Polyphenol Compound 18a Modulates UCP1-Dependent Thermogenesis to Counteract Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
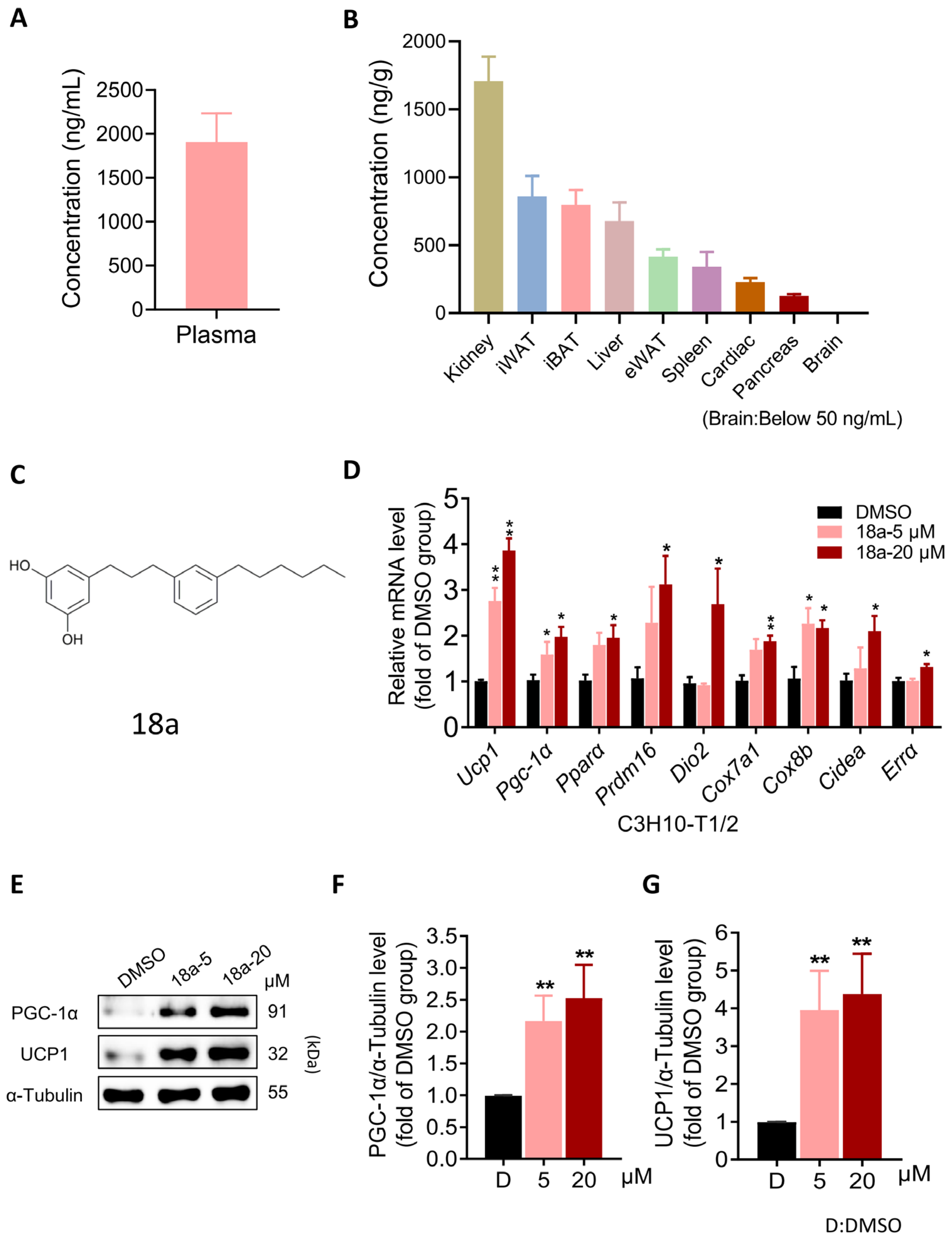
2.3. Tissue Distribution and Plasma Concentration
2.4. Cell Culture
2.5. Cell Transfection
2.6. Isolation of Primary Adipocytes
2.7. Quantitative RT-PCR Analysis
2.8. Western Blotting
2.9. Primary Adipocyte Respiratory Capacity Measurement
2.10. Animals
2.11. Body Composition Analysis
2.12. Glucose Tolerance Test (GTT)
2.13. Insulin Tolerance Test (ITT)
2.14. Immunohistochemical Staining
2.15. Histology
2.16. Statistical Analysis
3. Results
3.1. 18a Induces the Thermogenic Program in C3H10-T1/2 Cells While Exhibiting Favorable Adipose Tissue Distribution
3.2. 18a Enhances Thermogenesis and Mitochondrial Respiration in Primary Brown and Beige Adipocytes
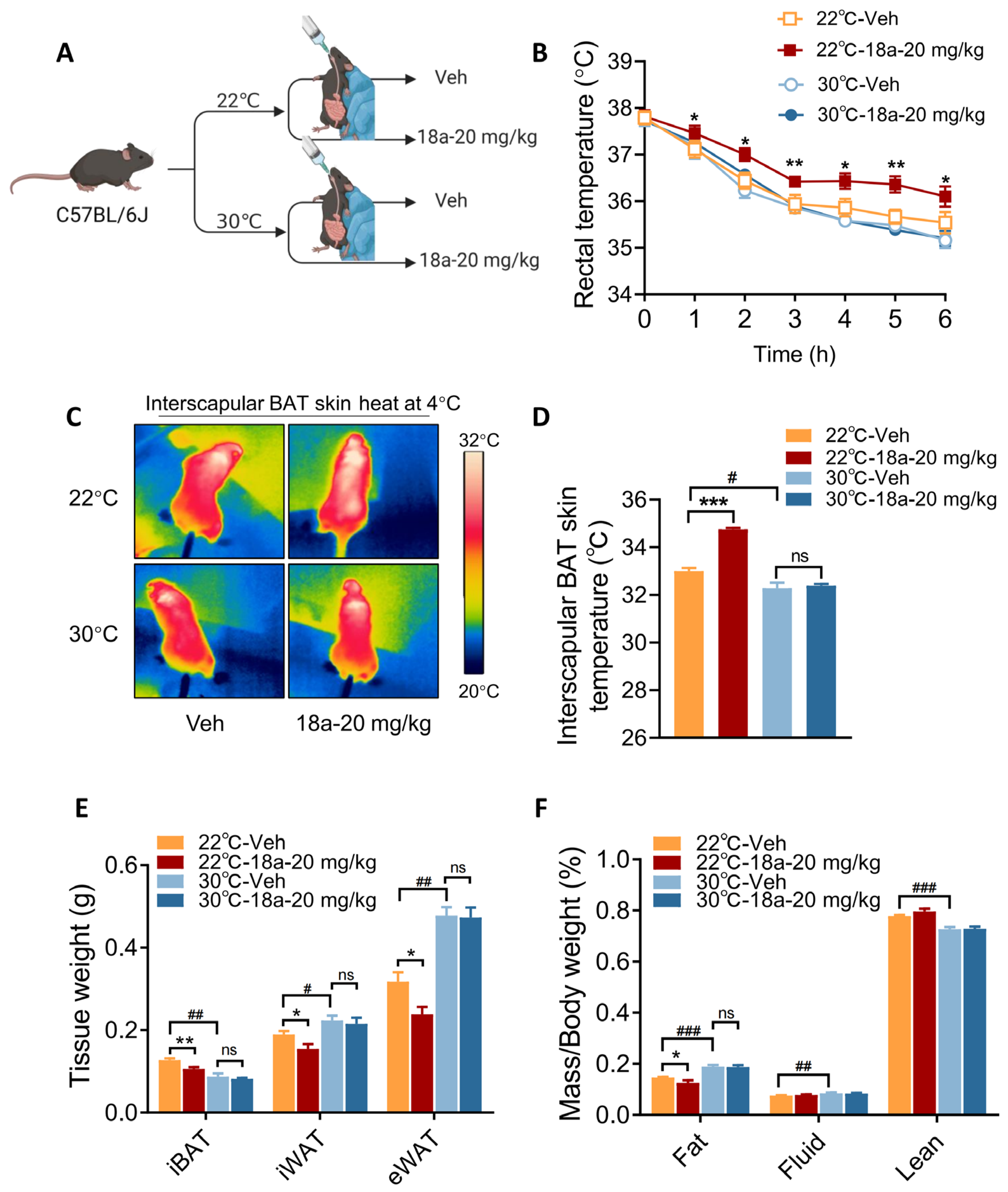
3.3. Short-Term 18a Treatment Significantly Activates Thermogenesis and Reduces Adipose Tissue Weight in Mice
3.4. Thermogenic Effects Induced by 18a Treatment Are Ablated in Thermoneutral Conditions
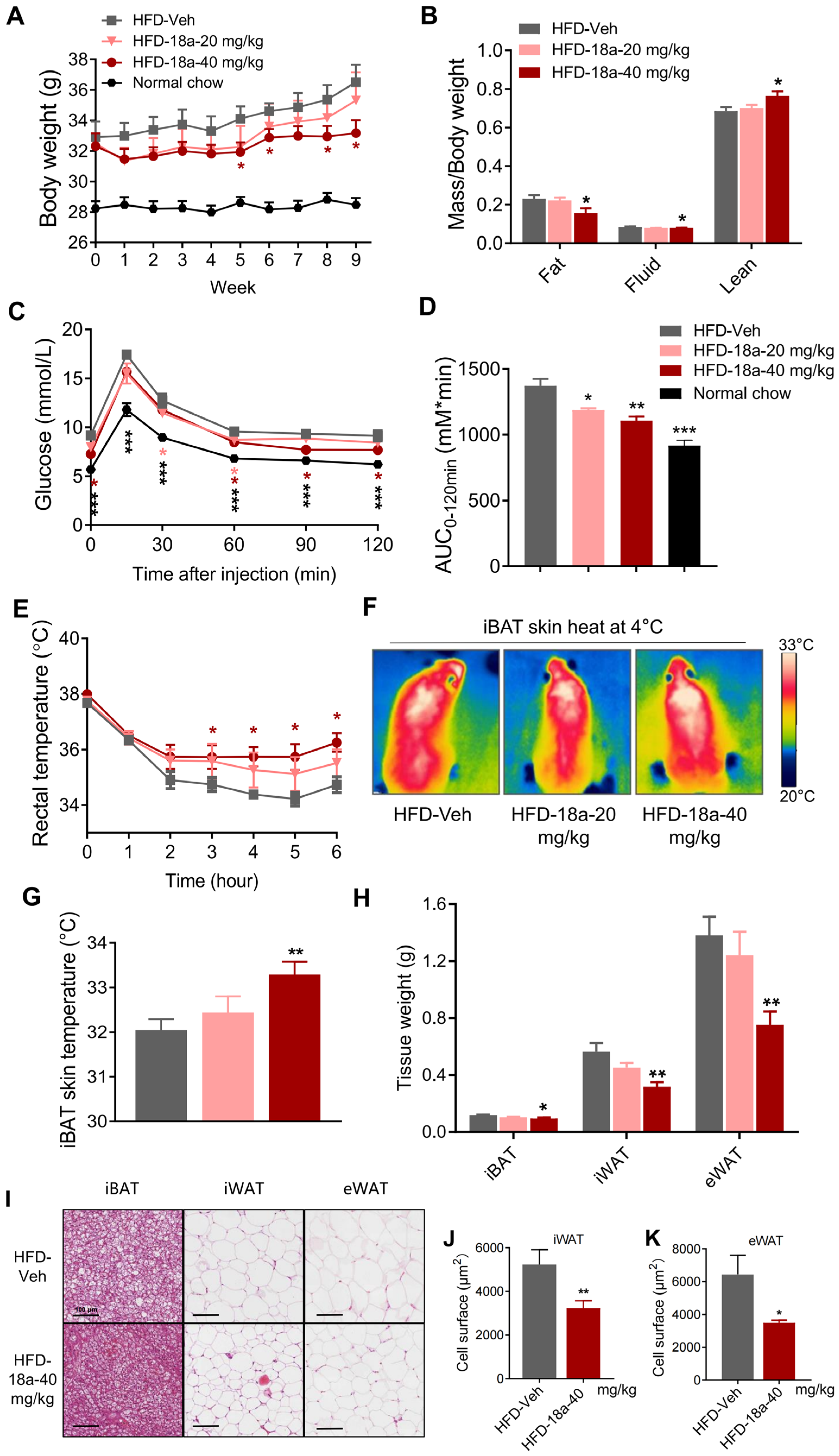
3.5. 18a Resists High-Fat-Induced Weight Gain and Enhances Glucose Tolerance in Mice
3.6. Deficiency in UCP1 in Mice Reverses the Metabolic Benefits of 18a
3.7. 18a Exerts Thermogenesis-Promoting Effects through AMPK-PGC-1α
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Ping, X.; Zhang, Y.; Zhang, T.; Wang, L.; Jin, L.; Zhao, W.; Guo, M.; Shen, F.; et al. Local hyperthermia therapy induces browning of white fat and treats obesity. Cell 2022, 185, 949–966.e919. [Google Scholar] [CrossRef]
- Chen, K.Y.; Brychta, R.J.; Abdul Sater, Z.; Cassimatis, T.M.; Cero, C.; Fletcher, L.A.; Israni, N.S.; Johnson, J.W.; Lea, H.J.; Linderman, J.D.; et al. Opportunities and challenges in the therapeutic activation of human energy expenditure and thermogenesis to manage obesity. J. Biol. Chem. 2020, 295, 1926–1942. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.J.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; van der Lans, A.A.; Cheng, C.C.; Adams, A.C.; van Marken Lichtenbelt, W.D.; Schrauwen, P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015, 5, 10275. [Google Scholar] [CrossRef]
- Jagtap, U.; Paul, A. UCP1 activation: Hottest target in the thermogenesis pathway to treat obesity using molecules of synthetic and natural origin. Drug Discov. Today 2023, 28, 103717. [Google Scholar] [CrossRef]
- Mitschke, M.M.; Hoffmann, L.S.; Gnad, T.; Scholz, D.; Kruithoff, K.; Mayer, P.; Haas, B.; Sassmann, A.; Pfeifer, A.; Kilic, A. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Yun, J.W. Prednisone stimulates white adipocyte browning via β3-AR/p38 MAPK/ERK signaling pathway. Life Sci. 2022, 288, 120204. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Zhang, H.; Ye, R.; Dong, M.; Lin, J.; Zhou, H.; Huang, Y.; Chen, L.; Jiang, X.; Nagaoka, K. Fluvastatin sodium ameliorates obesity through brown fat activation. Int. J. Mol. Sci. 2019, 20, 1622. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Dai, P. Capsaicin directly promotes adipocyte browning in the chemical compound-induced brown adipocytes converted from human dermal fibroblasts. Sci. Rep. 2022, 12, 6612. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Otieno, D.; Gu, I.; Lee, S.-O.; Parks, J.S.; Schimmel, K.; Kang, H.W. Effect of quercetin on nonshivering thermogenesis of brown adipose tissue in high-fat diet-induced obese mice. J. Nutr. Biochem. 2021, 88, 108532. [Google Scholar] [CrossRef] [PubMed]
- Reyad-ul-Ferdous, M.; Song, Y.; Zhang, H.; Pandey, V.K. Histone deacetylase (HDAC) inhibitor curcumin upregulates mitochondrial uncoupling protein1 (UCP1) and mitochondrial function in brown adipocytes, in-silico study and screening natural drug library. J. Saudi Chem. Soc. 2022, 26, 101571. [Google Scholar] [CrossRef]
- Puigserver, P.; Adelmant, G.; Wu, Z.; Fan, M.; Xu, J.; O‘Malley, B.; Spiegelman, B.M. Activation of PPARgamma coactivator-1 through transcription factor docking. Science 1999, 286, 1368–1371. [Google Scholar] [CrossRef]
- Halling, J.F.; Pilegaard, H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl. Physiol. Nutr. Metab. 2020, 45, 927–936. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Ha, J.; Guan, K.L.; Kim, J. AMPK and autophagy in glucose/glycogen metabolism. Mol. Asp. Med. 2015, 46, 46–62. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, J.I.; Boon, M.R.; Houtkooper, R.H. The role of AMPK signaling in brown adipose tissue activation. Cells 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St.-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Huang, Q.; Zou, L.; Wei, P.; Lu, J.; Zhang, Y. Methyl gallate: Review of pharmacological activity. Pharmacol. Res. 2023, 194, 106849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Xiang, J.Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit. Rev. Food Sci. Nutr. 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef]
- Saito, M.; Yoneshiro, T.; Matsushita, M. Food Ingredients as Anti-Obesity Agents. Trends Endocrinol. Metab. TEM 2015, 26, 585–587. [Google Scholar] [CrossRef]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Anti-Obesity and Anti-Inflammatory Synergistic Effects of Green Tea Catechins and Citrus β-Cryptoxanthin Ingestion in Obese Mice. Int. J. Mol. Sci. 2023, 24, 7054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Fang, H.; Guo, F.; Li, F.; Chen, A.; Huang, S. Flavonoids as inducers of white adipose tissue browning and thermogenesis: Signalling pathways and molecular triggers. Nutr. Metab. 2019, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.G.; Otieno, D.; Ha, K. Flavonoids, potential bioactive compounds, and non-shivering thermogenesis. Nutrients 2018, 10, 1168. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa polyphenols and gut microbiota interplay: Bioavailability, prebiotic effect, and impact on human health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Martín, J.; Asuero, A.G. State of the art of anthocyanins: Antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Ren, B.; Kwah, M.X.; Liu, C.; Ma, Z.; Shanmugam, M.K.; Ding, L.; Xiang, X.; Ho, P.C.; Wang, L.; Ong, P.S.; et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021, 515, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors (Oxf. Engl.) 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Chengqiu, D.; Honghong, W.; Jingya, L.; Fajun, N. Design and Synthesis of Alkyl Phenols Inhibitors of Death Associated Apoptotic Protein Kinase 2 (DRAK2). Chin. J. Org. Chem. 2021, 41, 3204. [Google Scholar] [CrossRef]
- Li, B.-H.; Zhang, M.; Duan, Y.-N.; Shuai, L.; Jiang, H.-W.; Li, J.; Nan, F.-J.; Li, J.-Y. Pyrazolone derivative C29 protects against HFD-induced obesity in mice via activation of AMPK in adipose tissue. Acta Pharmacol. Sin. 2021, 42, 964–974. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Q.; He, Y.; Lu, P.; Li, D.; Yang, M.; Gu, W.; Liu, R.; Hong, J.; Wang, J. IRX3 Overexpression Enhances Ucp1 Expression In Vivo. Front. Endocrinol. 2021, 12, 634191. [Google Scholar] [CrossRef]
- Zhi, X.; Wang, J.; Lu, P.; Jia, J.; Shen, H.B.; Ning, G. AdipoCount: A New Software for Automatic Adipocyte Counting. Front. Physiol. 2018, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Rodríguez, V.M.; Miranda, J.; Macarulla, M.T.; Churruca, I.; Portillo, M.P. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013, 141, 1530–1535. [Google Scholar] [CrossRef]
- Galmozzi, A.; Sonne, S.B.; Altshuler-Keylin, S.; Hasegawa, Y.; Shinoda, K.; Luijten, I.H.; Chang, J.W.; Sharp, L.Z.; Cravatt, B.F.; Saez, E. ThermoMouse: An in vivo model to identify modulators of UCP1 expression in brown adipose tissue. Cell Rep. 2014, 9, 1584–1593. [Google Scholar] [CrossRef]
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e535–e578. [Google Scholar] [CrossRef]
- Ikeda, K.; Kang, Q.; Yoneshiro, T.; Camporez, J.P.; Maki, H.; Homma, M.; Shinoda, K.; Chen, Y.; Lu, X.; Maretich, P.; et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Roesler, A.; Kazak, L. UCP1-independent thermogenesis. Biochem. J. 2020, 477, 709–725. [Google Scholar] [CrossRef]
- Keipert, S.; Lutter, D.; Schroeder, B.O.; Brandt, D.; Ståhlman, M.; Schwarzmayr, T.; Graf, E.; Fuchs, H.; de Angelis, M.H.; Tschöp, M.H.; et al. Endogenous FGF21-signaling controls paradoxical obesity resistance of UCP1-deficient mice. Nat. Commun. 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Pahlavani, M.; Ramalingam, L.; Jayarathne, S.; Andrade, J.; Scoggin, S.; Festuccia, W.T.; Kalupahana, N.S.; Moustaid-Moussa, N. Temperature-Dependent Effects of Eicosapentaenoic Acid (EPA) on Browning of Subcutaneous Adipose Tissue in UCP1 Knockout Male Mice. Int. J. Mol. Sci. 2023, 24, 8708. [Google Scholar] [CrossRef]
- Chen, S.; Liu, X.; Peng, C.; Tan, C.; Sun, H.; Liu, H.; Zhang, Y.; Wu, P.; Cui, C.; Liu, C.; et al. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity. Cell Metab. 2021, 33, 565–580.e567. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.G.; Moon, D.S.; Kim, H.J.; Shon, Y.H. Magnesium and calcium-enriched deep-sea water promotes mitochondrial biogenesis by AMPK-activated signals pathway in 3T3-L1 preadipocytes. Biomed. Pharmacother. 2016, 83, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.; Donado-Pestana, C.M.; Moura, M.H.; e Silva, R.R.; Pessoa, E.V.; Genovese, M.I. Phenolic compounds from jaboticaba (Plinia jaboticaba (Vell.) Berg) ameliorate intestinal inflammation and associated endotoxemia in obesity. Food Res. Int. 2021, 141, 110139. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.R.; Cho, Y.H.; Hong, Z.Y.; Lee, H.; Lee, S.J.; Hong, S.S.; Lee, E.J. The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes. Endocrinol. Metab. 2016, 31, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Crespillo, A.; Alonso, M.; Vida, M.; Pavón, F.J.; Serrano, A.; Rivera, P.; Romero-Zerbo, Y.; Fernández-Llebrez, P.; Martínez, A.; Pérez-Valero, V.; et al. Reduction of body weight, liver steatosis and expression of stearoyl-CoA desaturase 1 by the isoflavone daidzein in diet-induced obesity. Br. J. Pharmacol. 2011, 164, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Bagul, P.; Banerjee, S. Application of resveratrol in diabetes: Rationale, strategies and challenges. Curr. Mol. Med. 2015, 15, 312–330. [Google Scholar] [CrossRef]
- Pandareesh, M.; Mythri, R.; Bharath, M.S. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1178–1185. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic phytochemicals in cancer prevention and therapy: Bioavailability versus bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef]
- Mulholland, P.; Ferry, D.; Anderson, D.; Hussain, S.; Young, A.M.; Cook, J.; Hodgkin, E.; Seymour, L.; Kerr, D. Pre-clinical and clinical study of QC12, a water-soluble, pro-drug of quercetin. Ann. Oncol. 2001, 12, 245–248. [Google Scholar] [CrossRef]
- Pralhad, T.; Rajendrakumar, K. Study of freeze-dried quercetin–cyclodextrin binary systems by DSC, FT-IR, X-ray diffraction and SEM analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339. [Google Scholar] [CrossRef]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int. J. Pharm. 2012, 427, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-P.; Chen, L.-J.; Fan, L.-Y.; Tang, M.-H.; Yang, G.-L.; Yang, H.-S.; Du, X.-B.; Wang, G.-Q.; Yao, W.-X.; Zhao, Q.-M. Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin. Cancer Res. 2006, 12, 3193–3199. [Google Scholar] [CrossRef] [PubMed]
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Romano, M.R.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal encapsulation of the natural flavonoid fisetin improves bioavailability and antitumor efficacy. Int. J. Pharm. 2013, 444, 146–154. [Google Scholar] [CrossRef]
- Lee, H.S.; Heo, C.U.; Song, Y.H.; Lee, K.; Choi, C.I. Naringin promotes fat browning mediated by UCP1 activation via the AMPK signaling pathway in 3T3-L1 adipocytes. Arch. Pharmacal Res. 2023, 46, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK signaling by polyphenols: A novel strategy for tackling aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef]
- Hu, Z.; Li, M.; Cao, Y.; Akan, O.D.; Guo, T.; Luo, F. Targeting AMPK Signaling by Dietary Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2022, 66, e2100732. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Bolin, A.P.; Sousa-Filho, C.P.B.; Dos Santos, G.T.N.; Ferreira, L.T.; de Andrade, P.B.M.; Figueira, A.C.M.; Batista, F.A.H.; Otton, R. Adipogenic commitment induced by green tea polyphenols remodel adipocytes to a thermogenic phenotype. J. Nutr. Biochem. 2020, 83, 108429. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, A.; Hoffmann, L.S. Brown, beige, and white: The new color code of fat and its pharmacological implications. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Klein Hazebroek, M.; Keipert, S. Obesity-resistance of UCP1-deficient mice associates with sustained FGF21 sensitivity in inguinal adipose tissue. Front. Endocrinol. 2022, 13, 909621. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L.; et al. AMPK activation protects against diet induced obesity through Ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef]
- Kazak, L.; Chouchani, E.T.; Stavrovskaya, I.G.; Lu, G.Z.; Jedrychowski, M.P.; Egan, D.F.; Kumari, M.; Kong, X.; Erickson, B.K.; Szpyt, J.; et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc. Natl. Acad. Sci. USA 2017, 114, 7981–7986. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Song, Y.; Zhang, M.; Kang, Y.; Chen, D.; Ma, H.; Nan, F.; Duan, Y.; Li, J. Polyphenol Compound 18a Modulates UCP1-Dependent Thermogenesis to Counteract Obesity. Biomolecules 2024, 14, 618. https://doi.org/10.3390/biom14060618
Wen X, Song Y, Zhang M, Kang Y, Chen D, Ma H, Nan F, Duan Y, Li J. Polyphenol Compound 18a Modulates UCP1-Dependent Thermogenesis to Counteract Obesity. Biomolecules. 2024; 14(6):618. https://doi.org/10.3390/biom14060618
Chicago/Turabian StyleWen, Xueping, Yufei Song, Mei Zhang, Yiping Kang, Dandan Chen, Hui Ma, Fajun Nan, Yanan Duan, and Jingya Li. 2024. "Polyphenol Compound 18a Modulates UCP1-Dependent Thermogenesis to Counteract Obesity" Biomolecules 14, no. 6: 618. https://doi.org/10.3390/biom14060618
APA StyleWen, X., Song, Y., Zhang, M., Kang, Y., Chen, D., Ma, H., Nan, F., Duan, Y., & Li, J. (2024). Polyphenol Compound 18a Modulates UCP1-Dependent Thermogenesis to Counteract Obesity. Biomolecules, 14(6), 618. https://doi.org/10.3390/biom14060618






