Testosterone Reduces Myelin Abnormalities in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Ethical Statement
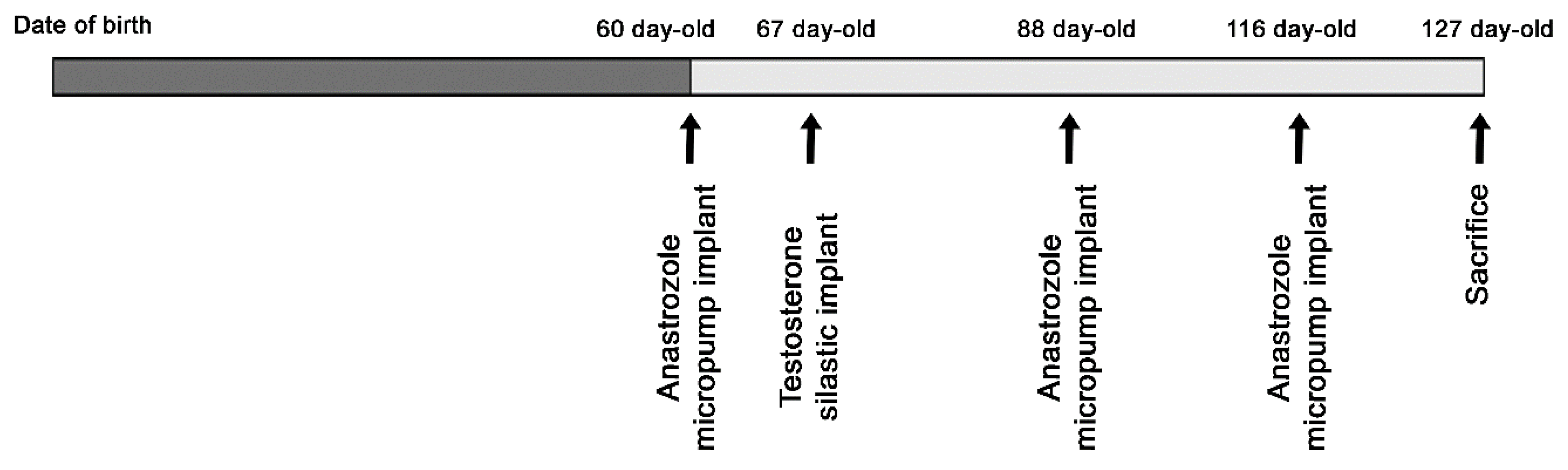
2.2. Treatment with Testosterone and Anastrozole, an Aromatase Inhibitor
2.3. Semithin Sections and Electron Microscopy Studies
2.4. Samples for Histological and Immunohistological Analyses
2.4.1. Luxol Fast Blue (LFB) Staining of Whole Myelin
2.4.2. Immunohistochemistry of Myelin Basic Protein (MBP) and Proteolipid Protein (PLP)
2.4.3. Immunohistochemical Analysis of Glutamine Synthetase (GS) and Its Colocalization with Glial Markers
2.4.4. Immunohistological Localization of Glutamate Transporter-1 (GLT-1)
2.4.5. Immunohistological Localization of the Oligodendrocyte Marker, CC1
2.5. Quantitative RT-PCR Analysis of mRNA Expression of Myelin Proteins, Inflammatory Factors and Glutamate Transporter
2.6. Clinical Assessments
2.7. Statistical Analysis
3. Results
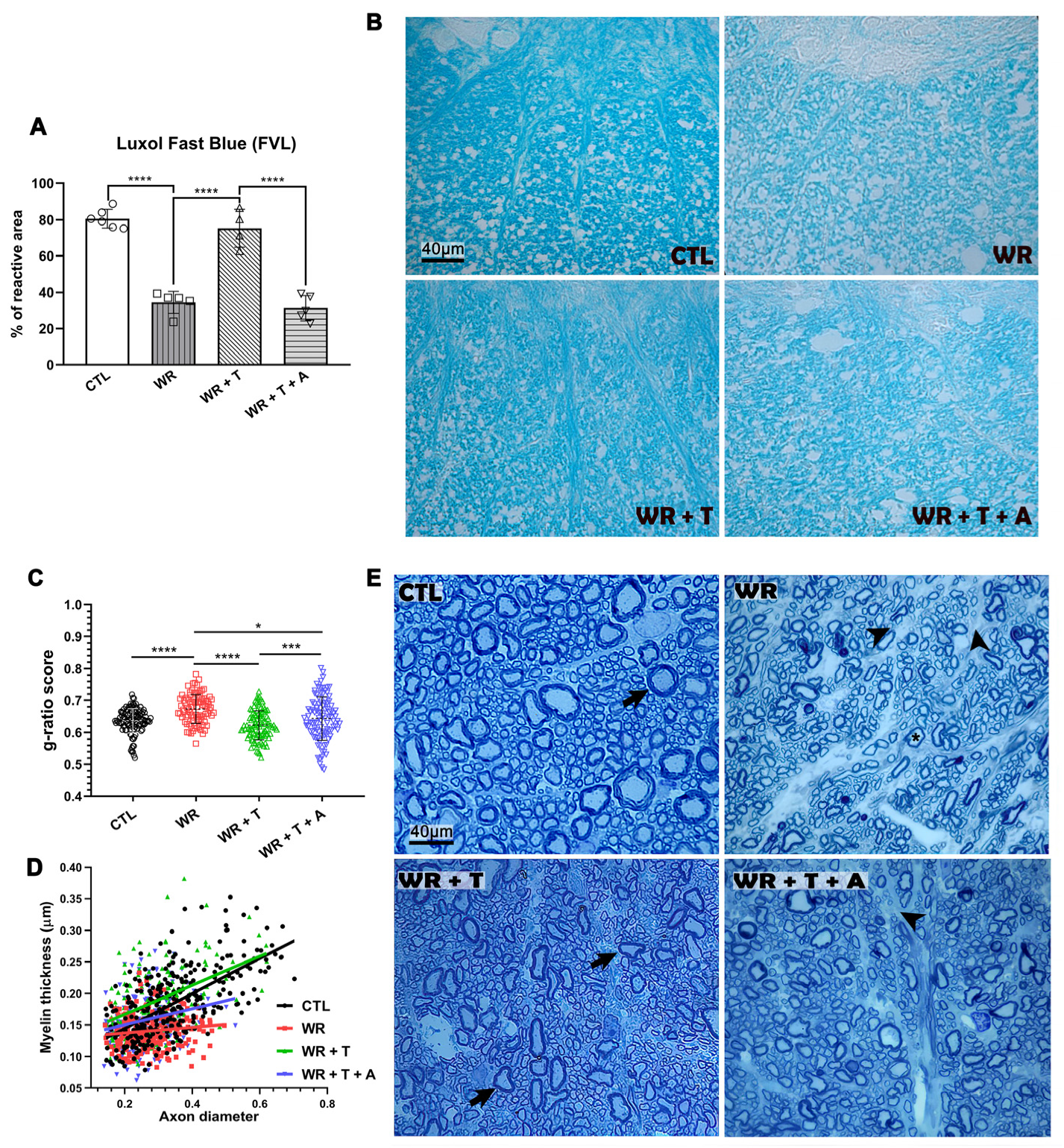
3.1. LFB Histochemistry Shows Preservation of Total Myelin in Testosterone-Treated WR Mice
3.2. Testosterone Restores the Thickness of Myelin Sheaths in Wobbler Mice
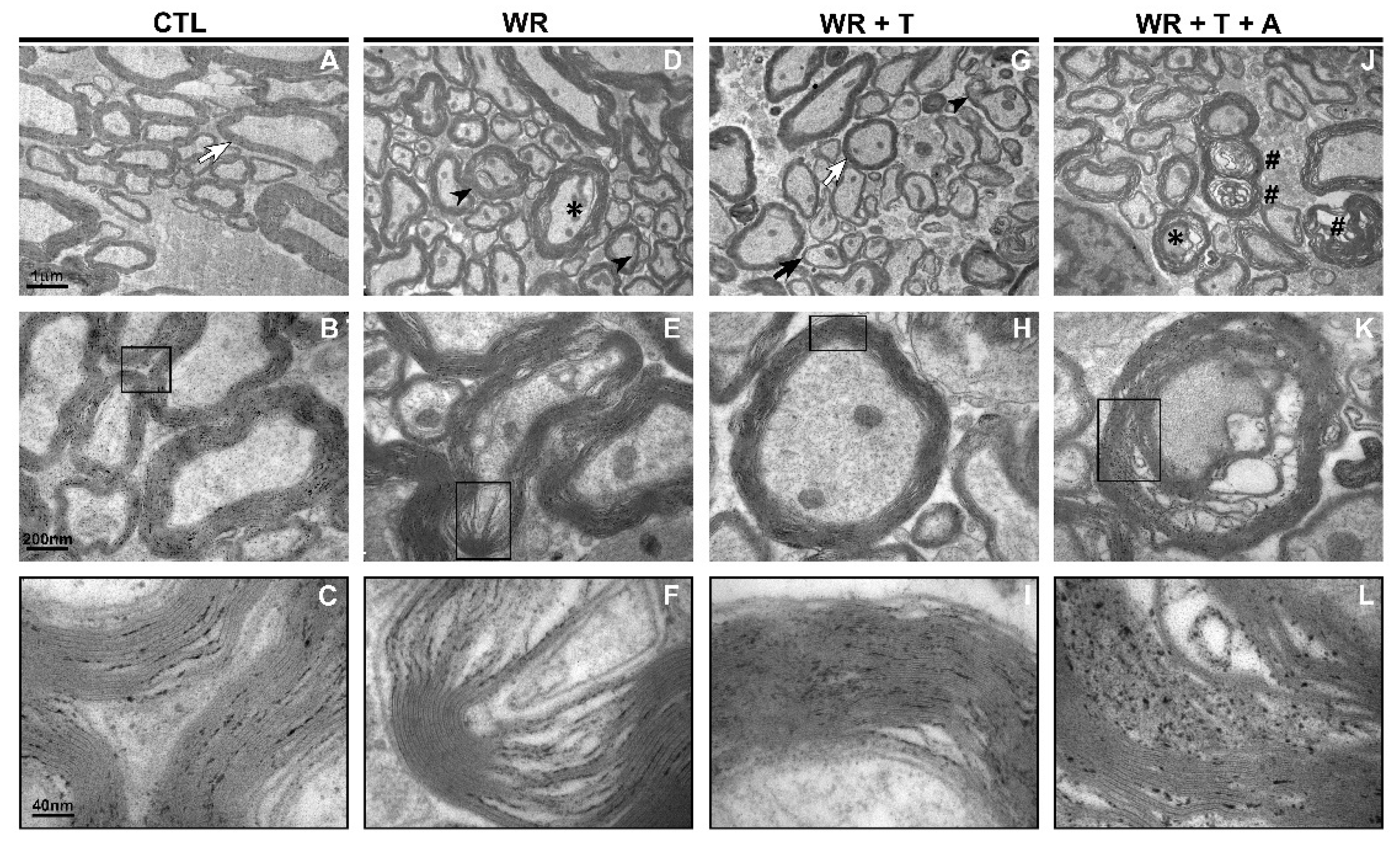
3.3. Testosterone Restores Axonal Myelination in WR Mice: Analysis by Using Electron Microscopy (EM)
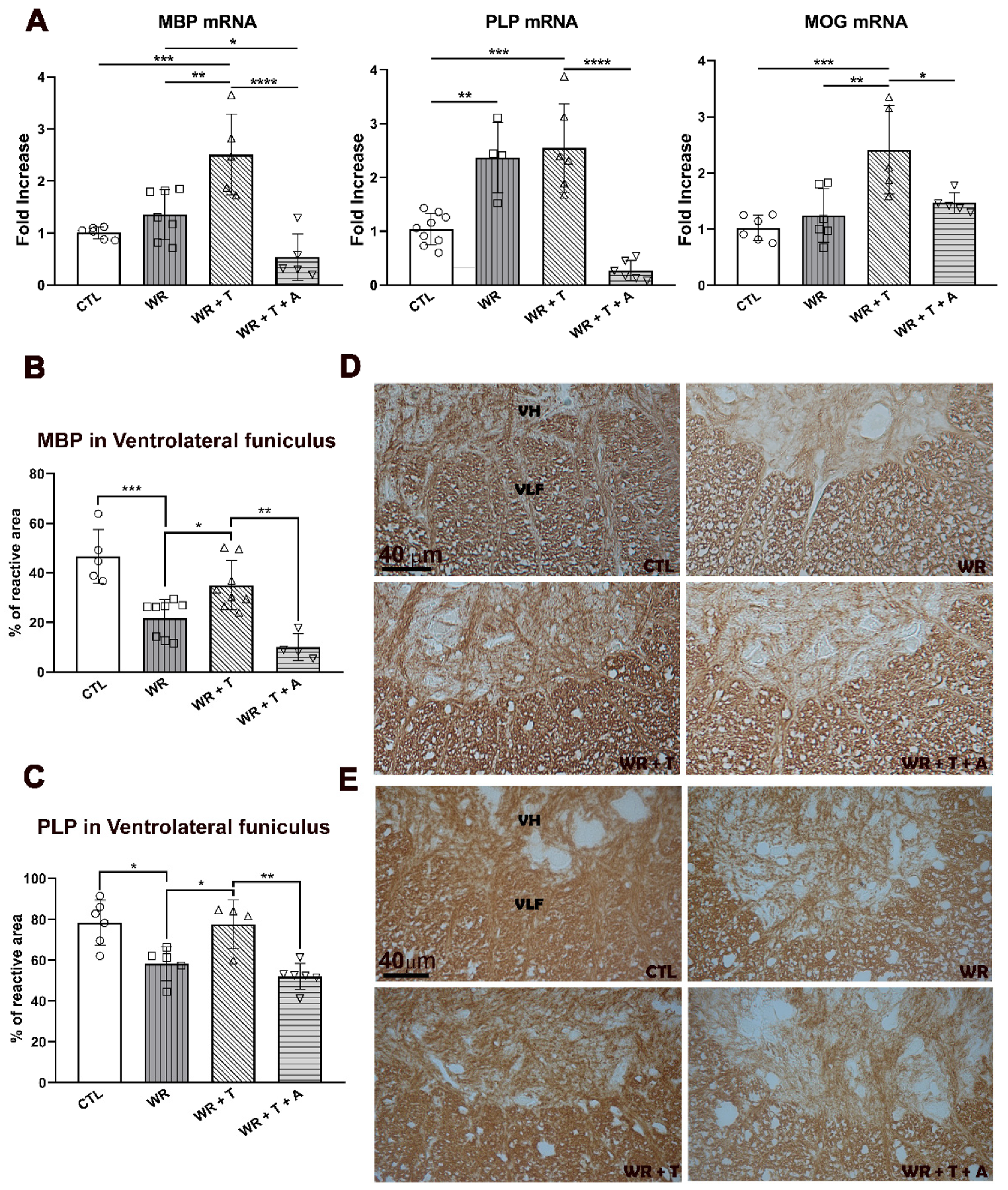
3.4. Testosterone Modulates the Expression of Myelin Proteins
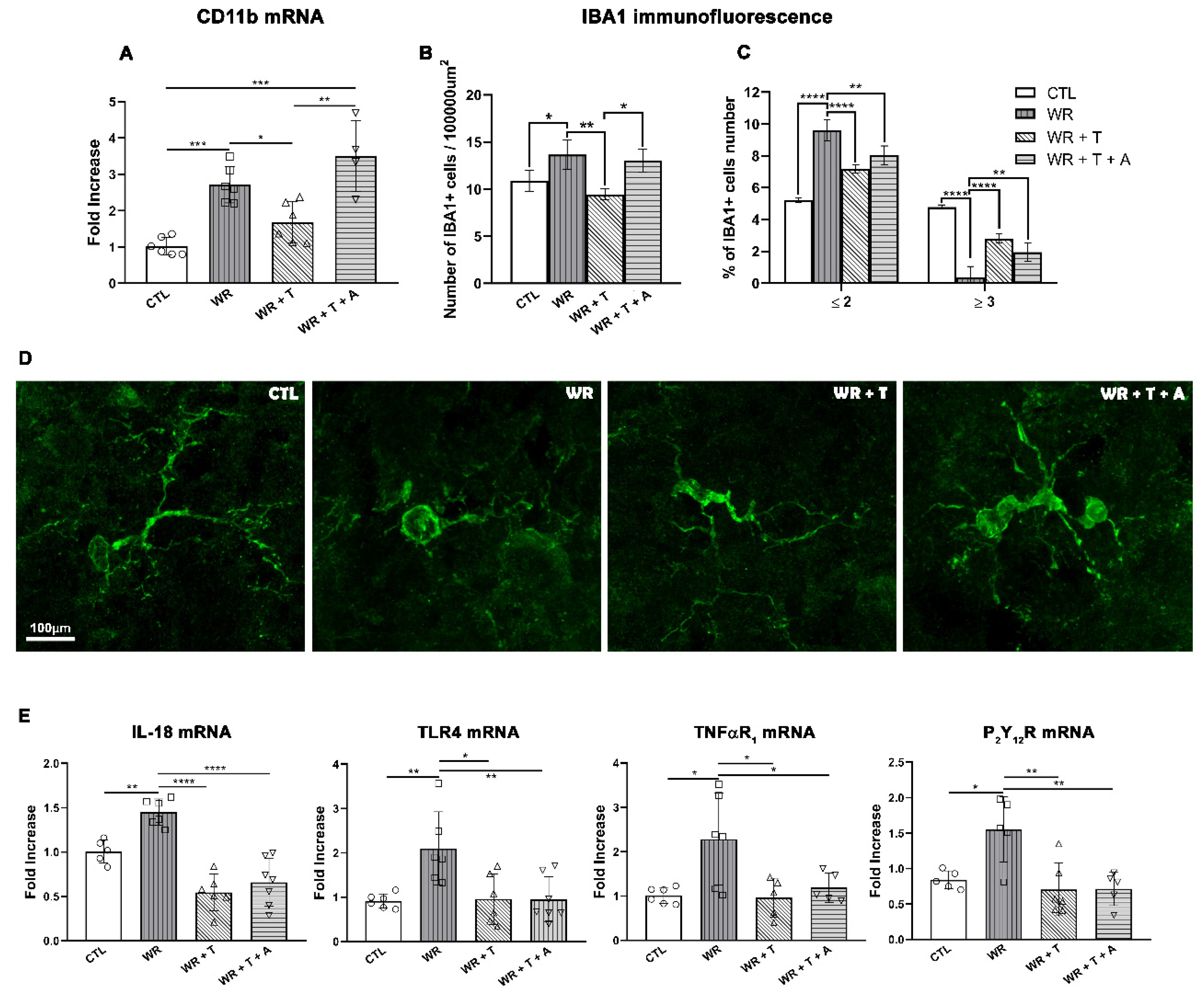
3.5. Testosterone Reduces the Activation of Microglia and the Expression of Proinflammatory Mediators in the Wobbler Spinal Cord
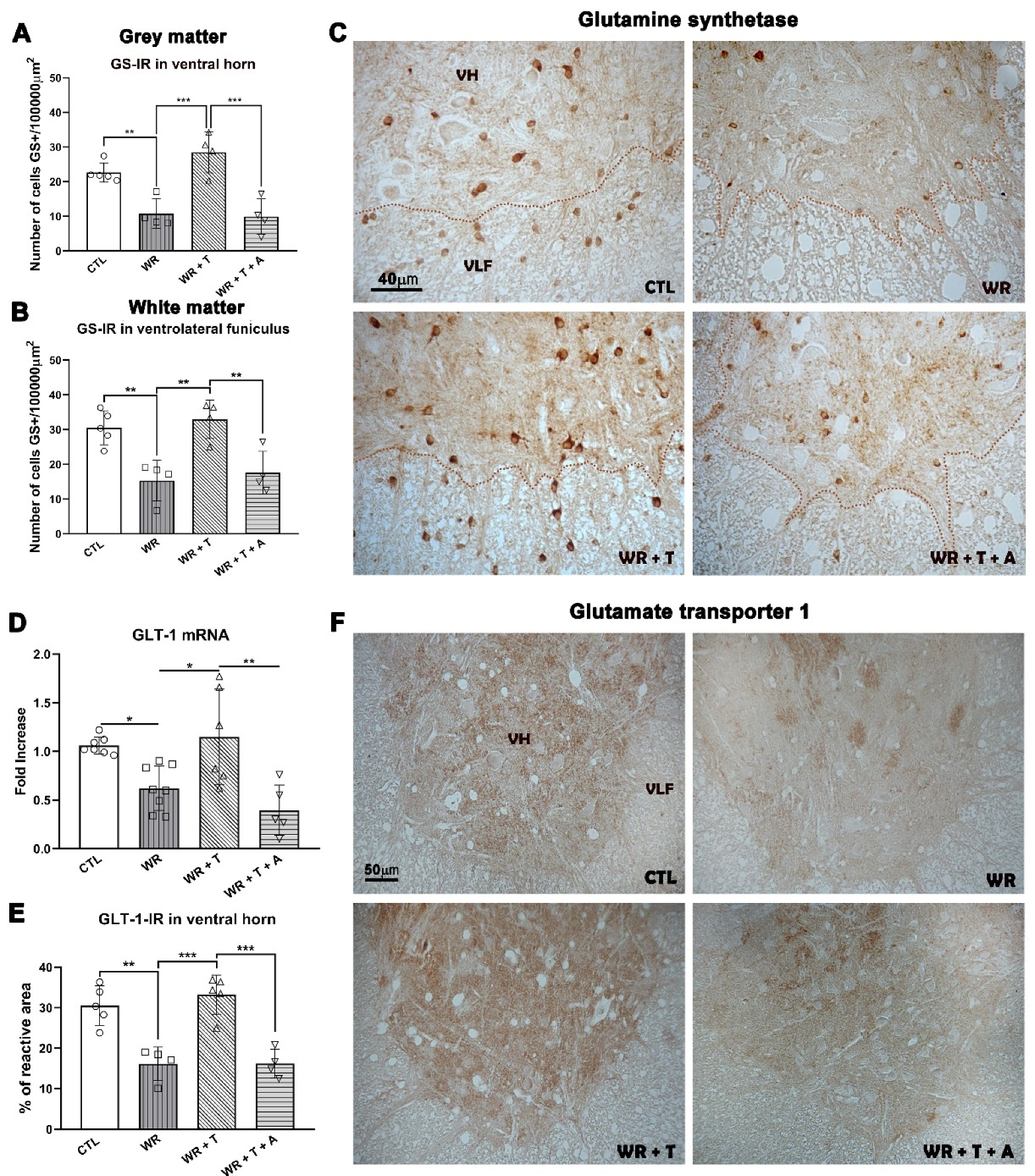
3.6. Effects of Testosterone and Anastrozole on Glutamine Synthetase in the Cervical Spinal Cord
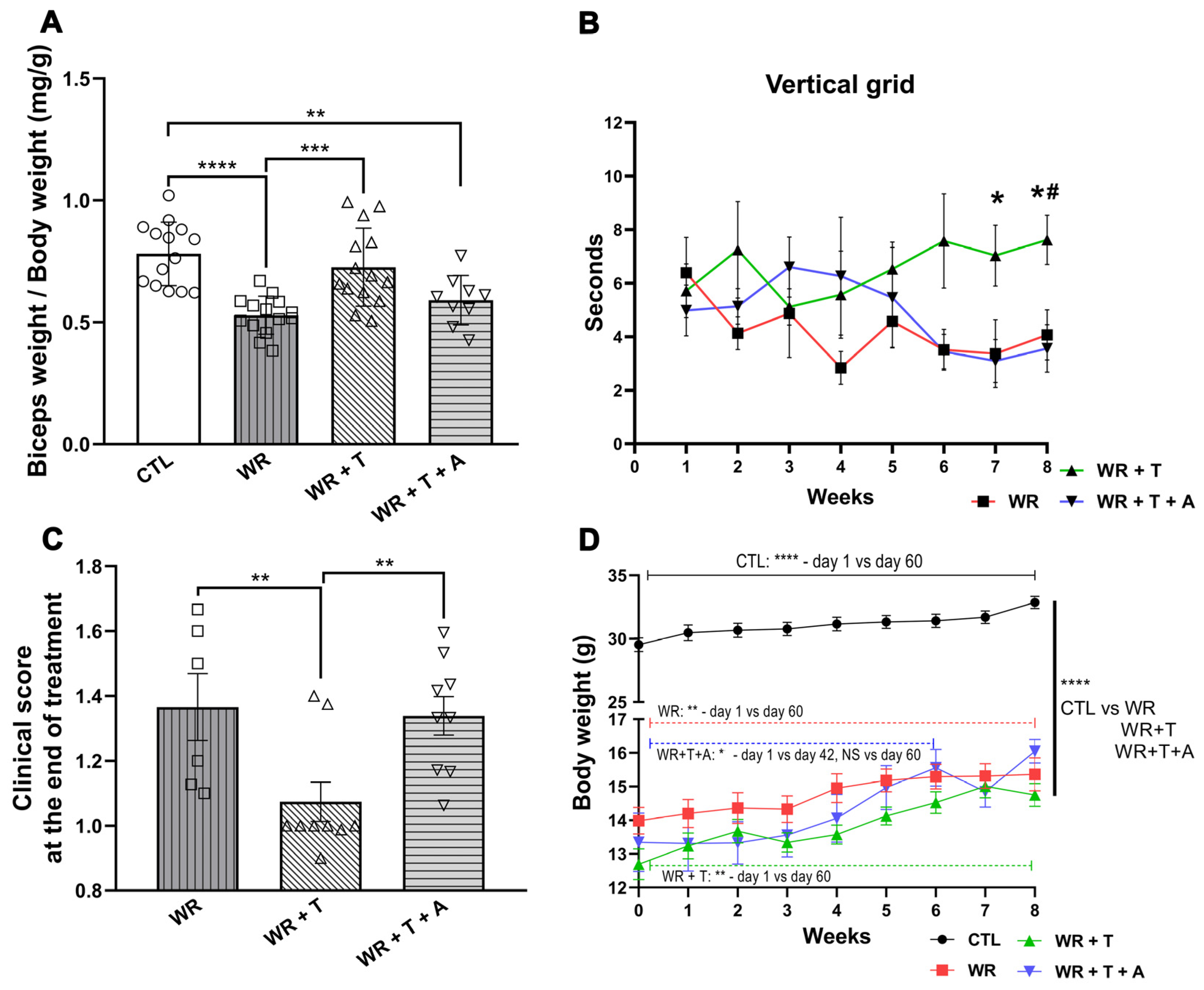
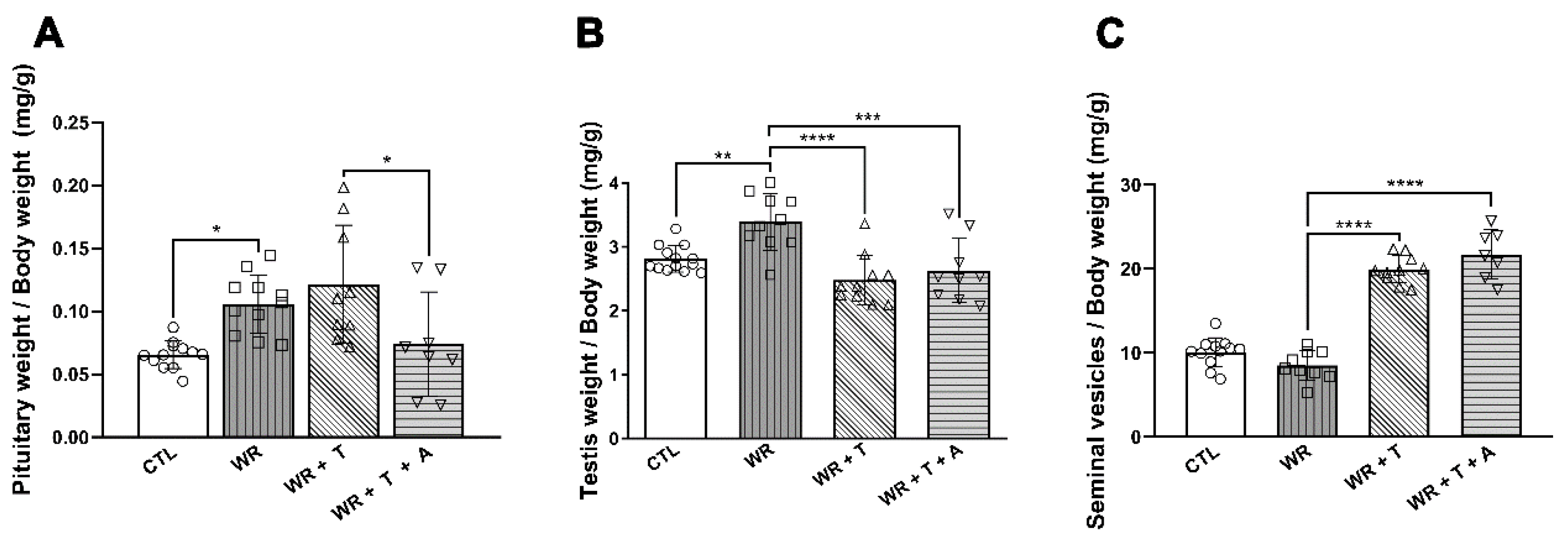
3.7. Effects of Testosterone and Anastrozole on the Biceps Weight, Body Weight, Clinical Parameters and Endocrine Glands
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhuri, A. Multiple sclerosis is primarily a neurodegenerative disease. J. Neural Transm. 2013, 120, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Sandi, D.; Fricska-Nagy, Z.; Bencsik, K.; Vécsei, L. Neurodegeneration in Multiple Sclerosis: Symptoms of Silent Progression, Biomarkers and Neuroprotective Therapy-Kynurenines Are Important Players. Molecules 2021, 26, 3423. [Google Scholar] [CrossRef]
- Moser, J.M.; Bigini, P.; Schmitt-John, T. The wobbler mouse, an ALS animal model. Mol. Genet. Genom. MGG 2013, 288, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Ferut, A.L.; Kurahashi, K.; McQuarrie, I.G. Impairment of retrograde axonal transport in wobbler mouse motor neuron disease. Muscle Nerve 1990, 13, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Raffaele, S.; Boccazzi, M.; Fumagalli, M. Oligodendrocyte Dysfunction in Amyotrophic Lateral Sclerosis: Mechanisms and Therapeutic Perspectives. Cells 2021, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Monachelli, G.G.; Meyer, M.; Rodríguez, G.E.; Garay, L.I.; Sica, R.E.P.; De Nicola, A.F.; Deniselle, M.C.G. Endogenous progesterone is associated to amyotrophic lateral sclerosis prognostic factors. Acta Neurol. Scand. 2011, 123, 60–67. [Google Scholar] [CrossRef]
- Meyer, M.; Garay, L.I.; Kruse, M.S.; Lara, A.; Gargiulo-Monachelli, G.; Schumacher, M.; Guennoun, R.; Coirini, H.; De Nicola, A.F.; Deniselle, M.C.G. Protective effects of the neurosteroid allopregnanolone in a mouse model of spontaneous motoneuron degeneration. J. Steroid Biochem. Mol. Biol. 2017, 174, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Deniselle, M.G.; Garay, L.; Sitruk-Ware, R.; Guennoun, R.; Schumacher, M.; De Nicola, A. The progesterone receptor agonist Nestorone holds back proinflammatory mediators and neuropathology in the wobbler mouse model of motoneuron degeneration. Neuroscience 2015, 308, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Lima, A.; Deniselle, M.C.G.; De Nicola, A.F. Early Signs of Neuroinflammation in the Postnatal Wobbler Mouse Model of Amyotrophic Lateral Sclerosis. Cell. Mol. Neurobiol. 2023, 43, 2149–2163. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Deniselle, M.C.; Liere, P.; Pianos, A.; Meyer, M.; Aprahamian, F.; Cambourg, A.; Di Giorgio, N.P.; Schumacher, M.; De Nicola, A.F.; Guennoun, R. Steroid Profiling in Male Wobbler Mouse, a Model of Amyotrophic Lateral Sclerosis. Endocrinology 2016, 157, 4446–4460. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-John, T.; Drepper, C.; Mußmann, A.; Hahn, P.; Kuhlmann, M.; Thiel, C.; Hafner, M.; Lengeling, A.; Heimann, P.; Jones, J.M.; et al. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat. Genet. 2005, 37, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Heimann, P.; Laage, S.; Jockusch, H. Defect of sperm assembly in a neurological mutant of the mouse, wobbler (WR). Differentiation 1991, 47, 77–83. [Google Scholar] [CrossRef]
- Niebroj-Dobosz, I.; Rafałowska, J.; Fidziańska, A.; Gadamski, R.; Grieb, P. Myelin composition of spinal cord in a model of amyotrophic lateral sclerosis (ALS) in SOD1G93A transgenic rats. Folia Neuropathol. 2007, 45, 236–241. [Google Scholar] [PubMed]
- Bianchi, V.E.; Rizzi, L.; Bresciani, E.; Omeljaniuk, R.J.; Torsello, A. Androgen Therapy in Neurodegenerative Diseases. J. Endocr. Soc. 2020, 4, bvaa120. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Ghoumari, A.; Mattern, C.; Bougnères, P.; Traiffort, E. Testosterone and Myelin Regeneration in the Central Nervous System. Androg. Clin. Res. Ther. 2021, 2, 231–251. [Google Scholar] [CrossRef]
- Gaignard, P.; Liere, P.; Thérond, P.; Schumacher, M.; Slama, A.; Guennoun, R. Role of Sex Hormones on Brain Mitochondrial Function, with Special Reference to Aging and Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, M.; Soon-Shiong, R.; French, L.; Kaplan, D.; Miller, F.; Paus, T. Axon diameter and axonal transport: In vivo and in vitro effects of androgens. Neuroimage 2015, 115, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hussain, R.; Ghoumari, A.M.; Bielecki, B.; Steibel, J.; Boehm, N.; Liere, P.; Macklin, W.B.; Kumar, N.; Habert, R.; Mhaouty-Kodja, S.; et al. The neural androgen receptor: A therapeutic target for myelin repair in chronic demyelination. Brain 2013, 136, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, B.; Mattern, C.; Ghoumari, A.M.; Javaid, S.; Smietanka, K.; Ghanem, C.A.; Mhaouty-Kodja, S.; Ghandour, M.S.; Baulieu, E.-E.; Franklin, R.J.M.; et al. Unexpected central role of the androgen receptor in the spontaneous regeneration of myelin. Proc. Natl. Acad. Sci. USA 2016, 113, 14829–14834. [Google Scholar] [CrossRef] [PubMed]
- Ghoumari, A.M.; Ghanem, C.A.; Asbelaoui, N.; Schumacher, M.; Hussain, R. Roles of Progesterone, Testosterone and Their Nuclear Receptors in Central Nervous System Myelination and Remyelination. Int. J. Mol. Sci. 2020, 21, 3163. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.P. Possible role of androgen receptors in amyotrophic lateral sclerosis: A hypothesis. Arch. Neurol. 1980, 37, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Cappello, V.; Vezzoli, E.; Righi, M.; Fossati, M.; Mariotti, R.; Crespi, A.; Patruno, M.; Bentivoglio, M.; Pietrini, G.; Francolini, M. Analysis of neuromuscular junctions and effects of anabolic steroid administration in the SOD1G93A mouse model of ALS. Mol. Cell. Neurosci. 2012, 51, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, M.; Onesto, E.; Zito, A.; Crippa, V.; Rusmini, P.; Mariotti, R.; Bentivoglio, M.; Bendotti, C.; Poletti, A. The anabolic/androgenic steroid nandrolone exacerbates gene expression modifications induced by mutant SOD1 in muscles of mice models of amyotrophic lateral sclerosis. Pharmacol. Res. 2012, 65, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, B.K.; Frost, L.M.; Christian, L.; Umapathi, P.; Gage, F.H. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann. Neurol. 2005, 57, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Zahaf, A.; Kassoussi, A.; Hutteau-Hamel, T.; Mellouk, A.; Marie, C.; Zoupi, L.; Tsouki, F.; Mattern, C.; Bobé, P.; Schumacher, M.; et al. Androgens show sex-dependent differences in myelination in immune and non-immune murine models of CNS demyelination. Nat. Commun. 2023, 14, 1592. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Ballabio, M.; Gonzalez, L.C.; Leonelli, E.; Motta, M.; Melcangi, R.C. The synthesis of glycoprotein Po and peripheral myelin protein 22 in sciatic nerve of male rats is modulated by testosterone metabolites. Brain Res. Mol. Brain Res. 2004, 126, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Roglio, I.; Bianchi, R.; Giatti, S.; Cavaletti, G.; Caruso, D.; Scurati, S.; Crippa, D.; Garcia-Segura, L.M.; Camozzi, F.; Lauria, G.; et al. Testosterone derivatives are neuroprotective agents in experimental diabetic neuropathy. Cell. Mol. Life Sci. CMLS 2007, 64, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Melcangi, R.; Ballabio, M.; Cavarretta, I.; Gonzalez, L.; Leonelli, E.; Veiga, S.; Martini, L.; Magnaghi, V. Effects of neuroactive steroids on myelin of peripheral nervous system. J. Steroid Biochem. Mol. Biol. 2003, 85, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Lara, A.; Esperante, I.; Meyer, M.; Liere, P.; Di Giorgio, N.; Schumacher, M.; Guennoun, R.; Gargiulo-Monachelli, G.; De Nicola, A.F.; Deniselle, M.C.G. Neuroprotective Effects of Testosterone in Male Wobbler Mouse, a Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2021, 58, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Deniselle, M.C.G.; Garay, L.I.; Monachelli, G.G.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Stage Dependent Effects of Progesterone on Motoneurons and Glial Cells of Wobbler Mouse Spinal Cord Degeneration. Cell. Mol. Neurobiol. 2010, 30, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Deniselle, M.C. Progesterone neuroprotection in the Wobbler mouse, a genetic model of spinal cord motor neuron disease. Neurobiol. Dis. 2002, 11, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo-Monachelli, G.; Meyer, M.; Lara, A.; Garay, L.; Lima, A.; Roig, P.; De Nicola, A.F.; Deniselle, M.C.G. Comparative effects of progesterone and the synthetic progestin norethindrone on neuroprotection in a model of spontaneous motoneuron degeneration. J. Steroid Biochem. Mol. Biol. 2019, 192, 105385. [Google Scholar] [CrossRef] [PubMed]
- Chomiak, T.; Hu, B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS ONE 2009, 4, e7754. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.; Allen, H.M.; Kwon, O.; Barak, B.; Wang, J.; He, Z.; Jiang, M.; Feng, G. MyelTracer: A Semi-Automated Software for Myelin g-Ratio Quantification. eNeuro 2021, 8, eneuro.0558-20.2021. [Google Scholar] [CrossRef] [PubMed]
- Dillenburg, A.; Ireland, G.; Holloway, R.K.; Davies, C.L.; Evans, F.L.; Swire, M.; Bechler, M.E.; Soong, D.; Yuen, T.J.; Su, G.H.; et al. Activin receptors regulate the oligodendrocyte lineage in health and disease. Acta Neuropathol. 2018, 135, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Budde, M.D.; Liang, H.-F.; Klein, R.S.; Russell, J.H.; Cross, A.H.; Song, S.-K. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol. Dis. 2006, 21, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Tüngler, V.; Deniselle, M.; Lima, A.; Roig, P.; De Nicola, A. Progesterone attenuates demyelination and microglial reaction in the lysolecithin-injured spinal cord. Neuroscience 2011, 192, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Labombarda, F.; Gonzalez, S.; Deniselle, M.C.G.; Garay, L.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone increases the expression of myelin basic protein and the number of cells showing NG2 immunostaining in the lesioned spinal cord. J. Neurotrauma 2006, 23, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Mironova, Y.A.; Shen, H.; Marino, R.A.; Waisman, A.; Lamers, W.H.; Bergles, D.E.; Bonci, A. Oligodendrocytes Support Neuronal Glutamatergic Transmission via Expression of Glutamine Synthetase. Cell Rep. 2019, 27, 2262–2271.e5. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Schirmer, L.; Zulji, A.; Sabeur, K.; Tiret, B.; Ribon, M.; Chang, S.; Lamers, W.H.; Boillée, S.; Chaumeil, M.M.; et al. Evidence for glutamine synthetase function in mouse spinal cord oligodendrocytes. Glia 2021, 69, 2812–2827. [Google Scholar] [CrossRef]
- Leicaj, M.L.; Pasquini, L.A.; Lima, A.; Deniselle, M.G.; Pasquini, J.M.; De Nicola, A.F.; Garay, L.I. Changes in neurosteroidogenesis during demyelination and remyelination in cuprizone-treated mice. J. Neuroendocr. 2018, 30, e12649. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Lara, A.; Hunt, H.; Belanoff, J.; de Kloet, E.R.; Deniselle, M.C.G.; De Nicola, A.F. The Selective Glucocorticoid Receptor Modulator Cort 113176 Reduces Neurodegeneration and Neuroinflammation in Wobbler Mice Spinal Cord. Neuroscience 2018, 384, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mitsumoto, H.; Ikeda, K.; Holmlund, T.; Greene, T.; Cedarbaum, J.M.; Wong, V.; Lindsay, R.M. The effects of ciliary neurotrophic factor on motor dysfunction in wobbler mouse motor neuron disease. Ann. Neurol. 1994, 36, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Krieger, C.; Perry, T.L.; Hansen, S.; Mitsumoto, H.; Honoré, T. Excitatory Amino Acid Receptor Antagonist in Murine Motoneuron Disease (The Wobbler Mouse). Can. J. Neurol. Sci. 1992, 19, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated microglia drive demyelination via CSF1R signaling. Glia 2021, 69, 1583–1604. [Google Scholar] [CrossRef] [PubMed]
- Traiffort, E.; Morisset-Lopez, S.; Moussaed, M.; Zahaf, A. Defective Oligodendroglial Lineage and Demyelination in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2021, 22, 3426. [Google Scholar] [CrossRef] [PubMed]
- Blondet, B.; Hantaz-Ambroise, D.; Aït-Ikhlef, A.; Cambier, D.; Murawsky, M.; Rieger, F. Astrocytosis in wobbler mouse spinal cord involves a population of astrocytes which is glutamine synthetase-negative. Neurosci. Lett. 1995, 183, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Diana, V.; Ottolina, A.; Botti, F.; Fumagalli, E.; Calcagno, E.; De Paola, M.; Cagnotto, A.; Invernici, G.; Parati, E.; Curti, D.; et al. Neural precursor-derived astrocytes of wobbler mice induce apoptotic death of motor neurons through reduced glutamate uptake. Exp. Neurol. 2010, 225, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Kruse, M.S.; Garay, L.; Lima, A.; Roig, P.; Hunt, H.; Belanoff, J.; de Kloet, E.R.; Deniselle, M.C.G.; De Nicola, A.F. Long-term effects of the glucocorticoid receptor modulator CORT113176 in murine motoneuron degeneration. Brain Res. 2020, 1727, 146551. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Ikenaka, K. Axonal and neuronal degeneration in myelin diseases. Neurosci. Res. 2019, 139, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Bigini, P.; Diana, V.; Barbera, S.; Fumagalli, E.; Micotti, E.; Sitia, L.; Paladini, A.; Bisighini, C.; De Grada, L.; Coloca, L.; et al. Longitudinal tracking of human fetal cells labeled with super paramagnetic iron oxide nanoparticles in the brain of mice with motor neuron disease. PLoS ONE 2012, 7, e32326. [Google Scholar] [CrossRef] [PubMed]
- De Paola, M.; Mariani, A.; Bigini, P.; Peviani, M.; Ferrara, G.; Molteni, M.; Gemma, S.; Veglianese, P.; Castellaneta, V.; Boldrin, V.; et al. Neuroprotective effects of toll-like receptor 4 antagonism in spinal cord cultures and in a mouse model of motor neuron degeneration. Mol. Med. 2012, 18, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, S.; Han, J.; Li, S.; Gao, X.; Wang, M.; Zhu, J.; Jin, T. Role of Toll-Like Receptors in Neuroimmune Diseases: Therapeutic Targets and Problems. Front. Immunol. 2021, 12, 777606. [Google Scholar] [CrossRef] [PubMed]
- Garay, L.; Deniselle, M.G.; Brocca, M.; Lima, A.; Roig, P.; De Nicola, A. Progesterone down-regulates spinal cord inflammatory mediators and increases myelination in experimental autoimmune encephalomyelitis. Neuroscience 2012, 226, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef] [PubMed]
- Thielsen, K.D.; Moser, J.M.; Schmitt-John, T.; Jensen, M.S.; Jensen, K.; Holm, M.M. The Wobbler Mouse Model of Amyotrophic Lateral Sclerosis (ALS) Displays Hippocampal Hyperexcitability, and Reduced Number of Interneurons, but No Presynaptic Vesicle Release Impairments. PLoS ONE 2013, 8, e82767. [Google Scholar] [CrossRef] [PubMed]
- Timmins, H.C.; Vucic, S.; Kiernan, M.C. Cortical hyperexcitability in amyotrophic lateral sclerosis: From pathogenesis to diagnosis. Curr. Opin. Neurol. 2023, 36, 353–359. [Google Scholar] [CrossRef]
- Saba, L.; Viscomi, M.T.; Caioli, S.; Pignataro, A.; Bisicchia, E.; Pieri, M.; Molinari, M.; Ammassari-Teule, M.; Zona, C. Altered Functionality, Morphology, and Vesicular Glutamate Transporter Expression of Cortical Motor Neurons from a Presymptomatic Mouse Model of Amyotrophic Lateral Sclerosis. Cereb. Cortex 2016, 26, 1512–1528. [Google Scholar] [CrossRef] [PubMed]
- Macrez, R.; Stys, P.K.; Vivien, D.; A Lipton, S.; Docagne, F. Mechanisms of glutamate toxicity in multiple sclerosis: Biomarker and therapeutic opportunities. Lancet Neurol. 2016, 15, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Alberdi, E.; Domercq, M.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Sánchez-Gómez, M.V. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001, 24, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Laouarem, Y.; Kassoussi, A.; Zahaf, A.; Hutteau-Hamel, T.; Mellouk, A.; Bobé, P.; Mattern, C.; Schumacher, M.; Traiffort, E. Functional cooperation of the hedgehog and androgen signaling pathways during developmental and repairing myelination. Glia 2021, 69, 1369–1392. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, C.A.; Degerny, C.; Hussain, R.; Liere, P.; Pianos, A.; Tourpin, S.; Habert, R.; Macklin, W.B.; Schumacher, M.; Ghoumari, A.M. Long-lasting masculinizing effects of postnatal androgens on myelin governed by the brain androgen receptor. PLoS Genet. 2017, 13, e1007049. [Google Scholar] [CrossRef] [PubMed]
- Sawal, N.; Kaur, J.; Kaur, K.; Gombar, S. Dihydrotestosterone in Amyotrophic lateral sclerosis-The missing link? Brain Behav. 2020, 10, e01645. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo-Monachelli, G.M.; Sivori, M.; Meyer, M.; Sica, R.E.P.; De Nicola, A.F.; Gonzalez-Deniselle, M.C. Circulating Gonadal and Adrenal Steroids in Amyotrophic Lateral Sclerosis: Possible Markers of Susceptibility and Outcome. Horm. Metab. Res. 2014, 46, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Scaricamazza, S.; Salvatori, I.; Ferri, A.; Valle, C. Skeletal Muscle in ALS: An Unappreciated Therapeutic Opportunity? Cells 2021, 10, 525. [Google Scholar] [CrossRef] [PubMed]

| Gene | Accession Number | Forward Primer (5→3) | Reverse Primer (5→3) |
|---|---|---|---|
| MBP | NM_001025100 | ATCCAAGTACCTGGCCACAG | CCTGTCACCGCTAAAGAAGC |
| PLP | NM_199478 | CTGGCTGAGGGCTTCTACAC | GACTGACAGGTGGTCCAGGT |
| MOG | NM_010814 | AAATGGCAAGGACCAAGATG | AGCAGGTGTAGCCTCCTTCA |
| CD11b | NM_008401 | AAACCACAGTCCCGCAGAGA | CGTGTTCACCAGCTGGCTTA |
| TLR4 | NM_021297 | GGCTCCTGGCTAGGACTCTGA | TCTGATCCATGCATTGGTAGGT |
| TNFαR1 | NM_011609 | GCTGACCCTCTGCTCTACGAA | GCCATCCACCACAGCATACA |
| IL-18 | NM_008360.2 | TGCCAAAAGGAAGATGATGC | ACACAAACCCTCCCCACCTA |
| P2Y12R | NM_027571.4 | TTTCAGATCCGCAGTAAATCCAA | GGCTCCAGTTTAGCATCACTA |
| GLT-1 | NM_001077514 | CAGTGCTGGAACTTTGCCTG | GGCTATGAAGATGGCTGCCA |
| Cyclophilin B | NM_022536 | GTGGCAAGATCGAAGTGGAGAAAC | TAAAAATCAGGCCTGTGGAATGTG |
| Location | Experimental Groups | |||
|---|---|---|---|---|
| Male Wobbler | Male Wobbler Testosterone | Male Wobbler Testosterone + Anastrozole | ||
| Myelin parameters | ||||
| Luxol fast blue | Ventrolateral funiculus |  |  |  |
| G-ratio | Ventrolateral funiculus |  |  |  |
| MBP mRNA MBP-IR | Whole CSC Ventrolateral funiculus |   |   |   |
| PLP mRNA PLP-IR | Whole CSC Ventrolateral funiculus |   |   |   |
| Inflammatory factors | ||||
| CD11b mRNA | Whole CSC |  |  |  |
| IBA1 cell density | Ventral region (Ventral horn + Ventrolateral funiculus) |  |  |  |
| IL-18 mRNA TLR4 mRNA TNFαR1 mRNA P2Y12R mRNA | Whole CSC |  |  |  |
| Glutamate metabolism | ||||
| GS-IR | Ventral horn Ventrolateral funiculus |   |   |   |
| GLT-1 mRNA GLT-1 IR | Whole CSC Ventral horn |   |   |   |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esperante, I.J.; Meyer, M.; Banzan, C.; Kruse, M.S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F.; Gonzalez Deniselle, M.C. Testosterone Reduces Myelin Abnormalities in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis. Biomolecules 2024, 14, 428. https://doi.org/10.3390/biom14040428
Esperante IJ, Meyer M, Banzan C, Kruse MS, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF, Gonzalez Deniselle MC. Testosterone Reduces Myelin Abnormalities in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis. Biomolecules. 2024; 14(4):428. https://doi.org/10.3390/biom14040428
Chicago/Turabian StyleEsperante, Ivan J., Maria Meyer, Carolina Banzan, Maria Sol Kruse, Analia Lima, Paulina Roig, Rachida Guennoun, Michael Schumacher, Alejandro F. De Nicola, and Maria Claudia Gonzalez Deniselle. 2024. "Testosterone Reduces Myelin Abnormalities in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis" Biomolecules 14, no. 4: 428. https://doi.org/10.3390/biom14040428
APA StyleEsperante, I. J., Meyer, M., Banzan, C., Kruse, M. S., Lima, A., Roig, P., Guennoun, R., Schumacher, M., De Nicola, A. F., & Gonzalez Deniselle, M. C. (2024). Testosterone Reduces Myelin Abnormalities in the Wobbler Mouse Model of Amyotrophic Lateral Sclerosis. Biomolecules, 14(4), 428. https://doi.org/10.3390/biom14040428







