Free Fatty Acids Correlate with the Interleukin-1 β and Interleukin-1 Receptor Antagonist in the Early Subacute Phase of Stroke
Abstract
1. Introduction
2. Material and Methods
2.1. Subjects
2.2. Free Fatty Acids Analysis
2.3. Interleukin-1 Beta and Interleukin-1 Receptor Antagonist Analysis
2.4. Statistical Analysis
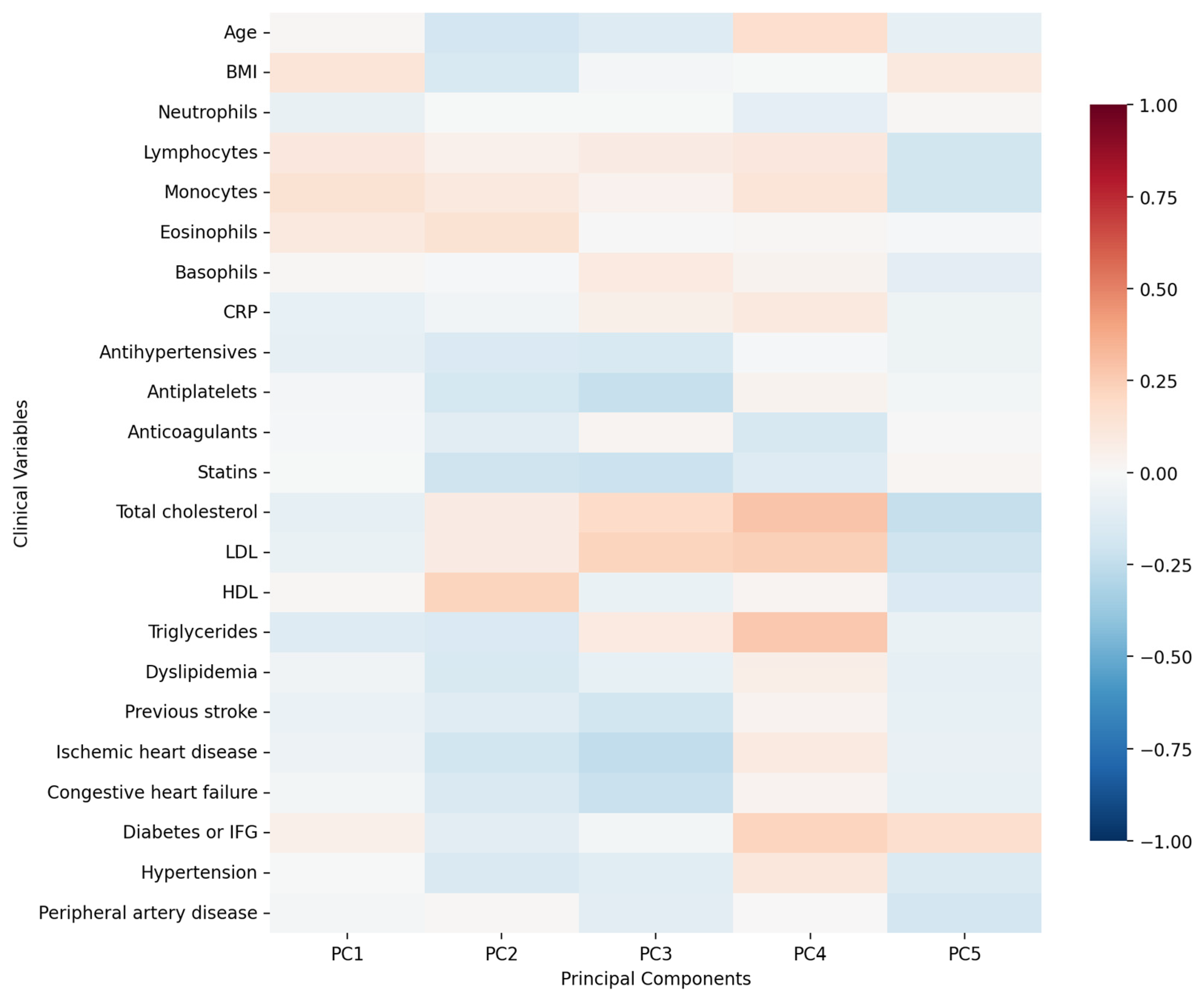
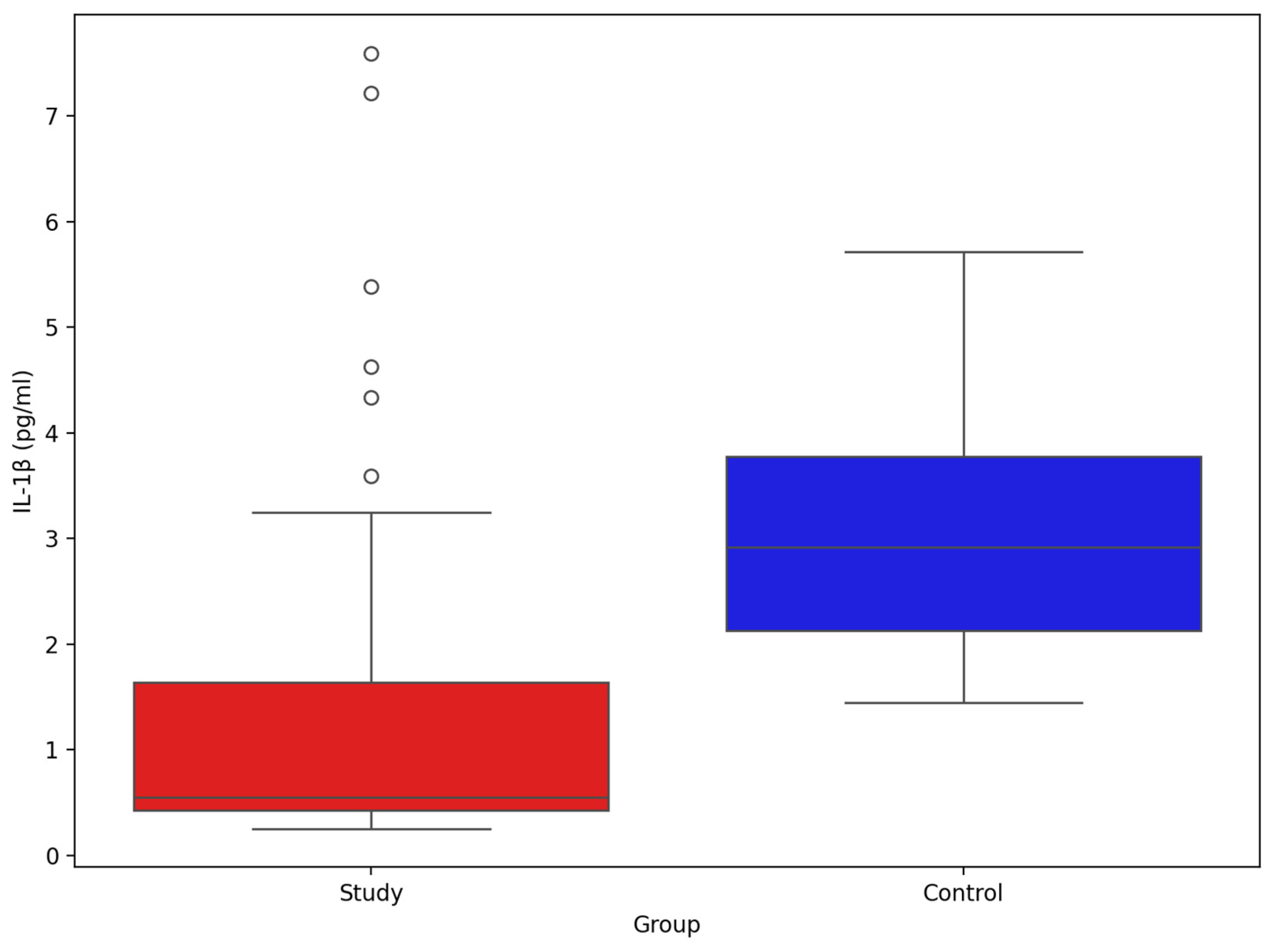
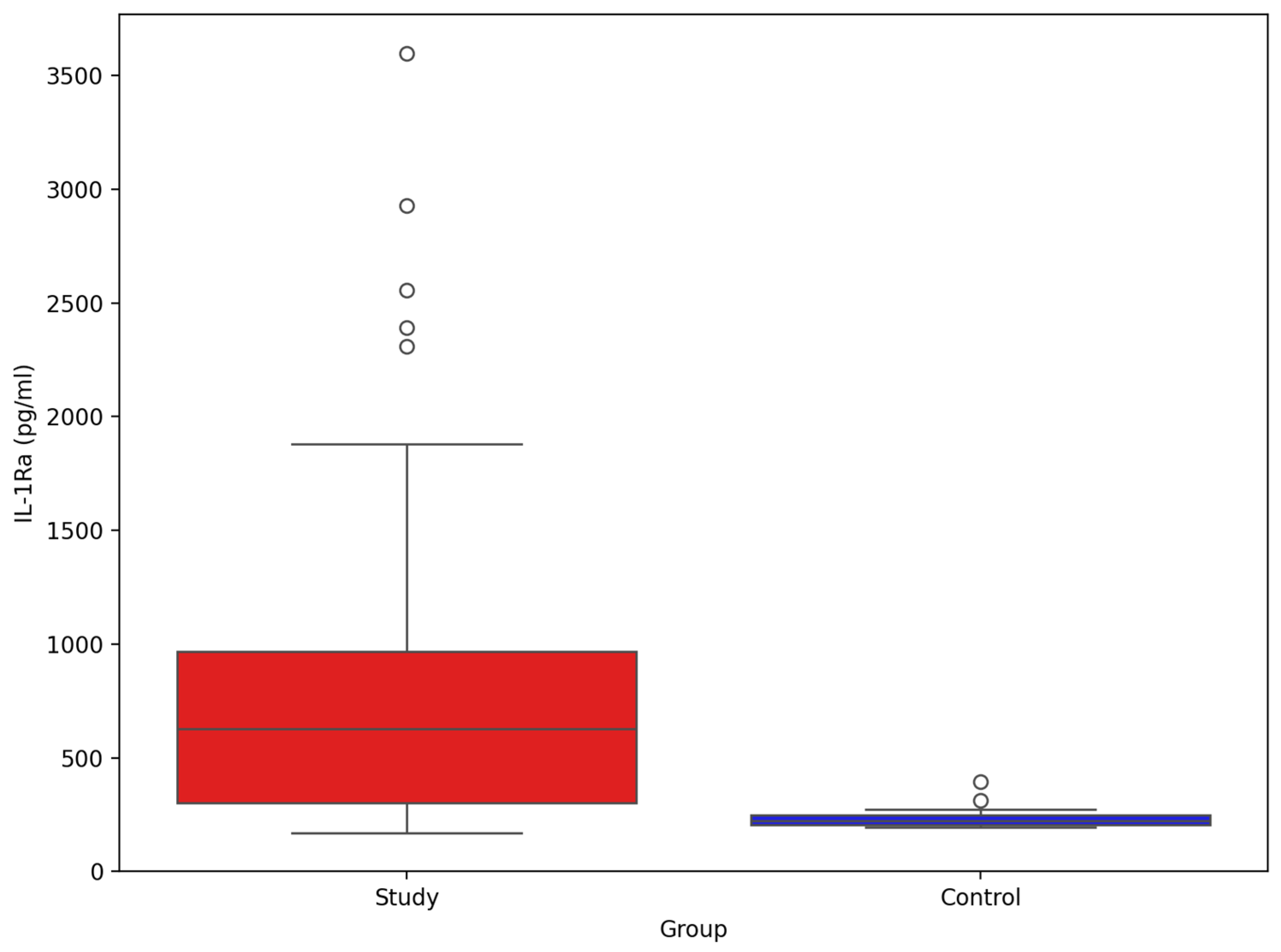
3. Results
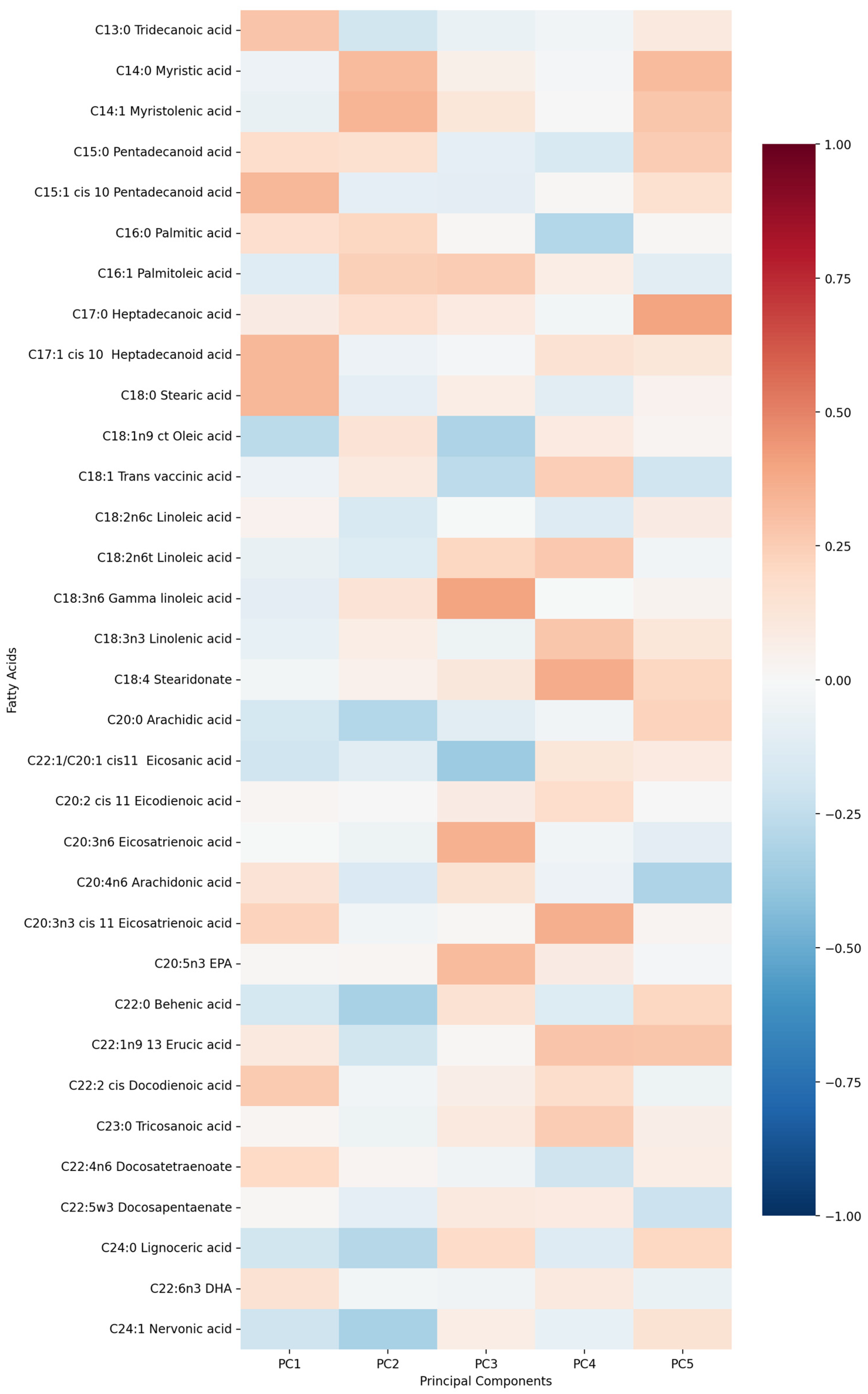
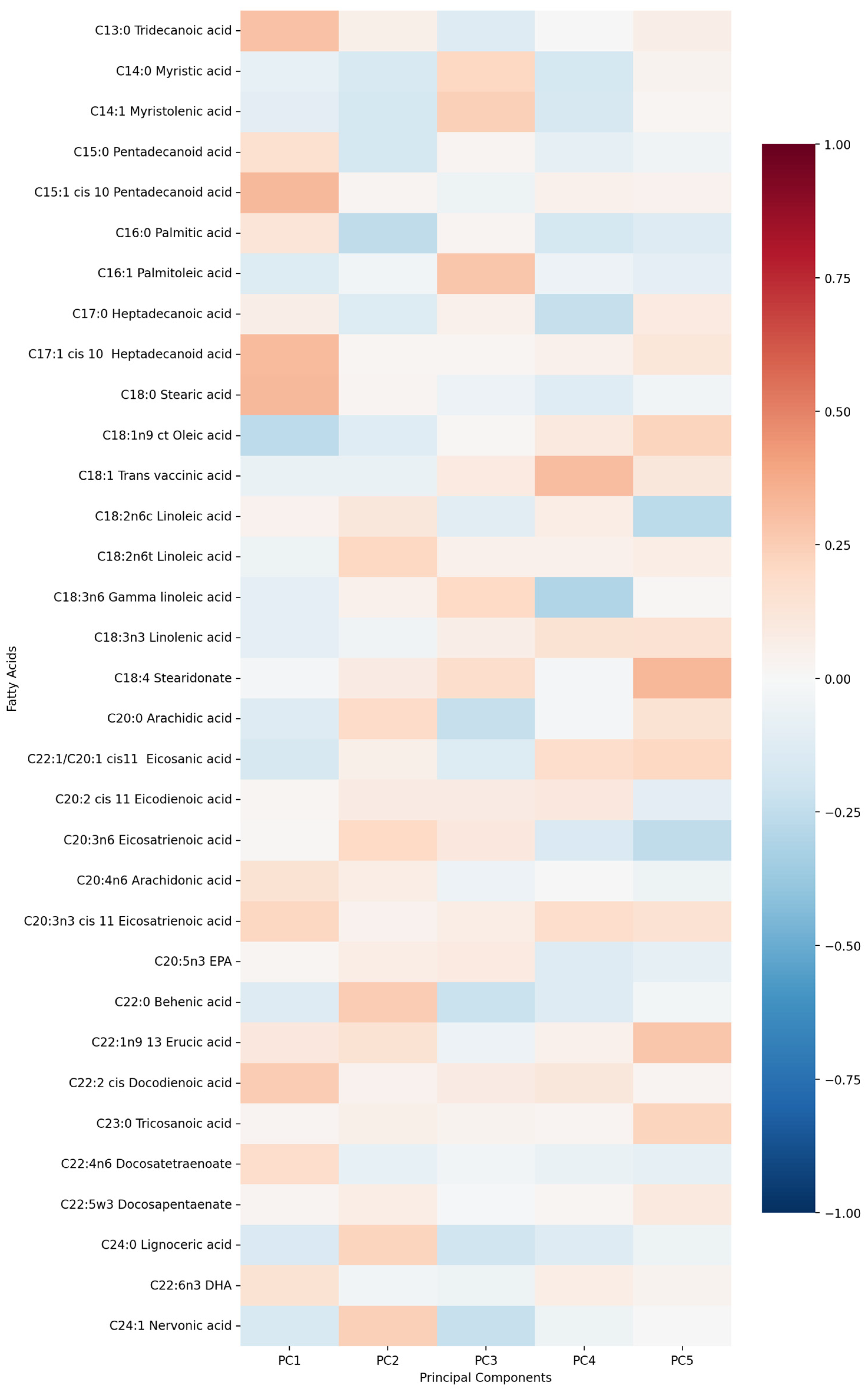
- PC1 (13.13%)—odd-chain fatty acids axis: C18:0 stearic acid, C17:1 heptadecanoid acid and C15:1 pentadecanoid acid.
- PC2 (9.74%)—cardiovascular medication/disease axis: statins, IHD and antiplatelets.
- PC3 (9.01%)—very long-chain fatty acids axis: C20:0 arachidic acid, C24:1 nervonic acid and C22:0 behenic acid.
- PC4 (7.20%)—omega-3/omega-6 fatty acids axis: C18:3n6 gamma linoleic acid, C20:5n3 EPA and triglycerides.
- PC5 (5.75%)—monounsaturated fatty acids axis: C18:1n9 oleic acid, C22:1 eicosanic acid and C18:4 stearidonate.
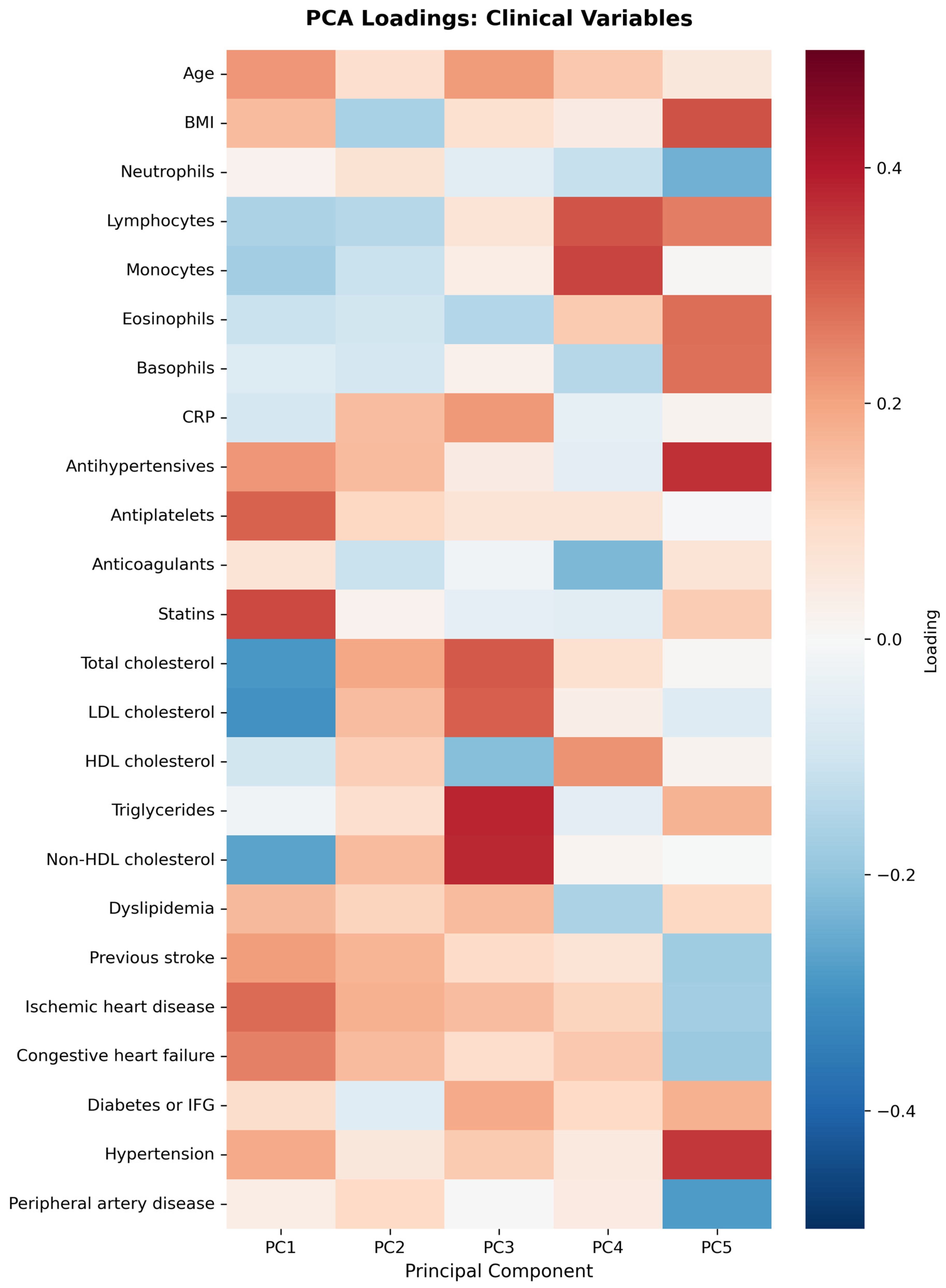
- PC1 (12.29%)—inflammation–lipids axis: C15:1 cis 10 pentadecanoid acid, C18:0 stearic acid, C17:1 cis 10 heptadecanoid acid.
- PC2 (9.94%)—lipids–metabolic axis: HDL, total cholesterol, LDL.
- PC3 (9.12%)—cell counts axis: neutrophils, lymphocytes, monocytes.
- PC4 (6.56%)—medications/comorbidities axis: statins, antiplatelets, anticoagulants.
- PC5 (5.95%)—fatty acids axis: C22:1n9 13 erucic acid, C18:2n6c linoleic acid, C18:4 stearidonate.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotlęga, D.; Ciećwież, S.; Turowska-Kowalska, J.; Nowacki, P. Pathogenetic Justification of Statin Use in Ischaemic Stroke Prevention According to Inflammatory Theory in Development of Atherosclerosis. Neurol. Neurochir. Pol. 2012, 46, 176–183. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Dempsey, R.; Hatcher, J.F. Integration of Cytokine Biology and Lipid Metabolism in Stroke. Front. Biosci. 2008, 13, 1250–1270. [Google Scholar] [CrossRef]
- Sobowale, O.A.; Parry-Jones, A.R.; Smith, C.J.; Tyrrell, P.J.; Rothwell, N.J.; Allan, S.M. Interleukin-1 in Stroke: From Bench to Bedside. Stroke 2016, 47, 2160–2167. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Denes, A.; Pinteaux, E.; Rothwell, N.J.; Allan, S.M. Interleukin-1 and Stroke: Biomarker, Harbinger of Damage, and Therapeutic Target. Cerebrovasc. Dis. 2011, 32, 517–527. [Google Scholar] [CrossRef]
- Alfadul, H.; Sabico, S.; Al-Daghri, N.M. The Role of Interleukin-1β in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 901616. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M. Interleukin-1 Inhibition and Ischaemic Stroke: Has the Time for a Major Outcomes Trial Arrived? Eur. Heart J. 2018, 39, 3518–3520. [Google Scholar] [CrossRef]
- Dinarello, C.A.; van der Meer, J.W. Treating Inflammation by Blocking Interleukin-1 in Humans. Semin. Immunol. 2013, 25, 469–484. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The Role of Interleukin-1 in General Pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Emsley, H.C.; Smith, C.J.; Georgiou, R.F.; Vail, A.; Hopkins, S.J.; Rothwell, N.J.; Tyrrell, P.J. Acute Stroke Investigators ARandomised Phase IIStudy of Interleukin-1 Receptor Antagonist in Acute Stroke Patients. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1366–1372. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Boller, S.; Debray, S.; Bouzakri, K.; Meier, D.T.; Prazak, R.; Kerr-Conte, J.; Pattou, F.; Ehses, J.A.; Schuit, F.C.; et al. Free Fatty Acids Induce a Proinflammatory Response in Islets via the Abundantly Expressed Interleukin-1 Receptor I. Endocrinology 2009, 150, 5218–5229. [Google Scholar] [CrossRef]
- Ferrucci, L.; Cherubini, A.; Bandinelli, S.; Bartali, B.; Corsi, A.; Lauretani, F.; Martin, A.; Andres-Lacueva, C.; Senin, U.; Guralnik, J.M. Relationship of Plasma Polyunsaturated Fatty Acids to Circulating Inflammatory Markers. J. Clin. Endocrinol. Metab. 2006, 91, 439–446. [Google Scholar] [CrossRef]
- Chait, A.; Kim, F. Saturated Fatty Acids and Inflammation: Who Pays the Toll? Arterioscler. Thromb. Vasc. Biol. 2010, 30, 692–693. [Google Scholar] [CrossRef]
- Nicholas, N.A.; Mbongue, J.C.; Garcia-Pérez, D.; Sorensen, D.; Bennit, H.F.; De Leon, M.; Langridge, W.H.R. Exploring the Interplay between Fatty Acids, Inflammation, and Type 2 Diabetes. Immuno 2024, 4, 91–107. [Google Scholar] [CrossRef]
- Amiri, P.; Baradaran, B.; Saghafi-Asl, M.; Naghizadeh, M.; Shanehbandi, D.; Karamzad, N.; Vahed, S.Z. Association of Proinflammatory Genes Expression with Serum Interleukin 1β and Free Fatty Acids in Metabolically Healthy and Unhealthy Abdominally Obese Individuals: A Case-Control Study. BMC Immunol. 2019, 20, 23. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, S.G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Szczuko, M.; Kotlęga, D.; Palma, J.; Zembroń-Łacny, A.; Tylutka, A.; Gołąb-Janowska, M.; Drozd, A. Lipoxins, RevD1, and 9,13 HODE as the Most Important Derivatives after an Early Incident of Ischemic Stroke. Sci. Rep. 2020, 10, 12849. [Google Scholar] [CrossRef]
- Ardisson Korat, A.V.; Malik, V.S.; Furtado, J.D.; Sacks, F.; Rosner, B.; Rexrode, K.M.; Willett, W.C.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain SFA Concentrations Are Inversely Associated with Incident Type 2 Diabetes in US Men and Women. J. Nutr. 2020, 150, 340–349. [Google Scholar] [CrossRef]
- Malik, V.S.; Chiuve, S.E.; Campos, H.; Rimm, E.B.; Mozaffarian, D.; Hu, F.B.; Sun, Q. Circulating Very-Long-Chain Saturated Fatty Acids and Incident Coronary Heart Disease in US Men and Women. Circulation 2015, 132, 260–268. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lee, C.H.; Tiep, S.; Yu, R.T.; Ham, J.; Kang, H.; Evans, R.M. Peroxisome-Proliferator-Activated Receptor Delta Activates Fat Metabolism to Prevent Obesity. Cell 2003, 113, 159–170. [Google Scholar] [CrossRef]
- Lemaitre, R.N.; King, I.B.; Rice, K.; McKnight, B.; Sotoodehnia, N.; Rea, T.D.; Johnson, C.O.; Raghunathan, T.E.; Cobb, L.A.; Mozaffarian, D.S.; et al. Erythrocyte Very Long-Chain Saturated Fatty Acids Associated with Lower Risk of Incident Sudden Cardiac Arrest. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 149–153. [Google Scholar] [CrossRef]
- Bockus, B.B.; Biggs, M.L.; Lai, H.T.M.; Otto, M.C.d.O.; Fretts, A.M.; McKnight, B.; Sotoodehnia, N.; King, I.B.; Song, X.; Siscovick, D.S.; et al. Assessment of Plasma Phospholipid Very-Long-Chain Saturated Fatty Acid Levels and Healthy Aging. JAMA Netw. Open 2021, 4, e2120616. [Google Scholar] [CrossRef]
- Håversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of Proinflammatory Cytokines by Long-Chain Saturated Fatty Acids in Human Macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef]
- Tsoukalas, D.; Fragoulakis, V.; Sarandi, E.; Docea, A.O.; Papakonstaninou, E.; Tsilimidos, G.; Anamaterou, C.; Fragkiadaki, P.; Aschner, M.; Tsatsakis, A.; et al. Targeted Metabolomic Analysis of Serum Fatty Acids for the Prediction of Autoimmune Diseases. Front. Mol. Biosci. 2019, 6, 120. [Google Scholar] [CrossRef]
- Oda, E.; Hatada, K.; Kimura, J.; Aizawa, Y.; Thanikachalam, P.V.; Watanabe, K. Relationships between Serum Unsaturated Fatty Acids and Coronary Risk Factors: Negative Relations between Nervonic Acid and Obesity-Related Risk Factors. Int. Heart J. 2005, 46, 975–985. [Google Scholar] [CrossRef]
- Namiecinska, M.; Piatek, P.; Lewkowicz, P. Nervonic Acid Synthesis Substrates as Essential Components in Profiled Lipid Supplementation for More Effective Central Nervous System Regeneration. Int. J. Mol. Sci. 2024, 25, 3792. [Google Scholar] [CrossRef]
- Eliton, C.; Bruce, D.; Kennedy, E. A Comparison of the Lipid and Fatty Acid Profiles from the Kernels of the Fruit (Nuts) of Ximenia caffra and Ricinodendron rautanenii from Zimbabwe. Ind. Crops Prod. 2008, 27, 29–32. [Google Scholar] [CrossRef]
- Minas, T.Z.; Lord, B.D.; Zhang, A.L.; Candia, J.; Dorsey, T.H.; Baker, F.S.; Tang, W.; Bailey-Whyte, M.; Smith, C.J.; Obadi, O.M.; et al. Circulating Trans Fatty Acids Are Associated with Prostate Cancer in Ghanaian and American Men. Nat. Commun. 2023, 14, 4322. [Google Scholar] [CrossRef]
- Kiage, J.N.; Merrill, P.D.; Judd, S.E.; He, K.; Lipworth, L.; Cushman, M.; Howard, V.J.; Kabagambe, E.K. Intake of Trans Fat and Incidence of Stroke in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Cohort. Am. J. Clin. Nutr. 2014, 99, 1071–1076. [Google Scholar] [CrossRef]
- Hadj Ahmed, S.; Kharroubi, W.; Kaoubaa, N.; Zarrouk, A.; Batbout, F.; Gamra, H.; Najjar, M.F.; Lizard, G.; Hininger-Favier, I.; Hammami, M. Correlation of Trans Fatty Acids with the Severity of Coronary Artery Disease Lesions. Lipids Health Dis. 2018, 17, 52. [Google Scholar] [CrossRef]
- Islam, M.A.; Amin, M.N.; Siddiqui, S.A.; Hossain, M.P.; Sultana, F.; Kabir, M.R. Trans Fatty Acids and Lipid Profile: A Serious Risk Factor to Cardiovascular Disease, Cancer, and Diabetes. Diabetes Metab. Syndr. 2019, 13, 1643–1647. [Google Scholar] [CrossRef]
- Brandt, E.J.; Myerson, R.; Perraillon, M.C.; Polonsky, T.S. Hospital Admissions for Myocardial Infarction and Stroke before and after the Trans-Fatty Acid Restrictions in New York. JAMA Cardiol. 2017, 2, 627–634. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rimm, E.B.; King, I.B.; Lawler, R.L.; McDonald, G.B.; Levy, W.C. Trans Fatty Acids and Systemic Inflammation in Heart Failure. Am. J. Clin. Nutr. 2004, 80, 1521–1525. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, H.; Ma, X.; Zhu, D.; Zhao, L.; Xiao, W. Adrenic Acid: A Promising Biomarker and Therapeutic Target (Review). Int. J. Mol. Med. 2025, 55, 20. [Google Scholar] [CrossRef]
- Mazidi, M.; Shekoohi, N.; Katsiki, N.; Banach, M. Omega-6 Fatty Acids and the Risk of Cardiovascular Disease: Insights from a Systematic Review and Meta-Analysis of Randomized Controlled Trials and a Mendelian Randomization Study. Arch. Med. Sci. 2021, 18, 466–479. [Google Scholar] [CrossRef]
- Galanty, A.; Grudzińska, M.; Paździora, W.; Paśko, P. Erucic Acid—Both Sides of the Story: A Concise Review on Its Beneficial and Toxic Properties. Molecules 2023, 28, 1924. [Google Scholar] [CrossRef]
- Farag, M.A.; Gad, M.Z. Omega-9 Fatty Acids: Potential Roles in Inflammation and Cancer Management. J. Genet. Eng. Biotechnol. 2022, 20, 48. [Google Scholar] [CrossRef]
- Imamura, F.; Lemaitre, R.N.; King, I.B.; Song, X.; Steffen, L.M.; Folsom, A.R.; Siscovick, D.S.; Mozaffarian, D. Long-Chain Monounsaturated Fatty Acids and Incidence of Congestive Heart Failure in 2 Prospective Cohorts. Circulation 2013, 127, 1512–1521. [Google Scholar] [CrossRef]
- Kapoor, R.; Huang, Y.-S. Gamma Linolenic Acid: An Anti-Inflammatory Omega-6 Fatty Acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Ramos, K.S.; Chapkin, R.S. Dietary Gamma-Linolenic Acid Modulates Macrophage–Vascular Smooth Muscle Cell Interactions: Evidence for a Macrophage-Derived Soluble Factor That Downregulates DNA Synthesis in Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1397–1403. [Google Scholar] [CrossRef]
- Kotlęga, D.; Peda, B.; Palma, J.; Zembroń-Łacny, A.; Gołąb-Janowska, M.; Masztalewicz, M.; Nowacki, P.; Szczuko, M. Free Fatty Acids Are Associated with the Cognitive Functions in Stroke Survivors. Int. J. Environ. Res. Public Health 2021, 18, 6500. [Google Scholar] [CrossRef]
- Ralston, J.C.; Lyons, C.L.; Kennedy, E.B.; Kirwan, A.M.; Roche, H.M. Fatty Acids and NLRP3 Inflammasome-Mediated Inflammation in Metabolic Tissues. Annu. Rev. Nutr. 2017, 37, 77–102. [Google Scholar] [CrossRef]
- Eguchi, K.; Cannito, S.; Morello, E.; Bocca, C.; Foglia, B.; Benetti, E.; Novo, E.; Chiazza, F.; Rogazzo, M.; Fantozzi, R.; et al. Microvesicles Released from Fat-Laden Cells Promote Activation of Hepatocellular NLRP3 Inflammasome: A Pro-Inflammatory Link between Lipotoxicity and Non-Alcoholic Steatohepatitis. PLoS ONE 2017, 12, e0172575. [Google Scholar] [CrossRef]
- Shen, L.; Yang, Y.; Ou, T.; Key, C.C.; Tong, S.H.; Sequeira, R.C.; Nelson, J.M.; Nie, Y.; Wang, Z.; Boudyguina, E.; et al. Dietary PUFAs Attenuate NLRP3 Inflammasome Activation via Enhancing Macrophage Autophagy. J. Lipid Res. 2017, 58, 1808–1821. [Google Scholar] [CrossRef]
- Martino, M.; Maruyama, K.; Kuhn, G.; Satoh, T.; Takeuchi, O.; Müller, R.; Akira, S. Inhibition of IL-1R1/MyD88 Signaling Promotes Mesenchymal Stem Cell-Driven Tissue Regeneration. Nat. Commun. 2016, 7, 11051. [Google Scholar] [CrossRef]
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Bézaire, V.; Langin, D. Regulation of Adipose Tissue Lipolysis Revisited: Symposium on ‘Frontiers in Adipose Tissue Biology’. Proc. Nutr. Soc. 2009, 68, 350–360. [Google Scholar] [CrossRef]
- Boi, R.; Ebefors, K.; Henricsson, M.; Borén, J.; Nyström, J. Modified Lipid Metabolism and Cytosolic Phospholipase A2 Activation in Mesangial Cells under Pro-Inflammatory Conditions. Sci. Rep. 2022, 12, 7322. [Google Scholar] [CrossRef]
- Fetsko, A.R.; Sebo, D.J.; Budzynski, L.B.; Scharbarth, A.; Taylor, M.R. IL-1β Disrupts Blood-Brain Barrier Development by Inhibiting Endothelial Wnt/β-Catenin Signaling. iScience 2024, 27, 109651. [Google Scholar] [CrossRef]
- Behm, C.; Nemec, M.; Blufstein, A.; Schubert, M.; Rausch-Fan, X.; Andrukhov, O.; Jonke, E. Interleukin-1β Induced Matrix Metalloproteinase Expression in Human Periodontal Ligament-Derived Mesenchymal Stromal Cells under In Vitro Simulated Static Orthodontic Forces. Int. J. Mol. Sci. 2021, 22, 1027. [Google Scholar] [CrossRef]
- Lee, Y.A.; Choi, H.; Lee, S.H.; Yang, H.-I.; Yoo, M.C.; Hong, S.-J.; Kim, K.S. Synergy between Adiponectin and Interleukin-1β on the Expression of Interleukin-6, Interleukin-8, and Cyclooxygenase-2 in Fibroblast-Like Synoviocytes. Exp. Mol. Med. 2012, 44, 440–447. [Google Scholar] [CrossRef]
- Gao, D.; Madi, M.; Ding, C.; Fok, M.; Steele, T.; Ford, C.; Hunter, L.; Chen, B. Interleukin-1β Mediates Macrophage-Induced Impairment of Insulin Signaling in Human Primary Adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E289–E304. [Google Scholar] [CrossRef]
- Stanimirovic, D.; Satoh, K. Inflammatory Mediators of Cerebral Endothelium: A Role in Ischemic Brain Inflammation. Brain Pathol. 2000, 10, 113–126. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and Foe for Ischemic Stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-Stroke Inflammation—Target or Tool for Therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef]
- Wang, X.; Barone, F.C.; Aiyar, N.V.; Feuerstein, G.Z. Interleukin-1 Receptor and Receptor Antagonist Gene Expression after Focal Stroke in Rats. Stroke 1997, 28, 155–161. [Google Scholar] [CrossRef]
- Juge-Aubry, C.E.; Somm, E.; Giusti, V.; Pernin, A.; Chicheportiche, R.; Verdumo, C.; Rohner-Jeanrenaud, F.; Burger, D.; Dayer, J.-M.; Meier, C.A. Adipose Tissue Is a Major Source of Interleukin-1 Receptor Antagonist: Upregulation in Obesity and Inflammation. Diabetes 2003, 52, 1104–1110. [Google Scholar] [CrossRef]
- Joesting, J.J.; Moon, M.L.; Gainey, S.J.; Tisza, B.L.; Blevins, N.A.; Freund, G.G. Fasting Induces IL-1 Resistance and Free-Fatty Acid-Mediated Up-Regulation of IL-1R2 and IL-1RA. Front. Immunol. 2014, 5, 315. [Google Scholar] [CrossRef]
- Xia, Y.Y.; Song, S.W.; Min, Y.; Zhong, Y.; Sheng, Y.C.; Li, R.P.; Liu, Q.H. The Effects of Anakinra on Focal Cerebral Ischemic Injury in Rats. CNS Neurosci. Ther. 2014, 20, 879–881. [Google Scholar] [CrossRef]
- Parry-Jones, A.R.; Stocking, K.; MacLeod, M.J.; Clarke, B.; Werring, D.J.; Muir, K.W.; Vail, A. Phase II Randomized, Placebo-Controlled, Clinical Trial of Interleukin-1 Receptor Antagonist in Intracerebral Hemorrhage: Blocking the Cytokine IL-1 in ICH (BLOC-ICH). Eur. Stroke J. 2023, 8, 819–827. [Google Scholar] [CrossRef]
- Cliteur, M.P.; van der Kolk, A.G.; Hannink, G.; Hofmeijer, J.; Jolink, W.; Klijn, C.; Schreuder, F. Anakinra in Cerebral Hemorrhage to Target Secondary Injury Resulting from Neuroinflammation (ACTION): Study Protocol of a Phase II Randomized Clinical Trial. Eur. Stroke J. 2024, 9, 265–273. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Gao, Y.; Jiang, J.; Li, M.; Li, S.; Hu, X.; Wang, J.; Wang, T.; Zhang, J.; et al. Impaired membrane lipids in ischemic stroke: A key player in inflammation and thrombosis. J. Neuroinflamm. 2025, 22, 144. [Google Scholar] [CrossRef]
- Nessel, I.; Whiley, L.; Dyall, S.C.; Michael-Titus, A.T. A plasma lipid signature in acute human traumatic brain injury: Link with neuronal injury and inflammation markers. J. Cereb. Blood Flow Metab. 2025, 45, 443–458. [Google Scholar] [CrossRef]
- Chen, X.; Wei, J.; Zhang, L.; Wang, H.; Zhang, Y.; Li, Z.; Wang, X.; Liu, L.; Zhang, Y.; Zhang, T. Association between plasma short-chain fatty acids and inflammation in human immunodeficiency virus-associated neurocognitive disorder: A pilot study. Lipids Health Dis. 2025, 24, 66. [Google Scholar] [CrossRef]
- Meier, P.; Glasmacher, S.; Salmen, A.; Chan, A.; Gertsch, J. Comparative targeted lipidomics between serum and cerebrospinal fluid of multiple sclerosis patients shows sex and age-specific differences of endocannabinoids and glucocorticoids. Acta Neuropathol. Commun. 2024, 12, 160. [Google Scholar] [CrossRef]
- Shi, J.; Nie, Z.; Wang, S.; Zhang, H.; Li, X.; Yao, J.; Jin, Y.; Yang, X.; Zhang, X.; Zhang, M.; et al. Serum lipidomics profiling to identify potential biomarkers of ischemic stroke: A pilot study in Chinese adults. Biomed. Environ. Sci. 2025, 38, 918–925. [Google Scholar] [CrossRef]
- Yuan, J.; Liao, Y.-S.; Zhang, T.-C.; Liu, S.; Ruan, M.-M.; Yang, T.-T.; Sun, S.-Q.; Xu, L.-Y.; Xie, X.-L.; Zhao, L. Disrupted Lipid Metabolism Aggravates Ischemic Brain Injury: Targeting FDFT1 for Stroke Therapy. Mol. Neurobiol. 2025, 62, 14227–14244. [Google Scholar] [CrossRef]


| Characteristic | Value |
|---|---|
| Age (years, mean ± SD) | 60.6 ± 11.9 |
| Sex (Male), n (%) | 32 (43.8) |
| BMI (mean ± SD) | 28.7 ± 4.9 |
| Hypertension, n (%) | 60 (82.2) |
| Diabetes/IFG, n (%) | 35 (47.9) |
| Current Smoking, n (%) | 25 (34.2) |
| Dyslipidaemia, n (%) | 41 (56.2) |
| Atrial Fibrillation, n (%) | 4 (5.5) |
| Previous Stroke/TIA, n (%) | 5 (6.8) |
| CRP (mg/L) | 2.6 ± 3.7 |
| Total Cholesterol (mg/dL) | 196.4 ± 53.0 |
| LDL (mg/dL) | 113.4 ± 45 |
| HDL (mg/dL) | 52.1 ± 15.2 |
| Triglycerides (mg/dL) | 156.1 ± 75.3 |
| Variable | Mean | SD | Spearman Correlation (rho) | p-Value |
|---|---|---|---|---|
| C15:0 pentadecanoid acid | 0.2172 | 0.1076 | 0.488 | <0.001 |
| C15:1 cis-10-pentadecanoid acid | 0.0811 | 0.036 | 0.4731 | <0.001 |
| C17:1 cis-10-heptadecanoid acid | 0.0909 | 0.0349 | 0.4113 | <0.001 |
| C18:0 stearic acid | 13.3136 | 1.9834 | 0.3019 | <0.05 |
| C24:0 lignoceric acid | 0.1527 | 0.0762 | −0.2799 | <0.05 |
| C24:1 nervonic acid | 0.3995 | 0.2469 | −0.2759 | <0.05 |
| C18:2n6t linoleic acid | 6.1411 | 1.9309 | −0.2718 | <0.05 |
| C17:0 heptadecanoic acid | 0.302 | 0.0503 | 0.2407 | <0.05 |
| C13:0 tridecanoic acid | 0.3071 | 0.0916 | 0.2382 | <0.05 |
| C23:0 tricosanoic acid | 0.2329 | 0.1517 | −0.2302 | 0.05 |
| C22:0 behenic acid | 0.2252 | 0.0979 | −0.224 | 0.0568 |
| C18:3n6 gammalinoleic acid | 0.3862 | 0.1921 | −0.2215 | 0.0597 |
| C22:4n6 docosatetraenoate | 0.2226 | 0.1165 | 0.2149 | 0.0678 |
| C20:3n6 eicosatrienoic acid | 1.2825 | 0.3089 | −0.2141 | 0.069 |
| C16:1 palmitoleic acid | 2.1342 | 0.7468 | −0.2034 | 0.0844 |
| C22:6n3 docosahexaenoic acid (DHA) | 1.7522 | 0.5305 | 0.1801 | 0.1273 |
| C16:0 palmitic acid | 26.8018 | 1.7418 | 0.1715 | 0.1469 |
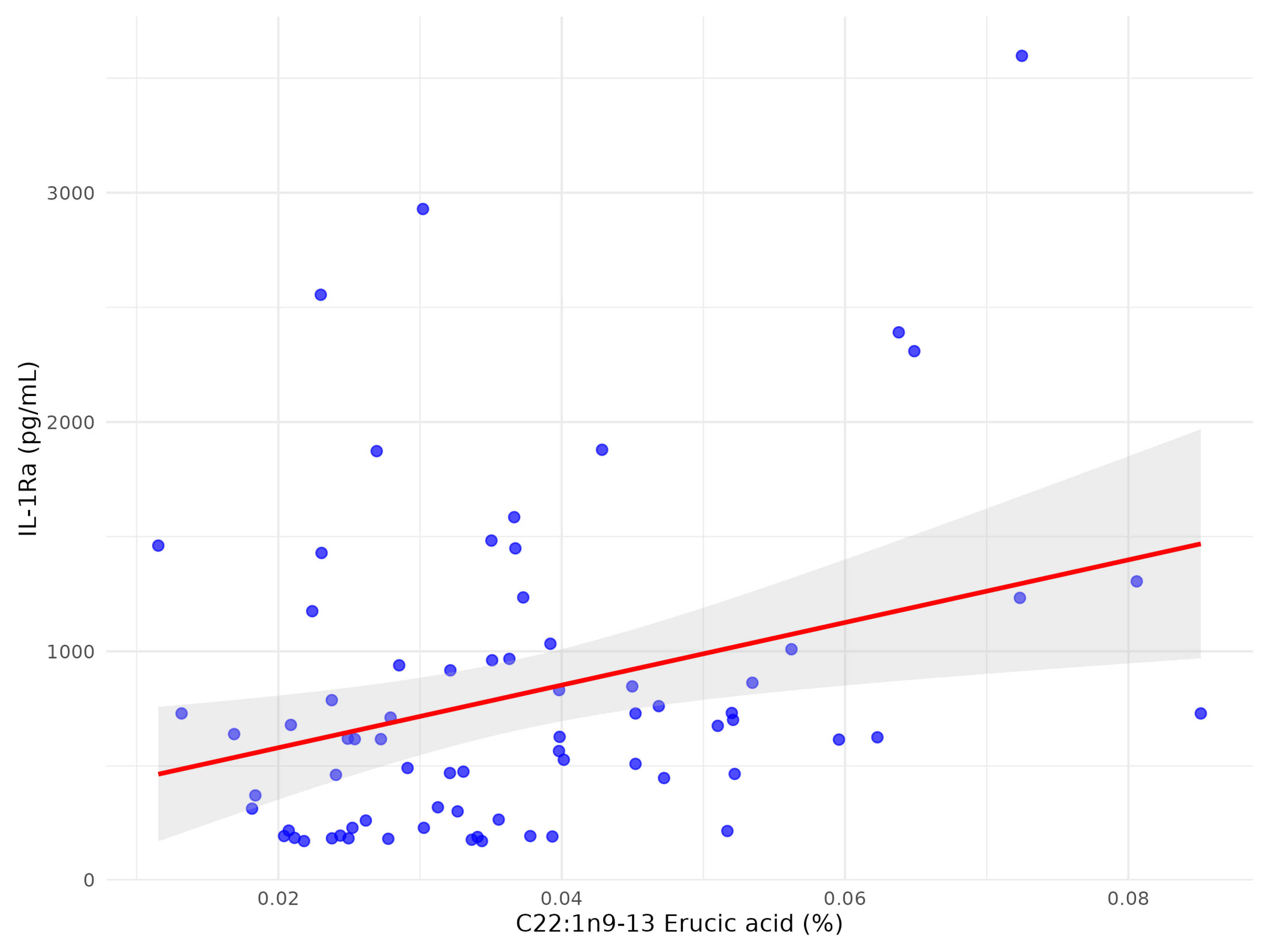
| C22:1n9 13 erucic acid | 0.037 | 0.0156 | −0.1684 | 0.1545 |
| C20:5n3 eicosapentaenoic acid (EPA) | 0.6033 | 0.2581 | −0.1549 | 0.1906 |
| C20:0 arachidic acid | 0.2061 | 0.073 | −0.1492 | 0.2078 |
| C14:1 myristolenic acid | 0.0698 | 0.0377 | 0.1389 | 0.2411 |
| C22:2-cis-docodienoic acid | 0.0169 | 0.0109 | 0.1291 | 0.2762 |
| C14:0 myristic acid | 1.2079 | 0.3812 | 0.1281 | 0.2802 |
| C20:2 cis-11-eicodienoic acid | 0.1506 | 0.0339 | −0.1277 | 0.2816 |
| C18:4 stearidonate | 0.0575 | 0.0273 | −0.1161 | 0.328 |
| C22:1/C20:1 cis11-eicosanic acid | 0.1785 | 0.0687 | 0.0966 | 0.4162 |
| C22:5w3 docosapentaenate | 0.4603 | 0.2315 | −0.0936 | 0.4311 |
| C18:3n3 linolenic acid | 0.5037 | 0.1586 | −0.0817 | 0.4918 |
| C20:3n3 cis-11-eicosatrienoic acid | 0.0309 | 0.0143 | 0.0471 | 0.6921 |
| C20:4n6 arachidonic acid | 6.3053 | 1.3142 | 0.0341 | 0.7746 |
| C18:1 vaccinic acid | 1.9785 | 0.3506 | 0.0143 | 0.9046 |
| C18:2n6c linoleic acid | 11.5384 | 2.3326 | 0.0087 | 0.9419 |
| C18:1n9 ct oleic acid | 22.5909 | 3.7134 | −0.0071 | 0.9522 |
| Free Fatty Acid | Mean | SD | Spearman Correlation (rho) | p-Value |
|---|---|---|---|---|
| C15:1 cis-10-pentadecanoid acid | 0.081 | 0.036 | −0.357 | <0.005 |
| C18:2n6t linoleic acid | 6.141 | 1.931 | 0.341 | <0.005 |
| C24:1 nervonic acid | 0.4 | 0.247 | 0.302 | <0.05 |
| C22:1n9 13 erucic acid | 0.037 | 0.016 | 0.299 | <0.05 |
| C18:3n6 gammalinoleic acid | 0.386 | 0.192 | 0.277 | <0.05 |
| C18:4 stearidonate | 0.057 | 0.027 | 0.255 | <0.05 |
| C24:0 lignoceric acid | 0.153 | 0.076 | 0.254 | <0.05 |
| C18:2n6c linoleic acid | 11.538 | 2.333 | −0.254 | <0.05 |
| C22:4n6 docosatetraenoate | 0.223 | 0.116 | −0.241 | <0.05 |
| C22:0 behenic acid | 0.225 | 0.098 | 0.236 | <0.05 |
| C17:1 cis-10-heptadecanoid acid | 0.091 | 0.035 | −0.228 | 0.052 |
| C15:0 pentadecanoid acid | 0.217 | 0.108 | −0.209 | 0.077 |
| C22:1/C20:1 cis-11-eicosanic acid | 0.179 | 0.069 | −0.205 | 0.082 |
| C20:0 arachidic acid | 0.206 | 0.073 | 0.202 | 0.086 |
| C20:5n3 eicosapentaenoic acid (EPA) | 0.603 | 0.258 | 0.193 | 0.102 |
| C20:2 cis-11-eicodienoic acid | 0.151 | 0.034 | −0.176 | 0.136 |
| C18:0 stearic acid | 13.314 | 1.983 | −0.166 | 0.16 |
| C23:0 tricosanoic acid | 0.233 | 0.152 | 0.162 | 0.17 |
| C13:0 tridecanoic acid | 0.307 | 0.092 | −0.144 | 0.225 |
| C18:1 trans vaccinic acid | 1.978 | 0.351 | −0.143 | 0.228 |
| C16:1 palmitoleic acid | 2.134 | 0.747 | 0.131 | 0.27 |
| C16:0 palmitic acid | 26.802 | 1.742 | −0.106 | 0.373 |
| C18:3n3 linolenic acid | 0.504 | 0.159 | 0.095 | 0.426 |
| C22:2 cis-docodienoic acid | 0.017 | 0.011 | −0.077 | 0.515 |
| C14:1 myristolenic acid | 0.07 | 0.038 | 0.072 | 0.544 |
| C22:6n3 docosahexaenoic acid (DHA) | 1.752 | 0.531 | −0.057 | 0.631 |
| C20:3n6 eicosatrienoic acid | 1.283 | 0.309 | 0.043 | 0.716 |
| C20:3n3 cis-11-eicosatrienoic acid | 0.031 | 0.014 | −0.043 | 0.716 |
| C22:5w3 docosapentaenate | 0.46 | 0.231 | 0.04 | 0.738 |
| C20:4n6 arachidonic acid | 6.305 | 1.314 | −0.038 | 0.751 |
| C17:0 heptadecanoic acid | 0.302 | 0.05 | −0.035 | 0.769 |
| C14:0 myristic acid | 1.208 | 0.381 | 0.013 | 0.914 |
| C18:1n9 ct oleic acid | 22.591 | 3.713 | −0.007 | 0.956 |
| Free Fatty Acid | Spearman (rho) | Beta Coefficient (95% CI) | R2 | p-Value |
|---|---|---|---|---|
| C18:3n6 gamma-linoleic acid | 0.277 | 1343 (−3114; 36.72) | 0.0581 | <0.05 |
| C22:1n9 13 erucic acid | 0.299 | 20,612.8 (8710; 32,515) | 0.1124 | <0.05 |
| C22:4n6 docosatetraenoate | −0.241 | 1538 (−3114; 36.72) | 0.0358 | 0.055 |
| Fatty Acid | Study Group (n = 73): Mean (SD) | Control Group (n = 33): Mean (SD) | p-Value (Mann–Whitney U Test) |
|---|---|---|---|
| C15:1 cis-10-pentadecanoid acid | 0.08 (0.04) | 0.31 (0.15) | <0.01 |
| C18:2n6t linoleic acid | 6.09 (1.92) | 16.22 (6.21) | <0.01 |
| C24:1 nervonic acid | 0.4 (0.25) | 0.16 (0.21) | <0.01 |
| C22:1n9 13 erucic acid | 0.04 (0.02) | 0.07 (0.03) | <0.01 |
| C18:3n6 gammalinoleic acid | 0.39 (0.19) | 0.6 (0.31) | <0.01 |
| C18:4 stearidonate | 0.06 (0.03) | 0.12 (0.06) | <0.01 |
| C24:0 lignoceric acid | 0.15 (0.08) | 0.11 (0.16) | <0.01 |
| C18:2n6c linoleic acid | 11.55 (2.36) | 30.2 (14.34) | <0.01 |
| C22:4n6 docosatetraenoate | 0.22 (0.12) | 0.63 (0.36) | <0.01 |
| C22:0 behenic acid | 0.23 (0.1) | 0.21 (0.2) | <0.01 |
| C17:1 cis-10-heptadecanoid acid | 0.09 (0.03) | 0.3 (0.13) | <0.01 |
| C15:0 pentadecanoid acid | 0.22 (0.11) | 0.49 (0.27) | <0.01 |
| C22:1/C20:1 cis-11-eicosanic acid | 0.18 (0.07) | 0.43 (0.22) | <0.01 |
| C20:0 arachidic acid | 0.21 (0.07) | 0.35 (0.19) | <0.01 |
| C20:5n3 eicosapentaenoic acid (EPA) | 0.61 (0.26) | 2.79 (2.66) | <0.01 |
| C20:2 cis-11-eicodienoic acid | 0.15 (0.03) | 0.41 (0.19) | <0.01 |
| C18:0 stearic acid | 13.36 (1.94) | 33.81 (13.16) | <0.01 |
| C23:0 tricosanoic acid | 0.23 (0.14) | 0.37 (0.45) | <0.01 |
| C13:0 tridecanoic acid | 0.31 (0.09) | 0.88 (0.45) | <0.01 |
| C18:1 trans vaccinic acid | 1.98 (0.35) | 3.99 (1.84) | <0.01 |
| C16:1 palmitoleic acid | 2.15 (0.75) | 4.02 (2.23) | <0.01 |
| C16:0 palmitic acid | 26.8 (1.76) | 60.19 (23.56) | <0.01 |
| C18:3n3 linolenic acid | 0.5 (0.15) | 1.68 (0.88) | <0.01 |
| C22:2 cis-docodienoic acid | 0.02 (0.01) | 0.04 (0.02) | <0.01 |
| C14:1 myristolenic acid | 0.07 (0.04) | 0.16 (0.13) | <0.01 |
| C22:6n3 docosahexaenoic acid (DHA) | 1.75 (0.54) | 5.48 (2.88) | <0.01 |
| C20:3n6 eicosatrienoic acid | 1.29 (0.31) | 2.8 (1.09) | <0.01 |
| C20:3n3 cis-11-eicosatrienoic acid | 0.03 (0.01) | 0.1 (0.04) | <0.01 |
| C22:5w3 docosapentaenate | 0.46 (0.23) | 1.35 (0.58) | <0.01 |
| C20:4n6 arachidonic acid | 6.29 (1.33) | 16.39 (7.3) | <0.01 |
| C17:0 heptadecanoic acid | 0.3 (0.05) | 0.77 (0.35) | <0.01 |
| C14:0 myristic acid | 1.22 (0.38) | 2.7 (1.67) | <0.01 |
| C18:1n9 ct oleic acid | 22.55 (3.74) | 42.84 (19.92) | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotlega, D.; Drozd, A.; Zembron-Lacny, A.; Morawin, B.; Ryterska, K.; Szczuko, M. Free Fatty Acids Correlate with the Interleukin-1 β and Interleukin-1 Receptor Antagonist in the Early Subacute Phase of Stroke. Biomolecules 2025, 15, 1537. https://doi.org/10.3390/biom15111537
Kotlega D, Drozd A, Zembron-Lacny A, Morawin B, Ryterska K, Szczuko M. Free Fatty Acids Correlate with the Interleukin-1 β and Interleukin-1 Receptor Antagonist in the Early Subacute Phase of Stroke. Biomolecules. 2025; 15(11):1537. https://doi.org/10.3390/biom15111537
Chicago/Turabian StyleKotlega, Dariusz, Arleta Drozd, Agnieszka Zembron-Lacny, Barbara Morawin, Karina Ryterska, and Malgorzata Szczuko. 2025. "Free Fatty Acids Correlate with the Interleukin-1 β and Interleukin-1 Receptor Antagonist in the Early Subacute Phase of Stroke" Biomolecules 15, no. 11: 1537. https://doi.org/10.3390/biom15111537
APA StyleKotlega, D., Drozd, A., Zembron-Lacny, A., Morawin, B., Ryterska, K., & Szczuko, M. (2025). Free Fatty Acids Correlate with the Interleukin-1 β and Interleukin-1 Receptor Antagonist in the Early Subacute Phase of Stroke. Biomolecules, 15(11), 1537. https://doi.org/10.3390/biom15111537








