Metabolomics in the Context of Exercise in Subjects with Multimorbidity: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. MultiPill-Exercise Pilot Study
2.2. Metabolomics Analysis from Dried Blood Spots
2.3. Statistical Analysis
3. Results
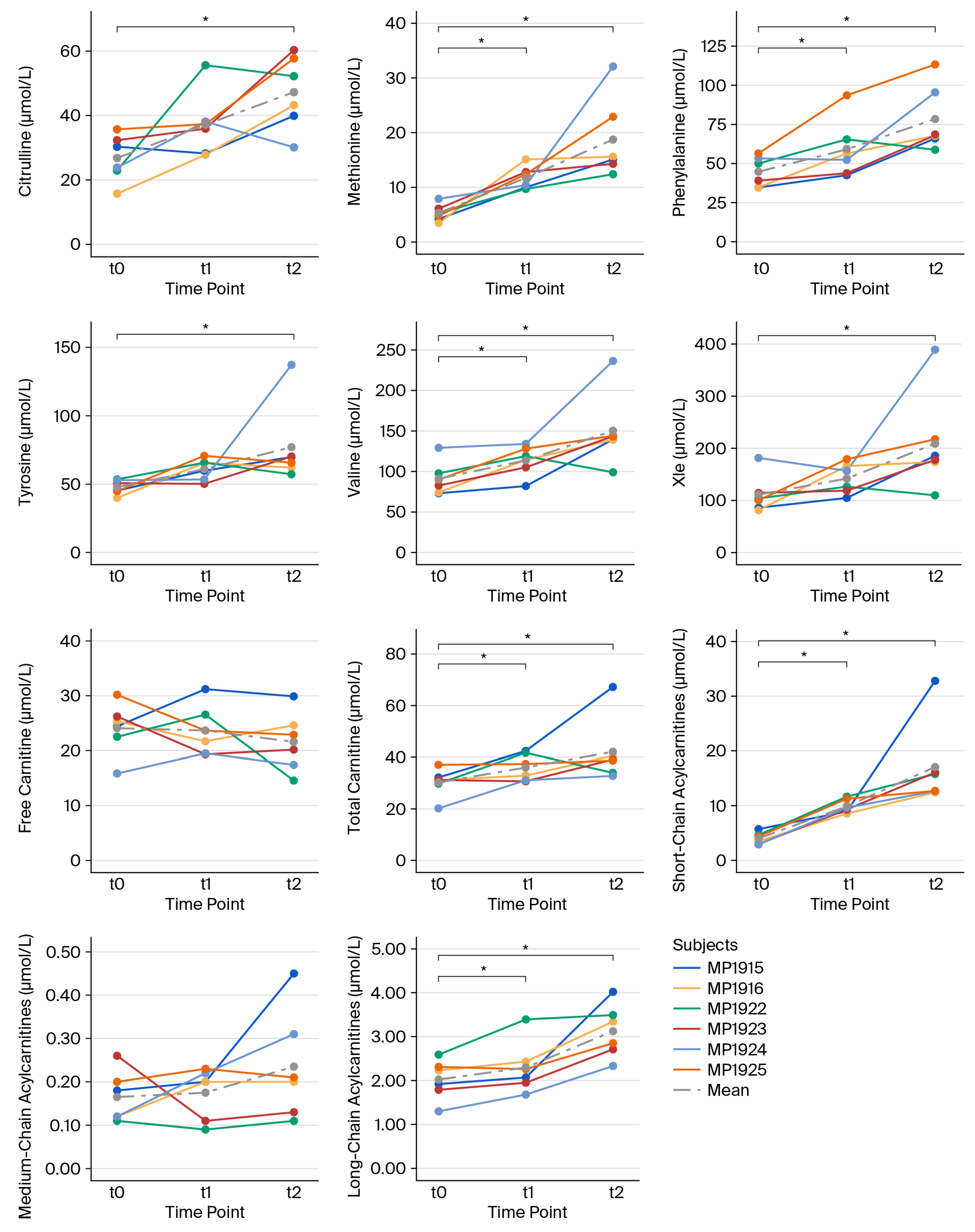
3.1. Individual Metabolite Kinetics Throughout the Intervention
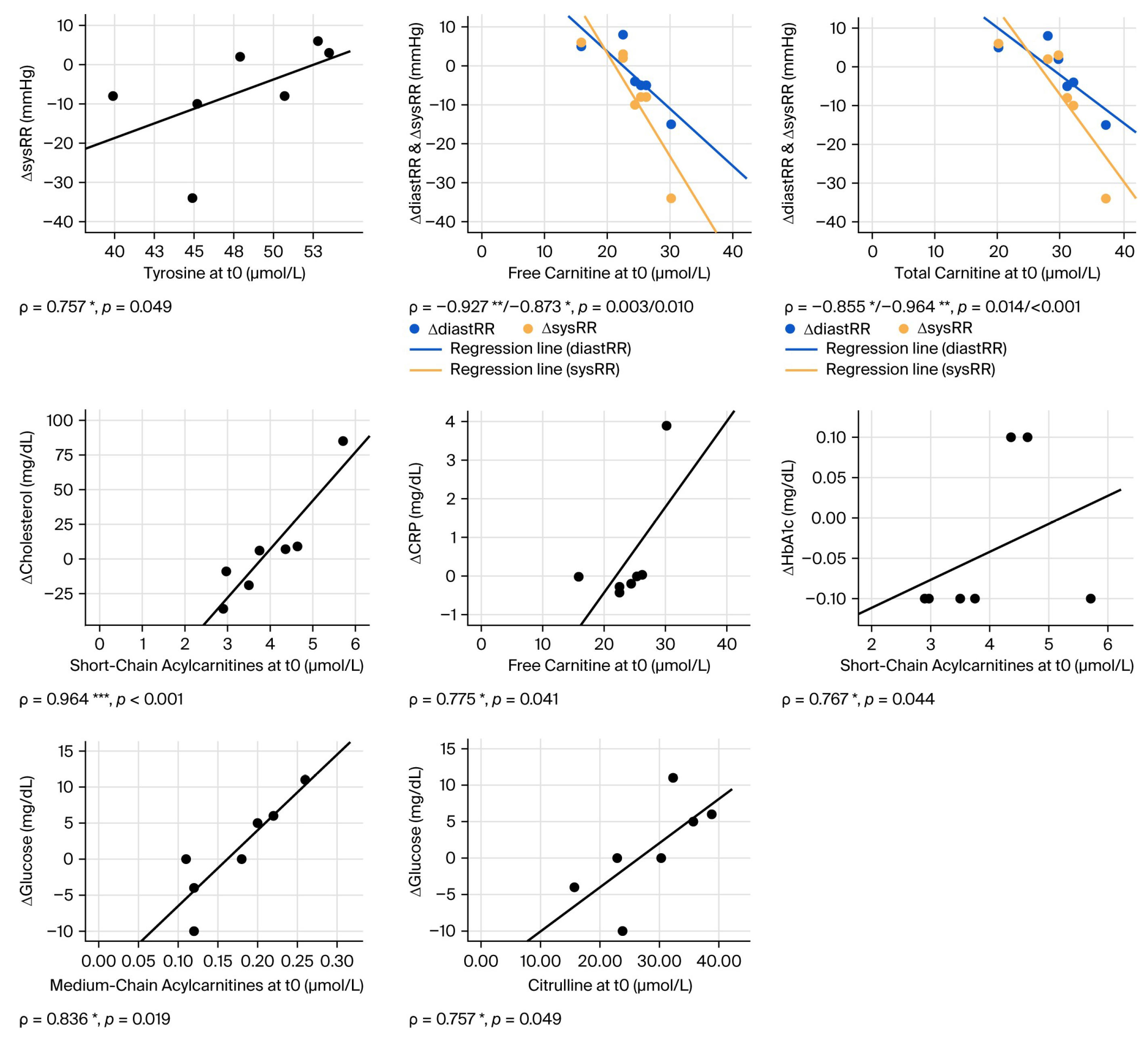
3.2. Correlation Analysis
3.3. Individual Acute Response at t2
4. Discussion
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, K.; Makovski, T.T.; Nagyova, I.; van den Akker, M.; Stranges, S. Strategies to improve health status among adults with multimorbidity: A scoping review. Maturitas 2023, 167, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Schweda, S.; Munz, B.; Burgstahler, C.; Niess, A.M.; Roesel, I.; Sudeck, G.; Krauss, I. Proof of Concept of a 6-Month Person-Oriented Exercise Intervention ‘MultiPill-Exercise’ among Patients at Risk of or with Multiple Chronic Diseases: Results of a One-Group Pilot Trial. Int. J. Environ. Res. Public Health 2022, 19, 9469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sakaguchi, C.A.; Nieman, D.C.; Signini, E.F.; Abreu, R.M.; Catai, A.M. Metabolomics-Based Studies Assessing Exercise-Induced Alterations of the Human Metabolome: A Systematic Review. Metabolites 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schranner, D.; Kastenmüller, G.; Schönfelder, M.; Römisch-Margl, W.; Wackerhage, H. Metabolite Concentration Changes in Humans After a Bout of Exercise: A Systematic Review of Exercise Metabolomics Studies. Sports Med. Open 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, Q.; Corkum, A.E.; Moaddel, R.; Ferrucci, L. Metabolomic profiles of being physically active and less sedentary: A critical review. Metabolomics 2021, 17, 68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khoramipour, K.; Sandbakk, Ø.; Keshteli, A.H.; Gaeini, A.A.; Wishart, D.S.; Chamari, K. Metabolomics in Exercise and Sports: A Systematic Review. Sports Med. 2022, 52, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Flynn, N.E.; Shaw, M.H.; Becker, J.T. Amino Acids in Health and Endocrine Function. Adv. Exp. Med. Biol. 2020, 1265, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.S.; Zhao, X.; Irmler, M.; Liu, X.; Hoene, M.; Scheler, M.; Li, Y.; Beckers, J.; Hrabĕ de Angelis, M.; Häring, H.U.; et al. Type 2 diabetes alters metabolic and transcriptional signatures of glucose and amino acid metabolism during exercise and recovery. Diabetologia 2015, 58, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, P.; Cowan, T.M.; Matern, D. Acylcarnitine profile analysis. Genet. Med. 2008, 10, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of l-carnitine for human health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCoin, C.S.; Knotts, T.A.; Adams, S.H. Acylcarnitines--old actors auditioning for new roles in metabolic physiology. Nat. Rev. Endocrinol. 2015, 11, 617–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huffman, K.M.; Slentz, C.A.; Bateman, L.A.; Thompson, D.; Muehlbauer, M.J.; Bain, J.R.; Stevens, R.D.; Wenner, B.R.; Kraus, V.B.; Newgard, C.B.; et al. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care 2011, 34, 174–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huffman, K.M.; Koves, T.R.; Hubal, M.J.; Abouassi, H.; Beri, N.; Bateman, L.A.; Stevens, R.D.; Ilkayeva, O.R.; Hoffman, E.P.; Muoio, D.M.; et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 2014, 57, 2282–2295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skogvold, H.B.; Rootwelt, H.; Reubsaet, L.; Elgstøen, K.B.P.; Wilson, S.R. Dried blood spot analysis with liquid chromatography and mass spectrometry: Trends in clinical chemistry. J. Sep. Sci. 2023, 46, e2300210. [Google Scholar] [CrossRef] [PubMed]
- Koal, T.; Deigner, H.P. Challenges in mass spectrometry based targeted metabolomics. Curr. Mol. Med. 2010, 10, 216–226. [Google Scholar] [CrossRef] [PubMed]
- la Marca, G. Mass spectrometry in clinical chemistry: The case of newborn screening. J. Pharm. Biomed. Anal. 2014, 101, 174–182. [Google Scholar] [CrossRef] [PubMed]
- NBS04-Ed2; Newborn Screening by Tandem Mass Spectrometry. 2nd Ed. Clinical Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2017.
- Millington, D.S. How mass spectrometry revolutionized newborn screening. J. Mass Spectrom. Adv. Clin. Lab. 2024, 32, 1–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, R.S.; Kelly, M.P.; Kelly, P. Metabolomics, physical activity, exercise and health: A review of the current evidence. Biochim. Biophys. Acta. Mol. Basis Dis. 2020, 1866, 165936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamaura, M.; Nishijima, K.; Takahashi, M.; Ando, T.; Mizushima, S.; Tochikubo, O. Lifestyle modification in metabolic syndrome and associated changes in plasma amino acid profiles. Circ. J. 2010, 4, 2434–2440. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Miller, S.L. Amino acid availability controls muscle protein metabolism. Diabetes Nutr. Metab. 1999, 12, 322–328. [Google Scholar] [PubMed]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.; DeLany, J.P. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeljkovic, A.; Mihajlovic, M.; Vujcic, S.; Guzonjic, A.; Munjas, J.; Stefanovic, A.; Kotur-Stevuljevic, J.; Rizzo, M.; Bogavac-Stanojevic, N.; Gagic, J.; et al. The Prospect of Genomic, Transcriptomic, Epigenetic and Metabolomic Biomarkers for The Personalized Prevention of Type 2 Diabetes and Cardiovascular Diseases. Curr. Vasc. Pharmacol. 2023, 21, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Carrard, J.; Guerini, C.; Appenzeller-Herzog, C.; Infanger, D.; Königstein, K.; Streese, L.; Hinrichs, T.; Hanssen, H.; Gallart-Ayala, H.; Ivanisevic, J.; et al. The Metabolic Signature of Cardiorespiratory Fitness: A Systematic Review. Sports Med. 2022, 52, 527–546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the factors that effect maximal fat oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, R.; Zhao, X.; Weigert, C.; Simon, P.; Fehrenbach, E.; Fritsche, J.; Machann, J.; Schick, F.; Wang, J.; Hoene, M.; et al. Medium chain acylcarnitines dominate the metabolite pattern in humans under moderate intensity exercise and support lipid oxidation. PLoS ONE 2010, 5, e11519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schweda, S.; Müller, G.; Munz, B.; Sudeck, G.; Martus, P.; Dierkes, K.; Krauss, I. Implementation and evaluation of an individualized physical exercise promotion program in people with manifested risk factors for multimorbidity (MultiPill-Exercise): A study protocol for a pragmatic randomized controlled trial. BMC Public Health 2022, 22, 1174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naja, K.; Hedaya, L.; Elashi, A.A.; Rizzo, M.; Elrayess, M.A. N-Lactoyl Amino Acids: Emerging Biomarkers in Metabolism and Disease. Diabetes Metab. Res. Rev. 2025, 41, e70060. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Abbreviations | Metabolites |
|---|---|
| Carnitine, C0 | free/unconjugated carnitine |
| Cx | total carnitine (sum of free carnitine and all acylcarnitines) |
| C2 | acetylcarnitine |
| C3-DC+C4-OH | malonylcarnitine (C3-DC) + 3-hydroxybutyrylcarnitine (C4-OH) |
| C3 | propionylcarnitine (C3) |
| C4 | butyrylcarnitine + isobutyrylcarnitine (C4) |
| C4-DC+C5-OH | methylmalonylcarnitine (C4-DC) + 3-hydroxyisovalerylcarnitine (C5-OH) |
| C5 | isovalerylcarnitine + methylbutyrylcarnitine (C5) |
| C5-DC+C6-OH | glutarylcarnitine (C5-DC) + 3-hydroxyhexanoylcarnitine (C6-OH) |
| C6 | hexanoylcarnitine (C6) |
| C6-DC | methylglutarylcarnitine (C6-DC) |
| C8 | octanoylcarnitine (C8) |
| C10 | decanoylcarnitine (C10) |
| C10:1 | decenoylcarnitine (C10:1) |
| C12 | dodecanoylcarnitine (C12) |
| C12:1 | dodecenoylcarnitine (C12:1) |
| C14 | tetradecanoylcarnitine (C14) |
| C14-OH | 3-hydroxytetradecanoylcarnitine (C14-OH) |
| C14:1-OH | 3-hydroxytetradecenoylcarnitine (C14:1-OH) |
| C14:1 | tetradecenoylcarnitine (C14:1) |
| C14:2 | tetradecadienoylcarnitine (C14:2) |
| C16 | palmitoylcarnitine (C16) |
| C18 | stearoylcarnitine (C18) |
| C18:1 | oleoylcarnitine (C18:1) |
| C18:2 | linoleoylcarnitine (C18:2) |
| C16-OH | 3-hydroxypalmitoylcarnitine (C16-OH) |
| C18:1-OH | 3-hydroxyoleoylcarnitine (C18:1-OH) |
| C18:2-OH | 3-hydroxylinoleoylcarnitine (C18:2-OH) |
| SC-ACs | short-chain acylcarnitines (C2-C6) |
| MC-ACs | medium-chain acylcarnitines (C8-C12) |
| LC-ACs | long-chain acylcarnitines (>C12) |
| Cit | citrulline |
| Met | methionine |
| Phe | phenylalanine |
| Tyr | tyrosine |
| Val | valine |
| Xle | leucine + isoleucine |
| t | n | Min | Max | MD | ME | SD | Δ (t0–t1) p | Δ (t0–t2) p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| amino acids | Cit (µmol/L) | t0 | 6 | 15.7 | 35.7 | 27.05 | 26.78 | 7.34 | 10.37 0.156 | 20.45 0.031 * |
| t1 | 6 | 27.8 | 55.6 | 36.60 | 37.15 | 10.10 | ||||
| t2 | 6 | 30.1 | 60.3 | 47.70 | 47.23 | 11.56 | ||||
| Met (µmol/L) | t0 | 6 | 3.5 | 7.9 | 5.05 | 5.30 | 1.56 | 6.43 0.016 * | 13.43 0.031 * | |
| t1 | 6 | 9.7 | 15.1 | 11.40 | 11.73 | 2.09 | ||||
| t2 | 6 | 12.4 | 32.1 | 15.35 | 18.73 | 7.47 | ||||
| Phe (µmol/L) | t0 | 6 | 34.8 | 56.6 | 44.65 | 44.83 | 9.63 | 14.10 0.031 * | 33.27 0.013 * | |
| t1 | 6 | 42.6 | 93.8 | 54.15 | 58.93 | 19.04 | ||||
| t2 | 6 | 58.8 | 112.9 | 68.10 | 78.10 | 21.11 | ||||
| Tyr (µmol/L) | t0 | 6 | 39.9 | 53.5 | 47.95 | 47.83 | 5.36 | 13.07 0.109 | 29.20 0.031 * | |
| t1 | 6 | 50.2 | 70.7 | 62.70 | 60.90 | 7.91 | ||||
| t2 | 6 | 57.2 | 137.2 | 67.75 | 77.03 | 29.88 | ||||
| Val (µmol/L) | t0 | 6 | 73.1 | 129.2 | 86.25 | 91.12 | 20.87 | 22.60 0.016 * | 59.17 0.031 * | |
| t1 | 6 | 82.0 | 134.0 | 116.55 | 113.72 | 18.58 | ||||
| t2 | 6 | 99.0 | 236.4 | 141.55 | 150.28 | 45.54 | ||||
| Xle (µmol/L) | t0 | 6 | 81.4 | 181.6 | 102.15 | 111.32 | 36.50 | 30.75 0.109 | 97.63 0.031 * | |
| t1 | 6 | 104.9 | 179.1 | 141.75 | 142.07 | 29.43 | ||||
| t2 | 6 | 109.9 | 389.1 | 181.80 | 208.95 | 94.96 | ||||
| (acyl)carnitines | Carnitine (µmol/L) | t0 | 6 | 15.9 | 30.2 | 24.88 | 24.09 | 4.76 | −0.43 0.844 | −2.50 0.313 |
| t1 | 6 | 19.3 | 31.2 | 22.68 | 23.66 | 4.59 | ||||
| t2 | 6 | 14.6 | 29.9 | 21.53 | 21.59 | 5.44 | ||||
| Cx (µmol/L) | t0 | 6 | 20.2 | 37.1 | 31.20 | 30.28 | 5.54 | 5.75 0.047 * | 11.78 0.031 * | |
| t1 | 6 | 30.7 | 42.4 | 35.15 | 36.03 | 5.24 | ||||
| t2 | 6 | 32.8 | 67.2 | 38.90 | 42.07 | 12.69 | ||||
| SC-ACs (µmol/L) | t0 | 6 | 2.9 | 5.7 | 3.93 | 4.01 | 1.09 | 5.88 0.016 * | 13.07 0.031 * | |
| t1 | 6 | 8.6 | 11.7 | 9.47 | 9.89 | 1.27 | ||||
| t2 | 6 | 12.4 | 32.8 | 14.26 | 17.08 | 7.87 | ||||
| MC-ACs (µmol/L) | t0 | 6 | 0.1 | 0.3 | 0.15 | 0.17 | 0.06 | 0.01 0.484 | 0.07 0.313 | |
| t1 | 6 | 0.1 | 0.2 | 0.20 | 0.18 | 0.06 | ||||
| t2 | 6 | 0.1 | 0.5 | 0.21 | 0.24 | 0.13 | ||||
| LC-ACs (µmol/L) | t0 | 6 | 1.3 | 2.6 | 2.08 | 2.02 | 0.45 | 0.27 0.031 * | 1.10 0.031 * | |
| t1 | 6 | 1.7 | 3.4 | 2.17 | 2.30 | 0.59 | ||||
| t2 | 6 | 2.3 | 4.0 | 3.10 | 3.12 | 0.61 |
| Δphysiological/Clinical Parameters (t0–t1) | Statistics | AA Concentration (t0) | AC Concentration (t0) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cit | Met | Phe | Tyr | Val | Xle | C0 | Cx | SC-ACs | MC-ACs | LC-ACs | |||
| sports | ΔrelVO2max [mL/kgxmin] | correlation [ρ] | −0.624 | −0.459 | −0.532 | −0.184 | −0.716 | −0.514 | 0.019 | 0.167 | 0.239 | −0.278 | 0.184 |
| p (2-sided) | 0.134 | 0.300 | 0.219 | 0.694 | 0.070 | 0.238 | 0.969 | 0.721 | 0.606 | 0.546 | 0.694 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| ΔPPO [W/kg] | correlation [ρ] | −0.144 | 0.180 | −0.487 | 0.487 | −0.054 | 0.018 | −0.245 | −0.373 | 0.108 | −0.055 | 0.162 | |
| p (2-sided) | 0.758 | 0.699 | 0.268 | 0.268 | 0.908 | 0.969 | 0.596 | 0.41 | 0.818 | 0.908 | 0.728 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| body weight | ΔBMI [kg/m2] | correlation [ρ] | −0.679 | 0.107 | −0.036 | 0.214 | −0.036 | 0.071 | −0.577 | −0.360 | −0.179 | −0.577 | −0.321 |
| p (2-sided) | 0.094 | 0.819 | 0.939 | 0.645 | 0.939 | 0.879 | 0.175 | 0.427 | 0.702 | 0.175 | 0.482 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| inflammation | ΔIL6 [ng/L] | correlation[ρ] | 0.643 | −0.250 | 0.071 | −0.143 | 0.036 | −0.214 | 0.342 | 0.414 | 0.643 | 0.306 | 0.464 |
| p (2-sided) | 0.119 | 0.589 | 0.879 | 0.760 | 0.939 | 0.645 | 0.452 | 0.355 | 0.119 | 0.504 | 0.294 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| ΔCRP [mg/dL] | correlation[ρ] | 0.000 | −0.071 | 0.536 | −0.429 | −0.071 | 0.143 | 0.775 * | 0.595 | −0.321 | 0.270 | 0.179 | |
| p (2-sided) | 1.000 | 0.879 | 0.215 | 0.337 | 0.879 | 0.76 | 0.041 | 0.159 | 0.482 | 0.558 | 0.702 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| Δfibrinogen [mg/dL] | correlation[ρ] | 0.257 | 0.086 | −0.029 | 0.429 | 0.029 | 0.086 | 0.261 | 0.174 | 0.429 | 0.116 | 0.486 | |
| p (2-sided) | 0.623 | 0.872 | 0.957 | 0.397 | 0.957 | 0.872 | 0.618 | 0.742 | 0.397 | 0.827 | 0.329 | ||
| n | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | ||
| diabetes | Δinsulin [pmol/L] | correlation[ρ] | 0.000 | 0.107 | −0.250 | 0.321 | 0.000 | 0.036 | 0.144 | −0.072 | 0.143 | 0.090 | 0.429 |
| p (2-sided) | 1.000 | 0.819 | 0.589 | 0.482 | 1.000 | 0.939 | 0.758 | 0.878 | 0.76 | 0.848 | 0.337 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| ΔHbA1c [%] | correlation[ρ] | 0.356 | −0.225 | 0.337 | −0.019 | 0.206 | −0.131 | 0.198 | 0.415 | 0.767 * | −0.076 | 0.636 | |
| p (2-sided) | 0.434 | 0.628 | 0.460 | 0.968 | 0.658 | 0.78 | 0.67 | 0.354 | 0.044 | 0.872 | 0.125 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| Δglucose [mg/dL] | correlation[ρ] | 0.757 * | 0.090 | −0.180 | 0.000 | −0.234 | 0.054 | 0.500 | 0.291 | 0.126 | 0.836 * | 0.018 | |
| p (2-sided) | 0.049 | 0.848 | 0.699 | 1.000 | 0.613 | 0.908 | 0.253 | 0.527 | 0.788 | 0.019 | 0.969 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| lipoprotein and fatty acid metabolism | Δcholesterol [mg/dL] | correlation[ρ] | 0.214 | −0.357 | 0.000 | 0.000 | −0.286 | −0.321 | 0.180 | 0.523 | 0.964 *** | −0.018 | 0.571 |
| p (2-sided) | 0.645 | 0.432 | 1.000 | 1.000 | 0.535 | 0.482 | 0.699 | 0.229 | <0.001 | 0.969 | 0.18 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| Δtriglycerides [mg/dL] | correlation[ρ] | −0.018 | 0.270 | −0.450 | 0.468 | −0.198 | 0.126 | −0.073 | −0.245 | 0.000 | 0.209 | 0.018 | |
| p (2-sided) | 0.969 | 0.558 | 0.31 | 0.289 | 0.67 | 0.788 | 0.877 | 0.596 | 1 | 0.653 | 0.969 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| ΔLDL [mg/dL] | correlation[ρ] | 0.679 | −0.214 | −0.143 | −0.071 | 0.000 | −0.250 | 0.180 | 0.234 | 0.607 | 0.342 | 0.321 | |
| p (2-sided) | 0.094 | 0.645 | 0.76 | 0.879 | 1 | 0.589 | 0.699 | 0.613 | 0.148 | 0.452 | 0.482 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| ΔHDL [mg/dL] | correlation[ρ] | 0.144 | −0.108 | 0.360 | −0.018 | 0.559 | −0.036 | −0.018 | 0.027 | 0.324 | −0.309 | 0.505 | |
| p (2-sided) | 0.758 | 0.818 | 0.427 | 0.969 | 0.192 | 0.939 | 0.969 | 0.954 | 0.478 | 0.5 | 0.248 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| cardiovascular parameters | ΔdiastRR [mmHg] | correlation[ρ] | 0.072 | 0.414 | −0.360 | 0.577 | 0.342 | 0.198 | −0.927 ** | −0.855 * | −0.090 | −0.191 | −0.541 |
| p (2-sided) | 0.878 | 0.355 | 0.427 | 0.175 | 0.452 | 0.67 | 0.003 | 0.014 | 0.848 | 0.682 | 0.21 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| ΔsysRR [mmHg] | correlation[ρ] | −0.324 | 0.667 | −0.036 | 0.757 * | 0.631 | 0.523 | −0.873 * | −0.964 *** | −0.450 | −0.445 | −0.396 | |
| p (2-sided) | 0.478 | 0.102 | 0.939 | 0.049 | 0.129 | 0.229 | 0.01 | <0.001 | 0.31 | 0.317 | 0.379 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| Δresting HR [bpm] | correlation[ρ] | −0.074 | −0.296 | −0.111 | −0.037 | 0.111 | −0.296 | 0.187 | 0.112 | 0.408 | −0.224 | 0.741 | |
| p (2-sided) | 0.875 | 0.518 | 0.812 | 0.937 | 0.812 | 0.518 | 0.688 | 0.811 | 0.364 | 0.629 | 0.057 | ||
| n | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | ||
| t2 | n | Min | Max | MD | ME | SD | p | ||
|---|---|---|---|---|---|---|---|---|---|
| amino acids | Cit (µmol/L) | pre | 26 | 23.3 | 60.3 | 36.00 | 37.20 | 10.76 | |
| post | 26 | 23.5 | 90.2 | 32.60 | 36.03 | 13.70 | |||
| Δ | 26 | −24.6 | 29.9 | −0.85 | −1.17 | 11.40 | 0.464 | ||
| Met (µmol/L) | pre | 26 | 5.3 | 32.1 | 8.70 | 11.89 | 6.73 | ||
| post | 26 | 5.1 | 31.0 | 9.20 | 12.57 | 7.13 | |||
| Δ | 26 | −5.3 | 8.3 | 0.00 | 0.67 | 3.20 | 0.579 | ||
| Phe (µmol/L) | pre | 26 | 42.6 | 112.9 | 59.30 | 63.22 | 15.97 | ||
| post | 26 | 45.5 | 136.3 | 58.45 | 64.23 | 18.47 | |||
| Δ | 26 | −15.4 | 23.4 | −0.30 | 1.00 | 9.67 | 0.886 | ||
| Tyr (µmol/L) | pre | 26 | 38.9 | 137.2 | 62.90 | 65.99 | 20.07 | ||
| post | 26 | 37.6 | 117.8 | 64.85 | 69.34 | 18.76 | |||
| Δ | 26 | −19.4 | 37.5 | 0.75 | 3.35 | 11.32 | 0.210 | ||
| Val (µmol/L) | pre | 26 | 91.7 | 324.5 | 139.35 | 145.65 | 48.15 | ||
| post | 26 | 91.4 | 277.7 | 137.15 | 144.50 | 39.17 | |||
| Δ | 26 | −46.8 | 32.7 | 0.45 | −1.14 | 18.37 | 0.920 | ||
| Xle (µmol/L) | pre | 26 | 101.8 | 389.1 | 161.00 | 175.87 | 72.28 | ||
| post | 26 | 100.0 | 320.3 | 155.85 | 171.62 | 58.62 | |||
| Δ | 26 | −84.8 | 37.5 | −2.80 | −4.26 | 29.30 | 0.745 | ||
| (acyl-)carnitines | C0 (µmol/L) | pre | 26 | 14.6 | 58.1 | 30.64 | 32.57 | 11.77 | |
| post | 26 | 15.3 | 56.7 | 27.98 | 31.35 | 10.67 | |||
| Δ | 26 | −13.8 | 4.9 | −0.40 | −1.22 | 4.76 | 0.437 | ||
| Cx (µmol/L) | pre | 26 | 5.1 | 71.9 | 41.55 | 43.66 | 14.52 | ||
| post | 26 | 30.9 | 75.5 | 44.35 | 47.12 | 12.03 | |||
| Δ | 26 | −12.4 | 52.8 | 3.20 | 3.46 | 11.35 | 0.070 | ||
| SC-ACs (µmol/L) | pre | 26 | 2.6 | 32.8 | 8.12 | 10.16 | 7.29 | ||
| post | 26 | 3.6 | 35.0 | 10.55 | 12.36 | 7.06 | |||
| Δ | 26 | −3.2 | 8.1 | 2.19 | 2.20 | 2.27 | <0.001 ** | ||
| MC-ACs (µmol/L) | pre | 26 | 0.1 | 0.6 | 0.21 | 0.23 | 0.12 | ||
| post | 26 | 0.1 | 0.8 | 0.23 | 0.26 | 0.14 | |||
| Δ | 26 | −0.1 | 0.2 | 0.03 | 0.03 | 0.06 | 0.027 * | ||
| LC-ACs (µmol/L) | pre | 26 | 1.7 | 4.0 | 2.95 | 2.96 | 0.67 | ||
| post | 26 | 1.9 | 5.0 | 2.98 | 3.13 | 0.90 | |||
| Δ | 26 | −0.9 | 1.7 | 0.15 | 0.17 | 0.54 | 0.147 |
| Δphysiological/Clinical Parameters (t0–t2) | Statistics | Amino Acid Mobilization During Acute Bout of Exercise at t2 | (Acyl-)carnitine Mobilization During Acute Bout of Exercise at t2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cit | Met | Phe | Tyr | Val | Xle | C0 | Cx | SC- ACs | MC-ACs | LC- ACs | |||
| sports | ΔrelVO2max [mL/kgxmin] | correlation [ρ] | −0.047 | 0.012 | −0.002 | −0.034 | 0.014 | −0.087 | −0.079 | −0.040 | 0.230 | −0.170 | 0.124 |
| p (2-sided) | 0.819 | 0.955 | 0.993 | 0.867 | 0.945 | 0.672 | 0.703 | 0.846 | 0.258 | 0.407 | 0.547 | ||
| n | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | ||
| ΔPPO [W/kg] | correlation [ρ] | 0.024 | −0.073 | −0.170 | −0.077 | −0.121 | −0.158 | −0.001 | −0.011 | 0.044 | −0.480 * | 0.113 | |
| p (2-sided) | 0.910 | 0.735 | 0.427 | 0.719 | 0.573 | 0.460 | 0.997 | 0.960 | 0.838 | 0.018 | 0.599 | ||
| n | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | 24 | ||
| body weight | ΔBMI [kg/m2] | correlation [ρ] | −0.090 | −0.324 | −0.080 | 0.015 | −0.169 | −0.032 | 0.017 | −0.028 | −0.266 | −0.124 | −0.240 |
| p (2-sided) | 0.663 | 0.107 | 0.699 | 0.941 | 0.409 | 0.875 | 0.935 | 0.893 | 0.188 | 0.545 | 0.238 | ||
| n | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | 26 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankamp, R.; Schweda, S.; Janzen, N.; Nieß, A.M.; Krauß, I.; Munz, B. Metabolomics in the Context of Exercise in Subjects with Multimorbidity: A Pilot Study. Biomolecules 2025, 15, 1474. https://doi.org/10.3390/biom15101474
Bankamp R, Schweda S, Janzen N, Nieß AM, Krauß I, Munz B. Metabolomics in the Context of Exercise in Subjects with Multimorbidity: A Pilot Study. Biomolecules. 2025; 15(10):1474. https://doi.org/10.3390/biom15101474
Chicago/Turabian StyleBankamp, Rebecca, Simone Schweda, Nils Janzen, Andreas M. Nieß, Inga Krauß, and Barbara Munz. 2025. "Metabolomics in the Context of Exercise in Subjects with Multimorbidity: A Pilot Study" Biomolecules 15, no. 10: 1474. https://doi.org/10.3390/biom15101474
APA StyleBankamp, R., Schweda, S., Janzen, N., Nieß, A. M., Krauß, I., & Munz, B. (2025). Metabolomics in the Context of Exercise in Subjects with Multimorbidity: A Pilot Study. Biomolecules, 15(10), 1474. https://doi.org/10.3390/biom15101474






