Efficacy of the Probiotic L. brevis in Counteracting the Demineralizing Process of the Tooth Enamel Surface: Results from an In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Tooth Collection and Selection and Specimen Preparation
2.2. Preparation of the Artificial Saliva
2.3. Preparation of L. brevis Suspension
2.4. Preparation of the Demineralizing Solution
2.5. In Vitro Treatments
- Group 1: The specimens were immersed in 4 mL of artificial saliva, which was replaced daily with fresh artificial saliva. This protocol was repeated each day for five days.
- Group 2: The specimens were treated with the probiotic solution at 16.6 × 106 CFU/mL for 6 h/day, after which they were extensively washed and incubated in fresh artificial saliva for another 18 h. This protocol was repeated each day for five days.
- Group 3: The specimens were immersed for 2 min in the DA (6% citric acid), after which they were extensively washed and incubated in fresh artificial saliva for 24 h. This protocol was repeated each day for five days.
- Group 4: The specimens were immersed for 2 min in the DA (6% citric acid) and then in probiotic suspension (16.6 × 106 CFU/mL) for 6 h, after which they were extensively washed and incubated in artificial saliva for another 18 h. This protocol was repeated each day for five days.
2.6. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.7. Statistical Analysis
3. Results
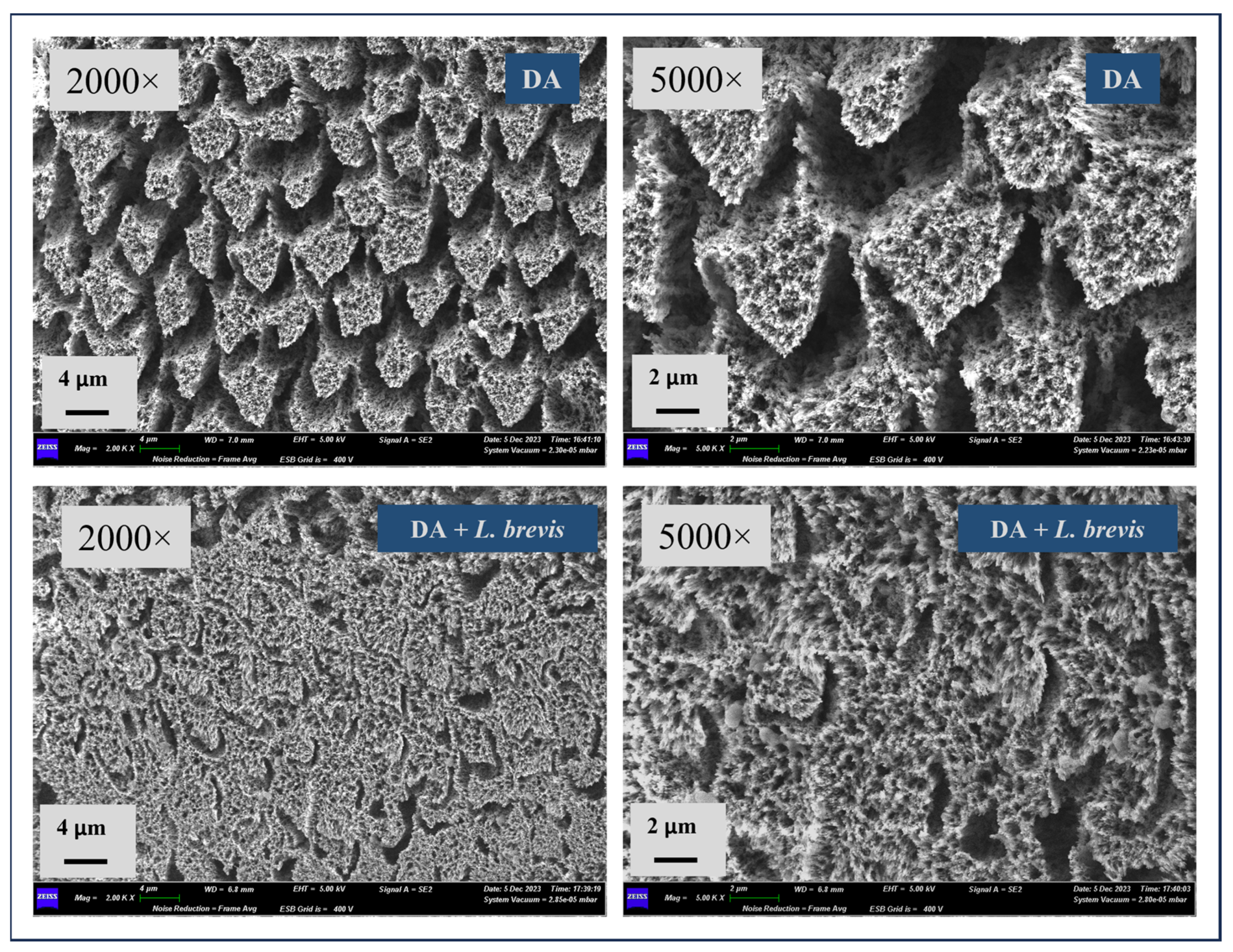
3.1. Effect of L. brevis on the Surface Morphology of Dental Enamel
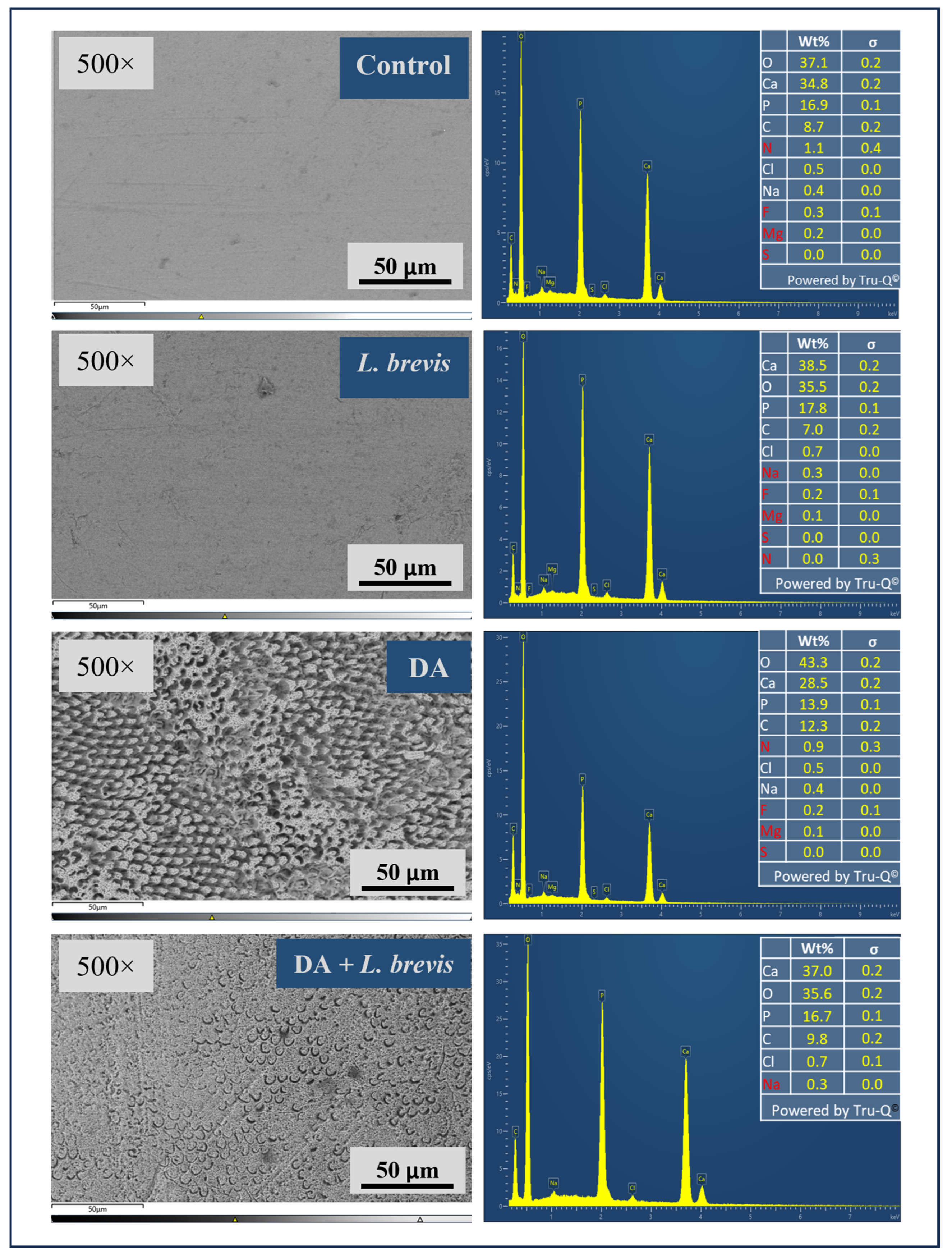
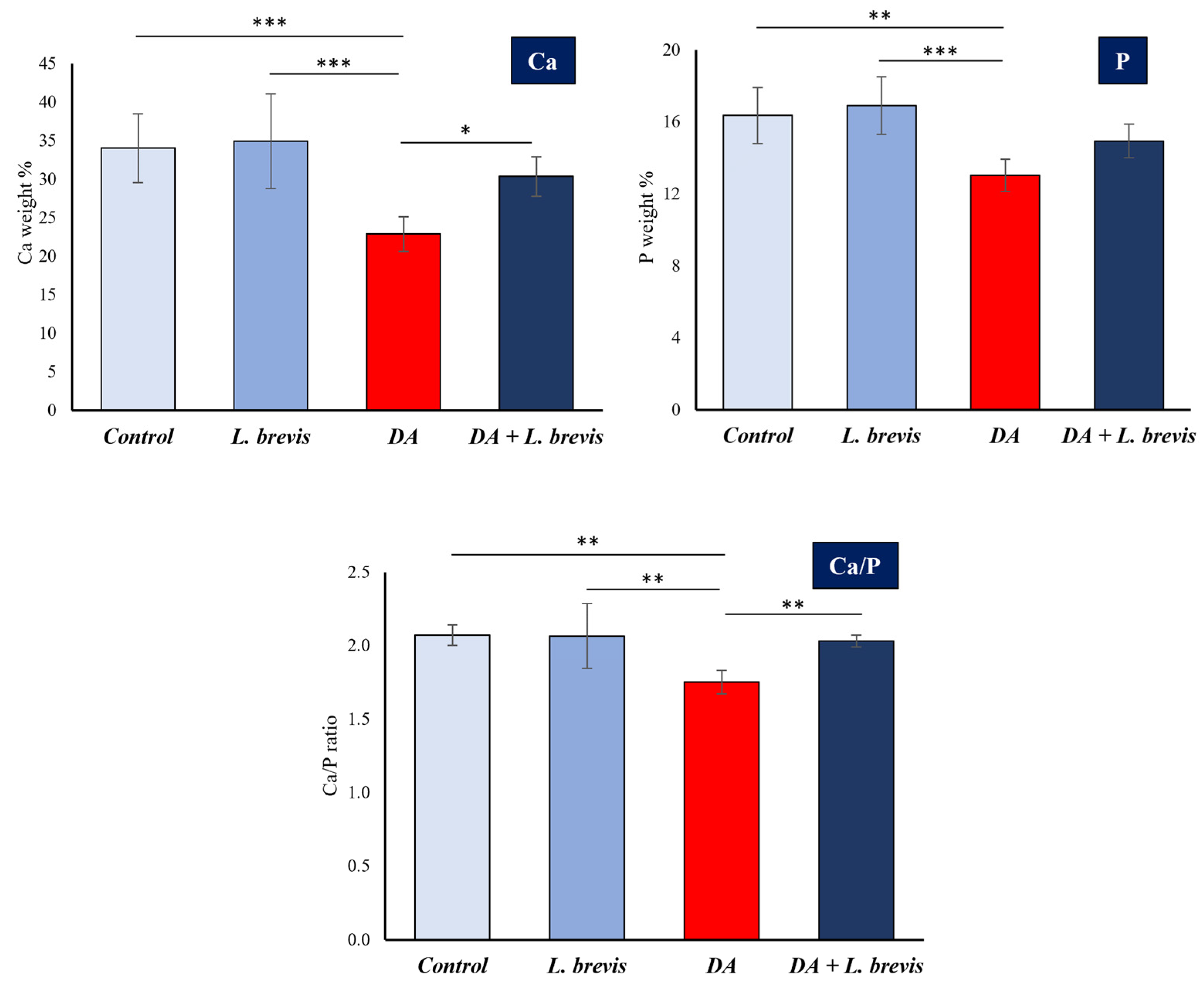
3.2. Effect of L. brevis on the Elemental Composition of Dental Enamel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitts, N.B.; Twetman, S.; Fisher, J.; Marsh, P.D. Understanding dental caries as a non-communicable disease. Br. Dent. J. 2021, 231, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Schreiner, H. Oral microbial interactions from an ecological perspective: A narrative review. Front. Oral Health 2023, 4, 1229118. [Google Scholar] [CrossRef] [PubMed]
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Bidlack, F.B. Tooth Enamel and its Dynamic Protein Matrix. Int. J. Mol. Sci. 2020, 21, 4458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Wang, W.; Ma, J.; Zhang, M.; Lu, X.; Liu, J.; Kou, Y. The rationale and potential for using Lactobacillus in the management of periodontitis. J. Microbiol. 2022, 60, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022, 10, e635. [Google Scholar] [CrossRef]
- Rwubuzizi, R.; Kim, H.; Holzapfel, W.H.; Todorov, S.D. Beneficial, safety, and antioxidant properties of lactic acid bacteria: A next step in their evaluation as potential probiotics. Heliyon 2023, 9, e15610. [Google Scholar] [CrossRef]
- Fijan, S. Probiotics and Their Antimicrobial Effect. Microorganisms 2023, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Petrariu, O.A.; Barbu, I.C.; Niculescu, A.G.; Constantin, M.; Grigore, G.A.; Cristian, R.E.; Mihaescu, G.; Vrancianu, C.O. Role of probiotics in managing various human diseases, from oral pathology to cancer and gastrointestinal diseases. Front. Microbiol. 2023, 14, 1296447. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Del Pinto, R.; Pietropaoli, D.; Ferri, C. Oral health as a modifiable risk factor for cardiovascular diseases. Trends Cardiovasc. Med. 2024, 34, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Altamura, S.; Pietropaoli, D.; Lombardi, F.; Del Pinto, R.; Ferri, C. An Overview of Chronic Kidney Disease Pathophysiology: The Impact of Gut Dysbiosis and Oral Disease. Biomedicines 2023, 11, 3033. [Google Scholar] [CrossRef] [PubMed]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef] [PubMed]
- Gruner, D.; Paris, S.; Schwendicke, F. Probiotics for managing caries and periodontitis: Systematic review and meta-analysis. J. Dent. 2016, 48, 16–25. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Campus, G.; Cocco, F.; Carta, G.; Cagetti, M.G.; Simark-Mattson, C.; Strohmenger, L.; Lingstrom, P. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin. Oral Investig. 2014, 18, 555–561. [Google Scholar] [CrossRef]
- Lai, S.; Lingstrom, P.; Cagetti, M.G.; Cocco, F.; Meloni, G.; Arrica, M.A.; Campus, G. Effect of Lactobacillus brevis CD2 containing lozenges and plaque pH and cariogenic bacteria in diabetic children: A randomised clinical trial. Clin. Oral Investig. 2021, 25, 115–123. [Google Scholar] [CrossRef]
- Riccia, D.N.; Bizzini, F.; Perilli, M.G.; Polimeni, A.; Trinchieri, V.; Amicosante, G.; Cifone, M.G. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007, 13, 376–385. [Google Scholar] [CrossRef]
- Ierardo, G.; Bossu, M.; Tarantino, D.; Trinchieri, V.; Sfasciotti, G.L.; Polimeni, A. The arginine-deiminase enzymatic system on gingivitis: Preliminary pediatric study. Ann. Stomatol. 2010, 1, 8–13. [Google Scholar]
- Maekawa, T.; Hajishengallis, G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014, 49, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, S.J.; Ko, S.H.; Ouwehand, A.C.; Ma, D.S. Modulation of the host response by probiotic Lactobacillus brevis CD2 in experimental gingivitis. Oral Dis. 2015, 21, 705–712. [Google Scholar] [CrossRef]
- Shah, M.P.; Gujjari, S.K.; Chandrasekhar, V.S. Long-term effect of Lactobacillus brevis CD2 (Inersan((R))) and/or doxycycline in aggressive periodontitis. J. Indian Soc. Periodontol. 2017, 21, 341–343. [Google Scholar] [CrossRef]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef]
- Sharma, A.; Tilak, T.; Bakhshi, S.; Raina, V.; Kumar, L.; Chaudhary, S.; Sahoo, R.; Gupta, R.; Thulkar, S. Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem cell transplantation. ESMO Open 2016, 1, e000138. [Google Scholar] [CrossRef] [PubMed]
- Tasli, L.; Mat, C.; De Simone, C.; Yazici, H. Lactobacilli lozenges in the management of oral ulcers of Behcet’s syndrome. Clin. Exp. Rheumatol. 2006, 24, S83–S86. [Google Scholar]
- Niscola, P.; Tendas, A.; Scaramucci, L.; Giovannini, M.; Trinchieri, V.; De Fabritiis, P. Aphthous oral ulceration and its successful management by Lactobacillus brevis CD2 extract in an adult haemophilic patient. Haemophilia 2012, 18, e78–e79. [Google Scholar] [CrossRef]
- Di Marzio, L.; Russo, F.P.; D’Alo, S.; Biordi, L.; Ulisse, S.; Amicosante, G.; De Simone, C.; Cifone, M.G. Apoptotic effects of selected strains of lactic acid bacteria on a human T leukemia cell line are associated with bacterial arginine deiminase and/or sphingomyelinase activities. Nutr. Cancer 2001, 40, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Marquis, R.E.; Bender, G.R.; Murray, D.R.; Wong, A. Arginine deiminase system and bacterial adaptation to acid environments. Appl. Environ. Microbiol. 1987, 53, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.M.; Gordan, V.V.; Garvan, C.W.; Browngardt, C.M.; Burne, R.A. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol. Immunol. 2009, 24, 89–95. [Google Scholar] [CrossRef]
- Gordan, V.V.; Garvan, C.W.; Ottenga, M.E.; Schulte, R.; Harris, P.A.; McEdward, D.; Magnusson, I. Could alkali production be considered an approach for caries control? Caries Res. 2010, 44, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Reyes, E.; Martin, J.; Moncada, G.; Neira, M.; Palma, P.; Gordan, V.; Oyarzo, J.F.; Yevenes, I. Caries-free subjects have high levels of urease and arginine deiminase activity. J. Appl. Oral Sci. 2014, 22, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Sezen, M.; Ow-Yang, C.; Karahan, O.; Kitiki, B. Micro and nanostructural analysis of a human tooth using correlated focused ion beam (FIB) and transmission Electron microscopy (TEM) investigations. Micron 2018, 115, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sarna-Bos, K.; Skic, K.; Boguta, P.; Adamczuk, A.; Vodanovic, M.; Chalas, R. Elemental mapping of human teeth enamel, dentine and cementum in view of their microstructure. Micron 2023, 172, 103485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Boyes, V.; Festy, F.; Lynch, R.J.M.; Watson, T.F.; Banerjee, A. In-vitro subsurface remineralisation of artificial enamel white spot lesions pre-treated with chitosan. Dent. Mater. 2018, 34, 1154–1167. [Google Scholar] [CrossRef]
- Bakry, A.S.; Abbassy, M.A.; Alharkan, H.F.; Basuhail, S.; Al-Ghamdi, K.; Hill, R. A Novel Fluoride Containing Bioactive Glass Paste is Capable of Re-Mineralizing Early Caries Lesions. Materials 2018, 11, 1636. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F.; Rafiee, A.; Soltani, M.; Dashti, M.H. Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral Health 2019, 19, 92. [Google Scholar] [CrossRef]
- Thimmaiah, C.; Shetty, P.; Shetty, S.B.; Natarajan, S.; Thomas, N.A. Comparative analysis of the remineralization potential of CPP-ACP with Fluoride, Tri-Calcium Phosphate and Nano Hydroxyapatite using SEM/EDX—An in vitro study. J. Clin. Exp. Dent. 2019, 11, e1120–e1126. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.M.; Erfan, D.; Abou El Fadl, R.K. Comparative evaluation of the effects of human breast milk and plain and probiotic-containing infant formulas on enamel mineral content in primary teeth: An in vitro study. Eur. Arch. Paediatr. Dent. 2020, 21, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Balhaddad, A.A.; Garcia, I.M.; Collares, F.M.; Weir, M.D.; Xu, H.H.K.; Melo, M.A.S. pH-responsive calcium and phosphate-ion releasing antibacterial sealants on carious enamel lesions in vitro. J. Dent. 2020, 97, 103323. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; Pessan, J.P.; Romero, G.D.A.; Cannon, M.L.; Danelon, M. Effect of fluoride, casein phosphopeptide-amorphous calcium phosphate and sodium trimetaphosphate combination treatment on the remineralization of caries lesions: An in vitro study. Arch. Oral Biol. 2021, 122, 105001. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, F.; Tosco, V.; Monterubbianesi, R.; Orilisi, G.; Gatto, M.L.; Sparabombe, S.; Meme, L.; Mengucci, P.; Putignano, A.; Orsini, G. Remineralization Efficacy of Four Remineralizing Agents on Artificial Enamel Lesions: SEM-EDS Investigation. Materials 2022, 15, 4398. [Google Scholar] [CrossRef] [PubMed]
- Rodemer, T.; Putz, N.; Hannig, M. Influence of hydroxyapatite nanoparticles on the formation of calcium fluoride surface layer on enamel and dentine in vitro. Sci. Rep. 2022, 12, 17612. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.M.; El-Sayed, S.M.; El-Sayed, H.S.; Youssef, A.M. Laboratory evaluation of anti-plaque and remineralization efficacy of sugarless probiotic jelly candy supplemented with natural nano prebiotic additive. Sci. Rep. 2023, 13, 10977. [Google Scholar] [CrossRef] [PubMed]
- Coradin, T.; Porporatti, A.; Bosco, J. Assessing in vitro remineralization of primary artificial caries: A systematic review of multi-techniques characterization approaches. Dent. Rev. 2023, 3, 100073. [Google Scholar] [CrossRef]
- Kucukyilmaz, E.; Savas, S. Measuring the remineralization potential of different agents with quantitative light-induced fluorescence digital Biluminator. J. Appl. Biomater. Funct. Mater. 2017, 15, e101–e106. [Google Scholar] [CrossRef]
- Amaechi, B.T. Protocols to Study Dental Caries In Vitro: pH Cycling Models. Methods Mol. Biol. 2019, 1922, 379–392. [Google Scholar] [CrossRef]
- Beighton, D. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent. Oral Epidemiol. 2005, 33, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chopra, A.; Kamath, S.U.; Kashyap, N.N. Can acid produced from probiotic bacteria alter the surface roughness, microhardness, and elemental composition of enamel? An in vitro study. Odontology 2023, 111, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xu, J.; Li, Q.; Xia, X.; Du, G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018, 18, 221. [Google Scholar] [CrossRef] [PubMed]
- Vuotto, C.; Barbanti, F.; Mastrantonio, P.; Donelli, G. Lactobacillus brevis CD2 inhibits Prevotella melaninogenica biofilm. Oral Dis. 2014, 20, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.J. A comparative study of the effect of probiotics on cariogenic biofilm model for preventing dental caries. Arch. Microbiol. 2014, 196, 601–609. [Google Scholar] [CrossRef]
- Alfano, A.; Perillo, F.; Fusco, A.; Savio, V.; Corsaro, M.M.; Donnarumma, G.; Schiraldi, C.; Cimini, D. Lactobacillus brevis CD2: Fermentation Strategies and Extracellular Metabolites Characterization. Probiotics Antimicrob. Proteins 2020, 12, 1542–1554. [Google Scholar] [CrossRef]
- Cunha-Cruz, J.; Scott, J.; Rothen, M.; Mancl, L.; Lawhorn, T.; Brossel, K.; Berg, J.; Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry. Salivary characteristics and dental caries: Evidence from general dental practices. J. Am. Dent. Assoc. 2013, 144, e31–e40. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamura, S.; Augello, F.R.; Ortu, E.; Pietropaoli, D.; Cinque, B.; Giannoni, M.; Lombardi, F. Efficacy of the Probiotic L. brevis in Counteracting the Demineralizing Process of the Tooth Enamel Surface: Results from an In Vitro Study. Biomolecules 2024, 14, 605. https://doi.org/10.3390/biom14050605
Altamura S, Augello FR, Ortu E, Pietropaoli D, Cinque B, Giannoni M, Lombardi F. Efficacy of the Probiotic L. brevis in Counteracting the Demineralizing Process of the Tooth Enamel Surface: Results from an In Vitro Study. Biomolecules. 2024; 14(5):605. https://doi.org/10.3390/biom14050605
Chicago/Turabian StyleAltamura, Serena, Francesca Rosaria Augello, Eleonora Ortu, Davide Pietropaoli, Benedetta Cinque, Mario Giannoni, and Francesca Lombardi. 2024. "Efficacy of the Probiotic L. brevis in Counteracting the Demineralizing Process of the Tooth Enamel Surface: Results from an In Vitro Study" Biomolecules 14, no. 5: 605. https://doi.org/10.3390/biom14050605
APA StyleAltamura, S., Augello, F. R., Ortu, E., Pietropaoli, D., Cinque, B., Giannoni, M., & Lombardi, F. (2024). Efficacy of the Probiotic L. brevis in Counteracting the Demineralizing Process of the Tooth Enamel Surface: Results from an In Vitro Study. Biomolecules, 14(5), 605. https://doi.org/10.3390/biom14050605







