Non-Vesicular Extracellular Particle (NVEP) Proteomes from Diverse Biological Sources Reveal Specific Marker Composition with Varying Enrichment Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Profiling Across NVEPs Isolated from Different Species and Specimens
2.1.1. Ethical Approval and Specimens
2.1.2. Isolation of NVEPs
2.1.3. Characterization
2.2. Nano-Tracking Analysis (NTA)
2.3. Column Separation
2.4. Transmission Electron Microscopy (TEM)
2.5. Proteomic Analysis
2.6. Statistical Analysis
2.7. Data Mining and Visualization
2.8. Cell Lines
2.9. Internalization of NVEPs
3. Results
3.1. Isolation and Characterization of the Physical Properties of Non-Vesicular Extracellular Particles (NVEPs) from Blood, Brain, and Semen Samples from Different Species
3.2. NEVP Particles Are Amembranous and Devoid of Tetraspanins
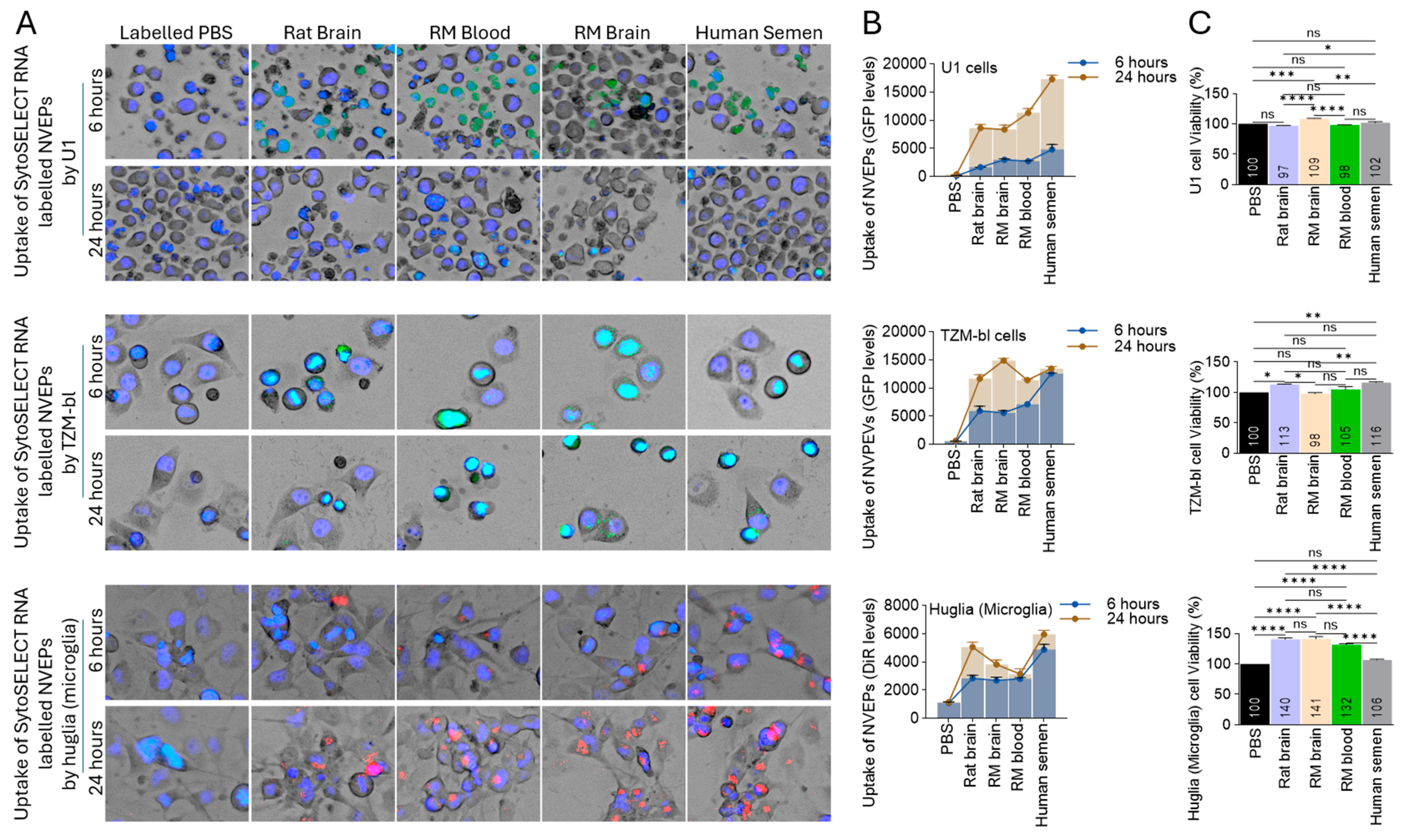
3.3. Species-Independent Internalization of NVEPs by Human Cells
3.4. Extracellular Condensates (NVEPs) Are Enriched with Proteins
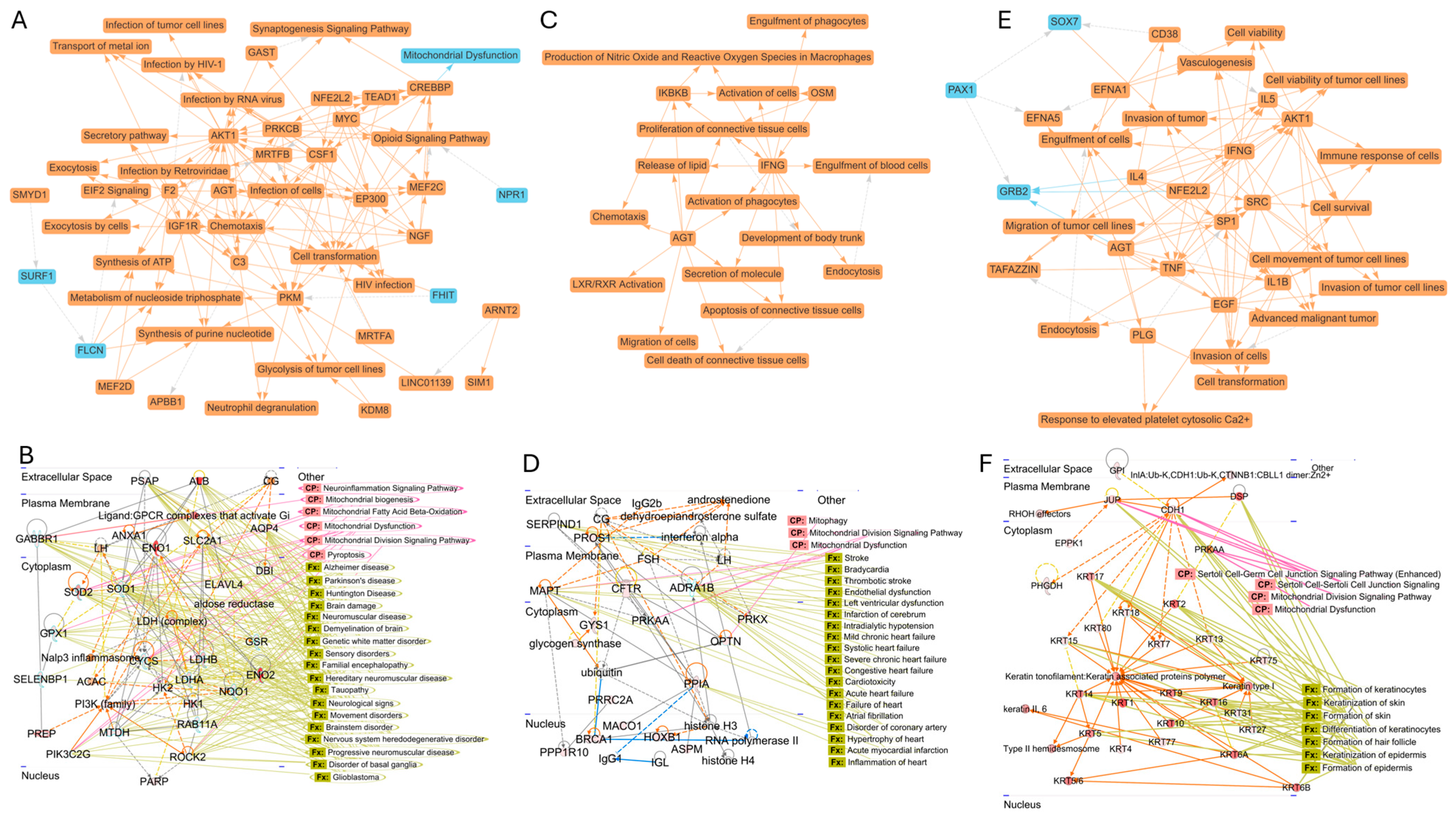
3.5. Species-Specific Network Properties of Proteins Associated with NVEPs
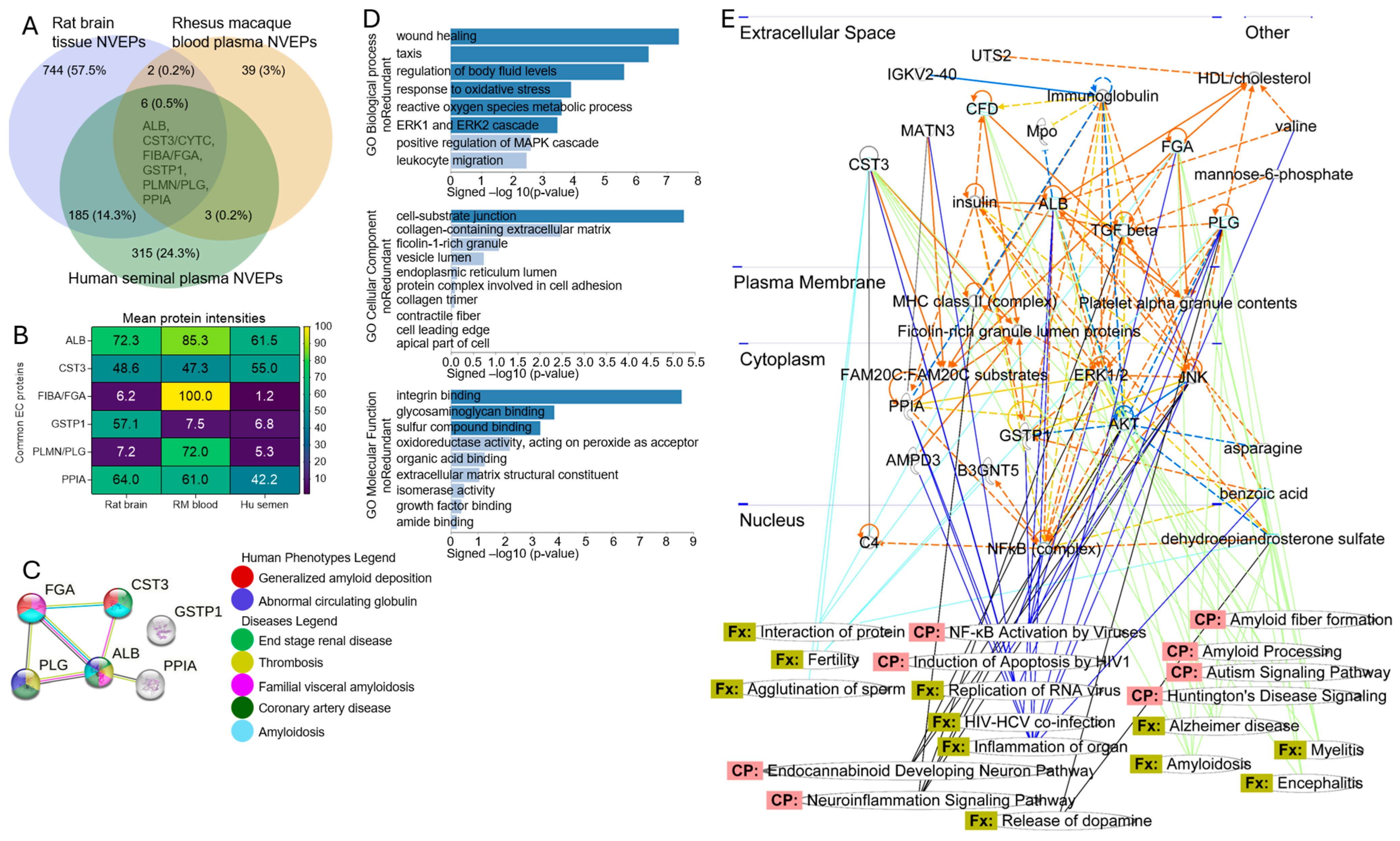
3.6. Protein–Protein Interactome (PPI) Network and Functional Enrichment of Common Human–Rat–Rhesus Proteins Associated with NVEPs
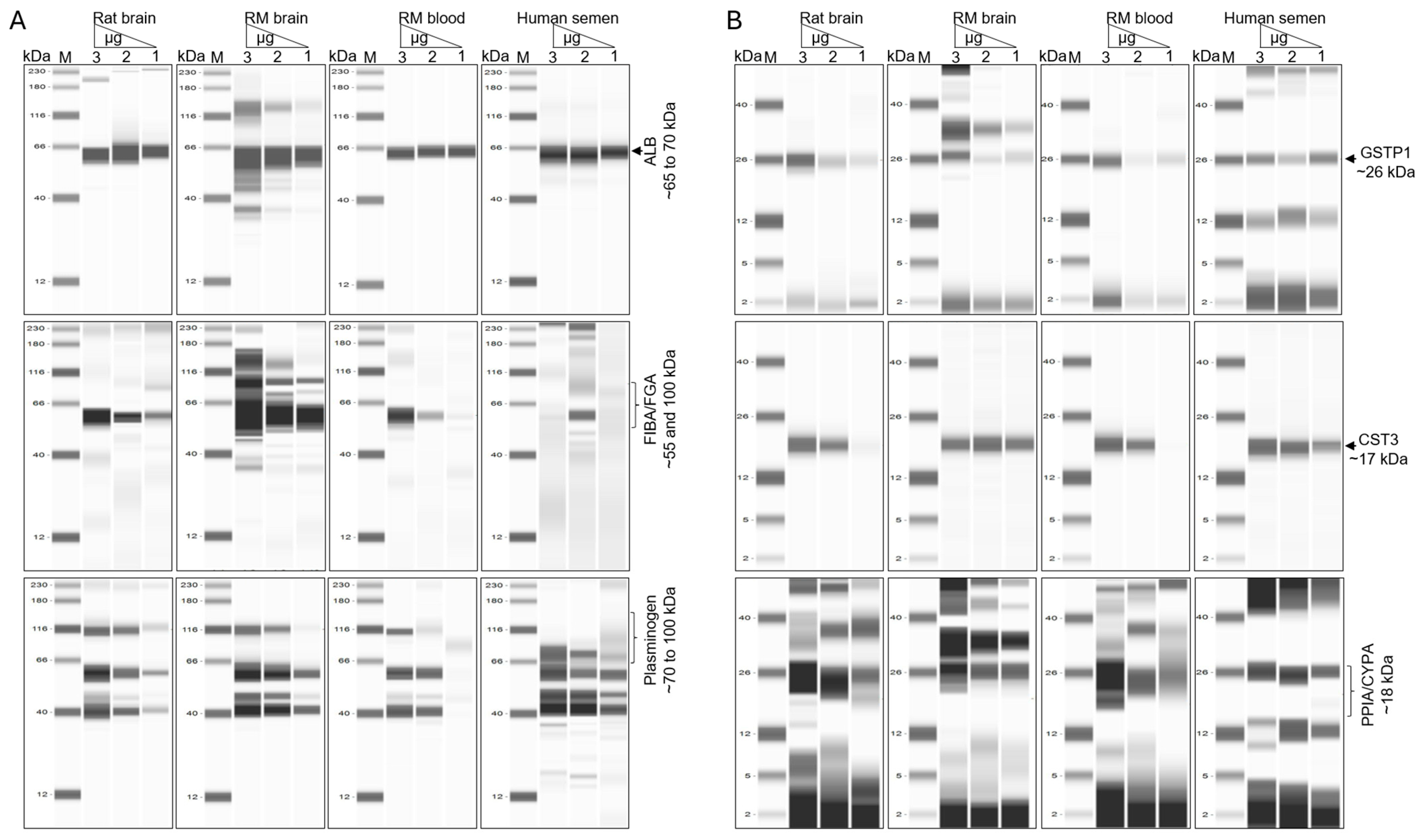
3.7. Six NVEP-Associated Proteins as Markers of NVEPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Kaddour, H.; Lyu, Y.; Shouman, N.; Mohan, M.; Okeoma, C.M. Development of Novel High-Resolution Size-Guided Turbidimetry-Enabled Particle Purification Liquid Chromatography (PPLC): Extracellular Vesicles and Membraneless Condensates in Focus. Int. J. Mol. Sci. 2020, 21, 5361. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, H.; McDew-White, M.; Madeira, M.M.; Tranquille, M.A.; Tsirka, S.E.; Mohan, M.; Okeoma, C.M. Chronic delta-9-tetrahydrocannabinol (THC) treatment counteracts SIV-induced modulation of proinflammatory microRNA cargo in basal ganglia-derived extracellular vesicles. J. Neuroinflamm. 2022, 19, 225. [Google Scholar] [CrossRef]
- Kopcho, S.; McDew-White, M.; Naushad, W.; Mohan, M.; Okeoma, C.M. SIV Infection Regulates Compartmentalization of Circulating Blood Plasma miRNAs within Extracellular Vesicles (EVs) and Extracellular Condensates (ECs) and Decreases EV-Associated miRNA-128. Viruses 2023, 15, 622. [Google Scholar]
- Kopcho, S.; McDew-White, M.; Naushad, W.; Mohan, M.; Okeoma, C.M. Alterations in Abundance and Compartmentalization of miRNAs in Blood Plasma Extracellular Vesicles and Extracellular Condensates during HIV/SIV Infection and Its Modulation by Antiretroviral Therapy (ART) and Delta-9-Tetrahydrocannabinol (Δ9-THC). Viruses 2023, 15, 623. [Google Scholar]
- Okeoma, B.C.; Kaddour, H.; Naushad, W.; Paromov, V.; Chaudhary, A.; Noghero, A.; Stapleton, J.T.; Okeoma, C.M. Identification of proteins in semen-derived extracellular vesicles that bind to Tat and NF-κB and that may impair HIV replication. Sci. Signal 2025, 18, eado9243. [Google Scholar] [CrossRef] [PubMed]
- Okeoma, C.M.; Naushad, W.; Okeoma, B.C.; Gartner, C.; Santos-Ortega, Y.; Vary, C.; Lima-Bastos, S.; Carregari, V.C.; Larsen, M.R.; Noghero, A.; et al. Lipidomic and proteomic insights from extracellular vesicles in the postmortem dorsolateral prefrontal cortex reveal substance use disorder-induced brain changes. Transl. Psychiatry 2025, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Coffey, R.J. Extracellular vesicles and nanoparticles at a glance. J. Cell Sci. 2024, 137, jcs260201. [Google Scholar] [CrossRef]
- Yu, L.; Shi, H.; Gao, T.; Xu, W.; Qian, H.; Jiang, J.; Yang, X.; Zhang, X. Exomeres and supermeres: Current advances and perspectives. Bioact. Mater. 2025, 50, 322–343. [Google Scholar] [CrossRef] [PubMed]
- Naushad, W.; Premadasa, L.S.; Okeoma, B.C.; Mohan, M.; Okeoma, C.M. Extracellular condensates (ECs) are endogenous modulators of HIV transcription and latency reactivation. bioRxiv 2024. 2024.2009.2014.613037. [Google Scholar] [CrossRef]
- Kaddour, H.; Panzner, T.D.; Welch, J.L.; Shouman, N.; Mohan, M.; Stapleton, J.T.; Okeoma, C.M. Electrostatic Surface Properties of Blood and Semen Extracellular Vesicles: Implications of Sialylation and HIV-Induced Changes on EV Internalization. Viruses 2020, 12, 1117. [Google Scholar] [CrossRef]
- Anyanwu, N.C.J.; Premadasa, L.S.; Naushad, W.; Okeoma, B.C.; Mahesh, M.; Okeoma, C.M. Rigorous process for isolation of gut-derived extracellular vesicles and the effect on latent HIV. bioRxiv 2025. 2025.2001.2009.632234. [Google Scholar] [CrossRef]
- Kaddour, H.; Lyu, Y.; Welch, J.L.; Paromov, V.; Mandape, S.N.; Sakhare, S.S.; Pandhare, J.; Stapleton, J.T.; Pratap, S.; Dash, C.; et al. Proteomics Profiling of Autologous Blood and Semen Exosomes from HIV-infected and Uninfected Individuals Reveals Compositional and Functional Variabilities. Mol. Cell Proteom. 2020, 19, 78–100. [Google Scholar] [CrossRef]
- Lyu, Y.; Kaddour, H.; Kopcho, S.; Panzner, T.D.; Shouman, N.; Kim, E.-Y.; Martinson, J.; McKay, H.; Martinez-Maza, O.; Margolick, J.B.; et al. Human Immunodeficiency Virus (HIV) Infection and Use of Illicit Substances Promote Secretion of Semen Exosomes that Enhance Monocyte Adhesion and Induce Actin Reorganization and Chemotactic Migration. Cells 2019, 8, 1027. [Google Scholar] [CrossRef]
- Soucy, A.; Potts, C.; Kaija, A.; Harrington, A.; McGilvrey, M.; Sutphin, G.L.; Korstanje, R.; Tero, B.; Seeker, J.; Pinz, I.; et al. Effects of a Global Rab27a Null Mutation on Murine PVAT and Cardiovascular Function. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 7. [Google Scholar] [CrossRef]
- Gal, J.; Vary, C.; Gartner, C.A.; Jicha, G.A.; Abner, E.L.; Ortega, Y.S.; Choucair, I.; Wilcock, D.M.; Nelson, R.; Nelson, P. Exploratory mass spectrometry of cerebrospinal fluid from persons with autopsy-confirmed LATE-NC. Res. Sq. 2023, 74, 65. [Google Scholar] [CrossRef]
- Young, K.; Conley, B.; Romero, D.; Tweedie, E.; O’Neill, C.; Pinz, I.; Brogan, L.; Lindner, V.; Liaw, L.; Vary, C.P. BMP9 regulates endoglin-dependent chemokine responses in endothelial cells. Blood 2012, 120, 4263–4273. [Google Scholar] [CrossRef] [PubMed]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef]
- Zhang, Y.; Bilbao, A.; Bruderer, T.; Luban, J.; Strambio-De-Castillia, C.; Lisacek, F.; Hopfgartner, G.; Varesio, E. The Use of Variable Q1 Isolation Windows Improves Selectivity in LC-SWATH-MS Acquisition. J. Proteome Res. 2015, 14, 4359–4371. [Google Scholar] [CrossRef]
- Ivosev, G.; Burton, L.; Bonner, R. Dimensionality reduction and visualization in principal component analysis. Anal. Chem. 2008, 80, 4933–4944. [Google Scholar] [CrossRef]
- Lambert, J.P.; Ivosev, G.; Couzens, A.L.; Larsen, B.; Taipale, M.; Lin, Z.Y.; Zhong, Q.; Lindquist, S.; Vidal, M.; Aebersold, R.; et al. Mapping differential interactomes by affinity purification coupled with data-independent mass spectrometry acquisition. Nat. Methods 2013, 10, 1239–1245. [Google Scholar] [CrossRef]
- Moore, M.; Ryzhov, S.; Sawyer, D.B.; Gartner, C.; Vary, C.P.H. ALK1 Signaling in Human Cardiac Progenitor Cells Promotes a Pro-angiogenic Secretome. J. Cell Signal 2024, 5, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, J.C. VENNY; An interactive tool for comparing lists with Venn Diagrams; BioinfoGP: Madrid, Spain, 2007. [Google Scholar]
- Folks, T.M.; Clouse, K.A.; Justement, J.; Rabson, A.; Duh, E.; Kehrl, J.H.; Fauci, A.S. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. USA 1989, 86, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Rothschild, M.A.; Oratz, M.; Schreiber, S.S. Serum albumin. Hepatology 1988, 8, 385–401. [Google Scholar] [CrossRef]
- Alcaraz-Quiles, J.; Casulleras, M.; Oettl, K.; Titos, E.; Flores-Costa, R.; Duran-Güell, M.; López-Vicario, C.; Pavesi, M.; Stauber, R.E.; Arroyo, V.; et al. Oxidized Albumin Triggers a Cytokine Storm in Leukocytes Through P38 Mitogen-Activated Protein Kinase: Role in Systemic Inflammation in Decompensated Cirrhosis. Hepatology 2018, 68, 1937–1952. [Google Scholar] [CrossRef]
- Casulleras, M.; Flores-Costa, R.; Duran-Güell, M.; Alcaraz-Quiles, J.; Sanz, S.; Titos, E.; López-Vicario, C.; Fernández, J.; Horrillo, R.; Costa, M.; et al. Albumin internalizes and inhibits endosomal TLR signaling in leukocytes from patients with decompensated cirrhosis. Sci. Transl. Med. 2020, 12, eaax5135. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Kulkarni, M.; Bowman, E.; Shan, L.; Sattar, A.; Funderburg, N.; McComsey, G.A. Serum Albumin Is Associated with Higher Inflammation and Carotid Atherosclerosis in Treated Human Immunodeficiency Virus Infection. Open Forum Infect. Dis. 2018, 5, ofy291. [Google Scholar] [CrossRef]
- Butler, J.M.; Sharif, U.; Ali, M.; McKibbin, M.; Thompson, J.P.; Gale, R.; Yang, Y.C.; Inglehearn, C.; Paraoan, L. A missense variant in CST3 exerts a recessive effect on susceptibility to age-related macular degeneration resembling its association with Alzheimer’s disease. Hum. Genet. 2015, 134, 705–715. [Google Scholar] [CrossRef]
- Zurdel, J.; Finckh, U.; Menzer, G.; Nitsch, R.M.; Richard, G. CST3 genotype associated with exudative age related macular degeneration. Br. J. Ophthalmol. 2002, 86, 214–219. [Google Scholar] [CrossRef]
- Chabas, D.; Baranzini, S.E.; Mitchell, D.; Bernard, C.C.; Rittling, S.R.; Denhardt, D.T.; Sobel, R.A.; Lock, C.; Karpuj, M.; Pedotti, R.; et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science 2001, 294, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; de Moerloose, P.; Neerman-Arbez, M. Clinical Features and Management of Congenital Fibrinogen Deficiencies. Semin. Thromb. Hemost. 2016, 42, 366–374. [Google Scholar] [CrossRef]
- Casini, A.; Blondon, M.; Lebreton, A.; Koegel, J.; Tintillier, V.; de Maistre, E.; Gautier, P.; Biron, C.; Neerman-Arbez, M.; de Moerloose, P. Natural history of patients with congenital dysfibrinogenemia. Blood 2015, 125, 553–561. [Google Scholar] [CrossRef]
- Casini, A.; Brungs, T.; Lavenu-Bombled, C.; Vilar, R.; Neerman-Arbez, M.; de Moerloose, P. Genetics, diagnosis and clinical features of congenital hypodysfibrinogenemia: A systematic literature review and report of a novel mutation. J. Thromb. Haemost. 2017, 15, 876–888. [Google Scholar] [CrossRef]
- Peyvandi, F.; Haertel, S.; Knaub, S.; Mannucci, P.M. Incidence of bleeding symptoms in 100 patients with inherited afibrinogenemia or hypofibrinogenemia. J. Thromb. Haemost. 2006, 4, 1634–1637. [Google Scholar] [CrossRef]
- Rowczenio, D.; Stensland, M.; de Souza, G.A.; Strøm, E.H.; Gilbertson, J.A.; Taylor, G.; Rendell, N.; Minogue, S.; Efebera, Y.A.; Lachmann, H.J.; et al. Renal Amyloidosis Associated with 5 Novel Variants in the Fibrinogen A Alpha Chain Protein. Kidney Int. Rep. 2017, 2, 461–469. [Google Scholar] [CrossRef]
- Bogaards, J.J.; Venekamp, J.C.; van Bladeren, P.J. Stereoselective conjugation of prostaglandin A2 and prostaglandin J2 with glutathione, catalyzed by the human glutathione S-transferases A1-1, A2-2, M1a-1a, and P1-1. Chem. Res. Toxicol. 1997, 10, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Brunnström, A.; Hamberg, M.; Griffiths, W.J.; Mannervik, B.; Claesson, H.E. Biosynthesis of 14,15-hepoxilins in human l1236 Hodgkin lymphoma cells and eosinophils. Lipids 2011, 46, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Raum, D.; Marcus, D.; Alper, C.A.; Levey, R.; Taylor, P.D.; Starzl, T.E. Synthesis of human plasminogen by the liver. Science 1980, 208, 1036–1037. [Google Scholar] [CrossRef]
- Schmidt, A.; Echtermeyer, F.; Alozie, A.; Brands, K.; Buddecke, E. Plasmin- and thrombin-accelerated shedding of syndecan-4 ectodomain generates cleavage sites at Lys(114)-Arg(115) and Lys(129)-Val(130) bonds. J. Biol. Chem. 2005, 280, 34441–34446. [Google Scholar] [CrossRef]
- Liotta, L.A.; Goldfarb, R.H.; Brundage, R.; Siegal, G.P.; Terranova, V.; Garbisa, S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981, 41, 4629–4636. [Google Scholar]
- Tjwa, M.; Moura, R.; Moons, L.; Plaisance, S.; De Mol, M.; Jansen, S.; Dewerchin, M.; Verfaillie, C.; Carmeliet, P. Fibrinolysis-independent role of plasmin and its activators in the haematopoietic recovery after myeloablation. J. Cell Mol. Med. 2009, 13, 4587–4595. [Google Scholar] [CrossRef]
- Gechtman, Z.; Sharma, R.; Kreizman, T.; Fridkin, M.; Shaltiel, S. Synthetic peptides derived from the sequence around the plasmin cleavage site in vitronectin. Use in mapping the PAI-1 binding site. FEBS Lett. 1993, 315, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Stanton, H.; Cowell, S.; Butler, G.; Knäuper, V.; Atkinson, S.; Gavrilovic, J. Mechanisms for pro matrix metalloproteinase activation. Apmis 1999, 107, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, P.A.; Egelund, R.; Petersen, H.H. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol. Life Sci. 2000, 57, 25–40. [Google Scholar] [CrossRef]
- Schmid, F.X. Protein folding. Prolyl isomerases join the fold. Curr. Biol. 1995, 5, 993–994. [Google Scholar] [CrossRef]
- Liao, Y.; Luo, D.; Peng, K.; Zeng, Y. Cyclophilin A: A key player for etiological agent infection. Appl. Microbiol. Biotechnol. 2021, 105, 1365–1377. [Google Scholar] [CrossRef]
- Zhou, D.; Mei, Q.; Li, J.; He, H. Cyclophilin A and viral infections. Biochem. Biophys. Res. Commun. 2012, 424, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Braaten, D.; Luban, J. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 2001, 20, 1300–1309. [Google Scholar] [CrossRef]
- Madlala, P.; Singh, R.; An, P.; Werner, L.; Mlisana, K.; Abdool Karim, S.S.; Winkler, C.A.; Ndung’u, T. Association of Polymorphisms in the Regulatory Region of the Cyclophilin a Gene (PPIA) with Gene Expression and HIV/AIDS Disease Progression. J. Acquir. Immune Defic. Syndr. 2016, 72, 465–473. [Google Scholar] [CrossRef]
- Liu, C.; Perilla, J.R.; Ning, J.; Lu, M.; Hou, G.; Ramalho, R.; Himes, B.A.; Zhao, G.; Bedwell, G.J.; Byeon, I.-J.; et al. Cyclophilin A stabilizes the HIV-1 capsid through a novel non-canonical binding site. Nat. Commun. 2016, 7, 10714. [Google Scholar] [CrossRef] [PubMed]
- Molina-García, L.; Gasset-Rosa, F.; Moreno-Del Álamo, M.; Fernández-Tresguerres, M.E.; Moreno-Díaz de la Espina, S.; Lurz, R.; Giraldo, R. Functional amyloids as inhibitors of plasmid DNA replication. Sci. Rep. 2016, 6, 25425. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef]
- Adda, C.G.; Murphy, V.J.; Sunde, M.; Waddington, L.J.; Schloegel, J.; Talbo, G.H.; Vingas, K.; Kienzle, V.; Masciantonio, R.; Howlett, G.J.; et al. Plasmodium falciparum merozoite surface protein 2 is unstructured and forms amyloid-like fibrils. Mol. Biochem. Parasitol. 2009, 166, 159–171. [Google Scholar] [CrossRef]
- Rauceo, J.M.; Gaur, N.K.; Lee, K.G.; Edwards, J.E.; Klotz, S.A.; Lipke, P.N. Global cell surface conformational shift mediated by a Candida albicans adhesin. Infect. Immun. 2004, 72, 4948–4955. [Google Scholar] [CrossRef]
- Gour, S.; Kaushik, V.; Kumar, V.; Bhat, P.; Yadav, S.C.; Yadav, J.K. Antimicrobial peptide (Cn-AMP2) from liquid endosperm of Cocos nucifera forms amyloid-like fibrillar structure. J. Pept. Sci. 2016, 22, 201–207. [Google Scholar] [CrossRef]
- Whelly, S.; Johnson, S.; Powell, J.; Borchardt, C.; Hastert, M.C.; Cornwall, G.A. Nonpathological extracellular amyloid is present during normal epididymal sperm maturation. PLoS ONE 2012, 7, e36394. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef]
- Angehrn, F.N.; Duan, P.; Zhang, J.Y.; Hong, M. Binding Sites of a PET Ligand in Tau Fibrils with the Alzheimer’s Disease Fold from 19F and 13C Solid-State NMR. Biochemistry 2025, 64, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Kanyo, J.; Wilson, R.; Bathla, S.; Cardozo, P.L.; Tong, L.; Qin, S.; Fuentes, L.A.; Pinheiro-de-Sousa, I.; Huynh, T.; et al. Subcellular proteomics and iPSC modeling uncover reversible mechanisms of axonal pathology in Alzheimer’s disease. Nat. Aging 2025, 5, 504–527. [Google Scholar] [CrossRef]
- Gilbert, S.; Pacheco, R.; Armien, A.G.; Garner, M. An investigation of an amyloid-like deposition disorder in reptiles. J. Zoo Wildl. Med. 2025, 56, 135–140. [Google Scholar] [CrossRef]
- Puangmalai, N.; Aday, A.E.; Samples, M.; Bhatt, N.; Cascio, F.L.; Marcatti, M.; Park, S.J.; Fung, L.; Jerez, C.; Penalva, L.O.; et al. Pathogenic Oligomeric Tau alters Neuronal RNA Processes through the Formation of Nuclear Heteromeric Amyloids with RNA-Binding Protein Musashi1. Prog. Neurobiol. 2025, 247, 102742. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Fenu, S.; Izzo, A.; Montano, N.; Polli, F.M.; Rapisarda, A.; Costa, F.; Schiariti, M.P.; Gessi, M.; Marucci, G.; et al. Transthyretin amyloid deposition in the ligamentum flavum of an Italian cohort of patients with lumbar spinal stenosis. Neurol. Sci. 2025, 46, 3295–3298. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.; Madison, M.N.; Margolick, J.B.; Galvin, S.; Gupta, P.; Martínez-Maza, O.; Dash, C.; Okeoma, C.M. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci. Rep. 2017, 7, 45034. [Google Scholar] [CrossRef] [PubMed]




| Rat Brain (PFC) | RM Brain (BG) | RM Blood Plasma | Human Seminal Plasma | |
|---|---|---|---|---|
| Total Number # | 6 | 6 | 6 | 6 |
| # used for TEM | 6 (pooled into 3) | 6 (pooled into 3) | 6 (pooled into 3) | 6 (pooled into 3) |
| # used for proteomics | 3 | 3 | 3 | 3 |
| # used for capillary Western blot | 3 | 3 | 3 | 3 |
| Volume (mL) or weight (mg) of specimens | ~100 mg | ~100 mg | 1 mL | 1 mL |
| Rat Brain | RM Brain | RM Blood | Human Semen | |
|---|---|---|---|---|
| Number of Values | 30 | 30 | 30 | 30 |
| Minimum (nm) | 26 | 19 | 19 | 19 |
| 25% Percentile (nm) | 79 | 55.25 | 53 | 40.75 |
| Median (nm) | 93 | 67 | 57.5 | 56 |
| 75% Percentile (nm) | 100 | 83 | 93.5 | 59 |
| Maximum (nm) | 134 | 119 | 118 | 79 |
| Range (nm) | 108 | 119 | 99 | 60 |
| Mean (nm) | 91.63 | 67.03 | 64.83 | 51.6 |
| Std. Deviation | 20.45 | 23.98 | 26.49 | 15.85 |
| Std. Error of Mean | 3.733 | 4.377 | 4.836 | 2.894 |
| Coefficient of Variation | 22.31% | 35.77% | 40.85% | 30.72% |
| Rat Brain (Figure 4A) | RM Blood (Figure 4B) | Human Semen (Figure 4C) | |
|---|---|---|---|
| Theme 1 | Infection and immune response | Immune response and inflammation | Tumor growth and malignancy |
| Theme 2 | Cancer and cell transformation | Apoptosis and cell death | Invasion and metastasis |
| Theme 3 | Metabolism and energy production | Connective tissue regulation and development | Cell viability and cell survival |
| Theme 4 | Chemotaxis and cellular movement | Lipid metabolism and secretion | Immune response |
| Theme 5 | Synaptic signaling and neurobiology | Cell migration and chemotaxis | Cellular motility and migration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naushad, W.; Okeoma, B.C.; Gartner, C.; Santos-Ortega, Y.; Vary, C.P.H.; Premadasa, L.S.; Noghero, A.; Stapleton, J.T.; Ghiran, I.C.; Mohan, M.; et al. Non-Vesicular Extracellular Particle (NVEP) Proteomes from Diverse Biological Sources Reveal Specific Marker Composition with Varying Enrichment Levels. Biomolecules 2025, 15, 1487. https://doi.org/10.3390/biom15111487
Naushad W, Okeoma BC, Gartner C, Santos-Ortega Y, Vary CPH, Premadasa LS, Noghero A, Stapleton JT, Ghiran IC, Mohan M, et al. Non-Vesicular Extracellular Particle (NVEP) Proteomes from Diverse Biological Sources Reveal Specific Marker Composition with Varying Enrichment Levels. Biomolecules. 2025; 15(11):1487. https://doi.org/10.3390/biom15111487
Chicago/Turabian StyleNaushad, Wasifa, Bryson C. Okeoma, Carlos Gartner, Yulica Santos-Ortega, Calvin P. H. Vary, Lakmini S. Premadasa, Alessio Noghero, Jack T. Stapleton, Ionita C. Ghiran, Mahesh Mohan, and et al. 2025. "Non-Vesicular Extracellular Particle (NVEP) Proteomes from Diverse Biological Sources Reveal Specific Marker Composition with Varying Enrichment Levels" Biomolecules 15, no. 11: 1487. https://doi.org/10.3390/biom15111487
APA StyleNaushad, W., Okeoma, B. C., Gartner, C., Santos-Ortega, Y., Vary, C. P. H., Premadasa, L. S., Noghero, A., Stapleton, J. T., Ghiran, I. C., Mohan, M., & Okeoma, C. M. (2025). Non-Vesicular Extracellular Particle (NVEP) Proteomes from Diverse Biological Sources Reveal Specific Marker Composition with Varying Enrichment Levels. Biomolecules, 15(11), 1487. https://doi.org/10.3390/biom15111487






