Single-Nucleus RNA-Seq Reveals Spermatogonial Stem Cell Developmental Pattern in Shaziling Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Information
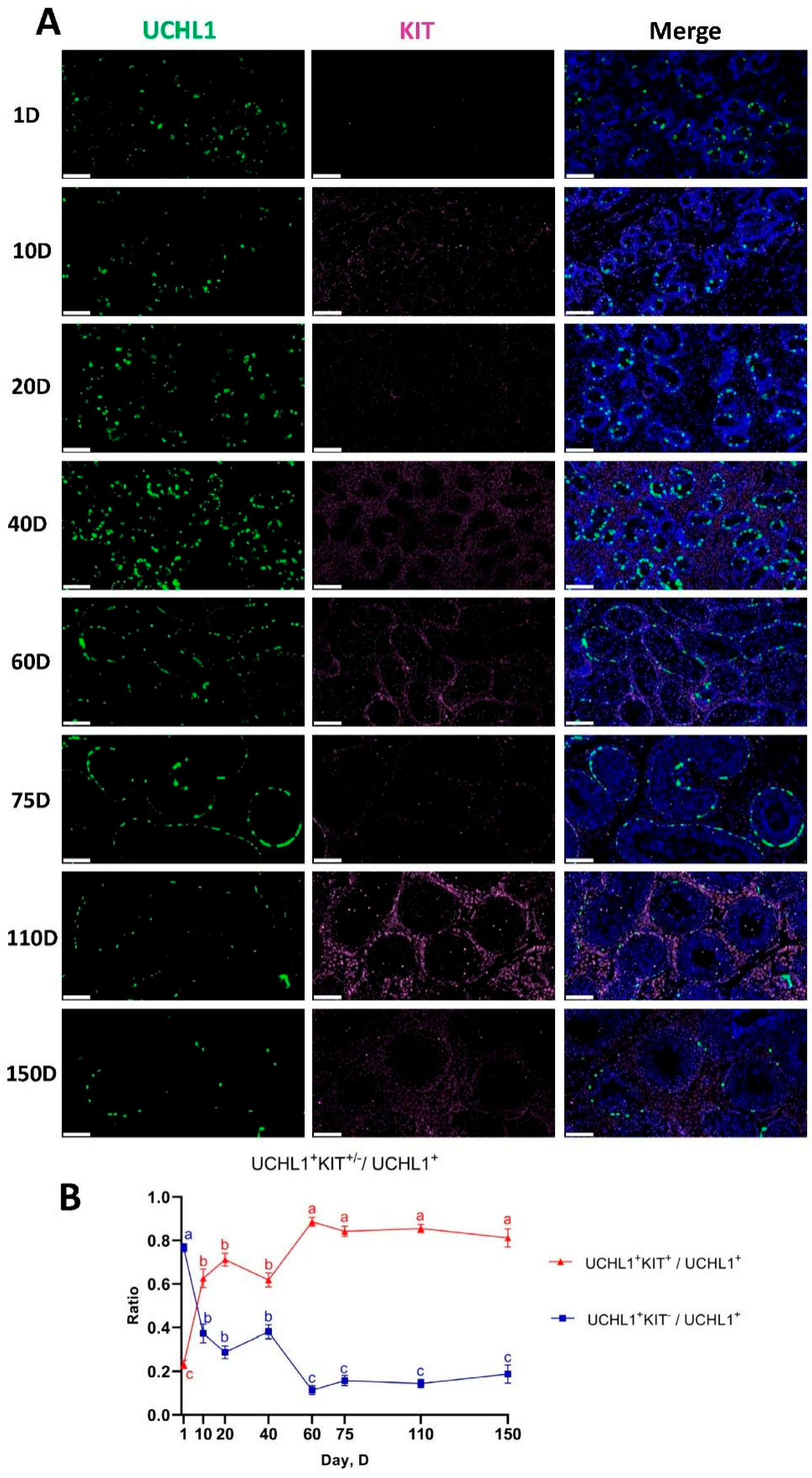
2.2. Histological Analysis and Immunofluorescence Staining of Testicular Tissue Sections
2.3. Conducting snRNA-Seq
2.4. Data Mapping, Quality Control, and Cell Clustering Analysis
2.5. Functional Enrichment and GSVA Pathway Analysis
2.6. Cell Trajectory Analysis
3. Results
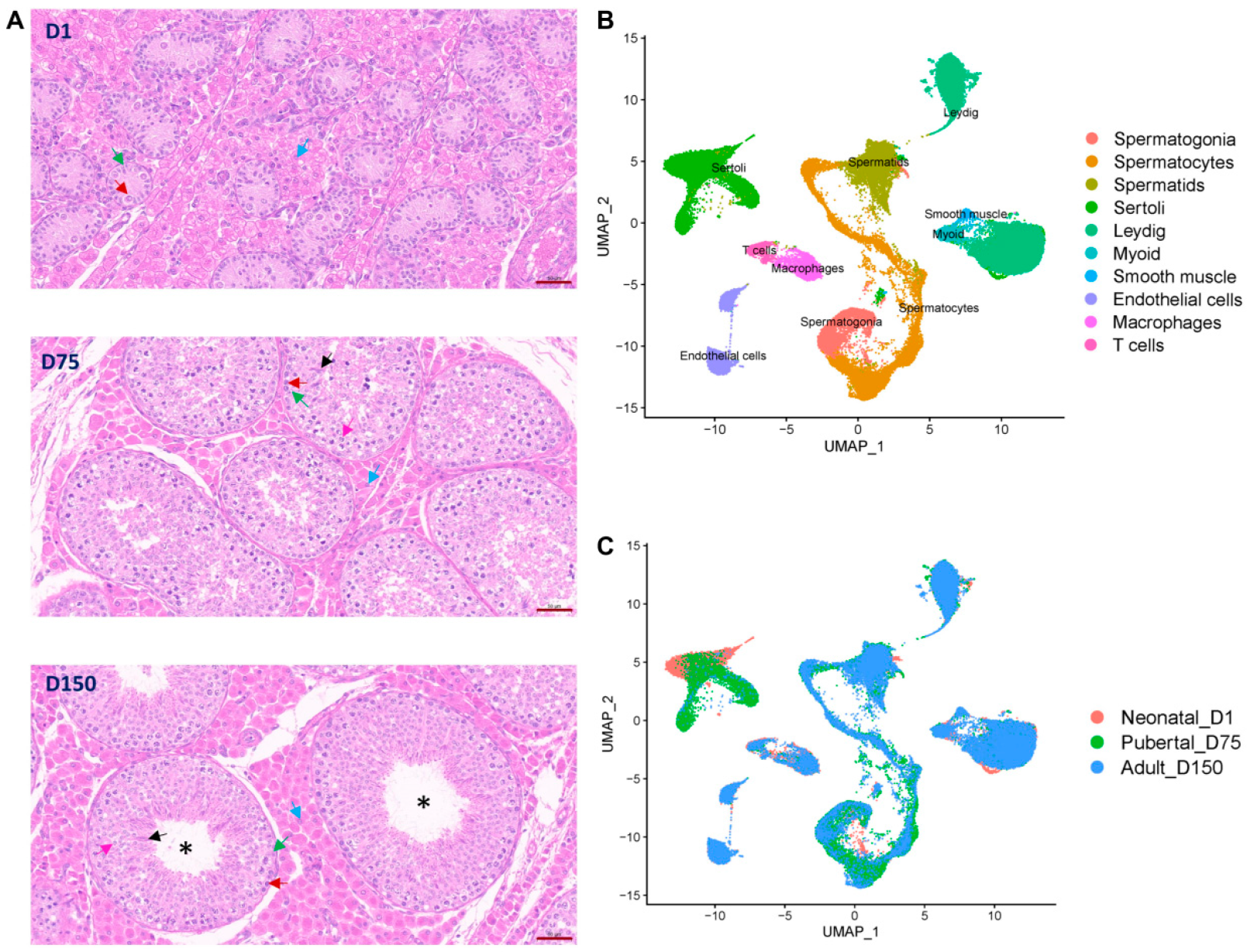
3.1. Overview of Single-Cell Transcriptomes and Histomorphological Analysis of Shaziling Pig Testes at Different Ages
3.2. Identification of Testicular Cell Types by Cell Cluster Analysis
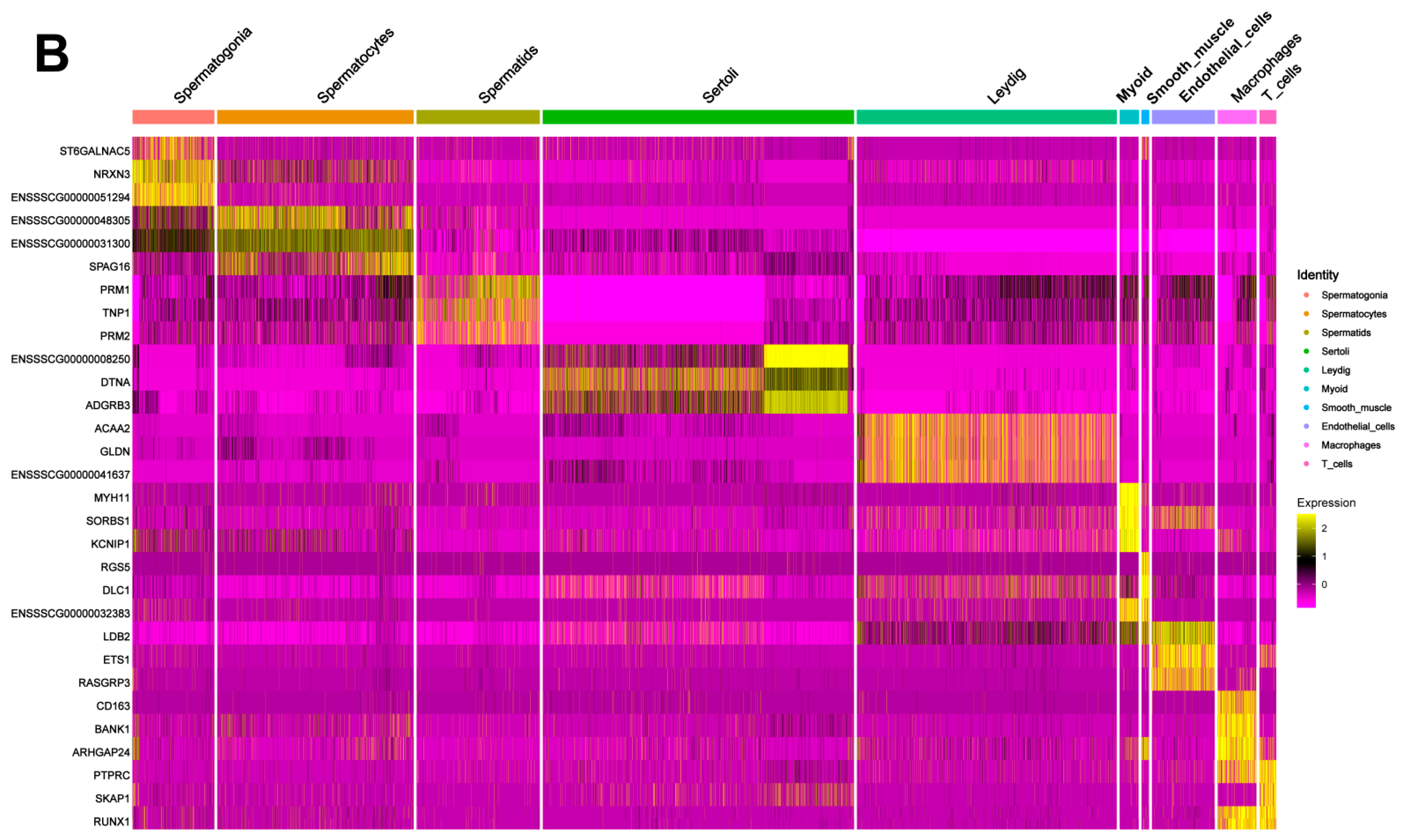
3.3. Discovery of Marker Genes for Each Cell Type in Shaziling Pig Testes
3.4. Germ Cell Lineage Development in Shaziling Pig Testes
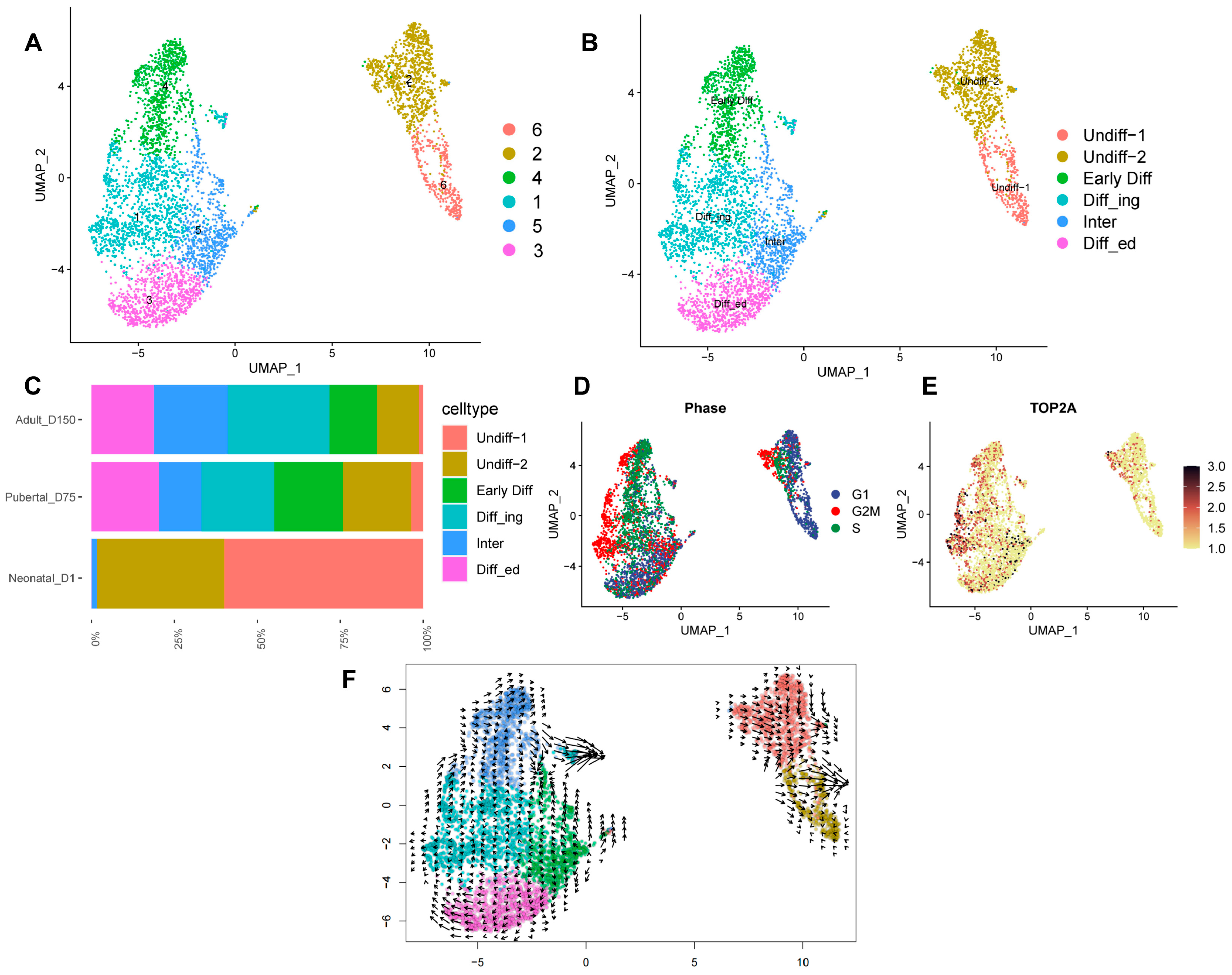
3.5. Re-Clustering of Shaziling Pig Spermatogonial Cells
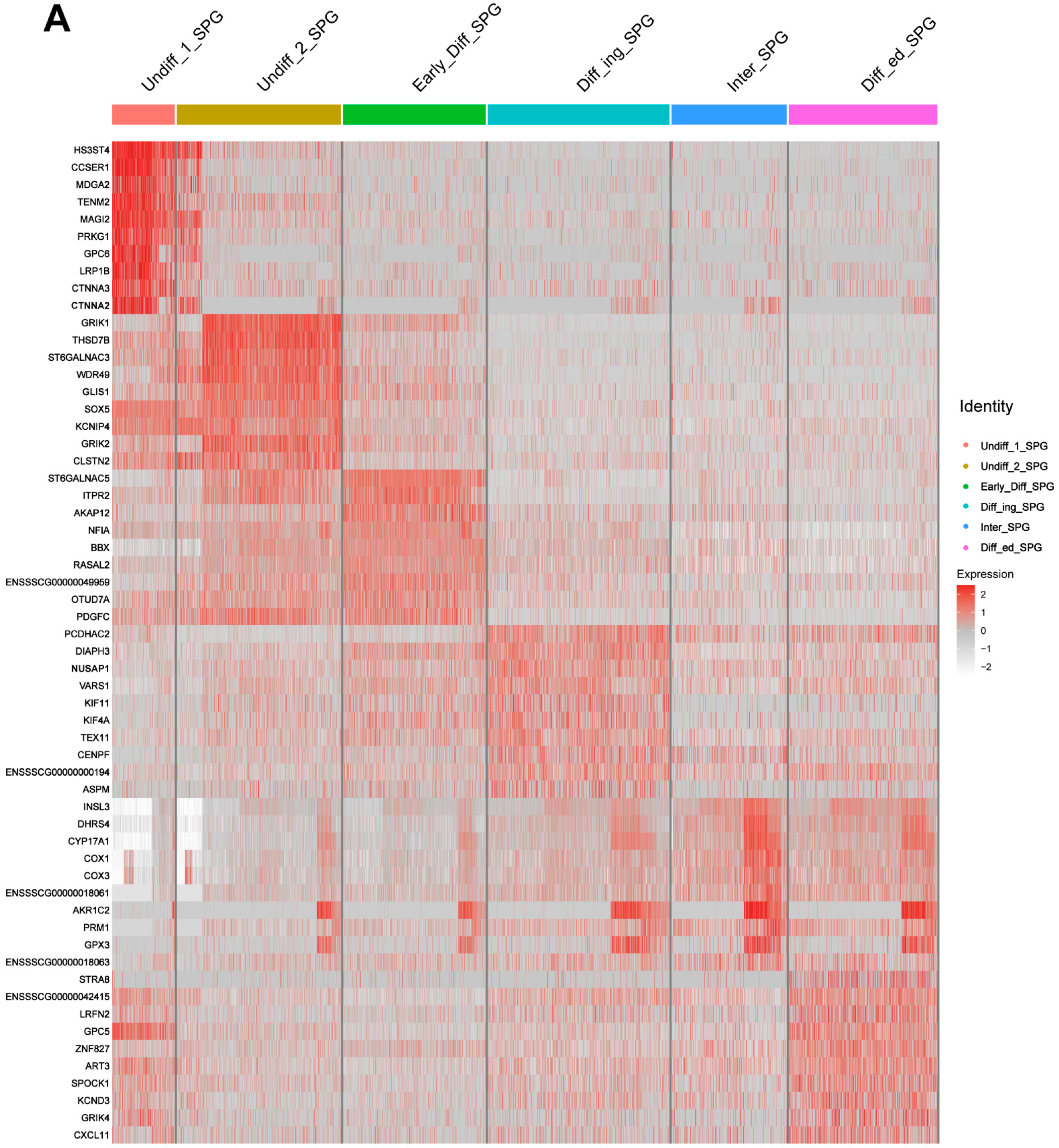
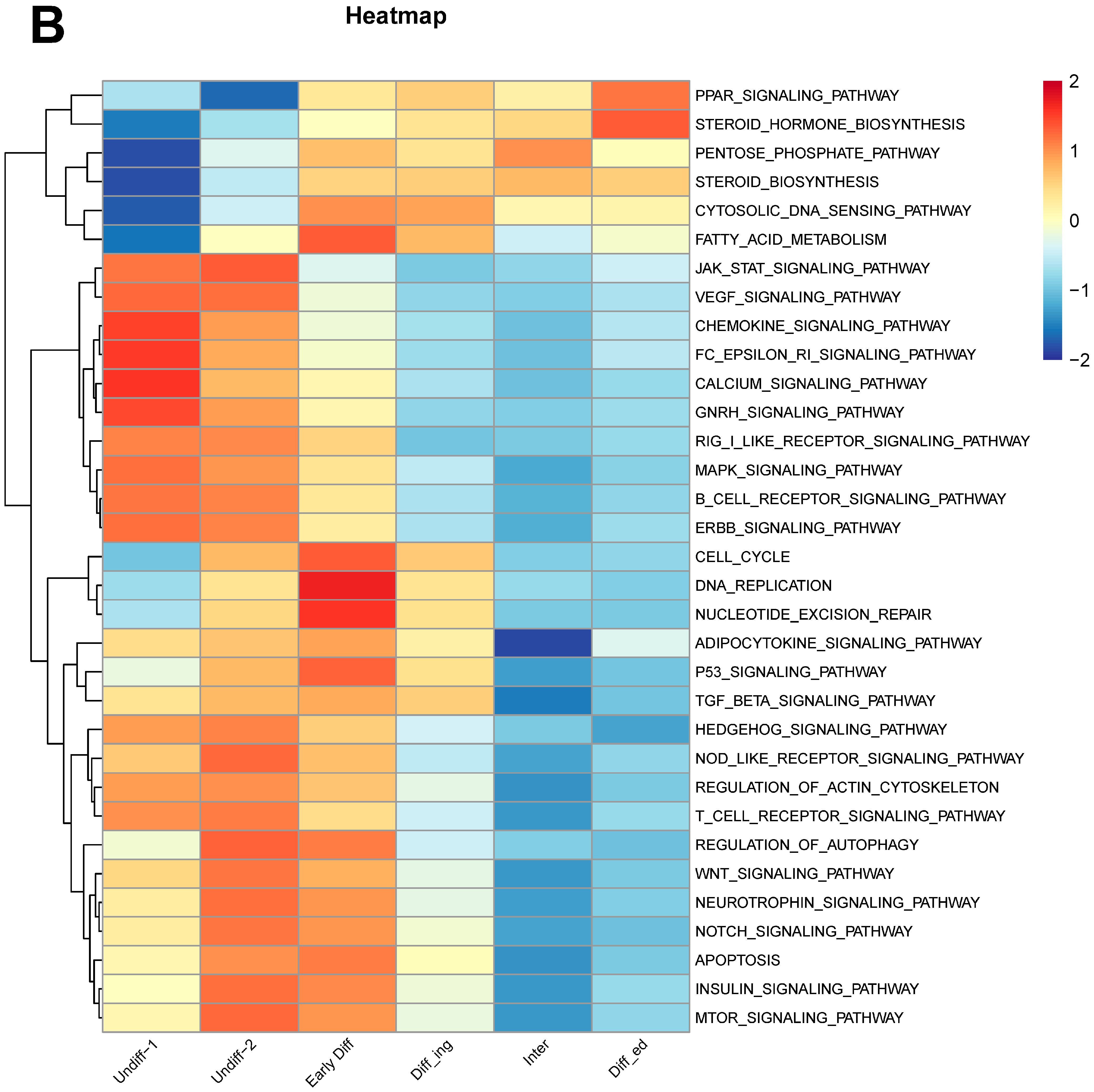
3.6. Potential SSC Marker Genes and Important Pathways for Maintenance and Self-Renewal of SSCs
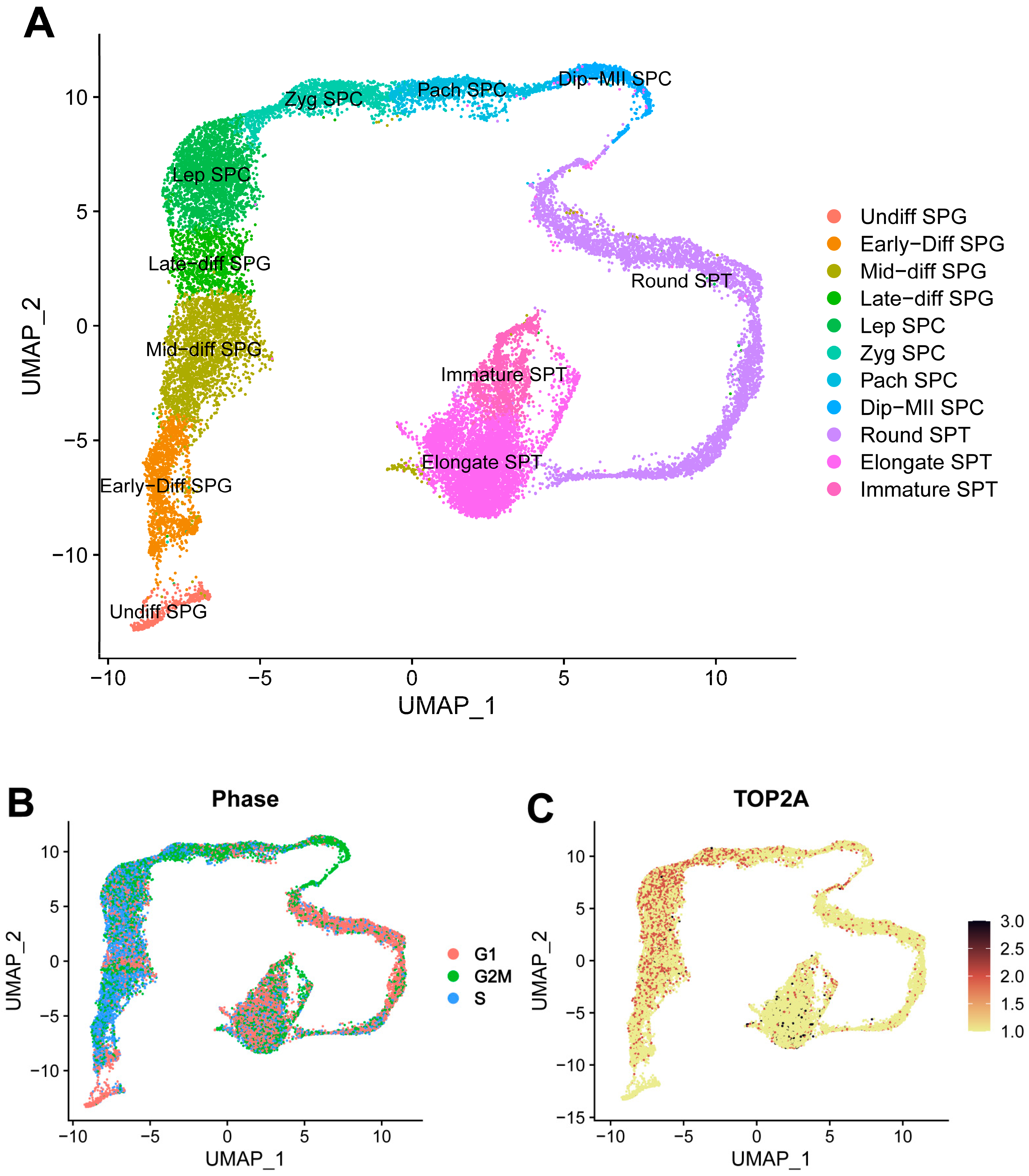
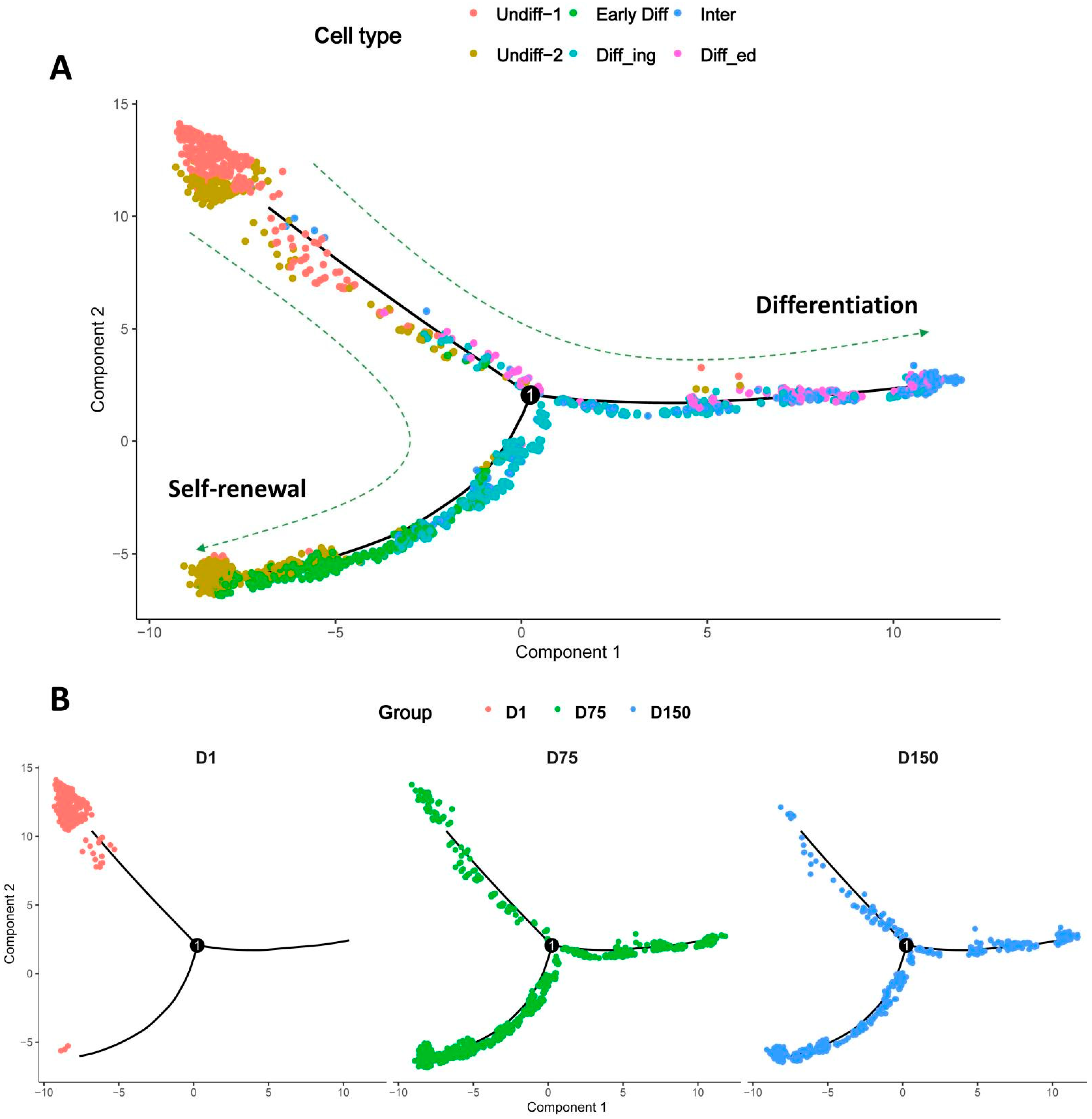
3.7. Pseudotime Trajectory Analysis of Shaziling Pig Spermatogonial Cells
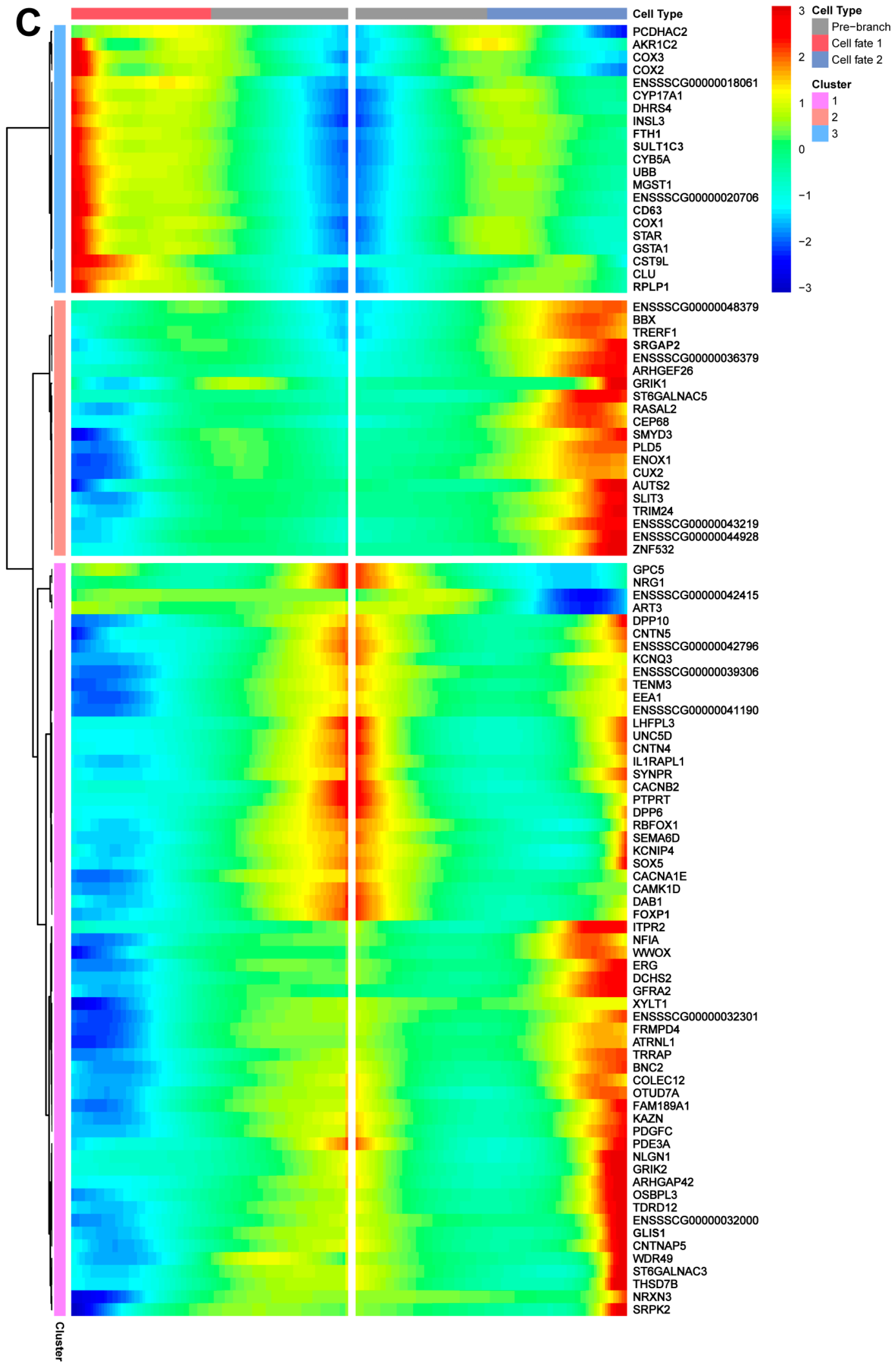
3.8. Developmental Pattern of Spermatogonia in Shaziling Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makela, J.A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef] [PubMed]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ohmura, M.; Ohbo, K. The niche for spermatogonial stem cells in the mammalian testis. Int. J. Hematol. 2005, 82, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Di Persio, S.; Neuhaus, N. Human spermatogonial stem cells and their niche in male (in)fertility: Novel concepts from single-cell RNA-sequencing. Hum. Reprod. 2023, 38, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, W.; Ye, K.; He, L.; Ge, Q.; Huang, Y.; Zhao, X. Single-Nucleus RNA-Seq: Open the Era of Great Navigation for FFPE Tissue. Int. J. Mol. Sci. 2023, 24, 13744. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, W.; Zhou, Z.; Chen, Z.; Lan, Z.; Bo, H.; Fan, L. Single-cell RNA sequencing technology in human spermatogenesis: Progresses and perspectives. Mol. Cell. Biochem. 2 Sep. 2023. [Google Scholar] [CrossRef]
- Suzuki, T. Overview of single-cell RNA sequencing analysis and its application to spermatogenesis research. Reprod. Med. Biol. 2023, 22, e12502. [Google Scholar] [CrossRef]
- Tan, K.; Wilkinson, M.F. A single-cell view of spermatogonial stem cells. Curr. Opin. Cell Biol. 2020, 67, 71–78. [Google Scholar] [CrossRef]
- Estermann, M.A.; Williams, S.; Hirst, C.E.; Roly, Z.Y.; Serralbo, O.; Adhikari, D.; Powell, D.; Major, A.T.; Smith, C.A. Insights into Gonadal Sex Differentiation Provided by Single-Cell Transcriptomics in the Chicken Embryo. Cell Rep. 2020, 31, 107491. [Google Scholar] [CrossRef]
- Yu, X.W.; Li, T.T.; Du, X.M.; Shen, Q.Y.; Zhang, M.F.; Wei, Y.D.; Yang, D.H.; Xu, W.J.; Chen, W.B.; Bai, C.L.; et al. Single-cell RNA sequencing reveals atlas of dairy goat testis cells. Zool. Res. 2021, 42, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Xi, H.; Qiao, P.; Li, Y.; Xian, M.; Zhu, D.; Hu, J. Single-cell transcriptomics reveals male germ cells and Sertoli cells developmental patterns in dairy goats. Front. Cell Dev. Biol. 2022, 10, 944325. [Google Scholar] [CrossRef]
- Chen, M.; Wang, N.; Yang, H.; Liu, D.; Gao, Y.; Duo, L.; Cui, X.; Hao, F.; Ye, J.; Gao, F.; et al. Single-cell transcriptome analysis of the germ cells and somatic cells during mitotic quiescence stage in goats. FASEB J. 2023, 37, e23244. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sun, P.; Liu, W.X.; Shan, L.Y.; Hu, Y.T.; Fan, H.T.; Shen, W.; Liu, Y.B.; Zhou, Y.; Zhang, T. Single-cell RNA sequencing of the Mongolia sheep testis reveals a conserved and divergent transcriptome landscape of mammalian spermatogenesis. FASEB J. 2022, 36, e22348. [Google Scholar] [CrossRef]
- Yang, H.; Ma, J.; Wan, Z.; Wang, Q.; Wang, Z.; Zhao, J.; Wang, F.; Zhang, Y. Characterization of sheep spermatogenesis through single-cell RNA sequencing. FASEB J. 2021, 35, e21187. [Google Scholar] [CrossRef] [PubMed]
- Mipam, T.; Chen, X.; Zhao, W.; Zhang, P.; Chai, Z.; Yue, B.; Luo, H.; Wang, J.; Wang, H.; Wu, Z.; et al. Single-cell transcriptome analysis and in vitro differentiation of testicular cells reveal novel insights into male sterility of the interspecific hybrid cattle-yak. BMC Genom. 2023, 24, 149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, J.; Xiong, L.; Kang, Y.; Guo, S.; Cao, M.; Ding, Z.; Bao, P.; Chu, M.; Liang, C.; et al. Single-cell RNA sequencing and UPHLC-MS/MS targeted metabolomics offer new insights into the etiological basis for male cattle-yak sterility. Int. J. Biol. Macromol. 2023, 253, 126831. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, J.; Xiong, L.; Guo, S.; Cao, M.; Kang, Y.; Ding, Z.; La, Y.; Liang, C.; Yan, P.; et al. Single-Cell RNA Sequencing Reveals Atlas of Yak Testis Cells. Int. J. Mol. Sci. 2023, 24, 7982. [Google Scholar] [CrossRef]
- Zhang, L.; Li, F.; Lei, P.; Guo, M.; Liu, R.; Wang, L.; Yu, T.; Lv, Y.; Zhang, T.; Zeng, W.; et al. Single-cell RNA-sequencing reveals the dynamic process and novel markers in porcine spermatogenesis. J. Anim. Sci. Biotechnol. 2021, 12, 122. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, M.; Liu, Z.; Liu, R.; Zheng, Y.; Yu, T.; Lv, Y.; Lu, H.; Zeng, W.; Zhang, T.; et al. Single-cell RNA-seq analysis of testicular somatic cell development in pigs. J. Genet. Genom. 2022, 49, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, X.; Yan, S.; Yang, A.; Xiang, J.; Deng, Y.; Yin, Y.; Chen, B.; Gu, J. Comprehensive Analysis of the Transcriptome-Wide m(6)A Methylome in Shaziling Pig Testicular Development. Int. J. Mol. Sci. 2023, 24, 14475. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Kerns, K. Sperm capacitation as a predictor of boar fertility. Mol. Reprod. Dev. 2023, 90, 594–600. [Google Scholar] [CrossRef]
- Ding, H.; Luo, Y.; Liu, M.; Huang, J.; Xu, D. Histological and transcriptome analyses of testes from Duroc and Meishan boars. Sci. Rep. 2016, 6, 20758. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Avraham-Davidi, I.; Basu, A.; Burks, T.; Shekhar, K.; Hofree, M.; Choudhury, S.R.; Aguet, F.; Gelfand, E.; Ardlie, K.; et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 2017, 14, 955–958. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, C.S.; Murrow, L.M.; Gartner, Z.J. DoubletFinder: Doublet Detection in Single-Cell RNA Sequencing Data Using Artificial Nearest Neighbors. Cell Syst. 2019, 8, 329–337.e4. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdottir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Mao, Q.; Tang, Y.; Wang, L.; Chawla, R.; Pliner, H.A.; Trapnell, C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 2017, 14, 979–982. [Google Scholar] [CrossRef]
- Qiu, X.; Hill, A.; Packer, J.; Lin, D.; Ma, Y.A.; Trapnell, C. Single-cell mRNA quantification and differential analysis with Census. Nat. Methods 2017, 14, 309–315. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lonnerberg, P.; Furlan, A.; et al. RNA velocity of single cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Turley, H.; Comley, M.; Houlbrook, S.; Nozaki, N.; Kikuchi, A.; Hickson, I.D.; Gatter, K.; Harris, A.L. The distribution and expression of the two isoforms of DNA topoisomerase II in normal and neoplastic human tissues. Br. J. Cancer 1997, 75, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Di Persio, S.; Saracino, R.; Fera, S.; Muciaccia, B.; Esposito, V.; Boitani, C.; Berloco, B.P.; Nudo, F.; Spadetta, G.; Stefanini, M.; et al. Spermatogonial kinetics in humans. Development 2017, 144, 3430–3439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, S.; Xiao, W.; Song, Y.; Tang, L.; Cao, M.; Yang, J.; Wang, S.; Li, Z.; Xu, C.; et al. A single-cell transcriptomic landscape of mouse testicular aging. J. Adv. Res. 2023, 53, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Pawar, R.M.; Makala, H.; Goel, S. Conserved expression of ubiquitin carboxyl-terminal esterase L1 (UCHL1) in mammalian testes. Indian J. Exp. Biol. 2015, 53, 305–312. [Google Scholar] [PubMed]
- Kim, Y.H.; Choi, Y.R.; Kim, B.J.; Jung, S.E.; Kim, S.M.; Jin, J.H.; Yun, M.H.; Kim, S.U.; Kim, Y.H.; Hwang, S.; et al. GDNF family receptor alpha 1 is a reliable marker of undifferentiated germ cells in bulls. Theriogenology 2019, 132, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Megee, S.; Dobrinski, I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell Physiol. 2009, 220, 460–468. [Google Scholar] [CrossRef]
- Rossi, P.; Sette, C.; Dolci, S.; Geremia, R. Role of c-kit in mammalian spermatogenesis. J. Endocrinol. Investig. 2000, 23, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Schrans-Stassen, B.H.; van de Kant, H.J.; de Rooij, D.G.; van Pelt, A.M. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 1999, 140, 5894–5900. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, R.; Lee, W.Y.; Kim, J.H.; Do, J.T.; Park, C.; Song, H. Stage-specific expression of Sal-like protein 4 in boar testicular germ cells. Theriogenology 2017, 101, 44–52. [Google Scholar] [CrossRef]
- Heidari, B.; Rahmati-Ahmadabadi, M.; Akhondi, M.M.; Zarnani, A.H.; Jeddi-Tehrani, M.; Shirazi, A.; Naderi, M.M.; Behzadi, B. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J. Assist. Reprod. Genet. 2012, 29, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testicular growth and development in puberty. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Makela, J.A.; Hobbs, R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 158, R169–R187. [Google Scholar] [CrossRef] [PubMed]
- Frankenhuis, M.T.; Kramer, M.F.; de Rooij, D.G. Spermatogenesis in the boar. Vet. Q. 1982, 4, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Grive, K.J.; Hu, Y.; Shu, E.; Grimson, A.; Elemento, O.; Grenier, J.K.; Cohen, P.E. Dynamic transcriptome profiles within spermatogonial and spermatocyte populations during postnatal testis maturation revealed by single-cell sequencing. PLoS Genet. 2019, 15, e1007810. [Google Scholar] [CrossRef] [PubMed]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-Cell RNA Sequencing of Human, Macaque, and Mouse Testes Uncovers Conserved and Divergent Features of Mammalian Spermatogenesis. Dev. Cell 2020, 54, 529–547.e512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, F.; Zhang, L.; Lei, P.; Zheng, Y.; Zeng, W. Stage-specific embryonic antigen 4 is a membrane marker for enrichment of porcine spermatogonial stem cells. Andrology 2020, 8, 1923–1934. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, W.Y.; Park, C.; Hong, K.; Song, H. CD14 is a unique membrane marker of porcine spermatogonial stem cells, regulating their differentiation. Sci. Rep. 2019, 9, 9980. [Google Scholar] [CrossRef] [PubMed]
- Joset, P.; Wacker, A.; Babey, R.; Ingold, E.A.; Andermatt, I.; Stoeckli, E.T.; Gesemann, M. Rostral growth of commissural axons requires the cell adhesion molecule MDGA2. Neural Dev. 2011, 6, 22. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.; Lee, S.J.; Qiang, Y.; Lee, D.; Lee, H.W.; Kim, H.; Je, H.S.; Sudhof, T.C.; Ko, J. MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc. Natl. Acad. Sci. USA 2013, 110, 336–341. [Google Scholar] [CrossRef]
- Silva, J.P.; Lelianova, V.G.; Ermolyuk, Y.S.; Vysokov, N.; Hitchen, P.G.; Berninghausen, O.; Rahman, M.A.; Zangrandi, A.; Fidalgo, S.; Tonevitsky, A.G.; et al. Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc. Natl. Acad. Sci. USA 2011, 108, 12113–12118. [Google Scholar] [CrossRef]
- Hofmann, F.; Wegener, J.W. cGMP-dependent protein kinases (cGK). Methods Mol. Biol. 2013, 1020, 17–50. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, M.; De Cat, B.; Ceulemans, H.; Bruystens, A.M.; Coomans, C.; Durr, J.; Vermeesch, J.; Marynen, P.; David, G. Glypican-6, a new member of the glypican family of cell surface heparan sulfate proteoglycans. J. Biol. Chem. 1999, 274, 26968–26977. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Yan, R.; Yang, G.; Xiang, S.; Zhao, F. Hedgehog signaling activation required for glypican-6-mediated regulation of invasion, migration, and epithelial-mesenchymal transition of gastric cancer cells. Biosci. Rep. 2020, 40, BSR20193181. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Shen, Z.; Sun, P. Downregulation of Low-density lipoprotein receptor-related protein 1B (LRP1B) inhibits the progression of hepatocellular carcinoma cells by activating the endoplasmic reticulum stress signaling pathway. Bioengineered 2022, 13, 9467–9481. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Beer, A.G.; Widschwendter, P.; Oberdanner, J.; Salzmann, K.; Sarg, B.; Lindner, H.; Herz, J.; Patsch, J.R.; Marschang, P. LRP1b shows restricted expression in human tissues and binds to several extracellular ligands, including fibrinogen and apoE-carrying lipoproteins. Atherosclerosis 2011, 216, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Vite, A.; Li, J.; Radice, G.L. New functions for alpha-catenins in health and disease: From cancer to heart regeneration. Cell Tissue Res. 2015, 360, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.C.; Bach, E.A. JAK/STAT signaling in stem cells and regeneration: From Drosophila to vertebrates. Development 2019, 146, dev167643. [Google Scholar] [CrossRef]
- Stine, R.R.; Matunis, E.L. JAK-STAT signaling in stem cells. Adv. Exp. Med. Biol. 2013, 786, 247–267. [Google Scholar] [CrossRef]
- Brawley, C.; Matunis, E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 2004, 304, 1331–1334. [Google Scholar] [CrossRef]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 161, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Caires, K.C.; de Avila, J.M.; Cupp, A.S.; McLean, D.J. VEGFA family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology 2012, 153, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Yang, S.; Zhu, Y.; Zou, S.; Li, P.; Wang, J.; Zhu, Z.; Huang, Y.; He, Z.; Li, Z. VEGF/VEGFR2 Signaling Regulates Germ Cell Proliferation in vitro and Promotes Mouse Testicular Regeneration in vivo. Cells Tissues Organs 2016, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.E.; Kim, D.; Kaucher, A.; Oatley, M.J.; Oatley, J.M. CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J. Cell Sci. 2013, 126, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, S.; Fissore, R. Vertebrate Reproduction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006064. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ signaling in mammalian spermatozoa. Mol. Cell. Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef] [PubMed]
- Gorczynska-Fjalling, E. The role of calcium in signal transduction processes in Sertoli cells. Reprod. Biol. 2004, 4, 219–241. [Google Scholar]
- Wang, G.; Shao, S.H.; Weng, C.C.; Wei, C.; Meistrich, M.L. Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol. Sci. 2010, 117, 225–237. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, S.; Meistrich, M.L. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J. Cell. Physiol. 2007, 211, 149–158. [Google Scholar] [CrossRef]
- Shetty, G.; Mitchell, J.M.; Meyer, J.M.; Wu, Z.; Lam, T.N.A.; Phan, T.T.; Zhang, J.; Hill, L.; Tailor, R.C.; Peters, K.A.; et al. Restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation. Andrology 2020, 8, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Uthamanthil, R.K.; Zhou, W.; Shao, S.H.; Weng, C.C.; Tailor, R.C.; Hermann, B.P.; Orwig, K.E.; Meistrich, M.L. Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology 2013, 1, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Ichtchenko, K.; Sudhof, T.C.; Brose, N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc. Natl. Acad. Sci. USA 1999, 96, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Ichtchenko, K.; Hata, Y.; Nguyen, T.; Ullrich, B.; Missler, M.; Moomaw, C.; Sudhof, T.C. Neuroligin 1: A splice site-specific ligand for beta-neurexins. Cell 1995, 81, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Motazacker, M.M.; Rost, B.R.; Hucho, T.; Garshasbi, M.; Kahrizi, K.; Ullmann, R.; Abedini, S.S.; Nieh, S.E.; Amini, S.H.; Goswami, C.; et al. A defect in the ionotropic glutamate receptor 6 gene (GRIK2) is associated with autosomal recessive mental retardation. Am. J. Hum. Genet. 2007, 81, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Low, C.M.; Moody, O.A.; Jenkins, A.; Traynelis, S.F. Ionotropic GABA and Glutamate Receptor Mutations and Human Neurologic Diseases. Mol. Pharmacol. 2015, 88, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Guzman, Y.F.; Ramsey, K.; Stolz, J.R.; Craig, D.W.; Huentelman, M.J.; Narayanan, V.; Swanson, G.T. A gain-of-function mutation in the GRIK2 gene causes neurodevelopmental deficits. Neurol. Genet. 2017, 3, e129. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lewandoski, M.; Perantoni, A.O.; Kurebayashi, S.; Nakanishi, G.; Jetten, A.M. Identification of Glis1, a novel Gli-related, Kruppel-like zinc finger protein containing transactivation and repressor functions. J. Biol. Chem. 2002, 277, 30901–30913. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, X.; Li, H.; Fan, J.; Qian, X.; Li, H.; Xu, Y. Phospholipase D as a key modulator of cancer progression. Biol. Rev. Camb. Philos. Soc. 2020, 95, 911–935. [Google Scholar] [CrossRef]
- Foster, D.A.; Xu, L. Phospholipase D in cell proliferation and cancer. Mol. Cancer Res. 2003, 1, 789–800. [Google Scholar]
- Jing, S.; Yu, Y.; Fang, M.; Hu, Z.; Holst, P.L.; Boone, T.; Delaney, J.; Schultz, H.; Zhou, R.; Fox, G.M. GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family. J. Biol. Chem. 1997, 272, 33111–33117. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xie, J.; Fei, Y.; Gao, P.; Xie, Q.; Gao, W.; Xu, Z. GDNF family receptor alpha 2 promotes neuroblastoma cell proliferation by interacting with PTEN. Biochem. Biophys. Res. Commun. 2019, 510, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Chen, C.; Yan, S.; Yang, A.; Deng, Y.; Chen, B.; Gu, J. Single-Nucleus RNA-Seq Reveals Spermatogonial Stem Cell Developmental Pattern in Shaziling Pigs. Biomolecules 2024, 14, 607. https://doi.org/10.3390/biom14060607
Tang X, Chen C, Yan S, Yang A, Deng Y, Chen B, Gu J. Single-Nucleus RNA-Seq Reveals Spermatogonial Stem Cell Developmental Pattern in Shaziling Pigs. Biomolecules. 2024; 14(6):607. https://doi.org/10.3390/biom14060607
Chicago/Turabian StyleTang, Xiangwei, Chujie Chen, Saina Yan, Anqi Yang, Yanhong Deng, Bin Chen, and Jingjing Gu. 2024. "Single-Nucleus RNA-Seq Reveals Spermatogonial Stem Cell Developmental Pattern in Shaziling Pigs" Biomolecules 14, no. 6: 607. https://doi.org/10.3390/biom14060607
APA StyleTang, X., Chen, C., Yan, S., Yang, A., Deng, Y., Chen, B., & Gu, J. (2024). Single-Nucleus RNA-Seq Reveals Spermatogonial Stem Cell Developmental Pattern in Shaziling Pigs. Biomolecules, 14(6), 607. https://doi.org/10.3390/biom14060607




