Proteomic Signatures of Hippocampal Nonsynaptic and Synaptosome-Enriched Mitochondria in Rats Resilient to Chronic Social Isolation
Abstract
1. Introduction
2. Proteomic Sample Preparation and Analysis
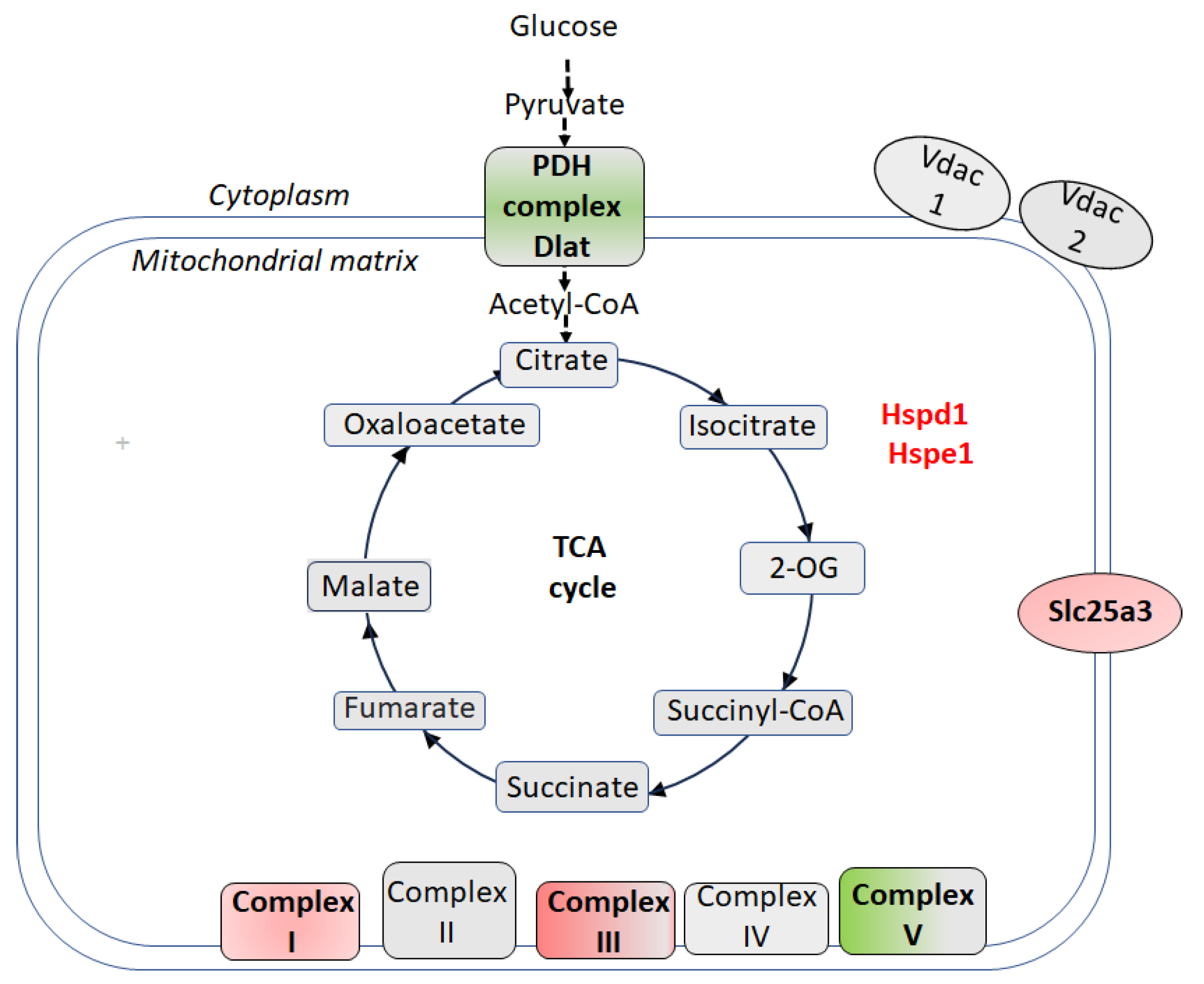
3. Proteomic Signatures of Hippocampal Nonsynaptic Mitochondria in CSIS-Resilient vs. CSIS-Susceptible Rats
4. Proteomic Signatures of Hippocampal Nonsynaptic Mitochondria in CSIS-Resilient vs. Control Rats
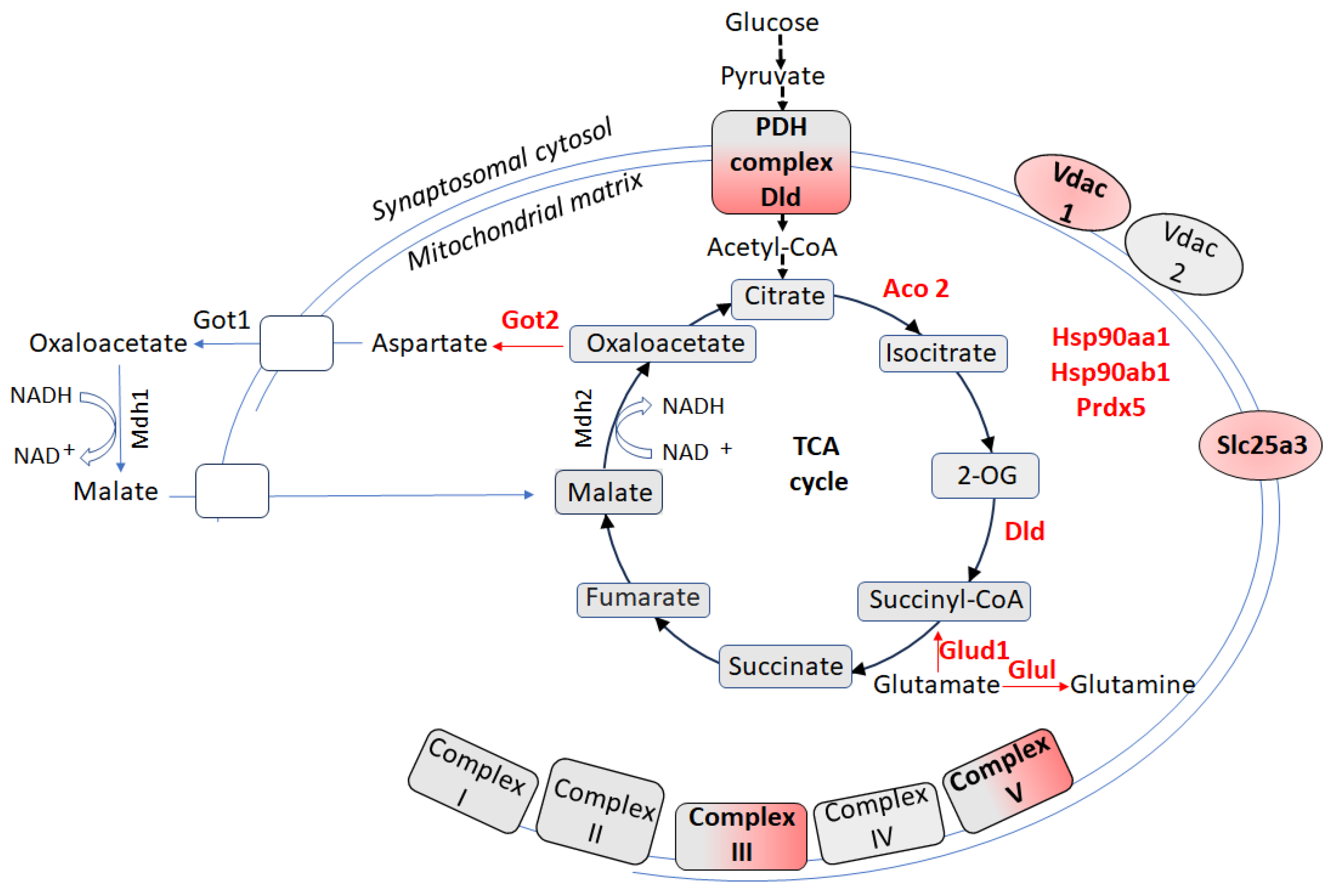
5. Proteomic Signatures of Hippocampal Synaptosome-Enriched Mitochondria in CSIS-Resilient vs. CSIS-Susceptible Rats
6. Proteomic Signatures of Hippocampal Synaptosome-Enriched Mitochondria in CSIS-Resilient vs. Control Rats
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cacioppo, J.T.; Hughes, M.E.; Waite, L.J.; Hawkley, L.C.; Thisted, R.A. Loneliness as a Specific Risk Factor for Depressive Symptoms: Cross-Sectional and Longitudinal Analyses. Psychol. Aging 2006, 21, 140–151. [Google Scholar] [CrossRef]
- Brandt, L.; Liu, S.; Heim, C.; Heinz, A. The Effects of Social Isolation Stress and Discrimination on Mental Health. Transl. Psychiatry 2022, 12, 398. [Google Scholar] [CrossRef]
- Courtin, E.; Knapp, M. Social Isolation, Loneliness and Health in Old Age: A Scoping Review. Health Soc. Care Community 2017, 25, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, I.; Filipović, D.; Puškaš, N. Hippocampal BDNF in Physiological Conditions and Social Isolation. Rev. Neurosci. 2017, 28, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, J.T.; Cacioppo, S.; Capitanio, J.P.; Cole, S.W. The Neuroendocrinology of Social Isolation. Annu. Rev. Psychol. 2015, 66, 733–767. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, G.A.; Pavlova, I.V.; Zaichenko, M.I. Effects of Social Isolation on the Development of Anxiety and Depression-Like Behavior in Model Experiments in Animals. Neurosci. Behav. Physiol. 2022, 52, 722–738. [Google Scholar] [CrossRef]
- Brenes, J.C.; Fornaguera, J. The Effect of Chronic Fluoxetine on Social Isolation-Induced Changes on Sucrose Consumption, Immobility Behavior, and on Serotonin and Dopamine Function in Hippocampus and Ventral Striatum. Behav. Brain Res. 2009, 198, 199–205. [Google Scholar] [CrossRef]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and Consequences of Social Isolation Stress in Animal Model-A Comprehensive Review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef]
- Filipović, D.; Todorović, N.; Bernardi, R.E.; Gass, P. Oxidative and Nitrosative Stress Pathways in the Brain of Socially Isolated Adult Male Rats Demonstrating Depressive- and Anxiety-like Symptoms. Brain Struct. Funct. 2017, 222, 1–20. [Google Scholar] [CrossRef]
- Zlatković, J.; Todorović, N.; Bošković, M.; Pajović, S.B.; Demajo, M.; Filipović, D. Different Susceptibility of Prefrontal Cortex and Hippocampus to Oxidative Stress Following Chronic Social Isolation Stress. Mol. Cell. Biochem. 2014, 393, 43–57. [Google Scholar] [CrossRef]
- Filipović, D.; Novak, B.; Xiao, J.; Yan, Y.; Yeoh, K.; Turck, C.W. Chronic Fluoxetine Treatment of Socially Isolated Rats Modulates Prefrontal Cortex Proteome. Neuroscience 2022, 501, 52–71. [Google Scholar] [CrossRef]
- Willner, P.; Muscat, R.; Papp, M. Chronic Mild Stress-Induced Anhedonia: A Realistic Animal Model of Depression. Neurosci. Biobehav. Rev. 1992, 16, 525–534. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Perić, I.; Costina, V.; Stanisavljević, A.; Findeisen, P.; Filipović, D. Proteomic Characterization of Hippocampus of Chronically Socially Isolated Rats Treated with Fluoxetine: Depression-like Behaviour and Fluoxetine Mechanism of Action. Neuropharmacology 2018, 135, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Perić, I.; Stanisavljević, A.; Gass, P.; Filipović, D. Fluoxetine Reverses Behavior Changes in Socially Isolated Rats: Role of the Hippocampal GSH-Dependent Defense System and Proinflammatory Cytokines. Eur. Arch. Psychiatry Clin. Neurosci. 2017, 267, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Mitic, M.; Lukic, I.; Radojcic, M.B. Alterations in the Nrf2–Keap1 Signaling Pathway and Its Downstream Target Genes in Rat Brain under Stress. Brain Res. 2015, 1602, 20–31. [Google Scholar] [CrossRef]
- Carrier, N.; Kabbaj, M. Testosterone and Imipramine Have Antidepressant Effects in Socially Isolated Male but Not Female Rats. Horm. Behav. 2012, 61, 678–685. [Google Scholar] [CrossRef]
- Wood, S.K.; Bhatnagar, S. Resilience to the Effects of Social Stress: Evidence from Clinical and Preclinical Studies on the Role of Coping Strategies. Neurobiol. Stress 2015, 1, 164–173. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Henningsen, K.; Palmfeldt, J.; Christiansen, S.; Baiges, I.; Bak, S.; Jensen, O.N.; Gregersen, N.; Wiborg, O. Candidate Hippocampal Biomarkers of Susceptibility and Resilience to Stress in a Rat Model of Depression. Mol. Cell. Proteom. 2012, 11, M111.016428. [Google Scholar] [CrossRef]
- Karatoreos, I.N.; McEwen, B.S. Annual Research Review: The Neurobiology and Physiology of Resilience and Adaptation across the Life Course. J. Child Psychol. Psychiatry Allied Discip. 2013, 54, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Murrough, J.W.; Han, M.H.; Charney, D.S.; Nestler, E.J. Neurobiology of Resilience. Nat. Neurosci. 2012, 15, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Cathomas, F.; Murrough, J.W.; Nestler, E.J.; Han, M.H.; Russo, S.J. Neurobiology of Resilience: Interface Between Mind and Body. Biol. Psychiatry 2019, 86, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Larosa, A.; Wong, T.P. The Hippocampus in Stress Susceptibility and Resilience: Reviewing Molecular and Functional Markers. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2022, 119, 110601. [Google Scholar] [CrossRef]
- McEwen, B.S.; Bowles, N.P.; Gray, J.D.; Hill, M.N.; Hunter, R.G.; Karatsoreos, I.N.; Nasca, C. Mechanisms of Stress in the Brain. Nat. Neurosci. 2015, 18, 1353–1363. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Mayberg, H.S. Limbic-Cortical Dysregulation: A Proposed Model of Depression. J. Neuropsychiatry Clin. Neurosci. 1997, 9, 471–481. [Google Scholar] [CrossRef]
- Kube, T.; Schwarting, R.; Rozenkrantz, L.; Glombiewski, J.A.; Rief, W. Distorted Cognitive Processes in Major Depression: A Predictive Processing Perspective. Biol. Psychiatry 2020, 87, 388–398. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Ji, Y.; Yang, L. Biological and Psychological Perspectives of Resilience: Is It Possible to Improve Stress Resistance? Front. Hum. Neurosci. 2018, 12, 326. [Google Scholar] [CrossRef]
- Mirescu, C.; Gould, E. Stress and Adult Neurogenesis. Hippocampus 2006, 16, 233–238. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal Neurogenesis Confers Stress Resilience by Inhibiting the Ventral Dentate Gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Manji, H.; Kato, T.; Di Prospero, N.A.; Ness, S.; Beal, M.F.; Krams, M.; Chen, G. Impaired Mitochondrial Function in Psychiatric Disorders. Nat. Rev. Neurosci. 2012, 13, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Schinder, A.F.; Christle, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional Neurogenesis in the Adult Hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Morava, É.; Kozicz, T. Mitochondria and the Economy of Stress (Mal)Adaptation. Neurosci. Biobehav. Rev. 2013, 37, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141. [Google Scholar] [CrossRef]
- Hollis, F.; van der Kooij, M.A.; Zanoletti, O.; Lozano, L.; Cantó, C.; Sandi, C. Mitochondrial Function in the Brain Links Anxiety with Social Subordination. Proc. Natl. Acad. Sci. USA 2015, 112, 15486–15491. [Google Scholar] [CrossRef]
- Allen, J.; Romay-Tallon, R.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Mitochondria and Mood: Mitochondrial Dysfunction as a Key Player in the Manifestation of Depression. Front. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef]
- Khan, M.; Baussan, Y.; Hebert-Chatelain, E. Connecting Dots between Mitochondrial Dysfunction and Depression. Biomolecules 2023, 13, 695. [Google Scholar] [CrossRef]
- Kasahara, T.; Kato, T. What Can Mitochondrial DNA Analysis Tell Us About Mood Disorders? Biol. Psychiatry 2018, 83, 731–738. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An Energetic View of Stress: Focus on Mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Villa, R.F. The Neurobiology of Depression: An Integrated Overview from Biological Theories to Clinical Evidence. Mol. Neurobiol. 2017, 54, 4847–4865. [Google Scholar] [CrossRef] [PubMed]
- Zuccoli, G.S.; Saia-Cereda, V.M.; Nascimento, J.M.; Martins-de-Souza, D. The Energy Metabolism Dysfunction in Psychiatric Disorders Postmortem Brains: Focus on Proteomic Evidence. Front. Neurosci. 2017, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Pekkurnaz, G.; Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 2022, 4, 802–812. [Google Scholar] [CrossRef]
- Sheng, Z.H. The interplay of axonal energy homeostasis and mitochondrial trafficking and anchoring. Trends Cell Biol. 2017, 27, 403–416. [Google Scholar] [CrossRef]
- Duarte, F.V.; Ciampi, D.; Duarte, C.B. Mitochondria as central hubs in synaptic modulation. Cell. Mol. Life Sci. 2023, 80, 173. [Google Scholar] [CrossRef]
- Devine, M.J.; Kittler, J.T. Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Stauch, K.L.; Purnell, P.R.; Fox, H.S. Quantitative Proteomics of Synaptic and Nonsynaptic Mitochondria: Insights for Synaptic Mitochondrial Vulnerability. J. Proteome Res. 2014, 13, 2620–2636. [Google Scholar] [CrossRef]
- Davey, G.P.; Clark, J.B. Threshold Effects and Control of Oxidative Phosphorylation in Nonsynaptic Rat Brain Mitochondria. J. Neurochem. 1996, 66, 1617–1624. [Google Scholar] [CrossRef]
- Ilaria, S.; Tamara, D.; Antonella, D.J.; Elena, M. Role of mitochondria-endoplasmic reticulum contacts in neurodegenerative, neurodevelopmental and neuropsychiatric conditions. Eur. J. Neurosci. 2024, 60, 5040–5068. [Google Scholar] [CrossRef]
- Villa, R.F.; Gorini, A.; Ferrari, F.; Hoyer, S. Energy Metabolism of Cerebral Mitochondria during Aging, Ischemia and Post-Ischemic Recovery Assessed by Functional Proteomics of Enzymes. Neurochem. Int. 2013, 63, 765–781. [Google Scholar] [CrossRef]
- Villa, R.F.; Gorini, A.; Lo Faro, A.; Dell’Orbo, C. A Critique on the Preparation and Enzymatic Characterization of Synaptic and Nonsynaptic Mitochondria from Hippocampus. Cell. Mol. Neurobiol. 1989, 9, 247–262. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Gorini, A. Energy Metabolism of Rat Cerebral Cortex, Hypothalamus and Hypophysis during Ageing. Neuroscience 2012, 227, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Pathak, D.; Shields, L.Y.; Mendelsohn, B.A.; Haddad, D.; Lin, W.; Gerencser, A.A.; Kim, H.; Brand, M.D.; Edwards, R.H.; Nakamura, K. The role of mitochondrially derived ATP in synaptic vesicle recycling. J. Biol. Chem. 2015, 290, 22325–22336. [Google Scholar] [CrossRef] [PubMed]
- Myeong, J.; Stunault, M.I.; Klyachko, V.A.; Ashrafi, G. Metabolic regulation of single synaptic vesicle exo- and endocytosis in hippocampal synapses. Cell Rep. 2024, 43, 114218. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Manzaneda, M.; Fuentes-Moliz, A.; Tabares, L. Presynaptic Mitochondria Communicate With Release Sites for Spatio-Temporal Regulation of Exocytosis at the Motor Nerve Terminal. Front. Synaptic Neurosci. 2022, 14, 858340. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Gorini, A.; Brunello, N.; Tascedda, F. Effect of Desipramine and Fluoxetine on Energy Metabolism of Cerebral Mitochondria. Neuroscience 2016, 330, 326–334. [Google Scholar] [CrossRef]
- Graham, L.C.; Eaton, S.L.; Brunton, P.J.; Atrih, A.; Smith, C.; Lamont, D.J.; Gillingwater, T.H.; Pennetta, G.; Skehel, P.; Wishart, T.M. Proteomic profiling of neuronal mitochondria reveals modulators of synaptic architecture. Mol. Neurodegener. 2017, 12, 77. [Google Scholar] [CrossRef]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef]
- Cicali, K.A.; Tapia-Rojas, C. Synaptic Mitochondria: A Crucial Factor in the Aged Hippocampus. Ageing Res. Rev. 2024, 101, 10254. [Google Scholar] [CrossRef]
- Rangaraju, V.; Calloway, N.; Ryan, T.A. Activity-Driven Local ATP Synthesis Is Required for Synaptic Function. Cell 2014, 156, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Hollenbeck, P.J.; Saxton, W.M. The axonal transport of mitochondria. J. Cell Sci. 2005, 118, 5411–5419. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Perić, I.; Costina, V.; Stanisavljević, A.; Gass, P.; Findeisen, P. Social Isolation Stress-Resilient Rats Reveal Energy Shift from Glycolysis to Oxidative Phosphorylation in Hippocampal Nonsynaptic Mitochondria. Life Sci. 2020, 254, 117790. [Google Scholar] [CrossRef]
- Perić, I.; Costina, V.; Gass, P.; Findeisen, P.; Filipović, D. Hippocampal Synaptoproteomic Changes of Susceptibility and Resilience of Male Rats to Chronic Social Isolation. Brain Res. Bull. 2021, 166, 128–141. [Google Scholar] [CrossRef]
- Kristian, T. Isolation of mitochondria from the CNS. Curr. Protoc. Neurosci. 2010, 7, 7.22.1–7.22.12. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Watanabe, S.; Al Omran, A.J.; Shao, A.S.; Zhang, Z.; Xue, C.; Zhang, J.; Watanabe, J.; Liang, J. Social Isolation Induces Succinate Dehydrogenase Dysfunction in Anxious Mice. Neurochem. Int. 2022, 161, 105434. [Google Scholar] [CrossRef]
- Watanabe, S.; Omran, A.A.; Shao, A.S.; Xue, C.; Zhang, Z.; Zhang, J.; Davies, D.L.; Shao, X.M.; Watanabe, J.; Liang, J. Dihydromyricetin Improves Social Isolation-Induced Cognitive Impairments and Astrocytic Changes in Mice. Sci. Rep. 2022, 12, 5899. [Google Scholar] [CrossRef] [PubMed]
- Khodagholi, F.; Shaerzadeh, F.; Montazeri, F. Mitochondrial Aconitase in Neurodegenerative Disorders: Role of a Metabolism- Related Molecule in Neurodegeneration. Curr. Drug Targets 2017, 19, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S. Mitochondrial DNA, Aconitase “wraps” It Up. Trends Biochem. Sci. 2005, 30, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Brivio, P.; Audano, M.; Gallo, M.T.; Gruca, P.; Lason, M.; Litwa, E.; Fumagalli, F.; Papp, M.; Mitro, N.; Calabrese, F. Metabolomic Signature and Mitochondrial Dynamics Outline the Difference between Vulnerability and Resilience to Chronic Stress. Transl. Psychiatry 2022, 12, 87. [Google Scholar] [CrossRef]
- Gorini, A.; Canosi, U.; Devecchi, E.; Geroldi, D.; Villa, R.F. ATPases Enzyme Activities during Ageing in Different Types of Somatic and Synaptic Plasma Membranes from Rat Frontal Cerebral Cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 81–90. [Google Scholar] [CrossRef]
- Al Omran, A.J.; Shao, A.S.; Watanabe, S.; Zhang, Z.; Zhang, J.; Xue, C.; Watanabe, J.; Davies, D.L.; Shao, X.M.; Liang, J. Social Isolation Induces Neuroinflammation and Microglia Overactivation, While Dihydromyricetin Prevents and Improves Them. J. Neuroinflammation 2022, 19, 2. [Google Scholar] [CrossRef]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The Diverse Functions of GAPDH: Views from Different Subcellular Compartments. Cell. Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Yogalingam, G.; Hwang, S.; Ferreira, J.C.B.; Mochly-Rosen, D. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) Phosphorylation by Protein Kinase Cδ (PKCδ) Inhibits Mitochondria Elimination by Lysosomal-like Structures Following Ischemia and Reoxygenation-Induced Injury. J. Biol. Chem. 2013, 288, 18947–18960. [Google Scholar] [CrossRef]
- Colell, A.; Ricci, J.-E.; Tait, S.; Milasta, S.; Maurer, U.; Bouchier-Hayes, L.; Fitzgerald, P.; Guio-Carrion, A.; Waterhouse, N.J.; Li, C.W.; et al. GAPDH and Autophagy Preserve Survival after Apoptotic Cytochrome c Release in the Absence of Caspase Activation. Cell 2007, 129, 983–997. [Google Scholar] [CrossRef]
- Ramzan, R.; Weber, P.; Linne, U.; Vogt, S. GAPDH: The Missing Link between Glycolysis and Mitochondrial Oxidative Phosphorylation? Biochem. Soc. Trans. 2013, 41, 1294–1297. [Google Scholar] [CrossRef]
- Gambill, B.D.; Voos, W.; Kang, P.J.; Miao, B.; Langer, T.; Craig, E.A.; Pfanner, N. A Dual Role for Mitochondrial Heat Shock Protein 70 in Membrane Translocation of Preproteins. J. Cell Biol. 1993, 123, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Cuervo, A.M. Chaperone-Mediated Autophagy: A Unique Way to Enter the Lysosome World. Trends Cell Biol. 2012, 22, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Terlecky, S.R.; Dice, J.F.; Knecht, E. Selective Binding and Uptake of Ribonuclease A and Glyceraldehyde-3-Phosphate Dehydrogenase by Isolated Rat Liver Lysosomes. J. Biol. Chem. 1994, 269, 26374–26380. [Google Scholar] [CrossRef] [PubMed]
- Audano, M.; Schneider, A.; Mitro, N. Mitochondria, Lysosomes, and Dysfunction: Their Meaning in Neurodegeneration. J. Neurochem. 2018, 147, 291–309. [Google Scholar] [CrossRef]
- Willner, P. The Chronic Mild Stress (CMS) Model of Depression: History, Evaluation and Usage. Neurobiol. Stress 2016, 6, 78. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, L.; Lu, S.; Li, C.; Bai, M.; Xu, E.; Shen, J.; Li, Y. Baicalin Ameliorates CUMS-Induced Depression-like Behaviors through Activating AMPK/PGC-1α Pathway and Enhancing NIX-Mediated Mitophagy in Mice. Eur. J. Pharmacol. 2023, 938, 175435. [Google Scholar] [CrossRef]
- Ko, Y.H.; Delannoy, M.; Hullihen, J.; Chiu, W.; Pedersen, P.L. Mitochondrial ATP Synthasome: Cristae-Enriched Membranes and a Multiwell Detergent Screening Assay Yield Dispersed Single Complexes Containing the ATP Synthase and Carriers for Pi and ADP/ATP. J. Biol. Chem. 2003, 278, 12305–12309. [Google Scholar] [CrossRef]
- Kolbe, H.V.J.; Costello, D.; Wong, A. Mitochondrial Phosphate Transport. Large Scale Isolation and Characterization of the Phosphate Transport Protein from Beef Heart Mitochondria. J. Biol. Chem. 1984, 259, 9115–9120. [Google Scholar] [CrossRef]
- Filiou, M.D.; Zhang, Y.; Teplytska, L.; Reckow, S.; Gormanns, P.; Maccarrone, G.; Frank, E.; Kessler, M.S.; Hambsch, B.; Nussbaumer, M.; et al. Proteomics and Metabolomics Analysis of a Trait Anxiety Mouse Model Reveals Divergent Mitochondrial Pathways. Biol. Psychiatry 2011, 70, 1074–1082. [Google Scholar] [CrossRef]
- Gokhale, A.; Hartwig, C.; Freeman, A.A.H.; Bassell, J.L.; Zlatic, S.A.; Sapp Savas, C.; Vadlamudi, T.; Abudulai, F.; Pham, T.T.; Crocker, A.; et al. Systems Analysis of the 22q11.2 Microdeletion Syndrome Converges on a Mitochondrial Interactome Necessary for Synapse Function and Behavior. J. Neurosci. 2019, 39, 3561–3581. [Google Scholar] [CrossRef]
- Palmieri, F. The Mitochondrial Transporter Family (SLC25): Physiological and Pathological Implications. Pflugers Arch. 2004, 447, 689–709. [Google Scholar] [CrossRef]
- Balasse, E.O.; Féry, F. Ketone Body Production and Disposal: Effects of Fasting, Diabetes, and Exercise. Diabetes. Metab. Rev. 1989, 5, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Korotchkina, L.G. Regulation of the Pyruvate Dehydrogenase Complex. Biochem. Soc. Trans. 2006, 34, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Bie, A.S.; Cömert, C.; Körner, R.; Corydon, T.J.; Palmfeldt, J.; Hipp, M.S.; Hartl, F.U.; Bross, P. An Inventory of Interactors of the Human HSP60/HSP10 Chaperonin in the Mitochondrial Matrix Space. Cell Stress Chaperones 2020, 25, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; King, M.S.; Pryde, K.R. The Production of Reactive Oxygen Species by Complex I. Biochem. Soc. Trans. 2008, 36, 976–980. [Google Scholar] [CrossRef]
- Bleier, L.; Dröse, S. Superoxide Generation by Complex III: From Mechanistic Rationales to Functional Consequences. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 1320–1331. [Google Scholar] [CrossRef]
- Smith, H.L.; Bourne, J.N.; Cao, G.; Chirillo, M.A.; Ostroff, L.E.; Watson, D.J.; Harris, K.M. Mitochondrial Support of Persistent Presynaptic Vesicle Mobilization with Age-Dependent Synaptic Growth after LTP. Elife 2016, 5, e15275. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Jarvie, P.E.; Robinson, P.J. A Rapid Percoll Gradient Procedure for Preparation of Synaptosomes. Nat. Protoc. 2008, 3, 1718–1728. [Google Scholar] [CrossRef]
- Evans, G.J.O. The Synaptosome as a Model System for Studying Synaptic Physiology. Cold Spring Harb. Protoc. 2015, 2015, 421–424. [Google Scholar] [CrossRef]
- Vos, M.; Lauwers, E.; Verstreken, P. Synaptic Mitochondria in Synaptic Transmission and Organization of Vesicle Pools in Health and Disease. Front. Synaptic Neurosci. 2010, 2, 139. [Google Scholar] [CrossRef]
- Hollenbeck, P.J.; Street, W.S.; Lafayette, W. Mitochondria and Neurotransmission: Evacuating the Synapse. Neuron 2005, 47, 331–333. [Google Scholar] [CrossRef]
- Yan, L.J.; Thangthaeng, N.; Forster, M.J. Changes in Dihydrolipoamide Dehydrogenase Expression and Activity during Postnatal Development and Aging in the Rat Brain. Mech. Ageing Dev. 2008, 129, 282–290. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the Hub of Protein Homeostasis: Emerging Mechanistic Insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef]
- Altieri, D.C. Hsp90 Regulation of Mitochondrial Protein Folding: From Organelle Integrity to Cellular Homeostasis. Cell. Mol. Life Sci. C 2012, 70, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The Chaperone-Mediated Autophagy Receptor Organizes in Dynamic Protein Complexes at the Lysosomal Membrane. Mol. Cell. Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nie, T.; Xu, H.; Yang, J.; Yang, Q.; Mao, Z. Chaperone-Mediated Autophagy: Advances from Bench to Bedside. Neurobiol. Dis. 2019, 122, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Karplus, P.A.; Poole, L.B. Typical 2-Cys Peroxiredoxins-Structures, Mechanisms and Functions. FEBS J. 2009, 276, 2469–2477. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Harper, M.E. Uncoupling Proteins and the Control of Mitochondrial Reactive Oxygen Species Production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 Chaperone Machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef]
- Lackie, R.E.; Maciejewski, A.; Ostapchenko, V.G.; Marques-Lopes, J.; Choy, W.Y.; Duennwald, M.L.; Prado, V.F.; Prado, M.A.M. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, J.; Lu, S.; Li, H.; Chen, J.; Wu, X.; Yan, Q.; Liu, H.; Li, H.; Shi, Y. CKB Promotes Mitochondrial ATP Production by Suppressing Permeability Transition Pore. Adv. Sci. 2024, 11, e2403093. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.E.; Machado, L.B.; Santiago, A.P.S.A.; Da-Silva, W.S.; De Felice, F.G.; Holub, O.; Oliveira, M.F.; Galina, A. Mitochondrial Creatine Kinase Activity Prevents Reactive Oxygen Species Generation: Antioxidant Role of Mitochondrial Kinase-Dependent ADP Re-Cycling Activity. J. Biol. Chem. 2006, 281, 37361–37371. [Google Scholar] [CrossRef]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as Key Components of the Stress Response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Woo, H.A.; Kang, D. The Role of Peroxiredoxins in the Transduction of H2O2 Signals. Antioxidants Redox Signal. 2018, 28, 537–557. [Google Scholar] [CrossRef]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Suárez, I.; Bodega, G.; Fernández, B. Glutamine Synthetase in Brain: Effect of Ammonia. Neurochem. Int. 2002, 41, 123–142. [Google Scholar] [CrossRef]
- Son, H.; Baek, J.H.; Go, B.S.; Jung, D.H.; Sontakke, S.B.; Chung, H.J.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; et al. Glutamine Has Antidepressive Effects through Increments of Glutamate and Glutamine Levels and Glutamatergic Activity in the Medial Prefrontal Cortex. Neuropharmacology 2018, 143, 143–152. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, H.; Baek, J.H.; Song, M.; Park, H.; Jeong, W.; Chung, H.J.; Yoo, D.Y.; Lee, D.K.; Park, S.W.; et al. Activation of Glutamine Synthetase (GS) as a New Strategy for the Treatment of Major Depressive Disorder and Other GS-Related Diseases. Acta Pharmacol. Sin. 2025, 46, 880–891. [Google Scholar] [CrossRef]
- Choudary, P.V.; Molnar, M.; Evans, S.J.; Tomita, H.; Li, J.Z.; Vawter, M.P.; Myers, R.M.; Bunney, W.E.; Akil, H.; Watson, S.J.; et al. Altered Cortical Glutamatergic and GABAergic Signal Transmission with Glial Involvement in Depression. Proc. Natl. Acad. Sci. USA 2005, 102, 15653. [Google Scholar] [CrossRef]
- Filiou, M.D.; Sandi, C. Anxiety and Brain Mitochondria: A Bidirectional Crosstalk. Trends Neurosci. 2019, 42, 573–588. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef]
- van der Werff, S.J.A.; van den Berg, S.M.; Pannekoek, J.N.; Elzinga, B.M.; van der Wee, N.J.A. Neuroimaging Resilience to Stress: A Review. Front. Behav. Neurosci. 2013, 7, 39. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipović, D.; Turck, C.W. Proteomic Signatures of Hippocampal Nonsynaptic and Synaptosome-Enriched Mitochondria in Rats Resilient to Chronic Social Isolation. Biomolecules 2025, 15, 1358. https://doi.org/10.3390/biom15101358
Filipović D, Turck CW. Proteomic Signatures of Hippocampal Nonsynaptic and Synaptosome-Enriched Mitochondria in Rats Resilient to Chronic Social Isolation. Biomolecules. 2025; 15(10):1358. https://doi.org/10.3390/biom15101358
Chicago/Turabian StyleFilipović, Dragana, and Christoph W. Turck. 2025. "Proteomic Signatures of Hippocampal Nonsynaptic and Synaptosome-Enriched Mitochondria in Rats Resilient to Chronic Social Isolation" Biomolecules 15, no. 10: 1358. https://doi.org/10.3390/biom15101358
APA StyleFilipović, D., & Turck, C. W. (2025). Proteomic Signatures of Hippocampal Nonsynaptic and Synaptosome-Enriched Mitochondria in Rats Resilient to Chronic Social Isolation. Biomolecules, 15(10), 1358. https://doi.org/10.3390/biom15101358





