Synthesis of a Side Chain Alkyne Analogue of Sitosterol as a Chemical Probe for Imaging in Plant Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
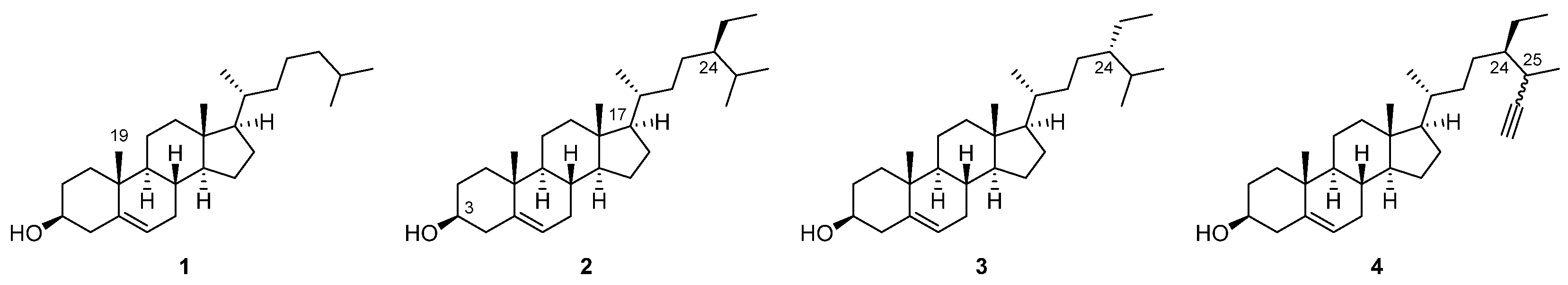
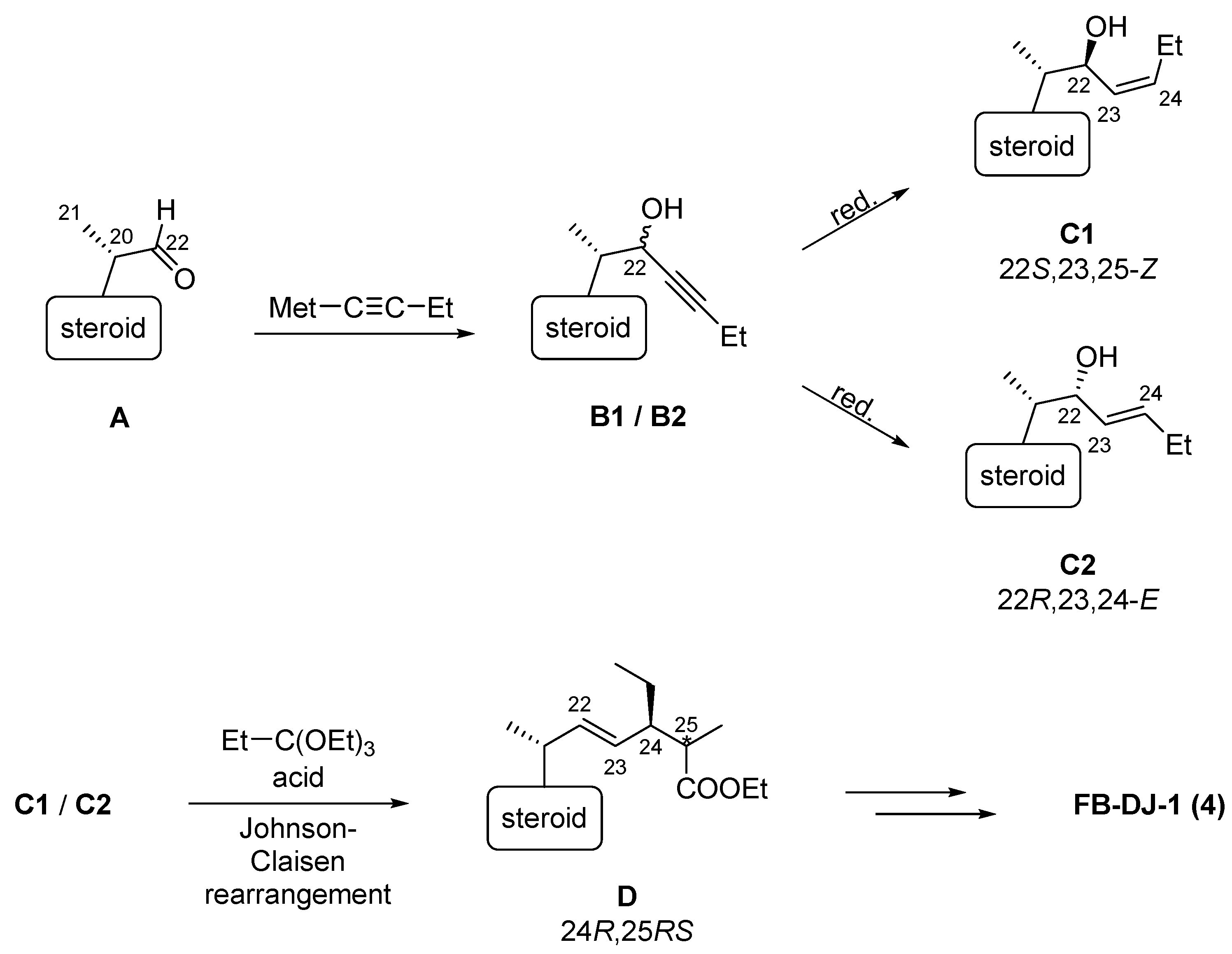
3.1. Synthesis Strategy
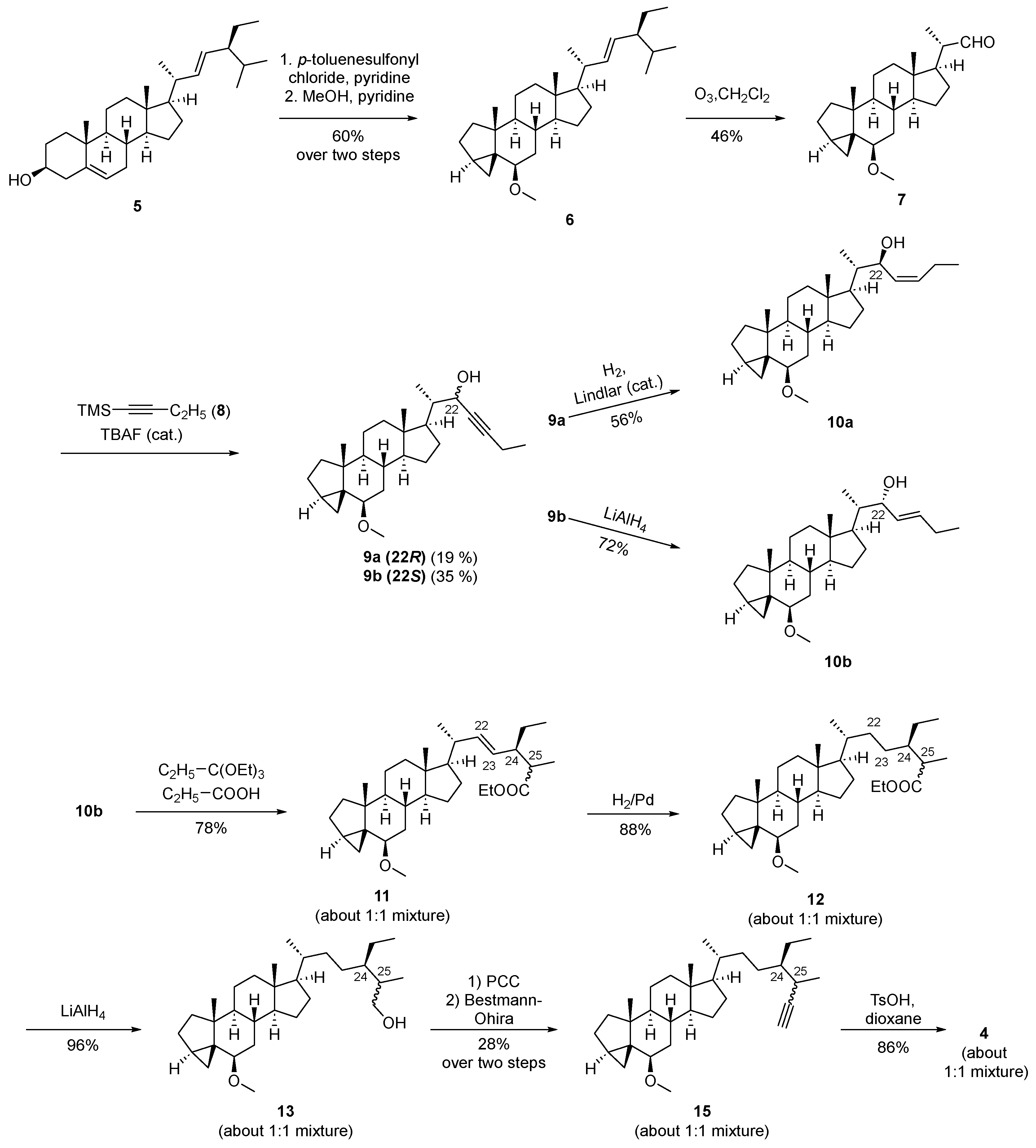
3.2. Chemistry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Binder, U.; Bracher, F.; Giera, M. Antifungal drug testing by combining minimal inhibitory concentration testing with target identification by gas chromatography-mass spectrometry. Nat. Protoc. 2017, 12, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Barghchi, H.; Pahlavani, N.; Barati, M.; Amiri, Y.; Fadel, A.; Khosravi, M.; Talebi, S.; Arzhang, P.; Ziaei, R.; et al. Biological and pharmacological effects and nutritional impact of phytosterols: A comprehensive review. Phytother. Res. 2022, 36, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, K.; Chen, X.; Wang, J.; Shu, G.; Ping, Z.; Zhang, S. Health outcomes associated with phytosterols: An umbrella review of systematic reviews and meta-analyses of randomized controlled trials. Phytomedicine 2024, 122, 155151. [Google Scholar] [CrossRef] [PubMed]
- Jao, C.Y.; Nedelcu, D.; Lopez, L.V.; Samarakoon, T.N.; Welti, R.; Salic, A. Bioorthogonal probes for imaging sterols in cells. ChemBiochem 2015, 16, 611–617. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc. Chem. Res. 2011, 44, 666–676. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Kacprzak, K.; Skiera, I.; Piasecka, M.; Paryzek, Z. Alkaloids and Isoprenoids Modification by Copper(I)-Catalyzed Huisgen 1,3-Dipolar Cycloaddition (Click Chemistry): Toward New Functions and Molecular Architectures. Chem. Rev. 2016, 116, 5689–5743. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhao, S.; Wang, X.; Liu, B.; Xu, H. Click Chemistry in Natural Product Modification. Front. Chem. 2021, 9, 774977. [Google Scholar] [CrossRef]
- Hu, J.; Lu, J.R.; Ju, Y. Steroid/triterpenoid functional molecules based on “click chemistry”. Chem. Asian J. 2011, 6, 2636–2647. [Google Scholar] [CrossRef]
- Chauhan, N.; Sere, Y.Y.; Sokol, A.M.; Graumann, J.; Menon, A.K. A PhotoClick cholesterol-based quantitative proteomics screen for cytoplasmic sterol-binding proteins in Saccharomyces cerevisiae. Yeast 2020, 37, 15–25. [Google Scholar] [CrossRef]
- Tallman, K.A.; Kim, H.H.; Korade, Z.; Genaro-Mattos, T.C.; Wages, P.A.; Liu, W.; Porter, N.A. Probes for protein adduction in cholesterol biosynthesis disorders: Alkynyl lanosterol as a viable sterol precursor. Redox Biol. 2017, 12, 182–190. [Google Scholar] [CrossRef]
- Windsor, K.; Genaro-Mattos, T.C.; Kim, H.-Y.H.; Liu, W.; Tallman, K.A.; Miyamoto, S.; Korade, Z.; Porter, N.A. Probing lipid-protein adduction with alkynyl surrogates: Application to Smith-Lemli-Opitz syndrome. J. Lipid Res. 2013, 54, 2842–2850. [Google Scholar] [CrossRef]
- Hofmann, K.; Thiele, C.; Schött, H.F.; Gaebler, A.; Schoene, M.; Kiver, Y.; Friedrichs, S.; Lütjohann, D.; Kuerschner, L. A novel alkyne cholesterol to trace cellular cholesterol metabolism and localization. J. Lipid Res. 2014, 55, 583–591. [Google Scholar] [CrossRef]
- Seck, I.; Fall, A.; Lago, C.; Sène, M.; Gaye, M.; Seck, M.; Gómez, G.; Fall, Y. Synthesis of a Cholesterol Side-Chain Triazole Analogue via ‘Click’ Chemistry. Synthesis 2015, 47, 2826–2830. [Google Scholar] [CrossRef][Green Version]
- Xiao, X.; Tang, J.J.; Peng, C.; Wang, Y.; Fu, L.; Qiu, Z.P.; Xiong, Y.; Yang, L.F.; Cui, H.W.; He, X.L.; et al. Cholesterol Modification of Smoothened Is Required for Hedgehog Signaling. Mol. Cell 2017, 66, 154–162.e10. [Google Scholar] [CrossRef]
- Sheng, R.; Luo, T.; Li, H.; Sun, J.; Wang, Z.; Cao, A. ‘Click’ synthesized sterol-based cationic lipids as gene carriers, and the effect of skeletons and headgroups on gene delivery. Bioorg. Med. Chem. 2013, 21, 6366–6377. [Google Scholar] [CrossRef]
- Hajdaś, G.; Kawka, A.; Koenig, H.; Kułaga, D.; Sosnowska, K.; Mrówczyńska, L.; Pospieszny, T. Click chemistry as a method for the synthesis of steroid bioconjugates of bile acids derivatives and sterols. Steroids 2023, 199, 109282. [Google Scholar] [CrossRef]
- Kawka, A.; Hajdaś, G.; Kułaga, D.; Koenig, H.; Kowalczyk, I.; Pospieszny, T. Molecular structure, spectral and theoretical study of new type bile acid–sterol conjugates linked via 1,2,3-triazole ring. J. Mol. Struct. 2023, 1273, 134313. [Google Scholar] [CrossRef]
- Darnet, S.; Blary, A.; Chevalier, Q.; Schaller, H. Phytosterol Profiles, Genomes and Enzymes—An Overview. Front. Plant Sci. 2021, 12, 665206. [Google Scholar] [CrossRef]
- Lomenick, B.; Shi, H.; Huang, J.; Chen, C. Identification and characterization of β-sitosterol target proteins. Bioorg. Med. Chem. Lett. 2015, 25, 4976–4979. [Google Scholar] [CrossRef]
- Yuan, J.-W.; Qu, L.-B. Efficient synthesis of novel β-sitosterol scaffolds containing 1,2,3-triazole via copper(I)-catalyzed click reaction under microwave irradiation. Z. Naturforsch. B 2017, 72, 717–724. [Google Scholar] [CrossRef]
- Ilkar Erdagi, S.; Uyanik, C. Biological evaluation of bioavailable amphiphilic polymeric conjugate based-on natural products: Diosgenin and curcumin. Int. J. Polym. Mater. Polym. Biomat. 2020, 69, 73–84. [Google Scholar] [CrossRef]
- Chapelon, A.-S.; Moraléda, D.; Rodriguez, R.; Ollivier, C.; Santelli, M. Enantioselective synthesis of steroids. Tetrahedron 2007, 63, 11511–11616. [Google Scholar] [CrossRef]
- Brill, Z.G.; Condakes, M.L.; Ting, C.P.; Maimone, T.J. Navigating the Chiral Pool in the Total Synthesis of Complex Terpene Natural Products. Chem. Rev. 2017, 117, 11753–11795. [Google Scholar] [CrossRef]
- Jastrzębska, I.; Covey, D.F. Chiral Building Blocks for Total Steroid Synthesis and the Use of Steroids as Chiral Building Blocks in Organic Synthesis. In Chiral Building Blocks in Asymmetric Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 367–387. [Google Scholar] [CrossRef]
- Wiersig, J.R.; Waespe-Sarcevic, N.; Djerassi, C. Stereospecific synthesis of the side chain of the steroidal plant sex hormone oogoniol. J. Org. Chem. 1979, 44, 3374–3382. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Kimura, M.; Khalifa, F.M.; Ikekawa, N. Side Chain Structural Requirement for Utilization of Sterols by the Silkworm for Growth and Development. Non-stereoselective Utilization of the 24-Stereoisomeric Pairs of 24-Alkylsterols. Chem. Pharm. Bull. 1984, 32, 4372–4381. [Google Scholar] [CrossRef]
- Partridge, J.J.; Faber, S.; Uskoković, M.R. Vitamin D3 metabolites. I. Synthesis of 25-hydroxycholesterol. Helv. Chim. Acta 1974, 57, 764–771. [Google Scholar] [CrossRef]
- Litvinovskaya, R.P.; Raiman, M.E.; Khripach, V.A. Synthesis of 28-homobrassinosteroids modified in the 26-position. Chem. Nat. Comp. 2009, 45, 647–652. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 7846, 1-Butyne. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1-Butyne (accessed on 12 February 2024).
- Nakamura, E.; Kuwajima, I. Fluoride Catalyzed Addition of Silylacetylenes to Carbonyl Compounds. Angew. Chem. Int. Ed. 1976, 15, 498–499. [Google Scholar] [CrossRef]
- Capani, J.S., Jr.; Cochran, J.E.; Liang, J. CsF-Mediated in Situ Desilylation of TMS-Alkynes for Sonogashira Reaction. J. Org. Chem. 2019, 84, 9378–9384. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Zercher, C.K. Studies on DNA-cleaving agents: Synthesis of a functional dynemicin analogue. J. Am. Chem. Soc. 1991, 113, 2311–2313. [Google Scholar] [CrossRef]
- Nishikawa, T.; Ino, A.; Isobe, M.; Goto, T. Synthesis of a Simple Model Compound of Dynemicin and Cycloaromatization with Pinacol-Pinacolone Rearrangement in the Strained Enediyne Medium Ring. Chem. Lett. 2006, 20, 1271–1274. [Google Scholar] [CrossRef]
- Nishikawa, T.; Yoshikai, M.; Obi, K.; Isobe, M. Synthesis of both enantiomers of dynemicin a model compound. New remote asymmetric induction in acetylide addition into quinoline nucleus as key step. Tetrahedron Lett. 1994, 35, 7997–8000. [Google Scholar] [CrossRef]
- Horibe, I.; Nakai, H.; Sato, T.; Seo, S.; Takeda, K.i.; Takatsuto, S. Stereoselective synthesis of the C-24 and C-25 stereoisomeric pairs of 24-ethyl-26-hydroxy- and 24-ethyl-[26-2H]sterols and their Δ22-derivatives: Reassignment of 13C n.m.r. signals of the pro-R and the pro-S methyl groups at C-25 of 24-ethylsterols. J. Chem. Soc. Perkin Trans. 1989, 1, 1957–1967. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Ohyama, K.; Kushiro, T.; Nakamura, K.; Fujimoto, Y. Biosynthesis of 20-hydroxyecdysone in Ajuga hairy roots: Fate of 6α- and 6β-hydrogens of lathosterol. Bioorg. Med. Chem. 1999, 7, 2925–2930. [Google Scholar] [CrossRef]
- Suto, T.; Yanagita, Y.; Nagashima, Y.; Takikawa, S.; Kurosu, Y.; Matsuo, N.; Sato, T.; Chida, N. Unified Total Synthesis of Madangamines A, C, and E. J. Am. Chem. Soc. 2017, 139, 2952–2955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollweck, M.; Jordan, D.; Bracher, F. Synthesis of a Side Chain Alkyne Analogue of Sitosterol as a Chemical Probe for Imaging in Plant Cells. Biomolecules 2024, 14, 542. https://doi.org/10.3390/biom14050542
Hollweck M, Jordan D, Bracher F. Synthesis of a Side Chain Alkyne Analogue of Sitosterol as a Chemical Probe for Imaging in Plant Cells. Biomolecules. 2024; 14(5):542. https://doi.org/10.3390/biom14050542
Chicago/Turabian StyleHollweck, Miriam, David Jordan, and Franz Bracher. 2024. "Synthesis of a Side Chain Alkyne Analogue of Sitosterol as a Chemical Probe for Imaging in Plant Cells" Biomolecules 14, no. 5: 542. https://doi.org/10.3390/biom14050542
APA StyleHollweck, M., Jordan, D., & Bracher, F. (2024). Synthesis of a Side Chain Alkyne Analogue of Sitosterol as a Chemical Probe for Imaging in Plant Cells. Biomolecules, 14(5), 542. https://doi.org/10.3390/biom14050542






