Pro-Inflammatory Protein PSCA Is Upregulated in Neurological Diseases and Targets β2-Subunit-Containing nAChRs
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatic Analysis
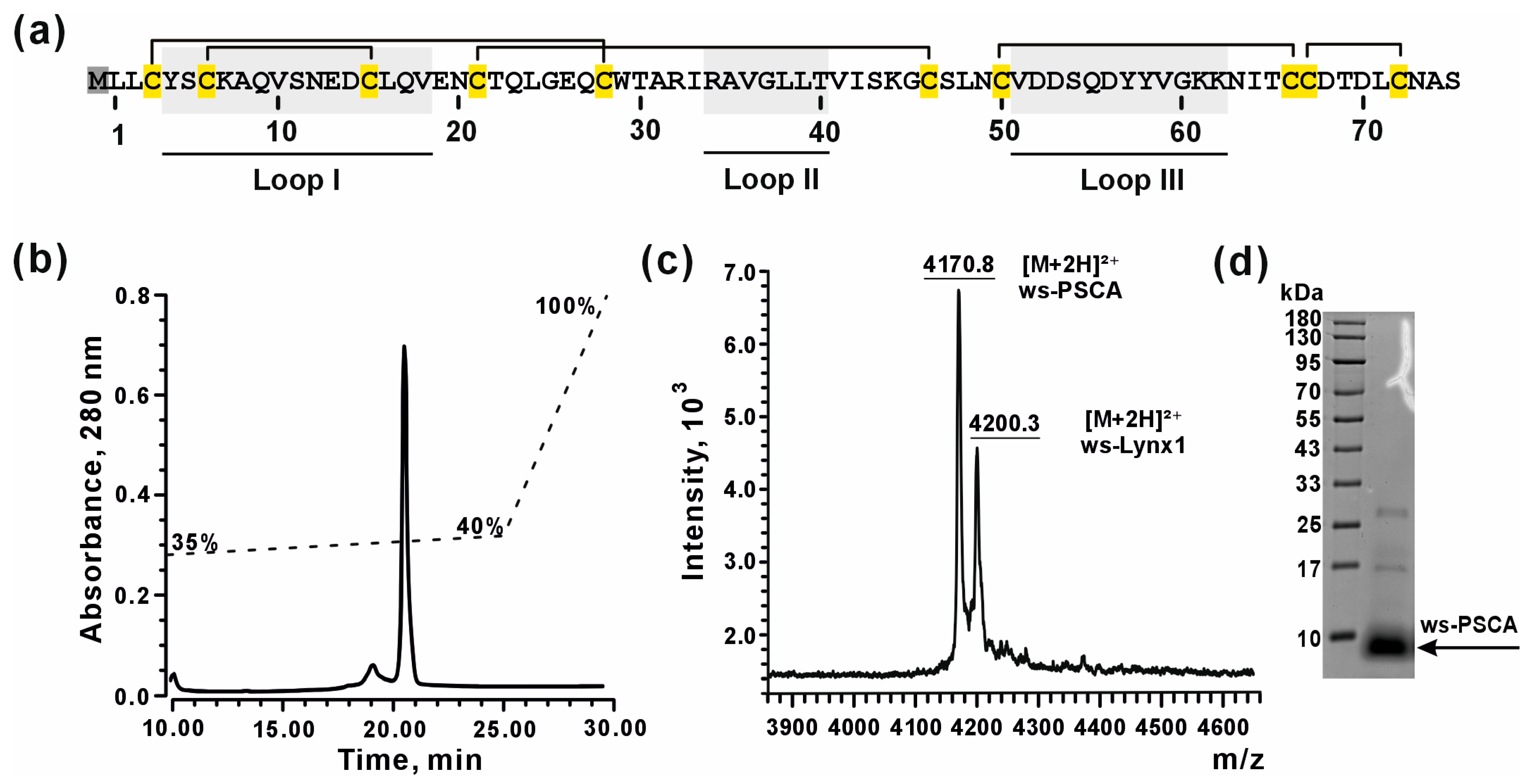
2.2. Design of ws-PSCA Gene for Recombinant Production
2.3. Production and Characterization of Recombinant ws-PSCA
2.4. Hippocampal Neurons and Astrocytes Isolation
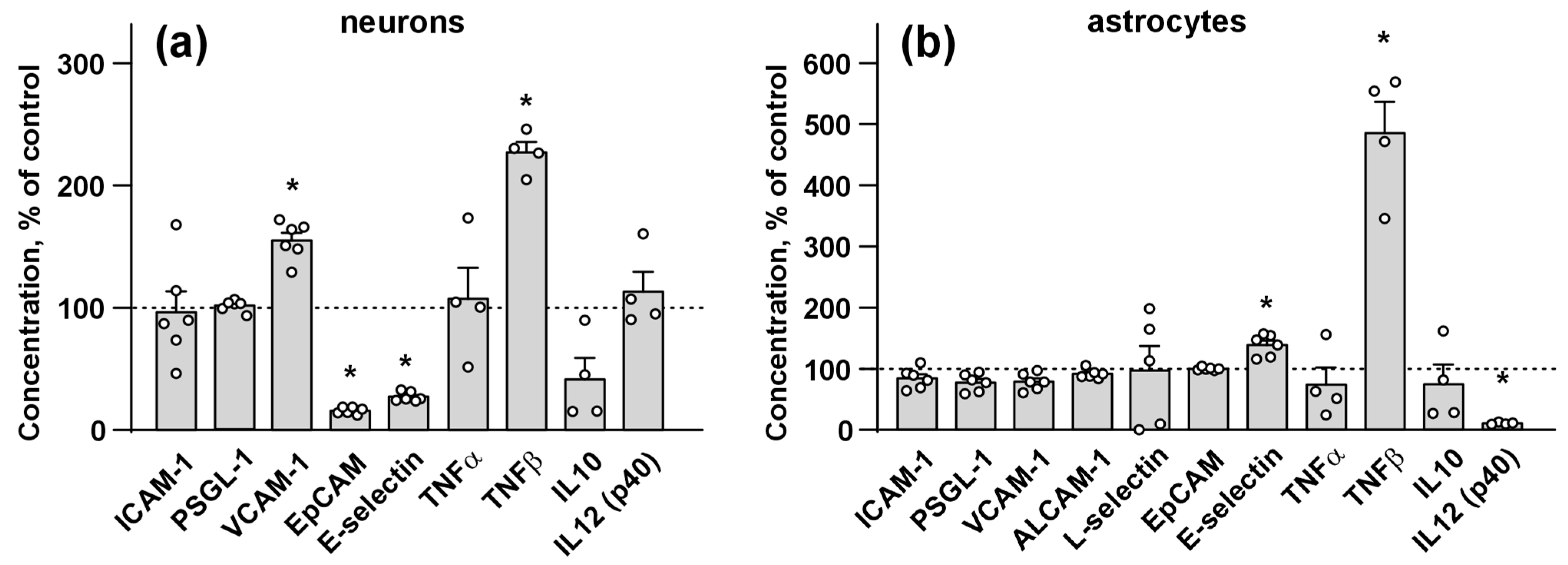
2.5. Analysis of Inflammatory Cytokines Secretion by Neurons and Astrocytes
2.6. Study of Structure and Dynamics by NMR Spectroscopy
2.7. Accession Codes
2.8. Electrophysiology Recordings in X. laevis Oocytes
2.9. Statistical Analysis
2.10. Computer Modeling of HS and LS α4β2-nAChR Stoichiometries
2.11. Molecular Dynamics Simulations
- The first type of MD simulations served for conformational sampling of isolated ws-PSCA and nAChRs to generate unlike conformations as an input for the ensemble docking (Figure S4, steps 1–3):
- For PSCA, four NMR-derived representative conformations were selected and each were subjected to 200 ns MD simulations in aqueous solution; resulting aggregated 800 ns trajectory was clustered using the gmx cluster utility and gromos clustering method, with a cutoff value of 0.250 nm, yielding 93 clusters of conformations (Figure S4, steps 1–3a).
- For nAChRs, two 600 ns MD simulations for HS and LS stoichiometries were calculated (Figure S4, steps 2b, 2c), from which individual dimeric interaction interfaces were extracted and concatenated: β2(+)/β2(−) (600 ns), α4(+)/β2(−) (2400 ns), and β2(+)/α4(−) (2400 ns); this was done using the gmx trjconv and gmx trjcat utilities. These trajectories were clustered using the following cutoff values: 0.140 nm (β2(+)/β2(−); 53 clusters), 0.150 nm (α4(+)/β2(−); 54 clusters), and 0.172 nm (β2(+)/α4(−); 57 clusters) (Figure S4, steps 3b–d). Clustering was performed using the defined binding site residues (see Section 2.12 below), excluding the M4 helix terminus (residues 455–477) of the β2-subunit due to its high flexibility.
- Secondly, two MD replicas (500 ns) were aimed to assess the stability of the proposed model of the HS (α4)2(β2)3-nAChR complex with ws-PSCA after the ensemble docking and to characterize intermolecular contacts (Figure S4, step 7). An in-house Impulse software [59] was used for the latter, omitting the first 30 ns of each trajectory estimated to equilibrate the system.
2.12. Ensemble Docking of the nAChR/PSCA Complex
- β2(+): 21–35, 46–50, 60–64, 94–103, 110–120, 129–167, 177–206, 261–271, and 455–477;
- β2(−): 31–45, 52–63, 76–83, 106–125, 136–142, 158–184, 204–210, and 455–477;
- α4(+): 24–38, 49–53, 62–67, 97–105, 113–123, 132–172, 182–214, and 267–277;
- α4(−): 33–49, 54–66, 77–87, 109–128, 139–146, 161–187, and 210–216.
- Buried surface area ≥ 2750 Å2;
- Molecular hydrophobic potential’s [62] complementarity score ≥ 0.5;
- Number of intermolecular ionic bonds ≥ 6;
- Number of intermolecular hydrogen bonds ≥ 7.
- at the β2(+)/β2(−) interface,
- K147(+), R186(+) → E13, D14, D52, D53, D68
- D192(+), D193(+) → R32, R34
- D170(−) → R32, R34
- D171(−) → R32, R34
- at the α4(+)/β2(−) interface,
- R193(+), K194(+) → E13, D14, D52, D53, D68
- D170(−) → R32, R34
- D171(−), E196(+) → R32, R34
3. Results
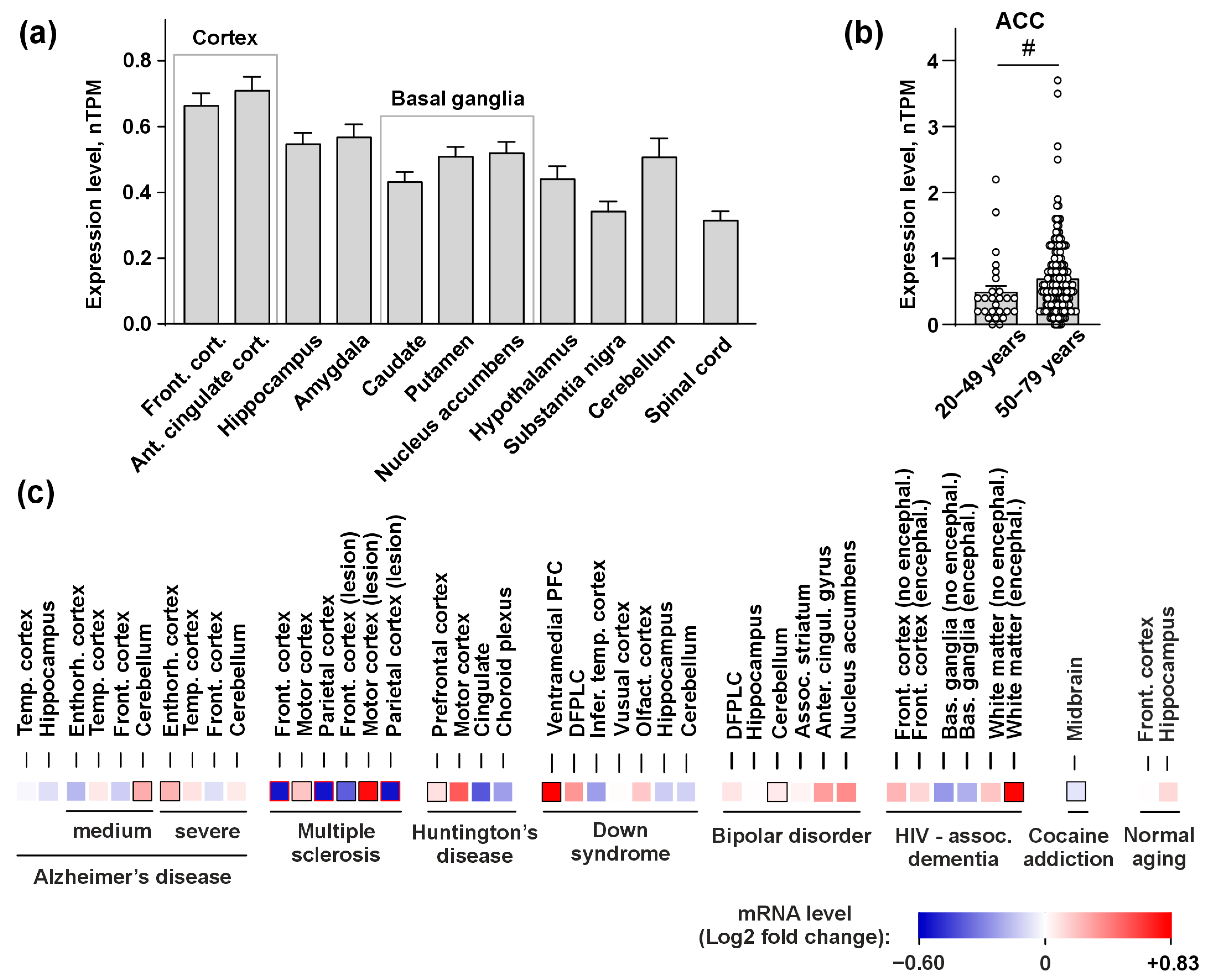
3.1. PSCA Expression in the Human Brain
3.2. Bacterial Production of Recombinant ws-PSCA
3.3. Ws-PSCA Regulates Secretion of Inflammatory Factors and Adhesion Molecules by Neurons and Astrocytes
3.4. NMR Structure and Dynamics of ws-PSCA in Aqueous Solution
3.5. Ws-PSCA Inhibits α4β2- and α3β2-nAChRs, but Not α4β4-nAChRs
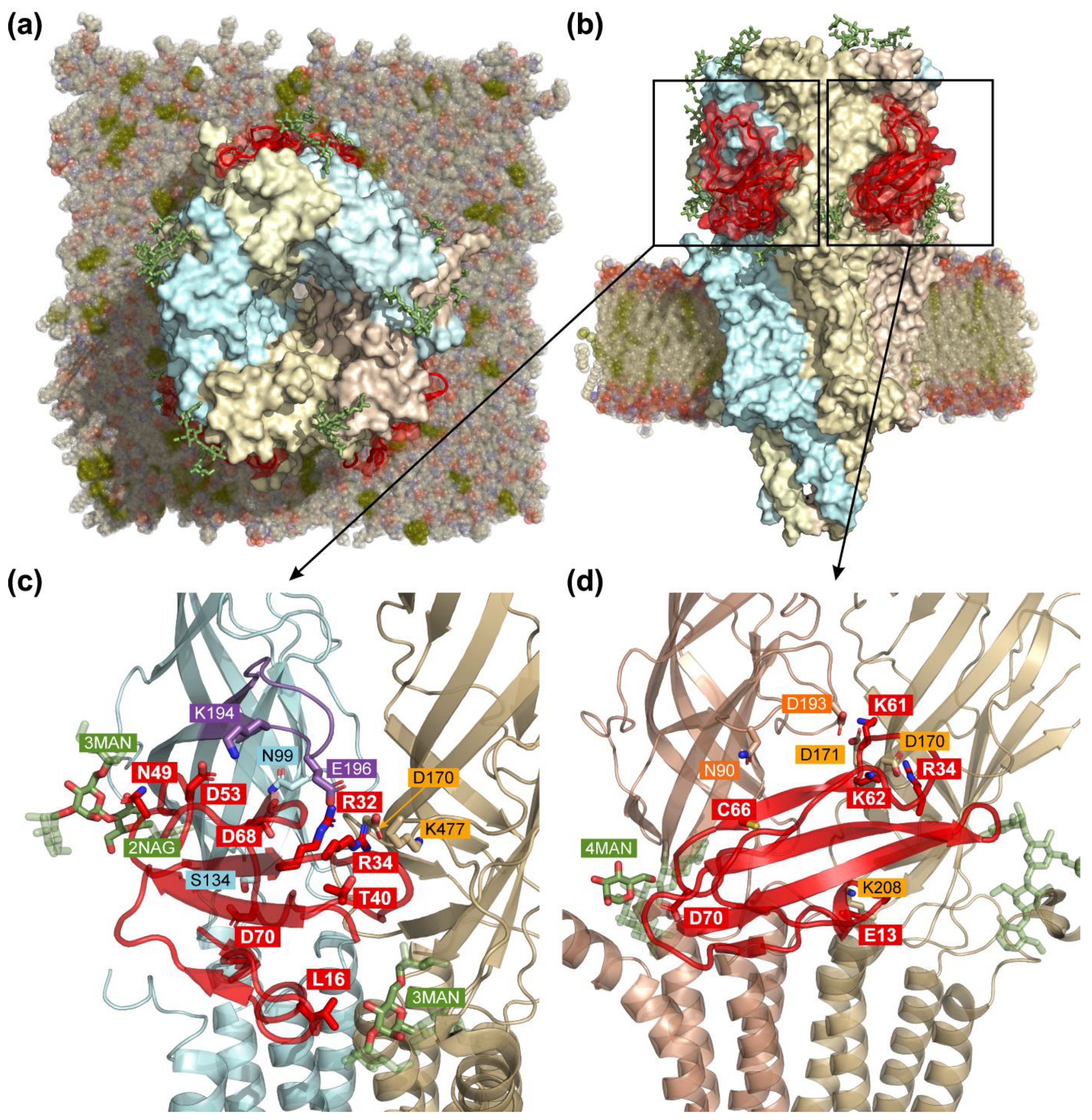
3.6. Computer Modeling of the α4β2-nAChR/ws-PSCA Complex
3.7. MD of nAChR/ws-PSCA Complex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The Global Burden of Neurological Disorders: Translating Evidence into Policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Olazarán, J.; Carnero-Pardo, C.; Fortea, J.; Sánchez-Juan, P.; García-Ribas, G.; Viñuela, F.; Martínez-Lage, P.; Boada, M. Prevalence of Treated Patients with Alzheimer’s Disease: Current Trends and COVID-19 Impact. Alzheimers Res. Ther. 2023, 15, 130. [Google Scholar] [CrossRef]
- Hangya, B.; Ranade, S.P.; Lorenc, M.; Kepecs, A. Central Cholinergic Neurons Are Rapidly Recruited by Reinforcement Feedback. Cell 2015, 162, 1155–1168. [Google Scholar] [CrossRef]
- Drever, B.D.; Riedel, G.; Platt, B. The Cholinergic System and Hippocampal Plasticity. Behav. Brain Res. 2011, 221, 505–514. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Sambeth, A.; Blokland, A. Acetylcholine and Attention. Behav. Brain Res. 2011, 221, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Platt, B.; Riedel, G. The Cholinergic System and Spatial Learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Mitsushima, D.; Sano, A.; Takahashi, T. A Cholinergic Trigger Drives Learning-Induced Plasticity at Hippocampal Synapses. Nat. Commun. 2013, 4, 2760. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Albin, R.L. The Cholinergic System and Parkinson Disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef]
- Du, Y.; Chen, L.; Yan, M.-C.; Wang, Y.-L.; Zhong, X.-L.; Xv, C.-X.; Li, Y.-B.; Cheng, Y. Neurometabolite Levels in the Brains of Patients with Autism Spectrum Disorders: A Meta-Analysis of Proton Magnetic Resonance Spectroscopy Studies (N = 1501). Mol. Psychiatry 2023, 28, 3092–3103. [Google Scholar] [CrossRef]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a Neuromodulator: Cholinergic Signaling Shapes Nervous System Function and Behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Wallace, T.L.; Bertrand, D. Importance of the Nicotinic Acetylcholine Receptor System in the Prefrontal Cortex. Biochem. Pharmacol. 2013, 85, 1713–1720. [Google Scholar] [CrossRef]
- Crestini, A.; Carbone, E.; Rivabene, R.; Ancidoni, A.; Rosa, P.; Tata, A.M.; Fabrizi, E.; Locuratolo, N.; Vanacore, N.; Lacorte, E.; et al. A Systematic Review on Drugs Acting as Nicotinic Acetylcholine Receptor Agonists in the Treatment of Dementia. Cells 2024, 13, 237. [Google Scholar] [CrossRef]
- Wittenberg, R.E.; Wolfman, S.L.; De Biasi, M.; Dani, J.A. Nicotinic Acetylcholine Receptors and Nicotine Addiction: A Brief Introduction. Neuropharmacology 2020, 177, 108256. [Google Scholar] [CrossRef]
- Terry, A.V.; Jones, K.; Bertrand, D. Nicotinic Acetylcholine Receptors in Neurological and Psychiatric Diseases. Pharmacol. Res. 2023, 191, 106764. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Moretti, M.; Gaimarri, A.; Zanardi, A.; Clementi, F.; Zoli, M. Heterogeneity and Complexity of Native Brain Nicotinic Receptors. Biochem. Pharmacol. 2007, 74, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.P.; Stokoe, S.A.; Sathler, M.F.; Nichols, R.A.; Kim, S. Selective Coactivation of A7- and A4β2-Nicotinic Acetylcholine Receptors Reverses Beta-Amyloid-Induced Synaptic Dysfunction. J. Biol. Chem. 2021, 296, 100402. [Google Scholar] [CrossRef]
- Vasilyeva, N.A.; Loktyushov, E.V.; Bychkov, M.L.; Shenkarev, Z.O.; Lyukmanova, E.N. Three-Finger Proteins from the Ly6/uPAR Family: Functional Diversity within One Structural Motif. Biochemistry 2017, 82, 1702–1715. [Google Scholar] [CrossRef] [PubMed]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, Evolution and Functions of the Human and Mouse Ly6/uPAR Family Genes. Hum. Genom. 2016, 10, 10. [Google Scholar] [CrossRef]
- Morgan, B.P.; Boyd, C.; Bubeck, D. Molecular Cell Biology of Complement Membrane Attack. Semin. Cell Dev. Biol. 2017, 72, 124–132. [Google Scholar] [CrossRef]
- Arredondo, J.; Chernyavsky, A.I.; Grando, S.A. Overexpression of SLURP-1 and -2 Alleviates the Tumorigenic Action of Tobacco-Derived Nitrosamine on Immortalized Oral Epithelial Cells. Biochem. Pharmacol. 2007, 74, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Shlepova, O.V.; Shulepko, M.A.; Shipunova, V.O.; Bychkov, M.L.; Kukushkin, I.D.; Chulina, I.A.; Azev, V.N.; Shramova, E.I.; Kazakov, V.A.; Ismailova, A.M.; et al. Selective Targeting of A7 Nicotinic Acetylcholine Receptor by Synthetic Peptide Mimicking Loop I of Human SLURP-1 Provides Efficient and Prolonged Therapy of Epidermoid Carcinoma in Vivo. Front. Cell Dev. Biol. 2023, 11, 1256716. [Google Scholar] [CrossRef] [PubMed]
- Isaev, A.B.; Bychkov, M.L.; Kulbatskii, D.S.; Andreev-Andrievskiy, A.A.; Mashkin, M.A.; Shulepko, M.A.; Shlepova, O.V.; Loktyushov, E.V.; Latanov, A.V.; Kirpichnikov, M.P.; et al. Upregulation of Cholinergic Modulators Lypd6 and Lypd6b Associated with Autism Drives Anxiety and Cognitive Decline. Cell Death Discov. 2024, 10, 444. [Google Scholar] [CrossRef]
- Palumbo, T.B.; Miwa, J.M. Lynx1 and the Family of Endogenous Mammalian Neurotoxin-like Proteins and Their Roles in Modulating nAChR Function. Pharmacol. Res. 2023, 194, 106845. [Google Scholar] [CrossRef]
- Shenkarev, Z.O.; Shulepko, M.A.; Bychkov, M.L.; Kulbatskii, D.S.; Shlepova, O.V.; Vasilyeva, N.A.; Andreev-Andrievskiy, A.A.; Popova, A.S.; Lagereva, E.A.; Loktyushov, E.V.; et al. Water-Soluble Variant of Human Lynx1 Positively Modulates Synaptic Plasticity and Ameliorates Cognitive Impairment Associated with A7-nAChR Dysfunction. J. Neurochem. 2020, 155, 45–61. [Google Scholar] [CrossRef]
- Ibañez-Tallon, I.; Miwa, J.M.; Wang, H.-L.; Adams, N.C.; Crabtree, G.W.; Sine, S.M.; Heintz, N. Novel Modulation of Neuronal Nicotinic Acetylcholine Receptors by Association with the Endogenous Prototoxin Lynx1. Neuron 2002, 33, 893–903. [Google Scholar] [CrossRef]
- Dessaud, E.; Salaün, D.; Gayet, O.; Chabbert, M.; deLapeyrière, O. Identification of Lynx2, a Novel Member of the Ly-6/Neurotoxin Superfamily, Expressed in Neuronal Subpopulations during Mouse Development. Mol. Cell. Neurosci. 2006, 31, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Darvas, M.; Morsch, M.; Racz, I.; Ahmadi, S.; Swandulla, D.; Zimmer, A. Modulation of the Ca2+ Conductance of Nicotinic Acetylcholine Receptors by Lypd6. Eur. Neuropsychopharmacol. 2009, 19, 670–681. [Google Scholar] [CrossRef]
- Ochoa, V.; George, A.A.; Nishi, R.; Whiteaker, P. The Prototoxin LYPD6B Modulates Heteromeric A3β4-Containing Nicotinic Acetylcholine Receptors, but Not A7 Homomers. FASEB J. 2016, 30, 1109–1119. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Cinar, B.; Jensen, M.M.; Lyukmanova, E.N.; Shulepko, M.A.; Tsetlin, V.; Klein, A.B.; Mikkelsen, J.D. Expression of the Ly-6 Family Proteins Lynx1 and Ly6H in the Rat Brain Is Compartmentalized, Cell-Type Specific, and Developmentally Regulated. Brain Struct. Funct. 2014, 219, 1923–1934. [Google Scholar] [CrossRef]
- Wu, M.; Puddifoot, C.A.; Taylor, P.; Joiner, W.J. Mechanisms of Inhibition and Potentiation of A4β2 Nicotinic Acetylcholine Receptors by Members of the Ly6 Protein Family. J. Biol. Chem. 2015, 290, 24509–24518. [Google Scholar] [CrossRef]
- Hruska, M.; Keefe, J.; Wert, D.; Tekinay, A.B.; Hulce, J.J.; Ibañez-Tallon, I.; Nishi, R. Prostate Stem Cell Antigen Is an Endogenous Lynx1-like Prototoxin That Antagonizes Alpha7-Containing Nicotinic Receptors and Prevents Programmed Cell Death of Parasympathetic Neurons. J. Neurosci. 2009, 29, 14847–14854. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Sakamoto, H.; Yoshida, T.; Saeki, N. Prostate Stem Cell Antigen Is Expressed in Normal and Malignant Human Brain Tissues. Oncol. Lett. 2018, 15, 3081–3084. [Google Scholar] [CrossRef]
- Reiter, R.E.; Gu, Z.; Watabe, T.; Thomas, G.; Szigeti, K.; Davis, E.; Wahl, M.; Nisitani, S.; Yamashiro, J.; Le Beau, M.M.; et al. Prostate Stem Cell Antigen: A Cell Surface Marker Overexpressed in Prostate Cancer. Proc. Natl. Acad. Sci. USA 1998, 95, 1735–1740. [Google Scholar] [CrossRef]
- Jensen, M.M.; Arvaniti, M.; Mikkelsen, J.D.; Michalski, D.; Pinborg, L.H.; Härtig, W.; Thomsen, M.S. Prostate Stem Cell Antigen Interacts with Nicotinic Acetylcholine Receptors and Is Affected in Alzheimer’s Disease. Neurobiol. Aging 2015, 36, 1629–1638. [Google Scholar] [CrossRef]
- Bychkov, M.L.; Isaev, A.B.; Andreev-Andrievskiy, A.A.; Petrov, K.; Paramonov, A.S.; Kirpichnikov, M.P.; Lyukmanova, E.N. Aβ1-42 Accumulation Accompanies Changed Expression of Ly6/uPAR Proteins, Dysregulation of the Cholinergic System, and Degeneration of Astrocytes in the Cerebellum of Mouse Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2023, 24, 14852. [Google Scholar] [CrossRef]
- Lyukmanova, E.; Kirichenko, A.; Kulbatskii, D.; Isaev, A.; Kukushkin, I.; Che, Y.; Kirpichnikov, M.; Bychkov, M. Water-Soluble Lynx1 Upregulates Dendritic Spine Density and Stimulates Astrocytic Network and Signaling. Mol. Neurobiol. 2024, 62, 5531–5545. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A Scaling Normalization Method for Differential Expression Analysis of RNA-Seq Data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Shulepko, M.A.; Lyukmanova, E.N.; Shenkarev, Z.O.; Dubovskii, P.V.; Astapova, M.V.; Feofanov, A.V.; Arseniev, A.S.; Utkin, Y.N.; Kirpichnikov, M.P.; Dolgikh, D.A. Towards Universal Approach for Bacterial Production of Three-Finger Ly6/uPAR Proteins: Case Study of Cytotoxin I from Cobra N. Oxiana. Protein Expr. Purif. 2017, 130, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Suntsova, M.; Gogvadze, E.V.; Salozhin, S.; Gaifullin, N.; Eroshkin, F.; Dmitriev, S.E.; Martynova, N.; Kulikov, K.; Malakhova, G.; Tukhbatova, G.; et al. Human-Specific Endogenous Retroviral Insert Serves as an Enhancer for the Schizophrenia-Linked Gene PRODH. Proc. Natl. Acad. Sci. USA 2013, 110, 19472–19477. [Google Scholar] [CrossRef]
- Schildge, S.; Bohrer, C.; Beck, K.; Schachtrup, C. Isolation and Culture of Mouse Cortical Astrocytes. J. Vis. Exp. 2013, 71, e50079. [Google Scholar] [CrossRef]
- Kazimierczuk, K.; Orekhov, V.Y. Accelerated NMR Spectroscopy by Using Compressed Sensing. Angew. Chem. Int. Ed. Engl. 2011, 50, 5556–5559. [Google Scholar] [CrossRef]
- Sattler, M.; Schleucher, J.; Griesinger, C. Heteronuclear Multidimensional NMR Experiments for the Structure Determination of Proteins in Solution Employing Pulsed Field Gradients. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 93–158. [Google Scholar] [CrossRef]
- Bax, A.; Vuister, G.W.; Grzesiek, S.; Delaglio, F.; Wang, A.C.; Tschudin, R.; Zhu, G. Measurement of Homo- and Heteronuclear J Couplings from Quantitative J Correlation. Methods Enzymol. 1994, 239, 79–105. [Google Scholar] [CrossRef] [PubMed]
- Korzhnev, D.M.; Billeter, M.; Arseniev, A.S.; Orekhov, V.Y. NMR Studies of Brownian Tumbling and Internal Motions in Proteins. Prog. Nucl. Magn. Reson. Spectrosc. 2001, 38, 197–266. [Google Scholar] [CrossRef]
- Wüthrich, K. NMR with Proteins and Nucleic Acids. Europhys. News 1986, 17, 11–13. [Google Scholar] [CrossRef]
- Shen, Y.; Bax, A. Protein Structural Information Derived from NMR Chemical Shift with the Neural Network Program TALOS-N. Methods Mol. Biol. 2015, 1260, 17–32. [Google Scholar] [CrossRef]
- Schmidt, E.; Güntert, P. Automated Structure Determination from NMR Spectra. Methods Mol. Biol. 2015, 1261, 303–329. [Google Scholar] [CrossRef] [PubMed]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A Program for Display and Analysis of Macromolecular Structures. J. Mol. Graph. 1996, 14, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.M.; Akke, M.; Palmer, A.G. Backbone Dynamics of Escherichia Coli Ribonuclease HI: Correlations with Structure and Function in an Active Enzyme. J Mol Biol 1995, 246, 144–163. [Google Scholar] [CrossRef]
- Cole, R.; Loria, J.P. FAST-Modelfree: A Program for Rapid Automated Analysis of Solution NMR Spin-Relaxation Data. J. Biomol. NMR 2003, 26, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kulbatskii, D.; Shenkarev, Z.; Bychkov, M.; Loktyushov, E.; Shulepko, M.; Koshelev, S.; Povarov, I.; Popov, A.; Peigneur, S.; Chugunov, A.; et al. Human Three-Finger Protein Lypd6 Is a Negative Modulator of the Cholinergic System in the Brain. Front. Cell Dev. Biol. 2021, 9, 662227. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Kang, G.; Natarajan, K.; Hibbs, R.E.; Sine, S.M. Structural Bases for Stoichiometry-Selective Calcium Potentiation of a Neuronal Nicotinic Receptor. Br. J. Pharmacol. 2024, 181, 1973–1992. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively Expanding the Structural Coverage of Protein-Sequence Space with High-Accuracy Models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Sali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Park, S.-J.; Lee, J.; Qi, Y.; Kern, N.R.; Lee, H.S.; Jo, S.; Joung, I.; Joo, K.; Lee, J.; Im, W. CHARMM-GUI Glycan Modeler for Modeling and Simulation of Carbohydrates and Glycoconjugates. Glycobiology 2019, 29, 320–331. [Google Scholar] [CrossRef]
- Krylov, N.A.; Efremov, R.G. Libxtc: An Efficient Library for Reading XTC-Compressed MD Trajectory Data. BMC Res. Notes 2021, 14, 124. [Google Scholar] [CrossRef]
- Shulepko, M.A.; Bychkov, M.L.; Shenkarev, Z.O.; Kulbatskii, D.S.; Makhonin, A.M.; Paramonov, A.S.; Chugunov, A.O.; Kirpichnikov, M.P.; Lyukmanova, E.N. Biochemical Basis of Skin Disease Mal de Meleda: SLURP-1 Mutants Differently Affect Keratinocyte Proliferation and Apoptosis. J. Investig. Dermatol. 2021, 141, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Ohue, M.; Shimoda, T.; Suzuki, S.; Matsuzaki, Y.; Ishida, T.; Akiyama, Y. MEGADOCK 4.0: An Ultra-High-Performance Protein-Protein Docking Software for Heterogeneous Supercomputers. Bioinformatics 2014, 30, 3281–3283. [Google Scholar] [CrossRef]
- Pyrkov, T.V.; Chugunov, A.O.; Krylov, N.A.; Nolde, D.E.; Efremov, R.G. PLATINUM: A Web Tool for Analysis of Hydrophobic/Hydrophilic Organization of Biomolecular Complexes. Bioinformatics 2009, 25, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Kritsilis, M.; V Rizou, S.; Koutsoudaki, P.N.; Evangelou, K.; Gorgoulis, V.G.; Papadopoulos, D. Ageing, Cellular Senescence and Neurodegenerative Disease. Int. J. Mol. Sci. 2018, 19, 2937. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Mineev, K.S.; D’Hoedt, D.; Kasheverov, I.E.; Filkin, S.Y.; Krivolapova, A.P.; Janickova, H.; Dolezal, V.; et al. NMR Structure and Action on Nicotinic Acetylcholine Receptors of Water-Soluble Domain of Human LYNX1. J. Biol. Chem. 2011, 286, 10618–10627. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shulepko, M.A.; Shenkarev, Z.O.; Bychkov, M.L.; Paramonov, A.S.; Chugunov, A.O.; Kulbatskii, D.S.; Arvaniti, M.; Dolejsi, E.; Schaer, T.; et al. Secreted Isoform of Human Lynx1 (SLURP-2): Spatial Structure and Pharmacology of Interactions with Different Types of Acetylcholine Receptors. Sci. Rep. 2016, 6, 30698. [Google Scholar] [CrossRef] [PubMed]
- Shulepko, M.; Lyukmanova, E.; Paramonov, A.; Lobas, A.; Shenkarev, Z.; Kasheverov, I.; Dolgikh, D.; Tsetlin, V.; Arseniev, A.; Kirpichnikov, M. Human Neuromodulator SLURP-1: Bacterial Expression, Binding to Muscle-Type Nicotinic Acetylcholine Receptor, Secondary Structure, and Conformational Heterogeneity in Solution. Biochemistry 2013, 78, 204–211. [Google Scholar] [CrossRef]
- Paramonov, A.S.; Kulbatskii, D.S.; Loktyushov, E.V.; Tsarev, A.V.; Dolgikh, D.A.; Shenkarev, Z.O.; Kirpichnikov, M.P.; Lyukmanova, E.N. Recombinant Production and Structural Studies of the Human Lypd6 and Lypd6b Proteins. Russ. J. Bioorganic Chem. 2017, 43, 644–652. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a Central Mechanism in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Johnson, N.R.; Boyd, T.D.; Coughlan, C.; Chial, H.J.; Potter, H. Innate Immune System Activation and Neuroinflammation in Down Syndrome and Neurodegeneration: Therapeutic Targets or Partners? Front. Aging Neurosci. 2021, 13, 718426. [Google Scholar] [CrossRef]
- Field, S.E.; Curle, A.J.; Barker, R.A. Inflammation and Huntington’s Disease—A Neglected Therapeutic Target? Expert Opin. Investig. Drugs 2024, 33, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. Bipolar Disorder: Role of Inflammation and the Development of Disease Biomarkers. Psychiatry Investig. 2015, 13, 18. [Google Scholar] [CrossRef]
- Mustafa, M.; Musselman, D.; Jayaweera, D.; da Fonseca Ferreira, A.; Marzouka, G.; Dong, C. HIV-Associated Neurocognitive Disorder (HAND) and Alzheimer’s Disease Pathogenesis: Future Directions for Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 11170. [Google Scholar] [CrossRef]
- Shen, Y.; Bax, A. Protein Backbone and Sidechain Torsion Angles Predicted from NMR Chemical Shifts Using Artificial Neural Networks. J. Biomol. NMR 2013, 56, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Kneller, J.M.; Lu, M.; Bracken, C. An Effective Method for the Discrimination of Motional Anisotropy and Chemical Exchange. J. Am. Chem. Soc. 2002, 124, 1852–1853. [Google Scholar] [CrossRef]
- Tsetlin, V.I. Three-Finger Snake Neurotoxins and Ly6 Proteins Targeting Nicotinic Acetylcholine Receptors: Pharmacological Tools and Endogenous Modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Lyukmanova, E.N.; Shulepko, M.A.; Buldakova, S.L.; Kasheverov, I.E.; Shenkarev, Z.O.; Reshetnikov, R.V.; Filkin, S.Y.; Kudryavtsev, D.S.; Ojomoko, L.O.; Kryukova, E.V.; et al. Water-Soluble LYNX1 Residues Important for Interaction with Muscle-Type and/or Neuronal Nicotinic Receptors. J. Biol. Chem. 2013, 288, 15888–15899. [Google Scholar] [CrossRef]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Court, J.A.; Martin-Ruiz, C.; Graham, A.; Perry, E. Nicotinic Receptors in Human Brain: Topography and Pathology. J. Chem. Neuroanat. 2000, 20, 281–298. [Google Scholar] [CrossRef]
- Mann, S.L.; Hazlett, E.A.; Byne, W.; Hof, P.R.; Buchsbaum, M.S.; Cohen, B.H.; Goldstein, K.E.; Haznedar, M.M.; Mitsis, E.M.; Siever, L.J.; et al. Anterior and Posterior Cingulate Cortex Volume in Healthy Adults: Effects of Aging and Gender Differences. Brain Res. 2011, 1401, 18–29. [Google Scholar] [CrossRef]
- Gu, Z.; Alexander, G.M.; Dudek, S.M.; Yakel, J.L. Hippocampus and Entorhinal Cortex Recruit Cholinergic and NMDA Receptors Separately to Generate Hippocampal Theta Oscillations. Cell Rep. 2017, 21, 3585–3595. [Google Scholar] [CrossRef]
- Yu, W.; Krook-Magnuson, E. Cognitive Collaborations: Bidirectional Functional Connectivity Between the Cere-bellum and the Hippocampus. Front. Syst. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.T.; Wimmer, I.; Höftberger, R.; Gerlach, S.; Haider, L.; Zrzavy, T.; Hametner, S.; Mahad, D.; Binder, C.J.; Krumbholz, M.; et al. Disease-Specific Molecular Events in Cortical Multiple Sclerosis Lesions. Brain 2013, 136, 1799–1815. [Google Scholar] [CrossRef]
- Thiruvady, D.R.; Georgiou-Karistianis, N.; Egan, G.F.; Ray, S.; Sritharan, A.; Farrow, M.; Churchyard, A.; Chua, P.; Bradshaw, J.L.; Brawn, T.-L.; et al. Functional Connectivity of the Prefrontal Cortex in Huntington’s Disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sabec, M.H.; Wonnacott, S.; Warburton, E.C.; Bashir, Z.I. Nicotinic Acetylcholine Receptors Control Encoding and Retrieval of Associative Recognition Memory through Plasticity in the Medial Prefrontal Cortex. Cell Rep. 2018, 22, 3409–3415. [Google Scholar] [CrossRef]
- Anderson, J.S.; Nielsen, J.A.; Ferguson, M.A.; Burback, M.C.; Cox, E.T.; Dai, L.; Gerig, G.; Edgin, J.O.; Korenberg, J.R. Abnormal Brain Synchrony in Down Syndrome. Neuroimage Clin. 2013, 2, 703–715. [Google Scholar] [CrossRef]
- Tai, H.; Kandeel, N.; Menon, M.; Ibrahim, A.; Choo, B.; Santana, R.; Jolayemi, A. Role of the Cerebellum in Bipolar Disorder: A Systematic Literature Review. Cureus 2024, 16, e56044. [Google Scholar] [CrossRef]
- Lee, M.; Martin-Ruiz, C.; Graham, A.; Court, J.; Jaros, E.; Perry, R.; Iversen, P.; Bauman, M.; Perry, E. Nicotinic Receptor Abnormalities in the Cerebellar Cortex in Autism. Brain 2002, 125, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.K.; Roth, L.M.; Grinspan, J.B.; Jordan-Sciutto, K.L. White Matter Loss and Oligodendrocyte Dysfunction in HIV: A Consequence of the Infection, the Antiretroviral Therapy or Both? Brain Res. 2019, 1724, 146397. [Google Scholar] [CrossRef]
- Tang, W.-X.; Fasulo, W.H.; Mash, D.C.; Hemby, S.E. Molecular Profiling of Midbrain Dopamine Regions in Cocaine Overdose Victims. J. Neurochem. 2003, 85, 911–924. [Google Scholar] [CrossRef]
- Utkin, Y.N. Aging Affects Nicotinic Acetylcholine Receptors in Brain. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 119–124. [Google Scholar] [CrossRef]
- Tregellas, J.R.; Wylie, K.P. Alpha7 Nicotinic Receptors as Therapeutic Targets in Schizophrenia. Nicotine Tob. Res. 2019, 21, 349–356. [Google Scholar] [CrossRef]
- Ray, M.A.; Graham, A.J.; Lee, M.; Perry, R.H.; Court, J.A.; Perry, E.K. Neuronal Nicotinic Acetylcholine Receptor Subunits in Autism: An Immunohistochemical Investigation in the Thalamus. Neurobiol. Dis. 2005, 19, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Lee, M.L.; Martin-Ruiz, C.M.; Court, J.A.; Volsen, S.G.; Merrit, J.; Folly, E.; Iversen, P.E.; Bauman, M.L.; Perry, R.H.; et al. Cholinergic Activity in Autism: Abnormalities in the Cerebral Cortex and Basal Forebrain. Am. J. Psychiatry 2001, 158, 1058–1066. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Weyn, A.; Mikkelsen, J.D. Hippocampal A7 Nicotinic Acetylcholine Receptor Levels in Patients with Schizophrenia, Bipolar Disorder, or Major Depressive Disorder. Bipolar Disord. 2011, 13, 701–707. [Google Scholar] [CrossRef]
- Hesse, S.; Rullmann, M.; Günnewig, T.; Schweickert de Palma, E.; Burmeister, L.; van Grinsven, M.; Zientek, F.; Luthardt, J.; Hankir, M.K.; Meyer, P.M.; et al. Cholinergic Network Modulation in Disinhibited Eating Behavior. Commun. Biol. 2025, 8, 1347. [Google Scholar] [CrossRef]
- Gatta, V.; Mengod, G.; Reale, M.; Tata, A.M. Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis. Biomedicines 2020, 8, 153. [Google Scholar] [CrossRef]
- D’Souza, G.X.; Waldvogel, H.J. Targeting the Cholinergic System to Develop a Novel Therapy for Huntington’s Disease. J. Huntingt. Dis. 2016, 5, 333–342. [Google Scholar] [CrossRef]
- Capó-Vélez, C.M.; Delgado-Vélez, M.; Báez-Pagán, C.A.; Lasalde-Dominicci, J.A. Nicotinic Acetylcholine Receptors in HIV: Possible Roles During HAND and Inflammation. Cell Mol. Neurobiol. 2018, 38, 1335–1348. [Google Scholar] [CrossRef]
- MacDowell, K.S.; Díaz-Marsá, M.; Güemes, I.; Rodríguez, A.; Leza, J.C.; Carrasco, J.L. Inflammatory Activation and Cholinergic Anti-Inflammatory System in Eating Disorders. Brain Behav. Immun. 2013, 32, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Minami, S.S.; Shen, V.; Le, D.; Krabbe, G.; Asgarov, R.; Perez-Celajes, L.; Lee, C.-H.; Li, J.; Donnelly-Roberts, D.; Gan, L. Reducing Inflammation and Rescuing FTD-Related Behavioral Deficits in Progranulin-Deficient Mice with A7 Nicotinic Acetylcholine Receptor Agonists. Biochem. Pharmacol. 2015, 97, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Rollema, H.; Hurst, R.S. The Contribution of Agonist and Antagonist Activities of A4β2* nAChR Ligands to Smoking Cessation Efficacy: A Quantitative Analysis of Literature Data. Psychopharmacology 2018, 235, 2479–2505. [Google Scholar] [CrossRef]
- Mitra, S.; Mucha, M.; Khatri, S.N.; Glenon, R.; Schulte, M.K.; Bult-Ito, A. Attenuation of Compulsive-Like Behavior Through Positive Allosteric Modulation of A4β2 Nicotinic Acetylcholine Receptors in Non-Induced Compulsive-Like Mice. Front. Behav. Neurosci. 2016, 10, 244. [Google Scholar] [CrossRef]
- Philip, N.S.; Carpenter, L.L.; Tyrka, A.R.; Price, L.H. Nicotinic Acetylcholine Receptors and Depression: A Review of the Preclinical and Clinical Literature. Psychopharmacology 2010, 212, 1–12. [Google Scholar] [CrossRef]
- Parikh, V.; Kutlu, M.G.; Gould, T.J. nAChR Dysfunction as a Common Substrate for Schizophrenia and Comorbid Nicotine Addiction: Current Trends and Perspectives. Schizophr. Res. 2016, 171, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kamens, H.M.; Flarend, G.; Horton, W.J. The Role of Nicotinic Receptors in Alcohol Consumption. Pharmacol. Res. 2023, 190, 106705. [Google Scholar] [CrossRef] [PubMed]
- Alasmari, F.; Ahmad, A.; Alsanea, S.; Hammad, A.M.; Al-Qerem, W. Current Insights and Prospects for the Pathogenesis and Treatment of Clinical Manifestations Associated with Down Syndrome through Neurotransmitter, Inflammatory, and Oxidative Stress Pathways. Front. Pharmacol. 2025, 16, 1592277. [Google Scholar] [CrossRef]
- Dawson, A.; Miles, M.F.; Damaj, M.I. The Β2 Nicotinic Acetylcholine Receptor Subunit Differentially Influences Ethanol Behavioral Effects in the Mouse. Alcohol 2013, 47, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Zhang, L.; Zhou, F.; Gong, S.; Gu, H.; De Biasi, M.; Zhou, F.-M.; Dani, J.A. Cocaine Inhibition of Nicotinic Acetylcholine Receptors Influences Dopamine Release. Front. Synaptic Neurosci. 2014, 6, 19. [Google Scholar] [CrossRef]
- Mendez, I.A.; Damborsky, J.C.; Winzer-Serhan, U.H.; Bizon, J.L.; Setlow, B. A4β2* and A7 Nicotinic Acetylcholine Receptor Binding Predicts Choice Preference in Two Cost Benefit Decision Making Tasks. Neuroscience 2013, 230, 121–131. [Google Scholar] [CrossRef]
- Luo, F.; Liu, L.; Guo, M.; Liang, J.; Chen, L.; Shi, X.; Liu, H.; Cheng, Y.; Du, Y. Deciphering and Targeting the ESR2–miR-10a-5p–BDNF Axis in the Prefrontal Cortex: Advancing Postpartum Depression Understanding and Therapeutics. Research 2024, 7, 0537. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Xie, L.; Xiao, Z.; Li, M.; Li, Y.; Luo, T. Proteomic Analysis Reveals Chromatin Remodeling as a Potential Therapeutical Target in Neuroblastoma. J. Transl. Med. 2025, 23, 234. [Google Scholar] [CrossRef]
- Yin, M.; Feng, C.; Yu, Z.; Zhang, Y.; Li, Y.; Wang, X.; Song, C.; Guo, M.; Li, C. sc2GWAS: A Comprehensive Platform Linking Single Cell and GWAS Traits of Human. Nucleic Acids Res. 2025, 53, D1151–D1161. [Google Scholar] [CrossRef]
- Sil, S.; Niu, F.; Tom, E.; Liao, K.; Periyasamy, P.; Buch, S. Cocaine Mediated Neuroinflammation: Role of Dysregulated Autophagy in Pericytes. Mol. Neurobiol. 2019, 56, 3576–3590. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional Roles of Reactive Astrocytes in Neuroinflammation and Neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Andersson, U.; Brines, M. Neurons Are a Primary Driver of Inflammation via Release of HMGB1. Cells 2021, 10, 2791. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Baddal, B.; Haarsma, R.; O’Seaghdha, M.; Yang, N.J.; Blake, K.J.; Portley, M.; Verri, W.A.; Dale, J.B.; Wessels, M.R.; et al. Blocking Neuronal Signaling to Immune Cells Treats Streptococcal Invasive Infection. Cell 2018, 173, 1083–1097.e22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dai, A.-X.; Tang, H.-L.; Lu, C.-H.; Liu, H.-X.; Hou, T.; Lu, Z.-J.; Kong, N.; Peng, X.-Y.; Lin, K.-X.; et al. Increase of ALCAM and VCAM-1 in the Plasma Predicts the Alzheimer’s Disease. Front. Immunol. 2022, 13, 1097409. [Google Scholar] [CrossRef]
- Petersen, E.; Søndergaard, H.; Oturai, A.; Jensen, P.; Sorensen, P.; Sellebjerg, F.; Börnsen, L. Soluble Serum VCAM-1, Whole Blood mRNA Expression and Treatment Response in Natalizumab-Treated Multiple Sclerosis. Mult. Scler. Relat. Disord. 2016, 10, 66–72. [Google Scholar] [CrossRef]
- Elovaara, I.; Ukkonen, M.; Leppäkynnäs, M.; Lehtimäki, T.; Luomala, M.; Peltola, J.; Dastidar, P. Adhesion Molecules in Multiple Sclerosis: Relation to Subtypes of Disease and Methylprednisolone Therapy. Arch. Neurol. 2000, 57, 546–551. [Google Scholar] [CrossRef]
- Li, G.; Xiong, K.; Korff, A.; Pan, C.; Quinn, J.F.; Galasko, D.R.; Liu, C.; Montine, T.J.; Peskind, E.R.; Zhang, J. Increased CSF E-Selectin in Clinical Alzheimer’s Disease without Altered CSF Aβ42 and Tau. J. Alzheimers Dis. 2015, 47, 883–887. [Google Scholar] [CrossRef]
- Giovannoni, G.; Thorpe, J.W.; Kidd, D.; Kendall, B.E.; Moseley, I.F.; Thompson, A.J.; Keir, G.; Miller, D.H.; Feldmann, M.; Thompson, E.J. Soluble E-Selectin in Multiple Sclerosis: Raised Concentrations in Patients with Primary Progressive Disease. J. Neurol. Neurosurg. Psychiatry 1996, 60, 20–26. [Google Scholar] [CrossRef]
- Richard, S.; Lagerstedt, L.; Burkhard, P.R.; Debouverie, M.; Turck, N.; Sanchez, J.-C. E-Selectin and Vascular Cell Adhesion Molecule-1 as Biomarkers of 3-Month Outcome in Cerebrovascular Diseases. J. Inflamm. 2015, 12, 61. [Google Scholar] [CrossRef]
- Schnell, U.; Cirulli, V.; Giepmans, B.N.G. EpCAM: Structure and Function in Health and Disease. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2013, 1828, 1989–2001. [Google Scholar] [CrossRef]
- Selmaj, K.; Raine, C.S.; Cannella, B.; Brosnan, C.F. Identification of Lymphotoxin and Tumor Necrosis Factor in Multiple Sclerosis Lesions. J. Clin. Investig. 1991, 87, 949–954. [Google Scholar] [CrossRef]
- Saba, J.; Couselo, F.L.; Bruno, J.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Neuroinflammation in Huntington’s Disease: A Starring Role for Astrocyte and Microglia. Curr. Neuropharmacol. 2022, 20, 1116–1143. [Google Scholar] [CrossRef]
- James Bates, R.E.; Browne, E.; Schalks, R.; Jacobs, H.; Tan, L.; Parekh, P.; Magliozzi, R.; Calabrese, M.; Mazarakis, N.D.; Reynolds, R. Lymphotoxin-Alpha Expression in the Meninges Causes Lymphoid Tissue Formation and Neurodegeneration. Brain 2022, 145, 4287–4307. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.H. TNF Receptors: Structure-Function Relationships and Therapeutic Targeting Strategies. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2025, 1867, 184394. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.; Hoyer, D. Astrocytes: Adhesion Molecules and Immunomodulation. Curr. Drug Targets 2016, 17, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, S.; Kim, E.; Jang, Y.; Han, B.-S. VCAM1-Mediated Regulation of Dopaminergic Neuron Function in Parkinson’s Disease. BMB Rep. 2025, 58, 217–223. [Google Scholar] [CrossRef]
- Brown, T.C.; Sankpal, N.V.; Gillanders, W.E. Functional Implications of the Dynamic Regulation of EpCAM during Epithelial-to-Mesenchymal Transition. Biomolecules 2021, 11, 956. [Google Scholar] [CrossRef]
- Chauhan, R.; Mohan, M.; Mannan, A.; Devi, S.; Singh, T.G. Unravelling the Role of Interleukin-12 in Neuroinflammatory Mechanisms: Pathogenic Pathways Linking Neuroinflammation to Neuropsychiatric Disorders. Int. Immunopharmacol. 2025, 156, 114654. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Arvaniti, M.; Jensen, M.M.; Shulepko, M.A.; Dolgikh, D.A.; Pinborg, L.H.; Härtig, W.; Lyukmanova, E.N.; Mikkelsen, J.D. Lynx1 and Aβ1-42 Bind Competitively to Multiple Nicotinic Acetylcholine Receptor Subtypes. Neurobiol. Aging 2016, 46, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Paramonov, A.S.; Kocharovskaya, M.V.; Tsarev, A.V.; Kulbatskii, D.S.; Loktyushov, E.V.; Shulepko, M.A.; Kirpichnikov, M.P.; Lyukmanova, E.N.; Shenkarev, Z.O. Structural Diversity and Dynamics of Human Three-Finger Proteins Acting on Nicotinic Acetylcholine Receptors. Int. J. Mol. Sci. 2020, 21, 7280. [Google Scholar] [CrossRef] [PubMed]
- Shenkarev, Z.O.; Chesnokov, Y.M.; Zaigraev, M.M.; Chugunov, A.O.; Kulbatskii, D.S.; Kocharovskaya, M.V.; Paramonov, A.S.; Bychkov, M.L.; Shulepko, M.A.; Nolde, D.E.; et al. Membrane-Mediated Interaction of Non-Conventional Snake Three-Finger Toxins with Nicotinic Acetylcholine Receptors. Commun. Biol. 2022, 5, 1344. [Google Scholar] [CrossRef] [PubMed]
- Gotti, C.; Zoli, M.; Clementi, F. Brain Nicotinic Acetylcholine Receptors: Native Subtypes and Their Relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef]
- Gozlan, E.; Lewit-Cohen, Y.; Frenkel, D. Sex Differences in Astrocyte Activity. Cells 2024, 13, 1724. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Addendum: Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 636, E4. [Google Scholar] [CrossRef]


| Receptor Type | IC50, μM | Maximal Inhibition, % | nH |
|---|---|---|---|
| HS α4β2 | 27 ± 13 | 31 ± 7 1 | 1.4 ± 0.6 |
| LS α4β2 | 15 ± 13 | 0.7 ± 0.2 | |
| α3β2 | 50 ± 25 | 1.2 ± 0.4 |
| ws-PSCA Residue | High-Sensitive α4β2-nAChR, Primary (+)/Complementary (−) Subunits, MD Time 1 | |||||
|---|---|---|---|---|---|---|
| α4(+)/β2(−), 30–500 ns | β2(+)/β2(−), 30–400 ns | β2(+)/β2(−), 450–500 ns | ||||
| (+) | (−) | (+) | (−) | (+) | (−) | |
| ws-PSCA Loop I | ||||||
| E13 | K208 (I, H) | |||||
| L16 | MAN (H; N460) | |||||
| ws-PSCA Loop II | ||||||
| R32 | E196 (I, H) | D170 (I, H) | ||||
| R34 | E196 (I, H) | D170 (I, H) | D170 (I, H) | |||
| T40 | K477 (H) | |||||
| ws-PSCA Loop III | ||||||
| N49 | MAN (H; N146) NAG (H; N146) | |||||
| D53 | K194 (I, H) | |||||
| Y58 | V181 (H) | |||||
| G60 | D170 (H) | |||||
| K61 | D193 (I, H) | D171 (I, H) | D193 (H, I) | D170 (H, I) D171 (H, I) | ||
| K62 | D193 (I, H) | |||||
| N63 | K194 (H) | |||||
| C66 | N190 (I, H) | |||||
| D68 | N99 (H) S132 (H) | |||||
| D70 | S134 (H) | MAN (H; N143) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shulepko, M.A.; Che, Y.; Paramonov, A.S.; Kocharovskaya, M.V.; Kulbatskii, D.S.; Ivanova, A.A.; Chugunov, A.O.; Bychkov, M.L.; Kirichenko, A.V.; Shenkarev, Z.O.; et al. Pro-Inflammatory Protein PSCA Is Upregulated in Neurological Diseases and Targets β2-Subunit-Containing nAChRs. Biomolecules 2025, 15, 1381. https://doi.org/10.3390/biom15101381
Shulepko MA, Che Y, Paramonov AS, Kocharovskaya MV, Kulbatskii DS, Ivanova AA, Chugunov AO, Bychkov ML, Kirichenko AV, Shenkarev ZO, et al. Pro-Inflammatory Protein PSCA Is Upregulated in Neurological Diseases and Targets β2-Subunit-Containing nAChRs. Biomolecules. 2025; 15(10):1381. https://doi.org/10.3390/biom15101381
Chicago/Turabian StyleShulepko, Mikhail A., Yuqi Che, Alexander S. Paramonov, Milita V. Kocharovskaya, Dmitrii S. Kulbatskii, Anisia A. Ivanova, Anton O. Chugunov, Maxim L. Bychkov, Artem V. Kirichenko, Zakhar O. Shenkarev, and et al. 2025. "Pro-Inflammatory Protein PSCA Is Upregulated in Neurological Diseases and Targets β2-Subunit-Containing nAChRs" Biomolecules 15, no. 10: 1381. https://doi.org/10.3390/biom15101381
APA StyleShulepko, M. A., Che, Y., Paramonov, A. S., Kocharovskaya, M. V., Kulbatskii, D. S., Ivanova, A. A., Chugunov, A. O., Bychkov, M. L., Kirichenko, A. V., Shenkarev, Z. O., Kirpichnikov, M. P., & Lyukmanova, E. N. (2025). Pro-Inflammatory Protein PSCA Is Upregulated in Neurological Diseases and Targets β2-Subunit-Containing nAChRs. Biomolecules, 15(10), 1381. https://doi.org/10.3390/biom15101381









