Chemerin in Pulmonary Fibrosis: Advances in Mechanistic and Fundamental Research
Abstract
1. Introduction
2. Elevated Chemerin May Promote Pulmonary Fibrosis
3. Functional Role of Chemerin in Pulmonary Fibrosis
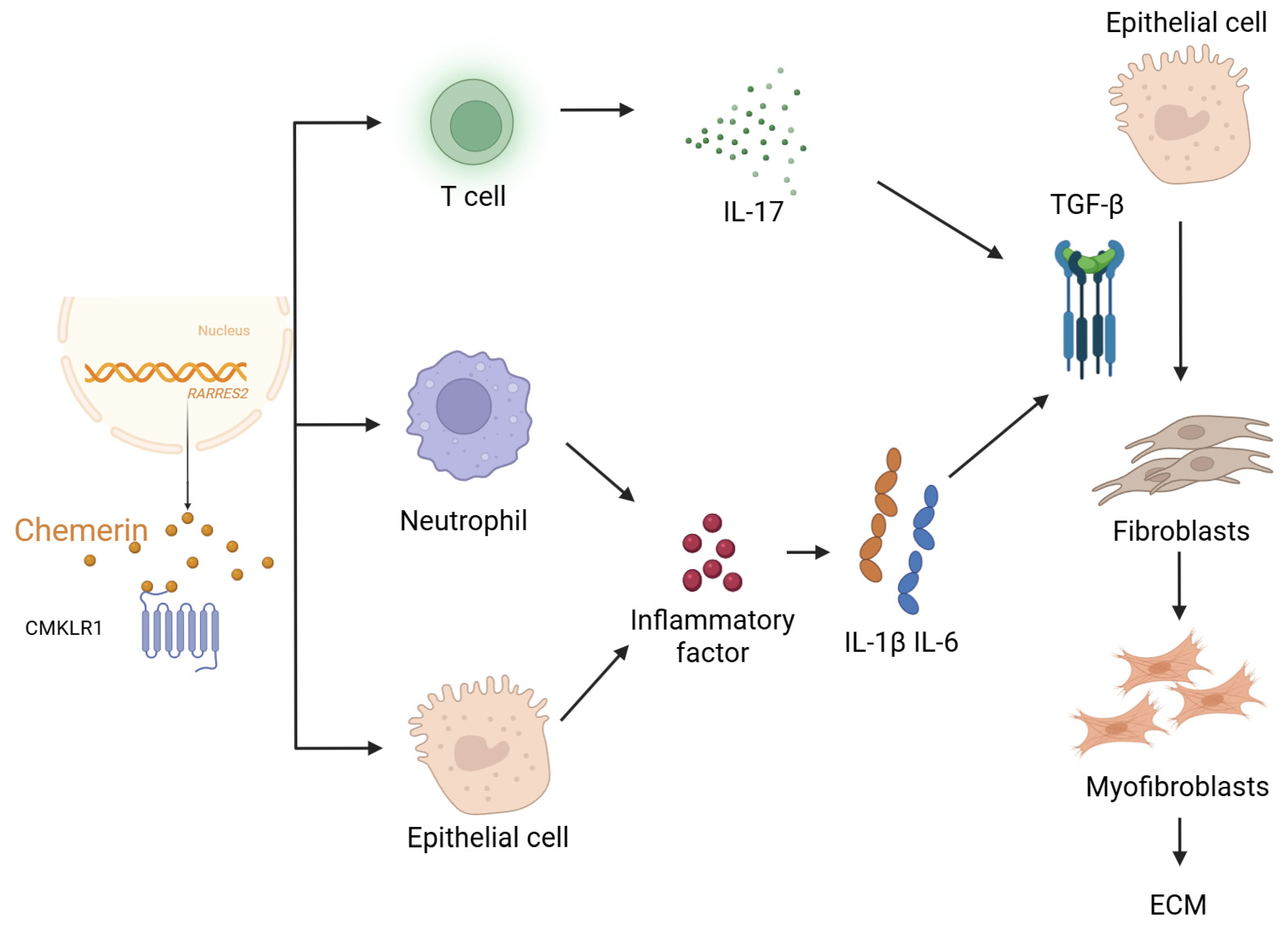
3.1. Mechanistic Basis for Chemerin’s Involvement in Pulmonary Fibrosis
3.2. Etiological and Risk Factors Associated with Chemerin in Pulmonary Fibrosis
3.3. Clinical Relevance of Chemerin in Pulmonary Fibrosis
3.4. Therapeutic Implications of Targeting Chemerin in Pulmonary Fibrosis
4. Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavis, P.; Bondue, B.; Cardozo, A.K. The Dual Role of Chemerin in Lung Diseases. Cells 2024, 13, 171. [Google Scholar] [CrossRef]
- Su, X.; Cheng, Y.; Zhang, G.; Wang, B. Chemerin in inflammatory diseases. Clin. Chim. Acta 2021, 517, 41–47. [Google Scholar] [CrossRef]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; Le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific Recruitment of Antigen-Presenting Cells by Chemerin, a Novel Processed Ligand from Human Inflammatory Fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balan, P.; Popovich, D.G. The Effects of New Zealand Grown Ginseng Fractions on Cytokine Production from Human Monocytic THP-1 Cells. Molecules 2021, 26, 1158. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor. Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Ham-Karim, H.; Negm, O.; Ahmad, N.; Ilyas, M. Investigating genomic, proteomic, and post-transcriptional regulation profiles in colorectal cancer: A comparative study between primary tumors and associated metastases. Cancer Cell Int. 2023, 23, 192. [Google Scholar] [CrossRef]
- Ben Dhaou, C.; Mandi, K.; Frye, M.; Acheampong, A.; Radi, A.; De Becker, B.; Antoine, M.; Baeyens, N.; Wittamer, V.; Parmentier, M. Chemerin regulates normal angiogenesis and hypoxia-driven neovascularization. Angiogenesis 2022, 25, 159–179. [Google Scholar] [CrossRef]
- Kennedy, A.J.; Davenport, A.P. International Union of Basic and Clinical Pharmacology CIII: Chemerin Receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2018, 70, 174–196. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Estecha, A.; Samaniego, R.; Sánchez-Ramón, S.; Vega, M.Á.; Sánchez-Mateos, P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 2011, 117, 88–97. [Google Scholar] [CrossRef]
- Wood, B.M.; Bossuyt, J. Emergency Spatiotemporal Shift: The Response of Protein Kinase D to Stress Signals in the Cardiovascular System. Front. Pharmacol. 2017, 8, 9. [Google Scholar] [CrossRef]
- Wheeler, A.M.; Baker, J.F.; Riley, T.R., IV; Johnson, T.M.; Yang, Y.; Roul, P.; Wysham, K.D.; Cannon, G.W.; Kunkel, G.; Kerr, G.; et al. Peripheral Biomarker Signatures in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2025, 77, 971–980. [Google Scholar] [CrossRef]
- Kubota, K.; Ohtake, N.; Ohbuchi, K.; Mase, A.; Imamura, S.; Sudo, Y.; Miyano, K.; Yamamoto, M.; Kono, T.; Uezono, Y. Hydroxy-alpha sanshool induces colonic motor activity in rat proximal colon: A possible involvement of KCNK9. Am. J. Physiol. Liver Physiol. 2015, 308, G579–G590. [Google Scholar] [CrossRef]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef]
- Kim, K.; Lee, J.; Jo, Y.S. Factors for progressive pulmonary fibrosis in connective tissue disease-related interstitial lung disease. Ther. Adv. Respir. Dis. 2023, 17, 17534666231212301. [Google Scholar] [CrossRef] [PubMed]
- Mottais, A.; Riberi, L.; Falco, A.; Soccal, S.; Gohy, S.; De Rose, V. Epithelial–Mesenchymal Transition Mechanisms in Chronic Airway Diseases: A Common Process to Target? Int. J. Mol. Sci. 2023, 24, 12412. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- DeCuzzi, N.L.; Oberbauer, D.P.; Chmiel, K.J.; Pargett, M.; Ferguson, J.M.; Murphy, D.; Zeki, A.A.; Albeck, J.G. Spatiotemporal Clusters of ERK Activity Coordinate Cytokine-induced Inflammatory Responses in Human Airway Epithelial Cells. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cai, R.; Cao, R.; Liu, Y.; Zhang, N.; Zhang, Y.; Wang, Y. Chemerin Promotes Proliferation of Cardiac Fibroblasts via CMKLR1/PI3k/Akt/NF-κB Signaling Pathway. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Pozharskaya, V.; Torres-González, E.; Rojas, M.; Gal, A.; Amin, M.; Dollard, S.; Roman, J.; Stecenko, A.A.; Mora, A.L. Twist: A regulator of epithelial-mesenchymal transition in lung fibrosis. PLoS ONE 2009, 4, e7559. [Google Scholar] [CrossRef]
- Zhao, D.; Bi, G.; Feng, J.; Huang, R.; Chen, X. Association of Serum Chemerin Levels with Acute Ischemic Stroke and Carotid Artery Atherosclerosis in a Chinese Population. Med. Sci. Monit. 2015, 21, 3121–3128. [Google Scholar] [CrossRef]
- Er, L.-K.; Wu, S.; Hsu, L.-A.; Teng, M.-S.; Sun, Y.-C.; Ko, Y.-L. Pleiotropic Associations of RARRES2 Gene Variants and Circulating Chemerin Levels: Potential Roles of Chemerin Involved in the Metabolic and Inflammation-Related Diseases. Mediat. Inflamm. 2018, 2018, 4670521. [Google Scholar] [CrossRef]
- Hsu, L.-A.; Chou, H.-H.; Teng, M.-S.; Wu, S.; Ko, Y.-L. Circulating chemerin levels are determined through circulating platelet counts in nondiabetic Taiwanese people: A bidirectional Mendelian randomization study. Atherosclerosis 2021, 320, 61–69. [Google Scholar] [CrossRef]
- Mannes, P.Z.; Adams, T.S.; Farsijani, S.; Barnes, C.E.; Latoche, J.D.; Day, K.E.; Nedrow, J.R.; Ahangari, F.; Kaminski, N.; Lee, J.S.; et al. Noninvasive assessment of the lung inflammation-fibrosis axis by targeted imaging of CMKLR1. Sci Adv. 2024, 10, eadm9817. [Google Scholar] [CrossRef]
- Peng, L.; Wen, L.; Shi, Q.-F.; Gao, F.; Huang, B.; Meng, J.; Hu, C.-P.; Wang, C.-M. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-kappaB/NLRP3-mediated epithelial–mesenchymal transition and inflammation. Cell Death Dis. 2020, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Kiczmer, P.; Terenowicz, M.; Katra, M.; Mielcarska, S.; Ziora, P.; Rydel, M.; Czyżewski, D.; Drozdzowska, B. Expression of chemerin and B7 family proteins in lung adenocarcinoma—Pilot study. Med. Res. J. 2024, 9, 148–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Wang, C.; Sun, J.; Lai, X.; Xu, Y.; Lan, X.; Lei, C.; Zhang, C.; Yang, D.; et al. Exploring polymorphisms of the bovine RARRES2 gene and their associations with growth traits. Mol. Biol. Rep. 2012, 39, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Sanges, S.; Rice, L.; Tu, L.; Cracowski, J.L.; Montani, D.; Mantero, J.; Ternynck, C.; Marot, G.; Hachlla, E.; Launay, D.; et al. Biomarkers of Hemodynamic Severity of Systemic Sclerosis-Associated Pulmonary Arterial Hypertension by Serum Proteome Analysis. Ann. Rheum. Dis. 2023, 82, 365–373. [Google Scholar] [CrossRef]
- Huang, W.J.; Tang, X.X. Virus infection induced pulmonary fibrosis. J. Transl. Med. 2021, 19, 496. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Y.; Ling, X.; Qin, H.; Wang, M.; Luo, B. Chemerin/CMKLR1 Axis Promotes Inflammation and Pyroptosis by Activating NLRP3 Inflammasome in Diabetic Cardiomyopathy Rat. Front. Physiol. 2020, 11, 381. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-beta Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef]
- Domvri, K.; Organtzis, I.; Apostolopoulos, A.; Fouka, E.; Kontakiotis, T.; Papakosta, D. Prognostic Value of Serum Biomarkers in Patients with Idiopathic Pulmonary Fibrosis in Relation to Disease Progression. J. Pers. Med. 2023, 13, 1307. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, A.; Washimkar, K.R.; Mugale, M.N. Unraveling the interplay between vital organelle stress and oxidative stress in idiopathic pulmonary fibrosis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119676. [Google Scholar] [CrossRef] [PubMed]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, T.; Bai, Y.; Sun, J.; Guo, J.; Yuan, H.; Shan, Z. Targeting myocardial inflammation: Investigating the therapeutic potential of atrial natriuretic peptide in atrial fibrosis. Mol. Biol. Rep. 2024, 51, 506. [Google Scholar] [CrossRef]
- Paranage, K. The Mahaweli Development Project and the ‘rendering technical’ of agrarian development in Sri Lanka. Heliyon 2019, 5, e01811. [Google Scholar] [CrossRef]
- Blatner, N.R.; Mulcahy, M.F.; Dennis, K.L.; Scholtens, D.; Bentrem, D.J.; Phillips, J.D.; Ham, S.; Sandall, B.P.; Khan, M.W.; Mahvi, D.M.; et al. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci. Transl. Med. 2012, 4, 164ra159. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, H.; Ke, S.; Mo, L.; Qiu, M.; Zhu, G.; Zhu, W.; Liu, L. Identifying potential biomarkers of idiopathic pulmonary fibrosis through machine learning analysis. Sci. Rep. 2023, 13, 16559. [Google Scholar] [CrossRef]
- Liu, J.; Xu, M.; Han, L.; Rao, Y.; Han, H.; Zheng, H.; Wu, J.; Sun, X. Chelerythrine Inhibits TGF-beta-Induced Epithelial–Mesenchymal Transition in A549 Cells via RRM2. Pharmaceuticals 2025, 18, 1036. [Google Scholar] [CrossRef]
- Finch, P.W.; Cross, L.J.M.; McAuley, D.F.; Farrell, C.L. Palifermin for the protection and regeneration of epithelial tissues following injury: New findings in basic research and pre-clinical models. J. Cell. Mol. Med. 2013, 17, 1065–1087. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, S.; Bettaieb, A.; Matsuo, K.; Matsuo, I.; Hosein, E.; Chahed, S.; Wiede, F.; Zhang, S.; Zhang, Z.-Y.; et al. Pancreatic T cell protein–tyrosine phosphatase deficiency affects beta cell function in mice. Diabetologia 2015, 58, 122–131. [Google Scholar] [CrossRef]
- El-Khodary, N.M.; Ghoneim, A.I.; El-Tayaar, A.A.; El-Touny, E.M. The Impact of Trimetazidine on Cardiac Fibrosis, Inflammation, and Function in Ischemic Cardiomyopathy Patients. Cardiovasc. Drugs Ther. 2023, 37, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Bai, B.; Li, H.; Feng, Y.; Sun, J.; Fang, Y.; Zheng, P.; Zhang, G. The role of oxidative stress-related genes in idiopathic pulmonary fibrosis. Sci. Rep. 2025, 15, 5954. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; O’leary, E.M.; Witt, L.J.; Tian, Y.; Gökalp, G.A.; Meliton, A.Y.; Dulin, N.O.; Mutlu, G.M. Glutamine Metabolism Is Required for Collagen Protein Synthesis in Lung Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019, 61, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Mannes, P.Z.; Barnes, C.E.; Biermann, J.; Latoche, J.D.; Day, K.E.; Zhu, Q.; Tabary, M.; Xiong, Z.; Nedrow, J.R.; Izar, B.; et al. Molecular imaging of chemokine-like receptor 1 (CMKLR1) in experimental acute lung injury. Proc. Natl. Acad. Sci. USA 2023, 120, e2216458120. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar]
- Jacenik, D.; Fichna, J. Chemerin in immune response and gastrointestinal pathophysiology. Clin. Chim. Acta 2020, 504, 146–153. [Google Scholar] [CrossRef]
- Kis, K.; Liu, X.; Hagood, J.S. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev. Mol. Med. 2011, 13, e27. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Li, N.; Li, P.; Wang, Z.; Ting, W.; Liu, X.; Wu, W. Chemerin: A Potential Regulator of Inflammation and Metabolism for Chronic Obstructive Pulmonary Disease and Pulmonary Rehabilitation. BioMed Res. Int. 2020, 2020, 4574509. [Google Scholar] [CrossRef]
- Yue, G.; An, Q.; Xu, X.; Jin, Z.; Ding, J.; Hu, Y.; Du, Q.; Xu, J.; Xie, R. The role of Chemerin in human diseases. Cytokine 2023, 162, 156089. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Xu, H.; Wei, Z.; Yang, Y.; Jin, F.; Zhang, M.; Wang, C.; Song, W.; Huo, J.; et al. ACE2 Attenuates Epithelial-Mesenchymal Transition in MLE-12 Cells Induced by Silica. Drug Des. Dev. Ther. 2020, 14, 1547–1559. [Google Scholar] [CrossRef]
- Ren, L.-L.; Li, X.-J.; Duan, T.-T.; Li, Z.-H.; Yang, J.-Z.; Zhang, Y.-M.; Zou, L.; Miao, H.; Zhao, Y.-Y. Transforming growth factor-beta signaling: From tissue fibrosis to therapeutic opportunities. Chem. Biol. Interact. 2023, 369, 110289. [Google Scholar] [CrossRef]
- Jayachandran, J.; Srinivasan, H.; Mani, K.P. Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 2021, 710, 108984. [Google Scholar] [CrossRef]
- Feng, J.; Liu, H.; Jiang, K.; Gong, X.; Huang, R.; Zhou, C.; Mao, J.; Chen, Y.; Xu, H.; Zhang, X.; et al. Enhanced oxidative stress aggravates BLM-induced pulmonary fibrosis by promoting cellular senescence through enhancing NLRP3 activation. Life Sci. 2024, 358, 123128. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Arndt, P. The Role of Chemerin in Neutrophil Activation and Diseases of the Lung. Biomedicines 2025, 13, 1354. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Kwitniewski, M.; Banas, M.; Zabieglo, K.; Murzyn, K.; Cichy, J. Chemerin regulation and role in host defense. Am. J. Clin. Exp. Immunol. 2014, 3, 1–19. [Google Scholar]
- Hu, H.; Bajorath, J. Data set of activity cliffs with single-atom modification and associated X-ray structure information for medicinal and computational chemistry applications. Data Brief 2020, 33, 106364. [Google Scholar] [CrossRef]
- Toukourou, H.; Uwambayinema, F.; Yakoub, Y.; Mertens, B.; Laleye, A.; Lison, D.; Quetin-Leclercq, J.; Gbaguidi, F. In Vitro and In Vivo Toxicity Studies on Cymbopogon giganteus Chiov. Leaves Essential Oil from Benin. J. Toxicol. 2020, 2020, 8261058. [Google Scholar] [CrossRef]
- Wang, H.; Sun, K.; Peng, H.; Wang, Y.; Zhang, L. Emerging roles of noncoding RNAs in idiopathic pulmonary fibrosis. Cell Death Discov. 2024, 10, 443. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wu, K.-J. Epigenetic regulation of epithelial-mesenchymal transition: Focusing on hypoxia and TGF-β signaling. J. Biomed. Sci. 2020, 27, 39. [Google Scholar] [CrossRef]
- Wu, F.; Yang, J.; Liu, J.; Wang, Y.; Mu, J.; Zeng, Q.; Deng, S.; Zhou, H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct. Target. Ther. 2021, 6, 218. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X. Crosstalk between Wnt/beta-catenin signaling pathway and DNA damage response in cancer: A new direction for overcoming therapy resistance. Front. Pharmacol. 2023, 14, 1230822. [Google Scholar] [CrossRef]
- Karampela, I.; Christodoulatos, G.S.; Vallianou, N.; Tsilingiris, D.; Chrysanthopoulou, E.; Skyllas, G.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Armaganidis, A.; et al. Circulating Chemerin and Its Kinetics May Be a Useful Diagnostic and Prognostic Biomarker in Critically Ill Patients with Sepsis: A Prospective Study. Biomolecules 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Kosałka-Węgiel, J.; Lichołai, S.; Pacholczak-Madej, R.; Dziedzina, S.; Milewski, M.; Kuszmiersz, P.; Korona, A.; Gąsior, J.; Matyja-Bednarczyk, A.; Kwiatkowska, H.; et al. Serum IL-17 and TNFalpha as prognostic biomarkers in systemic sclerosis patients: A prospective study. Rheumatol. Int. 2024, 44, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Dvorska, D.; Sebova, D.; Kajo, K.; Kapinova, A.; Svajdlenka, E.; Goga, M.; Frenak, R.; Treml, J.; Mersakova, S.; Strnadel, J.; et al. Chemopreventive and therapeutic effects of Hippophae rhamnoides L. fruit peels evaluated in preclinical models of breast carcinoma. Front. Pharmacol. 2025, 16, 1561436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, F.; Kang, L.; Wang, Z.; Wang, Y. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm. Med. 2017, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Omori, A.; Goshima, M.; Kakuda, C.; Kodama, T.; Otani, K.; Okada, M.; Yamawaki, H. Chemerin-9-induced contraction was enhanced through the upregulation of smooth muscle chemokine-like receptor 1 in isolated pulmonary artery of pulmonary arterial hypertensive rats. Pflugers Arch. Eur. J. Physiol. 2020, 472, 335–342. [Google Scholar] [CrossRef]
- Kumar, V.; LaJevic, M.; Pandrala, M.; Jacobo, S.A.; Malhotra, S.V.; Zabel, B.A. Novel CMKLR1 Inhibitors for Application in Demyelinating Disease. Sci. Rep. 2019, 9, 7178. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, L. Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction. J. Transl. Med. 2022, 20, 141. [Google Scholar] [CrossRef]
- Xia, H.; Diebold, D.; Nho, R.; Perlman, D.; Kleidon, J.; Kahm, J.; Avdulov, S.; Peterson, M.; Nerva, J.; Bitterman, P.; et al. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J. Exp. Med. 2008, 205, 1659–1672. [Google Scholar] [CrossRef]
- Meng, C.; Fan, G.; Liu, J.; Tao, N.; Sun, T. Pirfenidone and nintedanib exert additive antifibrotic effects by the SPP1-AKT pathway in macrophages and fibroblasts. Biochem. Biophys. Res. Commun. 2024, 716, 150020. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Y.; Zhang, Y.; Li, S.; Zhao, M.; Xia, T.; Meng, H. Pulmonary Delivery of Specialized Pro-Resolving Mediators-Based Nanotherapeutics Attenuates Pulmonary Fibrosis in Preclinical Animal Models. ACS Nano 2023, 17, 15354–15370. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, Z.; Huang, Z.; Dong, J.; Qian, L. Chemerin in Pulmonary Fibrosis: Advances in Mechanistic and Fundamental Research. Biomolecules 2025, 15, 1469. https://doi.org/10.3390/biom15101469
Jiang Y, Li Z, Huang Z, Dong J, Qian L. Chemerin in Pulmonary Fibrosis: Advances in Mechanistic and Fundamental Research. Biomolecules. 2025; 15(10):1469. https://doi.org/10.3390/biom15101469
Chicago/Turabian StyleJiang, Yongshuai, Ziyang Li, Zhenghang Huang, Junsheng Dong, and Li Qian. 2025. "Chemerin in Pulmonary Fibrosis: Advances in Mechanistic and Fundamental Research" Biomolecules 15, no. 10: 1469. https://doi.org/10.3390/biom15101469
APA StyleJiang, Y., Li, Z., Huang, Z., Dong, J., & Qian, L. (2025). Chemerin in Pulmonary Fibrosis: Advances in Mechanistic and Fundamental Research. Biomolecules, 15(10), 1469. https://doi.org/10.3390/biom15101469





