Short-Chain Fatty Acid Profiles in Amyotrophic Lateral Sclerosis: Longitudinal Effects of Disease and Mediterranean Diet Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients Selection
2.2. Clinical Assessment
2.3. Nutritional Intervention
2.4. Plasma SCFA Analysis
2.4.1. Standard Plasma Samples and Real Samples Derivatization Protocol
2.4.2. Liquid Chromatography Mass Spectrometry Analytical Method
2.5. Statistical Analysis of SFCAs Longitudinal Study Dynamics
3. Results
3.1. Clinical and Demographical Characteristics of ALS and Control Groups
3.2. Longitudinal Analysis of Plasma SCFA in ALS Patients and Healthy Controls
3.3. Correlation Between Plasma Levels of SCFAs and Patients Clinical Characteristics
3.4. Correlations Between Expression of SCFAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-NPH | 3-Nitrophenylhydrazine Hydrochloride |
| 3OH-BA | 3-Hydroxybutyric Acid |
| 4-MVA | 4-Methyl-Valeric Acid |
| AA | Acetic Acid |
| ALS | Amyotrophic Lateral Sclerosis |
| BA | Butyric Acid |
| BBB | Blood-Brain Barrier |
| CAL | Calibration |
| CNS | Central Nervous System |
| DHA | Docosahexaenoic Acid |
| EDC | N-(-3-Dimethylaminopropyl)-N’-Ehtylcarbodiimde Hydrochloride |
| EPA | Eicosapentaenoic Acid |
| ESI | Electrospray Ionization Mode |
| FA | Formic Acid |
| GBA | Gut Microbiota-Brain Axis |
| GPCR(s) | G Protein-Coupled Receptor(S) |
| HA | Hexanoic Acid |
| HC | Healthy Control |
| HDAC(s) | Histone Deacetylase(S) |
| ISTDs | Internal Standards—Isotope Labeled |
| LBP | Lower Lipopolysaccharide-Binding Protein |
| LC-MS/MS | Liquid Chromatography (LC) (Tandem) Mass Spectrometry |
| MCFA | Medium-Chain Fatty Acid |
| MCTs | Monocarboxylate Transporters |
| MeOH | Methanol |
| MS | Mass Spectrometer |
| PA | Propanoic Acid |
| PD | Parkinson’s Disease |
| Py | Pyridine |
| QC | Quality Controls |
| SCFAs | Short-Chain Fatty Acids |
| T0 | Baseline |
| T1 | Observation Period—Six Months of Natural Disease Progression |
| T2 | Dietary Intervention Phase—Six Months of Dietary Intervention |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Shaw, P.J.; Simmons, Z.; Van Den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, W.; Wei, C.; Jiang, S.; Li, S.; Wang, X.; Xu, R. Pathological mechanisms of amyotrophic lateral sclerosis. Neural Regen. Res. 2024, 19, 1036–1044. [Google Scholar] [CrossRef]
- Morgan, S.; Orrell, R.W. Pathogenesis of amyotrophic lateral sclerosis. Br. Med. Bull. 2016, 119, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.N.; Houser, M.; Tansey, M.G.; Glass, J.D.; Hertzberg, V.S. The gut microbiome and neuroinflammation in amyotrophic lateral sclerosis? Emerging clinical evidence. Neurobiol. Dis. 2020, 135, 104300. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Henderson, R.D.; Lee, A.; Lee, J.D.; Woodruff, T.M.; Restuadi, R.; McRae, A.; Wray, N.R.; Ngo, S.; Steyn, F.J. Gut microbiota in ALS: Possible role in pathogenesis? Expert Rev. Neurother. 2019, 19, 785–805. [Google Scholar] [CrossRef]
- Boddy, S.L.; Giovannelli, I.; Sassani, M.; Cooper-Knock, J.; Snyder, M.P.; Segal, E.; Elinav, E.; Barker, L.A.; Shaw, P.J.; McDermott, C.J. The gut microbiome: A key player in the complexity of amyotrophic lateral sclerosis (ALS). BMC Med. 2021, 19, 13. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Moțățăianu, A.; Șerban, G.; Andone, S. The role of short-chain fatty acids in microbiota-gut-brain cross-talk with a focus on amyotrophic lateral sclerosis: A systematic review. Int. J. Mol. Sci. 2023, 24, 15094. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 508738. [Google Scholar] [CrossRef]
- Morris, M.C. The role of nutrition in Alzheimer’s disease: Epidemiological evidence. Eur. J. Neurol. 2009, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Gouinguenet, P.; Ashor, A.; Stewart, C.; Deighton, K.; Matu, J.; Griffiths, A.; Malcomson, F.C.; Joel, A.; Houghton, D.; et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: A systematic review of randomized controlled trials and observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 8698–8719. [Google Scholar] [CrossRef]
- Brooks, B.R.; Miller, R.G.; Swash, M.; Munsat, T.L. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Mot. Neuron Disord. 2000, 1, 293–299. [Google Scholar] [CrossRef]
- Cedarbaum, J.M.; Stambler, N.; Malta, E.; Fuller, C.; Hilt, D.; Thurmond, B.; Nakanishi, A.; BDNF ALS Study Group (Phase III). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J. Neurol. Sci. 1999, 169, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Labra, J.; Menon, P.; Byth, K.; Morrison, S.; Vucic, S. Rate of disease progression: A prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 2016, 87, 628–632. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; et al. Modern vision of the Mediterranean diet. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E36. [Google Scholar] [CrossRef]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252–1266. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; González, S.; Nogacka, A.M.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de Los Reyes-Gavilán, C.G. An Overview on Fecal Branched Short-Chain Fatty Acids Along Human Life and as Related with Body Mass Index: Associated Dietary and Anthropometric Factors. Front. Microbiol. 2020, 11, 973. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short-and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short-and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Szabó, R.T.; Kovács-Weber, M.; Zimborán, Á.; Kovács, L.; Erdélyi, M. Effects of short-and medium-chain fatty acids on production, meat quality, and microbial attributes—A review. Molecules 2023, 28, 4956. [Google Scholar] [CrossRef]
- Xiao, X.Q.; Zhao, Y.; Chen, G.Q. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 2007, 28, 3608–3616. [Google Scholar] [CrossRef]
- Wang, B.L.; Wu, J.F.; Xiao, D.; Wu, B.; Wei, D.X. 3-hydroxybutyrate in the brain: Biosynthesis, function, and disease therapy. Brain-X 2023, 1, e6. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of blood-brain barrier protection by microbiota-derived short-chain fatty acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Mei, C.; Li, W.; Zhao, B.; He, Y.; Li, Q.; Zhang, T.; Li, X.; Zhang, K.; Zhang, Y.; Zhong, Z. Short-chain fatty acids mediate gut microbiota-brain communication and protect the blood-brain barrier integrity. Ann. N. Y. Acad. Sci. 2025, 1545, 116–131. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Oleskin, A.V.; Shenderov, B.A. Neuromodulatory effects and targets of the SCFAs and gasotransmitters produced by the human symbiotic microbiota. Microb. Ecol. Health Dis. 2016, 27, 30971. [Google Scholar] [CrossRef]
- Stilling, R.M.; Van De Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, D.W.; Chung, J.Y.; Choi, S.Y.; Yoon, Y.S.; Won, M.-H.; Hwang, I.K. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 1850–1857. [Google Scholar] [CrossRef]
- Suganya, K.; Koo, B.S. Gut-brain axis: Role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int. J. Mol. Sci. 2020, 21, 7551. [Google Scholar] [CrossRef] [PubMed]
- Motataianu, A.; Barcutean, L.; Balasa, R. Neuroimmunity in amyotrophic lateral sclerosis: Focus on microglia. Amyotroph. Lateral Scher. Front. Degener. 2020, 21, 159–166. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Guo, C.; Huo, Y.J.; Li, Y.; Han, Y.; Zhou, D. Gut-brain axis: Focus on gut metabolites short-chain fatty acids. World J. Clin. Cases 2022, 10, 1754. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am. J. Physiol. 1973, 224, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Kumar, A.; Bala, L.; Kalita, J.; Misra, U.K.; Singh, R.L.; Khetrapal, C.L.; Babu, G.N. Metabolomic analysis of serum by (1) H NMR spectroscopy in amyotrophic lateral sclerosis. Clin. Chim. Acta. 2010, 411, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Błaszczyński, J.; Billaut, J.C.; Nadal-Desbarats, L.; Pradat, P.F.; Devos, D.; Moreau, C.; Andres, C.; Emond, P.; Corcia, P.; et al. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 2015, 53, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.-S.; Kuo, C.-H.; Lin, C.H. Association of fecal and plasma levels of short-chain fatty acids with gut microbiota and clinical severity in patients with Parkinson disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Marchese, E.; Serrano, A.; Michetti, F.; D’Ambrosi, N. The dual role of microglia in ALS: Mechanisms and therapeutic approaches. Front. Aging Neurosci. 2017, 9, 242. [Google Scholar] [CrossRef]
- Henkel, J.S.; Beers, D.R.; Zhao, W.; Appel, S.H. Microglia in ALS: The good, the bad, and the resting. J. Neuroimmune Pharm. 2009, 4, 389–398. [Google Scholar] [CrossRef]
- Trend, S.; Leffler, J.; Jones, A.P.; Cha, L.; Gorman, S.; Brown, D.A.; Breit, S.N.; Kermode, A.G.; French, M.A.; Ward, N.C.; et al. Associations of serum short-chain fatty acids with circulating immune cells and serum biomarkers in patients with multiple sclerosis. Sci. Rep. 2021, 11, 5244. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, Z.; Pu, Y.; Chen, S.; Wang, T.; Wang, Y.; Xu, X.; Wang, S.; Jin, M.; Yao, Y.; et al. Serum short-chain fatty acids and its correlation with motor and non-motor symptoms in Parkinson’s disease patients. BMC Neurol. 2022, 22, 13. [Google Scholar] [CrossRef]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Koppel, S.J.; Swerdlow, R.H. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem. Int. 2018, 117, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Møller, N. Ketone body, 3-hydroxybutyrate: Minor metabolite-major medical manifestations. J. Clin. Endocrinol. Metab. 2020, 105, 2884–2892. [Google Scholar] [CrossRef]
- Skonieczna-Żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef]
- Pavlin, M.; Repič, M.; Vianello, R.; Mavri, J. The Chemistry of Neurodegeneration: Kinetic Data and Their Implications. Mol. Neurobiol. 2016, 53, 3400–3415. [Google Scholar] [CrossRef]
- Cai, B.; Zhong, L.; Wang, Q.; Xu, W.; Li, X.; Chen, T. Curcumin Alleviates 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinson’s Disease in Mice via Modulating Gut Microbiota and Short-Chain Fatty Acids. Front. Pharmacol. 2023, 14, 1198335. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Pagliai, G.; Russo, E.; Niccolai, E.; Dinu, M.; Di Pilato, V.; Magrini, A.; Bartolucci, G.; Baldi, S.; Menicatti, M.; Giusti, B.; et al. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: The CARDIVEG Study. Eur. J. Nutr. 2020, 59, 2011–2024. [Google Scholar] [CrossRef]
- Zhang, C.; Björkman, A.; Cai, K.; Liu, G.; Wang, C.; Li, Y.; Xia, H.; Sun, L.; Kristiansen, K.; Wang, J.; et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front. Immunol. 2018, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on gut microbiota associated to mediterranean diet adherence and specific dietary intakes on general adult population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Seethaler, B.; Nguyen, N.K.; Basrai, M.; Kiechle, M.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Short-chain fatty acids are key mediators of the favorable effects of the Mediterranean diet on intestinal barrier integrity: Data from the randomized controlled LIBRE trial. Am. J. Clin. Nutr. 2022, 116, 928–942. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076. [Google Scholar] [CrossRef]
- Perna, S.; Allehdan, S.; Rafique, A.; Saad, S.; AlGhareeb, F.; Riso, P. Does the Mediterranean diet have any effect on lipid profile, central obesity and liver enzymes in non-alcoholic fatty liver disease (NAFLD) subjects? A systematic review and Meta-analysis of randomized control trials. Nutrients 2023, 15, 2250. [Google Scholar] [CrossRef]
- Anania, C.; Perla, F.M.; Olivero, F.; Pacifico, L.; Chiesa, C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018, 24, 2083. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, W.; Cai, L.; Zeng, G.; Fang, W.; Dai, X.; Ye, Q.; Chen, X.; Zhang, J. Acetate supplementation produces antidepressant-like effect via enhanced histone acetylation. J. Affect. Disord. 2021, 281, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef]
- Grüter, T.; Mohamad, N.; Rilke, N.; Blusch, A.; Sgodzai, M.; Demir, S.; Pedreiturria, X.; Lemhoefer, K.; Gisevius, B.; Haghikia, A.; et al. Propionate exerts neuroprotective and neuroregenerative effects in the peripheral nervous system. Proc. Natl. Acad. Sci. USA 2023, 120, e2216941120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, M.; Liu, D.; Zheng, C. Metabolic rescue of α-synuclein-induced neurodegeneration through propionate supplementation and intestine-neuron signaling in C. elegans. Cell Rep. 2024, 43, 113865. [Google Scholar] [CrossRef]
- Li, X.; Dong, L.; Li, A.; Yi, J.; Brotto, M.; Zhou, J. Butyrate ameliorates mitochondrial respiratory capacity of the motor-neuron-like cell line NSC34-G93A, a cellular model for ALS. Biomolecules 2022, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yi, J.; Zhang, Y.G.; Zhou, J.; Sun, J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 2015, 3, e12356. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Wang, F.; Ding, G.; Chen, W.; Fang, R.; Yao, Y.; Pang, M.; Lu, Z.-Q.; Liu, J. Sodium butyrate exerts neuroprotective effects by restoring the blood-brain barrier in traumatic brain injury mice. Brain Res. 2016, 1642, 70–78. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wu, S.; Yi, J.; Xia, Y.; Jin, D.; Zhou, J.; Sun, J. Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 2017, 39, 322–336. [Google Scholar] [CrossRef]
- Schiweck, C.; Dalile, B.; Balliet, A.; Aichholzer, M.; Reinken, H.; Erhardt, F.; Thanarajah, S.E. Circulating Short Chain Fatty Acids Are Associated with Depression Severity and Predict Remission from Major Depressive Disorder. Brain Behav. Immun.-Health 2025, 48, 101070. [Google Scholar] [CrossRef]
- Fontdevila, L.; Povedano, M.; Domínguez, R.; Boada, J.; Serrano, J.C.; Pamplona, R.; Portero-Otín, M. Examining the complex interplay between gut microbiota abundance and short-chain fatty acid production in amyotrophic lateral sclerosis patients shortly after onset of disease. Sci. Rep. 2024, 14, 23497. [Google Scholar] [CrossRef] [PubMed]
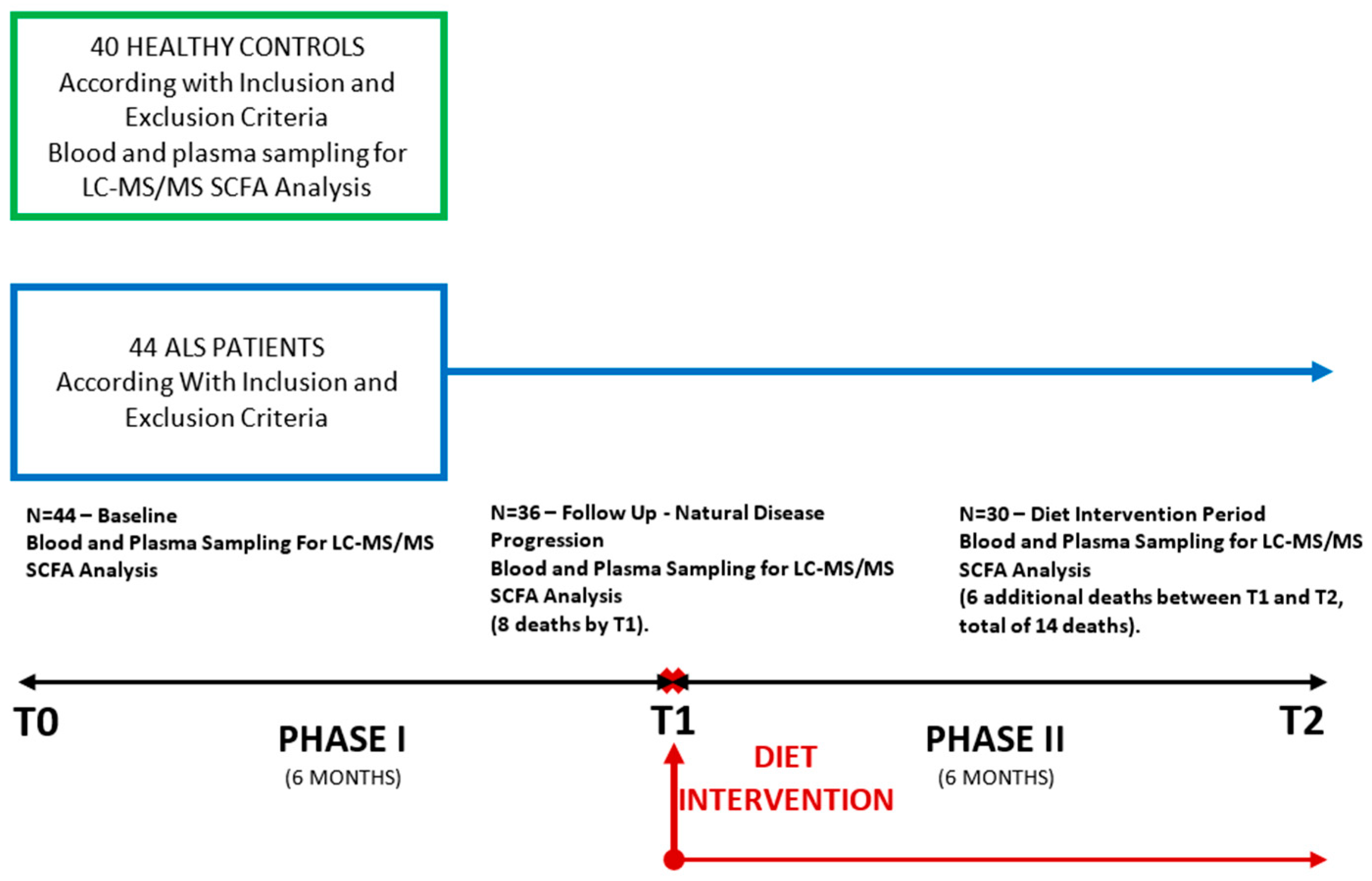
| Control | ALS | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | |||
| Patients number | 40 | 44 | 36 | 30 | |
| Sex ratio (M:F) | 24:16 | 28:16 | 23:13 | 20:10 | |
| Age (mean ± SD) (years) | 56.97 ± 11.83 | 58.05 ± 12.14 | 58.17 ± 11.78 | 57.43 ± 12.39 | |
| ΔPR | NA | 0.92 ± 1.05 | 0.75 ± 0.67 | 0.54 ± 0.34 | |
| ALS-FRS-R subscore | Respiratory | NA | 10.95 ± 1.61 | 11.00 ± 1.33 | 10.23 ± 2.34 |
| Bulbar | NA | 9.64 ± 2.63 | 9.39 ± 2.71 | 9.27 ± 2.88 | |
| Gross motor | NA | 6.42 ± 3.23 | 8.00 ± 2.82 | 5.57 ± 3.14 | |
| Fine motor | NA | 7.24 ± 3.42 | 6.53 ± 3.39 | 6.23 ± 3.50 | |
| Total | NA | 33.95 ± 7.92 | 32.69 ± 7.00 | 31.58 ± 8.91 | |
| Beck Depression score | NA | NA | 13.69 ± 8.17 | 13.17 ± 9.65 | |
| SCFA | Control | ALS | |||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | C vs. T0 | T0 vs. T1 | T1 vs. T2 | ||
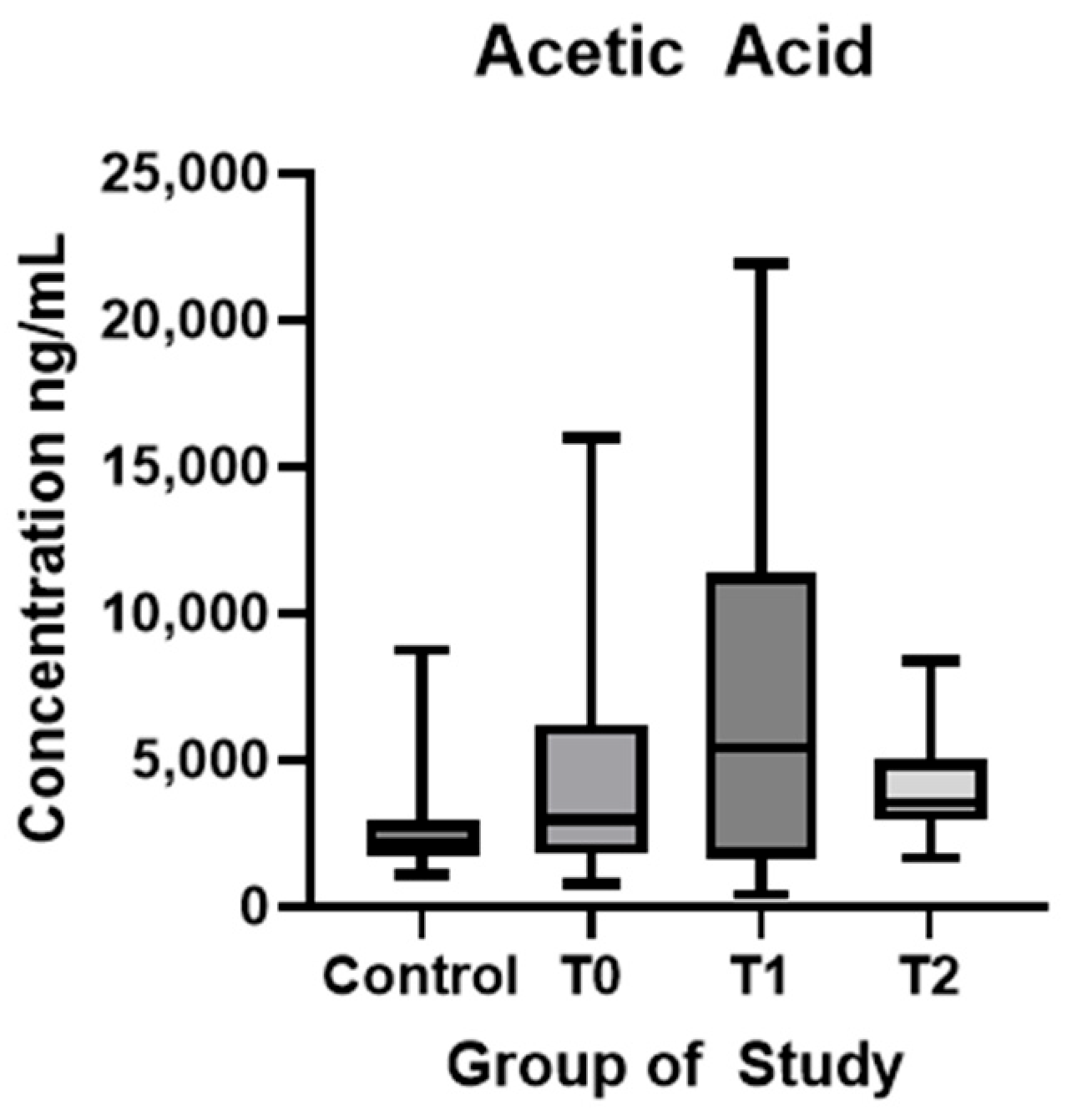
| Acetic acid (ng/mL) (mean ± SD) | 2622 ± 1724 | 4633 ± 3948 | 7057 ± 6445 | 4223 ± 1757 | p < 0.01 * | p = 0.05 | p = 0.01 * |
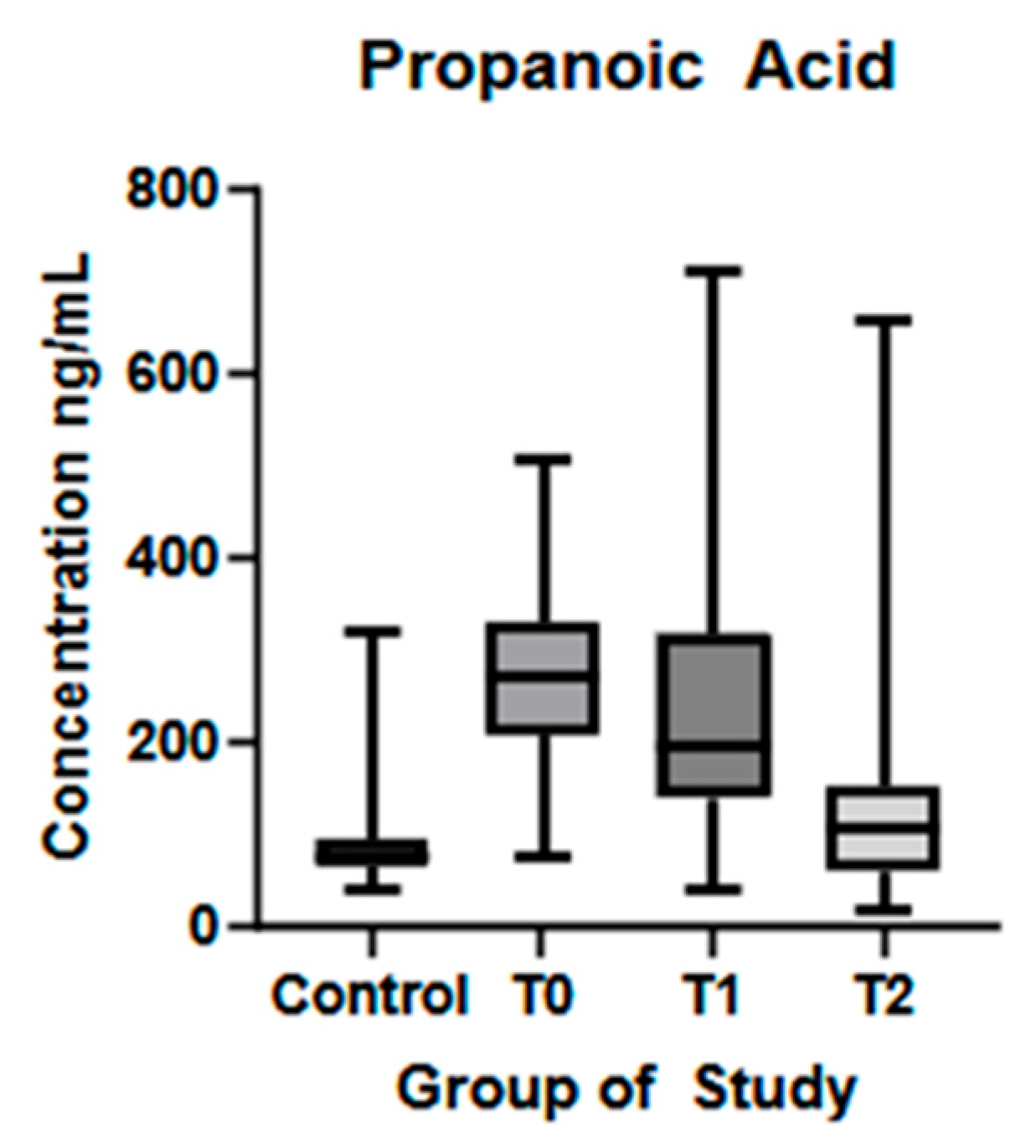
| Propanoic acid (ng/mL) (mean ± SD) | 85.11 ± 44.08 | 269.9 ± 105.7 | 231.9 ± 137.9 | 154.4 ± 169.5 | p < 0.01 * | p = 0.17 | p = 0.04 * |
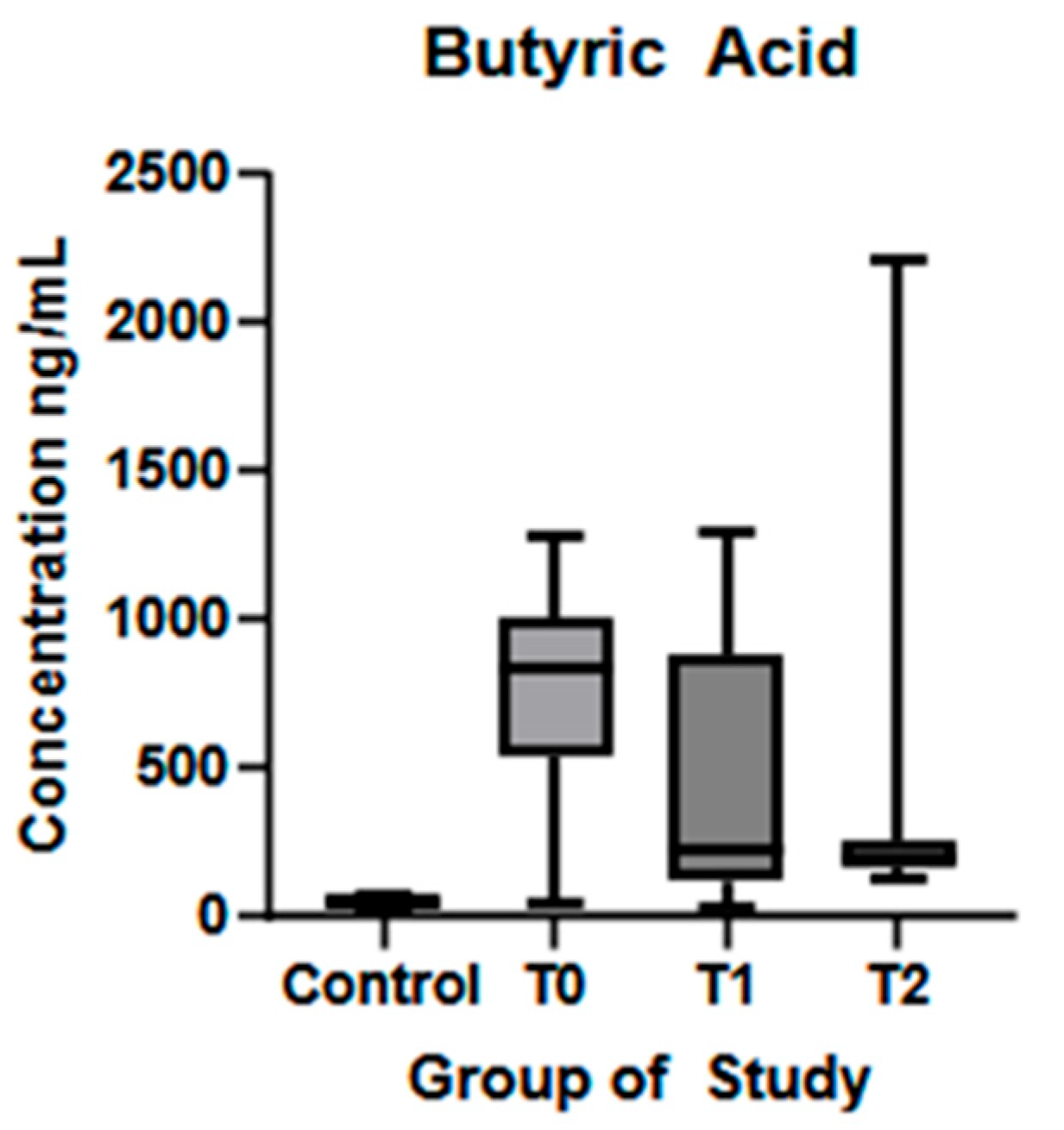
| Butyric acid (ng/mL) (mean ± SD) | 51.55 ± 12.44 | 743.8 ± 367 | 519.6 ± 429.3 | 367.4 ± 505.3 | p < 0.01 * | p = 0.01 * | p = 0.19 |
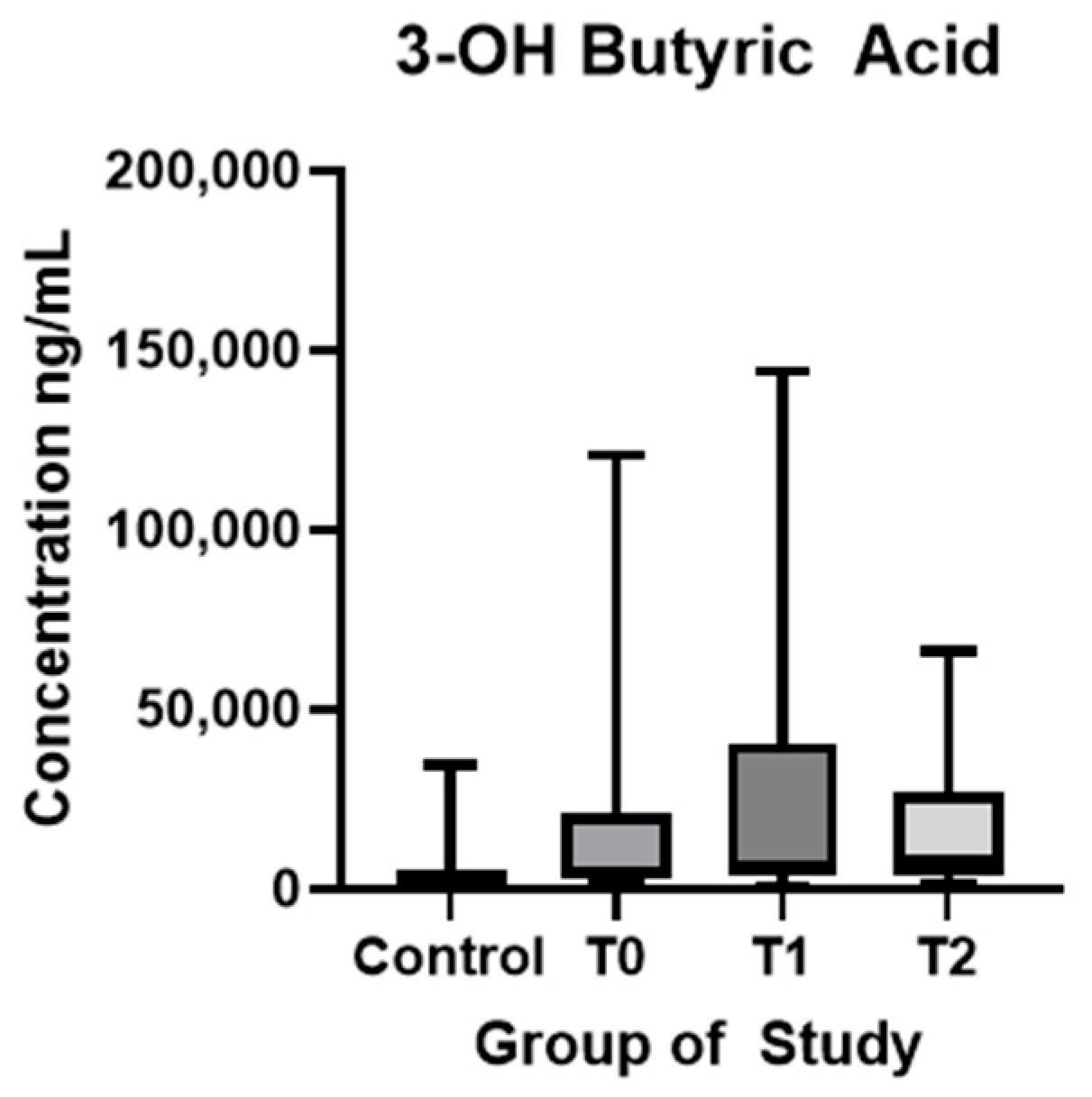
| 3-OH-butyric acid (ng/mL) (mean ± SD) | 5635 ± 7672 | 15,850 ± 23,738 | 24,049 ± 31,527 | 15,429 ± 15,525 | p < 0.01 * | p = 0.20 | p = 0.15 |
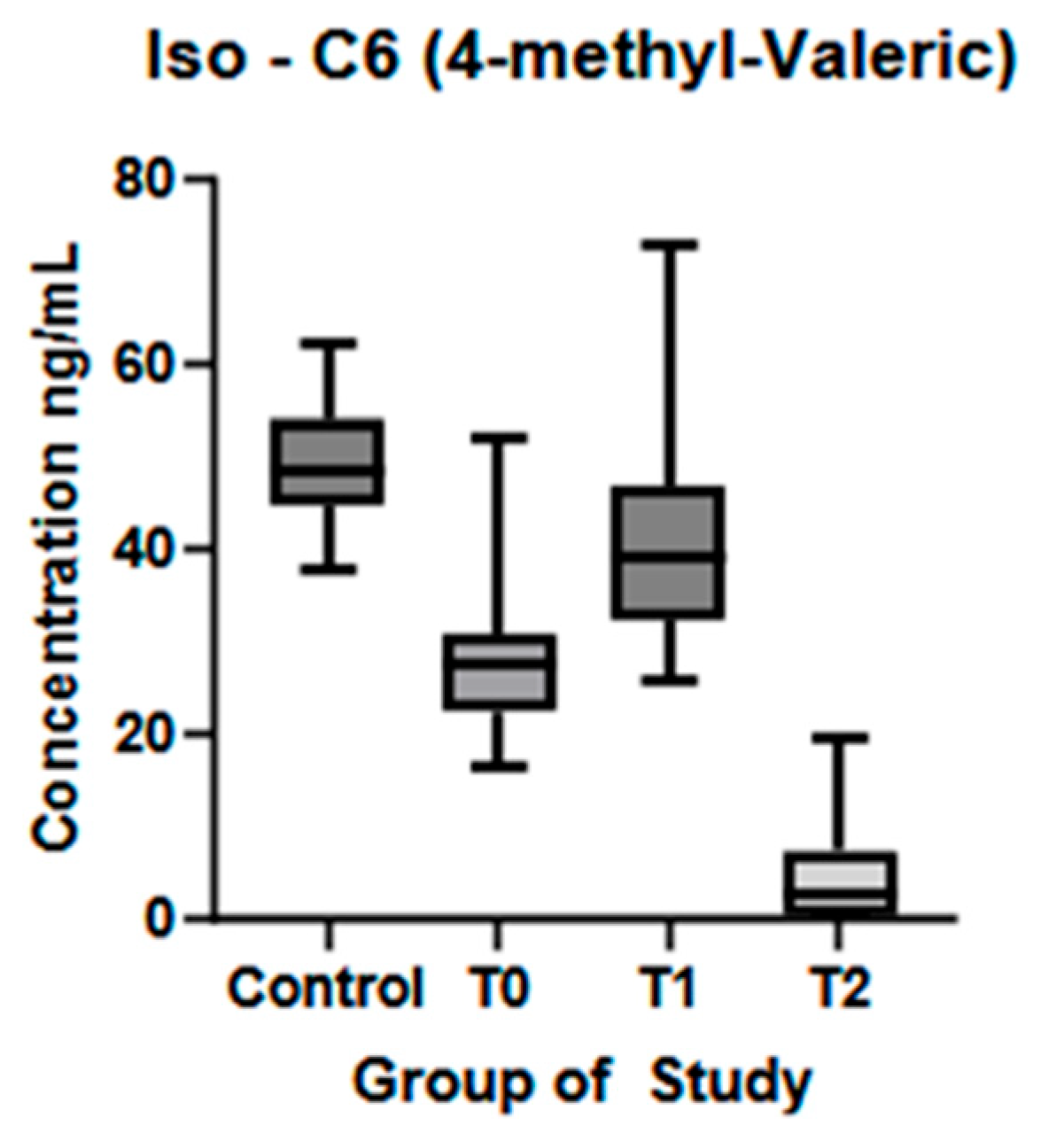
| 4-methyl-valeric acid (ng/mL) (mean ± SD) | 49.32 ± 6.30 | 20.15 ± 8.42 | 40.67 ± 10.80 | 4.55 ± 5.11 | p < 0.01 * | p < 0.01 * | p < 0.01 * |
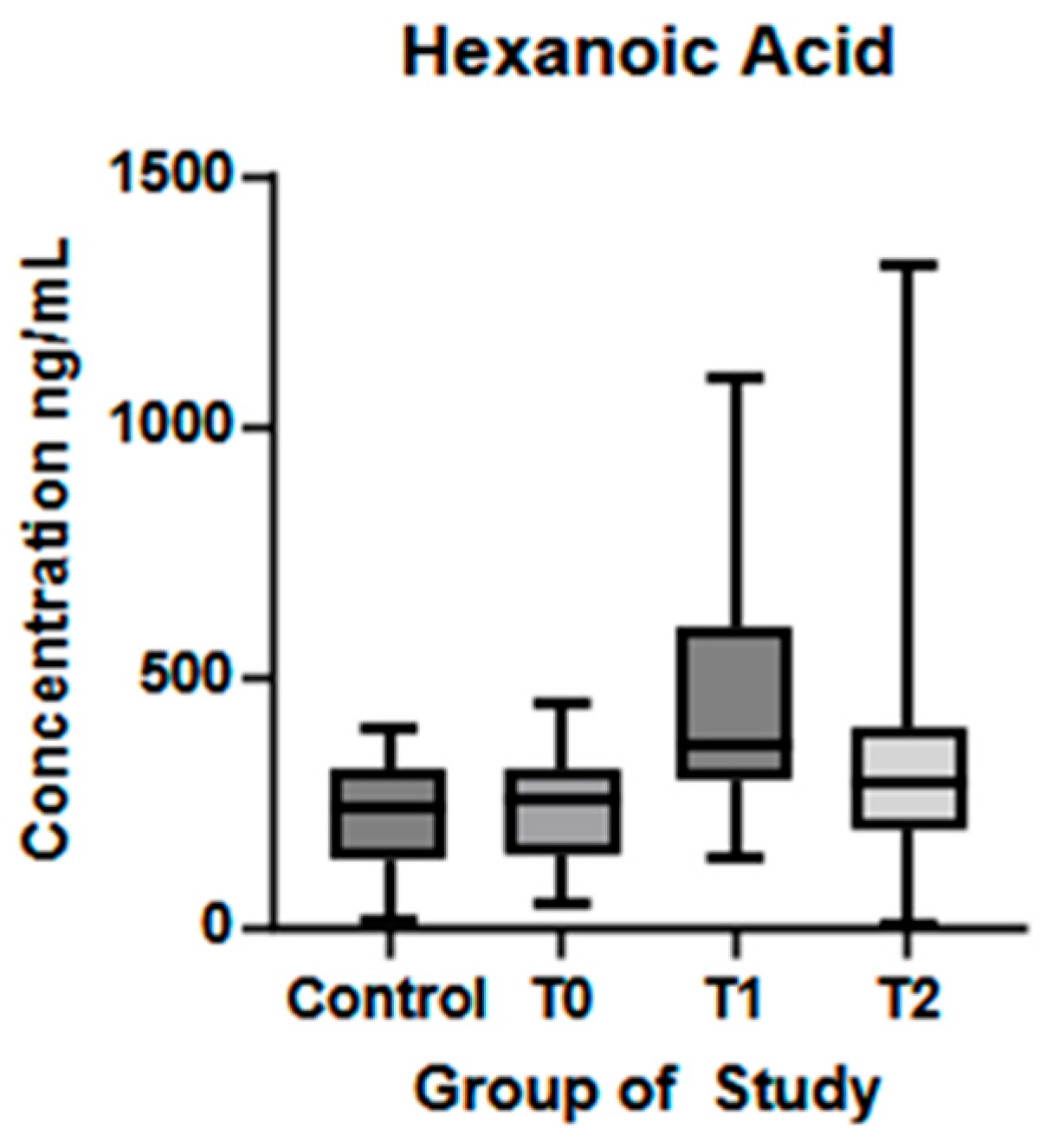
| Hexanoic acid (ng/mL) (mean ± SD) | 219.7 ± 112.3 | 242.3 ± 112 | 449.1 ± 255.7 | 382.1 ± 297.3 | p = 0.35 | p < 0.01 * | p = 0.33 |
| SCFA | T1 | T2 | |
|---|---|---|---|
| Acetic acid | 3-OH butyric acid | r = 0.68, p < 001 | r = 0.80, p < 0.01 |
| Propanoic acid | Butyric acid | r = 0.78, p < 0.01 | r = 0.52, p < 0.01 |
| 4-methyl-valeric acid | Hexanoic acid | r = 0.53, p < 0.01 | r = 0.44, p = 0.01 |
| 3-OH butyric acid | Hexanoic acid | r = −0.42, p < 0.01 | r = 0.07, p = 0.71 |
| Propanoic acid | Hexanoic acid | r = −0.03, p = 0.82 | r = 0.46, p < 0.01 |
| Butyric acid | Hexanoic acid | r = −0.03, p = 0.83 | r = 0.46, p = 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moțățăianu, A.; Ion, V.; Dumitreasă, M.; Ormenișan, I.; Farczadi, L.; Andone, S.; Bălașa, R.; Roman, M.M. Short-Chain Fatty Acid Profiles in Amyotrophic Lateral Sclerosis: Longitudinal Effects of Disease and Mediterranean Diet Intervention. Biomolecules 2025, 15, 1380. https://doi.org/10.3390/biom15101380
Moțățăianu A, Ion V, Dumitreasă M, Ormenișan I, Farczadi L, Andone S, Bălașa R, Roman MM. Short-Chain Fatty Acid Profiles in Amyotrophic Lateral Sclerosis: Longitudinal Effects of Disease and Mediterranean Diet Intervention. Biomolecules. 2025; 15(10):1380. https://doi.org/10.3390/biom15101380
Chicago/Turabian StyleMoțățăianu, Anca, Valentin Ion, Mihai Dumitreasă, Ioana Ormenișan, Lenard Farczadi, Sebastian Andone, Rodica Bălașa, and Medeea Maria Roman. 2025. "Short-Chain Fatty Acid Profiles in Amyotrophic Lateral Sclerosis: Longitudinal Effects of Disease and Mediterranean Diet Intervention" Biomolecules 15, no. 10: 1380. https://doi.org/10.3390/biom15101380
APA StyleMoțățăianu, A., Ion, V., Dumitreasă, M., Ormenișan, I., Farczadi, L., Andone, S., Bălașa, R., & Roman, M. M. (2025). Short-Chain Fatty Acid Profiles in Amyotrophic Lateral Sclerosis: Longitudinal Effects of Disease and Mediterranean Diet Intervention. Biomolecules, 15(10), 1380. https://doi.org/10.3390/biom15101380







