does hypoleptinemia trigger entrapment in anorexia nervosa? Etiological and clinical considerations. Eur. Eat. Disord. Rev. 2024, 32, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Burghardt, R.; Schneider, N.; Broecker-Preuss, M.; Weiss, D.; Merle, J.V.; Craciun, E.M.; Pfeiffer, E.; Mann, K.; Lehmkuhl, U.; et al. The role of leptin and cortisol in hyperactivity in patients with acute and weight-recovered anorexia nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 658–662. [Google Scholar] [CrossRef]
- Batury, V.L.; Walton, E.; Tam, F.; Wronski, M.L.; Buchholz, V.; Frieling, H.; Ehrlich, S. DNA methylation of ghrelin and leptin receptors in underweight and recovered patients with anorexia nervosa. J. Psychiatr. Res. 2020, 131, 271–278. [Google Scholar] [CrossRef]
- Tolle, V.; du Montcel, C.T.; Mattioni, J.; Schéle, E.; Viltart, O.; Dickson, S.L. To eat or not to eat: A role for ghrelin and LEAP2 in eating disorders? Neurosci. Appl. 2024, 3, 104045. [Google Scholar] [CrossRef]
- Tural, U.; Iosifescu, D.V. Adiponectin in anorexia nervosa and its modifiers: A meta-regression study. Int. J. Eat. Disord. 2022, 55, 1279–1290. [Google Scholar] [CrossRef]
- Makimura, H.; Stanley, T.L.; Chen, C.Y.; Branch, K.L.; Grinspoon, S.K. Relationship of adiponectin to endogenous GH pulse secretion parameters in response to stimulation with a growth hormone releasing factor. Growth Horm. IGF Res. 2011, 21, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Meskers, C.G.M.; Trappenburg, M.C.; Verlaan, S.; Reijnierse, E.M.; Whittaker, A.C.; Maier, A.B. Malnutrition is associated with dynamic physical performance. Aging Clin. Exp. Res. 2020, 32, 1085–1092. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Eddy, K.T.; Rutkove, S.B.; Breithaupt, L. Anorexia nervosa and muscle health: A systematic review of our current understanding and future recommendations for study. Int. J. Eat. Disord. 2023, 56, 483–500. [Google Scholar] [CrossRef]
- de Araújo, I.M.; Rebolho, M.V.F.; Gomes, M.M.; Suen, V.M.; de Paula, F.J.A. Bone evaluation and relationship between body composition and bone mass in anorexia nervosa followed up by a multidisciplinary team. Endocrine 2025, 89, 259–266. [Google Scholar] [CrossRef] [PubMed]
- DiVasta, A.D.; Stamoulis, C.; Rubin, C.T.; Gallagher, J.S.; Kiel, D.P.; Snyder, B.D.; Gordon, C.M. Low-Magnitude Mechanical Signals to Preserve Skeletal Health in Female Adolescents with Anorexia Nervosa: A Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2441779. [Google Scholar] [CrossRef]
- Legroux, I.; Cortet, B. Factors influencing bone loss in anorexia nervosa: Assessment and therapeutic options. RMD Open 2019, 5, e001009. [Google Scholar] [CrossRef]
- Gibson, D.; Filan, Z.; Westmoreland, P.; Mehler, P.S. Loss of Bone Density in Patients with Anorexia Nervosa Food That Alone Will Not Cure. Nutrients 2024, 16, 3593. [Google Scholar] [CrossRef]
- Donat, A.; Knapstein, P.R.; Jiang, S.; Baranowsky, A.; Ballhause, T.M.; Frosch, K.H.; Keller, J. Glucose Metabolism in Osteoblasts in Healthy and Pathophysiological Conditions. Int. J. Mol. Sci. 2021, 22, 4120. [Google Scholar] [CrossRef]
- Hediger, C.; Rost, B.; Itin, P. Cutaneous manifestations in anorexia nervosa. Schweiz. Med. Wochenschr. 2000, 130, 565–575. [Google Scholar]
- Strumia, R. Skin signs in anorexia nervosa. Dermatoendocrinology 2009, 1, 268–270. [Google Scholar] [CrossRef]
- Strumia, R. Eating disorders and the skin. Clin. Dermatol. 2013, 31, 80–85. [Google Scholar] [CrossRef]
- Safer, J.D. Thyroid hormone action on skin. Dermatoendocrinology 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Lackner, S.; Meier-Allard, N.; Mörkl, S.; Müller, W.; Fürhapter-Rieger, A.; Mangge, H.; Zelzer, S.; Holasek, S. Hypercarotenemia in Anorexia Nervosa Patients May Influence Weight Balance: Results of a Clinical Cross-Sectional Cohort Study. Front. Psychiatry 2021, 12, 758300. [Google Scholar] [CrossRef]
- Rallis, E.; Lotsaris, K.; Grech, V.S.; Tertipi, N.; Sfyri, E.; Kefala, V. The Nutrient-Skin Connection: Diagnosing Eating Disorders Through Dermatologic Signs. Nutrients 2024, 16, 4354. [Google Scholar] [CrossRef] [PubMed]
- Stamu-O’Brien, C.; Shivakumar, S.; Messas, T.; Kroumpouzos, G. Through the looking glass: Skin signs that help diagnose eating disorders. Clin. Dermatol. 2023, 41, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cleary, B.S.; Gaudiani, J.L.; Mehler, P.S. Interpreting the complete blood count in anorexia nervosa. Eat. Disord. 2010, 18, 132–139. [Google Scholar] [CrossRef]
- De Filippo, E.; Marra, M.; Alfinito, F.; Di Guglielmo, M.L.; Majorano, P.; Cerciello, G.; De Caprio, C.; Contaldo, F.; Pasanisi, F. Hematological complications in anorexia nervosa. Eur. J. Clin. Nutr. 2016, 70, 1305–1308. [Google Scholar] [CrossRef]
- Hütter, G.; Ganepola, S.; Hofmann, W.K. The hematology of anorexia nervosa. Int. J. Eat. Disord. 2009, 42, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.K.; Grinspoon, S.K.; Ciampa, J.; Hier, J.; Herzog, D.; Klibanski, A. Medical findings in outpatients with anorexia nervosa. Arch. Intern. Med. 2005, 165, 561–566. [Google Scholar] [CrossRef]
- Walsh, K.; Blalock, D.V.; Mehler, P.S. Hematologic findings in a large sample of patients with anorexia nervosa and bulimia nervosa. Am. J. Hematol. 2020, 95, E98–E101. [Google Scholar] [CrossRef]
- Salari, N.; Razavizadeh, S.; Abdolmaleki, A.; Heidarian, P.; Rahimi, A.; Shohaimi, S.; Mohammadi, M. The global prevalence of anemia in patients with anorexia nervosa: A systematic review and meta-analysis. BMC Psychol. 2025, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Ishikawa, H.; Kitadate, A.; Sasaki, R.; Kobayashi, T.; Nanjyo, H.; Kanbayashi, T.; Shimizu, T. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: A case report. BMC Psychiatry 2018, 18, 150. [Google Scholar] [CrossRef] [PubMed]
- Funayama, M.; Koreki, A.; Mimura, Y.; Takata, T.; Ogino, S.; Kurose, S.; Shimizu, Y.; Kudo, S. Restrictive type and infectious complications might predict nadir hematological values among individuals with anorexia nervosa during the refeeding period: A retrospective study. J. Eat. Disord. 2022, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Lambert, M.; Rodhain, J.; Lefebvre, C.; Coche, E. Haematological changes and infectious complications in anorexia nervosa: A case-control study. QJM Int. J. Med. 1993, 86, 791–799. [Google Scholar]
- Palmblad, J.; Fohlin, L.; Lundström, M. Anorexia nervosa and polymorphonuclear (PMN) granulocyte reactions. Scand. J. Haematol. 1977, 19, 334–342. [Google Scholar] [CrossRef]
- Brown, R.F.; Bartrop, R.; Beumont, P.; Birmingham, C.L. Bacterial infections in anorexia nervosa: Delayed recognition increases complications. Int. J. Eat. Disord. 2005, 37, 261–265. [Google Scholar] [CrossRef]
- Mashimoto, M.; Higuchi, F.; Okazaki, S.; Hoshii, Y.; Nakagawa, S. Decreased Volume of Bone Marrow Adipocytes With Sparse Gelatinous Marrow Transformation in a Patient With Pancytopenia With Anorexia Nervosa: A Case Report. Cureus 2024, 16, e58390. [Google Scholar] [CrossRef]
- Oświęcimska, J.; Malczyk, Ż.; Szymlak, A.; Mikołajczak, A.; Ziora, K.; Zamlynski, J.; Machura, E.; Zajac, P.; Koczy, B.; Kasperska-Zajac, A. Changes in Platelet Count and Size Indices in Adolescent Patients with Anorexia Nervosa. Clin. Appl. Thromb./Hemost. 2017, 23, 562–566. [Google Scholar] [CrossRef]
- Mitchell, O.; Feldman, D.M.; Diakow, M.; Sigal, S.H. The pathophysiology of thrombocytopenia in chronic liver disease. Hepatic Med.: Evid. Res. 2016, 8, 39–50. [Google Scholar] [CrossRef]
- Bourke, C.D.; Berkley, J.A.; Prendergast, A.J. Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol. 2016, 37, 386–398. [Google Scholar] [CrossRef]
- Marcos, A.; Nova, E.; Montero, A. Changes in the immune system are conditioned by nutrition. Eur. J. Clin. Nutr. 2003, 57, 66–69. [Google Scholar] [CrossRef]
- Gibson, D.; Mehler, P.S. Anorexia Nervosa and the Immune System—A Narrative Review. J. Clin. Med. 2019, 8, 1915. [Google Scholar] [CrossRef]
- Dostálová, I.; Kaválková, P.; Papežová, H.; Domluvilová, D.; Zikán, V.; Haluzík, M. Association of macrophage inhibitory cytokine-1 with nutritional status, body composition and bone mineral density in patients with anorexia nervosa: The influence of partial realimentation. Nutr. Metab. 2010, 7, 34. [Google Scholar] [CrossRef]
- Karczewska-Kupczewska, M.; Kowalska, I.; Nikolajuk, A.; Adamska, A.; Otziomek, E.; Gorska, M.; Straczkowski, M. Hyperinsulinemia acutely increases serum macrophage inhibitory cytokine-1 concentration in anorexia nervosa and obesity. Clin. Endocrinol. 2012, 76, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Omodei, D.; Pucino, V.; Labruna, G.; Procaccini, C.; Galgani, M.; Perna, F.; Pirozzi, D.; De Caprio, C.; Marone, G.; Fontana, L.; et al. Immune-metabolic profiling of anorexic patients reveals an anti-oxidant and anti-inflammatory phenotype. Metabolism 2015, 64, 396–405. [Google Scholar] [CrossRef]
- Elegido, A.; Graell, M.; Andrés, P.; Gheorghe, A.; Marcos, A.; Nova, E. Increased naive CD4+ and B lymphocyte subsets are associated with body mass loss and drive relative lymphocytosis in anorexia nervosa patients. Nutr. Res. 2017, 39, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Freff, J.; Bröker, L.; Leite Dantas, R.; Schwarte, K.; Bühlmeier, J.; Kraft, I.; Hinney, A.; Buhlmann, U.; Arolt, V.; Dannlowski, U.; et al. Expression of CXCR4 on CD4+ T cells predicts body composition parameters in female adolescents with anorexia nervosa. Front. Psychiatry 2022, 13, 960905. [Google Scholar] [CrossRef]
- Pászthy, B.; Svec, P.; Vásárhelyi, B.; Túry, F.; Mazzag, J.; Tulassay, T.; Treszl, A. Investigation of regulatory T cells in anorexia nervosa. Eur. J. Clin. Nutr. 2007, 61, 1245–1249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freff, J.; Schwarte, K.; Bröker, L.; Bühlmeier, J.; Kraft, I.; Öztürk, D.; Hinney, A.; Arolt, V.; Dannlowski, U.; Romer, G.; et al. Alterations in B cell subsets correlate with body composition parameters in female adolescents with anorexia nervosa. Sci. Rep. 2021, 11, 1125. [Google Scholar] [CrossRef]
- Xiao, K.; Gillissie, E.S.; Lui, L.M.; Ceban, F.; Teopiz, K.M.; Gill, H.; Cao, B.; Ho, R.; Rosenblat, J.D.; McIntyre, R.S. Immune response to vaccination in adults with mental disorders: A systematic review. J. Affect. Disord. 2022, 304, 66–77. [Google Scholar] [CrossRef]
- Caldiroli, A.; La Tegola, D.; Affaticati, L.M.; Manzo, F.; Cella, F.; Scalia, A.; Capuzzi, E.; Nicastro, M.; Colmegna, F.; Buoli, M.; et al. Clinical and peripheral biomarkers in female patients affected by Anorexia: Does the Neutrophil/Lymphocyte Ratio (NLR) affect severity? Nutrients 2023, 15, 1133. [Google Scholar] [CrossRef]
- Słotwińska, S.M.; Słotwiński, R. Immune disorders in anorexia. Cent. Eur. J. Immunol. 2017, 42, 294–300. [Google Scholar] [CrossRef]
- Nilsson, A.; Elander, L.; Hallbeck, M.; Kugelberg, U.Ö.; Engblom, D.; Blomqvist, A. The involvement of prostaglandin E2 in interleukin-1β evoked anorexia is strain dependent. Brain Behav. Immun. 2017, 60, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Pomeroy, C.; Eckert, E.; Hu, S.; Eiken, B.; Mentink, M.; Crosby, R.D.; Chao, C.C. Role of interleukin-6 and transforming growth factor-beta in anorexia nervosa. Biol. Psychiatry 1994, 36, 836–839. [Google Scholar] [CrossRef]
- Polack, E.; Nahmod, V.E.; Emeric-Sauval, E.; Bello, M.; Costas, M.; Finkielman, S.; Arzt, E. Low lymphocyte interferon-gamma production and variable proliferative response in anorexia nervosa patients. J. Clin. Immunol. 1993, 13, 445–451. [Google Scholar] [CrossRef]
- Di Paolo, A.; Membrino, V.; Alia, S.; Nanetti, L.; Svarca, L.E.; Perrone, M.L.; Aquilanti, L.; Mazzanti, L.; Vignini, A.; Salvolini, E.; et al. Pro-inflammatory cytokine alterations in recent onset anorexia nervosa adolescent female patients before and after 6 months of integrated therapy: A case-control study. J. Investig. Med. 2024, 72, 522–531. [Google Scholar] [CrossRef]
- Tyszkiewicz-Nwafor, M.; Jowik, K.; Paszynska, E.; Dutkiewicz, A.; Słopien, A.; Dmitrzak-Weglarz, M. Expression of immune-related proteins and their association with neuropeptides in adolescent patients with anorexia nervosa. Neuropeptides 2022, 91, 102214. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Gaudiani, J.L.; Sabel, A.L.; Long, C.S.; Stahel, P.F.; Mehler, P.S. Complement C3 serum levels in anorexia nervosa: A potential biomarker for the severity of disease? Ann. Gen. Psychiatry 2011, 10, 16. [Google Scholar] [CrossRef]
- Wagner-Skacel, J.; Haidacher, F.; Wiener, M.; Pahsini, K.; Marinschek, S.; Lahousen, T.; Wonisch, W.; Bengesser, S.; Butler, M.I.; Lackner, S.; et al. Oxidative Status in Adult Anorexia Nervosa Patients and Healthy Controls-Results from a Cross-Sectional Pilot Study. Antioxidants 2022, 11, 842. [Google Scholar] [CrossRef]
- Amerio, A.; Martino, E.; Strangio, A.; Aguglia, A.; Escelsior, A.; Conio, B.; Sukkar, S.G.; Saverino, D. Autoantibodies, Oxidative Stress, and Nutritional State in Anorexia Nervosa. Antibodies 2024, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Yuan, Y.; Chen, J. Celiac Disease, Gluten-Free Diet, and Eating Disorders: From Bench to Bedside. Foods 2024, 14, 74. [Google Scholar] [CrossRef]
- Larsen, J.T.; Yilmaz, Z.; Vilhjálmsson, B.J.; Thornton, L.M.; Benros, M.E.; Musliner, K.L.; Eating Disorders Working Group of the Psychiatric Genomics Consortium; Werge, T.; Hougaard, D.M.; Mortensen, P.B.; et al. Anorexia nervosa and inflammatory bowel diseases—Diagnostic and genetic associations. JCPP Adv. 2021, 1, e12036. [Google Scholar] [CrossRef]
- Su, Q.; Li, J.; Lu, Y.; Wu, M.; Liang, J.; An, Z. Anorexia and bulimia in relation to ulcerative colitis: A Mendelian randomization study. Front. Nutr. 2024, 11, 1400713. [Google Scholar] [CrossRef]
- Ilzarbe, L.; Fàbrega, M.; Quintero, R.; Bastidas, A.; Pintor, L.; García-Campayo, J.; Gomollón, F.; Ilzarbe, D. Inflammatory Bowel Disease and Eating Disorders: A systematized review of comorbidity. J. Psychosom. Res. 2017, 102, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Joshkon, A.; Reytier, C.; Bertin, D.; Castets, S.; Reynaud, R.; Bat, F.; Mege, J.L.; Blot-Chabaud, M.; Darmon, P.; Bardin, N. Identification of autoantibodies against HIF1a in patients with anorexia nervosa and their potential role in hepatic cytolysis. Sci. Rep. 2025, 15, 21347. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, G.; Guadagno, A.; Herzum, A.; Amicarelli, V.; Drago, F. Purpuric pityriasis rosea in patients with anorexia nervosa. Dermatol. Rep. 2025, 17, 10053. [Google Scholar] [CrossRef]
- Paszynska, E.; Hernik, A.; Slopien, A.; Boucher, Y.; Tyszkiewicz-Nwafor, M.; Roszak, M.; Bilska, K.; Dmitrzak-Weglarz, M. Expression of salivary immunoglobulins and their association with analgesic neuropeptide opiorphin in anorexia nervosa during adolescence. J. Eat. Disord. 2022, 10, 118. [Google Scholar] [CrossRef]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.M.; Barnett, J.A.; Gibson, D.L. A critical analysis of eating disorders and the gut microbiome. J. Eat. Disord. 2022, 10, 154. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Troisi, J.; Serena, G.; Fasano, A.; Dalle Grave, R.; Cascino, G.; Marciello, F.; Calugi, S.; Scala, G.; Corrivetti, G.; et al. The Gut Microbiome and Metabolomics Profiles of Restricting and Binge-Purging Type Anorexia Nervosa. Nutrients 2021, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Støving, R.K.; Berreira Ibraim, S.; Hyötyläinen, T.; Thirion, F.; Arora, T.; Lyu, L.; Stankevic, E.; Hansen, T.H.; Déchelotte, P.; et al. The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Nat. Microbiol. 2023, 8, 787–802. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Panagopoulos, F.; Evangelopoulos, A.; Stamatopoulos, V.; Papagiorgos, A.; Geladari, E.; Dalamaga, M. Gut Microbiota and Its Role in the Brain-Gut-Kidney Axis in Hypertension. Curr. Hypertens. Rep. 2023, 25, 367–376. [Google Scholar] [CrossRef]
- Shahid, F.; Afridi, Z.; Ali, M.; Saddique, M.N.; Iqbal, J.; Jha, A. Beneath the surface: Unveiling the gut-brain axis in anorexia and depression. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2025, 30, 14. [Google Scholar] [CrossRef]
- Rousseau, L.; Salaün, C.; Langlois, L.; Achamrah, N.; Coëffier, M. Burden of negative energy balance in anorexia nervosa: Role of circadian rhythm, mitochondrial dynamics, microbiota-gut-brain axis and their interactions. Clin. Nutr. 2025, 52, 27–45. [Google Scholar] [CrossRef]
- Frintrop, L.; Trinh, S.; Seitz, J.; Kipp, M. The Role of Glial Cells in Regulating Feeding Behavior: Potential Relevance to Anorexia Nervosa. J. Clin. Med. 2021, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Nguyen, K.H.; Ball, J.B.; Hopkins, S.; Kelley, T.; Baratta, M.V.; Fleshner, M.; Maier, S.F. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain Behav. Immun. 2022, 100, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Nunez, S.G.; Rabelo, S.P.; Subotic, N.; Caruso, J.W.; Knezevic, N.N. Chronic Stress and Autoimmunity: The Role of HPA Axis and Cortisol Dysregulation. Int. J. Mol. Sci. 2025, 26, 9994. [Google Scholar] [CrossRef]
- Wei, Y.; Peng, S.; Lian, C.; Kang, Q.; Chen, J. Anorexia nervosa and gut microbiome: Implications for weight change and novel treatments. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, P.; Bagherieh, S.; Mirzayi, P.; Kalantari, A.; Mirmosayyeb, O.; Teixeira, A.L.; Rezaei, N. Serum and plasma levels of brain-derived neurotrophic factor in individuals with eating disorders (EDs): A systematic review and meta-analysis. J. Eat. Disord. 2022, 10, 105. [Google Scholar] [CrossRef]
- Chen, H.; Martins, I.; Kroemer, G. The autophagy-repressive tissue hormone DBI/ACBP (diazepam binding inhibitor, acyl-CoA binding protein) suppresses anorexia. Autophagy 2024, 20, 2827–2829. [Google Scholar] [CrossRef]
- Haleem, D.J. Improving therapeutics in anorexia nervosa with tryptophan. Life Sci. 2017, 178, 87–93. [Google Scholar] [CrossRef]
- Jenkins, Z.M.; Eikelis, N.; Phillipou, A.; Castle, D.J.; Wilding, H.E.; Lambert, E.A. Autonomic Nervous System Function in Anorexia Nervosa: A Systematic Review. Front. Neurosci. 2021, 15, 682208. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Ramírez-Goerke, M.I.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Navarro-Jiménez, E.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. The Impact of Anorexia Nervosa and the Basis for Non-Pharmacological Interventions. Nutrients 2023, 15, 2594. [Google Scholar] [CrossRef]
- Brennan, C.; Green, G.; Morgan, A.; Baudinet, J. The Role of the Dietitian Within a Day Programme for Adolescent Anorexia Nervosa: A Reflexive Thematic Analysis of Child and Adolescent Eating Disorder Clinician Perspectives. J. Hum. Nutr. Diet. 2025, 38, e70070. [Google Scholar] [CrossRef] [PubMed]
- Bohon, C.; Le Grange, D.; Attia, E.; Golden, N.H.; Steinberg, D. United States-based practice guidelines for children and adolescents with eating disorders: Synthesis of clinical practice guidelines. J. Eat. Disord. 2025, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Dozio, E.; Alonge, M.; Tori, G.; Caumo, A.; Russo, R.G.; Scuttari, E.; Fringuelli, L.; Terruzzi, I. Dynamic Nutrition Strategies for Anorexia Nervosa: Marker-Based Integration of Calories and Proteins. Nutrients 2025, 17, 560. [Google Scholar] [CrossRef]
- Brennan, C.; Felemban, L.; McAdams, E.; Walsh, K.; Baudinet, J. An Exploration of Clinical Characteristics and Treatment Outcomes Associated with Dietetic Intervention in Adolescent Anorexia Nervosa. Nutrients 2024, 16, 4117. [Google Scholar] [CrossRef]
- Ayrolles, A.; Clarke, J.; Godart, N.; André-Carletti, C.; Barbe, C.; Bargiacchi, A.; Blanchet, C.; Bergametti, F.; Bertrand, V.; Caldagues, E.; et al. Early-onset anorexia nervosa: A scoping review and management guidelines. J. Eat. Disord. 2024, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Teo, D.E.S.Y.; Teong, V.W.L.; Ramachandran, R.; Lim, S.L.; Lin, C.X. Characteristics and outcome of patients with anorexia nervosa on medical nutritional therapy: An institutional study with review of literature. Singap. Med. J. 2024, 65, 564–570. [Google Scholar] [CrossRef]
- Del Portillo, R.C.; Milla, S.P.; Martín, P.M.; Loria-Kohen, V.; Olmos, M.Á.M.; Álvarez, M.T.M.; Alija, M.J.C.; Palmero, M.Á.M.; Lozano, E.C.; Valero-Pérez, M.; et al. A consensus report by the Working Group on Eating Disorders of Sociedad Española de Nutrición Clínica y Metabolismo (GTTCA-SENPE). Evaluation, medical and nutritional management of anorexia nervosa. Update 2023. Nutr. Hosp. 2024, 41, 1–60. [Google Scholar] [CrossRef]
- Hunter, R.; Platygeni, M.; Moore, E. Plant-based recovery from restrictive eating disorder: A qualitative enquiry. Appetite 2024, 194, 107137. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, G.; Croci, I.; De Santis, G.L.; Chianello, I.; Miller, K.P.; Gualtieri, P.; Di Renzo, L.; De Lorenzo, A.; Tozzi, A.E.; Zanna, V. Adherence to a Mediterranean Diet, Body Composition and Energy Expenditure in Outpatients Adolescents Diagnosed with Anorexia Nervosa: A Pilot Study. Nutrients 2023, 15, 3223. [Google Scholar] [CrossRef] [PubMed]
- Stheneur, C.; Hanachi, M. Somatic Outcomes and Nutritional Management of Anorexia Nervosa. Nutrients 2023, 15, 2541. [Google Scholar] [CrossRef]
- van den Hoek Ostende, M.M.; Brizzi, G.; Meregalli, V.; Schroeder, P.A.; Collantoni, E. Psychological distance affects real movements in virtual reality: Distance to food in anorexia nervosa. J. Eat. Disord. 2025, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Herpertz-Dahlmann, B.; Mörkl, S. Biological Therapies and Eating Disorders. Eur. Eat. Disord. Rev. 2025, 33, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Thorey, S.; Blanchet, C.; Guessoum, S.B.; Moro, M.R.; Ludot, M.; Carretier, E. Efficacy and tolerance of second-generation antipsychotics in anorexia nervosa: A systematic scoping review. PLoS ONE 2023, 18, e0278189. [Google Scholar] [CrossRef]
- Peck, S.K.; Shao, S.; Gruen, T.; Yang, K.; Babakanian, A.; Trim, J.; Finn, D.M.; Kaye, W.H. Psilocybin therapy for females with anorexia nervosa: A phase 1, open-label feasibility study. Nat. Med. 2023, 29, 1947–1953. [Google Scholar] [CrossRef]
- Rodan, S.C.; Maguire, S.; Meez, N.; Greenstien, K.; Zartarian, G.; Mills, K.L.; Suraev, A.; Bedoya-Pérez, M.A.; McGregor, I.S. Prescription and Nonprescription Drug Use Among People with Eating Disorders. JAMA Netw. Open 2025, 8, e2522406. [Google Scholar] [CrossRef]
- Andries, A.; Frystyk, J.; Flyvbjerg, A.; Stoning, R.K. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int. J. Eat. Dis. 2014, 47, 18–23. [Google Scholar] [CrossRef]
- Ragnhildstveit, A.; Slayton, M.; Jackson, L.K.; Brendle, M.; Ahuja, S.; Holle, W.; Moore, C.; Sollars, K.; Seli, P.; Robison, R. Ketamine as a Novel Psychopharmacotherapy for Eating Disorders: Evidence and Future Directions. Brain Sci. 2022, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, R.; Millischer, V.; Stiernborg, M.; Tume, C.E.; Mehdinia, S.; Barker, P.; Yilmaz, Z.; Gonçalves, V.F.; Lavebratt, C.; et al. Elevated plasma GDF15 combined with FGF21 suggests mitochondrial dysfunction in a subgroup of anorexia nervosa patients. Transl. Psychiatry 2025, 15, 215. [Google Scholar] [CrossRef]
- Borner, T.; Shaulson, E.D.; Ghidewon, M.Y.; Barnett, A.B.; Horn, C.C.; Doyle, R.P.; Grill, H.J.; Hayes, M.R.; De Jonghe, B.C. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020, 31, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.M.; Jagarlapudi, S.; Patel, A.; Zou, C.; Joaquim, S.; Li, X.; Kang, L.; Pang, J.; Hales, K.; Ziso-Qejvanaj, E.; et al. Growth differentiation factor 15 neutralization does not impact anorexia or survival in lipopolysaccharide-induced inflammation. Iscience 2021, 24, 102554. [Google Scholar] [CrossRef]
- Hebebrand, J.; Milos, G.; Seitz, J.; Matthews, A. Recombinant human leptin for the treatment of anorexia nervosa. Lancet 2024, 404, 1639–1640. [Google Scholar] [CrossRef]
- Brown, R.J.; Oral, E.A.; Cochran, E.; Araújo-Vilar, D.; Savage, D.B.; Long, A.; Fine, G.; Salinardi, T.; Gorden, P. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 2018, 60, 479–489. [Google Scholar] [CrossRef]
- Welt, C.K.; Chan, J.L.; Bullen, J.; Murphy, R.; Smith, P.; DePaoli, A.M.; Karalis, A.; Mantzoros, C.S. Recombinant human leptin in women with hypothalamic amenorrhea. N. Engl. J. Med. 2004, 351, 987–997. [Google Scholar] [CrossRef]
- Hebebrand, J.; Antel, J.; Peters, T. Case report: Clinical improvements observed in first off-label metreleptin treatment of a patient with atypical anorexia nervosa. Eur. Child Adolesc. Psychiatry 2024, 33, 2267–2272. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Karampela, I.; Grivakou, E.; Dalamaga, M. The intricate role of adipokines in cancer-related signaling and the tumor microenvironment: Insights for future research. Semin. Cancer Biol. 2025, 113, 130–150. [Google Scholar] [CrossRef]
- Gradl-Dietsch, G.; Milos, G.; Wabitsch, M.; Bell, R.; Tschöpe, F.; Antel, J.; Hebebrand, J. Rapid Emergence of Appetite and Hunger Resulting in Weight Gain and Improvement of Eating Disorder Symptomatology during and after Short-Term Off-Label Metreleptin Treatment of a Patient with Anorexia Nervosa. Obes. Facts 2023, 16, 99–107. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef]
- Dhopatkar, N.; Keeler, J.L.; Mutwalli, H.; Whelan, K.; Treasure, J.; Himmerich, H. Gastrointestinal symptoms, gut microbiome, probiotics and prebiotics in anorexia nervosa: A review of mechanistic rationale and clinical evidence. Psychoneuroendocrinology 2023, 147, 105959. [Google Scholar] [CrossRef]
- Žaja, O.; Fiolić, M.; Ćuk, M.C.; Tiljak, M.K. The role of L. reuteri DSM17938 in nutritional recovery and treatment of constipation in children and adolescents with anorexia nervosa—A randomized, double blind, placebo controlled study. Clin. Nutr. ESPEN 2021, 46, 47–53. [Google Scholar] [CrossRef]
- Maschek, S.; Østergaard, T.H.; Krych, L.; Zachariassen, L.F.; Sørensen, D.B.; Junker Mentzel, C.M.; Hansen, A.K.; Sjögren, J.M.; Barfod, K.K. Investigating fecal microbiota transplants from individuals with anorexia nervosa in antibiotic-treated mice using a cross-over study design. J. Eat. Disord. 2025, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Kooij, K.L.; Andreani, N.A.; van der Gun, L.L.; Keller, L.; Trinh, S.; van der Vijgh, B.; Luijendijk, M.; Dempfle, A.; Herpertz-Dahlmann, B.; Seitz, J.; et al. Fecal microbiota transplantation of patients with anorexia nervosa did not alter flexible behavior in rats. Int. J. Eat. Disord. 2024, 57, 1868–1881. [Google Scholar] [CrossRef] [PubMed]
- Quagebeur, R.; Dalile, B.; Raes, J.; Van Oudenhove, L.; Verbeke, K.; Vrieze, E. The role of short-chain fatty acids (SCFAs) in regulating stress responses, eating behavior, and nutritional state in anorexia nervosa: Protocol for a randomized controlled trial. J. Eat. Disord. 2023, 11, 191. [Google Scholar] [CrossRef]
- Hemmings, A.; Gallop, L.; İnce, B.; Cutinha, D.; Kan, C.; Simic, M.; Zadeh, E.; Malvisi, I.; McKenzie, K.; Zocek, L.; et al. A randomised controlled feasibility trial of intermittent theta burst stimulation with an open longer-term follow-up for young people with persistent anorexia nervosa (RaISE): Study protocol. Eur. Eat. Disord. Rev. 2024, 32, 575–588. [Google Scholar] [CrossRef]
- Himmerich, H.; Lewis, Y.D.; Conti, C.; Mutwalli, H.; Karwautz, A.; Sjögren, J.M.; Uribe Isaza, M.M.; Tyszkiewicz-Nwafor, M.; Aigner, M.; McElroy, S.L.; et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines update 2023 on the pharmacological treatment of eating disorders. World J. Biol. Psychiatry 2023, 24, 643–706. [Google Scholar] [CrossRef]
| Author, Year | Type of Study | Findings | Remarks |
|---|---|---|---|
| Brennan et al., 2025 [184] | Semi-structured interviews were undertaken by 11 clinicians who had been working with adolescents aged 12–18 y.o. with anorexia nervosa (AN) who had been on a daily program. | The role of the dietician was pointed out as a crucial component of this intervention daily program. | The personalized nature of each nutritional interventionist turned out to be the most important determinant between the different phases of the nutritional program, especially between the weight restoration and maintenance phase. |
| Bohon et al., 2025 [185] | The authors have summarized key recommendations for AN management in the United States. | Early recognition of restrictive AN in the population together with family interventions and the help from a multidisciplinary team is of utmost importance. | The importance of screening tools for eating disorders in the general population has been stressed in this review manuscript. |
| Dozio et al., 2025 [186] | Observational study involving 79 patients with AN in Italy who had been admitted for management of AN. | The results after 6 months of medical management of these patients supported that early fat and protein restoration in these patients together with their close monitoring were very helpful. | The authors concluded that sustained medical nutritionists care and the implementation of a multidisciplinary team for as long as 6 months revealed encouraging results. |
| Brennan et al., 2024 [187] | A retrospective cohort study involving 92 patients with AN from Maudsley Center for Child and Adolescent Eating Disorders in London, United Kingdom. | The results of this study supported that there was no difference regarding weight gain among patients who received dietician’s guidance compared to those who did not. | The authors concluded that more information is needed in order to timely intervene with the consultation of a registered dietician among patients with AN. |
| Ayrolles et al., 2024 [188] | A scoping review from France regarding patients with early-onset pre-pubertal AN. | The authors have pointed out that there is lack of information regarding this particular group of early-onset AN. | The authors concluded that much more research is mandatory in this early-onset pre-pubertal group of patients with AN to better characterize and manage them. |
| Teo et al., 2024 [189] | A retrospective cohort study involving 47 patients with AN in Singapore. | Patients with AN can achieve weight restoration with a more dynamic management and for less duration of in-hospital stay with safety. | The authors concluded that in Asia and from their patient cohort, a shorter and more dynamic calorie administration could result in a lower duration of in-hospital stay. However, they have highlighted that the incidence of the refeeding syndrome should be further studied in this Asian group of patients. |
| Campos del Portillo et al., 2024 [190] | A consensus report from Spain. | The authors reported that outpatient management was most often preferred. However, there should be frequent re-assessment even at out of hospital care of these patients by a multidisciplinary team. | The authors point out the significance of alertness for the refeeding syndrome, especially among very malnourished patients with AN. |
| Hunter et al., 2024 [191] | A qualitative study interviewing 14 female patients with AN in the UK to evaluate the effects of a plant-based diet. | The authors proposed that a plant-based diet could be motivating for patients with AN. | The authors concluded that the participants found this plant-based diet motivating and that it was giving them good support for healthier eating habits. |
| Cinelli et al., 2023 [192] | An observational study including 8 outpatients with AN in Rome, Italy. | The patients were seen at baseline and after 6 months. The Mediterranean Diet was reported to be a good choice regarding weight management among these patients with AN. | The authors concluded that the Mediterranean Diet could offer a very promising dietary plan in patients with AN. However, the group consisted of only 8 patients. |
| Sthener et al., 2023 [193] | An editorial manuscript. | The authors reported that the initiation of nutritional management should be gradually increased during the first days due to the increased risk of refeeding syndrome. | The authors pointed out that the use of parenteral nutrition should be restricted among patients with AN due to increased risk for infections and metabolic adverse effects. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kounatidis, D.C.; Vallianou, N.G. Decoding the Spectrum of Anorexia Nervosa: Clinical Impact, Molecular Insights, and Therapeutic Perspectives. Biomolecules 2025, 15, 1559. https://doi.org/10.3390/biom15111559
Kounatidis DC, Vallianou NG. Decoding the Spectrum of Anorexia Nervosa: Clinical Impact, Molecular Insights, and Therapeutic Perspectives. Biomolecules. 2025; 15(11):1559. https://doi.org/10.3390/biom15111559
Chicago/Turabian StyleKounatidis, Dimitris C., and Natalia G. Vallianou. 2025. "Decoding the Spectrum of Anorexia Nervosa: Clinical Impact, Molecular Insights, and Therapeutic Perspectives" Biomolecules 15, no. 11: 1559. https://doi.org/10.3390/biom15111559
APA StyleKounatidis, D. C., & Vallianou, N. G. (2025). Decoding the Spectrum of Anorexia Nervosa: Clinical Impact, Molecular Insights, and Therapeutic Perspectives. Biomolecules, 15(11), 1559. https://doi.org/10.3390/biom15111559







