Dissecting the Long-Term Effect of Stress Early in Life on FKBP5: The Role of miR-20b-5p and miR-29c-3p
Abstract
1. Introduction
2. Materials and Methods
2.1. Prenatal Stress Model
2.2. In Vitro Cell Model
- Short-term treatment: HIP-009 cells were treated for 3 days during proliferation with cortisol (100 μM) or the vehicle, and cells were harvested immediately after the end of the treatment.
- Long-term treatment: HIP-009 cells were treated with cortisol (100 μM) or the vehicle during 3 days of proliferation, grown in transition medium for 3 days, and then harvested after 21 days of differentiation without any treatment.
2.3. RNA Isolation
2.4. Real-Time PCR
2.5. Statistical Analyses
3. Results
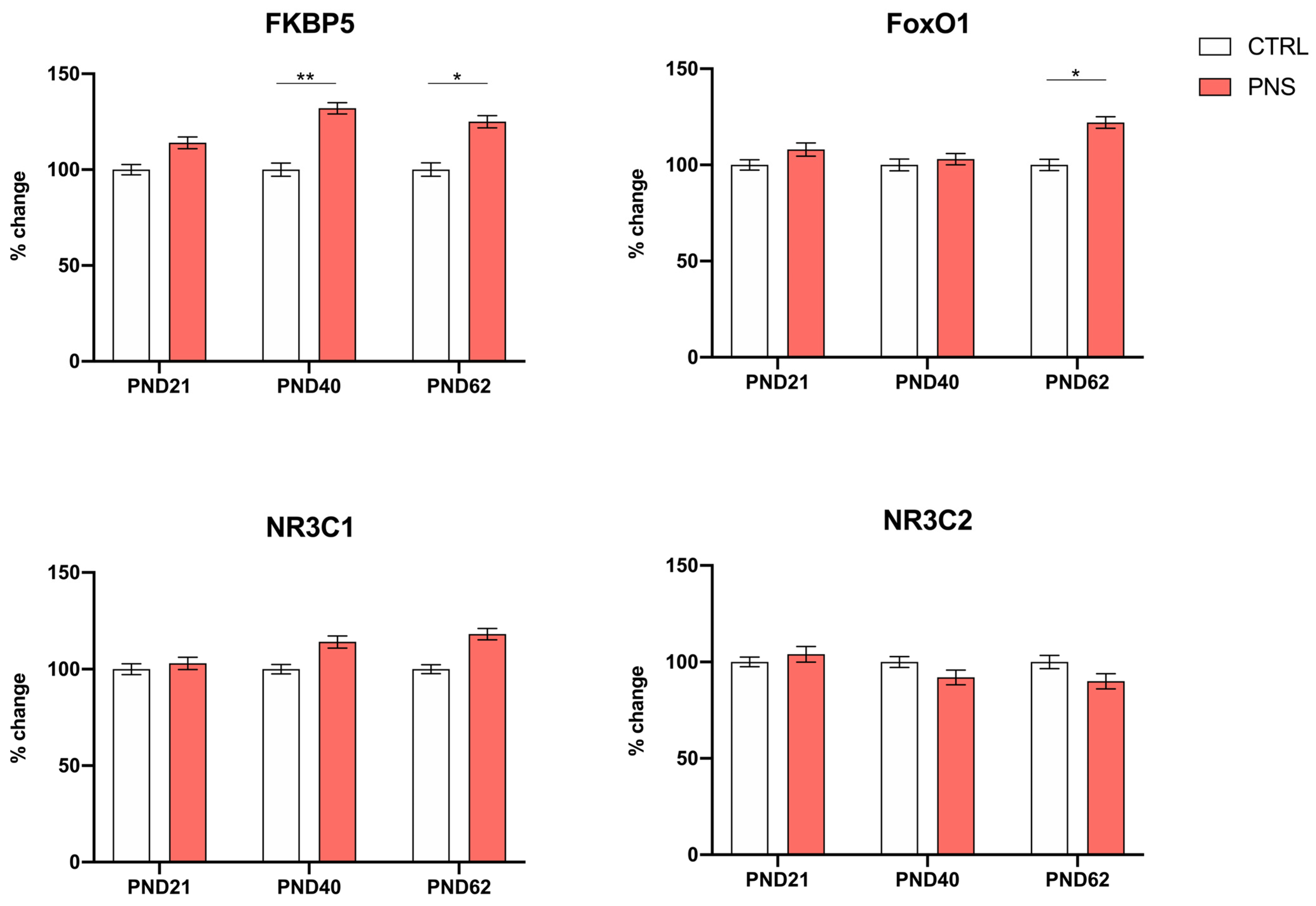
3.1. Effects of Prenatal Stress on the Modulation of Stress-Responsive Genes during Neurodevelopment
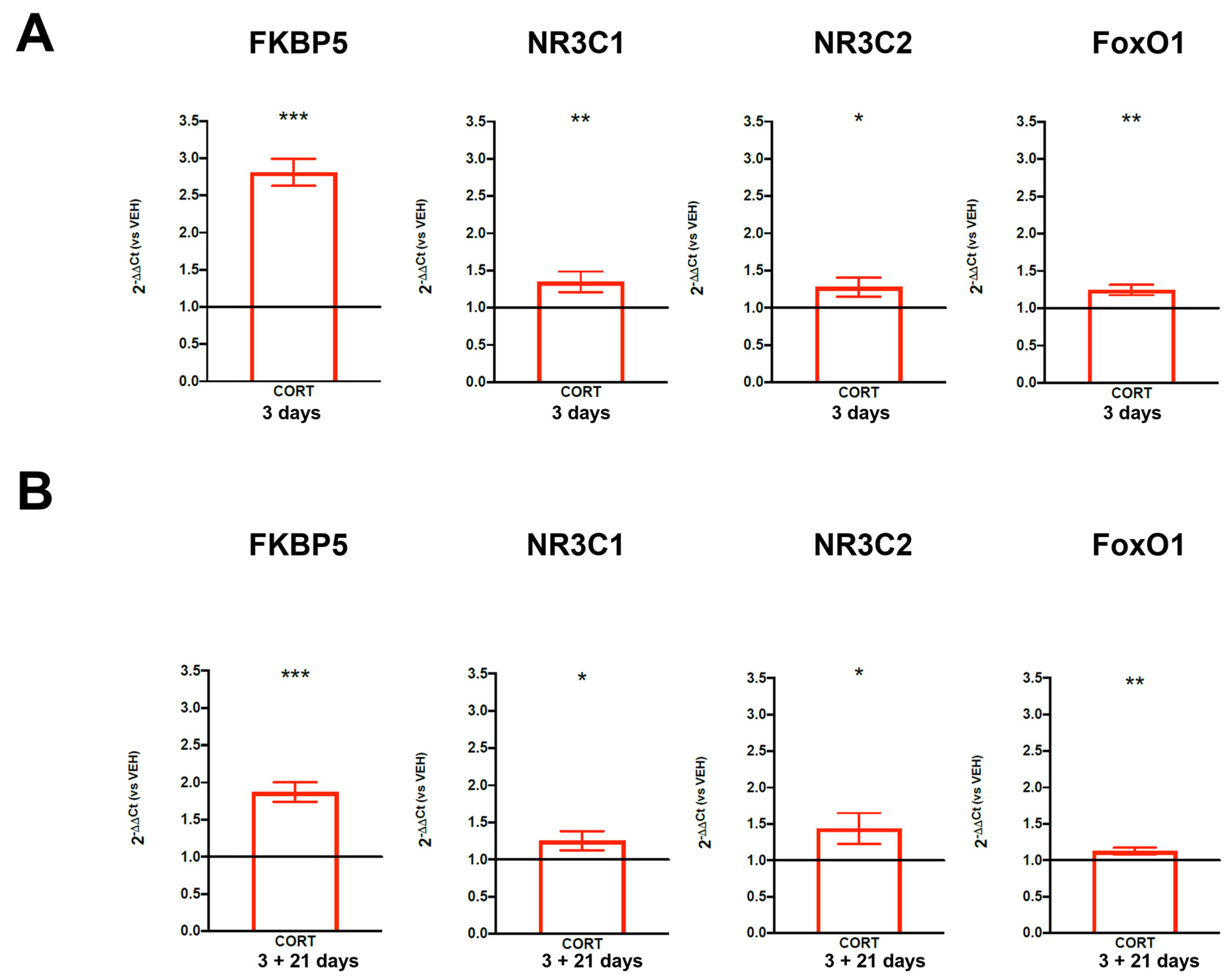
3.2. Short- and Long-Term Effects of Cortisol Treatment in an In Vitro Model of Human Hippocampal Progenitor Cells
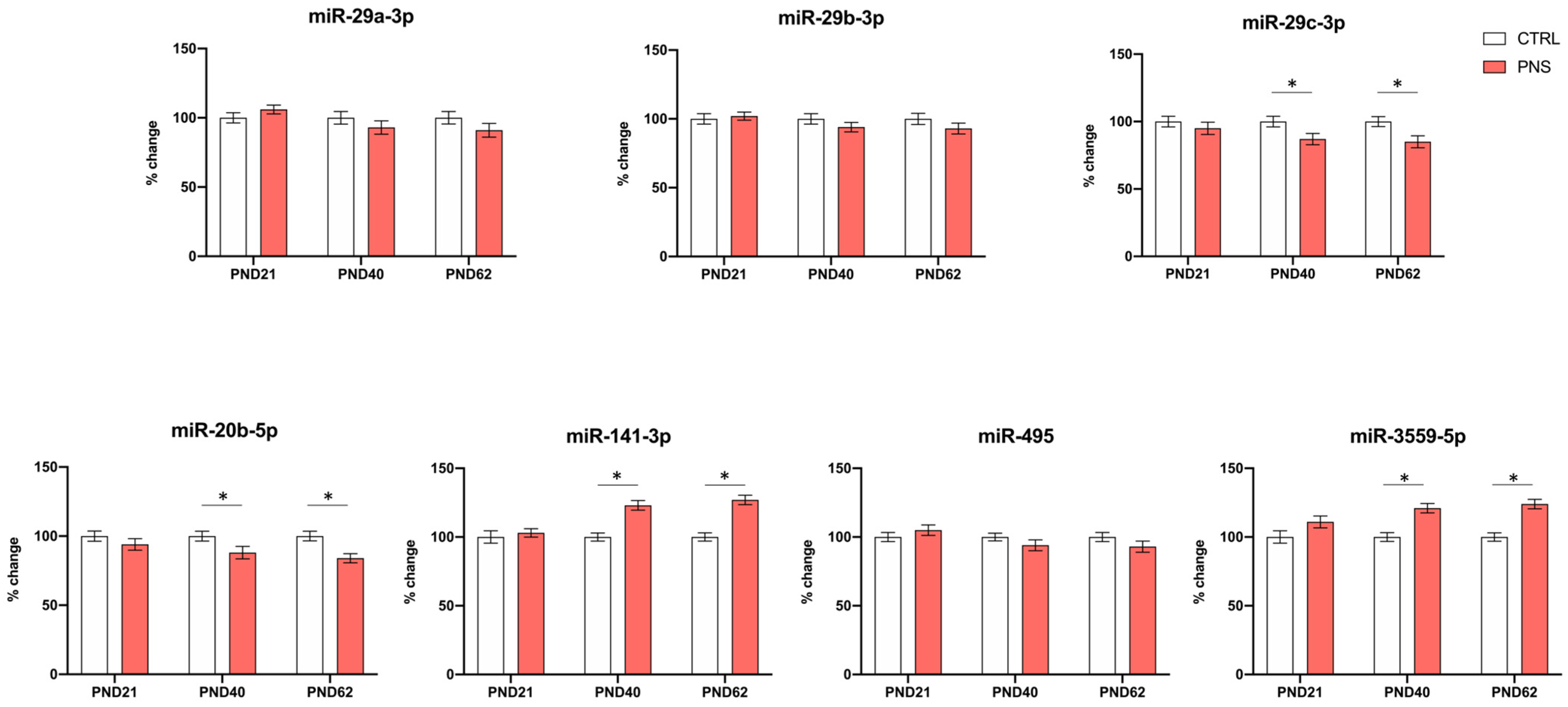
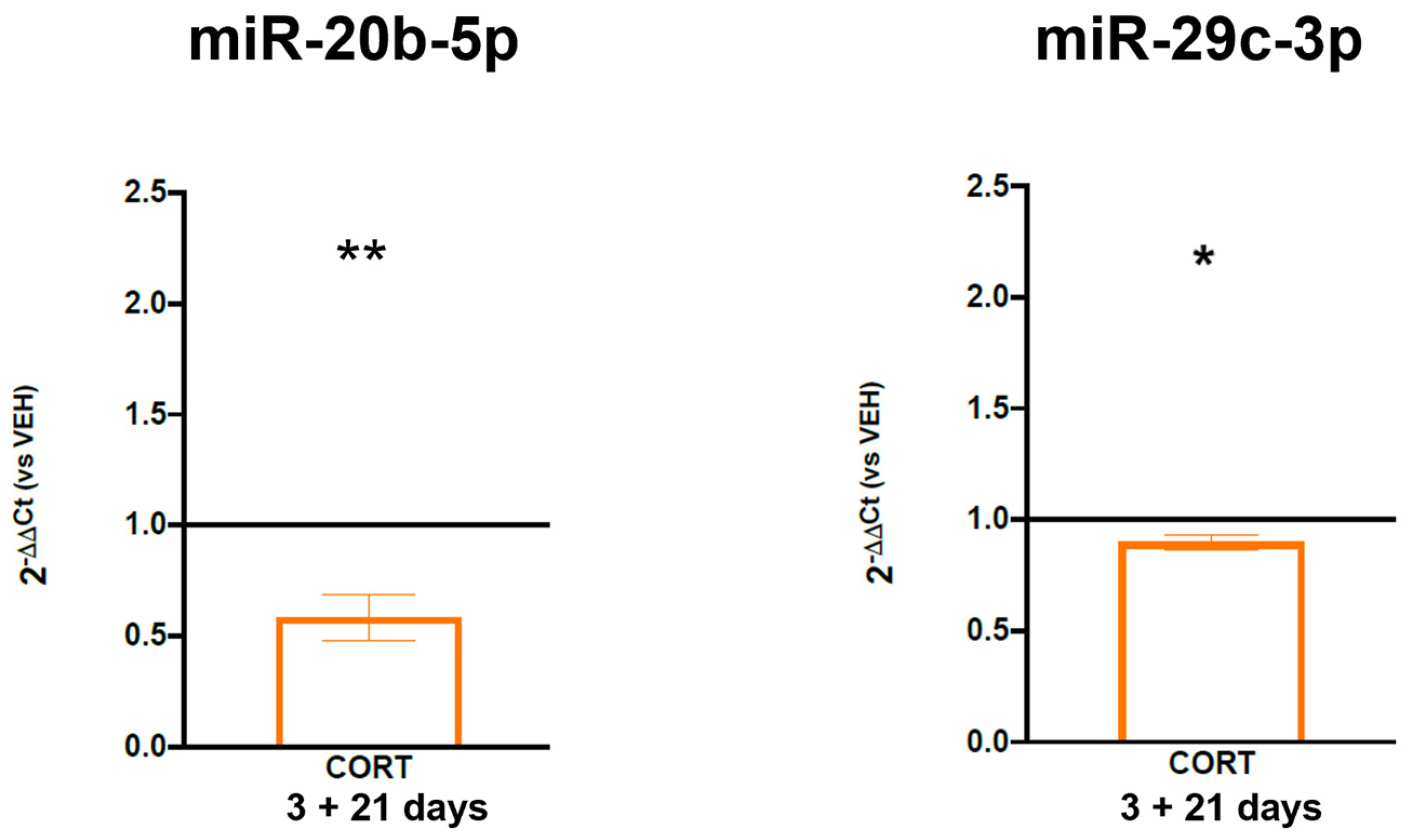
3.3. Role of miRNAs Targeting FKBP5 in the Long-Lasting Effect of ELS
4. Discussion
5. Conclusions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reemst, K.; Kracht, L.; Kotah, J.M.; Rahimian, R.; van Irsen, A.A.S.; Congrains Sotomayor, G.; Verboon, L.N.; Brouwer, N.; Simard, S.; Turecki, G.; et al. Early-life stress lastingly impacts microglial transcriptome and function under basal and immune-challenged conditions. Transl. Psychiatry 2022, 12, 507. [Google Scholar] [CrossRef]
- Mourtzi, N.; Sertedaki, A.; Charmandari, E. Glucocorticoid Signaling and Epigenetic Alterations in Stress-Related Disorders. Int. J. Mol. Sci. 2021, 22, 5964. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; De Kloet, E.R.; Yehuda, R.; Malaspina, D.; Kranz, T.M. Early Life Stress Effects on Glucocorticoid—BDNF Interplay in the Hippocampus. Front. Mol. Neurosci. 2015, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Matosin, N.; Halldorsdottir, T.; Binder, E.B. Understanding the Molecular Mechanisms Underpinning Gene by Environment Interactions in Psychiatric Disorders: The FKBP5 Model. Biol. Psychiatry 2018, 83, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Klengel, T.; Binder, E.B. Gene—Environment interactions in major depressive disorder. Can. J. Psychiatry Rev. Can. Psychiatr. 2013, 58, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Provencal, N.; Arloth, J.; Cattaneo, A.; Anacker, C.; Cattane, N.; Wiechmann, T.; Roh, S.; Kodel, M.; Klengel, T.; Czamara, D.; et al. Glucocorticoid exposure during hippocampal neurogenesis primes future stress response by inducing changes in DNA methylation. Proc. Natl. Acad. Sci. USA 2020, 117, 23280–23285. [Google Scholar] [CrossRef] [PubMed]
- Comtois-Cabana, M.; Barr, E.; Provencal, N.; Ouellet-Morin, I. Association between child maltreatment and depressive symptoms in emerging adulthood: The mediating and moderating roles of DNA methylation. PLoS ONE 2023, 18, e0280203. [Google Scholar] [CrossRef]
- Zannas, A.S.; Wiechmann, T.; Gassen, N.C.; Binder, E.B. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 261–274. [Google Scholar] [CrossRef]
- Desmet, S.J.; De Bosscher, K. Glucocorticoid receptors: Finding the middle ground. J. Clin. Investig. 2017, 127, 1136–1145. [Google Scholar] [CrossRef]
- Malekpour, M.; Shekouh, D.; Safavinia, M.E.; Shiralipour, S.; Jalouli, M.; Mortezanejad, S.; Azarpira, N.; Ebrahimi, N.D. Role of FKBP5 and its genetic mutations in stress-induced psychiatric disorders: An opportunity for drug discovery. Front. Psychiatry 2023, 14, 1182345. [Google Scholar] [CrossRef]
- Tatro, E.T.; Everall, I.P.; Kaul, M.; Achim, C.L. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: Implications for major depressive disorder. Brain Res. 2009, 1286, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Young, K.A.; Thompson, P.M.; Cruz, D.A.; Williamson, D.E.; Selemon, L.D. BA11 FKBP5 expression levels correlate with dendritic spine density in postmortem PTSD and controls. Neurobiol. Stress 2015, 2, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.; Fillman, S.G.; Webster, M.J.; Weickert, C.S. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci. Rep. 2013, 3, 3539. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Malpighi, C.; Czamara, D.; Suarez, A.; Mariani, N.; Kajantie, E.; Luoni, A.; Eriksson, J.G.; Lahti, J.; et al. FoxO1, A2M, and TGF-beta1: Three novel genes predicting depression in gene X environment interactions are identified using cross-species and cross-tissues transcriptomic and miRNomic analyses. Mol. Psychiatry 2018, 23, 2192–2208. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.Y.; Jeong, S.Y.; Lee, J.W.; Kim, J.; Kim, J.H.; Chu, H.S.; Jeong, W.J.; Lee, B.J.; Ahn, B.; Kim, J.; et al. FOXO1 Is a Key Mediator of Glucocorticoid-Induced Expression of Tristetraprolin in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 13673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, H.; Liu, M.S.; Zhu, K.Y.; Hao, Y.; Zhu, D.L.; Li, P. Serum- and glucocorticoid-inducible kinase 1 promotes insulin resistance in adipocytes via degradation of insulin receptor substrate 1. Diabetes/Metab. Res. Rev. 2021, 37, e3451. [Google Scholar] [CrossRef]
- Yoo, H.S.; Rodriguez, A.; You, D.; Lee, R.A.; Cockrum, M.A.; Grimes, J.A.; Wang, J.C.; Kang, S.; Napoli, J.L. The glucocorticoid receptor represses, whereas C/EBPbeta can enhance or repress CYP26A1 transcription. iScience 2022, 25, 104564. [Google Scholar] [CrossRef]
- McCullumsmith, R.E.; Hammond, J.H.; Shan, D.; Meador-Woodruff, J.H. Postmortem brain: An underutilized substrate for studying severe mental illness. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2014, 39, 65–87. [Google Scholar] [CrossRef]
- Ferrer, I.; Martinez, A.; Boluda, S.; Parchi, P.; Barrachina, M. Brain banks: Benefits, limitations and cautions concerning the use of post-mortem brain tissue for molecular studies. Cell Tissue Bank. 2008, 9, 181–194. [Google Scholar] [CrossRef]
- Cattane, N.; Vernon, A.C.; Borsini, A.; Scassellati, C.; Endres, D.; Capuron, L.; Tamouza, R.; Benros, M.E.; Leza, J.C.; Pariante, C.M.; et al. Preclinical animal models of mental illnesses to translate findings from the bench to the bedside: Molecular brain mechanisms and peripheral biomarkers associated to early life stress or immune challenges. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2022, 58, 55–79. [Google Scholar] [CrossRef]
- Cattaneo, A.; Begni, V.; Malpighi, C.; Cattane, N.; Luoni, A.; Pariante, C.; Riva, M.A. Transcriptional Signatures of Cognitive Impairment in Rat Exposed to Prenatal Stress. Mol. Neurobiol. 2019, 56, 6251–6260. [Google Scholar] [CrossRef]
- Barzegar, M.; Sajjadi, F.S.; Talaei, S.A.; Hamidi, G.; Salami, M. Prenatal exposure to noise stress: Anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life. Hippocampus 2015, 25, 187–196. [Google Scholar] [CrossRef]
- Creutzberg, K.C.; Sanson, A.; Viola, T.W.; Marchisella, F.; Begni, V.; Grassi-Oliveira, R.; Riva, M.A. Long-lasting effects of prenatal stress on HPA axis and inflammation: A systematic review and multilevel meta-analysis in rodent studies. Neurosci. Biobehav. Rev. 2021, 127, 270–283. [Google Scholar] [CrossRef]
- Bassil, K.; De Nijs, L.; Rutten, B.P.F.; Van Den Hove, D.L.A.; Kenis, G. In vitro modeling of glucocorticoid mechanisms in stress-related mental disorders: Current challenges and future perspectives. Front. Cell Dev. Biol. 2022, 10, 1046357. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain A J. Neurol. 2020, 143, 3181–3213. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Suderman, M.; Cattane, N.; Mazzelli, M.; Begni, V.; Maj, C.; D’Aprile, I.; Pariante, C.M.; Luoni, A.; Berry, A.; et al. Long-term effects of stress early in life on microRNA-30a and its network: Preventive effects of lurasidone and potential implications for depression vulnerability. Neurobiol. Stress 2020, 13, 100271. [Google Scholar] [CrossRef] [PubMed]
- Lopizzo, N.; Mazzelli, M.; Zonca, V.; Begni, V.; D’Aprile, I.; Cattane, N.; Pariante, C.M.; Riva, M.A.; Cattaneo, A. Alterations in ‘inflammatory’ pathways in the rat prefrontal cortex as early biological predictors of the long-term negative consequences of exposure to stress early in life. Psychoneuroendocrinology 2021, 124, 104794. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Yamazaki, K.; Miyamoto, N.; Sawada, K. Functional Characterization of Acetylcholine Receptors Expressed in Human Neurons Differentiated from Hippocampal Neural Stem/Progenitor Cells. J. Biomol. Screen. 2016, 21, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Anacker, C.; Cattaneo, A.; Musaelyan, K.; Zunszain, P.A.; Horowitz, M.; Molteni, R.; Luoni, A.; Calabrese, F.; Tansey, K.; Gennarelli, M.; et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 8708–8713. [Google Scholar] [CrossRef]
- Criado-Marrero, M.; Smith, T.M.; Gould, L.A.; Kim, S.; Penny, H.J.; Sun, Z.; Gulick, D.; Dickey, C.A.; Blair, L.J. FKBP5 and early life stress affect the hippocampus by an age-dependent mechanism. Brain Behav. Immun. Health 2020, 9, 100143. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.L.; Kao, P.F.; Itriago, E.; Zhan, Y.; Kozubek, J.A.; Hoss, A.G.; Banigan, M.G.; Vanderburg, C.R.; Rezvani, A.H.; Latourelle, J.C.; et al. miR-149 and miR-29c as candidates for bipolar disorder biomarkers. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2017, 174, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Banigan, M.G.; Kao, P.F.; Kozubek, J.A.; Winslow, A.R.; Medina, J.; Costa, J.; Schmitt, A.; Schneider, A.; Cabral, H.; Cagsal-Getkin, O.; et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE 2013, 8, e48814. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chopra, N.; Nho, K.; Maloney, B.; Obukhov, A.G.; Nelson, P.T.; Counts, S.E.; Lahiri, D.K. Human microRNA (miR-20b-5p) modulates Alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences Alzheimer’s biomarkers. Mol. Psychiatry 2022, 27, 1256–1273. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, X.; Lu, Q.; Huang, K.; Tang, X.; He, Z. MiR-29c-3p May Promote the Progression of Alzheimer’s Disease through BACE1. J. Healthc. Eng. 2021, 2021, 2031407. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Gao, Y.; Liang, J.; Wang, B.; Xia, Q.; Xie, Y.; Shan, F.; Xia, Q. Comprehensive bioinformatics analysis and molecular validation of lncRNAs-mediated ceRNAs network in schizophrenia. Life Sci. 2023, 312, 121205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Meng, H.; Li, Y. Identification of target hub genes and construction of a novel miRNA regulatory network in autism spectrum disorder by integrated analysis. Medicine 2023, 102, e34420. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, D.A.; Cryan, J.F.; Nolan, Y.M. Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell 2019, 18, e13007. [Google Scholar] [CrossRef]
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the adolescent brain. Neuropsychiatr. Dis. Treat. 2013, 9, 449–461. [Google Scholar] [CrossRef]
- Raikkonen, K.; Gissler, M.; Kajantie, E. Associations Between Maternal Antenatal Corticosteroid Treatment and Mental and Behavioral Disorders in Children. JAMA 2020, 323, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Raikkonen, K.; Gissler, M.; Tapiainen, T.; Kajantie, E. Associations Between Maternal Antenatal Corticosteroid Treatment and Psychological Developmental and Neurosensory Disorders in Children. JAMA Netw. Open 2022, 5, e2228518. [Google Scholar] [CrossRef] [PubMed]
- Ninan, K.; Liyanage, S.K.; Murphy, K.E.; Asztalos, E.V.; McDonald, S.D. Evaluation of Long-term Outcomes Associated with Preterm Exposure to Antenatal Corticosteroids: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022, 176, e220483. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattane, N.; Di Benedetto, M.G.; D’Aprile, I.; Riva, M.A.; Cattaneo, A. Dissecting the Long-Term Effect of Stress Early in Life on FKBP5: The Role of miR-20b-5p and miR-29c-3p. Biomolecules 2024, 14, 371. https://doi.org/10.3390/biom14030371
Cattane N, Di Benedetto MG, D’Aprile I, Riva MA, Cattaneo A. Dissecting the Long-Term Effect of Stress Early in Life on FKBP5: The Role of miR-20b-5p and miR-29c-3p. Biomolecules. 2024; 14(3):371. https://doi.org/10.3390/biom14030371
Chicago/Turabian StyleCattane, Nadia, Maria Grazia Di Benedetto, Ilari D’Aprile, Marco Andrea Riva, and Annamaria Cattaneo. 2024. "Dissecting the Long-Term Effect of Stress Early in Life on FKBP5: The Role of miR-20b-5p and miR-29c-3p" Biomolecules 14, no. 3: 371. https://doi.org/10.3390/biom14030371
APA StyleCattane, N., Di Benedetto, M. G., D’Aprile, I., Riva, M. A., & Cattaneo, A. (2024). Dissecting the Long-Term Effect of Stress Early in Life on FKBP5: The Role of miR-20b-5p and miR-29c-3p. Biomolecules, 14(3), 371. https://doi.org/10.3390/biom14030371






