Natural Compounds with Antiviral Activity Against Clinically Relevant RNA Viruses: Advances of the Last Decade
Abstract
1. Introduction
2. RNA Viruses: Biology and Mechanisms of Action
Characteristics of RNA Viruses
3. The Secret to Therapeutic Targets: Replication Mechanisms
4. Materials and Methods
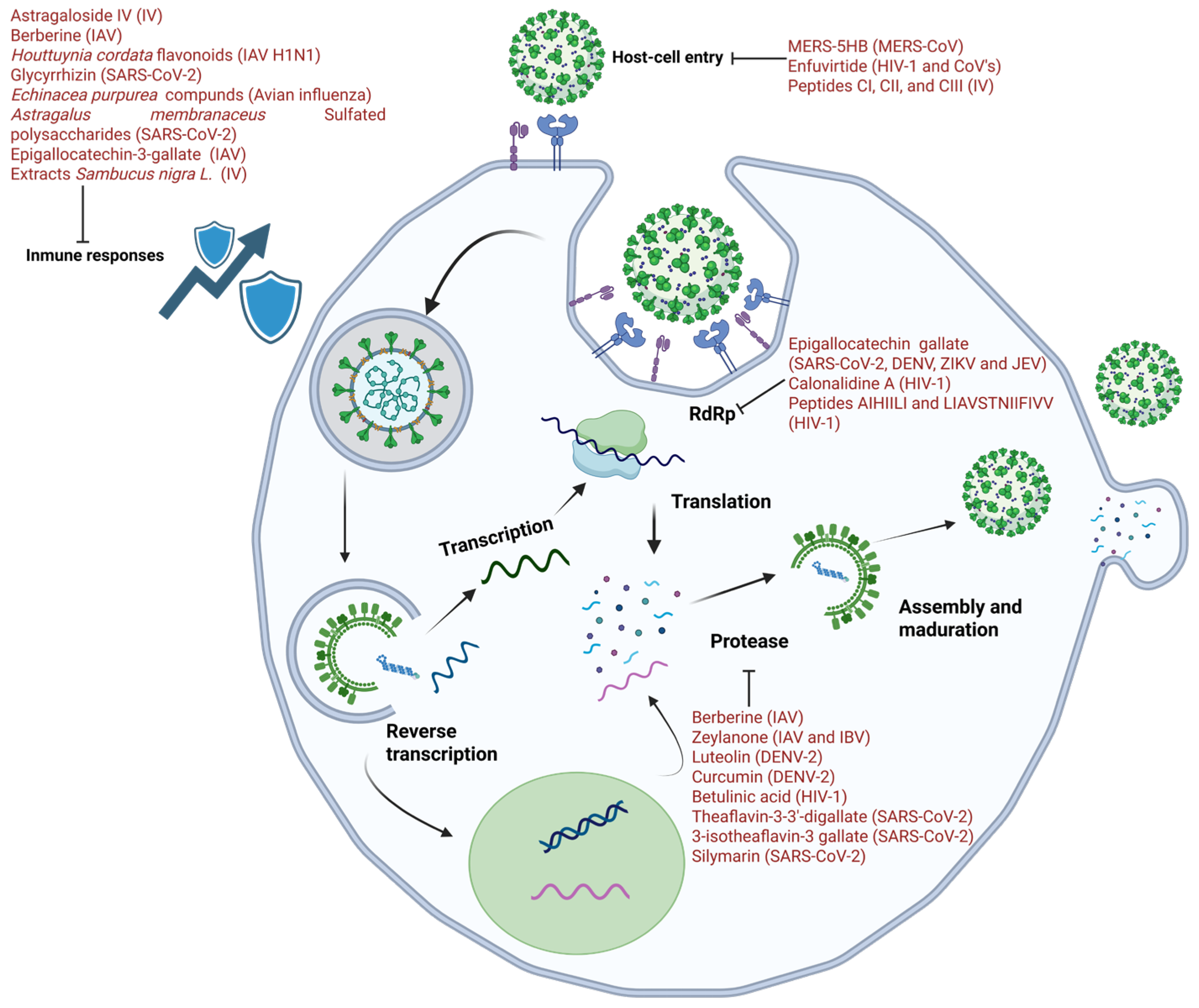
5. Natural Antivirals: Ten Years Ago
5.1. Inhibition of Replication
5.2. Inhibition of Host Cell Entry
5.3. Protein Processing Inhibitors
5.4. Favorable Immune Compounds
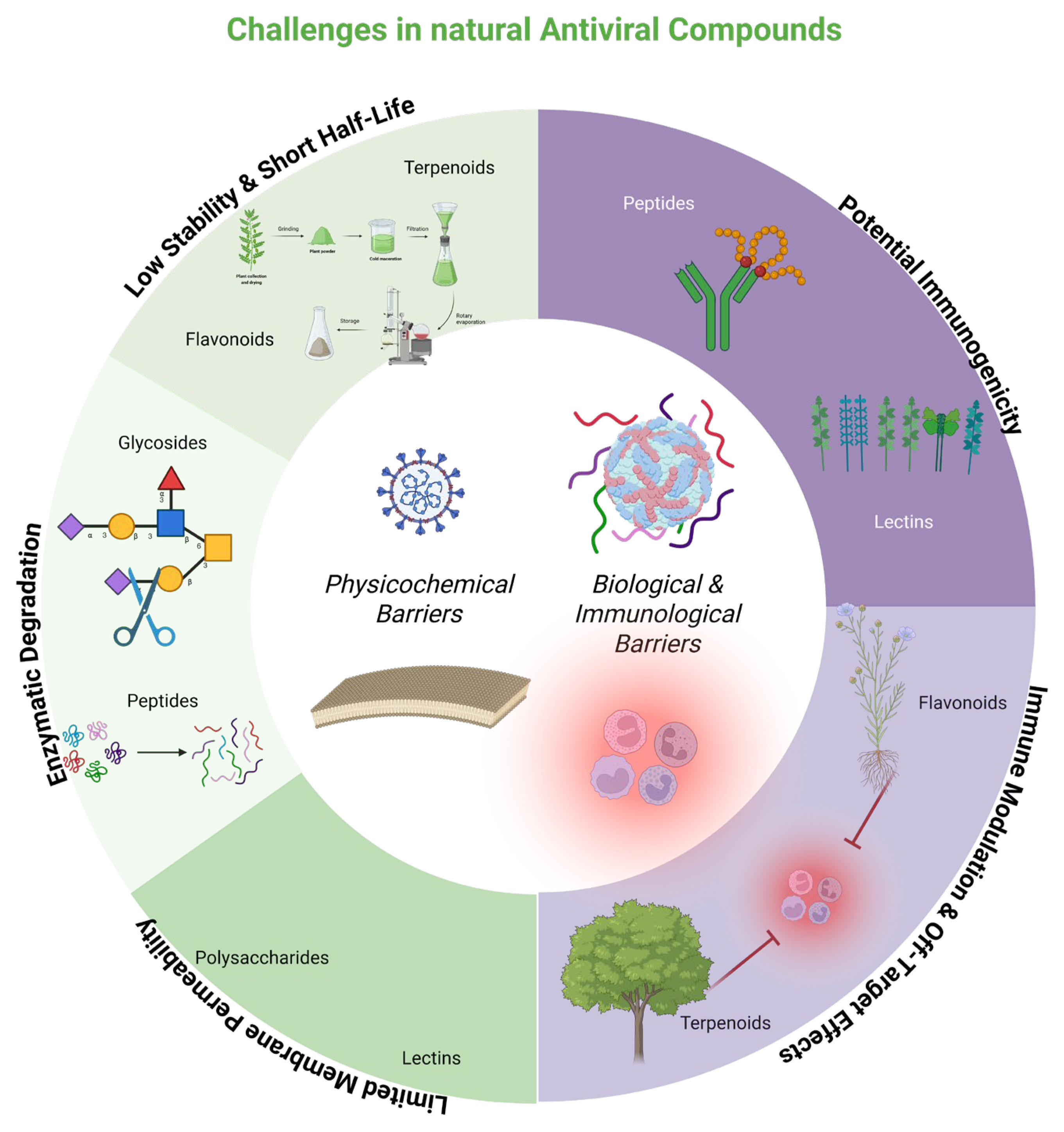
6. New Challenges
6.1. Nanodelivery of Natural Antivirals
6.2. Natural Compounds as Nanocarriers or Antivirals for Nanodelivery
- HBV: micelles of chito-oligosaccharides and derivatives enhance encapsulation efficiency and sustained release of lamivudine, and cell-penetrating peptide (CPP)-based approaches have demonstrated inhibition of Avihepadnavirus anatigruidae (DHBV) and HBV replication in cell and animal models [92,94].
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- England, C.; TrejoMartinez, J.; PerezSanchez, P.; Karki, U.; Xu, J. Plants as Biofactories for Therapeutic Proteins and Antiviral Compounds to Combat COVID-19. Life 2023, 13, 617. [Google Scholar] [CrossRef]
- Rosales-Mendoza, S.; Solís-Andrade, K.I.; Márquez-Escobar, V.A.; González-Ortega, O.; Bañuelos-Hernandez, B. Current Advances in the Algae-Made Biopharmaceuticals Field. Expert. Opin. Biol. Ther. 2020, 20, 751–766. [Google Scholar] [CrossRef]
- Zatla, I. Computational Screening of Natural Compounds as Antiviral Candidates Targeting the SARS-CoV-2 Main Protease. J. Integr. Omics 2024, 14, 1–12. [Google Scholar] [CrossRef]
- Alrasheid, A.A.; Babiker, M.Y.; Awad, T.A. Evaluation of Certain Medicinal Plants Compounds as New Potential Inhibitors of Novel Corona Virus (COVID-19) Using Molecular Docking Analysis. Silico Pharmacol. 2021, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. The Effect of Curcumin on the Risk of Mortality in Patients with COVID-19: A Systematic Review and Meta-analysis of Randomized Trials. Phytother. Res. 2022, 36, 3365. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.J.T.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and Immunomodulatory Activity of Curcumin: A Case for Prophylactic Therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Payne, S. Introduction to RNA Viruses. Viruses 2017, 97–105. [Google Scholar] [CrossRef]
- Poltronieri, P.; Sun, B.; Mallardo, M. RNA Viruses: RNA Roles in Pathogenesis, Coreplication and Viral Load. Curr. Genom. 2015, 16, 327–335. [Google Scholar] [CrossRef]
- Baltimore, D. Expression of Animal Virus Genomes. Bacteriol. Rev. 1971, 35, 235–241. [Google Scholar] [CrossRef]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. Origins and Evolution of Viruses of Eukaryotes: The Ultimate Modularity. Virology 2015, 479–480, 2–25. [Google Scholar] [CrossRef]
- Boudreault, S.; Roy, P.; Lemay, G.; Bisaillon, M. Viral Modulation of Cellular RNA Alternative Splicing: A New Key Player in Virus–Host Interactions? WIREs RNA 2019, 10, e1543. [Google Scholar] [CrossRef]
- Masters, P.S. The Molecular Biology of Coronaviruses. Adv. Virus Res. 2006, 66, 193–292. [Google Scholar] [CrossRef]
- García-Sastre, A.; Schmolke, M. Avian Influenza A H10N8—A Virus on the Verge? Lancet 2014, 383, 676–677. [Google Scholar] [CrossRef]
- Ng, T.I.; Correia, I.; Seagal, J.; DeGoey, D.A.; Schrimpf, M.R.; Hardee, D.J.; Noey, E.L.; Kati, W.M. Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses 2022, 14, 961. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Molecular Biology of Hepatitis B Virus Infection. Virology 2015, 479–480, 672–686. [Google Scholar] [CrossRef]
- Mohanty, P.; Panda, P.; Acharya, R.K.; Pande, B.; Bhaskar, L.; Verma, H.K. Emerging Perspectives on RNA Virus-Mediated Infections: From Pathogenesis to Therapeutic Interventions. World J. Virol. 2023, 12, 242–255. [Google Scholar] [CrossRef]
- Deng, H.; Cao, H.; Wang, Y.; Li, J.; Dai, J.; Li, L.-F.; Qiu, H.-J.; Li, S. Viral Replication Organelles: The Highly Complex and Programmed Replication Machinery. Front. Microbiol. 2024, 15, 1450060. [Google Scholar] [CrossRef]
- Tian, W.J.; Wang, X.J. Broad-Spectrum Antivirals Derived from Natural Products. Viruses 2023, 15, 1100. [Google Scholar] [CrossRef] [PubMed]
- Picarazzi, F.; Vicenti, I.; Saladini, F.; Zazzi, M.; Mori, M. Targeting the RdRp of Emerging RNA Viruses: The Structure-Based Drug Design Challenge. Molecules 2020, 25, 5695. [Google Scholar] [CrossRef] [PubMed]
- Melano, I.; Lo, Y.C.; Su, W.C. Characterization of Host Substrates of SARS-CoV-2 Main Protease. Front. Microbiol. 2023, 14, 1251705. [Google Scholar] [CrossRef] [PubMed]
- Miczi, M.; Golda, M.; Kunkli, B.; Nagy, T.; Tőzsér, J.; Mótyán, J.A. Identification of Host Cellular Protein Substrates of SARS-CoV-2 Main Protease. Int. J. Mol. Sci. 2020, 21, 9523. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Loh, Y.R.; Phoo, W.W.; Hung, A.W.; Kang, C.B.; Luo, D. Crystal Structure of Unlinked NS2B-NS3 Protease from Zika Virus. Science (1979) 2016, 354, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Choi, K.H.; Padmanabhan, R. Flavivirus Proteases: The Viral Achilles Heel to Prevent Future Pandemics. Antivir. Res. 2023, 210, 105516. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. Nuclear Localization of Non-Structural Protein 3 (NS3) during Dengue Virus Infection. Arch. Virol. 2021, 166, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Rápalo, S.N.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Meraz-Ríos, M.A.; Del Ángel, R.M. An Ivermectin—Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo. iScience 2023, 26, 108294. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Palacios-Rápalo, S.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Gutiérrez-Escolano, A.L.; Del Ángel, R.M. The Nuclear Pore Complex is a Key Target of Viral Proteases to Promote Viral Replication. Viruses 2021, 13, 706. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfán-Morales, C.N.; Cordero-Rivera, C.D.; Cisneros, B.; Gutiérrez-Escolano, A.L.; del Ángel, R.M. Nucleo-Cytoplasmic Transport of ZIKV Non-Structural 3 Protein Is Mediated by Importin-α/β and Exportin CRM-1. J. Virol. 2023, 97, e01773-22. [Google Scholar] [CrossRef]
- De Jesús-González, L.A.; Cervantes-Salazar, M.; Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; Farfán-Morales, C.N.; Palacios, S.; Pérez-Olais, J.H.; Rivera, C.D.C.; Monzón, A.M.H.; Ruíz-Jiménez, F.; et al. The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses. Viruses 2020, 12, 583. [Google Scholar] [CrossRef]
- Yi, J.; Peng, J.; Yang, W.; Zhu, G.; Ren, J.; Li, D.; Zheng, H. Picornavirus 3C—A Protease Ensuring Virus Replication and Subverting Host Responses. J. Cell Sci. 2021, 134, jcs253237. [Google Scholar] [CrossRef]
- Kim, K.H.; Rümenapf, T.; Strauss, E.G.; Strauss, J.H. Regulation of Semliki Forest Virus RNA Replication: A Model for the Control of Alphavirus Pathogenesis in Invertebrate Hosts. Virology 2004, 323, 153–163. [Google Scholar] [CrossRef]
- Li, X.D.; Sun, L.; Seth, R.B.; Pineda, G.; Chen, Z.J. Hepatitis C Virus Protease NS3/4A Cleaves Mitochondrial Antiviral Signaling Protein off the Mitochondria to Evade Innate Immunity. Proc. Natl. Acad. Sci. USA 2005, 102, 17717–17722. [Google Scholar] [CrossRef]
- Mondal, S.; Sarvari, G.; Boehr, D.D. Picornavirus 3C Proteins Intervene in Host Cell Processes through Proteolysis and Interactions with RNA. Viruses 2023, 15, 2413. [Google Scholar] [CrossRef]
- Sun, C.; Xie, C.; Bu, G.-L.; Zhong, L.-Y.; Zeng, M.-S. Molecular Characteristics, Immune Evasion, and Impact of SARS-CoV-2 Variants. Signal Transduct. Target. Ther. 2022, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Lundin, A.; Dijkman, R.; Bergström, T.; Kann, N.; Adamiak, B.; Hannoun, C.; Kindler, E.; Jónsdóttir, H.R.; Muth, D.; Kint, J.; et al. Targeting Membrane-Bound Viral RNA Synthesis Reveals Potent Inhibition of Diverse Coronaviruses Including the Middle East Respiratory Syndrome Virus. PLoS Pathog. 2014, 10, e1004166. [Google Scholar] [CrossRef] [PubMed]
- Childs, K.; Stock, N.; Ross, C.; Andrejeva, J.; Hilton, L.; Skinner, M.; Randall, R.; Goodbourn, S. Mda-5, but Not RIG-I, Is a Common Target for Paramyxovirus V Proteins. Virology 2007, 359, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Childs, K.S.; Andrejeva, J.; Randall, R.E.; Goodbourn, S. Mechanism of Mda-5 Inhibition by Paramyxovirus V Proteins. J. Virol. 2009, 83, 1465–1473. [Google Scholar] [CrossRef]
- Liu, Y.; Olagnier, D.; Lin, R. Host and Viral Modulation of RIG-I-Mediated Antiviral Immunity. Front. Immunol. 2017, 7, 662. [Google Scholar] [CrossRef]
- Burdick, R.C.; Li, C.; Munshi, M.; Rawson, J.M.O.; Nagashima, K.; Hu, W.-S.; Pathak, V.K. HIV-1 Uncoats in the Nucleus near Sites of Integration. Proc. Natl. Acad. Sci. USA 2020, 117, 5486–5493. [Google Scholar] [CrossRef]
- Kyriakou, E.; Magiorkinis, G. Interplay between Endogenous and Exogenous Human Retroviruses. Trends Microbiol. 2023, 31, 933–946. [Google Scholar] [CrossRef]
- Gu, S.-X.; Zhu, Y.-Y.; Wang, C.; Wang, H.-F.; Liu, G.-Y.; Cao, S.; Huang, L. Recent Discoveries in HIV-1 Reverse Transcriptase Inhibitors. Curr. Opin. Pharmacol. 2020, 54, 166–172. [Google Scholar] [CrossRef]
- El Sayed, K.A. Natural Products as Antiviral Agents. Stud. Nat. Prod. Chem. 2000, 24, 473–572. [Google Scholar]
- Kandeel, M.; Kitade, Y.; Almubarak, A. Repurposing FDA-Approved Phytomedicines, Natural Products, Antivirals and Cell Protectives against SARS-CoV-2 (COVID-19) RNA-Dependent RNA Polymerase. PeerJ 2020, 8, e10480. [Google Scholar] [CrossRef]
- Hong, S.; Seo, S.H.; Woo, S.-J.; Kwon, Y.; Song, M.; Ha, N.-C. Epigallocatechin Gallate Inhibits the Uridylate-Specific Endoribonuclease Nsp15 and Efficiently Neutralizes the SARS-CoV-2 Strain. J. Agric. Food Chem. 2021, 69, 5948–5954. [Google Scholar] [CrossRef]
- Usach, I.; Melis, V.; Peris, J. Non-nucleoside Reverse Transcriptase Inhibitors: A Review on Pharmacokinetics, Pharmacodynamics, Safety and Tolerability. J. Int. AIDS Soc. 2013, 16, 18567. [Google Scholar] [CrossRef] [PubMed]
- Seetaha, S.; Hannongbua, S.; Rattanasrisomporn, J.; Choowongkomon, K. Novel Peptides with HIV-1 Reverse Transcriptase Inhibitory Activity Derived from the Fruits of Quercus Infectoria. Chem. Biol. Drug Des. 2021, 97, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Goulding, L.V.; Kiss, E.; Goatley, L.; Vrancken, R.; Goris, N.E.J.; Dixon, L. In Vitro and in Vivo Antiviral Activity of Nucleoside Analogue CHPMPC against African Swine Fever Virus Replication. Antivir. Res. 2022, 208, 105433. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.D.; Winter, H.C.; Goldstein, I.J.; Markovitz, D.M. A Lectin Isolated from Bananas Is a Potent Inhibitor of HIV Replication. J. Biol. Chem. 2010, 285, 8646–8655. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K.; Shivkumar, M. Antiviral Plant-derived Natural Products to Combat RNA Viruses: Targets throughout the Viral Life Cycle. Lett. Appl. Microbiol. 2022, 75, 476–499. [Google Scholar] [CrossRef]
- Swanson, M.D.; Boudreaux, D.M.; Salmon, L.; Chugh, J.; Winter, H.C.; Meagher, J.L.; André, S.; Murphy, P.V.; Oscarson, S.; Roy, R.; et al. Engineering a Therapeutic Lectin by Uncoupling Mitogenicity from Antiviral Activity. Cell 2015, 163, 746–758. [Google Scholar] [CrossRef]
- Eggink, D.; Bontjer, I.; de Taeye, S.W.; Langedijk, J.P.M.; Berkhout, B.; Sanders, R.W. HIV-1 Anchor Inhibitors and Membrane Fusion Inhibitors Target Distinct but Overlapping Steps in Virus Entry. J. Biol. Chem. 2019, 294, 5736–5746. [Google Scholar] [CrossRef]
- Berillo, D.; Yeskendir, A.; Zharkinbekov, Z.; Raziyeva, K.; Saparov, A. Peptide-Based Drug Delivery Systems. Medicina 2021, 57, 1209. [Google Scholar] [CrossRef]
- Hopper, J.T.S.; Ambrose, S.; Grant, O.C.; Krumm, S.A.; Allison, T.M.; Degiacomi, M.T.; Tully, M.D.; Pritchard, L.K.; Ozorowski, G.; Ward, A.B.; et al. The Tetrameric Plant Lectin BanLec Neutralizes HIV through Bidentate Binding to Specific Viral Glycans. Structure 2017, 25, 773–782.e5. [Google Scholar] [CrossRef]
- Millet, J.K.; Séron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East Respiratory Syndrome Coronavirus Infection Is Inhibited by Griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Colunga Biancatelli, R.M.L.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and Vitamin C: An Experimental, Synergistic Therapy for the Prevention and Treatment of SARS-CoV-2 Related Disease (COVID-19). Front. Immunol. 2020, 11, 550247. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, J.; Xiao, S.; Zhang, L.; Zhou, D. Triterpenoid-Mediated Inhibition of Virus–Host Interaction: Is Now the Time for Discovering Viral Entry/Release Inhibitors from Nature? J. Med. Chem. 2020, 63, 15371–15388. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Zhan, Y.; Ta, W.; Tang, W.; Hua, R.; Wang, J.; Wang, C.; Lu, W. Potential Antiviral Activity of Isorhamnetin against SARS-CoV-2 Spike Pseudotyped Virus in Vitro. Drug Dev. Res. 2021, 82, 1124–1130. [Google Scholar] [CrossRef]
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm. Sin. B 2020, 10, 766–788. [Google Scholar] [CrossRef]
- Ahmadi, K.; Farasat, A.; Rostamian, M.; Johari, B.; Madanchi, H. Enfuvirtide, an HIV-1 Fusion Inhibitor Peptide, Can Act as a Potent SARS-CoV-2 Fusion Inhibitor: An in Silico Drug Repurposing Study. J. Biomol. Struct. Dyn. 2022, 40, 5566–5576. [Google Scholar] [CrossRef]
- Kannan, S.; Kolandaivel, P. Antiviral Potential of Natural Compounds against Influenza Virus Hemagglutinin. Comput. Biol. Chem. 2017, 71, 207–218. [Google Scholar] [CrossRef]
- Botwina, P.; Owczarek, K.; Rajfur, Z.; Ochman, M.; Urlik, M.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway. Viruses 2020, 12, 344. [Google Scholar] [CrossRef]
- Cetina-Montejo, L.; Ayora-Talavera, G.; Borges-Argáez, R. Zeylanone Epoxide Isolated from Diospyros Anisandra Stem Bark Inhibits Influenza Virus in Vitro. Arch. Virol. 2019, 164, 1543–1552. [Google Scholar] [CrossRef]
- Peng, M.; Watanabe, S.; Chan, K.W.K.; He, Q.; Zhao, Y.; Zhang, Z.; Lai, X.; Luo, D.; Vasudevan, S.G.; Li, G. Luteolin Restricts Dengue Virus Replication through Inhibition of the Proprotein Convertase Furin. Antivir. Res. 2017, 143, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New Insights into the Immunopathology and Control of Dengue Virus Infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L.R.F.; Wu, H.; Nebo, L.; Fernandes, J.B.; da Silva, M.F.d.G.F.; Kiefer, W.; Kanitz, M.; Bodem, J.; Diederich, W.E.; Schirmeister, T.; et al. Flavonoids as Noncompetitive Inhibitors of Dengue Virus NS2B-NS3 Protease: Inhibition Kinetics and Docking Studies. Bioorganic Med. Chem. 2015, 23, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Dang, M.; Roy, A.; Kang, J.; Song, J. Curcumin Allosterically Inhibits the Dengue NS2B-NS3 Protease by Disrupting Its Active Conformation. ACS Omega 2020, 5, 25677–25686. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of Dengue Virus by Curcuminoids. Antivir. Res. 2019, 162, 71–78. [Google Scholar] [CrossRef]
- Dwivedi, V.D.; Tripathi, I.P.; Mishra, S.K. In Silico Evaluation of Inhibitory Potential of Triterpenoids from Azadirachta Indica against Therapeutic Target of Dengue Virus, NS2B-NS3 Protease. J. Vector Borne Dis. 2016, 53, 156–161. [Google Scholar]
- Bharadwaj, S.; Lee, K.E.; Dwivedi, V.D.; Yadava, U.; Panwar, A.; Lucas, S.J.; Pandey, A.; Kang, S.G. Discovery of Ganoderma Lucidum Triterpenoids as Potential Inhibitors against Dengue Virus NS2B-NS3 Protease. Sci. Rep. 2019, 9, 19059. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, C.-H.; Morris-Natschke, S.L.; Lee, K.-H. Design, Synthesis, and Structure Activity Relationship Analysis of New Betulinic Acid Derivatives as Potent HIV Inhibitors. Eur. J. Med. Chem. 2021, 215, 113287. [Google Scholar] [CrossRef]
- Saraswat, J.; Singh, P.; Patel, R. A Computational Approach for the Screening of Potential Antiviral Compounds against SARS-CoV-2 Protease: Ionic Liquid vs Herbal and Natural Compounds. J. Mol. Liq. 2021, 326, 115298. [Google Scholar] [CrossRef]
- Alpert, P.T. The Role of Vitamins and Minerals on the Immune System. Home Health Care Manag. Pract. 2017, 29, 199–202. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Liang, D.; Quan, X.; Gu, R.; Meng, Z.; Gan, H.; Wu, Z.; Sun, Y.; Liu, S.; et al. Pharmacological Effects of Astragaloside IV: A Review. Molecules 2023, 28, 6118. [Google Scholar] [CrossRef]
- Huang, X.; Zhou, Y.; Li, Y.; Wang, T.; Chen, Y.; Zhou, Y.; Zhou, X.; Liu, Q. Astragaloside IV Inhibits Inflammation Caused by Influenza Virus via Reactive Oxygen Species/NOD-like Receptor Thermal Protein Domain Associated Protein 3/Caspase-1 Signaling Pathway. Immun. Inflamm. Dis. 2024, 12, e1309. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Wu, Y.; Wu, J.; Liu, H.; Zhou, S.; Ge, D.; Dong, R.; You, L.; Hao, Y. Berberine Ameliorates Pulmonary Inflammation in Mice with Influenza Viral Pneumonia by Inhibiting NLRP3 Inflammasome Activation and Gasdermin D-mediated Pyroptosis. Drug Dev. Res. 2022, 83, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Laldinsangi, C. The Therapeutic Potential of Houttuynia Cordata: A Current Review. Heliyon 2022, 8, e10386. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, J.; Kim, J.W.; Lee, S.G.; Kim, H.P. Flavonoids from the Aerial Parts of Houttuynia Cordata Attenuate Lung Inflammation in Mice. Arch. Pharm. Res. 2015, 38, 1304–1311. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An Alternative Drug for the Treatment of COVID-19 Infection and the Associated Respiratory Syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef]
- Al-Kamel, H.; Grundmann, O. Glycyrrhizin as a Potential Treatment for the Novel Coronavirus (COVID-19). Mini-Rev. Med. Chem. 2021, 21, 2204–2208. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Chrzanowska, A.; Graboń, W. Glycyrrhizin: An Old Weapon against a Novel Coronavirus. Phytother. Res. 2021, 35, 629–636. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Javed, H.; Sharma, C.; Goyal, S.N.; Kumar, S.; Jha, N.K.; Ojha, S. Can Echinacea Be a Potential Candidate to Target Immunity, Inflammation, and Infection—The Trinity of Coronavirus Disease 2019. Heliyon 2021, 7, e05990. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [PubMed]
- Shahsavandi, S.; Ebrahimi, M.M.; Farahani, A.H. Interfering With Lipid Raft Association: A Mechanism to Control Influenza Virus Infection By Sambucus Nigra. Iran. J. Pharm. Res. 2017, 16, 1147. [Google Scholar]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-Influenza Activity of Elderberry (Sambucus Nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Garber, A.; Barnard, L.; Pickrell, C. Review of Whole Plant Extracts With Activity Against Herpes Simplex Viruses In Vitro and In Vivo. J. Evid. Based Integr. Med. 2021, 26, 2515690X20978394. [Google Scholar] [CrossRef]
- Uwamahoro, H.; Collier, W.E.; Nashar, T.O.; Jaynes, J.M.; Mortley, D.G.; Davis, C.G.; Kanyairita, G.G.; Abdelazim, E.F.; Igiramaboko, J.F.R.; Habineza, C.; et al. Natural and Designed Cyclic Peptides as Potential Antiviral Drugs to Combat Future Coronavirus Outbreaks. Molecules 2025, 30, 1651. [Google Scholar] [CrossRef]
- Essa, R.Z.; Wu, Y.S.; Batumalaie, K.; Sekar, M.; Poh, C.L. Antiviral Peptides against SARS-CoV-2: Therapeutic Targets, Mechanistic Antiviral Activity, and Efficient Delivery. Pharmacol. Rep. 2022, 74, 1166–1181. [Google Scholar] [CrossRef]
- Alvizo-Báez, C.A.; de Jesús González-Escobedo, M.; Terrazas-Armendáriz, L.D.; Uscanga-Palomeque, A.C.; Luna-Cruz, I.E.; Martínez-Ruíz, A.M.; Ruíz-Robles, M.A.; Pérez-Tijerina, E.G.; Tamez-Guerra, R.; Rodríguez-Padilla, C.; et al. Quercetin Self-Assembly Nanoparticles with Antiviral Molecules Are Effective in Inhibiting SARS-CoV-2 Pseudovirus Infection. Front. Nanotechnol. 2025, 7, 1579997. [Google Scholar] [CrossRef]
- Abdi Syahputra, R.; Dalimunthe, A.; Utari, Z.D.; Halim, P.; Sukarno, M.A.; Zainalabidin, S.; Salim, E.; Gunawan, M.; Nurkolis, F.; Park, M.N.; et al. Nanotechnology and Flavonoids: Current Research and Future Perspectives on Cardiovascular Health. J. Funct. Foods 2024, 120, 106355. [Google Scholar] [CrossRef]
- Morsy, H.M.; Zaky, M.Y.; Yassin, N.Y.S.; Khalifa, A.Y.Z. Nanoparticle-Based Flavonoid Therapeutics: Pioneering Biomedical Applications in Antioxidants, Cancer Treatment, Cardiovascular Health, Neuroprotection, and Cosmeceuticals. Int. J. Pharm. 2025, 670, 125135. [Google Scholar] [CrossRef]
- Orosco, F.L.; Quimque, M.T.J. Antiviral Potential of Terpenoids against Major Viral Infections: Recent Advances, Challenges, and Opportunities. J. Adv. Biotechnol. Exp. Ther. 2024, 7, 221–238. [Google Scholar] [CrossRef]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers. Viruses 2023, 15, 647. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Ling, J. Medicinal Fungi with Antiviral Effect. Molecules 2022, 27, 4457. [Google Scholar] [CrossRef]
- Ndeboko, B.; Hantz, O.; Lemamy, G.J.; Cova, L. Developments in Cell-Penetrating Peptides as Antiviral Agents and as Vehicles for Delivery of Peptide Nucleic Acid Targeting Hepadnaviral Replication Pathway. Biomolecules 2018, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Negreanu-Pirjol, B.S.; Negreanu-Pirjol, T.; Popoviciu, D.R.; Anton, R.E.; Prelipcean, A.M. Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1781. [Google Scholar] [CrossRef]
- Yang, J.; An, H.W.; Wang, H. Self-Assembled Peptide Drug Delivery Systems. ACS Appl. Bio Mater. 2021, 4, 24–46. [Google Scholar] [CrossRef]
- Yang, L.Y.; Li, C.Q.; Zhang, Y.L.; Ma, M.W.; Cheng, W.; Zhang, G.J. Emerging Drug Delivery Vectors: Engineering of Plant-Derived Nanovesicles and Their Applications in Biomedicine. Int. J. Nanomed. 2024, 19, 2591–2610. [Google Scholar] [CrossRef]
- Rosa, V.; Ho, D.; Sabino-Silva, R.; Siqueira, W.L.; Silikas, N. Fighting Viruses with Materials Science: Prospects for Antivirus Surfaces, Drug Delivery Systems and Artificial Intelligence. Dent. Mater. 2021, 37, 496–507. [Google Scholar] [CrossRef]
- Gao, J.; Karp, J.M.; Langer, R.; Joshi, N. The Future of Drug Delivery. Chem. Mater. 2023, 35, 359–363. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Al-Nazawi, M. Virtual Screening and Repurposing of FDA Approved Drugs against COVID-19 Main Protease. Life Sci. 2020, 251, 117627. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Qureshi, A.; Kumar, M. AVPpred: Collection and Prediction of Highly Effective Antiviral Peptides. Nucleic Acids Res. 2012, 40, W199–W204. [Google Scholar] [CrossRef]
- Tao, S.; Chen, Y.; Wu, J.; Zhao, D.; Cai, H.; Wang, L. VDDB: A Comprehensive Resource and Machine Learning Tool for Antiviral Drug Discovery. MedComm–Future Med. 2023, 2, e32. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Sun, X.; Ma, T.; Lao, X.; Zheng, H. DRAVP: A Comprehensive Database of Antiviral Peptides and Proteins. Viruses 2023, 15, 820. [Google Scholar] [CrossRef]
| Group | Virus Type | Examples | Replication Mechanism | Features of Interest | Reference |
|---|---|---|---|---|---|
| III | dsRNA | Rotavirus | Replication occurs in the cytoplasm, where RdRp transcribes the negative strand to generate functional mRNA. | Because these viruses elicit strong innate immune responses, they are key models for studying antiviral immunity. | [11] |
| IV | +ssRNA | ZIKV, DENV, and SARS-CoV-2 | The genome can function as mRNA, allowing viral proteins to be translated quickly after entering the cytoplasm. | The viral RdRp synthesizes a −ssRNA intermediate that serves as a template for replication. Its high mutation rate and adaptability pose significant challenges for the development of antivirals. | [12] |
| V | −ssRNA | Lyssavirus rabies, IAV, and respiratory syncytial virus (RSV). | The genome cannot be translated directly; it must first be converted into mRNA by the RdRp before protein synthesis occurs. | Many viruses have segmented genomes, which promotes genetic reassortment and increases antigenic diversity. | [13] |
| VI | ssRNA retrovirus | HIV | They carry +ssRNA but do not use it directly as mRNA; instead, RT converts the RNA into DNA, which integrates into the host genome. | This replication strategy enables persistent infection and presents therapeutic challenges, while also providing targets for RT-directed antivirals. | [14] |
| VII | DsDNA retrovirus | Hepatitis B virus (HBV) | They use a pregenomic RNA intermediate during replication, which is subsequently reverse transcribed into DNA by a viral RT. | Their hybrid mechanism places them as a distinct category within the Baltimore system and raises therapeutic challenges like those of retroviruses. | [15] |
| Virus/Family | Compound/Extract | Biological Source | Type of Compound/Extract | CC50 (µM/µg/mL) | EC50 (µM/µg/mL) | Model (in vitro/in vivo) | Reference |
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 (Coronaviridae) | EGCG | Camellia sinensis (green tea) | Flavonoid | n.d. | IC5050 = 2.54 µg/mL; replication inhibition at 0.24 µg/mL | Vero cells (in vitro) | [43] |
| Quercetin + Vitamin C | Various plants | Flavonoid + vitamin | n.d. | Synergistic inhibition | In vitro; proposed clinical synergy | [54] | |
| Isorhamnetin | Various plants | Flavonoid | n.d. | Reduces pseudotyped virus entry | In vitro (HEK293/ACE2) | [57] | |
| Theaflavins (TF3DG, TF3G) | Camellia sinensis (tea) | Polyphenols | n.d. | Inhibit Mpro and RdRp (Nsp12) | In vitro (enzymatic/cell assays) | [58] | |
| Glycyrrhizin | Glycyrrhiza glabra (licorice) | Triterpenoid saponin | n.d. | ACE2 interaction; replication inhibition | In vitro (Vero cells) | [78,79,80] | |
| MERS-CoV (Coronaviridae) | Griffithsin lectin | Griffithsia sp. (red algae) | Lectin | n.d. | Inhibition of entry | In vitro (MRC-5, Vero, Huh-7) | [53] |
| HIV-1 (Retroviridae) | Calanolide A | Calophyllum spp. | Coumarin derivative | n.d. | Active in vitro; safe in phase I | In vivo/human volunteers (safety) | [44] |
| AIHIILI & LIAVSTNIIFIVV peptides | Quercus infectoria (acorn husks) | Peptides | n.d. | IC50 = 274 nM/236.3 nM | In vitro (RT inhibition) | [45] | |
| BanLec lectin (mutant H84T) | Musa spp. (banana) | Lectin | n.d. | Potent inhibition in vitro/in vivo | In vitro; in vivo (mice) | [47,48,49,52] | |
| Betulinic acid derivatives | Eucalyptus spp. | Pentacyclic triterpenoid | n.d. | Inhibits Gag maturation (capsid p24) | In vitro; Phase II halted | [70] | |
| HCV (Flaviviridae) | Oleanolic & echinocystic acids | Plants (pentacyclic triterpenoids) | Terpenoids | n.d. | Block viral envelope glycoproteins | In vitro (Huh-7) | [53] |
| DENV (Flaviviridae) | Luteolin | Vegetables (celery, carrots, broccoli) | Flavonoid | CC50 reported (n.d., exact value) | EC50 reported (n.d., exact value) | In vitro (Huh-7); in vivo (mice, ↓ viremia) | [63,64] |
| Agathisflavone | Poincianella pyramidalis (Tul.) | Biflavonoid | n.d. | IC50 = 15 µM | In vitro (NS2B-NS3 protease inhibition) | [65] | |
| Curcumin | Curcuma longa | Polyphenol | n.d. | Weak inhibition; derivative ↑ activity | In vitro (DENV2 NS3 protease); in silico | [66,67] | |
| Ganodermanontriol | Ganoderma lucidum (fungus) | Triterpenoid | n.d. | ↓ viral titre by ~40% at 50 µM | In vitro | [69] | |
| Influenza A/B (Orthomyxoviridae) | Zeylanone epoxide | Diospyros anisandra | Quinone derivative | n.d. | Entry & mid-stage replication inhibition | In vitro (IAV, IBV) | [62] |
| Influenza A (Orthomyxoviridae) | Quercetin | Multiple plants | Flavonoid | n.d. | Entry inhibition via HA2 interaction | In vitro (IAV) | [55] |
| Berberine | Berberis vulgaris; Coptis sp. | Alkaloid | n.d. | Inhibits ERK/MAPK ↓ replication | In vitro; in vivo (mice) | [61,75] | |
| Astragaloside IV | Astragalus membranaceus | Triterpenoid saponin | n.d. | Anti-inflammatory; ↓ viral titers | In vitro (A549); in vivo (mice) | [73,74] | |
| Flavonoids + polysaccharides (extract) | Houttuynia cordata | Mixed extract | n.d. | ↑ survival; ↓ lung inflammation | In vivo (mice) | [76,77] | |
| Echinacea extract | Echinacea purpurea | Extract (saponins, alkylamides, polysaccharides) | n.d. | ↑ NK cell activity; T lymphocyte production | In vivo/immunomodulatory | [81] | |
| EGCG (broad review) | Camellia sinensis | Flavonoid | n.d. | Inhibits neuraminidase; viral RNA synthesis | In vitro | [82] | |
| Elderberry extract | Sambucus nigra (berries) | Extract (lectins, polyphenols) | n.d. | Symptom improvement in a clinical trial | In vivo (patients) | [83,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cañedo-Figueroa, D.M.; Calderón-Sandate, D.N.; Hernández-Castillo, J.; Huerta-Garza, M.J.; Hernández-Rodríguez, X.; Velázquez-Cervantes, M.A.; Barrera-Aveleida, G.B.; Trujillo-Paez, J.V.; Lira-Hernández, F.I.; Marquez-Reyna, B.A.; et al. Natural Compounds with Antiviral Activity Against Clinically Relevant RNA Viruses: Advances of the Last Decade. Biomolecules 2025, 15, 1467. https://doi.org/10.3390/biom15101467
Cañedo-Figueroa DM, Calderón-Sandate DN, Hernández-Castillo J, Huerta-Garza MJ, Hernández-Rodríguez X, Velázquez-Cervantes MA, Barrera-Aveleida GB, Trujillo-Paez JV, Lira-Hernández FI, Marquez-Reyna BA, et al. Natural Compounds with Antiviral Activity Against Clinically Relevant RNA Viruses: Advances of the Last Decade. Biomolecules. 2025; 15(10):1467. https://doi.org/10.3390/biom15101467
Chicago/Turabian StyleCañedo-Figueroa, David Mauricio, Daniela Nahomi Calderón-Sandate, Jonathan Hernández-Castillo, Manuel Josafat Huerta-Garza, Ximena Hernández-Rodríguez, Manuel Adrián Velázquez-Cervantes, Giovanna Berenice Barrera-Aveleida, Juan Valentin Trujillo-Paez, Flor Itzel Lira-Hernández, Blanca Azucena Marquez-Reyna, and et al. 2025. "Natural Compounds with Antiviral Activity Against Clinically Relevant RNA Viruses: Advances of the Last Decade" Biomolecules 15, no. 10: 1467. https://doi.org/10.3390/biom15101467
APA StyleCañedo-Figueroa, D. M., Calderón-Sandate, D. N., Hernández-Castillo, J., Huerta-Garza, M. J., Hernández-Rodríguez, X., Velázquez-Cervantes, M. A., Barrera-Aveleida, G. B., Trujillo-Paez, J. V., Lira-Hernández, F. I., Marquez-Reyna, B. A., León-Juárez, M., García-Herrera, A. C., Osuna-Ramos, J. F., & De Jesús-González, L. A. (2025). Natural Compounds with Antiviral Activity Against Clinically Relevant RNA Viruses: Advances of the Last Decade. Biomolecules, 15(10), 1467. https://doi.org/10.3390/biom15101467










