Engineered N-TIMP2 Variant Specifically Targeting MMP-9 Exhibits Potent Anti-Glioblastoma Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. N-TIMP2 Expression and Purification
2.2. Cell Lines
2.3. Colony Formation Assay
2.4. Cell Invasion Assay
2.5. Spheroid Invasion Assay
2.6. MTT (3-(4,5-Dimethylthiazol-2-Vl)-2,5-Diphenyl Tetrazolium Bromide) Assay
2.7. Enzymatic Inhibition Assay
3. Results
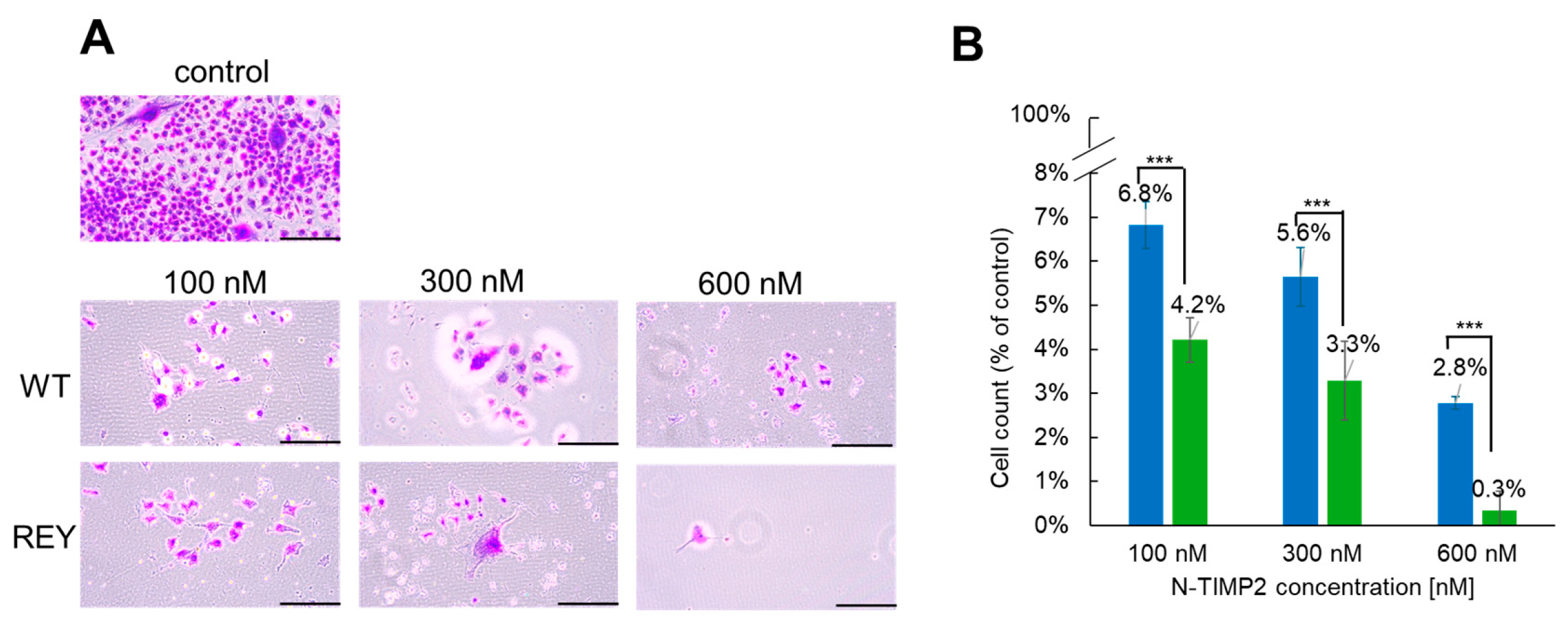
3.1. N-TIMP2 Variants Inhibit Cell Survival and Proliferation
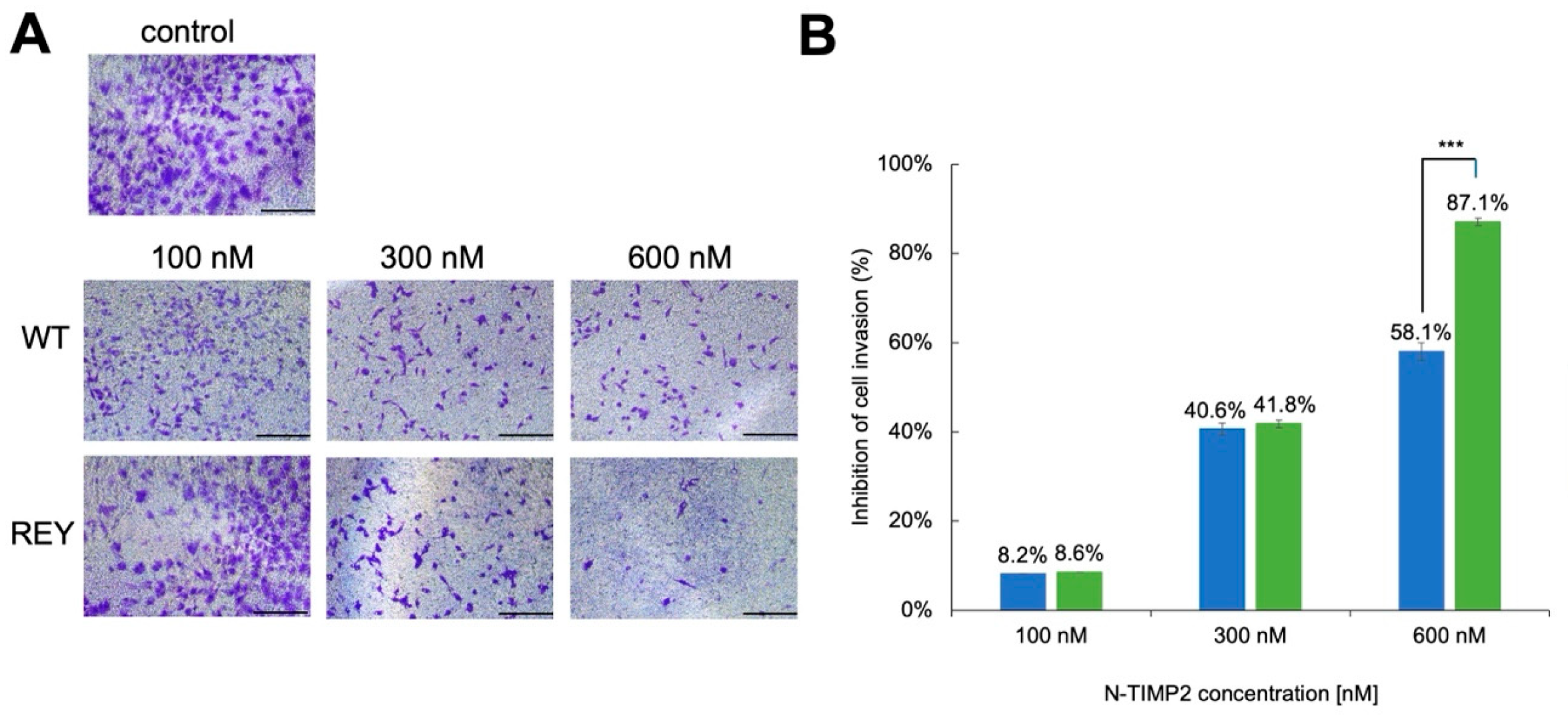
3.2. N-TIMP2 Variants Inhibit Cell Invasion
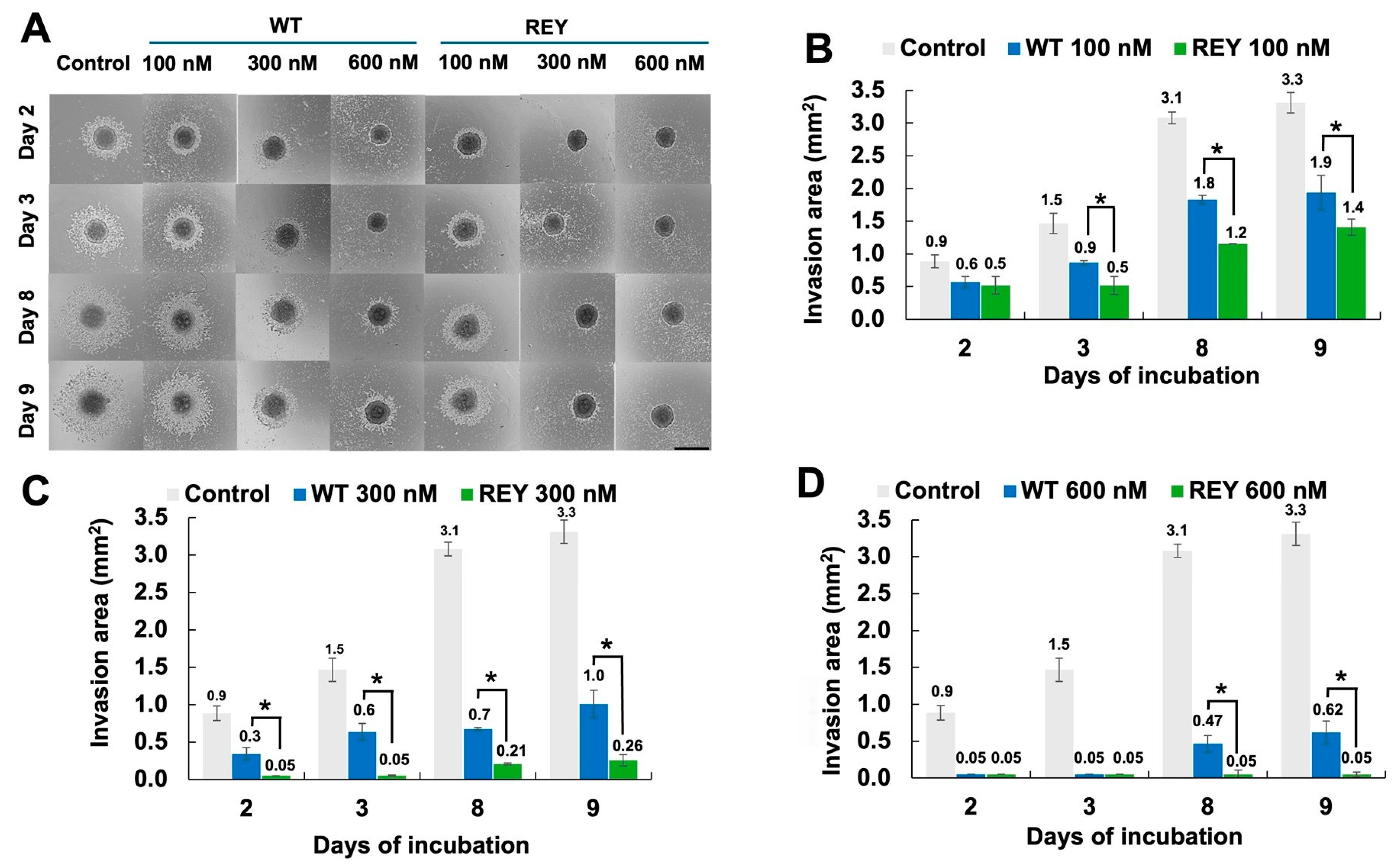
3.3. N-TIMP2 Variants Inhibit U251 Spheroid Spread
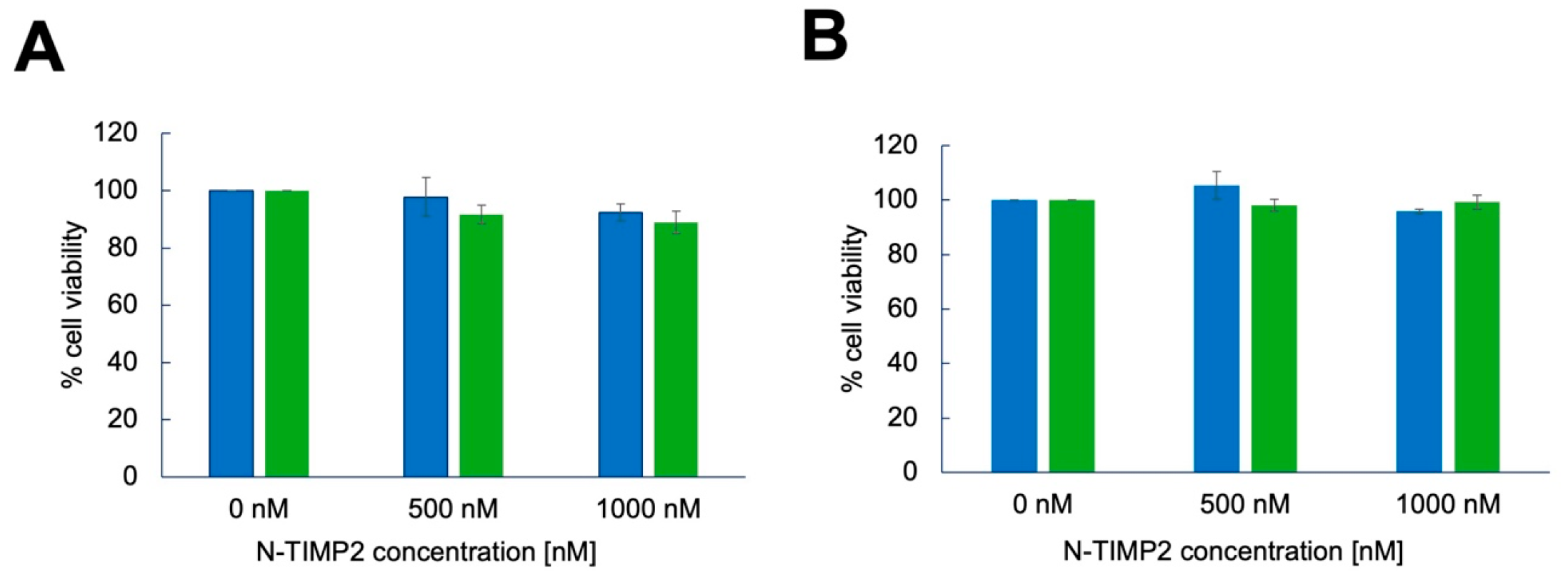
3.4. N-TIMP2 Variants Are Not Cytotoxic to U251 GB Cells and Healthy Vero Cells
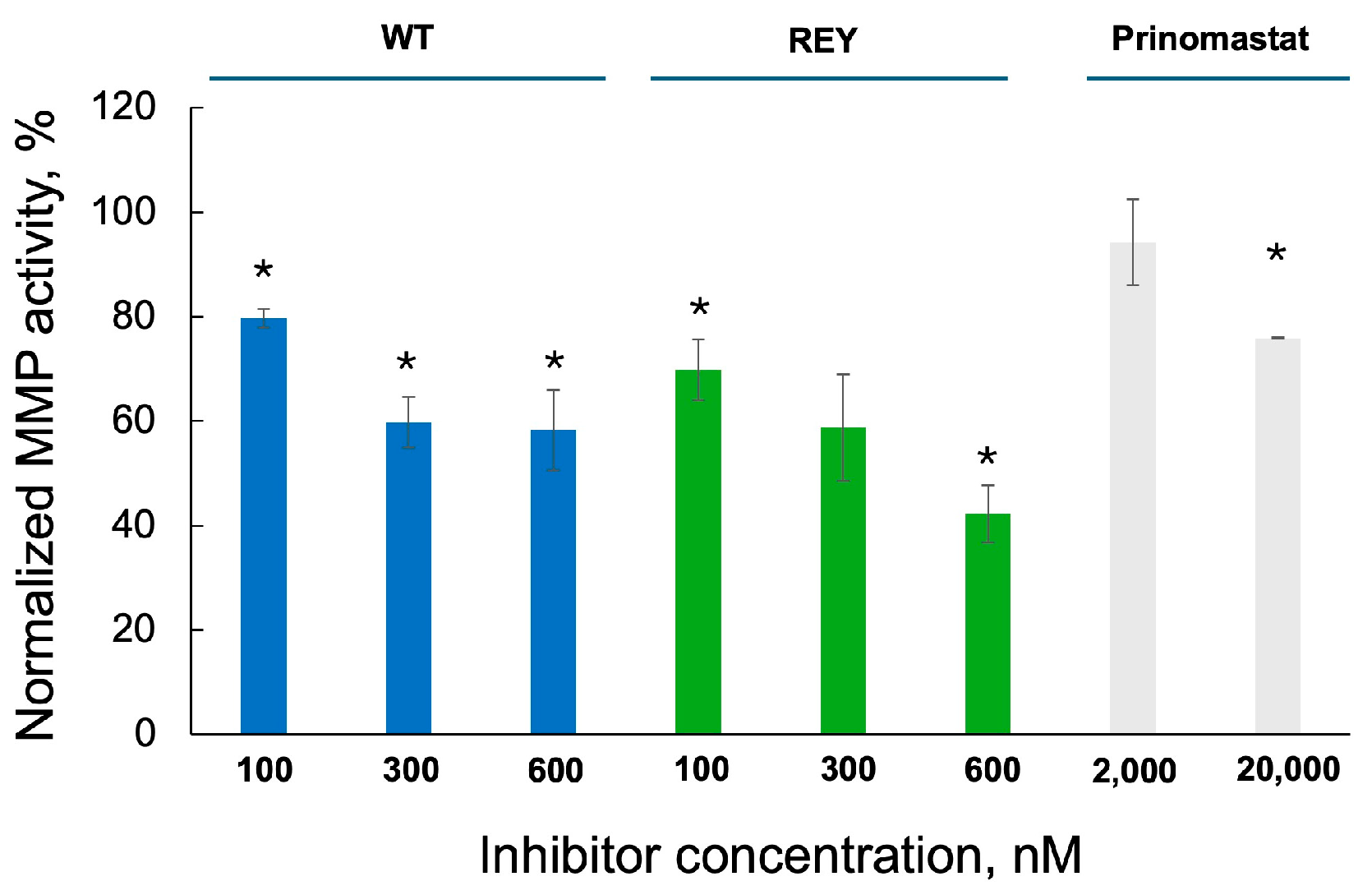
3.5. N-TIMP2 Variants Inhibit Enzymatic Activity of MMP-9 Released by U251 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GB | Glioblastoma |
| MMP | Matrix metalloproteinase |
| TMZ | Temozolomide |
| WT | Wild type |
| BBB | Blood–brain barrier |
| ECM | Extracellular matrix |
| N-TIMP2 | N-terminal domain of tissue inhibitor of metalloproteinase |
| MTT | 3-(4,5-dimethylthiazol-2-vl)-2,5-diphenyl tetrazolium bromide |
References
- Drappatz, J.; Norden, A.D.; Wen, P.Y. Therapeutic strategies for inhibiting invasion in glioblastoma. Expert Rev. Neurother. 2009, 9, 519–534. [Google Scholar]
- Hambardzumyan, D.; Bergers, G. Glioblastoma: Defining tumor niches. Trends Cancer 2015, 1, 252–265. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinases as breast cancer drivers and therapeutic targets. Front. Biosci. Landmark Ed. 2015, 20, 1144–1163. [Google Scholar] [CrossRef]
- Radisky, E.S.; Raeeszadeh-Sarmazdeh, M.; Radisky, D.C. Therapeutic Potential of Matrix Metalloproteinase Inhibition in Breast Cancer. J. Cell Biochem. 2017, 118, 3531–3548. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar]
- Bergers, G.; Brekken, R.; McMahon, G.; Vu, T.H.; Itoh, T.; Tamaki, K.; Tanzawa, K.; Thorpe, P.; Itohara, S.; Werb, Z.; et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2000, 2, 737–744. [Google Scholar] [CrossRef]
- Nozawa, H.; Chiu, C.; Hanahan, D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 12493–12498. [Google Scholar] [PubMed]
- Chantrain, C.F.; Shimada, H.; Jodele, S.; Groshen, S.; Ye, W.; Shalinsky, D.R.; Werb, Z.; Coussens, L.M.; DeClerck, Y.A. Stromal matrix metalloproteinase-9 regulates the vascular architecture in neuroblastoma by promoting pericyte recruitment. Cancer Res. 2004, 64, 1675–1686. [Google Scholar] [CrossRef]
- Jodele, S.; Chantrain, C.F.; Blavier, L.; Lutzko, C.; Crooks, G.M.; Shimada, H.; Coussens, L.M.; Declerck, Y.A. The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metalloproteinase-9 dependent. Cancer Res. 2005, 65, 3200–3208. [Google Scholar] [CrossRef]
- Acuff, H.B.; Carter, K.J.; Fingleton, B.; Gorden, D.L.; Matrisian, L.M. Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Res. 2006, 66, 259–266. [Google Scholar]
- Deryugina, E.I.; Quigley, J.P. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: Contrasting, overlapping and compensatory functions. Biochim. Et. Biophys. Acta 2010, 1803, 103–120. [Google Scholar] [CrossRef]
- Bauvois, B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: Outside-in signaling and relationship to tumor progression. Biochim. Et. Biophys. Acta 2012, 1825, 29–36. [Google Scholar] [CrossRef]
- Mehner, C.; Hockla, A.; Miller, E.; Ran, S.; Radisky, D.C.; Radisky, E.S. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014, 5, 2736–2749. [Google Scholar] [PubMed]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-infiltrating neutrophils constitute the major in vivo source of angiogenesis-inducing MMP-9 in the tumor microenvironment. Neoplasia 2014, 16, 771–788. [Google Scholar] [PubMed]
- Xue, Q.; Cao, L.; Chen, X.Y.; Zhao, J.; Gao, L.; Li, S.Z.; Fei, Z. High expression of MMP9 in glioma affects cell proliferation and is associated with patient survival rates. Oncol. Lett. 2017, 13, 1325–1330. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef] [PubMed]
- Thanh, H.D.; Lee, S.; Nguyen, T.T.; Huu, T.N.; Ahn, E.J.; Cho, S.H.; Kim, M.S.; Moon, K.S.; Jung, C. Temozolomide promotes matrix metalloproteinase 9 expression through p38 MAPK and JNK pathways in glioblastoma cells. Sci. Rep. 2024, 14, 14341. [Google Scholar] [CrossRef]
- Quesnel, A.; Coles, N.; Polvikoski, T.M.; Karagiannis, G.S.; Angione, C.; Islam, M.; Khundakar, A.A.; Filippou, P.S. The diagnostic and prognostic potential of the EGFR/MUC4/MMP9 axis in glioma patients. Sci. Rep. 2022, 12, 19868. [Google Scholar]
- Asuthkar, S.; Velpula, K.K.; Chetty, C.; Gorantla, B.; Rao, J.S. Epigenetic regulation of miRNA-211 by MMP-9 governs glioma cell apoptosis, chemosensitivity and radiosensitivity. Oncotarget 2012, 3, 1439. [Google Scholar] [CrossRef]
- Veeravalli, K.K.; Rao, J.S. MMP-9 and uPAR regulated glioma cell migration. Cell Adhes. Migr. 2012, 6, 509–512. [Google Scholar] [CrossRef]
- Dobra, G.; Gyukity-Sebestyén, E.; Bukva, M.; Harmati, M.; Nagy, V.; Szabó, Z.; Pankotai, T.; Klekner, Á.; Buzás, K. MMP-9 as prognostic marker for brain tumours: A comparative study on serum-derived small extracellular vesicles. Cancers 2023, 15, 712. [Google Scholar] [CrossRef]
- Taheri, E.; Raeeszadeh-Sarmazdeh, M. Effect of TIMPs and their minimally engineered variants in blocking invasion and migration of brain cancer cells. Oncotarget 2025, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Aitchison, E.; Dimesa, A.; Shoari, A. Matrix Metalloproteinases in Glioma: Drivers of Invasion and Therapeutic Targets. BioTech 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- DE Oliveira Rosario, L.V.; DA Rosa, B.G.; Goncalves, T.L.; Matias, D.I.L.; Freitas, C.; Ferrer, V.P. Glioblastoma factors increase the migration of human brain endothelial cells in vitro by increasing MMP-9/CXCR4 levels. Anticancer Res. 2020, 40, 2725–2737. [Google Scholar] [PubMed]
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Das, T.; Bhar, S.; Ghosh, D.; Kabi, B.; Kar, K.; Chandra, A. A promising future for breast cancer therapy with hydroxamic acid-based histone deacetylase inhibitors. Bioorganic Chem. 2025, 156, 108169. [Google Scholar] [CrossRef]
- Dragu, D.; Necula, L.G.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Soluble PD-L1: From Immune Evasion to Cancer Therapy. Life 2025, 15, 626. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B. Extracellular matrix stiffness: Mechanisms in tumor progression and therapeutic potential in cancer. Exp. Hematol. Oncol. 2025, 14, 54. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef]
- Overall, C.M.; Kleifeld, O. Tumour microenvironment-opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Cheng, J.; Li, P.; Tian, L.; Chen, Z.; Chen, Z.; Li, Y.; Song, J. Association study for the role of MMP8 gene polymorphisms in Colorectal cancer susceptibility. BMC Cancer 2023, 23, 1169. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Matrisian, L.M. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev. 2007, 26, 717–724. [Google Scholar] [CrossRef]
- Arkadash, V.; Yosef, G.; Shirian, J.; Cohen, I.; Horev, Y.; Grossman, M.; Sagi, I.; Radisky, E.S.; Shifman, J.M.; Papo, N. Development of high-affinity and high-specificity inhibitors of metalloproteinase 14 through computational design and directed evolution. J. Biol. Chem. 2017, 292, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Bonadio, A.; Wenig, B.L.; Hockla, A.; Radisky, E.S.; Shifman, J.M. Designed Loop Extension Followed by Combinatorial Screening Confers High Specificity to a Broad Matrix MetalloproteinaseInhibitor. J. Mol. Biol. 2023, 435, 168095. [Google Scholar]
- Raeeszadeh-Sarmazdeh, M.; Greene, K.A.; Sankaran, B.; Downey, G.P.; Radisky, D.C.; Radisky, E.S. Directed evolution of the metalloproteinase inhibitor TIMP-1 reveals that its N- and C-terminal domains cooperate in matrix metalloproteinase recognition. J. Biol. Chem. 2019, 294, 9476–9488. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Coban, M.; Mahajan, S.; Hockla, A.; Sankaran, B.; Downey, G.P.; Radisky, D.C.; Radisky, E.S. Engineering of tissue inhibitor of metalloproteinases TIMP-1 for fine discrimination between closely related stromelysins MMP-3 and MMP-10. J. Biol. Chem. 2022, 298, 101654. [Google Scholar] [CrossRef]
- Shirian, J.; Arkadash, V.; Cohen, I.; Sapir, T.; Radisky, E.S.; Papo, N.; Shifman, J.M. Converting a broad matrix metalloproteinase family inhibitor into a specific inhibitor of MMP-9 and MMP-14. FEBS Lett. 2018, 592, 1122–1134. [Google Scholar] [CrossRef]
- Rotenberg, N.; Feldman, M.; Shirian, J.; Hockla, A.; Radisky, E.S.; Shifman, J.M. Engineered TIMP2 with narrow MMP-9 specificity is an effective inhibitor of invasion and proliferation of triple-negative breast cancer cells. J. Biol. Chem. 2024, 300, 107867. [Google Scholar] [CrossRef]
- Zahn, I.; Braun, T.; Gögele, C.; Schulze-Tanzil, G. Minispheroids as a tool for ligament tissue engineering: Do the self-assembly techniques and spheroid dimensions influence the cruciate ligamentocyte phenotype? Int. J. Mol. Sci. 2021, 22, 11011. [Google Scholar] [CrossRef]
- Tao, F.; Kitamura, K.; Hanada, S.; Sugimoto, K.; Furihata, T.; Kojima, N. Rapid and stable formation method of human astrocyte spheroid in a high viscous methylcellulose medium and its functional advantages. Bioengineering 2023, 10, 349. [Google Scholar] [CrossRef]
- Dabkeviciute, G.; Maccioni, E.; Petrikaite, V. Effect of sunitinib derivatives on glioblastoma single-cell migration and 3D cell cultures. Am. J. Cancer Res. 2023, 13, 1377. [Google Scholar] [PubMed]
- Somasundaram, D.B.; Aravindan, S.; Major, R.; Natarajan, M.; Aravindan, N. MMP-9 reinforces radiation-induced delayed invasion and metastasis of neuroblastoma cells through second-signaling positive feedback with NFκB via both ERK and IKK activation. Cell Biol. Toxicol. 2023, 39, 1053–1076. [Google Scholar] [PubMed]
- Chen, X.-C.; Wei, X.-T.; Guan, J.-H.; Shu, H.; Chen, D. EGF stimulates glioblastoma metastasis by induction of matrix metalloproteinase-9 in an EGFR-dependent mechanism. Oncotarget 2017, 8, 65969. [Google Scholar] [CrossRef]
- Musumeci, G.; Magro, G.; Cardile, V.; Coco, M.; Marzagalli, R.; Castrogiovanni, P.; Imbesi, R.; Graziano, A.C.E.; Barone, F.; Di Rosa, M. Characterization of matrix metalloproteinase-2 and-9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res. 2015, 362, 45–60. [Google Scholar] [CrossRef]
- Mangani, S.; Kremmydas, S.; Karamanos, N.K. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers 2025, 17, 1161. [Google Scholar] [CrossRef]
- Blondeel, E.; Peirsman, A.; Vermeulen, S.; Piccinini, F.; De Vuyst, F.; Estêvão, D.; Al-Jamei, S.; Bedeschi, M.; Castellani, G.; Cruz, T. The Spheroid Light Microscopy Image Atlas for morphometrical analysis of three-dimensional cell cultures. Sci. Data 2025, 12, 283. [Google Scholar] [CrossRef]
- Welser-Alves, J.V.; Crocker, S.J.; Milner, R. A dual role for microglia in promoting tissue inhibitor of metalloproteinase (TIMP) expression in glial cells in response to neuroinflammatory stimuli. J. Neuroinflammation 2011, 8, 61. [Google Scholar] [CrossRef]
- Britton, R.; Wasley, T.; Harish, R.; Holz, C.; Hall, J.; Yee, D.C.; Melton Witt, J.; Booth, E.A.; Braithwaite, S.; Czirr, E.; et al. Noncanonical Activity of Tissue Inhibitor of Metalloproteinases 2 (TIMP2) Improves Cognition and Synapse Density in Aging. eNeuro 2023, 10, ENEURO.0031-23.2023. [Google Scholar] [CrossRef]
- Precilla, S.D.; Kuduvalli, S.S.; Praveena, E.A.; Thangavel, S.; Anitha, T. Integration of synthetic and natural derivatives revives the therapeutic potential of temozolomide against glioma-an in vitro and in vivo perspective. Life Sci. 2022, 301, 120609. [Google Scholar]
- Dario, A.; Tomei, G. The safety of the temozolomide in patients with malignant glioma. Curr. Drug Saf. 2006, 1, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Scaringi, C.; De Sanctis, V.; Minniti, G.; Enrici, R.M. Temozolomide-related hematologic toxicity. Oncol. Res. Treat. 2013, 36, 444–449. [Google Scholar] [CrossRef] [PubMed]
- De Vita, S.; De Matteis, S.; Laurenti, L.; Chiusolo, P.; Reddiconto, G.; Fiorini, A.; Leone, G.; Sica, S. Secondary Ph+ acute lymphoblastic leukemia after temozolomide. Ann. Hematol. 2005, 84, 760–762. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldman, M.; Rotenberg, N.; Shifman, J.M. Engineered N-TIMP2 Variant Specifically Targeting MMP-9 Exhibits Potent Anti-Glioblastoma Activity. Biomolecules 2025, 15, 1470. https://doi.org/10.3390/biom15101470
Feldman M, Rotenberg N, Shifman JM. Engineered N-TIMP2 Variant Specifically Targeting MMP-9 Exhibits Potent Anti-Glioblastoma Activity. Biomolecules. 2025; 15(10):1470. https://doi.org/10.3390/biom15101470
Chicago/Turabian StyleFeldman, Mark, Naama Rotenberg, and Julia M. Shifman. 2025. "Engineered N-TIMP2 Variant Specifically Targeting MMP-9 Exhibits Potent Anti-Glioblastoma Activity" Biomolecules 15, no. 10: 1470. https://doi.org/10.3390/biom15101470
APA StyleFeldman, M., Rotenberg, N., & Shifman, J. M. (2025). Engineered N-TIMP2 Variant Specifically Targeting MMP-9 Exhibits Potent Anti-Glioblastoma Activity. Biomolecules, 15(10), 1470. https://doi.org/10.3390/biom15101470






