The Emerging Role of Sialic Acids in Obesity and Diabetes: Molecular Mechanisms and Therapeutic Perspectives
Abstract
1. Introduction: The Emerging Role of Sialic Acids in Metabolic Disease
2. Clinical Associations of Sialic Acids in Obesity
3. Sialic Acids as Biomarkers for Diabetes and Insulin Resistance
3.1. Sialic Acids in Type 1 Diabetes
3.2. Sialic Acids in Type 2 Diabetes
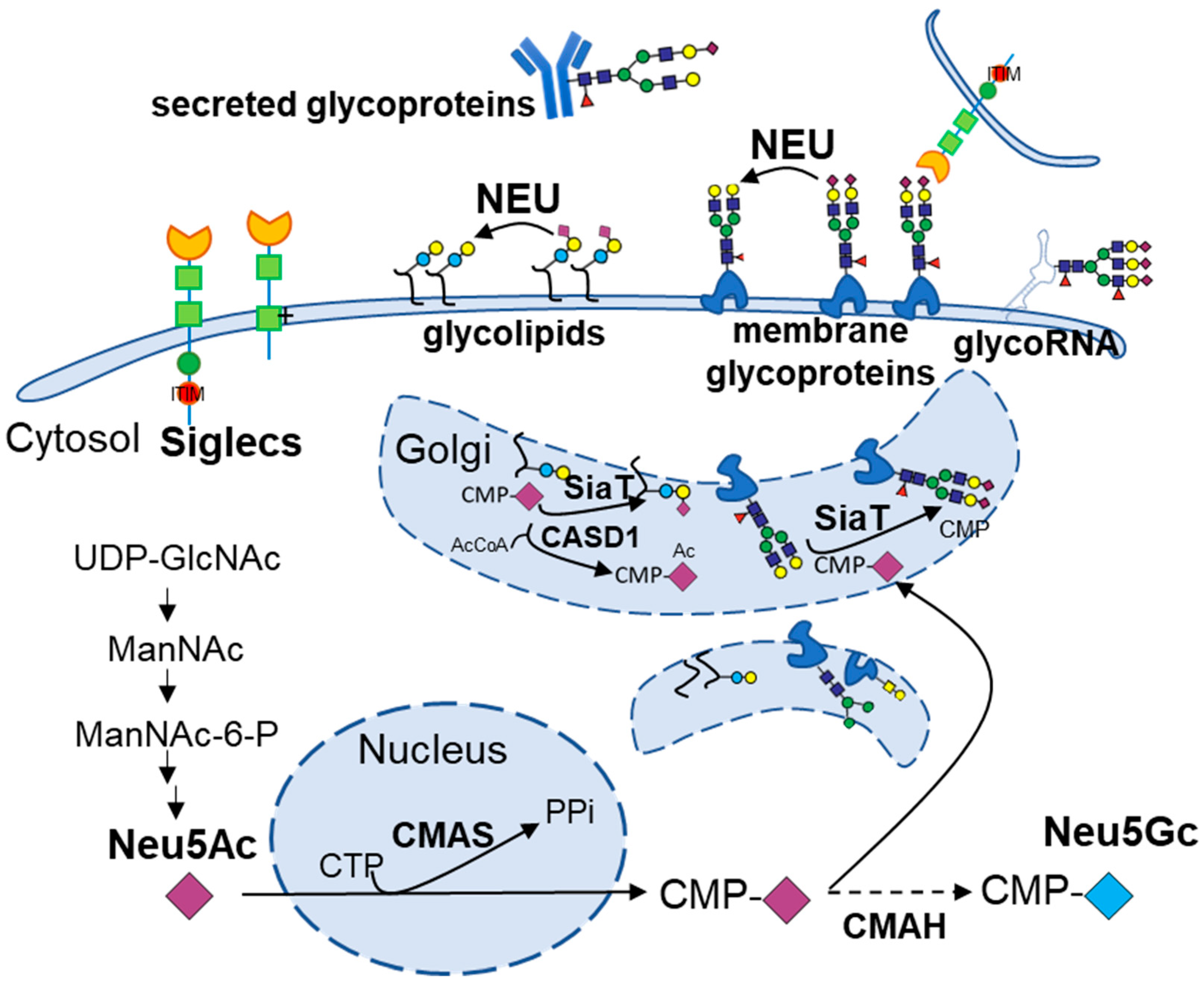
4. Molecular Insights into Sialic Acids and Obesity/Diabetes
4.1. Changes in IgG Glycoforms in Immune Dysfunction and Multiple Disorders
4.2. Regulation of Sialylation in Adipogenesis
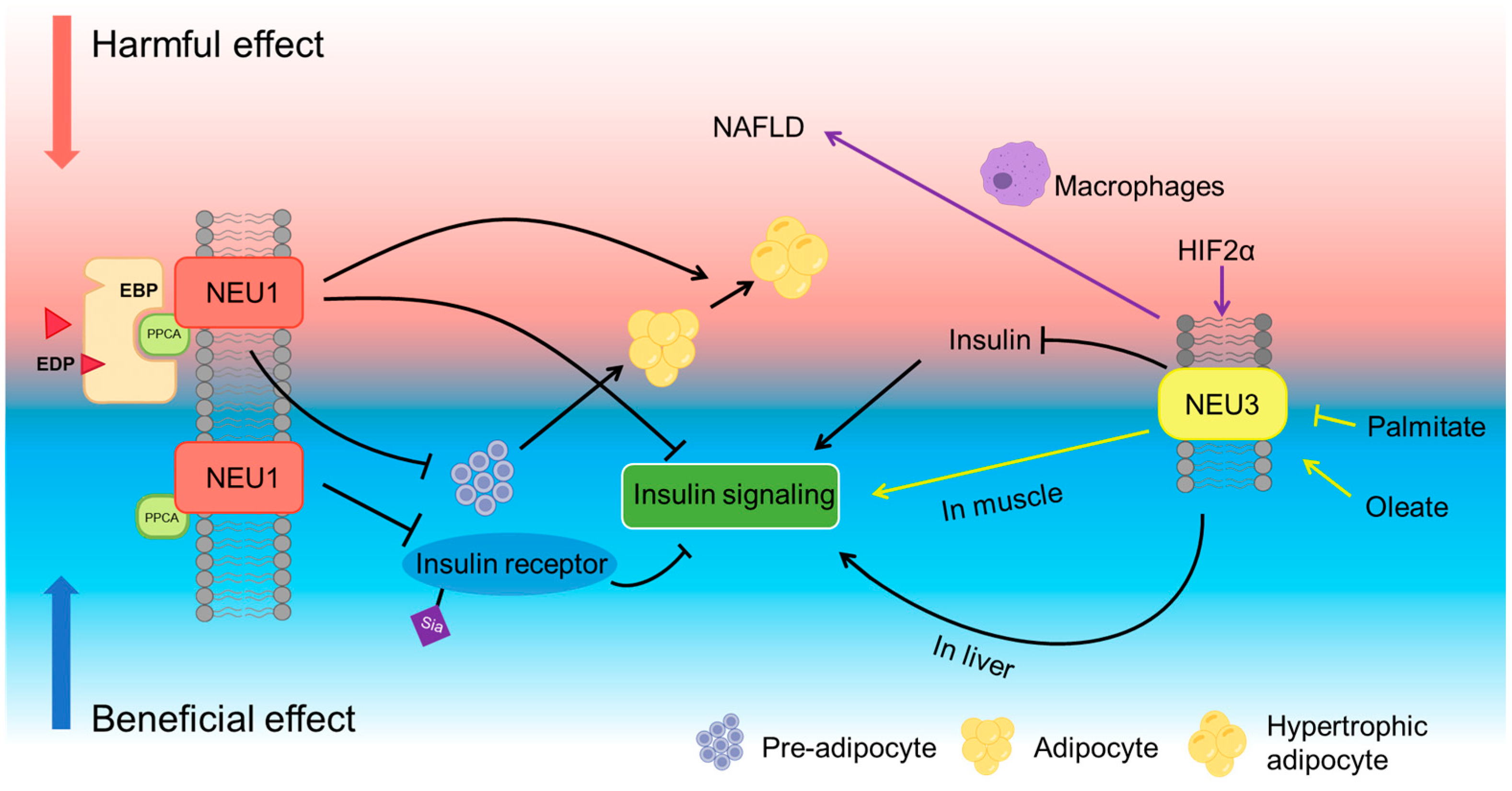
4.3. Neuraminidases and Metabolic Dysfunction
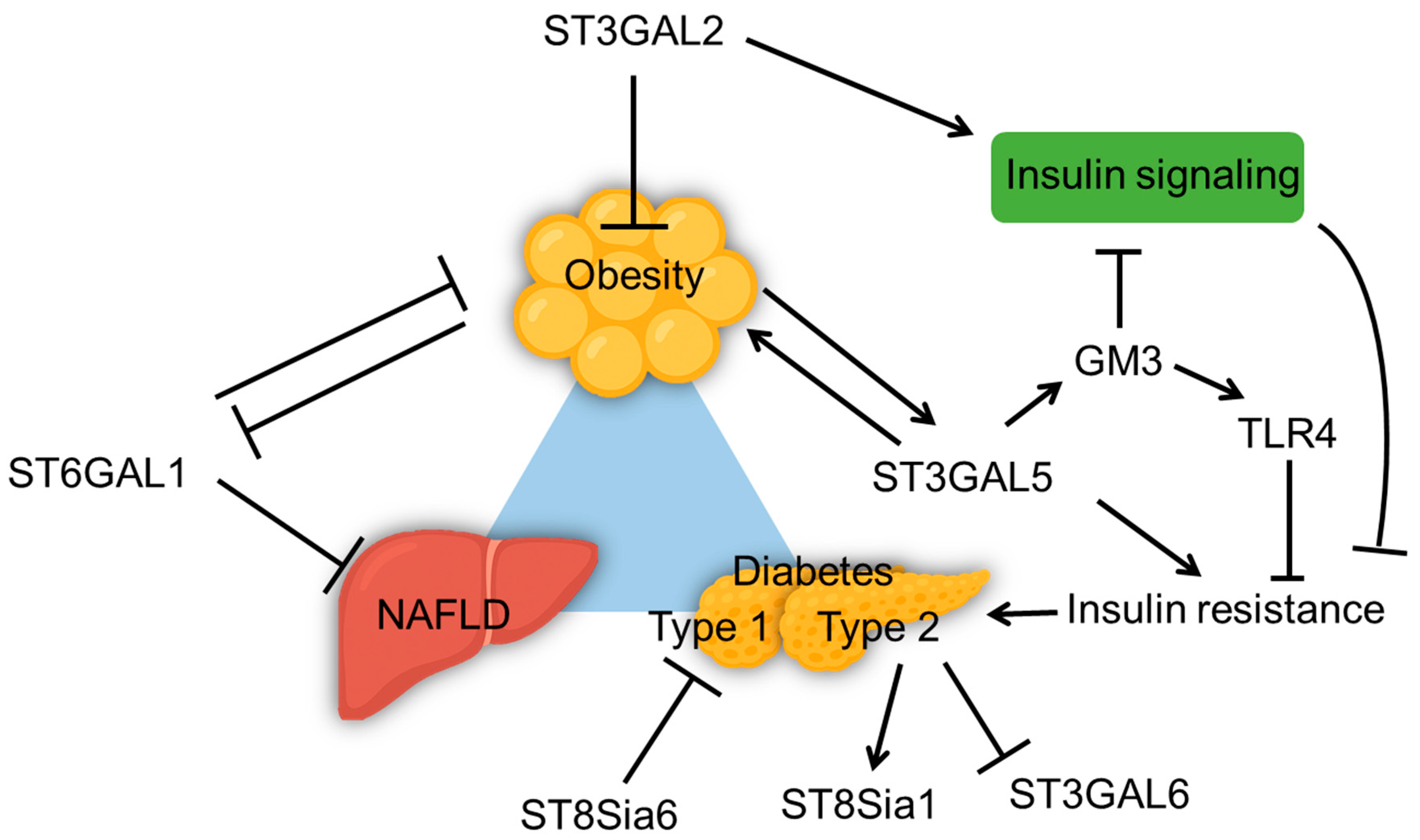
4.4. Sialyltransferase and Metabolic Dysfunction
4.5. Role of Siglecs in Metabolic Disorders
5. Therapeutic Interventions Targeting Sialic Acids
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CMAS | CMP-sialic acid synthetase |
| CRP | C-reactive protein |
| DANA | 2,3-dehydro-2-deoxy-N-acetylneuraminic acid |
| EBN | Edible Bird’s Nest |
| EDP | Elastin-derived peptide |
| ERC | Elastin receptor complex |
| ESC | Embryonic stem cells |
| GM3 | Monosialodihexosylganglioside (GM3 ganglioside) |
| HFD | High fat diet |
| HIF-2α | Hypoxia-inducible factor 2 alpha |
| IgG | Immunoglobulin G |
| IR | Insulin receptor |
| ITAMs | immunoreceptor tyrosine-based activation motifs |
| ITIMs | immunoreceptor tyrosine-based inhibitory motifs |
| LDL | Low density lipoprotein |
| MDSCs | Myeloid-derived suppressor cells |
| NAFLD | Non-alcoholic fatty liver disease |
| NASH | Non-alcoholic steatohepatitis |
| NEU | Neuraminidase |
| Neu5Ac | N-acetylneuraminic acid |
| SiaT | Sialyltransferase |
| Siglecs | Sialic acid-binding immunoglobulin-type lectins |
| STZ | Streptozotocin |
| T1DM | Type 1 diabetes |
| T2DM | Type 2 diabetes |
| TFEB | Transcription factor EB |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TSA | Total sialic acids |
References
- Lewis, A.L.; Chen, X.; Schnaar, R.L.; Varki, A. Sialic Acids and Other Nonulosonic Acids. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 75, pp. 1–213. ISBN 978-0-12-815202-7. [Google Scholar]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs Are Modified with N-Glycans and Displayed on the Surface of Living Cells. Cell 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef] [PubMed]
- Graziano, V.R.; Porat, J.; Ah Kioon, M.D.; Mejdrová, I.; Matz, A.J.; Lebedenko, C.G.; Chai, P.; Pluvinage, J.V.; Ricci-Azevedo, R.; Harrison, A.G.; et al. RNA N-Glycosylation Enables Immune Evasion and Homeostatic Efferocytosis. Nature 2025, 645, 784–792. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, W.; Torres, L.; Wang, X.; Ajaj, Y.; Zhu, L.; Luan, Y.; Zhou, H.; Wang, Y.; Zhang, D.; et al. Cell Surface RNAs Control Neutrophil Recruitment. Cell 2024, 187, 846–860.e17. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-H.; Hayakawa, T.; Diaz, S.; Krings, M.; Indriati, E.; Leakey, M.; Paabo, S.; Satta, Y.; Takahata, N.; Varki, A. Inactivation of CMP-N-Acetylneuraminic Acid Hydroxylase Occurred Prior to Brain Expansion during Human Evolution. Proc. Natl. Acad. Sci. USA 2002, 99, 11736–11741. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Oswald, D.M.; Oliva, K.D.; Kreisman, L.S.C.; Cobb, B.A. The Glycoscience of Immunity. Trends Immunol. 2018, 39, 523–535. [Google Scholar] [CrossRef]
- Duan, S.; Paulson, J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020, 38, 365–395. [Google Scholar] [CrossRef]
- Baumann, A.-M.T.; Bakkers, M.J.G.; Buettner, F.F.R.; Hartmann, M.; Grove, M.; Langereis, M.A.; de Groot, R.J.; Mühlenhoff, M. 9-O-Acetylation of Sialic Acids Is Catalysed by CASD1 via a Covalent Acetyl-Enzyme Intermediate. Nat. Commun. 2015, 6, 7673. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Bertozzi, C.R. The Clinical Impact of Glycobiology: Targeting Selectins, Siglecs and Mammalian Glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, D.; Ren, L.; Wang, N.; Jia, Y.; Zheng, Z.; Cai, W.; Fu, H.; Li, G. Sialylation Shields Glycoproteins from Oxidative Stress: Mechanistic Insights into Sialic Acid Oxidation and Structural Stability. J. Am. Chem. Soc. 2025, 147, 5828–5838. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Dong, C.; Chong, S.; Liu, Y.; Bian, Z.; Chen, X.; Fan, S. Sialyltransferase ST3GAL4 Directs a Dual Mechanism to Promote Pancreatic Ductal Adenocarcinoma Progression by Regulating Endoplasmic Reticulum Stress and Mitochondrial Homeostasis. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167900. [Google Scholar] [CrossRef]
- Jones, R.B.; Dorsett, K.A.; Hjelmeland, A.B.; Bellis, S.L. The ST6Gal-I Sialyltransferase Protects Tumor Cells against Hypoxia by Enhancing HIF-1α Signaling. J. Biol. Chem. 2018, 293, 5659–5667. [Google Scholar] [CrossRef]
- Yerlikaya, F.H.; Toker, A.; Çiçekler, H.; Arıbaş, A. The Association of Total Sialic Acid and Malondialdehyde Levels with Metabolic and Anthropometric Variables in Obesity. Biotech. Histochem. 2015, 90, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, F.M.; Yilmaz, G.; Savas Erdeve, S.; Dallar, Y.; Topkaya, B.C.; Yücel, D. Serum Sialic Acid, Hs-CRP and Oxidative Stress Parameters in Obese Children. J. Pediatr. Endocrinol. Metab. 2007, 20, 205–210. [Google Scholar] [CrossRef]
- Abdella, N.; Akanji, A.O.; Mojiminiyi, O.A.; Al Assoussi, A.; Moussa, M. Relation of Serum Total Sialic Acid Concentrations with Diabetic Complications and Cardiovascular Risk Factors in Kuwaiti Type 2 Diabetic Patients. Diabetes Res. Clin. Pract. 2000, 50, 65–72. [Google Scholar] [CrossRef]
- Akin, L.; Kurtoglu, S.; Muhtaroğlu, S.; Yikilmaz, A.; Kendirci, M.; Mazicioglu, M. The Association of Serum Sialic Acid with Carotid Intima-Media Thickness and Anthropometric and Metabolic Parameters in Obese Children and Adolescents. Ann. Nutr. Metab. 2011, 59, 139–144. [Google Scholar] [CrossRef]
- Rajappa, M.; Ikkruthi, S.; Nandeesha, H.; Satheesh, S.; Sundar, I.; Ananthanarayanan, P.H.; Harichandrakumar, K.T. Relationship of Raised Serum Total and Protein Bound Sialic Acid Levels with Hyperinsulinemia and Indices of Insulin Sensitivity and Insulin Resistance in Non-Diabetic Normotensive Obese Subjects. Diabetes Metab. Syndr. 2013, 7, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Kurtul, N.; Akarsu, E.; Aktaran, S. The Relationship between Serum Total Sialic Acid Levels and Adenosine Deaminase Activity in Obesity. Saudi Med. J. 2006, 27, 170–173. [Google Scholar] [PubMed]
- Crook, M.A.; Miell, J.; Ameerally, P.; Lumb, P.; Singh, N.; Russell-Jones, D.; Goldsmith, L. Serum Sialic Acid, a Reputed Cardiovascular Risk Factor, Is Related to Serum Leptin Concentrations in Fijians. Clin. Chim. Acta 2003, 331, 1–5. [Google Scholar] [CrossRef]
- Browning, L.M.; Jebb, S.A.; Mishra, G.D.; Cooke, J.H.; O’Connell, M.A.; Crook, M.A.; Krebs, J.D. Elevated Sialic Acid, but Not CRP, Predicts Features of the Metabolic Syndrome Independently of BMI in Women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1004–1010. [Google Scholar] [CrossRef]
- Crook, M.A.; Tutt, P.; Pickup, J.C. Elevated Serum Sialic Acid Concentration in NIDDM and Its Relationship to Blood Pressure and Retinopathy. Diabetes Care 1993, 16, 57–60. [Google Scholar] [CrossRef]
- Hong, Z.; Zhou, K.; Wei, Y.; Ma, B.; Xie, G.; Zhang, Z.; Liang, J. Associations of Plasma and Fecal Metabolites with Body Mass Index and Body Fat Distribution in Children. J. Clin. Endocrinol. Metab. 2024, 110, e1173–e1184. [Google Scholar] [CrossRef]
- Süer Gökmen, S.; Kazezoğlu, C.; Sunar, B.; Ozçelik, F.; Güngör, O.; Yorulmaz, F.; Gülen, S. Relationship between Serum Sialic Acids, Sialic Acid-Rich Inflammation-Sensitive Proteins and Cell Damage in Patients with Acute Myocardial Infarction. Clin. Chem. Lab. Med. 2006, 44, 199–206. [Google Scholar] [CrossRef]
- Suzzi, S.; Croese, T.; Ravid, A.; Gold, O.; Clark, A.R.; Medina, S.; Kitsberg, D.; Adam, M.; Vernon, K.A.; Kohnert, E.; et al. N-Acetylneuraminic Acid Links Immune Exhaustion and Accelerated Memory Deficit in Diet-Induced Obese Alzheimer’s Disease Mouse Model. Nat. Commun. 2023, 14, 1293. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The Effects of Intermittent or Continuous Energy Restriction on Weight Loss and Metabolic Disease Risk Markers: A Randomized Trial in Young Overweight Women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Koska, J.; Yassine, H.; Trenchevska, O.; Sinari, S.; Schwenke, D.C.; Yen, F.T.; Billheimer, D.; Nelson, R.W.; Nedelkov, D.; Reaven, P.D. Disialylated Apolipoprotein C-III Proteoform Is Associated with Improved Lipids in Prediabetes and Type 2 Diabetes. J. Lipid Res. 2016, 57, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Xie, Y.; Li, Q.; Artegoitia, V.M.; Lebrilla, C.B.; Keim, N.L.; Adams, S.H.; Krishnan, S. Diet Affects Glycosylation of Serum Proteins in Women at Risk for Cardiometabolic Disease. Eur. J. Nutr. 2021, 60, 3727–3741. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, R.; Zheng, S.; Wang, X.; Li, Q.; Ding, J.; Ma, X.; Zhuo, Z.; Li, Z.; Xin, Q.; Lu, X.; et al. Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner. Metabolites 2023, 13, 846. [Google Scholar] [CrossRef]
- Kurtoğlu, S.; Atabek, M.E.; Muhtaroglu, S.; Keskin, M. The Association of Serum Total Sialic Acid/Total Protein Ratio with Diabetic Parameters in Young Type 1 Diabetic Patients. Acta Diabetol. 2006, 43, 1–5. [Google Scholar] [CrossRef]
- Crook, M.; Cartwright, K.; Lumb, P.; Worsley, A. Serum Sialic Acid in Young Type-1 Diabetic Patients. Diabetes Res. Clin. Pract. 2000, 47, 119–122. [Google Scholar] [CrossRef]
- Moussa, M.A.A.; Alsaeid, M.; Refai, T.M.K.; Abdella, N.; Al-Sheikh, N.; Gomez, J.E. Association of Serum Sialic Acid with Cardiovascular Metabolic Risk Factors in Kuwaiti Children and Adolescents with Type 1 Diabetes. Metabolism 2004, 53, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Jensen, J.S.; Myrup, B.; Mathiesen, E.R.; Rønn, B.; Deckert, T. Raised Serum Sialic Acid Concentration Precedes Onset of Microalbuminuria in IDDM. A 10-Year Follow-up Study. Diabetes Care 1996, 19, 435–440. [Google Scholar] [CrossRef]
- Pickup, J.C.; Day, C.; Bailey, C.J.; Samuel, A.; Chusney, G.D.; Garland, H.O.; Hamilton, K.; Balment, R.J. Plasma Sialic Acid in Animal Models of Diabetes Mellitus: Evidence for Modulation of Sialic Acid Concentrations by Insulin Deficiency. Life Sci. 1995, 57, 1383–1391. [Google Scholar] [CrossRef]
- Tomofuji, Y.; Suzuki, K.; Kishikawa, T.; Shojima, N.; Hosoe, J.; Inagaki, K.; Matsubayashi, S.; Ishihara, H.; Watada, H.; Ishigaki, Y.; et al. Identification of Serum Metabolome Signatures Associated with Retinal and Renal Complications of Type 2 Diabetes. Commun. Med. 2023, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.; Kuhnle, G.; Stafford, G.; Gardner, R.A.; Spencer, D.I.; Osborn, H.M. Sialic Acid As A Potential Biomarker for Cardiovascular Disease, Diabetes and Cancer. Biomark. Med. 2021, 15, 911–928. [Google Scholar] [CrossRef]
- Schnaar, R.L.; Sandhoff, R.; Tiemeyer, M.; Kinoshita, T. Glycosphingolipids. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Wentworth, J.M.; Naselli, G.; Ngui, K.; Smyth, G.K.; Liu, R.; O’Brien, P.E.; Bruce, C.; Weir, J.; Cinel, M.; Meikle, P.J.; et al. GM3 Ganglioside and Phosphatidylethanolamine-Containing Lipids Are Adipose Tissue Markers of Insulin Resistance in Obese Women. Int. J. Obes. 2016, 40, 706–713. [Google Scholar] [CrossRef]
- Tagami, S.; Inokuchi, J.; Kabayama, K.; Yoshimura, H.; Kitamura, F.; Uemura, S.; Ogawa, C.; Ishii, A.; Saito, M.; Ohtsuka, Y.; et al. Ganglioside GM3 Participates in the Pathological Conditions of Insulin Resistance. J. Biol. Chem. 2002, 277, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, J.-I.; Kanoh, H. Pathophysiological Significance of GM3 Ganglioside Molecular Species with a Particular Attention to the Metabolic Syndrome Focusing on Toll-Like Receptor 4 Binding. Front. Mol. Biosci. 2022, 9, 918346. [Google Scholar] [CrossRef]
- Lipina, C.; Nardi, F.; Grace, H.; Hundal, H.S. NEU3 Sialidase as a Marker of Insulin Sensitivity: Regulation by Fatty Acids. Cell Signal 2015, 27, 1742–1750. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.L.; Thaysen-Andersen, M.; Fazakerley, D.J.; Holliday, M.; Packer, N.H.; James, D.E. Terminal Galactosylation and Sialylation Switching on Membrane Glycoproteins upon TNF-Alpha-Induced Insulin Resistance in Adipocytes. Mol. Cell Proteom. 2016, 15, 141–153. [Google Scholar] [CrossRef]
- Vattepu, R.; Sneed, S.L.; Anthony, R.M. Sialylation as an Important Regulator of Antibody Function. Front. Immunol. 2022, 13, 818736. [Google Scholar] [CrossRef]
- Thaçi, K.; Anthony, R.M. The Importance of IgG N-Glycosylation in Health, Disease, and Neonatal Hemochromatosis. Glycosci. Ther. 2025, 1, 100002. [Google Scholar] [CrossRef]
- Plomp, R.; Ruhaak, L.R.; Uh, H.-W.; Reiding, K.R.; Selman, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Beekman, M.; Wuhrer, M. Subclass-Specific IgG Glycosylation Is Associated with Markers of Inflammation and Metabolic Health. Sci. Rep. 2017, 7, 12325. [Google Scholar] [CrossRef]
- Russell, A.C.; Kepka, A.; Trbojević-Akmačić, I.; Ugrina, I.; Song, M.; Hui, J.; Hunter, M.; Laws, S.M.; Lauc, G.; Wang, W. Increased Central Adiposity Is Associated with Pro-Inflammatory Immunoglobulin G N-Glycans. Immunobiology 2019, 224, 110–115. [Google Scholar] [CrossRef]
- Tanigaki, K.; Sacharidou, A.; Peng, J.; Chambliss, K.L.; Yuhanna, I.S.; Ghosh, D.; Ahmed, M.; Szalai, A.J.; Vongpatanasin, W.; Mattrey, R.F.; et al. Hyposialylated IgG Activates Endothelial IgG Receptor FcγRIIB to Promote Obesity-Induced Insulin Resistance. J. Clin. Investig. 2018, 128, 309–322. [Google Scholar] [CrossRef]
- Šimunić-Briški, N.; Zekić, R.; Dukarić, V.; Očić, M.; Frkatović-Hodžić, A.; Deriš, H.; Lauc, G.; Knjaz, D. Physical Exercise Induces Significant Changes in Immunoglobulin G N-Glycan Composition in a Previously Inactive, Overweight Population. Biomolecules 2023, 13, 762. [Google Scholar] [CrossRef]
- Greto, V.L.; Cvetko, A.; Štambuk, T.; Dempster, N.J.; Kifer, D.; Deriš, H.; Cindrić, A.; Vučković, F.; Falchi, M.; Gillies, R.S.; et al. Extensive Weight Loss Reduces Glycan Age by Altering IgG N-Glycosylation. Int. J. Obes. 2021, 45, 1521–1531. [Google Scholar] [CrossRef]
- Deriš, H.; Tominac, P.; Vučković, F.; Briški, N.; Astrup, A.; Blaak, E.E.; Lauc, G.; Gudelj, I. Effects of Low-Calorie and Different Weight-Maintenance Diets on IgG Glycome Composition. Front. Immunol. 2022, 13, 995186. [Google Scholar] [CrossRef]
- Tijardović, M.; Marijančević, D.; Bok, D.; Kifer, D.; Lauc, G.; Gornik, O.; Keser, T. Intense Physical Exercise Induces an Anti-Inflammatory Change in IgG N-Glycosylation Profile. Front. Physiol. 2019, 10, 1522. [Google Scholar] [CrossRef]
- Sarin, H.V.; Gudelj, I.; Honkanen, J.; Ihalainen, J.K.; Vuorela, A.; Lee, J.H.; Jin, Z.; Terwilliger, J.D.; Isola, V.; Ahtiainen, J.P.; et al. Molecular Pathways Mediating Immunosuppression in Response to Prolonged Intensive Physical Training, Low-Energy Availability, and Intensive Weight Loss. Front. Immunol. 2019, 10, 907. [Google Scholar] [CrossRef]
- Kaburagi, T.; Kizuka, Y.; Kitazume, S.; Taniguchi, N. The Inhibitory Role of A2,6-Sialylation in Adipogenesis. J. Biol. Chem. 2017, 292, 2278–2286. [Google Scholar] [CrossRef]
- Grassot, V.; Bouchatal, A.; Da Silva, A.; Chantepie, S.; Papy-Garcia, D.; Maftah, A.; Gallet, P.-F.; Petit, J.-M. Heparan Sulfates and the Decrease of N-Glycans Promote Early Adipogenic Differentiation Rather than Myogenesis of Murine Myogenic Progenitor Cells. Differentiation 2017, 93, 15–26. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Liu, W.; Wang, Y.; Rao, Y.; Yu, H.; Cui, J.; Xie, X.; Sun, M.; Yin, L.; et al. Alterations of Protein Glycosylation in Embryonic Stem Cells during Adipogenesis. Int. J. Mol. Med. 2018, 41, 293–301. [Google Scholar] [CrossRef]
- Lipničanová, S.; Chmelová, D.; Ondrejovič, M.; Frecer, V.; Miertuš, S. Diversity of Sialidases Found in the Human Body—A Review. Int. J. Biol. Macromol. 2020, 148, 857–868. [Google Scholar] [CrossRef]
- Bourguet, E.; Figurska, S.; Fraączek, M.M. Human Neuraminidases: Structures and Stereoselective Inhibitors. J. Med. Chem. 2022, 65, 3002–3025. [Google Scholar] [CrossRef]
- Bonten, E.J.; Campos, Y.; Zaitsev, V.; Nourse, A.; Waddell, B.; Lewis, W.; Taylor, G.; d’Azzo, A. Heterodimerization of the Sialidase NEU1 with the Chaperone Protective Protein/Cathepsin A Prevents Its Premature Oligomerization. J. Biol. Chem. 2009, 284, 28430–28441. [Google Scholar] [CrossRef]
- Toussaint, K.; Appert-Collin, A.; Morjani, H.; Albrecht, C.; Sartelet, H.; Romier-Crouzet, B.; Maurice, P.; Duca, L.; Blaise, S.; Bennasroune, A. Neuraminidase-1: A Sialidase Involved in the Development of Cancers and Metabolic Diseases. Cancers 2022, 14, 4868. [Google Scholar] [CrossRef]
- Natori, Y.; Ohkura, N.; Nasui, M.; Atsumi, G.; Kihara-Negishi, F. Acidic Sialidase Activity Is Aberrant in Obese and Diabetic Mice. Biol. Pharm. Bull. 2013, 36, 1027–1031. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, H.; Shi, L.-X. MicroRNA-205 Ameliorates Lipid Accumulation in Non-Alcoholic Fatty Liver Disease through Targeting NEU1. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10072–10082. [Google Scholar] [CrossRef]
- Dridi, L.; Seyrantepe, V.; Fougerat, A.; Pan, X.; Bonneil, E.; Thibault, P.; Moreau, A.; Mitchell, G.A.; Heveker, N.; Cairo, C.W.; et al. Positive Regulation of Insulin Signaling by Neuraminidase 1. Diabetes 2013, 62, 2338–2346. [Google Scholar] [CrossRef]
- Fougerat, A.; Pan, X.; Smutova, V.; Heveker, N.; Cairo, C.W.; Issad, T.; Larrivée, B.; Medin, J.A.; Pshezhetsky, A.V. Neuraminidase 1 Activates Insulin Receptor and Reverses Insulin Resistance in Obese Mice. Mol. Metab. 2018, 12, 76–88. [Google Scholar] [CrossRef]
- Arabkhari, M.; Bunda, S.; Wang, Y.; Wang, A.; Pshezhetsky, A.V.; Hinek, A. Desialylation of Insulin Receptors and IGF-1 Receptors by Neuraminidase-1 Controls the Net Proliferative Response of L6 Myoblasts to Insulin. Glycobiology 2010, 20, 603–616. [Google Scholar] [CrossRef]
- Blaise, S.; Romier, B.; Kawecki, C.; Ghirardi, M.; Rabenoelina, F.; Baud, S.; Duca, L.; Maurice, P.; Heinz, A.; Schmelzer, C.E.H.; et al. Elastin-Derived Peptides Are New Regulators of Insulin Resistance Development in Mice. Diabetes 2013, 62, 3807–3816. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Ganglioside GM3 as a Gatekeeper of Obesity-Associated Insulin Resistance: Evidence and Mechanisms. FEBS Lett. 2015, 589, 3221–3227. [Google Scholar] [CrossRef]
- Sasaki, A.; Hata, K.; Suzuki, S.; Sawada, M.; Wada, T.; Yamaguchi, K.; Obinata, M.; Tateno, H.; Suzuki, H.; Miyagi, T. Overexpression of Plasma Membrane-Associated Sialidase Attenuates Insulin Signaling in Transgenic Mice. J. Biol. Chem. 2003, 278, 27896–27902. [Google Scholar] [CrossRef]
- Yoshizumi, S.; Suzuki, S.; Hirai, M.; Hinokio, Y.; Yamada, T.; Yamada, T.; Tsunoda, U.; Aburatani, H.; Yamaguchi, K.; Miyagi, T.; et al. Increased Hepatic Expression of Ganglioside-Specific Sialidase, NEU3, Improves Insulin Sensitivity and Glucose Tolerance in Mice. Metabolism 2007, 56, 420–429. [Google Scholar] [CrossRef]
- Minami, A.; Fujita, Y.; Shimba, S.; Shiratori, M.; Kaneko, Y.K.; Sawatani, T.; Otsubo, T.; Ikeda, K.; Kanazawa, H.; Mikami, Y.; et al. The Sialidase Inhibitor 2,3-Dehydro-2-Deoxy-N-Acetylneuraminic Acid Is a Glucose-Dependent Potentiator of Insulin Secretion. Sci. Rep. 2020, 10, 5198. [Google Scholar] [CrossRef]
- Pilling, D.; Martinez, T.C.; Gomer, R.H. Inhibition of CCl4-Induced Liver Inflammation and Fibrosis by a NEU3 Inhibitor. PLoS ONE 2024, 19, e0308060. [Google Scholar] [CrossRef]
- Xie, C.; Yagai, T.; Luo, Y.; Liang, X.; Chen, T.; Wang, Q.; Sun, D.; Zhao, J.; Ramakrishnan, S.K.; Sun, L.; et al. Activation of Intestinal Hypoxia-Inducible Factor 2α during Obesity Contributes to Hepatic Steatosis. Nat. Med. 2017, 23, 1298–1308. [Google Scholar] [CrossRef]
- Yang, W.H.; Aziz, P.V.; Heithoff, D.M.; Kim, Y.; Ko, J.Y.; Cho, J.W.; Mahan, M.J.; Sperandio, M.; Marth, J.D. Innate Mechanism of Mucosal Barrier Erosion in the Pathogenesis of Acquired Colitis. iScience 2023, 26, 107883. [Google Scholar] [CrossRef]
- Yang, W.H.; Westman, J.S.; Heithoff, D.M.; Sperandio, M.; Cho, J.W.; Mahan, M.J.; Marth, J.D. Neu3 Neuraminidase Induction Triggers Intestinal Inflammation and Colitis in a Model of Recurrent Human Food-Poisoning. Proc. Natl. Acad. Sci. USA 2021, 118, e2100937118. [Google Scholar] [CrossRef]
- Oh, M.; Ha, D.-I.; Son, C.; Kang, J.G.; Hwang, H.; Moon, S.B.; Kim, M.; Nam, J.; Kim, J.S.; Song, S.Y.; et al. Defect in Cytosolic Neu2 Sialidase Abrogates Lipid Metabolism and Impairs Muscle Function in Vivo. Sci. Rep. 2022, 12, 3216. [Google Scholar] [CrossRef]
- Glanz, V.Y.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Sialidase Activity in Human Pathologies. Eur. J. Pharmacol. 2019, 842, 345–350. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X. Sialic Acid Metabolism and Sialyltransferases: Natural Functions and Applications. Appl. Microbiol. Biotechnol. 2012, 94, 887–905. [Google Scholar] [CrossRef]
- Jones, M.B.; Oswald, D.M.; Joshi, S.; Whiteheart, S.W.; Orlando, R.; Cobb, B.A. B-Cell-Independent Sialylation of IgG. Proc. Natl. Acad. Sci. USA 2016, 113, 7207–7212. [Google Scholar] [CrossRef]
- Oswald, D.M.; Jones, M.B.; Cobb, B.A. Modulation of Hepatocyte Sialylation Drives Spontaneous Fatty Liver Disease and Inflammation. Glycobiology 2020, 30, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.H.; Aja, S.; Aoki, K.; Seldin, M.M.; Lei, X.; Ronnett, G.V.; Wong, G.W.; Schnaar, R.L. Mice Lacking Sialyltransferase ST3Gal-II Develop Late-Onset Obesity and Insulin Resistance. Glycobiology 2017, 27, 129–139. [Google Scholar] [CrossRef]
- Inokuchi, J.; Kanoh, H.; Inamori, K.; Nagafuku, M.; Nitta, T.; Fukase, K. Homeostatic and Pathogenic Roles of the GM3 Ganglioside. FEBS J. 2022, 289, 5152–5165. [Google Scholar] [CrossRef]
- Inamori, K.; Ito, H.; Tamura, Y.; Nitta, T.; Yang, X.; Nihei, W.; Shishido, F.; Imazu, S.; Tsukita, S.; Yamada, T.; et al. Deficient Ganglioside Synthesis Restores Responsiveness to Leptin and Melanocortin Signaling in Obese KKAy Mice. J. Lipid Res. 2018, 59, 1472–1481. [Google Scholar] [CrossRef]
- Strekalova, T.; Veniaminova, E.; Svirin, E.; Kopeikina, E.; Veremeyko, T.; Yung, A.W.Y.; Proshin, A.; Tan, S.Z.K.; Khairuddin, S.; Lim, L.W.; et al. Sex-Specific ADHD-like Behaviour, Altered Metabolic Functions, and Altered EEG Activity in Sialyltransferase ST3GAL5-Deficient Mice. Biomolecules 2021, 11, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Lee, S.; Wilson, H.; Seeger, M.; Iordanov, H.; Gatla, N.; Whittington, A.; Bach, D.; Lu, J.; Paller, A.S. Ganglioside GM3 Depletion Reverses Impaired Wound Healing in Diabetic Mice by Activating IGF-1 and Insulin Receptors. J. Investig. Dermatol. 2014, 134, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Randeria, P.S.; Seeger, M.A.; Wang, X.-Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. siRNA-Based Spherical Nucleic Acids Reverse Impaired Wound Healing in Diabetic Mice by Ganglioside GM3 Synthase Knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef]
- Choe, J.; Belmonte, P.; Crotts, S.; Nguyen, T.; Friedman, D.; Zastrow, A.; Rajcula, M.; Hammer, B.; Wilhelm, C.; Shapiro, M.J.; et al. ST8Sia6 Overexpression Protects Pancreatic β Cells from Spontaneous Autoimmune Diabetes in Nonobese Diabetic Mice. J. Clin. Investig. 2025, 135, e181207. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, P.J.; Shapiro, M.J.; Rajcula, M.J.; McCue, S.A.; Shapiro, V.S. Cutting Edge: ST8Sia6-Generated α-2,8-Disialic Acids Mitigate Hyperglycemia in Multiple Low-Dose Streptozotocin-Induced Diabetes. J. Immunol. 2020, 204, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, C.; Schug, J.; Canaday, P.S.; Russ, H.A.; Tarlow, B.D.; Grompe, M.T.; Horton, T.; Hebrok, M.; Streeter, P.R.; Kaestner, K.H.; et al. Human Islets Contain Four Distinct Subtypes of β Cells. Nat. Commun. 2016, 7, 11756. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Herrera, M.T.; Soldevila, G.; Garcia-Garcia, L.; Fabián, G.; Pérez-Armendariz, E.M.; Bobadilla, K.; Guzmán-Beltrán, S.; Sada, E.; Torres, M. High Glucose Concentrations Induce TNF-α Production through the down-Regulation of CD33 in Primary Human Monocytes. BMC Immunol. 2012, 13, 19. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-Induced Obesity Accelerates Oral Carcinogenesis by Recruitment and Functional Enhancement of Myeloid-Derived Suppressor Cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Li, S.; Zhao, J.; Meng, X.; Ma, L.; Wang, Y.; Li, C.; Zheng, L.; Ming, L. Obesity Accelerates Immune Evasion of Non-Small Cell Lung Carcinoma via TFEB-Dependent Upregulation of Siglec-15 and Glycolytic Reprogramming. Cancer Lett. 2022, 550, 215918. [Google Scholar] [CrossRef]
- Naujoks, W.; Quandt, D.; Hauffe, A.; Kielstein, H.; Bähr, I.; Spielmann, J. Characterization of Surface Receptor Expression and Cytotoxicity of Human NK Cells and NK Cell Subsets in Overweight and Obese Humans. Front. Immunol. 2020, 11, 573200. [Google Scholar] [CrossRef]
- Rosenstock, P.; Horstkorte, R.; Gnanapragassam, V.S.; Harth, J.; Kielstein, H. Siglec-7 Expression Is Reduced on a Natural Killer (NK) Cell Subset of Obese Humans. Immunol. Res. 2017, 65, 1017–1024. [Google Scholar] [CrossRef]
- Dharmadhikari, G.; Stolz, K.; Hauke, M.; Morgan, N.G.; Varki, A.; De Koning, E.; Kelm, S.; Maedler, K. Siglec-7 Restores β-Cell Function and Survival and Reduces Inflammation in Pancreatic Islets from Patients with Diabetes. Sci. Rep. 2017, 7, 45319. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Li, J.; Nie, L.; Hu, Y.; Wang, F.; Liu, H.; Fernandes, S.M.; Zhong, Q.; Li, X.; et al. Immunoregulatory Siglec Ligands Are Abundant in Human and Mouse Aorta and Are Up-Regulated by High Glucose. Life Sci. 2019, 216, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Mandal, M.; Al Mamun, M.A.; Kiran, S.; Yasmen, N.; Li, L.; Collier, D.M.; Jiang, J.; Park, F.; Singh, U.P. Siglec-E Augments Adipose Tissue Inflammation by Modulating TRAF3 Signaling and Monocytic Myeloid-Derived Suppressor Cells during Obesity. Front. Immunol. 2025, 16, 1501307. [Google Scholar] [CrossRef]
- Wang, X.; Liu, M.; Zhang, J.; Brown, N.K.; Zhang, P.; Zhang, Y.; Liu, H.; Du, X.; Wu, W.; Devenport, M.; et al. CD24-Siglec Axis Is an Innate Immune Checkpoint against Metaflammation and Metabolic Disorder. Cell Metab. 2022, 34, 1088–1103.e6. [Google Scholar] [CrossRef]
- Guo, M.; Guo, H.; Zhu, J.; Wang, F.; Chen, J.; Wan, C.; Deng, Y.; Wang, F.; Xu, L.; Chen, Y.; et al. A Novel Subpopulation of Monocytes with a Strong Interferon Signature Indicated by SIGLEC-1 Is Present in Patients with in Recent-Onset Type 1 Diabetes. Diabetologia 2024, 67, 623–640. [Google Scholar] [CrossRef]
- Jia, X.; Bai, X.; Yin, Z.; Zheng, Q.; Zhao, Y.; Lu, Y.; Shu, Y.; Wang, Y.; Zhang, Y.; Jin, S. Siglec-5 as a Novel Receptor Mediates Endothelial Cells oxLDL Transcytosis to Promote Atherosclerosis. Transl. Res. 2024, 274, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, X.; Wang, X.; Jia, X.; Wang, Z.; Deng, A.; Bai, X.; Zhu, L.; Li, B.; Feng, Z.; et al. Siglec-5 Is a Novel Marker of Critical Limb Ischemia in Patients with Diabetes. Sci. Rep. 2017, 7, 11272. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Ideris, A.; Abdullah, M.A. High Fat Diet-Induced Inflammation and Oxidative Stress Are Attenuated by N-Acetylneuraminic Acid in Rats. J. Biomed. Sci. 2015, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Kaburagi, T.; Otsuka, Y.; Oshiro, S. Antiobesity Effect of N-Acetylneuraminic Acid by Enhancing Antioxidative Capacity in Mice Fed a High-Fat Diet. J. Med. Food 2023, 26, 550–559. [Google Scholar] [CrossRef]
- Peng, J.; Vongpatanasin, W.; Sacharidou, A.; Kifer, D.; Yuhanna, I.S.; Banerjee, S.; Tanigaki, K.; Polasek, O.; Chu, H.; Sundgren, N.C.; et al. Supplementation with the Sialic Acid Precursor N-Acetyl-D-Mannosamine Breaks the Link Between Obesity and Hypertension. Circulation 2019, 140, 2005–2018. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, J.; Wang, Y.; Chen, Y.; Jiang, L. A Comprehensive Review of Edible Bird’s Nest. Food Res. Int. 2021, 140, 109875. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, M.; Liu, X.; Que, M.; Dekyi, K.; Zheng, L.; Zhang, Y.; Lv, Y.; Fan, Q.; Wang, X.; et al. Edible Bird’s Nest Regulates Glucose and Lipid Metabolic Disorders via the Gut-Liver Axis in Obese Mice. Food Funct. 2024, 15, 7577–7591. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.D.; Md Zain, Z.; Choy, K.W.; Zamakshshari, N.H.; Choong, M.J.; Lim, Y.M.; Mustafa, M.R. Edible Bird’s Nest Protects Against Hyperglycemia-Induced Oxidative Stress and Endothelial Dysfunction. Front. Pharmacol. 2020, 10, 1624. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Hou, Z.; Abdullah, M.A.; Ideris, A.; Ismail, N. Edible Bird’s Nest Attenuates High Fat Diet-Induced Oxidative Stress and Inflammation via Regulation of Hepatic Antioxidant and Inflammatory Genes. BMC Complement. Altern. Med. 2015, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Pilling, D.; Karhadkar, T.R.; Gomer, R.H. High-Fat Diet-Induced Adipose Tissue and Liver Inflammation and Steatosis in Mice Are Reduced by Inhibiting Sialidases. Am. J. Pathol. 2021, 191, 131–143. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Li, H.; Wang, Q.; Wang, P.G.; Ji, Y. The Emerging Role of Sialic Acids in Obesity and Diabetes: Molecular Mechanisms and Therapeutic Perspectives. Biomolecules 2025, 15, 1522. https://doi.org/10.3390/biom15111522
Peng X, Li H, Wang Q, Wang PG, Ji Y. The Emerging Role of Sialic Acids in Obesity and Diabetes: Molecular Mechanisms and Therapeutic Perspectives. Biomolecules. 2025; 15(11):1522. https://doi.org/10.3390/biom15111522
Chicago/Turabian StylePeng, Xinyi, Haojun Li, Qingwen Wang, Peng George Wang, and Yang Ji. 2025. "The Emerging Role of Sialic Acids in Obesity and Diabetes: Molecular Mechanisms and Therapeutic Perspectives" Biomolecules 15, no. 11: 1522. https://doi.org/10.3390/biom15111522
APA StylePeng, X., Li, H., Wang, Q., Wang, P. G., & Ji, Y. (2025). The Emerging Role of Sialic Acids in Obesity and Diabetes: Molecular Mechanisms and Therapeutic Perspectives. Biomolecules, 15(11), 1522. https://doi.org/10.3390/biom15111522






