Structural and Functional Characterization of the Vacuolar-Type Na+, K+/H+ Antiporter NHX1 from Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain, Medium, and Plasmids
2.2. Protein Structure Prediction
2.3. Evolutionary Conservation Analysis and Ion–Protein Interaction Analysis
2.4. Site-Directed Mutagenesis and Transformation of Yeast
2.5. Preparation of Yeast Microsomal Membranes and Western Blotting
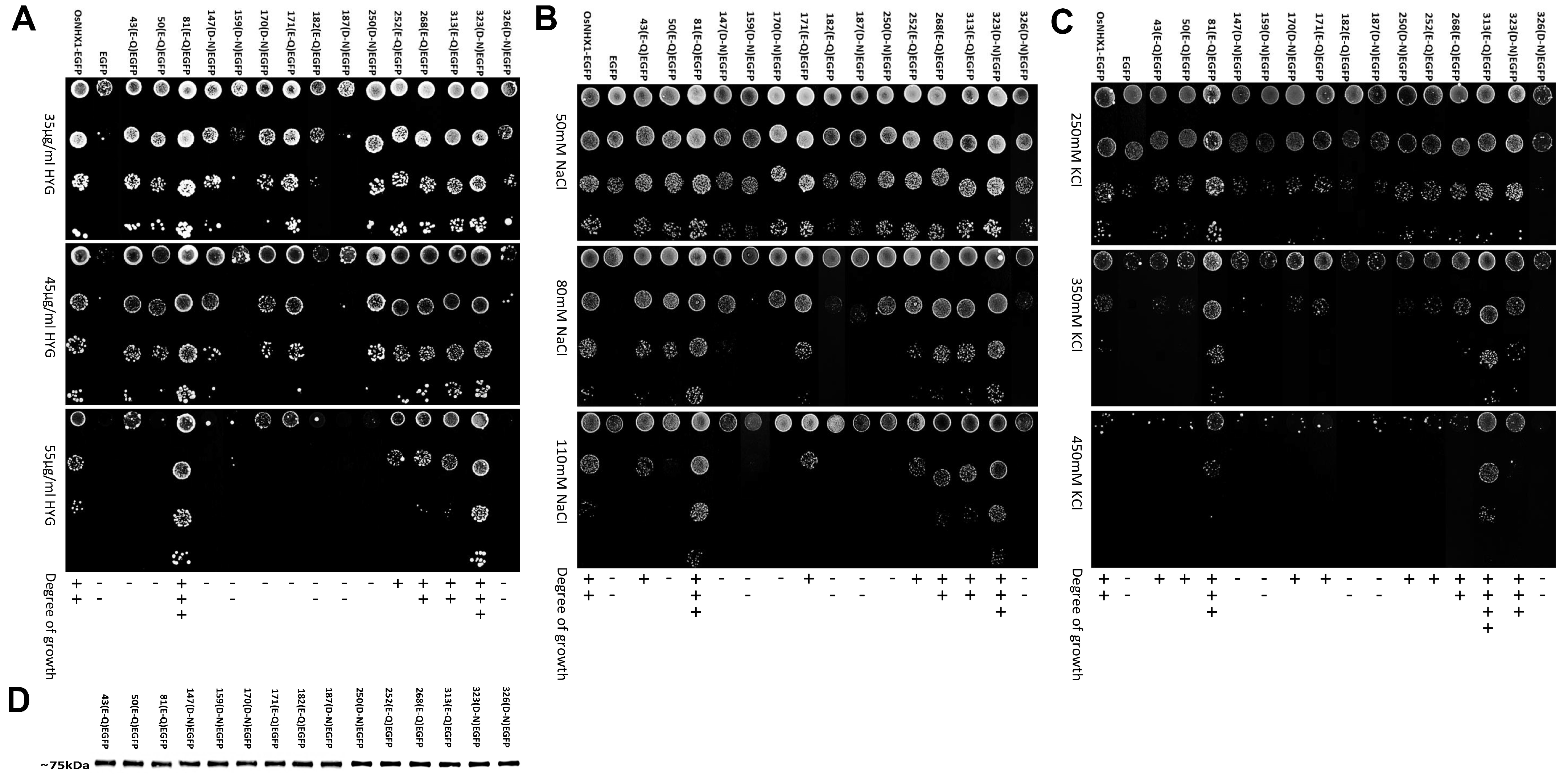
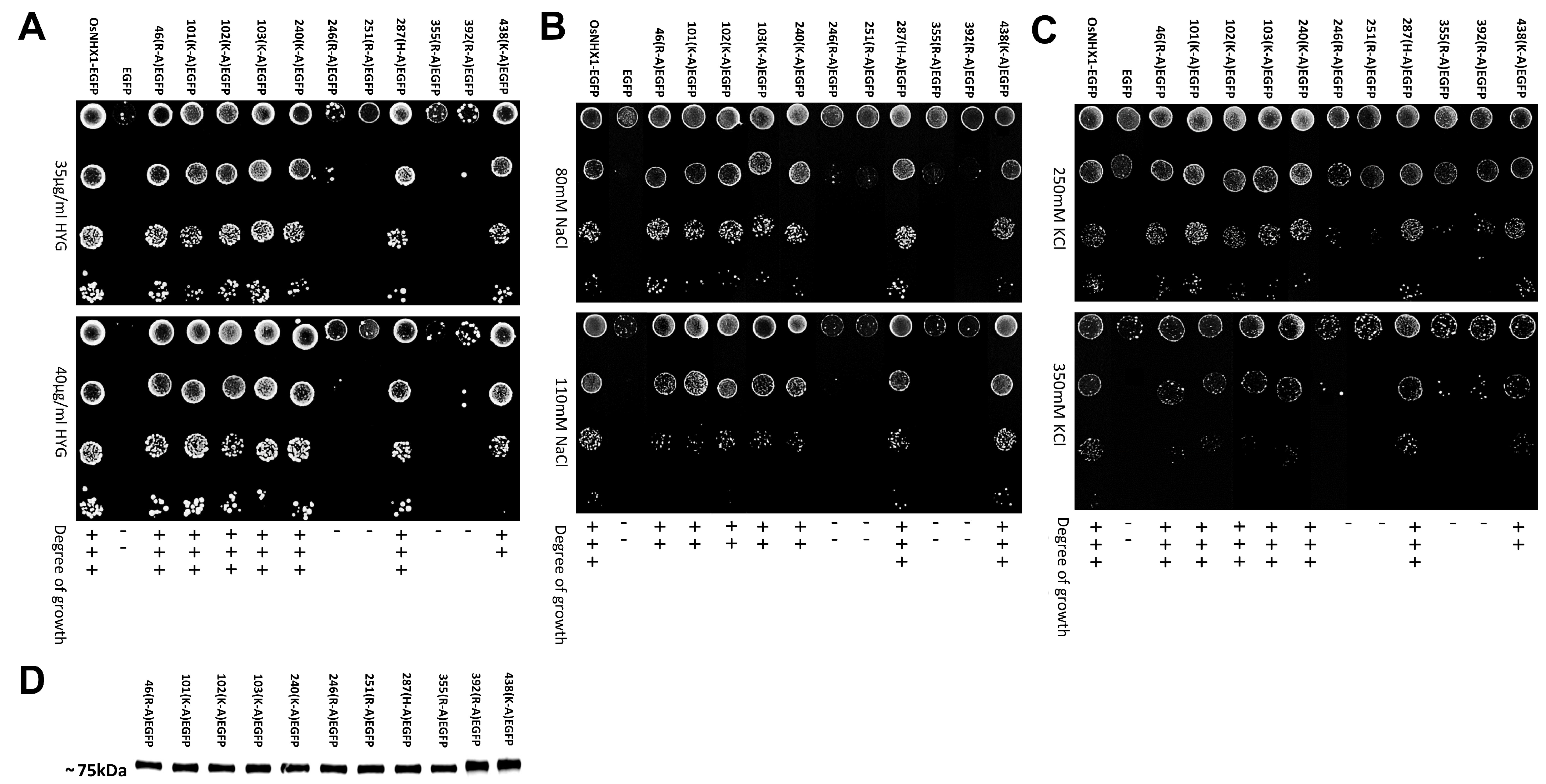
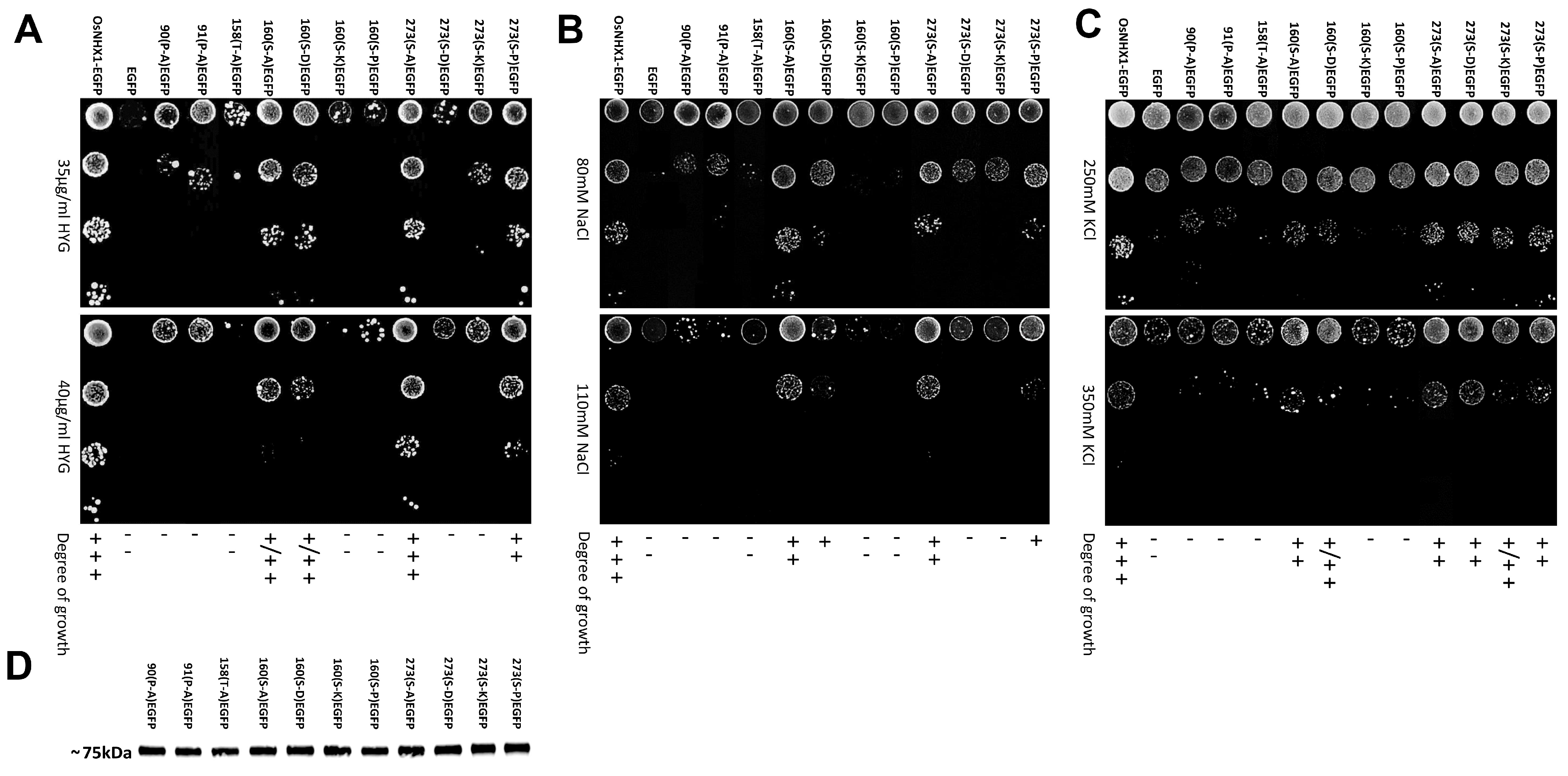
2.6. Mutant Functional Assays
3. Results and Discussion
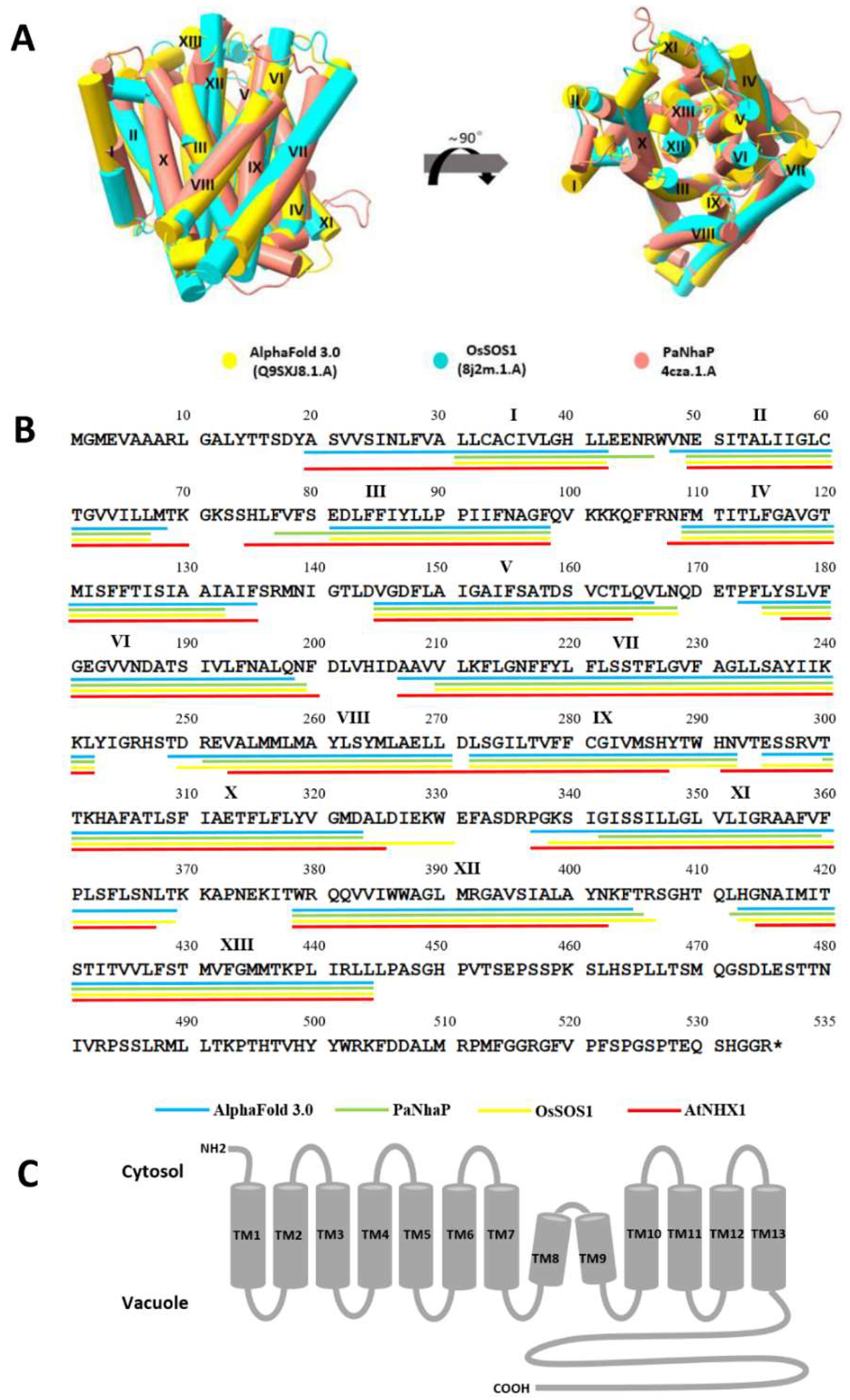
3.1. The 3D Model of OsNHX1 Shares a Typical “Funnel” Fold
3.2. The Predicted Topology of OsNHX1 Contains 13 Transmembrane Segments
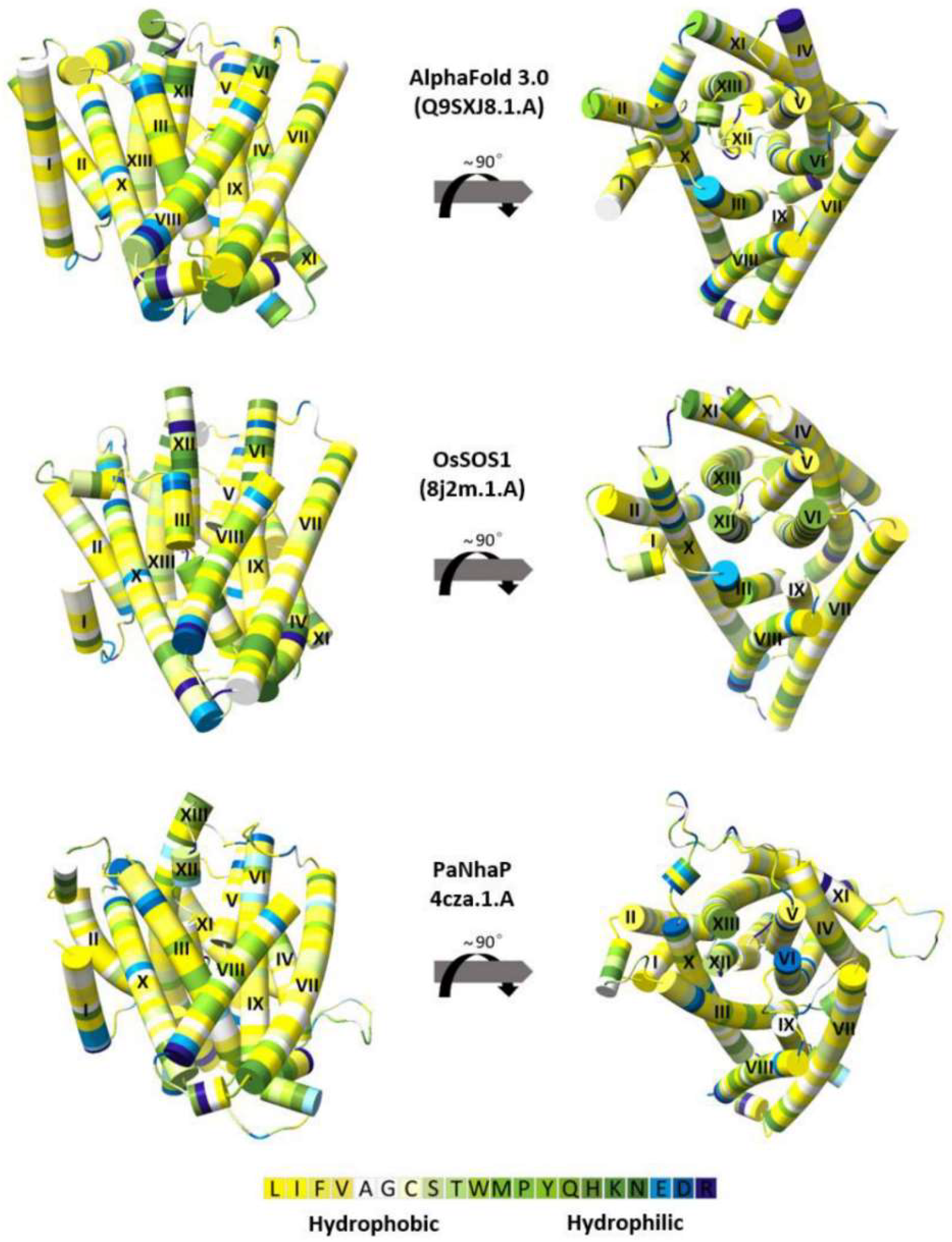
3.3. The OsNHX1 Model Structure Is Consistent with Hydrophobic Characteristics, the “Positive-Inside” Rule, and Evolutionary Conservation
3.4. Experimental Validation of the OsNHX1 Model by Structure-Guided Design of Mutations
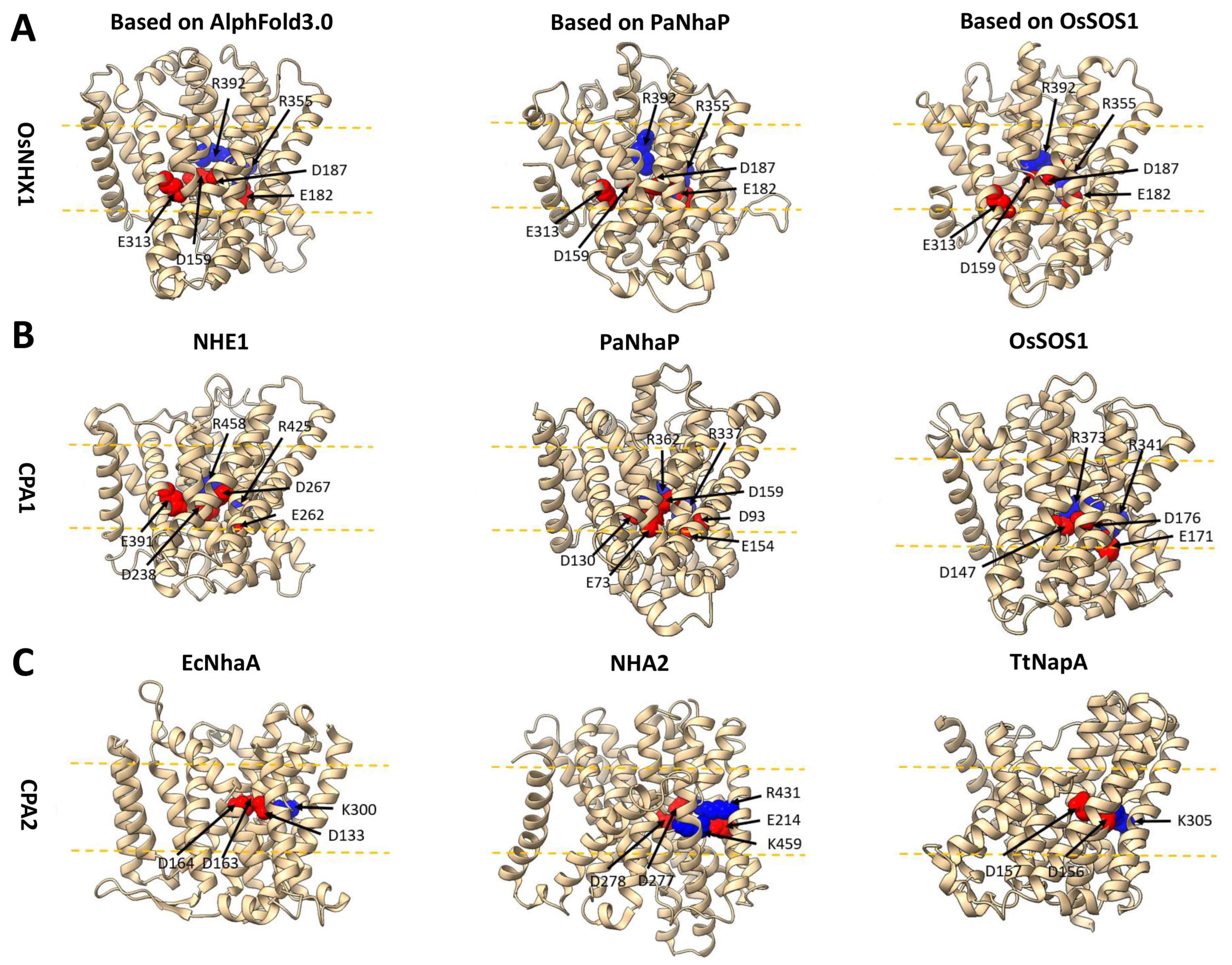
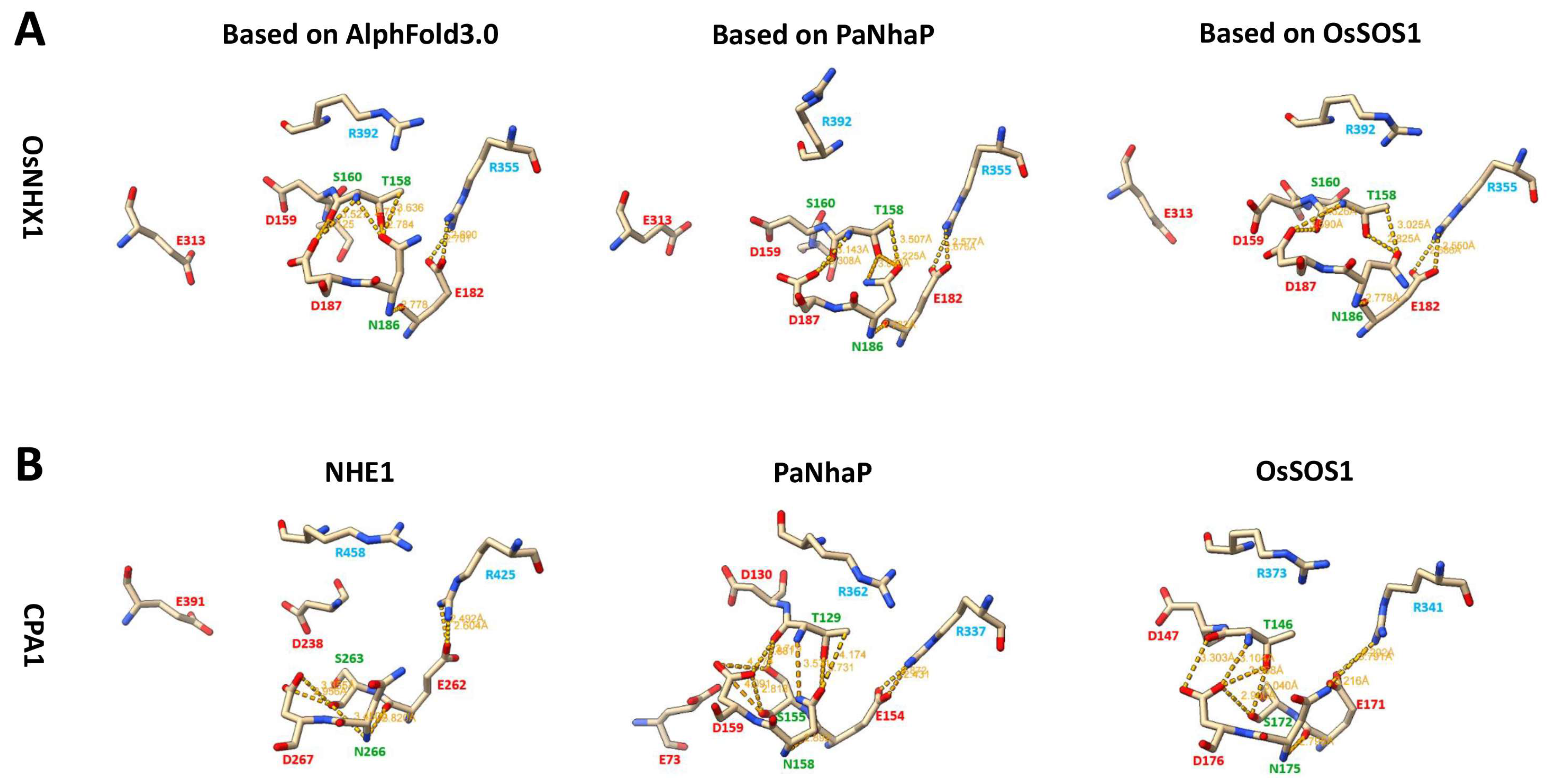
3.5. OsNHX1 Shares a Similar Transmembrane Charge-Compensated Pattern with Nhe1
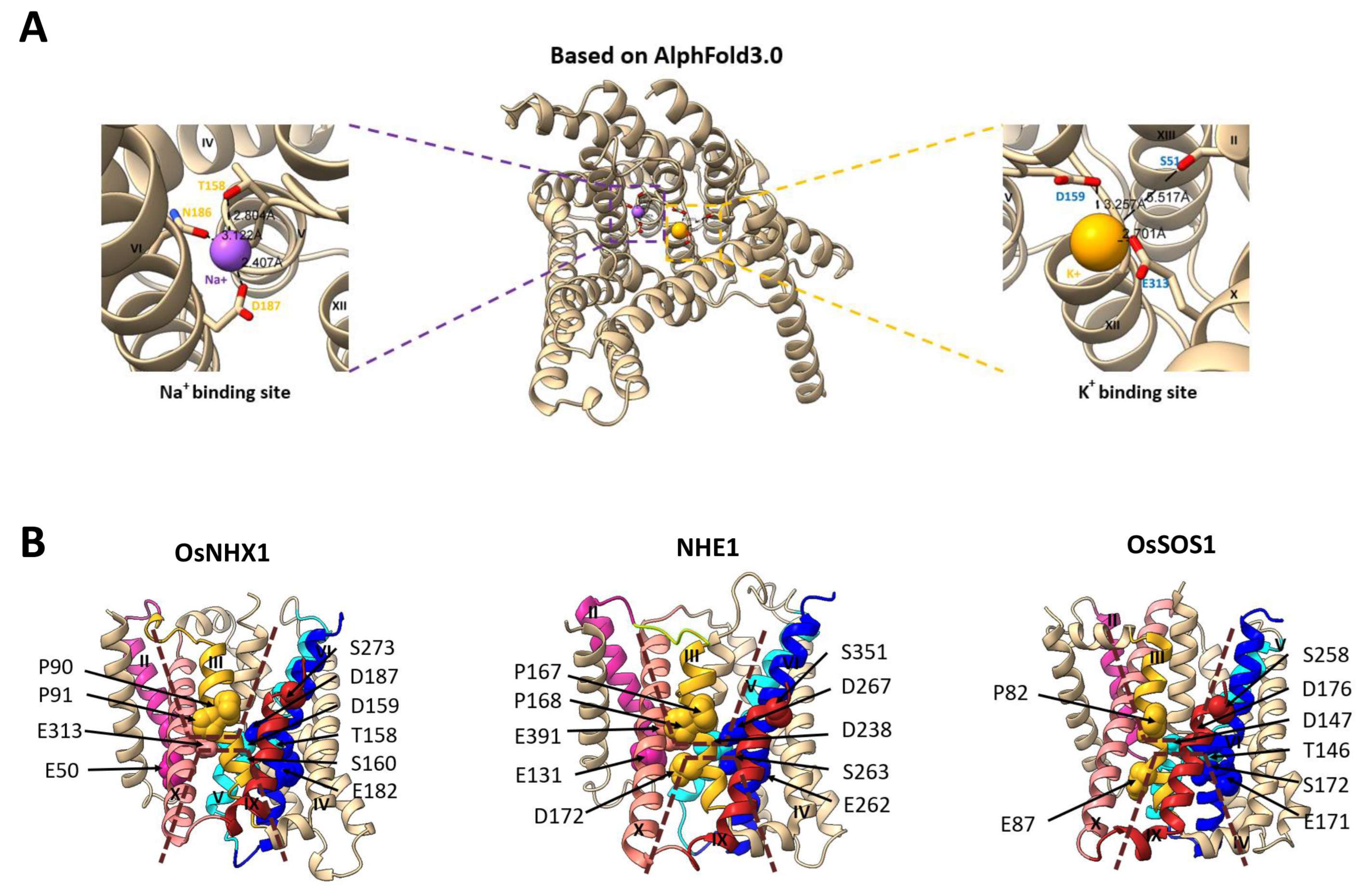
3.6. OsNHX1 Contains a Unique Charge Distribution on Both Sides of the Pore Domain
3.7. The Vacuolar-Type OsNHX1 Exhibits a Distinct Ion Transport Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.L.; Tukaye, D.N.; Mukherjee, S.; Rao, R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol. Biol. Cell 2005, 16, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Chanroj, S.; Wang, G.; Venema, K.; Zhang, M.W.; Delwiche, C.F.; Sze, H. Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front. Plant Sci. 2012, 3, 25. [Google Scholar] [CrossRef]
- Chanroj, S.; Padmanaban, S.; Czerny, D.D.; Jauh, G.Y.; Sze, H. K+ transporter AtCHX17 with its hydrophilic C tail localizes to membranes of the secretory/endocytic system: Role in reproduction and seed set. Mol. Plant 2013, 6, 1226–1246. [Google Scholar] [CrossRef]
- Mäser, P.; Sébastien, T.; Schroeder, J.I.; Ward, J.M.; Hirschi, K.; Sze, H.; Talke, I.N.; Amtmann, A.; Maathuis, F.J.M.; Sanders, D.; et al. Phylogenetic Relationships within Cation Transporter Families of Arabidopsis. Plant Physiol. 2001, 126, 1646–1667. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 354–411. [Google Scholar] [CrossRef]
- Apse, M.P.; Blumwald, E. Na+ transport in plants. FEBS Lett. 2007, 581, 2247–2254. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernandez, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef]
- Bassil, E.; Zhang, S.; Gong, H.; Tajima, H.; Blumwald, E. Cation specificity of vacuolar NHX-type cation/H+ antiporters. Plant Physiol. 2019, 179, 616–629. [Google Scholar] [CrossRef]
- Bassil, E.; Ohto, M.A.; Esumi, T.; Tajima, H.; Zhu, Z.; Cagnac, O.; Belmonte, M.; Peleg, Z.; Yamaguchi, T.; Blumwald, E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 2011, 23, 224. [Google Scholar] [CrossRef]
- Ashnest, J.R.; Huynh, D.L.; Dragwidge, J.M.; Ford, B.A.; Gendall, A.R. Arabidopsis intracellular NHX-type sodium-proton antiporters are required for seed storage protein processing. Plant Cell Physiol. 2015, 56, 2220–2233. [Google Scholar] [CrossRef] [PubMed]
- Reguera, M.; Bassil, E.; Tajima, H.; Wimmer, M.; Chanoca, A.; Otegui, M.S.; Paris, N.; Blumwald, E. pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. Plant Cell 2015, 27, 1200. [Google Scholar] [CrossRef]
- Dragwidge, J.M.; Ford, B.A.; Ashnest, J.R.; Das, P.; Gendall, A.R. Two endosomal NHX-type Na+/H+ antiporters are involved in auxin-mediated development in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1660–1669. [Google Scholar] [CrossRef]
- Quintero, F.J.; Pardo, J.M. Activation of the plasma membrane Na+/H+ antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl. Acad. Sci. USA 2011, 108, 2611–2616. [Google Scholar] [CrossRef]
- An, R.; Chen, Q.J.; Chai, M.F.; Lu, P.L.; Su, Z.; Qin, Z.X.; Chen, J.; Wang, X.C. AtNHX8, a member of the monovalent cation: Proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J. 2007, 49, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Nakamura, A.; Hara, N.; Toki, S.; Tanaka, Y. Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta 2011, 233, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Apse, M.P.; Shi, H.; Blumwald, E. Topological analysis of a plant vacuolar Na+/H+ antiporter reveals a luminal C terminus that regulates antiporter cation selectivity. Proc. Natl. Acad. Sci. USA 2003, 100, 12510–12515. [Google Scholar] [CrossRef]
- Slepkov, E.R.; Rainey, J.K.; Sykes, B.D.; Fliegel, L. Structural and functional analysis of the Na+/H+ exchanger. Biochem. J. 2007, 401, 623–633. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Zhao, H.; Wang, L.; An, L.; Qiu, Q.S. Functional analysis of the Na+, K+/H+ antiporter PeNHX3 from the tree halophyte Populus euphratica in yeast by model-guided mutagenesis. PLoS ONE 2014, 9, e104147. [Google Scholar] [CrossRef]
- Hunte, C.; Screpanti, E.; Venturi, M.; Rimon, A.; Padan, E.; Michel, H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature 2005, 435, 1197–1202. [Google Scholar] [CrossRef]
- Winkelmann, I.; Uzdavinys, P.; Kenney, I.M.; Brock, J.; Meier, P.F.; Wagner, L.M.; Gabriel, F.; Jung, S.; Matsuoka, R.; Ballmoos, C.V.; et al. Crystal structure of the Na+/H+ antiporter NhaA at active pH reveals the mechanistic basis for pH sensing. Nat. Commun. 2022, 13, 6383. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kang, H.J.; Von Ballmoos, C.; Newstead, S.; Uzdavinys, P.; Dotson, D.L.; Iwata, S.; Beckstein, O.; Cameron, A.D.; Drew, D. A two-domain elevator mechanism for sodium/proton antiport. Nature 2013, 501, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Paulino, C.; Wöhlert, D.; Kapotova, E.; Yildiz, Ö.; Kühlbrandt, W. Structure and transport mechanism of the sodium/proton antiporter MjNhaP1. eLife 2014, 3, e03583. [Google Scholar] [CrossRef]
- Wöhlert, D.; Kühlbrandt, W.; Yildiz, Ö. Structure and substrate ion binding in the sodium/proton antiporter PaNhaP. eLife 2014, 3, e03579. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.; Fudim, R.; Jung, S.; Zhang, C.; Bazzone, A.; Chatzikyriakidou, Y.; Robinson, C.V.; Nomura, N.; Iwata, S.; Landreh, M.; et al. Structure, mechanism and lipid-mediated remodeling of the mammalian Na+/H+ exchanger NHA2. Nat. Struct. Mol. Biol. 2022, 29, 108–120. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Y.; Ilie, A.; Kim, D.; Boucher, A.; Li, B.; Zhang, X.C.; Orlowski, J.; Zhao, Y. Structure and mechanism of the human NHE1-CHP1 complex. Nat. Commun. 2021, 12, 3474. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chen, Q.; Xie, Q.; Gao, Y.; He, L.; Dong, Y.; Jiang, X.; Zhao, Y. Architecture and autoinhibitory mechanism of the plasma membrane Na+/H+ antiporter SOS1 in Arabidopsis. Nat. Commun. 2023, 14, 4487. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, L.H.; Nie, J.W.; Zhang, C.R.; Han, X.; Li, Q.Y.; Qin, L.; Wang, M.H.; Huang, X.H.; Yu, F.F.; et al. Structure and activation mechanism of the rice Salt Overly Sensitive 1 (SOS1) Na+/H+ antiporter. Nat. Plants 2023, 9, 1924–1936. [Google Scholar] [CrossRef]
- Sato, Y.; Sakaguchi, M. Topogenic properties of transmembrane segments of Arabidopsis thaliana NHX1 reveal a common topology model of the Na+/H+ exchanger family. J. Biochem. 2005, 138, 425–431. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef]
- Atsunori, F.; Atsuko, N.; Akemi, T.; Hiroshi, T.; Akio, M.; Hirohiko, H.; Yoshiyuki, T. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004, 45, 146–159. [Google Scholar] [CrossRef]
- Fukuda, A.; Nakamura, A.; Tanaka, Y. Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. BBA-Gene Struct. Expr. 1999, 1446, 149–155. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera-a visualization system for exploratory research and analysis. J. Biol. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Mayrose, I.; Mitchell, A.; Pupko, T. Site-specific evolutionary rate inference: Taking phylogenetic uncertainty into account. J. Mol. Evol. 2005, 60, 345–353. [Google Scholar] [CrossRef]
- Heijne, G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the transmembrane topology. EMBO J. 1986, 5, 3021–3027. [Google Scholar] [CrossRef]
- Wallin, E.; Heijne, G.V. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998, 7, 1029–1038. [Google Scholar] [CrossRef]
- Mendoza, I.; Rubio, F.; Rodriguez-Navarro, A.; Pardo, J.M. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 1994, 269, 8792–8796. [Google Scholar] [CrossRef]
- Hernández, A.; Jiang, X.; Cubero, B.; Nieto, P.M.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Mutants of the Arabidopsis thaliana Cation/H+ antiporter AtNHX1 conferring increased salt tolerance in Yeast the endosome/prevacuolar compartment is a target for salt toxicity. J. Biol. Chem. 2009, 284, 14276–14285. [Google Scholar] [CrossRef]
- Chou, P.Y.; Fasman, G.D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 47, 45–148. [Google Scholar]
- Landau, M.; Herz, K.; Padan, E.; Ben-Tal, N. Model structure of the Na+/H+ exchanger 1 (NHE1) functional and clinical implications. J. Biol. Chem. 2007, 282, 37854–37863. [Google Scholar] [CrossRef]
- Slepkov, E.; Ding, J.; Han, J.; Fliegel, L. Mutational analysis of potential porelining amino acids in TM IV of the Na+/H+ exchanger. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2882–2889. [Google Scholar] [CrossRef]
- Murtazina, R.; Booth, B.J.; Bullis, B.L.; Singh, D.N.; Fliegel, L. Functional analysis of polar amino-acid residues in membrane associated regions of the NHE1 isoform of the mammalian Na+/H+ exchanger. Eur. J. Biochem. 2001, 268, 4674–4685. [Google Scholar] [CrossRef]
- Călinescu, O.; Paulino, C.; Kühlbrandt, W.; Fendler, K. Keeping it simple, transport mechanism and pH regulation in Na+/H+ exchangers. J. Biol. Chem. 2014, 289, 13168–13176. [Google Scholar] [CrossRef]
- Sun, M.H.; Ma, Q.J.; Hu, D.G.; Zhu, X.P.; You, C.X.; Shu, H.R. The glucose sensor MdHXK1 phosphorylates a tonoplast Na+/H+ exchanger to improve salt tolerance. Plant Physiol. 2018, 176, 2977–2990. [Google Scholar] [CrossRef]
- Kaufman, R.J.; Murtha-Riel, P.; Pittman, D.D.; Davies, M.V. Characterization of wild-type and ser53 mutant eukaryotic initiation factor 4E overexpression in mammalian cells. J. Biol. Chem. 1993, 268, 11902–11909. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, B.; Xing, Z.; Li, J.; Yuan, Y.; Lei, X.; Tang, H.; Wang, D.; Ma, J.; Heng, S.; Cheng, L. Structural and Functional Characterization of the Vacuolar-Type Na+, K+/H+ Antiporter NHX1 from Rice (Oryza sativa L.). Biomolecules 2025, 15, 1513. https://doi.org/10.3390/biom15111513
Cao B, Xing Z, Li J, Yuan Y, Lei X, Tang H, Wang D, Ma J, Heng S, Cheng L. Structural and Functional Characterization of the Vacuolar-Type Na+, K+/H+ Antiporter NHX1 from Rice (Oryza sativa L.). Biomolecules. 2025; 15(11):1513. https://doi.org/10.3390/biom15111513
Chicago/Turabian StyleCao, Boning, Zhiyong Xing, Jingxian Li, Ying Yuan, Xueru Lei, Hong Tang, Dan Wang, Jiali Ma, Shuangping Heng, and Lin Cheng. 2025. "Structural and Functional Characterization of the Vacuolar-Type Na+, K+/H+ Antiporter NHX1 from Rice (Oryza sativa L.)" Biomolecules 15, no. 11: 1513. https://doi.org/10.3390/biom15111513
APA StyleCao, B., Xing, Z., Li, J., Yuan, Y., Lei, X., Tang, H., Wang, D., Ma, J., Heng, S., & Cheng, L. (2025). Structural and Functional Characterization of the Vacuolar-Type Na+, K+/H+ Antiporter NHX1 from Rice (Oryza sativa L.). Biomolecules, 15(11), 1513. https://doi.org/10.3390/biom15111513





