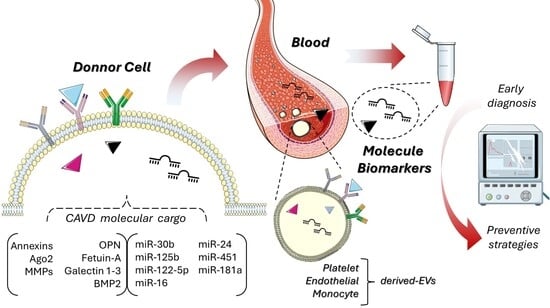
Extracellular Vesicles in Calcific Aortic Valve Disease: From Biomarkers to Drug Delivery Applications
Abstract
1. Introduction
2. Structural Stability of Extracellular Vesicles in CAVD
| Parameter | EV-Encapsulated RNA | Free Circulating RNA | Reference |
|---|---|---|---|
| RNase Resistance | High (lipid bilayer protection) | Low (direct exposure) | [25] |
| Stability in Storage | >6 months at −80 °C | Degrades within weeks | [26] |
| Signal-to-Noise Ratio | High (enriched cargo) | Low (diluted in biofluid) | [25] |
| Disease Specificity | Cell-of-origin signatures | Non-specific degradation products | [37] |
3. Extracellular Vesicles Across Biofluids Enhance Diagnostic Accessibility
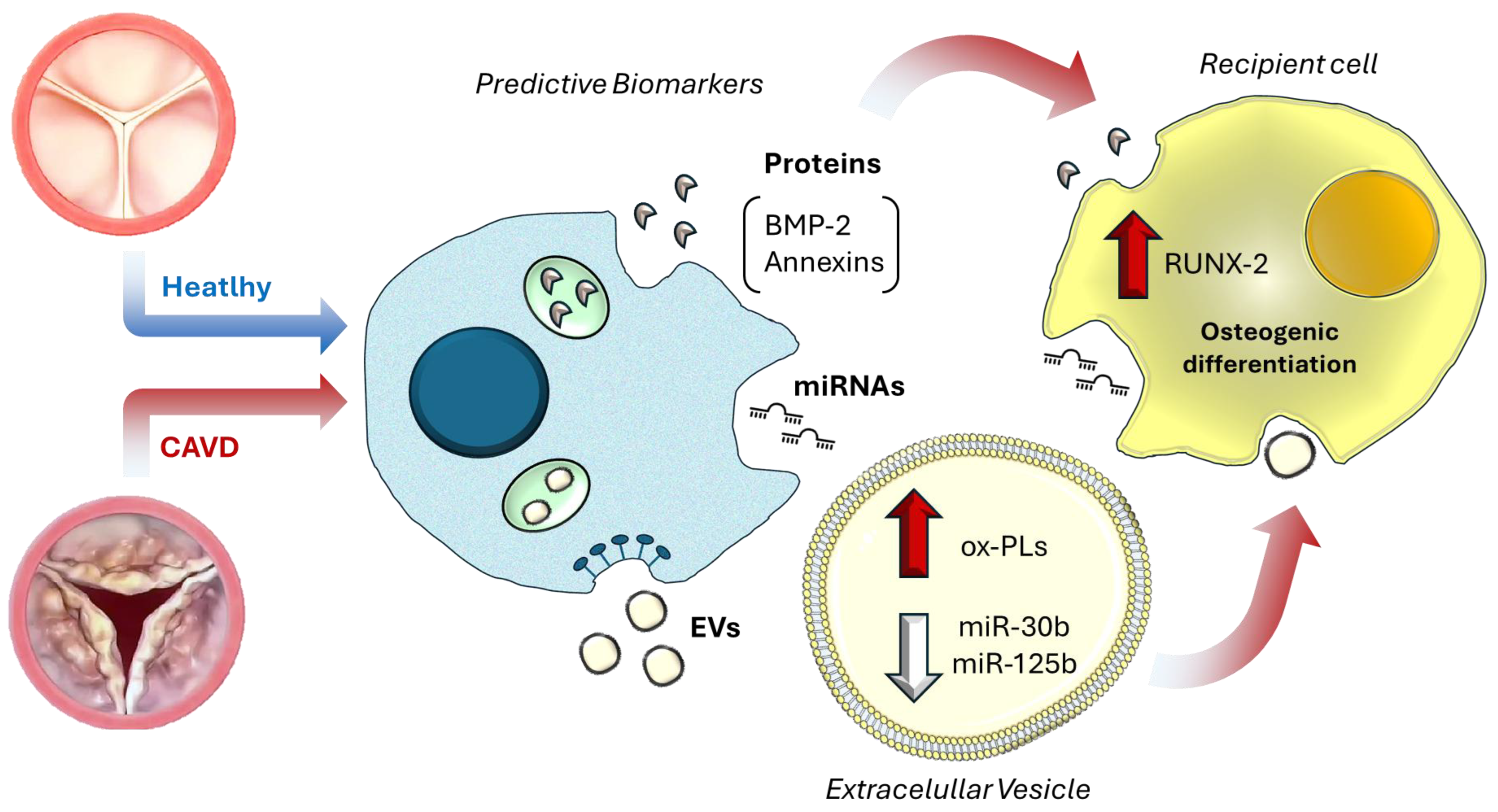
4. Extracellular Vesicles as Robust Biomarkers in CAVD
| CAVD Stage | miRNA | Expression in EVs | Biological Role | Clinical Utility | Refs. |
|---|---|---|---|---|---|
| Early Stage (Aortic Sclerosis) | miR-30b | ↓ in valve-derived EVs | Inhibits inflammation and osteogenic differentiation | Predicts early calcification risk; inversely correlates with Agatston scores | [77] |
| miR-125b | ↓ in plasma EVs | Suppresses VIC activation via TRAF6/NF-κB inhibition | Low levels linked to faster hemodynamic progression | [9,33] | |
| miR-146a | ↑ in macrophage EVs | Anti-inflammatory; targets TRAF6/IL-1R to reduce inflammation | Potential therapeutic target | [78,79] | |
| Intermediate Stage (Fibrosis/Calcification) | miR-214 | ↑ in VIC-derived EVs | Promotes calcification by inhibiting ATF4, an osteoclast activator | Correlates with ECM remodeling and valve stiffness | [80,81] |
| miR-122-5p | ↑ in VEC-derivedEVs | Drives inflammation via TLR4 signaling in VICs and cardiomyocytes | Elevated in early CAVD plasma EVs; predicts subclinical inflammation | [82,83] | |
| miR-148a | ↓ in circulating EVs | Normally inhibits osteogenic transition via Wnt/β-catenin suppression | Loss correlates with accelerated calcification and AS | [84,85] | |
| Advanced Stage (Severe Stenosis) | miR-21 | ↑ in platelet EVs | Promotes fibrosis via PTEN suppression and MMP-9 activation | Associated with the need for valve replacement | [86] |
| miR-221 | ↑ in endothelial EVs | Enhances angiogenesis and osteogenesis via p27/CDKN1B inhibition | Linked to adverse post-TAVR outcomes (e.g., paravalvular leaks) | [87,88] | |
| miR-155 | ↑ inflammatory EVs | Drives macrophage polarization to pro-calcific (M1) phenotype | Predicts MACE in CAVD patients (e.g., post-AVR heart failure) | [86,89,90] |
5. Bioengineering of EVs in CAVD
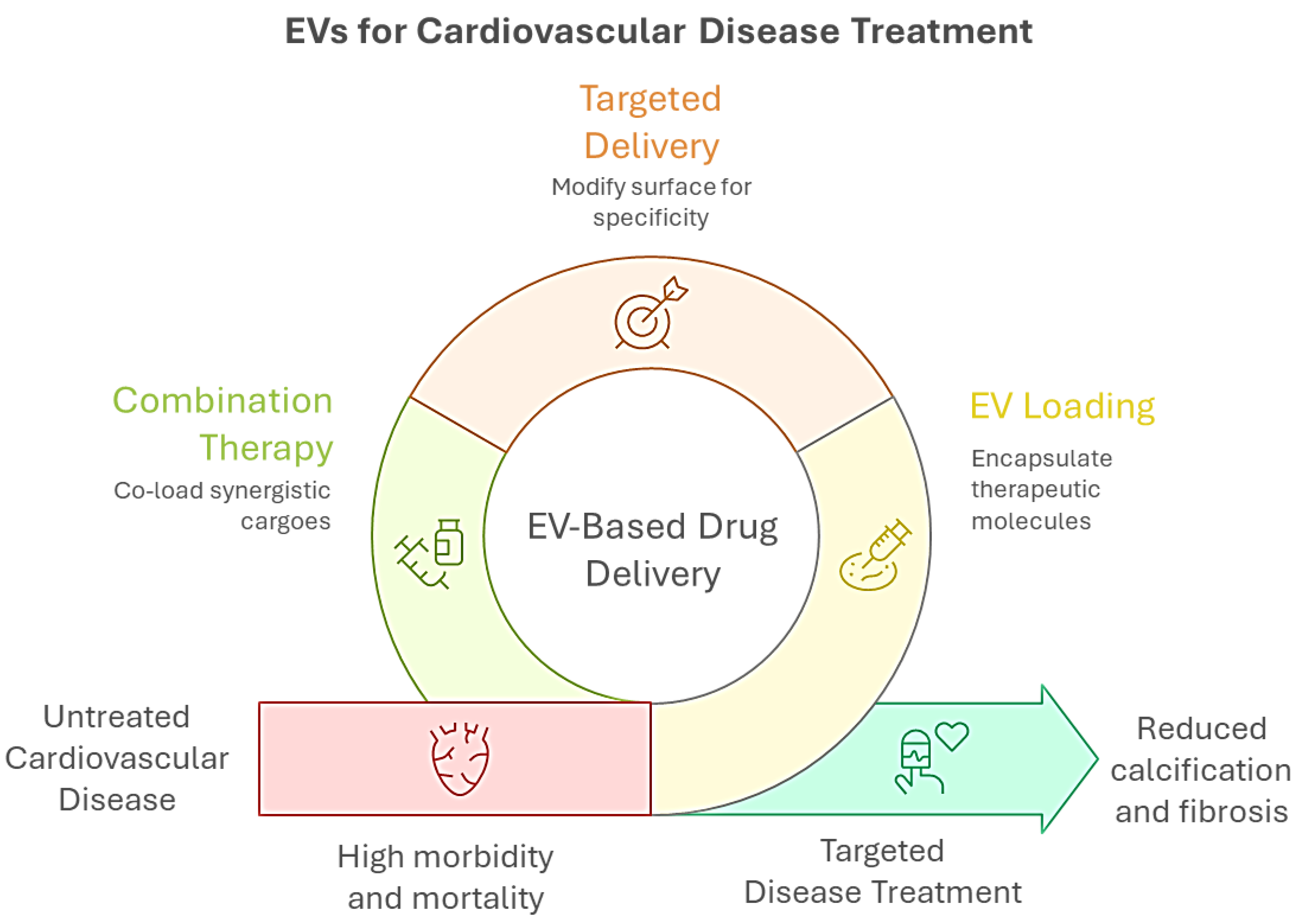
6. EVs as Drug Delivery Systems in CAVD
7. Clinical Translation of Extracellular Vesicles in CAVD
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAAs | Abdominal Aortic Aneurysms |
| Ago2 | Argonaute 2 |
| APP | Amyloid Precursor Protein |
| AS | Aortic Stenosis |
| ATF4 | Activating Transcription Factor 4 |
| AVR | Aortic Valve Replacement |
| BMP-2 | Bone Morphogenetic Protein 2 |
| CAVD | Calcific Aortic Valve Disease |
| CDKN1B | Cyclin Dependent Kinase Inhibitor 1B |
| CVD | Cardiovascular Disease |
| ECM | Extracellular Matrix |
| EV(s) | Extracellular Vesicle(s) |
| FLIM | Fluorescence Lifetime Imaging Microscopy |
| GDF-15 | Growth Differentiation Factor 15 |
| IL-1R | Interleukin-1 Receptor |
| MACE | Major Adverse Cardiac Events |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| miRNA(s) | microRNA(s) |
| mRNA(s) | messenger RNA(s) |
| MMP-9 | Matrix Metalloproteinase 9 |
| MPM | Multiphoton Microscopy |
| MSC(s) | Mesenchymal Stem Cell(s) |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NTA | Nanoparticle Tracking Analysis |
| OPN | Osteopontin |
| ox-PL(s) | Oxidized Phospholipid(s) |
| PON3 | Paraoxonase 3 |
| PTEN | Phosphatase and Tensin Homolog |
| RNA(s) | Ribonucleic Acid(s) |
| RNase(s) | Ribonuclease(s) |
| RUNX2 | Runt-related Transcription Factor 2 |
| SEC | Size-Exclusion Chromatography |
| SiNW | Silicon Nanowire |
| TAVR | Transcatheter Aortic Valve Replacement |
| TLR4 | Toll-like Receptor 4 |
| UC | Ultracentrifugation |
| VEC(s) | Valvular Endothelial Cell(s) |
| VIC(s) | Valvular Interstitial Cell(s) |
| VSMC(s) | Vascular Smooth Muscle Cell(s) |
References
- Blaser, M.C.; Bäck, M.; Lüscher, T.F.; Aikawa, E. Calcific aortic stenosis: Omics-based target discovery and therapy development. Eur. Heart J. 2025, 46, 620–634. [Google Scholar] [CrossRef]
- Hernandez-Vaquero, D.; Diaz, R.; Alperi, A.; Almendarez, M.G.; Escalera, A.; Cubero-Gallego, H.; Avanzas, P.; Moris, C.; Pascual, I. Life expectancy of patients undergoing surgical aortic valve replacement compared with that of the general population. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 394–399. [Google Scholar] [CrossRef]
- Ragni, E. Extracellular Vesicles: Recent Advances and Perspectives. Front. Biosci. 2025, 30, 36405. [Google Scholar] [CrossRef]
- Blaser, M.C.; Buffolo, F.; Halu, A.; Turner, M.E.; Schlotter, F.; Higashi, H.; Pantano, L.; Clift, C.L.; Saddic, L.A.; Atkins, S.K.; et al. Multiomics of Tissue Extracellular Vesicles Identifies Unique Modulators of Atherosclerosis and Calcific Aortic Valve Stenosis. Circulation 2023, 148, 661–678. [Google Scholar] [CrossRef]
- Di Vito, A.; Donato, A.; Presta, I.; Mancuso, T.; Brunetti, F.S.; Mastroroberto, P.; Amorosi, A.; Malara, N.; Donato, G. Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives. Int. J. Mol. Sci. 2021, 22, 913. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.; Vijayakumar, G.; Pillai, I.C. Non-coding RNAs mediating the regulation of genes and signaling pathways in aortic valve calcification. Gene 2025, 936, 149117. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Zhang, X.; Song, Z.; Han, L.; He, Y.; Xu, Z. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2014, 147, 1073–1080.e2. [Google Scholar] [CrossRef]
- Martin, P.J.; Haren, N.; Ghali, O.; Clabaut, A.; Chauveau, C.; Hardouin, P.; Broux, O. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, N.; Han, Y.; Zhang, C.; Zhang, H. miRNA Expression Profiling Uncovers a Role of miR-139-5p in Regulating the Calcification of Human Aortic Valve Interstitial Cells. Front. Genet. 2021, 12, 722564. [Google Scholar] [CrossRef] [PubMed]
- Resch, C.; Stamenkovic, A.; Surendran, A.; Zhang, A.; Oudit, G.Y.; Shah, A.; Ravandi, A. Valvular oxidized phospholipids correlate with severity of human aortic valvular stenosis. Free Radic. Biol. Med. 2025, 239, 219–229. [Google Scholar] [CrossRef]
- Niu, W.; Sun, B.; Li, M.; Cui, J.; Huang, J.; Zhang, L. TLR-4/microRNA-125a/NF-κB signaling modulates the immune response to Mycobacterium tuberculosis infection. Cell Cycle 2018, 17, 1931–1945. [Google Scholar] [CrossRef]
- Moreno, A.; Alarcón-Zapata, P.; Guzmán-Gútierrez, E.; Radojkovic, C.; Contreras, H.; Nova-Lampeti, E.; A Zúñiga, F.; Rodriguez-Alvárez, L.; Escudero, C.; Lagos, P.; et al. Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction. Biomedicines 2024, 12, 2119. [Google Scholar] [CrossRef]
- Ji, J.; He, Q.; Xia, Y.; Sha, X.; Liang, Q.; Xu, Y.; Chen, P.; Dong, C.; Zhao, R.; Yang, J.; et al. Circulating plasma derived exosomes from systemic lupus erythematosus aggravate lupus nephritis through miR-122-5p/FOXO3-mediated macrophage activation. J. Nanobiotechnol. 2024, 22, 779. [Google Scholar] [CrossRef]
- Lyu, J.; Sheng, M.; Cao, Y.; Jia, L.; Zhang, C.; Weng, Y.; Yu, W. Ischemia and reperfusion-injured liver-derived exosomes elicit acute lung injury through miR-122-5p regulated alveolar macrophage polarization. Int. Immunopharmacol. 2024, 131, 111853. [Google Scholar] [CrossRef] [PubMed]
- Hermann, S.; Grätz, C.; Kirchner, B.; Pfaffl, M.W. Extracellular vesicle-derived microRNA biomarkers: Goals and pitfalls. Trillium Exctracell. Vesicles 2020, 2, 42–47. [Google Scholar]
- Bernáth-Nagy, D.; Kalinyaprak, M.S.; Giannitsis, E.; Ábrahám, P.; Leuschner, F.; Frey, N.; Krohn, J.B. Circulating extracellular vesicles as biomarkers in the diagnosis, prognosis and therapy of cardiovascular diseases. Front. Cardiovasc. Med. 2024, 11, 1425159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Tong, L.; Hu, L.; Hong, Y.; Zhou, R.; Li, Z.; Dong, M.; Hou, J.; Xu, T. Systematic Evaluation of Isolation Techniques and Freeze-Thaw Effects on Plasma Extracellular Vesicle Heterogeneity and Subpopulation Profiling. J. Extracell. Biol. 2025, 4, e70058. [Google Scholar] [CrossRef]
- Newman, L.; Rowland, A. Detection and Isolation of Tissue-Specific Extracellular Vesicles From the Blood. J. Extracell. Biol. 2025, 4, e70059. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.C.; Kraler, S.; Lüscher, T.F.; Aikawa, E. Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis. Circ. Res. 2021, 128, 1371–1397. [Google Scholar] [CrossRef]
- Reventun, P.; Sánchez-Esteban, S.; Cook-Calvete, A.; Delgado-Marín, M.; Roza, C.; Jorquera-Ortega, S.; Hernandez, I.; Tesoro, L.; Botana, L.; Zamorano, J.L.; et al. Endothelial ILK induces cardioprotection by preventing coronary microvascular dysfunction and endothelial-to-mesenchymal transition. Basic Res. Cardiol. 2023, 118, 28. [Google Scholar]
- Viegas, C.; Carreira, J.; Maia, T.M.; Macedo, A.L.; Matos, A.P.; Neves, J.; Simes, D. Gla Rich Protein (GRP) Mediates Vascular Smooth Muscle Cell (VSMC) Osteogenic Differentiation, Extracellular Vesicle (EV) Calcification Propensity, and Immunomodulatory Properties. Int. J. Mol. Sci. 2024, 25, 12406. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles. 2022, 11, e12238. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Vago, R.; Radano, G.; Zocco, D.; Zarovni, N. Urine stabilization and normalization strategies favor unbiased analysis of urinary EV content. Sci. Rep. 2022, 12, 17663. [Google Scholar] [CrossRef]
- Sun, D.S.; Chang, H.H. Extracellular vesicles: Function, resilience, biomarker, bioengineering, and clinical implications. Tzu Chi Med. J. 2024, 36, 251–259. [Google Scholar]
- Kumari, S.; Lausted, C.; Scherler, K.; Ng, A.H.C.; Lu, Y.; Lee, I.; Hood, L.; Wang, K. Approaches and Challenges in Characterizing the Molecular Content of Extracellular Vesicles for Biomarker Discovery. Biomolecules 2024, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.H.; Reed, J.H. Extracellular vesicles as next-generation therapeutics and biomarkers in amyloidosis: A new frontier. Front. Biomater. Sci. 2024, 2, 1343658. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Tanase, D.M.; Valasciuc, E.; Gosav, E.M.; Floria, M.; Costea, C.F.; Dima, N.; Tudorancea, I.; Maranduca, M.A.; Serban, I.L. Contribution of Oxidative Stress (OS) in Calcific Aortic Valve Disease (CAVD): From Pathophysiology to Therapeutic Targets. Cells 2022, 11, 2663. [Google Scholar] [CrossRef]
- Fu, E.; Pan, K.; Li, Z. Engineering extracellular vesicles for targeted therapeutics in cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1503830. [Google Scholar] [CrossRef]
- Sánchez-Esteban, S.; Castro-Pinto, M.; Cook-Calvete, A.; Reventún, P.; Delgado-Marín, M.; Benito-Manzanaro, L.; Hernandez, I.; López-Menendez, J.; Zamorano, J.L.; Zaragoza, C.; et al. Integrin-Linked Kinase Expression in Human Valve Endothelial Cells Plays a Protective Role in Calcific Aortic Valve Disease. Antioxidants 2022, 11, 1736. [Google Scholar] [CrossRef]
- Yang, R.; Tang, Y.; Chen, X.; Yang, Y. Telocytes-derived extracellular vesicles alleviate aortic valve calcification by carrying miR-30b. ESC Heart Fail. 2021, 8, 3935–3946. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Q.; Wei, Z.; Xu, X.; Han, D.; Zhang, Y.; Chen, Z.; Liang, Q. MicroRNAs of extracellular vesicles derived from mesenchymal stromal cells alleviate inflammation in dry eye disease by targeting the IRAK1/TAB2/NF-κB pathway. Ocul. Surf. 2023, 28, 131–140. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.R.S.; Santos, N.L.; de Sousa Andrade, L.N. Extracellular vesicles as cancer biomarkers and drug delivery strategies in clinical settings: Advances, perspectives, and challenges. Clinics 2025, 80, 100635. [Google Scholar] [CrossRef]
- Novoa-Herrán, S. Retos y oportunidades en el estudio de vesículas extracelulares: Contexto institucional a nivel mundial y situación actual en Colombia. Biomédica 2021, 41, 555–589. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Cheng, Q.; Bai, L.; Huang, S.; Gao, J. Extracellular Vesicles in Cardiovascular Diseases: Diagnosis and Therapy. Front. Cell Dev. Biol. 2022, 10, 875376. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lucien, F.; Gustafson, D.; Lenassi, M.; Li, B.; Teske, J.J.; Boilard, E.; von Hohenberg, K.C.; Falcón-Perez, J.M.; Gualerzi, A.; Reale, A.; et al. MIBlood-EV: Minimal information to enhance the quality and reproducibility of blood extracellular vesicle research. J. Extracell. Vesicles 2023, 12, e12385. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, K.; Wang, Z.; Wang, Y.; Liu, J.; Lin, L.; Shao, Y.; Gao, L.; Yin, H.; Cui, C.; et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer 2016, 16, 753. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in Plasma Exosome is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef]
- Kumar, A.; Nader, M.A.; Deep, G. Emergence of Extracellular Vesicles as “Liquid Biopsy” for Neurological Disorders: Boom or Bust. Pharmacol. Rev. 2024, 76, 199–227. [Google Scholar] [CrossRef]
- Ma, C.; Ding, R.; Hao, K.; Du, W.; Xu, L.; Gao, Q.; Yu, C. Storage Stability of Blood Samples for miRNAs in Glycosylated Extracellular Vesicles. Molecules 2023, 29, 103. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Dai, Y.; Han, Y.; Wei, X.; Wei, G.; Chen, W.; Kong, S.; He, Y.; Liu, H.; et al. Neutrophil N1 polarization induced by cardiomyocyte-derived extracellular vesicle miR-9-5p aggravates myocardial ischemia/reperfusion injury. J. Nanobiotechnol. 2024, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, J.; Li, S.; Cui, T.; Ni, J.; Zhang, H.; Zhu, Y.; Mao, J.; Gao, X.; Midgley, A.C.; et al. M2 Macrophage-Derived sEV Regulate Pro-Inflammatory CCR2 + Macrophage Subpopulations to Favor Post-AMI Cardiac Repair. Adv. Sci. 2023, 10, 2202964. [Google Scholar] [CrossRef] [PubMed]
- Dang, G.; Li, T.; Yang, D.; Yang, G.; Du, X.; Yang, J.; Miao, Y.; Han, L.; Ma, X.; Song, Y.; et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox Biol. 2022, 50, 102257. [Google Scholar] [CrossRef]
- Saxena, S.; Volpe, M.C.; Agostinis, C.; Vodret, S.; Ring, N.A.R.; Colliva, A.; Vuerich, R.; Braga, L.; Cook-Calvete, A.; Romano, F.; et al. Anti-miRNA therapeutics for uterine fibroids. Biomed. Pharmacother. 2025, 185, 117946. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.; Eldh, M.; Offens, A.; Veerman, R.E.; Johansson, M.; Hemdan, T.; Netterling, H.; Huge, Y.; Abdul-Sattar Aljabery, F.; Alamdari, F.; et al. Protein profile in urinary extracellular vesicles is a marker of malignancy and correlates with muscle invasiveness in urinary bladder cancer. Cancer Lett. 2025, 609, 217352. [Google Scholar] [CrossRef]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef]
- Su, Y.; Chen, M.; Xu, W.; Gu, P.; Fan, X. Advances in Extracellular-Vesicles-Based Diagnostic and Therapeutic Approaches for Ocular Diseases. ACS Nano 2024, 18, 22793–22828. [Google Scholar] [CrossRef]
- Krohn, J.B.; Aikawa, E.; Aikawa, M.; Hutcheson, J.D.; Sahoo, S.; Fish, J.E. Editorial: Extracellular vesicles in cardiovascular inflammation and calcification. Front. Cardiovasc. Med. 2022, 9, 1077124. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, P.K.; Kashyap, V.; Kumar, S. Proteomics of Extracellular Vesicles: Recent Updates, Challenges and Limitations. Proteomes 2025, 13, 12. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Zhao, Z.; Wijerathne, H.; Godwin, A.K.; Soper, S.A. Isolation and analysis methods of extracellular vesicles (EVs). Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 80–103. [Google Scholar]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Ferguson, S.; Yang, K.S.; Peterson, H.M.; Carlson, J.C.T.; Weissleder, R. Multiplexed analysis of EV reveals specific biomarker composition with diagnostic impact. Nat. Commun. 2023, 14, 1239. [Google Scholar] [CrossRef]
- Botha, J.; Handberg, A.; Simonsen, J.B. Lipid-based strategies used to identify extracellular vesicles in flow cytometry can be confounded by lipoproteins: Evaluations of annexin V, lactadherin, and detergent lysis. J. Extracell. Vesicles 2022, 11, e12200. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Krohn, J.B.; Hutcheson, J.D.; Martínez-Martínez, E.; Aikawa, E. Extracellular vesicles in cardiovascular calcification: Expanding current paradigms. J. Physiol. 2016, 594, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Tandon, I.; Quinn, K.P.; Balachandran, K. Label-Free Multiphoton Microscopy for the Detection and Monitoring of Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2021, 8, 688513. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- Diehl, P.; Nagy, F.; Sossong, V.; Helbing, T.; Beyersdorf, F.; Olschewski, M.; Bode, C.; Moser, M. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb. Haemost. 2008, 99, 711–719. [Google Scholar] [CrossRef]
- Lorite, P.; Domínguez, J.N.; Palomeque, T.; Torres, M.I. Extracellular Vesicles: Advanced Tools for Disease Diagnosis, Monitoring, and Therapies. Int. J. Mol. Sci. 2024, 26, 189. [Google Scholar] [CrossRef]
- Hulanicka, M.; Garncarz, M.; Parzeniecka-Jaworska, M.; Jank, M. Plasma miRNAs as potential biomarkers of chronic degenerative valvular disease in Dachshunds. BMC Vet. Res. 2014, 10, 205. [Google Scholar] [CrossRef]
- Jung, R.G.; Duchez, A.-C.; Simard, T.; Dhaliwal, S.; Gillmore, T.; Di Santo, P.; Labinaz, A.; Ramirez, F.D.; Rasheed, A.; Robichaud, S.; et al. Plasminogen Activator Inhibitor-1–Positive Platelet-Derived Extracellular Vesicles Predicts MACE and the Proinflammatory SMC Phenotype. JACC Basic Transl. Sci. 2022, 7, 985–997. [Google Scholar] [CrossRef]
- Verwer, M.C.; Mekke, J.; Timmerman, N.; Waissi, F.; Boltjes, A.; Pasterkamp, G.; de Borst, G.J.; de Kleijn, D.P.V. Comparison of cardiovascular biomarker expression in extracellular vesicles, plasma and carotid plaque for the prediction of MACE in CEA patients. Sci. Rep. 2023, 13, 1010. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liang, J.; Witwer, K.W.; Zhang, Y.; Wang, Q.; Yin, H. Extracellular vesicle-associated microRNA-30b-5p activates macrophages through the SIRT1/ NF-κB pathway in cell senescence. Front. Immunol. 2022, 13, 955175. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yang, Y.; Cai, H.; Ao, Z.; Xing, Y.; Li, K.; Yang, K.; Guan, W.; Friend, J.; et al. Extracellular vesicle-based point-of-care testing for diagnosis and monitoring of Alzheimer’s disease. Microsyst. Nanoeng. 2025, 11, 65. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Rahimi, S.; Hosseinali, M.; Guilandokht, Z.; Raahemifar, K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: Applications and techniques. Biosens. Bioelectron. X 2024, 19, 100489. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, R.; Wang, B.; He, C.; Bai, S.; Yan, H.; Yu, J.; Li, H.; Peng, B.; Gao, Z.; et al. Electrochemical detection of extracellular vesicles for early diagnosis: A focus on disease biomarker analysis. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, M.; Xiao, Q.; Wang, H.; Chi, C.; Lin, T.; Wang, Y.; Yi, X.; Zhu, L. Recent Advances in Microfluidic-Based Extracellular Vesicle Analysis. Micromachines 2024, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Ha, T.; Gao, M.; Liu, L.; Wang, R.; Yu, K.; Kalbfleisch, J.H.; Kao, R.L.; Williams, D.L.; et al. MicroRNA-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor κB Activation and p53-Mediated Apoptotic Signaling. J. Infect. Dis. 2016, 214, 1773–1783. [Google Scholar] [CrossRef]
- Jiang, W.; Kong, L.; Ni, Q.; Lu, Y.; Ding, W.; Liu, G.; Pu, L.; Tang, W.; Kong, L. miR-146a Ameliorates Liver Ischemia/Reperfusion Injury by Suppressing IRAK1 and TRAF6. PLoS ONE 2014, 9, e101530. [Google Scholar] [CrossRef]
- Wu, H.; Fan, H.; Shou, Z.; Xu, M.; Chen, Q.; Ai, C.; Dong, Y.; Liu, Y.; Nan, Z.; Wang, Y.; et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019, 68, 204–212. [Google Scholar] [CrossRef]
- Li, N.; Bai, Y.; Zhou, G.; Ma, Y.; Tan, M.; Qiao, F.; Li, X.; Shen, M.; Song, X.; Zhao, X.; et al. miR-214 Attenuates Aortic Valve Calcification by Regulating Osteogenic Differentiation of Valvular Interstitial Cells. Mol. Ther. Nucl. Acids 2020, 22, 971–980. [Google Scholar] [CrossRef]
- Salim, M.T.; Esmerats, J.F.; Arjunon, S.; Villa-Roel, N.; Nerem, R.M.; Jo, H.; Yoganathan, A.P. miR-214 is Stretch-Sensitive in Aortic Valve and Inhibits Aortic Valve Calcification. Ann. Biomed. Eng. 2019, 47, 1106–1115. [Google Scholar] [CrossRef]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Xiang, X.; Niepmann, S.T.; Sedaghat, A.; Tiyerili, V.; Chennupati, R.; Moore, J.B.; Boon, R.A.; et al. Circulating MicroRNA-122-5p Is Associated with a Lack of Improvement in Left Ventricular Function After Transcatheter Aortic Valve Replacement and Regulates Viability of Cardiomyocytes Through Extracellular Vesicles. Circulation 2022, 146, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.; López, B.; Hermida, N.; Schroen, B.; José, G.S.; Heymans, S.; Valencia, F.; Gómez-Doblas, J.J.; De Teresa, E.; Díez, J.; et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin. Sci. 2014, 126, 497–506. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/β-catenin in ox-LDL -stimulated vascular smooth muscle cells. J. Biomed. Sci. 2018, 25, 11. [Google Scholar] [CrossRef]
- Yu, F.; Duan, Y.; Liu, C.; Huang, H.; Xiao, X.; He, Z. Extracellular vesicles in atherosclerosis and vascular calcification: The versatile non-coding RNAs from endothelial cells and vascular smooth muscle cells. Front. Med. 2023, 10, 1193660. [Google Scholar] [CrossRef] [PubMed]
- Mollajan, E.; Yazdani, S.; Ghasemzadeh, M.; Mozhgani, S.H. miR-21 in cardiovascular disease: New insights and emerging therapeutic potential. Discov. Appl. Sci. 2025, 7, 447. [Google Scholar] [CrossRef]
- Verjans, R.; Peters, T.; Beaumont, F.J.; van Leeuwen, R.; van Herwaarden, T.; Verhesen, W.; Munts, C.; Bijnen, M.; Henkens, M.; Diez, J.; et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload–Induced Heart Failure. Hypertension 2018, 71, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Qiao, E.; Huang, Z.; Wang, W. Exploring potential genes and pathways related to calcific aortic valve disease. Gene 2022, 808, 145987. [Google Scholar] [CrossRef]
- Chen, A.; Wen, J.; Lu, C.; Lin, B.; Xian, S.; Huang, F.; Wu, Y.; Zeng, Z. Inhibition of miR 155 5p attenuates the valvular damage induced by rheumatic heart disease. Int. J. Mol. Med. 2019, 45, 429–440. [Google Scholar] [CrossRef]
- Cable, J.; Witwer, K.W.; Coffey, R.J.; Milosavljevic, A.; von Lersner, A.K.; Jimenez, L.; Pucci, F.; Barr, M.M.; Dekker, N.; Barman, B.; et al. Exosomes, microvesicles, and other extracellular vesicles—A Keystone Symposia report. Ann. N. Y. Acad. Sci. 2023, 1523, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bavafa, A.; Izadpanahi, M.; Hosseini, E.; Hajinejad, M.; Abedi, M.; Forouzanfar, F.; Sahab-Negah, S. Exosome: An overview on enhanced biogenesis by small molecules. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 6473–6508. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Chae, C.W.; Choi, G.; Yoon, T.; Kwon, Y.W. Exosome-Based Therapy in Cardiovascular Diseases: A New Frontier in Cardiovascular Disease Treatment. Korean Circ. J. 2025, 55, 461–480. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; Du, S.; Gidda, S.; Bahatyrevich, N.; Hao, D.; Kumar, P.; Wang, A. Bioengineering Extracellular Vesicles for the Treatment of Cardiovascular Diseases. Adv. Biol. 2022, 6, e2200087. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Lincoln, J. Calcific Aortic Valve Disease: A Developmental Biology Perspective. Curr. Cardiol. Rep. 2018, 20, 21. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.Q.; Zhu, Y.; Han, X.Q.; Liu, N. Exosomes Derived from Mesenchymal Stromal Cells Pretreated with Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells. Front. Endocrinol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef]
- Nannan, L.; Decombis, S.; Terryn, C.; Audonnet, S.; Michel, J.; Brassart-Pasco, S.; Gsell, W.; Himmelreich, U.; Brassart, B. Dysregulation of intercellular communication in vitro and in vivo via extracellular vesicles secreted by pancreatic duct adenocarcinoma cells and generated under the influence of the AG9 elastin peptide-conditioned microenvironment. J. Extracell. Biol. 2024, 3, e145. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.S.; Jiang, W. Engineering therapeutical extracellular vesicles for clinical translation. Trends Biotechnol. 2025, 43, 61–82. [Google Scholar] [CrossRef]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2020, 72, 156–166. [Google Scholar] [CrossRef]
- Patel, S.; Guo, M.K.; Abdul Samad, M.; Howe, K.L. Extracellular vesicles as biomarkers and modulators of atherosclerosis pathogenesis. Front. Cardiovasc. Med. 2023, 10, 1202187. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dhawan, U.K.; Hussain, M.T.; Singh, P.; Bhagat, K.K.; Singhal, A.; Austin-Williams, S.; Sengupta, S.; Subramanian, M. Efferocytes release extracellular vesicles to resolve inflammation and tissue injury via prosaposin-GPR37 signaling. Cell Rep. 2023, 42, 112808. [Google Scholar] [CrossRef]
- Goody, P.R.; Christmann, D.; Goody, D.; Hildebrand, S.; Billig, H.; Nehl, D.; Chennupati, R.; Gladka, M.; Wilhelm-Jüngling, K.; Uchida, S.; et al. Calcific aortic valve disease augments vesicular microRNA-145-5p to regulate the calcification of valvular interstitial cells via cellular crosstalk. Basic Res. Cardiol. 2025, 120, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; Garibotti, H.G.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A.; et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv. Sci. 2023, 10, 202206187. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef]
- Park, D.J.; Duggan, E.; Ho, K.; Dorschner, R.A.; Dobke, M.; Nolan, J.P.; Eliceiri, B.P. Serpin-loaded extracellular vesicles promote tissue repair in a mouse model of impaired wound healing. J. Nanobiotechnol. 2022, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Iannotta, D.; Amruta, A.; Kijas, A.W.; Rowan, A.E.; Wolfram, J. Entry and exit of extracellular vesicles to and from the blood circulation. Nat. Nanotechnol. 2024, 19, 13–20. [Google Scholar] [CrossRef]
- Gupta, D.; Wiklander, O.P.B.; Wood, M.J.A.; El-Andaloussi, S. Biodistribution of therapeutic extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 170–190. [Google Scholar] [CrossRef]
- Miceli, R.T.; Chen, T.; Nose, Y.; Tichkule, S.; Brown, B.; Fullard, J.F.; Saulsbury, M.D.; Heyliger, S.O.; Gnjatic, S.; Kyprianou, N.; et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations. J. Extracell. Vesicles 2024, 13, e70005. [Google Scholar] [CrossRef]
- Cecchin, R.; Troyer, Z.; Witwer, K.; Morris, K.V. Extracellular vesicles: The next generation in gene therapy delivery. Mol. Ther. 2023, 31, 1225–1230. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef]
- Blaser, M.C.; Aikawa, E. Roles and Regulation of Extracellular Vesicles in Cardiovascular Mineral Metabolism. Front. Cardiovasc. Med. 2018, 5, 187. [Google Scholar] [CrossRef]
- Li, Y.; Xing, L.; Zhu, M.; Li, X.; Wei, F.; Sun, W.; Jia, Y. HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery. Biomolecules 2025, 15, 596. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Trębacz, H.; Barzycka, A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, X.; Liu, J.; Zhou, J. Biofunctionalized Decellularized Tissue-Engineered Heart Valve with Mesoporous Silica Nanoparticles for Controlled Release of VEGF and RunX2-siRNA against Calcification. Bioengineering 2023, 10, 859. [Google Scholar] [CrossRef]
- Voicu, G.; Mocanu, C.A.; Safciuc, F.; Anghelache, M.; Deleanu, M.; Cecoltan, S.; Pinteala, M.; Uritu, C.M.; Droc, I.; Simionescu, M.; et al. Nanocarriers of shRNA-Runx2 directed to collagen IV as a nanotherapeutic system to target calcific aortic valve disease. Mater. Today Bio 2023, 20, 100620. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wang, Z.; Zhang, L.; Xu, Y.; Li, Y.; Zhang, L.; Wang, G.; Yang, S.; Xue, G. Mesenchymal stem cell-derived extracellular vesicles protect against abdominal aortic aneurysm formation by inhibiting NET-induced ferroptosis. Exp. Mol. Med. 2023, 55, 939–951. [Google Scholar] [CrossRef]
- Ouyang, Y.; Hong, Y.; Mai, C.; Yang, H.; Wu, Z.; Gao, X.; Zeng, W.; Deng, X.; Liu, B.; Zhang, Y.; et al. Transcriptome analysis reveals therapeutic potential of NAMPT in protecting against abdominal aortic aneurysm in human and mouse. Bioact. Mater. 2024, 34, 17–36. [Google Scholar] [CrossRef]
- Stavrou, A.; Ortiz, A. Extracellular Vesicles: A Novel Tool in Nanomedicine and Cancer Treatment. Cancers 2022, 14, 4450. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Huang, W.; Wang, S.; Lou, A.; Wang, J.; Tu, Y.; Cui, W.; Zhou, W.; Zhang, W.; et al. Macrophage biomimetic nanoparticle-targeted functional extracellular vesicle micro-RNAs revealed via multiomics analysis alleviate sepsis-induced acute lung injury. J. Nanobiotechnol. 2024, 22, 362. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, M.; Kim, H.Y.; Kim, J.K.; Kim, W.K.; Lim, S.C.; Kang, K.W. Circulating small extracellular vesicles promote proliferation and migration of vascular smooth muscle cells via AXL and MerTK activation. Acta Pharmacol. Sin. 2023, 44, 984–998. [Google Scholar]
- Wang, Z.; Zhu, D.; Zhang, Y.; Xia, F.; Zhu, J.; Dai, J.; Zhuge, X. Extracellular vesicles produced by avian pathogenic Escherichia coli (APEC) activate macrophage proinflammatory response and neutrophil extracellular trap (NET) formation through TLR4 signaling. Microb. Cell Factories 2023, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, W.; Such, A.; Cichon, I.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Kolaczkowska, E. Large extracellular vesicle (EV) and neutrophil extracellular trap (NET) interaction captured in vivo during systemic inflammation. Sci. Rep. 2024, 14, 4680. [Google Scholar] [CrossRef]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, J.; Hu, H.; Zhu, Y.; Wang, X.; Zhou, S.; Wang, F.; Xiang, M. Engineered M2 macrophage-derived extracellular vesicles with platelet membrane fusion for targeted therapy of atherosclerosis. Bioact. Mater. 2024, 35, 447–460. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.; Wang, Q.; Chen, J.; Gao, J.; Tan, H.; Li, S.; Wu, Y.; Yang, H.; Huang, H.; et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics 2021, 11, 3916–3931. [Google Scholar] [CrossRef]
- Rodríguez, D.A.; Vader, P. Extracellular Vesicle-Based Hybrid Systems for Advanced Drug Delivery. Pharmaceutics 2022, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Choi, J.C.; Minard, C.G.; Hou, X.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. Matrix metalloproteinase levels in chronic thoracic aortic dissection. J. Surg. Res. 2014, 189, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular adipose tissue–derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Furmanik, M.; Chatrou, M.; van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic potential of RNA-enriched extracellular vesicles: The next generation in RNA delivery via biogenic nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Wu, Z.; Chen, Y. Diversity of extracellular vesicle sources in atherosclerosis: Role and therapeutic application. Angiogenesis 2025, 28, 34. [Google Scholar] [CrossRef]
- Fang, F.; Yang, H.; Wang, X.; Zhao, T.; Zhao, P.; Liu, X. Extracellular Vesicles in Atherosclerosis: From Pathogenesis to Theranostic Applications. Small 2025, 21, e2504761. [Google Scholar] [CrossRef] [PubMed]
- Favretto, G.; da Cunha, R.S.; Flores Santos, A.; Leitolis, A.; Schiefer, E.M.; Gregório, P.C.; Franco, C.R.C.; Massy, Z.; Dalboni, M.A.; Stinghen, A.E.M. Uremic endothelial-derived extracellular vesicles: Mechanisms of formation and their role in cell adhesion, cell migration, inflammation, and oxidative stress. Toxicol. Lett. 2021, 347, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jiapaer, Z.; Li, C.; Yang, X.; Sun, L.; Chatterjee, E.; Zhang, L.; Lei, J.; Li, G. Extracellular Non-Coding RNAs in Cardiovascular Diseases. Pharmaceutics 2023, 15, 155. [Google Scholar] [CrossRef]
- Gąsecka, A.; Szolc, P.; van der Pol, E.; Niewiara, Ł.; Guzik, B.; Kleczyński, P.; Tomaniak, M.; Figura, E.; Zaremba, M.; Grabowski, M.; et al. Endothelial Cell-Derived Extracellular Vesicles Allow to Differentiate Between Various Endotypes of INOCA: A Multicentre, Prospective, Cohort Study. J. Cardiovasc. Transl. Res. 2025, 18, 305–315. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Extracellular Vesicles from Cardiovascular Progenitor Cells in the Treatment of Non-Ischemic Dilated Cardiomyopathy. Identifier NCT05774509. Available online: https://clinicaltrials.gov/ct2/show/NCT05774509 (accessed on 22 October 2025).
- ClinicalTrials.gov. Salivary and Plasma Extracellular Vesicle-Associated Long Non-Coding RNAs in Acute and Chronic Heart Failure (SEAL-HF). Identifier NCT06169540. Available online: https://clinicaltrials.gov/ct2/show/NCT06169540 (accessed on 22 October 2025).
- ClinicalTrials.gov. Extracellular Vesicles in Obesity and Cardiometabolic Disease (EVOC). Identifier NCT06408961. Available online: https://clinicaltrials.gov/ct2/show/NCT06408961 (accessed on 22 October 2025).
- ClinicalTrials.gov. Early Valve Replacement in Severe Asymptomatic Aortic Stenosis (EASY-AS). Identifier NCT04204915. Available online: https://clinicaltrials.gov/ct2/show/NCT04204915 (accessed on 22 October 2025).
- ClinicalTrials.gov. Extracellular Vesicles from Mesenchymal Cells in the Treatment of Acute Respiratory Failure Syndrome. Identifier NCT06002841. Available online: https://clinicaltrials.gov/ct2/show/NCT06002841 (accessed on 22 October 2025).
- ClinicalTrials.gov. Safety and Efficacy of Mesenchymal Stem Cell Therapy in Patients with Cardiovascular Disease. Identifier NCT04897841. Available online: https://clinicaltrials.gov/ct2/show/NCT04897841 (accessed on 22 October 2025).
- Düsing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef]
- Small, A.; Kiss, D.; Giri, J.; Anwaruddin, S.; Siddiqi, H.; Guerraty, M.; Chirinos, J.A.; Ferrari, G.; Rader, D.J. Biomarkers of Calcific Aortic Valve Disease. Arter. Thromb. Vasc. Biol. 2017, 37, 623–632. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Ji, X.; Tan, Z.; Lubman, D.M. Column-based Technology for CD9-HPLC Immunoaffinity Isolation of Serum Extracellular Vesicles. J. Proteome Res. 2021, 20, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Luo, S.; Lu, P.; Cai, C.; Li, W.; Li, C. Composition, functions, and applications of exosomal membrane proteins. Front. Immunol. 2024, 15, 1408415. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Tang, S.; Lin, S.; Liao, M.; Chen, H.; Zhou, J. The role of extracellular vesicles in renal fibrosis. Cell Death Dis. 2019, 10, 367. [Google Scholar] [CrossRef]
- Havers, M.; Broman, A.; Lenshof, A.; Laurell, T. Advancement and obstacles in microfluidics-based isolation of extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1265–1285. [Google Scholar] [CrossRef]
- Chen, S.; Tang, R.; Liu, B. Current Understanding of Cardiovascular Calcification in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10225. [Google Scholar] [CrossRef]
- Qin, M.; Hu, J.; Li, X.; Liu, J.; Jiang, R.; Shi, Y.; Wang, Z.; Zhang, L.; Zhao, Y.; Gao, H.; et al. Exosomal membrane proteins analysis using a silicon nanowire field effect transistor biosensor. Talanta 2024, 278, 126534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, J.; Liu, J.; Li, X.; Sun, S.; Luan, X.; Zhao, Y.; Wei, S.; Li, M.; Zhang, Q.; et al. Si nanowire Bio-FET for electrical and label-free detection of cancer cell-derived exosomes. Microsyst. Nanoeng. 2022, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J. Silicon Nanowire Biosensor for Ultrasensitive and Label-Free Direct Detection of miRNAs. Methods Mol. Biol. 2011, 67, 111–121. [Google Scholar]
- Lo Faro, M.; Leonardi, A.; Priolo, F.; Fazio, B.; Irrera, A. Future Prospects of Luminescent Silicon Nanowires Biosensors. Biosensors 2022, 12, 1052. [Google Scholar] [CrossRef]
- Mishra, S.; Vaughn, A.D.; Devore, D.I.; Roth, C.M. Delivery of siRNA silencing Runx2 using a multifunctional polymer-lipid nanoparticle inhibits osteogenesis in a cell culture model of heterotopic ossification. Integr. Biol. 2012, 4, 1498–1507. [Google Scholar] [CrossRef]
- Voicu, G.; Rebleanu, D.; Constantinescu, C.A.; Fuior, E.V.; Ciortan, L.; Droc, I.; Uritu, C.M.; Pinteala, M.; Manduteanu, I.; Simionescu, M.; et al. Nano-Polyplexes Mediated Transfection of Runx2-shRNA Mitigates the Osteodifferentiation of Human Valvular Interstitial Cells. Pharmaceutics 2020, 12, 507. [Google Scholar] [CrossRef]
| Biomarker | Type | EV Source |
|---|---|---|
| Proteins | Annexin V | Plasma/Valvular EVs |
| BMP-2 | Valvular/VIC-derived EVs | |
| OPN | Plasma/Valvular EVs | |
| MMP-9 | Plasma/Valvular EVs | |
| GDF-15 | Plasma EVs | |
| PON3 | Plasma EVs | |
| TGF-β1 | Valvular/Plasma EVs | |
| NOTCH1 fragments | Tissue-derived EVs | |
| Lipids | Phosphatidylserine (PS) | Calcifying EVs (plasma/tissue) |
| Oxidized phospholipids | Plasma/Lp(a)-associated EVs | |
| Sphingomyelins/Cholesterol | Valvular/Plasma EVs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook-Calvete, A.; Delgado-Marin, M.; Fernandez-Rodriguez, B.; Zaragoza, C.; Saura, M. Extracellular Vesicles in Calcific Aortic Valve Disease: From Biomarkers to Drug Delivery Applications. Biomolecules 2025, 15, 1548. https://doi.org/10.3390/biom15111548
Cook-Calvete A, Delgado-Marin M, Fernandez-Rodriguez B, Zaragoza C, Saura M. Extracellular Vesicles in Calcific Aortic Valve Disease: From Biomarkers to Drug Delivery Applications. Biomolecules. 2025; 15(11):1548. https://doi.org/10.3390/biom15111548
Chicago/Turabian StyleCook-Calvete, Alberto, Maria Delgado-Marin, Blanca Fernandez-Rodriguez, Carlos Zaragoza, and Marta Saura. 2025. "Extracellular Vesicles in Calcific Aortic Valve Disease: From Biomarkers to Drug Delivery Applications" Biomolecules 15, no. 11: 1548. https://doi.org/10.3390/biom15111548
APA StyleCook-Calvete, A., Delgado-Marin, M., Fernandez-Rodriguez, B., Zaragoza, C., & Saura, M. (2025). Extracellular Vesicles in Calcific Aortic Valve Disease: From Biomarkers to Drug Delivery Applications. Biomolecules, 15(11), 1548. https://doi.org/10.3390/biom15111548