Alterations of Bioactive Lipid Profiles in the Retina Following Traumatic Optic Neuropathy in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Modeling TON in Mice
2.3. Assessment of PUFA Metabolites by Liquid Chromatography/Mass Spectrometry (LC/MS)
2.4. Statistical Analysis
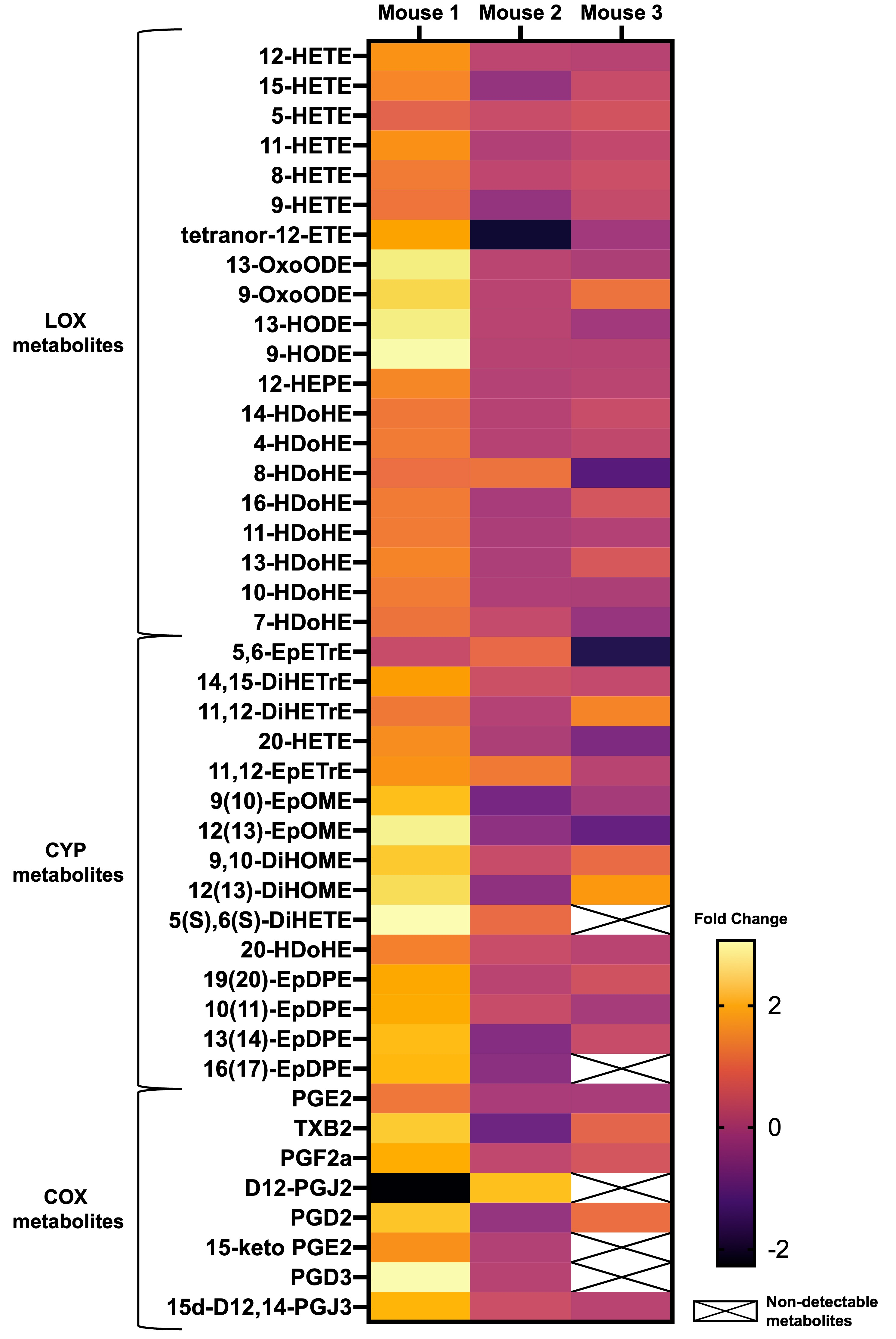
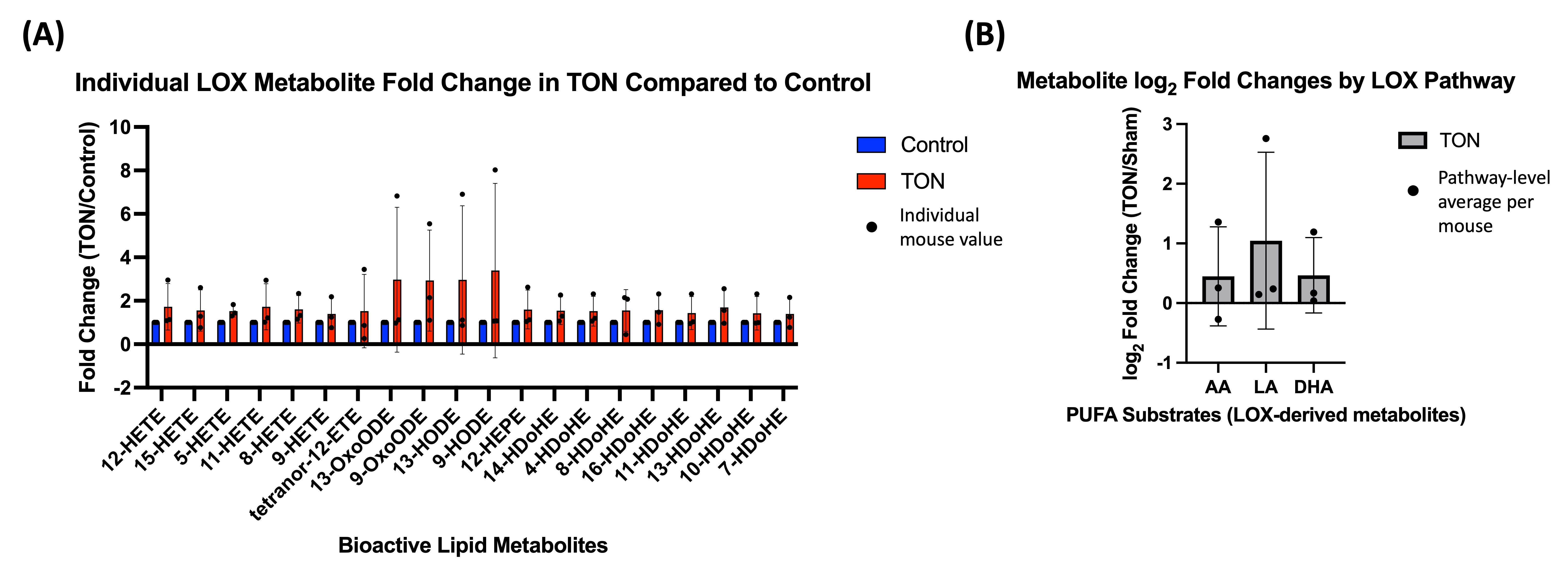
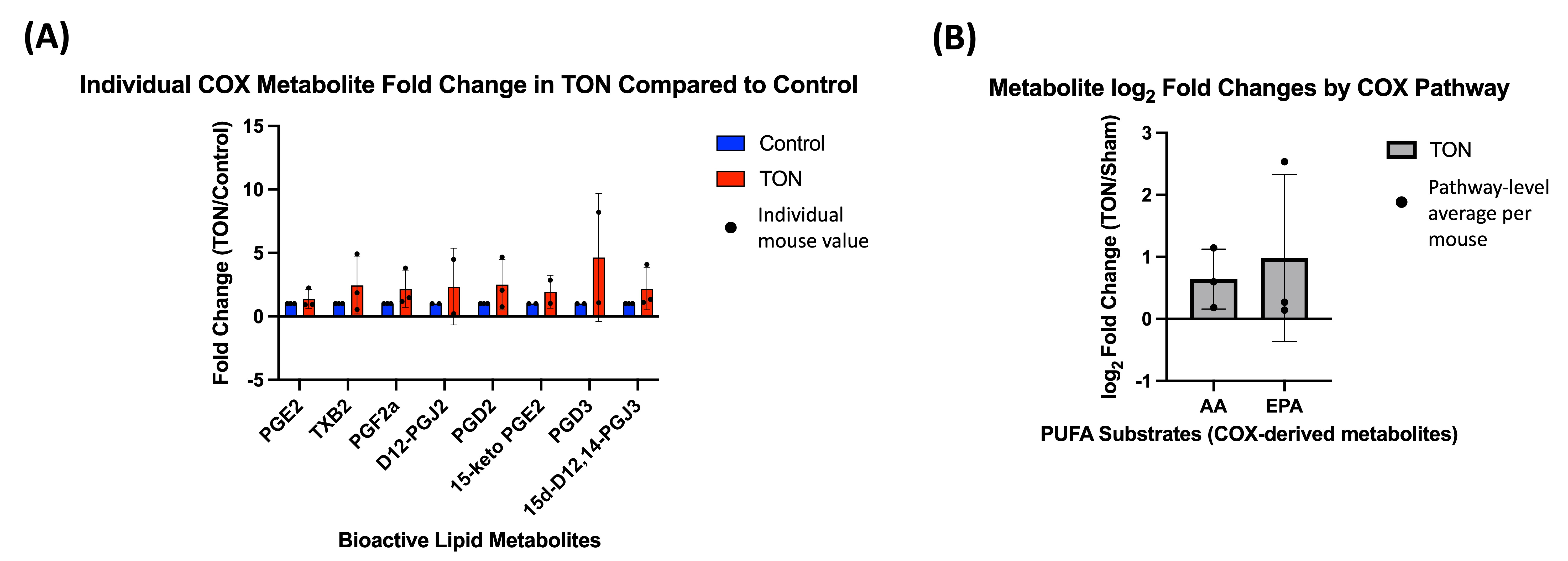
3. Results
4. Discussion
4.1. LOX Pathway
4.2. CYP and sEH Pathways
4.3. COX Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TON | Traumatic optic neuropathy |
| RGC | Retinal ganglion cell |
| ERG | Electroretinogram |
| BRN3A | Brain-specific homeobox/POU domain protein 3A |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| TBI | Traumatic brain injury |
| PUFA | Polyunsaturated fatty acid |
| AA | Arachidonic acid |
| LOX | Lipoxygenase |
| HETE | Hydroxyeicosatetraenoic acid |
| CYP | Cytochrome P450 |
| COX | Cyclooxygenase |
| COI | Controlled orbital impact |
| ARVO | Association for Research in Vision and Ophthalmology |
| LA | Linoleic acid |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FC | Fold change |
| EpOME | Epoxyoctadecamonoenoic acid |
| EpETrE | Epoxyeicosatrienoic acid |
| DiHOME | Dihydroxy-9Z-octadecenoic acid |
| DiHETrE | Dihydroxy-eicosatrienoic acid |
| sEH | Soluble epoxide hydrolase |
| HODE | Hydroxyoctadecadienoic acid |
| OxoODE | Oxoctadecadienoic acid |
| PPAR-γ | Peroxisome proliferator-activated receptor-γ |
| HDoHE | Hydroxydocosahexaenoic acid |
| HEPE | Hydroxyeicosapentaenoic acid |
| NF-ϰB | Nuclear factor-ϰB |
| EpDPE | Epoxydocosapentaenoic acid |
| DiHETE | Dihydroxy-5Z,8Z,11Z,17Z-eicosatetraenoic acid |
| PG | Prostaglandin |
References
- Atkins, E.J.; Newman, N.J.; Biousse, V. Post-Traumatic Visual Loss. Rev. Neurol. Dis. 2008, 5, 73–81. [Google Scholar]
- Bernardo-Colón, A.; Vest, V.; Cooper, M.L.; Naguib, S.A.; Calkins, D.J.; Rex, T.S. Progression and Pathology of Traumatic Optic Neuropathy From Repeated Primary Blast Exposure. Front. Neurosci. 2019, 13, 719. [Google Scholar] [CrossRef]
- McGrady, N.R.; Holden, J.M.; Ribeiro, M.; Boal, A.M.; Risner, M.L.; Calkins, D.J. Axon Hyperexcitability in the Contralateral Projection Following Unilateral Optic Nerve Crush in Mice. Brain Commun. 2022, 4, fcac251. [Google Scholar] [CrossRef]
- Alexandris, A.S.; Lee, Y.; Lehar, M.; Alam, Z.; McKenney, J.; Perdomo, D.; Ryu, J.; Welsbie, D.; Zack, D.J.; Koliatsos, V.E. Traumatic Axonal Injury in the Optic Nerve: The Selective Role of SARM1 in the Evolution of Distal Axonopathy. J. Neurotrauma 2023, 40, 1743–1761. [Google Scholar] [CrossRef]
- Xu, L.; Nguyen, J.V.; Lehar, M.; Menon, A.; Rha, E.; Arena, J.; Ryu, J.; Marsh-Armstrong, N.; Marmarou, C.R.; Koliatsos, V.E. Repetitive Mild Traumatic Brain Injury with Impact Acceleration in the Mouse: Multifocal Axonopathy, Neuroinflammation, and Neurodegeneration in the Visual System. Exp. Neurol. 2016, 275 Pt 3, 436–449. [Google Scholar] [CrossRef]
- Steinsapir, K.D.; Goldberg, R.A. Traumatic Optic Neuropathy: An Evolving Understanding. Am. J. Ophthalmol. 2011, 151, 928–933.e2. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P. Traumatic Optic Neuropathy—Clinical Features and Management Issues. Taiwan J. Ophthalmol. 2015, 5, 3–8. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, H.; Zhai, Q.; Li, H.; Wang, C.; Wang, Y. Traumatic Optic Neuropathy: A Review of Current Studies. Neurosurg. Rev. 2022, 45, 1895–1913. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Rajavi, Z.; Sedighi, N.; Daftarian, N.; Sanagoo, M. High-Dose Intravenous Methylprednisolone in Recent Traumatic Optic Neuropathy; a Randomized Double-Masked Placebo-Controlled Clinical Trial. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Guy, W.M.; Soparkar, C.N.S.; Alford, E.L.; Patrinely, J.R.; Sami, M.S.; Parke, R.B. Traumatic Optic Neuropathy and Second Optic Nerve Injuries. JAMA Ophthalmol. 2014, 132, 567. [Google Scholar] [CrossRef]
- Wladis, E.J.; Aakalu, V.K.; Sobel, R.K.; McCulley, T.J.; Foster, J.A.; Tao, J.P.; Freitag, S.K.; Yen, M.T. Interventions for Indirect Traumatic Optic Neuropathy. Ophthalmology 2021, 128, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Ford, R.L.; Xing, W.; Bunce, C.; Foot, B. Surveillance of Traumatic Optic Neuropathy in the UK. Eye 2010, 24, 240–250. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Elmasry, K.; Wan, M.; Abdulmoneim, S.; Still, A.; Khan, F.; Khalil, A.; Saul, A.; Hoda, M.N.; Al-Shabrawey, M. A Controlled Impact of Optic Nerve as a New Model of Traumatic Optic Neuropathy in Mouse. Investig. Opthalmology Vis. Sci. 2018, 59, 5548. [Google Scholar] [CrossRef]
- Saszik, S.M.; Robson, J.G.; Frishman, L.J. The Scotopic Threshold Response of the Dark-Adapted Electroretinogram of the Mouse. J. Physiol. 2002, 543, 899–916. [Google Scholar] [CrossRef]
- Sarkies, N. Traumatic Optic Neuropathy. Eye 2004, 18, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.E.; Zarini, S.; Precht, T.; Murphy, R.C.; Heidenreich, K.A. Transcellular Biosynthesis of Cysteinyl Leukotrienes in Rat Neuronal and Glial Cells. J. Neurochem. 2007, 103, 1310–1318. [Google Scholar] [CrossRef]
- Hariri, R.J.; Ghajar, J.B.; Pomerantz, K.B.; Hajjar, D.P.; Giannuzzi, R.F.; Tomich, E.; Andrews, D.W.; Patterson, R.H. Human Glial Cell Production of Lipoxygenase-Generated Eicosanoids: A Potential Role in the Pathophysiology of Vascular Changes Following Traumatic Brain Injury. J. Trauma 1989, 29, 1203–1210. [Google Scholar] [CrossRef]
- Ikeda, Y.; Long, D.M. Effects of the Arachidonate Lipoxygenase Inhibitor BW755C on Traumatic and Peritumoural Brain Oedema. Acta Neurochir. Suppl. 1990, 51, 68–70. [Google Scholar] [CrossRef]
- Kenny, E.M.; Fidan, E.; Yang, Q.; Anthonymuthu, T.S.; New, L.A.; Meyer, E.A.; Wang, H.; Kochanek, P.M.; Dixon, C.E.; Kagan, V.E.; et al. Ferroptosis Contributes to Neuronal Death and Functional Outcome After Traumatic Brain Injury. Crit. Care Med. 2019, 47, 410–418. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.-P.; Hu, H.; Wang, M.-L.; Sheng, W.-W.; Yao, H.-T.; Ding, W.; Chen, Z.; Wei, E.-Q. Expression Patterns of 5-Lipoxygenase in Human Brain with Traumatic Injury and Astrocytoma. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2006, 26, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Nadler, J.L.; Mirmira, R.G.; Casimiro, I. Regulation of tissue inflammation by 12-lipoxygenases. Biomolecules 2021, 11, 717. [Google Scholar] [CrossRef]
- Moustafa, M.; Khalil, A.; Darwish, N.H.E.; Zhang, D.-Q.; Tawfik, A.; Al-Shabrawey, M. 12-HETE Activates Müller Glial Cells: The Potential Role of GPR31 and miR-29. Prostaglandins Other Lipid Mediat. 2024, 171, 106805. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Dvoriantchikova, G.; Tse, B.C.; Pappas, S.; Chou, T.-H.; Tapia, M.; Porciatti, V.; Ivanov, D.; Tse, D.T.; Pelaez, D. A Novel Mouse Model of Traumatic Optic Neuropathy Using External Ultrasound Energy to Achieve Focal, Indirect Optic Nerve Injury. Sci. Rep. 2017, 7, 11779. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, S.; Lee, C.; Kumar, A.; Arjunan, P.; Li, Y.; Zhang, F.; Li, X. An Optic Nerve Crush Injury Murine Model to Study Retinal Ganglion Cell Survival. J. Vis. Exp. 2011, 50, 2685. [Google Scholar] [CrossRef]
- Magharious, M.M.; D’Onofrio, P.M.; Koeberle, P.D. Optic Nerve Transection: A Model of Adult Neuron Apoptosis in the Central Nervous System. J. Vis. Exp. 2011, 51, 2241. [Google Scholar] [CrossRef]
- Hines-Beard, J.; Marchetta, J.; Gordon, S.; Chaum, E.; Geisert, E.E.; Rex, T.S. A Mouse Model of Ocular Blast Injury That Induces Closed Globe Anterior and Posterior Pole Damage. Exp. Eye Res. 2012, 99, 63–70. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Chaemsaithong, P.; Zhou, S.-L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Gomez, R.; Docheva, N.; et al. Clinical Chorioamnionitis at Term: The Amniotic Fluid Fatty Acyl Lipidome. J. Lipid Res. 2016, 57, 1906–1916. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Zhou, S.-L. Stability and Analysis of Eicosanoids and Docosanoids in Tissue Culture Media. Prostaglandins Other Lipid Mediat. 2011, 94, 59–72. [Google Scholar] [CrossRef]
- Kim, M.Y.; Ibrahim, A.; Al-Shabrawey, M.A.-S. Altered Retinal Lipid Metabolites in a Mouse Model of Traumatic Optic Neuropathy. Investig. Ophthalmol. Vis. Sci. 2024, 65, 1690. [Google Scholar]
- Wang, D.; Liu, Y.; Chen, L.; Li, P.; Qu, Y.; Zhu, Y.; Zhu, Y. Key Role of 15-LO/15-HETE in Angiogenesis and Functional Recovery in Later Stages of Post-Stroke Mice. Sci. Rep. 2017, 7, 46698. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Li, Y.; Li, P.; Wang, D.; Liu, Y.; Qu, Y.; Zhu, D.; Zhu, Y. The 15-LO-1/15-HETE System Promotes Angiogenesis by Upregulating VEGF in Ischemic Brains. Neurol. Res. 2017, 39, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.; Frey, L.C.; Murphy, R.C.; Heidenreich, K.A. Injury-Related Production of Cysteinyl Leukotrienes Contributes to Brain Damage Following Experimental Traumatic Brain Injury. J. Neurotrauma 2009, 26, 1977–1986. [Google Scholar] [CrossRef]
- Rudhard, Y.; Sengupta Ghosh, A.; Lippert, B.; Böcker, A.; Pedaran, M.; Krämer, J.; Ngu, H.; Foreman, O.; Liu, Y.; Lewcock, J.W. Identification of 12/15-Lipoxygenase as a Regulator of Axon Degeneration through High-Content Screening. J. Neurosci. 2015, 35, 2927–2941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buland, J.R.; Wasserloos, K.J.; Tyurin, V.A.; Tyurina, Y.Y.; Amoscato, A.A.; Mallampalli, R.K.; Chen, B.B.; Zhao, J.; Zhao, Y.; Ofori-Acquah, S.; et al. Biosynthesis of Oxidized Lipid Mediators via Lipoprotein-Associated Phospholipase A2 hydrolysis of extracellular cardiolipin induces endothelial toxicity. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 311, L303–L316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chistyakov, D.V.; Astakhova, A.A.; Goriainov, S.V.; Sergeeva, M.G. Comparison of PPAR Ligands as Modulators of Resolution of Inflammation, via Their Influence on Cytokines and Oxylipins Release in Astrocytes. Int. J. Mol. Sci. 2020, 21, 9577. [Google Scholar] [CrossRef]
- Guryleva, M.V.; Chistyakov, D.V.; Lopachev, A.V.; Goriainov, S.V.; Astakhova, A.A.; Timoshina, Y.A.; Khutorova, A.V.; Fedorova, T.N.; Sergeeva, M.G. Modulation of the Primary Astrocyte-Enriched Cultures’ Oxylipin Profiles Reduces Neurotoxicity. Metabolites 2021, 11, 498. [Google Scholar] [CrossRef]
- Sapieha, P.; Stahl, A.; Chen, J.; Seaward, M.R.; Willett, K.L.; Krah, N.M.; Dennison, R.J.; Connor, K.M.; Aderman, C.M.; Liclican, E.; et al. 5-Lipoxygenase Metabolite 4-HDHA Is a Mediator of the Antiangiogenic Effect of ω-3 Polyunsaturated Fatty Acids. Sci. Transl. Med. 2011, 3, 69ra12. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, Y.; Hua, T.; Liu, X.-L.; Liu, T.; Yuan, R.-Y.; Li, G.-P.; Zhu, Y.; Zhang, X. Omega-3 Polyunsaturated Fatty Acid Supplementation Improves Lipid Metabolism and Endothelial Function by Providing a Beneficial Eicosanoid-Pattern in Patients with Acute Myocardial Infarction: A Randomized, Controlled Trial. Clin. Nutr. 2021, 40, 445–459. [Google Scholar] [CrossRef]
- Abdalla, H.B.; Alvarez, C.; Wu, Y.; Rojas, P.; Hammock, B.D.; Maddipati, K.R.; Trindade-da-Silva, C.A.; Soares, M.Q.S.; Clemente-Napimoga, J.T.; Kantarci, A.; et al. Soluble Epoxide Hydrolase Inhibition Enhances Production of Specialized Pro-resolving Lipid Mediator and Promotes Macrophage Plasticity. Br. J. Pharmacol. 2023, 180, 1597–1615. [Google Scholar] [CrossRef]
- Akiyama, S.; Nagai, H.; Oike, S.; Horikawa, I.; Shinohara, M.; Lu, Y.; Futamura, T.; Shinohara, R.; Kitaoka, S.; Furuyashiki, T. Chronic Social Defeat Stress Increases the Amounts of 12-Lipoxygenase Lipid Metabolites in the Nucleus Accumbens of Stress-Resilient Mice. Sci. Rep. 2022, 12, 11385. [Google Scholar] [CrossRef]
- Nagatake, T.; Shibata, Y.; Morimoto, S.; Node, E.; Sawane, K.; Hirata, S.; Adachi, J.; Abe, Y.; Isoyama, J.; Saika, A.; et al. 12-Hydroxyeicosapentaenoic Acid Inhibits Foam Cell Formation and Ameliorates High-Fat Diet-Induced Pathology of Atherosclerosis in Mice. Sci. Rep. 2021, 11, 10426. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Yuan, J.-J.; Chen, Q.; Xiong, X.-Y.; Zhang, Q.; Xie, Q.; Huang, J.-C.; Yang, G.-Q.; Gong, C.-X.; Qiu, Z.-M.; Sang, H.-F.; et al. Quantitative Profiling of Oxylipins in Acute Experimental Intracerebral Hemorrhage. Front. Neurosci. 2020, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Inceoglu, B.; Schmelzer, K.R.; Morisseau, C.; Jinks, S.L.; Hammock, B.D. Soluble Epoxide Hydrolase Inhibition Reveals Novel Biological Functions of Epoxyeicosatrienoic Acids (EETs). Prostaglandins Other Lipid Mediat. 2007, 82, 42–49. [Google Scholar] [CrossRef]
- Thompson, D.A.; Hammock, B.D. Dihydroxyoctadecamonoenoate Esters Inhibit the Neutrophil Respiratory Burst. J. Biosci. 2007, 32, 279–291. [Google Scholar] [CrossRef]
- Morisseau, C.; Inceoglu, B.; Schmelzer, K.; Tsai, H.-J.; Jinks, S.L.; Hegedus, C.M.; Hammock, B.D. Naturally Occurring Monoepoxides of Eicosapentaenoic Acid and Docosahexaenoic Acid Are Bioactive Antihyperalgesic Lipids. J. Lipid Res. 2010, 51, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Hamabata, T.; Nakamura, T.; Tachibana, Y.; Horikami, D.; Murata, T. 5,6-DiHETE Attenuates Vascular Hyperpermeability by Inhibiting Ca2+ Elevation in Endothelial Cells. J. Lipid Res. 2018, 59, 1864–1870. [Google Scholar] [CrossRef]
- Scher, J.U.; Pillinger, M.H. 15d-PGJ2: The Anti-Inflammatory Prostaglandin? Clin. Immunol. 2005, 114, 100–109. [Google Scholar] [CrossRef]
- Lefils-Lacourtablaise, J.; Socorro, M.; Géloën, A.; Daira, P.; Debard, C.; Loizon, E.; Guichardant, M.; Dominguez, Z.; Vidal, H.; Lagarde, M.; et al. The Eicosapentaenoic Acid Metabolite 15-Deoxy-Δ12,14-Prostaglandin J3 Increases Adiponectin Secretion by Adipocytes Partly via a PPARγ-Dependent Mechanism. PLoS ONE 2013, 8, e63997. [Google Scholar] [CrossRef]
- Wendling, M.G.; DuCharme, D.W. Cardiovascular Effects of Prostaglandin D3 and D2 in Anesthetized Dogs. Prostaglandins 1981, 22, 235–243. [Google Scholar] [CrossRef]
- Whitaker, M.O.; Wyche, A.; Fitzpatrick, F.; Sprecher, H.; Needleman, P. Triene Prostaglandins: Prostaglandin D3 and Icosapentaenoic Acid as Potential Antithrombotic Substances. Proc. Natl. Acad. Sci. USA 1979, 76, 5919–5923. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M. Orbital Compartment Syndrome with Traumatic Optic Neuropathy: A Case Report and Literature Review. Biomed. J. Sci. Tech. Res. 2019, 21, 16170–16172. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.Y.; Koneru, N.; Alahdab, G.; Risner, M.; Ibrahim, A.S.; Maddipati, K.R.; Al-Shabrawey, M. Alterations of Bioactive Lipid Profiles in the Retina Following Traumatic Optic Neuropathy in Mice. Biomolecules 2025, 15, 1450. https://doi.org/10.3390/biom15101450
Kim MY, Koneru N, Alahdab G, Risner M, Ibrahim AS, Maddipati KR, Al-Shabrawey M. Alterations of Bioactive Lipid Profiles in the Retina Following Traumatic Optic Neuropathy in Mice. Biomolecules. 2025; 15(10):1450. https://doi.org/10.3390/biom15101450
Chicago/Turabian StyleKim, Min Young, Nandini Koneru, Gieth Alahdab, Michael Risner, Ahmed S. Ibrahim, Krishna Rao Maddipati, and Mohamed Al-Shabrawey. 2025. "Alterations of Bioactive Lipid Profiles in the Retina Following Traumatic Optic Neuropathy in Mice" Biomolecules 15, no. 10: 1450. https://doi.org/10.3390/biom15101450
APA StyleKim, M. Y., Koneru, N., Alahdab, G., Risner, M., Ibrahim, A. S., Maddipati, K. R., & Al-Shabrawey, M. (2025). Alterations of Bioactive Lipid Profiles in the Retina Following Traumatic Optic Neuropathy in Mice. Biomolecules, 15(10), 1450. https://doi.org/10.3390/biom15101450




